Abstract
Objectives
To describe clinical characteristics, management and outcome of individuals with coronavirus disease 2019 (COVID-19); and to evaluate risk factors for all-cause in-hospital mortality.
Methods
This retrospective study from a University tertiary care hospital in northern Italy, included hospitalized adult patients with a diagnosis of COVID-19 between 25 February 2020 and 25 March 2020.
Results
Overall, 317 individuals were enrolled. Their median age was 71 years and 67.2% were male (213/317). The most common underlying diseases were hypertension (149/317; 47.0%), cardiovascular disease (63/317; 19.9%) and diabetes (49/317; 15.5%). Common symptoms at the time of COVID-19 diagnosis included fever (285/317; 89.9%), shortness of breath (167/317; 52.7%) and dry cough (156/317; 49.2%). An ‘atypical’ presentation including at least one among mental confusion, diarrhoea or nausea and vomiting was observed in 53/317 patients (16.7%). Hypokalaemia occurred in 25.8% (78/302) and 18.5% (56/303) had acute kidney injury. During hospitalization, 111/317 patients (35.0%) received non-invasive respiratory support, 65/317 (20.5%) were admitted to the intensive care unit (ICU) and 60/317 (18.5%) required invasive mechanical ventilation. All-cause in-hospital mortality, assessed in 275 patients, was 43.6% (120/275). On multivariable analysis, age (per-year increase OR 1.07; 95% CI 1.04–1.10; p < 0.001), cardiovascular disease (OR 2.58; 95% CI 1.07–6.25; p 0.03), and C-reactive protein levels (per-point increase OR 1.009; 95% CI 1.004–1.014; p 0.001) were independent risk factors for all-cause in-hospital mortality.
Conclusions
COVID-19 mainly affected elderly patients with predisposing conditions and caused severe illness, frequently requiring non-invasive respiratory support or ICU admission. Despite supportive care, COVID-19 remains associated with a substantial risk of all-cause in-hospital mortality.
Keywords: Acute respiratory distress syndrome, Coronavirus disease 2019, Interleukin-6, Mortality, Severe acute respiratory syndrome coronavirus 2
Introduction
As the incidence of coronavirus disease 2019 (COVID-19) has increased [1,2], so has the clinical understanding of the disease. However, data on clinical presentation, complications, management and outcome are mainly obtained from relatively small case series [1,[3], [4], [5], [6], [7], [8], [9]], mainly collected from China [1,[3], [4], [5],8,[10], [11], [12], [13], [14], [15], [16], [17]] or including selected groups of critically ill patients [6,7,[18], [19], [20]]. Consequently, they do not necessarily represent the current situation in European hospitals.
The main objective of this study was to describe clinical characteristics, management and outcome of individuals with COVID-19 who were admitted to our hospital in Genoa, northern Italy. We also sought to investigate risk factors associated with all-cause in-hospital mortality.
Materials and methods
With the first reported COVID-19 case in Genoa occurring on 25 February 2020, we created a collaborative study group (GECOVID-19) with the aim of improving the care of COVID-19 patients and conducting research at our hospital. This group prospectively recorded all consecutive patients with COVID-19 admitted to our hospital and collected data according to a pre-established clinical form.
Study design
For the purpose of this study, we established a retrospective cohort including all adults hospitalized with COVID-19 during the period from 25 February to 25 March 2020. The present report was designed after data collection, but the analysis was planned before starting the data analysis.
Setting
This study was conducted in a 1200-bed university-affiliated hospital (San Martino Policlinico Hospital) in Genoa, northern Italy, attending a population of approximately 400 000. During the study period, individuals with COVID-19 were admitted to the hospital if they presented Pao 2 <60 mmHg at rest in ambient air or if the exacerbation of their underlying diseases or severe symptoms were considered unmanageable at home.
Data collection and definitions
Detailed information regarding data collection and definitions used in this study are reported in the Supplementary material (Appendix S1). Briefly, the following data were collected from the patients' medical records at the time of COVID-19 diagnosis (i.e. at the time of the first clinical sample recorded as positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA and before any antiviral or antibiotic treatment): demographics, underlying diseases, and clinical, laboratory and radiological findings. Data on treatments, COVID-19-related complications and outcome were also collected during the course of the disease.
A confirmed case of COVID-19 was defined by a positive result of an RT-PCR assay of a respiratory sample. Cardiovascular disease was defined as a history of coronary artery disease, congestive heart failure, or severe valvular heart disease with or without valve replacement. Non-invasive respiratory support techniques included high-flow nasal cannula, continuous positive airway pressure and non-invasive positive-pressure ventilation.
Data regarding the number of patients who had died, had been discharged and were still hospitalized were recorded as of 19 April 2020. The primary outcome measure was all-cause in-hospital mortality.
Microbiology
Respiratory samples were tested for SARS-CoV-2 using RT-PCR targeted at open reading frame 1ab and nucleocapsid protein genes. A cycle threshold (Ct) value < 37 defined a positive test whereas a Ct value ≥ 40 defined a negative result. Possible co-infection with respiratory viruses was ruled out by means of multiplex RT-PCR on the same respiratory sample (Allplex TM Respiratory Panel Assay, Seoul, South Korea). Bacterial and/or fungal cultures were collected according to physicians' judgement, and microorganisms were identified with matrix-assisted laser desorption/ionization time-of-flight mass spectrometry and were tested for antimicrobial susceptibility with a Vitek 2 automated system (bioMérieux, Marcy l’Étoile, France).
Statistical analysis
No statistical sample size calculation was performed. Data were retrieved from an online database for anonymous and automatic data collection [21].
Quantitative variables were expressed as median and interquartile range (IQR), and qualitative variables as number and percentage. Qualitative variables were compared using the χ2 and Fisher's exact tests, as appropriate. Quantitative variables were compared by Wilcoxon rank sum test. Missing data for each variable were excluded from the denominator. Univariable analysis was used to identify potential predictors of all-cause in-hospital mortality. Potential significant baseline predictors (i.e. variables collected at the time of the first clinical sample positive for SARS-CoV-2 RNA) on univariable comparisons (p < 0.10) were considered for the multivariable logistic regression model. A stepwise backward selection approach was used to select the predictors to include in the final multivariable model. For easier graphical interpretation, age was grouped as follows: 31–40 years, 41–50 years, 51–60 years, 61–70 years, 71–80 years and >80 years. The analyses were performed using SPSS Statistics version 21.0 (IBM Corp., Armonk, NY, USA).
Ethical consideration
The study protocol was approved by the Ethics Committee of Liguria Region (N.CER Liguria 114/2020-ID10420) and the need for written informed consent was waived because of the retrospective nature of the study.
Results
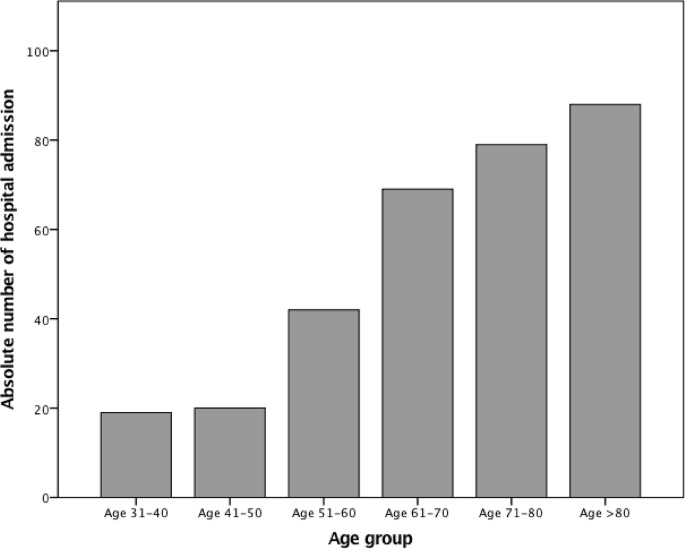
Overall, 317 COVID-19 patients were included in the study. The number of hospital admissions varied substantially according to the age group, with the highest number observed among patients aged >60 years (Fig. 1 ).
Fig. 1.
Number of admissions to hospital according to age group.
The median age was 71 years (IQR 60–82 years) and 213/317 (67.2%) were male. Overall, 65.3% (110/317) of patients had at least one underlying disease (Table 1 ). Hypertension was the most frequent underlying disease (149/317; 47.0%), followed by cardiovascular disease (63/317; 19.9%), diabetes mellitus (49/317; 15.5%) and neurological disease (28/317; 8.8%).
Table 1.
Clinical characteristics of the 317 hospitalized patients at the time of COVID-19 diagnosis
| Characteristicsa | Patients (n = 317 |
|---|---|
| Age, years | 71 (60–82) |
| Sex, male | 213 (67.2) |
| Underlying disease | |
| Hypertension | 149 (47.0) |
| Cardiovascular disease | 63 (19.9) |
| Diabetes mellitus | 49 (15.5) |
| Neurological disease | 28 (8.8) |
| Chronic kidney disease | 22 (6.9) |
| Chronic obstructive lung disease | 18 (5.7) |
| Solid cancer | 12 (3.8) |
| Haematological malignancy | 11 (3.5) |
| Number of underlying diseases | |
| 0 | 110 (34.7) |
| 1 | 95 (30.0) |
| 2 | 62 (19.6) |
| ≥3 | 50 (15.8) |
| Charlson co-morbidity index | 4 (2-5) |
| Time from illness onset to hospital admission, days | 5 (2-8) |
| Signs and symptoms | |
| Fever (Temperature >37.3°C) | 285 (89.9) |
| Shortness of breath | 167 (52.7) |
| Dry cough | 156 (49.2) |
| Asthenia | 57 (18.0) |
| Mental confusion | 29 (9.1) |
| Diarrhoea | 18 (5.7) |
| Myalgia | 18 (5.7) |
| Headache | 14 (4.4) |
| Nausea and vomiting | 14 (4.4) |
| Sputum | 9 (2.8) |
| Physical examination | |
| Tachypnoea | 140 (44.2) |
| Tachycardia | 89 (28.1) |
| Hypotension | 3 (0.9) |
| Laboratory findings | |
| Lymphopenia | 192/281 (68.3) |
| Thrombocytopenia | 114/272 (41.9) |
| ALT >40 U/L | 105/303 (34.7) |
| AST >40 U/L | 102/300 (32.2) |
| Hypokalaemia | 78/302 (25.8) |
| Leukopenia | 53/303 (17.4) |
| Inflammatory markersb | |
| C-reactive protein, mg/L (n = 301) | 79.3 (33.7-132.5), |
| D-dimer, μg/L (n = 278) | 1050 (630.7-1565.7) |
| Interleukin-6, ng/L (n = 252) | 46.7 (20.0-97.9) |
| Hypoxaemic respiratory failure | 199/310 (64.2) |
| Acute kidney injury | 56/303 (18.5) |
| Radiological findings | |
| Pulmonary consolidation | 200/294 (68.0) |
| Bilateral | 124/200 (62.0) |
| Monolateral | 76/200 (38.0) |
| Interstitial pattern | 124/294 (42.2) |
| Absence of lesions | 35/294 (11.9) |
| Pleural effusion | 11/294 (3.7) |
Abbreviations: ARDS, acute respiratory distress syndrome; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Continuous values are shown as median (interquartile range); all other values are n (%).
Normal C-reactive protein levels: 0.0–0.5 mg/L; normal D-dimer levels: 0.0–500.0 μg/L; normal IL-6 levels: 0.0–3.5 ng/L.
The median duration of symptoms before diagnosis was 5 days (IQR 2–8 days). At the time of COVID-19 diagnosis, the most common symptoms included fever (285/317; 89.9%), shortness of breath (167/317; 52.7%) and dry cough (156/317; 49.2%). Overall, 16.7% of the patients (53/317) presented with atypical clinical manifestations consisting of mental confusion, diarrhoea and nausea or vomiting in 9.1% (29/317), 5.7% (18/317) and 4.4% (14/317), respectively (3 out of 317 patients (0.9%) presented with only atypical manifestations at the time of COVID-19 diagnosis).
Lymphopenia was the most common haematological abnormality (198/281; 68.3%) followed by thrombocytopenia (114/272; 41.9%) and elevated serum transaminase levels. Hypokalaemia was also a common finding, observed in 25.8% of the patients (78/302) at the time of diagnosis, as was acute kidney injury (AKI) (56/303; 18.5%).
Of the 294/317 (92.7%) chest radiographs that were performed, 259/294 (88.1%) revealed abnormal results. The most common pattern was pulmonary consolidations in 200/294 (68.0%) patients, of which 124/200 (62.0%) were bilateral. One hundred and twenty-four patients out of 294 had an interstitial pattern (42.2%).
Of the patients with laboratory-confirmed COVID-19, nearly three-quarters (217/317; 68.5%) had urine sent to test for Streptococcus pneumoniae and Legionella pneumophila antigens but all were negative. Eighty-two of the 317 patients (25.8%) had blood cultures performed at the time of COVID-19 diagnosis, but there was no evidence of bacterial co-infection in any patients. Moreover, none of the 34 respiratory tract samples (7 sputum and 27 bronchoalveolar lavage fluid) tested for bacterial, viral or fungal pathogens at the time of diagnosis was positive.
As for treatment, most patients received hydroxychloroquine (225/317; 71.0%) and a combination treatment with darunavir/ritonavir (155/317; 48.9%) or oseltamivir (32/317; 10.1%). One-hundred and twenty-two individuals out of 317 (38.5%) were treated with methylprednisolone and 61/317 (19.2%) received tocilizumab (Table 2 ). Two-hundred and three patients out of 317 (64.0%) were treated with intravenous antimicrobials, most commonly a fifth-generation cephalosporin or a third-generation cephalosporin with either a macrolide or a fluoroquinolone.
Table 2.
Treatments and outcomes of hospitalized patients with COVID-19
| Characteristicsa | Patients (n = 317 |
|---|---|
| Anti-infectious drugs | |
| Hydroxychloroquine | 225 (71.0) |
| Darunavir/ritonavir | 155 (48.9) |
| Oseltamivir | 32 (10.1) |
| Lopinavir-ritonavir | 4 (1.3) |
| Remdesivir | 2 (0.6) |
| Anti-inflammatory drugs | |
| Corticosteroids | 122 (38.5) |
| Tocilizumab | 61 (19.2) |
| Antibioticsb | |
| Fifth-generation cephalosporin | 123 (38.8) |
| Third-generation cephalosporin | 46 (14.5) |
| Macrolides | 26 (8.2) |
| β-lactams/β-lactamase inhibitor | 24 (7.6) |
| Fluoroquinolones | 9 (2.8) |
| Others | 7 (2.2) |
| Complications during the hospitalization | |
| ARDS development | 116 (36.6) |
| Non-invasive respiratory support | 111 (35.0) |
| ICU admission | 65 (20.5) |
| Invasive mechanical ventilation | 60 (18.9) |
| Septic shock | 15 (4.7) |
| CRRT | 9 (2.8) |
| ICU length of stay, days (n = 46) | 12.0 (6.5–21.5) |
| Hospital length of stay, days, (n = 275) | 12.0 (5.0–19.0) |
| All-cause in hospital mortality | 120/275 (43.6) |
Abbreviations: ARDS, acute distress respiratory syndrome; CRRT, continuous renal replacement therapy; ICU intensive care unit.
Continuous values are shown as median (interquartile range); all other values are n (%).
Overall, 203/317 (64.0%) received antibiotics (32 combination therapy). Others include: two oxazolidinones; one nitroimidazole and one glycopeptide.
The majority of patients received oxygen therapy and 111 out of 317 (35.0%) required non-invasive respiratory support. Among them, 47.8% (53/111) subsequently required invasive mechanical ventilation (Table 2).
Overall, 20.5% (65/317) of patients needed intensive care and 18.9% (60/317) underwent invasive mechanical ventilation for a median of 9 days (IQR 4.5–16.5 days). None received extracorporeal membrane oxygenation. During hospitalization, 9/317 (2.8%) patients developed AKI requiring continuous renal replacement therapy.
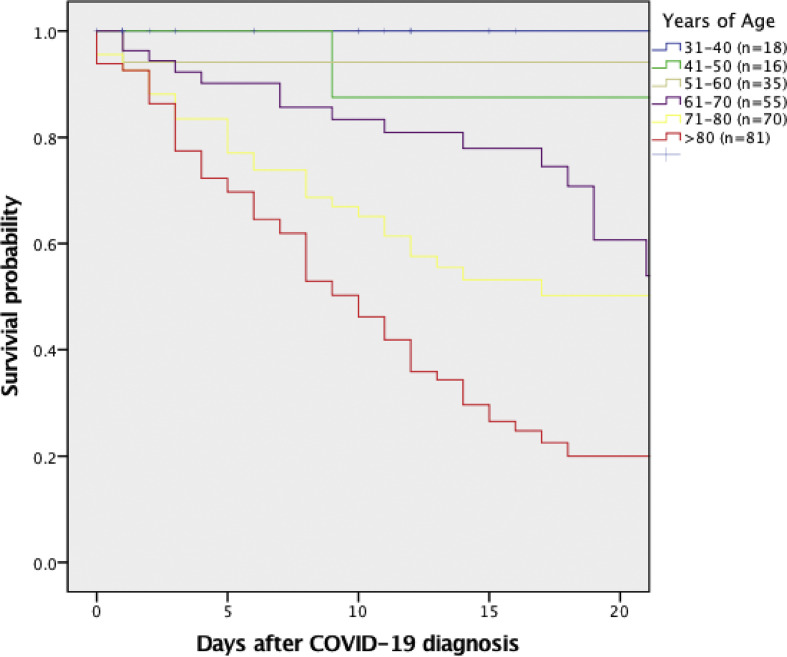
On 19 April 2020, a total of 275 (86.7%) patients were no longer hospitalized: 120/275 (43.6%) had died in the hospital and 155/275 (56.4%) had been discharged alive. Comparison between survivors and non-survivors is shown in Table 3 . On multivariable analysis, only age (per-year increase odds ratio OR 1.07; 95% CI 1.04–1.10; p < 0.001), cardiovascular disease (OR 2.58; 95% CI 1.07–6.25; p 0.03), and C-reactive protein (CRP) levels (per-point increase OR 1.009; 95% CI 1.004–1.014; p 0.001) retained an independent association with all-cause in-hospital mortality (Table 4 ). An additional multivariate model excluding CRP and IL-6 (i.e. those variables with the highest number of missing values) suggested that AKI at the time of COVID-19 diagnosis was a possible additional, independent predictor of increased in-hospital mortality (OR 3.31; 95% CI 1.53–7.16; p 0.002; complete model results are available in the Supplementary material, Table S1). Survival curves according to age groups for the entire study population and for those patients with no previous underlying disease are shown in Fig. 2 and see Supplementary material, Fig. S1, respectively.
Table 3.
Comparison of baseline clinical characteristics and laboratory findings of patients who had been discharged (n = 155) or had died (n = 120) as of 19 April 2020
| Characteristicsa | In-hospital survivors (n = 155) | In-hospital non-survivors (n = 120) | p |
|---|---|---|---|
| Age, years | 65 (52–76) | 81 (72–87) | <0.001 |
| Sex, male | 98 (63.2) | 85 (70.8) | 0.20 |
| Underlying disease | |||
| Hypertension | 57 (36.8) | 75 (62.5) | <0.001 |
| Diabetes mellitus | 16 (10.3) | 26 (21.7) | 0.01 |
| Cardiovascular disease | 16 (10.3) | 39 (32.5) | <0.001 |
| Chronic kidney disease | 5 (3.2) | 14 (11.7) | 0.01 |
| Neurological disease | 6 (3.9) | 20 (16.7) | <0.001 |
| Chronic obstructive lung disease | 4 (2.6) | 11 (9.2) | 0.03 |
| Solid cancer | 3 (1.9) | 7 (5.8) | 0.11 |
| Haematological malignancy | 3 (1.9) | 6 (5.0) | 0.19 |
| Number of underlying diseases | |||
| 0 | 80 (51.6) | 18 (15.0) | <0.001 |
| 1 | 44 (28.4) | 34 (28.3) | 1 |
| 2 | 20 (12.9) | 31 (25.8) | 0.08 |
| ≥3 | 11 (7.1) | 37 (30.8) | <0.001 |
| Charlson co-morbidity index | 2.0 (1.0–4.0) | 5.0 (4.0–6.0) | <0.001 |
| Time from illness onset to diagnosis, days | 6.0 (3.0–8.0) | 3.0 (2.0–7.0) | 0.001 |
| Signs and symptoms | |||
| Fever (temperature >37.3°C) | 146 (94.2) | 104 (86.7) | 0.04 |
| Dry cough | 86 (55.5) | 50 (41.7) | 0.03 |
| Shortness of breath | 67 (43.2) | 79 (65.8) | <0.001 |
| Asthenia | 34 (21.9) | 16 (13.3) | 0.08 |
| Myalgia | 15 (9.7) | 3 (2.5) | 0.03 |
| Headache | 12 (7.7) | 2 (1.7) | 0.03 |
| Diarrhoea | 8 (5.2) | 6 (5.0) | 1.00 |
| Nausea and vomiting | 8 (5.2) | 3 (2.5) | 0.36 |
| Sputum | 7 (4.5) | 1 (0.8) | 0.14 |
| Mental confusion | 3 (1.9) | 22 (18.3) | <0.001 |
| Physical examination | |||
| Tachypnoea | 55 (32.9) | 71 (59.2) | 0.01 |
| Tachycardia | 36 (23.2) | 37 (30.8) | 0.14 |
| Hypotension | 1 (0.6) | 2 (1.7) | 0.59 |
| Laboratory findings | |||
| Lymphopenia | 84/137 (61.3) | 81/104 (77.9) | 0.008 |
| Hypoxaemic respiratory failure | 72/152 (47.4) | 104/116 (89.7) | <0.001 |
| ALT >40 U/L | 49/145 (33.8) | 38/114 (33.3) | 1.00 |
| AST >40 U/L | 45/131 (34.4) | 54/103 (52.4) | 0.01 |
| Leukopenia | 38/145 (26.2) | 25/116 (21.6) | 0.47 |
| Hypokalaemia | 33/146 (22.6) | 56/116 (48.3) | <0.001 |
| Thrombocytopenia | 23/146 (15.8) | 19/116 (16.4) | 1.00 |
| Inflammatory markers | |||
| C-reactive protein, mg/L (n = 260) | 57.2 (20.0– 111.5) | 109.0 (73.1–169.0) | 0.001 |
| D-dimer, μg/L (n = 237) | 867.0 (480.6–1330.5) | 1317.0 (935.9–2284.0) | 0.22 |
| Interleukin-6, ng/L (n = 216) | 27.4 (15.0–54.3 | 74.0 (43.8–142.0) | 0.02 |
| Acute kidney injury | 12/146 (8.2) | 38/116 (32.8) | 0.001 |
| Treatments | |||
| Hydroxychloroquine | 109 (70.3) | 78 (65.0) | 0.36 |
| Antibiotics | 86 (55.5) | 81 (67.5) | 0.05 |
| Darunavir/ritonavir | 80 (51.6) | 52 (43.3) | 0.18 |
| Corticosteroids | 54 (34.8) | 40 (33.3) | 0.90 |
| Tocilizumab | 33 (21.3) | 17 (14.2) | 0.16 |
| Oseltamivir | 20 (12.9) | 9 (7.5) | 0.17 |
| Complications during hospitalization | |||
| Non-invasive respiratory support | 47 (30.3) | 39 (32.5) | 0.79 |
| ARDS development | 31 (20.0) | 63 (52.5) | <0.001 |
| ICU admission | 16 (10.3) | 30 (25.0) | <0.001 |
| Invasive mechanical ventilation | 14 (9.0) | 28 (23.3) | <0.001 |
| Septic shock | 2 (1.3) | 8 (6.7) | 0.02 |
| CRRT | 1 (0.6) | 2 (1.7) | 0.58 |
| ICU length of stay (n = 46) | 14.0 (9.3–20.0) | 8.5 (4.5–17.0) | 0.05 |
| Hospital length of stay (n = 275) | 13.0 (6.0–19.3) | 10.0 (4.0–17.0) | 0.04 |
Abbreviations: ARDS, acute respiratory distress syndrome; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRRT, continuous renal replacement therapy; ICU, intensive care unit.
Continuous values are shown as median (interquartile range); all other values are n (%).
Table 4.
Multivariable analysis for risk factors associated with all-cause in-hospital mortality
| Characteristics | OR | 95% CI | p |
|---|---|---|---|
| Cardiovascular disease | 2.58 | 1.07–6.25 | 0.03 |
| Acute kidney injury | 2.32 | 0.87–6.23 | 0.09 |
| Age (per-year increase) | 1.07 | 1.04–1.10 | <0.001 |
| CRP, mg/L (per-point increase) | 1.009 | 1.004–1.014 | 0.001 |
| IL-6, ng/L (per-point increase) | 1.002 | 1.000–1.004 | 0.07 |
Overall, 211 out of 275 patients (76.3%) were included in the multivariate model because C-reactive protein (CRP) and interleukin-6 (IL-6) values were missing in 15/275 (5%) and 59/275 (21%) individuals, respectively.
Fig. 2.
All-cause hospital mortality of individuals with COVID-19 according to age. Median days (interquartile range) after COVID-19 diagnosis in each age group were as follows: 10.5 (1.5–17.8), 8 (2.0–18.7), 11.0 (5.0–16.0), 13.5 (5.8–19-0), 11.0 (4.5–18.5) and 8.0 (3.0–14.0) days for patients aged 31–40, 41–50, 51–60, 61–70, 71–80 and > 80 years, respectively.
Discussion
The findings of the present cohort study conducted in a teaching hospital in northern Italy can be summarized as follows: (a) COVID-19 is mainly a disease of the elderly, with multiple underlying conditions and frequent atypical presentations; (b) overall in-hospital mortality is particularly high (120/275; 43.6%) and complications are common; and (c) age, cardiovascular disease and increased CRP were associated with all-cause in-hospital mortality in our study.
In our series, we observed an uneven age distribution among hospitalized COVID-19 patients, with more than 50% being aged ≥70 years. This percentage is significantly higher than that observed in China [1,3,4,8,[10], [11], [12], [13], [14], [15], [16], [17]]. Indeed, the pooled mean age of patients hospitalized with COVID-19 in 14 previous Chinese studies that included unselected patients was only 50 years [1,3,4,8,[10], [11], [12], [13], [14], [15], [16], [17]]. Reasons for the predominance of old patients in our report are not clear, although they may be attributable to the ageing Italian population [22], or the fact that, compared with young patients, older ones have an increasing risk of chronic co-morbidities that predispose them to more severe form of COVID-19 [4,6].
The clinical manifestations most commonly observed in our study included fever, shortness of breath and dry cough. Fever and shortness of breath were also common findings in case series of COVID-19 patients from China and the USA [1,3,4,8,[10], [11], [12], [13], [14], [15], [16], [17],23,24], and these findings have been considered as prognostic indicators for adult respiratory distress syndrome and higher mortality [18]. Surprisingly, our data show that 16.7% of patients (53/317) with COVID-19 had ‘atypical’ clinical manifestations such as mental confusion, diarrhoea or nausea and vomiting. Although these atypical manifestations could potentially lead to a delay in diagnosis [25] with possible uncontrolled transmission of the infection, it should be addressed that in our cohort there were only 3/317 patients (0.9%) who presented with only atypical manifestations. Accordingly, even in our outbreak setting, the level of COVID-19 suspicion should be low in the absence of fever, cough or shortness of breath.
With regards to laboratory findings, we observed a high proportion of patients with AKI at the time of COVID-19 diagnosis. This finding was only occasionally reported in previous series [6,12]; we believe that the likely contributing factors include dehydration due to diarrhoea and poor oral feeding and side effects of symptomatic drugs (i.e. non-steroidal anti-inflammatory drugs). Whether coronavirus has a pathogenic effect on kidneys warrants further investigation [26]. Contrary to what was expected, the rate of hypokalaemia in our population was also high, reaching 25.8% (78/302). This is consistent with that previously reported for the SARS-CoV outbreak in 2003 in Singapore [27]. Although the mechanism of hypokalaemia is still unknown, a role of diarrhoea or vomiting might be postulated. Of note, hypokalaemia could predispose patients to develop cardiac arrhythmias, which previous authors observed in about 7%–16% of individuals with COVID-19 [5,24].
In our cohort, the number of antibiotics prescribed to COVID-19 patients was particularly high, suggesting substantial inappropriate prescription of antibiotics. This finding might reflect the lack of scientific evidence on how to properly manage patients with COVID-19 in the early stages of the pandemic. Moreover, the high number of patients presenting with radiological images suggestive for pulmonary consolidation (200/294; 68.0%), could have increased the suspicion of bacterial co-infection. However, in our study, which was based on routine clinical practice, we were not able to document any bacterial co-infection. On the basis of these results, we think improving the differential diagnosis between SARS-CoV-2 and bacterial respiratory pathogens should become a critical research priority, to reduce, in line with antimicrobial stewardship principles, the rates of unnecessary antibiotic prescriptions in individuals with COVID-19.
With the exception of remdesivir [28], no other specific antiviral therapy has been found to provide benefit for COVID-19 to date, and treatment mainly consists of supportive care [5,19,29,30]. In the present study, 71.0% (225/317) and 48.9% (155/317) of the patients received hydroxychloroquine and darunavir/ritonavir, respectively. Although no effective outcomes were observed for hydroxychloroquine [31] and protease inhibitors [32] in two recent studies, future randomized studies should be performed to clarify the impact of antiviral drugs on the natural history of the disease.
Despite aggressive supportive treatment of respiratory and renal complications, we observed a striking all-cause in-hospital mortality of 43.6% (120/275), which is greater than previously reported rates from Chinese and US patients [1,3,4,8,[10], [11], [12], [13], [14], [15], [16], [17],23,24]. Our main hypothesis was that the high mortality associated with COVID-19 probably reflects the old age of our patients and the severity of underlying diseases. However, increased CRP levels were also associated with death. High levels of CRP have also been associated with the severity of the disease [18], possibly suggesting the involvement of cytokine storm in the clinical outcome of the patients [18,33,34]. The implication of the host immune response in COVID-19 suggests a potential role of anti-inflammatory drugs as adjunctive therapy. However, the role of corticosteroids or tocilizumab—a recombinant humanized monoclonal antibody inhibiting membrane-bound and soluble interleukin-6 receptors [35]—remains controversial [29], even if case reports [36,37] and case series [38] have reported benefits. Although we cannot advocate the universal use of corticosteroids and tocilizumab in individuals with COVID-19, follow-up studies evaluating the role of anti-inflammatory drugs are warranted [30]. Finally, the independent association we found between AKI and increased mortality in the additional multivariable model may reflect the unfavourable prognostic effect of organ dysfunction and/or severe disease presentation.
Our study has several limitations. First, it is a retrospective analysis and we did not examine all aspects of care that potentially could influence the outcome. For example, data are lacking about some important clinical characteristics that have been found associated with COVID-19 severity, such as weight, body mass index and smoking status. Second, this study was performed at a single institution of northern Italy and the results may not be representative of other Italian or European centres. Third, some data were missing for certain patients included in this study. Therefore, studies with the inclusion of more patients would be needed to increase the statistical power and lend support to inflammatory markers (e.g. CRP, interleukin-6) as risk of in-hospital death. Finally, among our study population, 42/317 were still hospitalized at the time of writing this report. Therefore, all-cause in-hospital mortality could be underestimated.
In conclusion, in our cohort, COVID-19 mainly affected elderly individuals with predisposing conditions and caused severe illness that frequently required non-invasive respiratory support or admission to intensive care. Despite supportive care, COVID-19 remains associated with a substantial risk of all-cause in-hospital mortality.
Transparency declaration
M. Bassetti serves on scientific advisory boards for Angelini, AstraZeneca, Bayer, Cubist, Pfizer, Menarini, MSD, Nabriva, Paratek, Roche, Shionogi, Tetraphase, The Medicine Company and Astellas Pharma Inc. and has received funding for travel or speaker honoraria from Algorithm, Angelini, Astellas Pharma Inc., AstraZeneca, Cubist, Pfizer, MSD, Gilead Sciences, Menarini, Novartis, Ranbaxy, and Teva; D.R. Giacobbe reports honoraria from Stepstone Pharma GmbH and unconditional grants from MSD Italia and Correvio Italia. All other authors declare no competing interests.
Funding
This research received no external funding.
Authors' contributions
Conceptualization was by A. Vena, D.R. Giacobbe, A.Di Biagio, M. Mikulska, L. Taramasso, A. De Maria, L. Ball, I. Brunetti, M. Loconte, N. A. Patroniti, C.Robba, E. Delfino, C. Dentone and L. Magnasco; methodology was by A. Vena, D.R. Giacobbe, M. Bavastro, M. Cerchiaro, P. Pelosi and M. Bassetti; the software programming was by M. Giacomini and S. Mora. A.Di Biagio, M. Mikulska, L. Taramasso, A. De Maria, L. Ball, I. Brunetti, M. Loconte, N. A. Patroniti, C.Robba, E. Delfino, C. Dentone, L. Magnasco, P. Pelosi, M. Bassetti, L. Nicolini, F.Toscanini, E. Barisione, F. Baldi, E. Balletto, M. Berruti, F. Briano, C. Sepulcri, S. Dettori, L. Labate, M. Mirabella, F. Portunato, R. Pincino, C. Russo and S. Tutino were responsible for validation and the formal analysis was by A. Vena, D.R. Giacobbe, M. Bavastro, M. Cerchiaro, M. Giacomini and S. Mora. A. Vena, D.R. Giacobbe, M. Bavastro, M. Cerchiaro, M. Giacomini and S. Mora contributed to the investigation and data curation was by A. Vena, D.R. Giacobbe, A.Di Biagio, M. Mikulska, L. Taramasso, A. De Maria, L. Ball, I. Brunetti, M. Loconte, N. A. Patroniti, C.Robba, E. Delfino, C. Dentone, L. Magnasco, L. Nicolini, F.Toscanini, E. Barisione, F. Baldi, E. Balletto, M. Berruti, F. Briano, C. Sepulcri, S. Dettori, L. Labate, M. Mirabella, F. Portunato, R. Pincino, C. Russo, S. Tutino. The original draft was written by A. Vena and D.R. Giacobbe, and review and editing were by A. Vena, D.R. Giacobbe, P. Pelosi, M. Bassetti A., Di Biagio, M. Mikulska, L. Taramasso, A. De Maria, L. Ball, I. Brunetti, M. Loconte, N. A. Patroniti, C.Robba, E. Delfino, C. Dentone and L. Magnasco. P. Pelosi and M. Bassetti supervised the study.
Acknowledgements
The authors thank Alessio Signori for statistical analysis.
Editor: M. Paul
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2020.07.049.
Contributor Information
GECOVID study group:
Anna Alessandrini, Marco Camera, Emanuele Delfino, Andrea De Maria, Chiara Dentone, Antonio Di Biagio, Ferdinando Dodi, Antonio Ferrazin, Giovanni Mazzarello, Malgorzata Mikulska, Laura Nicolini, Federica Toscanini, Daniele R. Giacobbe, Antonio Vena, Lucia Taramasso, Elisa Balletto, Federica Portunato, Eva Schenone, Nirmala Rosseti, Federico Baldi, Marco Berruti, Federica Briano, Silvia Dettori, Laura Labate, Laura Magnasco, Michele Mirabella, Rachele Pincino, Chiara russo, Giovanni Sarteschi, Chiara sepulcri, Stefania Tutino, Roberto Pontremoli, Valentina Beccati, Salvatore Casciaro, Massimo Casu, Francesco Gavaudan, Maria Ghinatti, Elisa Gualco, Giovanna Leoncini, Paola pitto, Kassem salam, Angelo Gratarola, Mattia Bixio, Annalisa Amelia, Andrea Balestra, Paola Ballarino, Nicholas Bardi, Roberto Boccafogli, Francesca Caserza, Elisa Calzolari, Marta Castelli, Elisabetta Cenni, Paolo Cortese, Giuseppe Cuttone, Sara Feltrin, Stefano Giovinazzo, Patrizia Giuntini, Letizia Natale, Davide Orsi, Matteo Pastorino, Tommaso Perazzo, Fabio Pescetelli, Federico Schenone, Maria G. Serra, Marco Sottano, Roberto Tallone, Massimo Amelotti, Marie J. Majabò, Massimo Merlini, Federica Perazzo, Nidal Ahamd, Paolo Barbera, Marta Bovio, Paola Campodonico, Andrea Collidà, Ombretta Cutuli, Agnese Lomeo, Francesca Fezza, Nicola Gentilucci, Nadia Hussein, Emanuele Malvezzi, Laura Massobrio, Giula Motta, Laura Pastorino, Nicoletta Pollicardo, Stefano Sartini, Paola Vacca, Valentina Virga, Italo Porto, Giampaolo Bezante, Roberta Della Bona, Giovanni La Malfa, Alberto Valbusa, Vered G. Ad, Emanuela Barisione, Michele Bellotti, Aloe’ Teresita, Alessandro Blanco, Marco Grosso, Maria Grazia Piroddi, Paolo Moscatelli, Paola Ballarino, Matteo Caiti, Elisabetta Cenni, Patrizia Giuntini, Ottavia Magnani, Samir Sukkar, Ludovica Cogorno, Raffaella Gradaschi, Erica Guiddo, Eleonora Martino, Livia Pisciotta, Bruno Cavagliere, Rossi Cristina, Farina Francesca, Giacomo Garibotto, Pasquale Esposito, Carmen Bellezza, Emirjona Harusha, Francesca Rossi, Eleonora Arboscello, Laura Arzani, Laura De Mattei, Marzia Spadaro, Giovanni Passalacqua, Diego Bagnasco, Fulvio Braido, Annamaria Riccio, Elena Tagliabue, Claudio Gustavino, Antonella Ferraiolo, Fiammetta Monacelli, Mona Mahmoud, Luca Tagliafico, Armando Napolitano, Maria Fiorio, Monica Pizzonia, Chiara Giannotti, Alessio Nencioni, Salvatore Giuffrida, Nicola Rosso, Alessandra Morando, Riccardo Papalia, Donata Passerini, Gabriella Tiberio, Giovanni Orengo, Alberto Battaglini, Silvano Ruffoni, and Sergio Caglieris
Appendix. GECOVID-19 Study group
Anna Alessandrini, Marco Camera, Emanuele Delfino, Andrea De Maria, Chiara Dentone, Antonio Di Biagio, Ferdinando Dodi, Antonio Ferrazin, Giovanni Mazzarello, Malgorzata Mikulska, Laura Nicolini, Federica Toscanini, Daniele Roberto Giacobbe, Antonio Vena, Lucia Taramasso, Elisa Balletto, Federica Portunato, Eva Schenone, Nirmala Rosseti, Federico Baldi, Marco Berruti, Federica Briano, Silvia Dettori, Laura Labate, Laura Magnasco, Michele Mirabella, Rachele Pincino, Chiara russo, Giovanni Sarteschi, Chiara sepulcri, Stefania Tutino (Clinica di Malattie Infettive); Roberto Pontremoli, Valentina Beccati, Salvatore Casciaro, Massimo Casu, Francesco Gavaudan, Maria Ghinatti, Elisa Gualco, Giovanna Leoncini, Paola pitto, Kassem salam (Clinica di Medicina interna 2); Angelo Gratarola, Mattia Bixio, Annalisa Amelia, Andrea Balestra, Paola Ballarino, Nicholas Bardi, Roberto Boccafogli, Francesca Caserza, Elisa Calzolari, Marta Castelli, Elisabetta Cenni, Paolo Cortese, Giuseppe Cuttone, Sara Feltrin, Stefano Giovinazzo, Patrizia Giuntini, Letizia Natale, Davide Orsi, Matteo Pastorino, Tommaso Perazzo, Fabio Pescetelli, Federico Schenone, Maria Grazia Serra, Marco Sottano (Anestesia e Rianimazione, Emergenza Covid padiglione 64 “Fagiolone”); Roberto Tallone, Massimo Amelotti, Marie Jeanne Majabò, Massimo Merlini, Federica Perazzo (Cure intermedie); Nidal Ahamd, Paolo Barbera, Marta Bovio, Paola Campodonico, Andrea Collidà, Ombretta Cutuli, Agnese Lomeo, Francesca Fezza, Nicola Gentilucci, Nadia Hussein, Emanuele Malvezzi, Laura Massobrio, Giula Motta, Laura Pastorino, Nicoletta Pollicardo, Stefano Sartini, Paola Vacca, Valentina Virga (Dipartimento di Emergenza ed accettazione); Italo Porto, Giampaolo Bezante, Roberta Della Bona, Giovanni La Malfa, Alberto Valbusa, Vered Gil Ad (Clinica Malattie Cardiovascolari); Emanuela Barisione, Michele Bellotti, Aloe’ Teresita, Alessandro Blanco, Marco Grosso, Maria Grazia Piroddi, Maria Grazia Piroddi (Pneumologia ad Indirizzo Interventistico); Paolo Moscatelli, Paola Ballarino, Matteo Caiti, Elisabetta Cenni, Patrizia Giuntini, Ottavia Magnani (Medicine d’Urgenza); Samir Sukkar, Ludovica Cogorno, Raffaella Gradaschi, Erica Guiddo, Eleonora Martino, Livia Pisciotta (Dietetica e nutrizione clinica); Bruno Cavagliere, Rossi Cristina, Farina Francesca (Direzione delle Professioni sanitarie); Giacomo Garibotto, Pasquale Esposito (clinica nefrologica, dialisi e trapianto); Carmen Bellezza, Emirjona Harusha, Francesca Rossi, Eleonora Arboscello, Laura Arzani, Laura De Mattei: Marzia Spadaro (Area medica critica, Pronto Soccorso); Giovanni Passalacqua, Diego Bagnasco, Fulvio Braido, Annamaria Riccio, Elena Tagliabue (Clinica Malattie Respiratorie ed Allergologia); Claudio Gustavino, Antonella Ferraiolo (Ostetricia e Ginecologia); Fiammetta Monacelli, Mona Mahmoud, Luca Tagliafico, Armando Napolitano, Maria Fiorio, Monica Pizzonia, Chiara Giannotti, Alessio Nencioni (Geriatria); Salvatore Giuffrida, Nicola Rosso (Direzione Amministrativa); Alessandra Morando, Riccardo Papalia, Donata Passerini, Gabriella Tiberio (Direzione di presidio); Giovanni Orengo, Alberto Battaglini (Gestione del rischio clinico); Silvano Ruffoni, Sergio Caglieris.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bassetti M., Vena A., Giacobbe D.R. The novel Chinese coronavirus (2019-nCoV) infections: challenges for fighting the storm. Eur J Clin Invest. 2020;50 doi: 10.1111/eci.13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao J., Tu W.J., Cheng W., Yu L., Liu Y.K., Hu X., et al. Clinical features and short-term outcomes of 102 patients with corona virus disease 2019 in Wuhan, China. Clin Infect Dis. 2020;71:748–755. doi: 10.1093/cid/ciaa243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatraju P.K., Ghassemieh B.J., Nichols M., Kim R., Jerome K.R., Nalla A.K., et al. Covid-19 in critically ill patients in the seattle Region—case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang R., Pan M., Zhang X., Fan X., Han M., Zhao F., et al. Epidemiological and clinical features of 125 hospitalized patients with COVID-19 in Fuyang, Anhui, China. Int J Infect Dis. 2020;95:421–428. doi: 10.1016/j.ijid.2020.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Godaert L., Proye E., Demoustier-Tampere D., Coulibaly P.S., Hequet F. Drame M Clinical characteristics of older patients: the experience of a geriatric short-stay unit dedicated to patients with COVID-19 in France. J Infect. 2020;81:e93–e94. doi: 10.1016/j.jinf.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X., Fang J., Zhu Y., Chen L., Ding F., Zhou R., et al. Clinical characteristics of non-critically ill patients with novel coronavirus infection (COVID-19) in a Fangcang Hospital. Clin Microbiol Infect. 2020;26:1063–1068. doi: 10.1016/j.cmi.2020.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L., He W., Yu X., Hu D., Bao M., Liu H., et al. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect. 2020;80:639–645. doi: 10.1016/j.jinf.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan W.J., Ni Z.Y., Hu Y., Liang W., Ou C., He J., et al. Clinical characteristics of Covid-19 in China. Reply. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMc2005203. [DOI] [PubMed] [Google Scholar]
- 14.Liang W.H., Guan W.J., Li C.C., Li Y.M., Liang H.R., Zhao Y., et al. Clinical characteristics and outcomes of hospitalised patients with COVID-19 treated in Hubei (epicenter) and outside Hubei (non-epicenter): a Nationwide Analysis of China. Eur Respir J. 2020 doi: 10.1183/13993003.00562-2020. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng Y., Ling Y., Bai T., Xie Y., Huang J., Li J., et al. COVID-19 with different severity: a multi-center study of clinical features. Am J Respir Crit Care Med. 2020;201:1380–1388. doi: 10.1164/rccm.202002-0445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y., Du X., Chen J., Jin Y., Peng L., Wang H.H.X., et al. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J Infect. 2020;81:e6–e12. doi: 10.1016/j.jinf.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang G., Hu C., Luo L., Fang F., Chen Y., Li J., et al. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J Clin Virol. 2020;127:104364. doi: 10.1016/j.jcv.2020.104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:1–11. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., et al. Network, baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region. Italy JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y., Lu X., Chen H., Chen T., Su N., Huang F., et al. Clinical course and outcomes of 344 intensive care patients with COVID-19. Am J Respir Crit Care Med. 2020;201:1430–1434. doi: 10.1164/rccm.202003-0736LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giannini B., Riccardi N., Cenderello G., Di Biagio A., Dentone C. Giacomini M from Liguria HIV Web to Liguria Infectious Diseases Network: how a digital platform improved doctors' work and patients' care. AIDS Res Hum Retroviruses. 2018;34:239–240. doi: 10.1089/aid.2017.0064. [DOI] [PubMed] [Google Scholar]
- 22.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020 doi: 10.1001/jama.2020.4683. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 23.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goyal P., Choi J.J., Pinheiro L.C., Schenck E.J., Chen R., Jabri A., et al. Clinical characteristics of Covid-19 in New York City. N Engl J Med. 2020;382:2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janssens J.P., Krause K.H. Pneumonia in the very old. Lancet Infect Dis. 2004;4:112–124. doi: 10.1016/S1473-3099(04)00931-4. [DOI] [PubMed] [Google Scholar]
- 26.Fanelli V., Fiorentino M., Cantaluppi V., Gesualdo L., Stallone G., Ronco C., et al. Acute kidney injury in SARS-CoV-2 infected patients. Crit Care. 2020;24:155. doi: 10.1186/s13054-020-02872-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leong H.N., Chan K.P., Oon L.L., Koay E., Ng L.C., Lee M.A., et al. Clinical and laboratory findings of SARS in Singapore. Ann Acad Med Singapore. 2006;35:332–339. [PubMed] [Google Scholar]
- 28.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., et al. Remdesivir for the treatment of Covid-19 – preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMc2022236. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 29.Alhazzani W., Moller M.H., Arabi Y.M., Loeb M., Gong M.N., Fan E., et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Crit Care Med. 2020 doi: 10.1097/CCM.0000000000004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bassetti M., Giaccobe D.R., Aliberti S., Barisione E., Centanni S., De Rosa F.G., et al. Balancing evidence and frontline experience in the early phases of the COVID-19 pandemic: current position of the Italian Society of Anti-Infective Therapy (SITA) and the Italian Society of Pulmonology (SIP) Clin Microbiol Infect. 2020;26:880–894. doi: 10.1016/j.cmi.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Magagnoli J., Narendran S., Pereira F., Cummings T., Harnd J.W., Sutton S.S., et al. 2020. Outcomes of hydroxycloroquine usage in United States veterans hospitalized with COVID-19. Med NY. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., et al. UK Hlh across Speciality Collaboration. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brunner H.I., Ruperto N., Zuber Z., Keane C., Harari O., Kenwright A., et al. Paediatric Rheumatology International Trials Organisation, G. Pediatric Rheumatology Collaborative Study, Efficacy and safety of tocilizumab in patients with polyarticular-course juvenile idiopathic arthritis: results from a phase 3, randomised, double-blind withdrawal trial. Ann Rheum Dis. 2015;74:1110–1117. doi: 10.1136/annrheumdis-2014-205351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang X., Song K., Tong F., Fei M., Guo H., Lu Z., et al. First case of COVID-19 in a patient with multiple myeloma successfully treated with tocilizumab. Blood Adv. 2020;4:1307–1310. doi: 10.1182/bloodadvances.2020001907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mihai C., Dobrota R., Schroder M., Garaiman A., Jordan S., Becker M.O., et al. COVID-19 in a patient with systemic sclerosis treated with tocilizumab for SSc-ILD. Ann Rheum Dis. 2020;79:668–669. doi: 10.1136/annrheumdis-2020-217442. [DOI] [PubMed] [Google Scholar]
- 38.Xu X., Han M., Li T., Sun W., Wang D., Fu B., et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020;117:10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.