Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2) can cause various mild to severe neurologic symptoms, leading to significant morbidity and mortality. We hereby present a fatal case of a 50-year-old male health care provider, admitted due to altered mental status due to encephalopathy, cerebral edema, and fulminant cerebral vasoconstriction caused by SARS-Cov-2. Our case highlights the importance of considering SARS-Cov-2 infection in the differential diagnosis for patients with unexplained central nervous system dysfunction and cerebral edema to prevent delayed diagnosis and render rapid treatment.
Keywords: COVID19, SARS-Cov-2, Encephalopathy, Pandemic, Vasoconstriction
Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2), was first identified in December 2019 in Wuhan, China. On March 11, 2020 it was declared a pandemic by the World Health Organization [1]. As of early August 2020, there are more than 17.5 million cases with over 680,000 confirmed deaths, affecting 188 countries [2].
SARS-Cov-2 is thought to spread among humans primarily through respiratory droplets. A large percentage of the patients remain completely asymptomatic or show minimal symptoms. However, some of the patients become severely ill, leading to significant morbidity and mortality. Typical symptoms include fever, shortness of breath, cough, and fatigue. Less common presentations include headaches, alerted mental status, syncope, chest pain, abdominal pain, diarrhea, nausea, and vomiting [3,4]. We report a presumptive case of COVID-19 associated encephalopathy with diffuse cerebral vasoconstriction which resulted in rapid patient decline and ultimate demise.
Case presentation
A 50-year-old male healthcare provider with high exposure to COVID-19 patients presented to the emergency room with altered mental status, following 1-2-day history of fatigue, severe headache, nausea, vomiting, and worsening lethargy. Past medical and surgical histories were unremarkable.
On initial physical examination, the patient did not follow commands or respond to painful stimuli. Pupils were in fixed mydriasis with deviation towards the left. Vital signs were within normal limits.
Given the neurological symptoms, patient was immediately intubated for airway protection. Initial chest x-ray and brain computed tomography without intravenous (IV) contrast was normal (Fig. 1). Urine and blood toxicology tests were negative for cocaine, opioids, marijuana, phencyclidine, ethanol, methanol, salicylate, and barbiturates. An influenza test was negative. COVID-19 infection was subsequently confirmed using Real-Time Reverse Transcriptase-Polymerase Chain Reaction assay to detect SARS-CoV-2 viral nucleic acid in a nasopharyngeal swab specimen. C-reactive protein was elevated at admission measuring 355 mg/L (reference range: 0-10 mg/L). Lumbar puncture and cerebral spinal fluid analysis were not performed due to the patient's rapidly declining neurological function, abnormal pupillary findings on physical examination, and increasing risk for herniation.
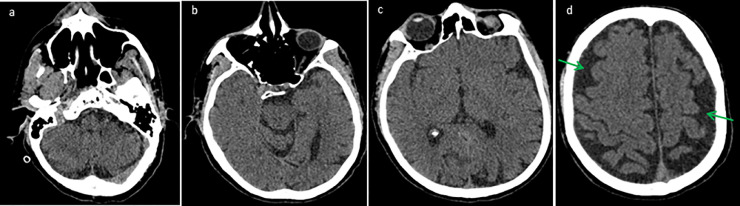
Fig. 1.
Axial brain CT without IV contrast (a, b, c, d) obtained at initial presentation is remarkable only for mild cerebral generalized parenchymal volume loss with sulcal enlargement (green arrows).
On admission, the patient was started on IV Meropenem and Vancomycin. After confirmation of COVID-19, he was started on IV Hydroxychloroquine treatment without significant response. Brain magnetic resonance imaging (MRI), angiography, and venography without IV contrast was performed 2 days following admission due to the patient's unstable and worsening hemodynamic and neurologic status. The brain MRI was remarkable for severe cerebral edema with mass effect, diffuse cerebral sulcal effacement, and downward cerebellar tonsillar herniation resulting in brainstem compression and narrowing of the 4th ventricle (Fig. 2). There was no evidence for acute territorial infarction. Magnetic resonance angiography and Magnetic resonance venography demonstrated severe diffuse cerebral arterial and dural venous sinus constriction (Figs. 2 and 3).
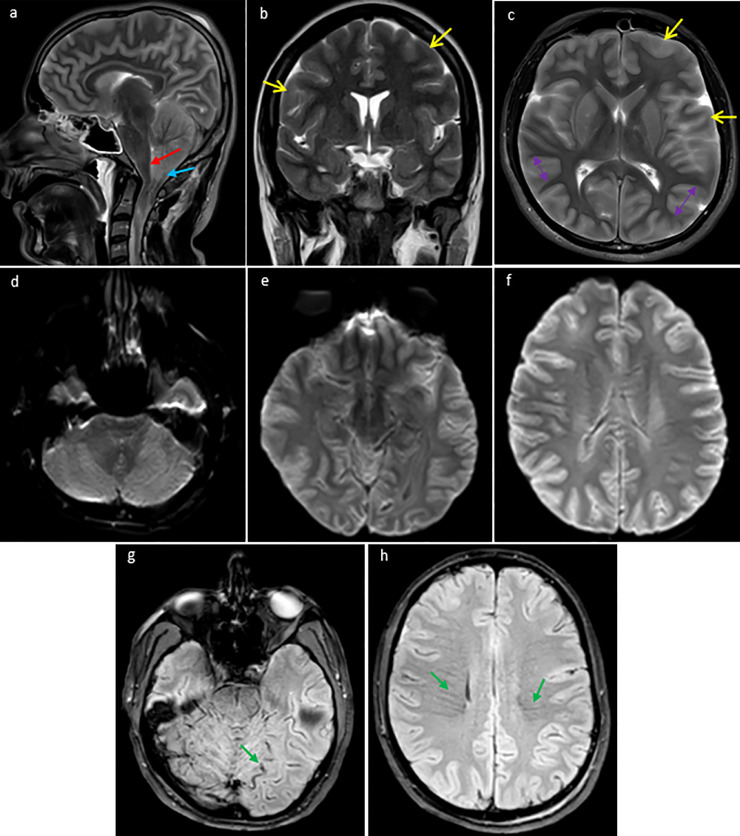
Fig. 2.
Brain MRI without IV contrast (obtained 2 days after the initial brain CT): sagital (a), coronal (b), and axial (c) T2 weighted sequences of the brain demonstrate interval development of extensive gyral swelling (double head arrows), diffuse cerebral sulcal effacement (yellow arrow), downward cerebellar tonsillar herniation (blue arrow), and compression and ventral displacement of the brainstem and fourth ventricle (red arrows). Axial DWI sequences (d, e, f) demonstrate no evidence of restricted diffusion to suggest acute ischemia. Axial T2 gradient-echo sequence (g, h) demonstrates prominent collateral tortuous superficial cortical veins and prominent engorged collateral deep medullary veins coursing through the centrum semiovale bilaterally (green arrows).
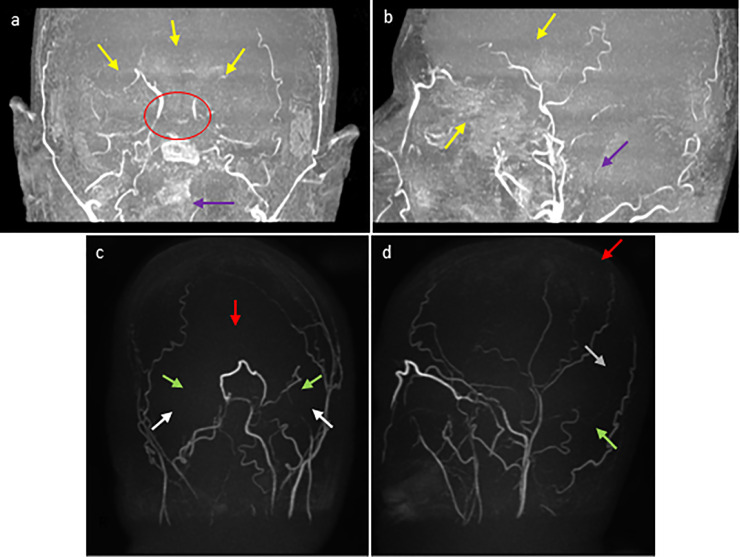
Fig. 3.
MRA of the head without IV contrast (obtained 2 days after the initial brain CT): coronal (a) and sagittal (b) maximum intensity projection (MIP) views demonstrate diffuse narrowing and diminished flow related enhancement throughout the major branches of the anterior (yellow arrows) and posterior (purple arrows) cerebral arterial circulations and Circle of Willis (red circle). MRV of the head without IV contrast (same day): coronal (c) and sagittal (d) MIP views demonstrate absence of the major branches of the posterior superior dural venous sinuses including absence of the superior sagittal sinus (red arrows), transverse sinus (green arrows), sigmoid sinus (white arrows), and straight sinus (gray arrow).
The patient's hemodynamic condition deteriorated rapidly, and he expired 2 days after the MRI, following asystolic cardiac arrest. Autopsy was not performed due to family's refusal.
Discussion
Angiotensin converting enzyme 2 receptors are present in many body systems including the central nervous, cardiovascular, respiratory, gastrointestinal, and musculoskeletal systems and are believed to be the main functional receptor for SARS-Cov-2 [3], [4], [5]. SARS-Cov-2 can cause central nervous system injury via direct or indirect mechanisms including severe cytokine-mediated inflammation, disruption of the blood brain barrier, viral encephalitis, and acute cerebrovascular ischemia. Neurologic manifestations have also been reported in other human coronaviruses such as middle eastern respiratory syndrome corona virus and SARS-CoV, and postmortem autopsies have demonstrated the presence of SARS-CoV nucleic acid in patients’ CSF and brain tissue [5], [6], [7], [8], [9].
In our case, severe intracranial edema eventually leading to cerebellar herniation, brain stem compression, and diffuse intracranial vasoconstriction may be explained by a combination of severe cytokine-mediated immune response, development of vasculitis/vasculopathy, and direct viral invasion into the brain parenchyma [7], [8], [9].
Our case highlights the importance of considering SARS-Cov-2 infection in the differential diagnosis for patients with unexplained central nervous system dysfunction and cerebral edema to prevent delayed diagnosis and render rapid treatment. It also signifies the role of imaging in the management of COVID-19 patients with unexplained altered mental status.
Ethics approval
Approval from the Ethics Committee of Isfahan University of Medical Sciences was obtained according to the 1964 Declaration of Helsinki and its later amendments.
Footnotes
Conflicts of Interest: Authors declare they have no conflict of interest.
Funding: None.
References
- 1.World Health OrganizationDirector-General's opening remarks at the media briefing on COVID-19. March11, 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—11-march-2020.
- 2.Johns Hopkins UniversityCOVID-19 Dashboard by the Center for Systems Science and Engineering. https://coronavirus.jhu.edu/map.html. Accessed August 1st, 2020.
- 3.Filatov A, Sharma P, Hindi F, Espinosa PS. Neurological complications of coronavirus (COVID-19): encephalopathy. Cureus. 2020;12(3):e7352. doi: 10.7759/cureus.7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hellinger JC, Sirous R, Hellinger RL, Krauthamer A. Abdominal presentation of COVID-19. Appl Radiol. 2020;49(3):24–26. [Google Scholar]
- 5.Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID-19–associated Acute Hemorrhagic Necrotizing Encephalopathy: CT and MRI Features. Radiology. 2020;296(2):E119–E120. doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pleasure SJ, Green AJ, Josephson SA. The spectrum of neurologic disease in the severe acute respiratory syndrome coronavirus 2 pandemic infection: neurologists move to the frontlines. JAMA Neurol. 2020;10 doi: 10.1001/jamaneurol.2020.1065. Published online April. [DOI] [PubMed] [Google Scholar]
- 7.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):1–9. doi: 10.1001/jamaneurol.2020.1127. Published online April. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, Yang L. Nervous system involvement after infection with covid-19 and other corona virus. Brain Behav Immune. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11(7):995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]