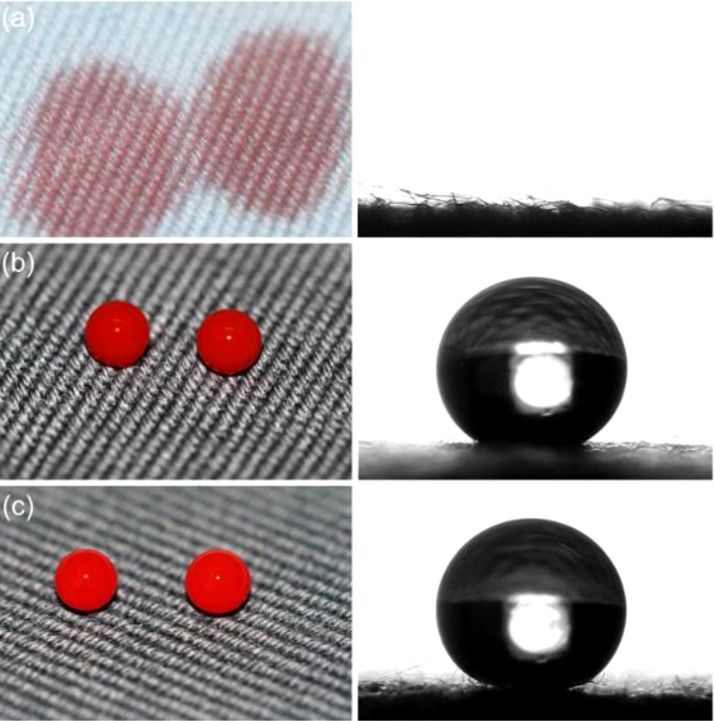
Graphical abstract
Keywords: Graphene, Superhydrophobic, Contact angle, Self-cleaning, Anti-Corrosion
Abstract
Lotus like materials having superhydrophobicity is attaining greater demand due to the possibility of molding them into different high end applications. The major issue related to self-cleaning superhydrophobic surfaces is their restricted mechanical properties. The development of nanotechnology has brought many advantages in the fabrication and properties of superhydrophobic surfaces and thus it enhanced the demand of superhydrophobic surfaces. Many scientific groups have studied and reported about the superhydrophobicity exhibited by graphene and its analogous derivatives. The fabrication of the devices having properties ranging from anti-sticking and self-cleaning to anti-corrosion and low friction is made possible by the incorporation of this wonderful two-dimensional material. This review focuses on the preparation and properties of graphene based superhydrophobic coating materials with special mention to the wide range of applications rendered by them.
1. Introduction
Composite materials with superhydrophobic traits have got extensive consideration owing to their excellent characteristic properties such as self-cleaning, hindered corrosion [1], and application in water resistant electronic devices [[2], [3], [4]]. The excellent water repellent properties observed for several natural objects such as lotus leaf, butterfly wings, rice plants etc. motivated scientists towards developing materials having exceptional water repellency [5]. Superhydrophobic surfaces are those facets having a water contact angle of 150 or more [6] and these materials are prepared with sufficient surface abrasion and minimum surface energy [7]. Both industrial and domestic applications are possible with “superhydrophobic surfaces” which made them ranked 7th in journals of material science discipline between 2006 and 2010 [8]. Superhydrophobic nanocoatings extend the area of nanotechnology and superhydrophobicity into a new dimension which has an essential role in improving the properties of coatings.
The technologies and materials in coating industry are advancing day by day to improvise the efficiency of coatings by the incorporation of cost effective and greener concepts. Undoubtedly, coating is one of the finest methods to alter the solid interface properties by paving a new protecting layer over the substrate via chemical or physical process. So the development of superhydrophobic nanocoatings are attaining more attraction due to its possible extended high end application [9].
The surface wetting performance can be generally classified into 4 categories hydrophilic, hydrophobic, superhydrophilic and superhydrophobic according to their water contact angle (WCA). Hydrophilic and hydrophobic have their WCA within the range of 10° < θ < 90° and 90° < θ < 150° respectively. Superhydrophilic and superhydrophobic domains are of considerable interest owing to the utmost surface wetting traits having WCA differing in the stretch of 0° < θ < 10°and 150° < θ < 180° respectively. The Superhydrophobic regime is important as it experiences a perfect non wetting capability with an exceptionally elevated water contact angle that result in the rolling of water droplets easily [10].
The work of Jiang et al. [11] on nanostructure based superhydrophobicity triggered the curiosity of scientific community and it was followed by several works on superhydrophobic nano materials. Wenzel model gives a fair explanation of the contact angle of a substrate which is in contact with a liquid. Cassie and Baxter further proposed a model which deals with heterogeneous surfaces comprising of two fractions, the former deals with solid liquid and latter with the liquid air interface. The composition of the surface together with its roughness regulate the surface dampening and the water contact angle of the surface [12,13]. The nano-micro hierarchical roughness plays an eminent role in manipulating the characteristics of superhydrophobic nanocoatings and they are classified as inorganic, organic and inorganic-organic materials [9].
Silica encompassed materials are the most common choices for superhydrophobic nanocoating area. In fact, they are hydrophilic, but through chemical treatment they obtain superhydrophobicity [14,15]. Carbonaceous materials such as carbon nanotubes, carbon nanofibers and fullerenes have gained focus in superhydrophobic nanocoatings in the recent years [9]. Inorganic and organic nanomaterials are also utilized as promising materials for enhancing the flexibility and molecular framework of these coatings [[16], [17], [18]]. Yuan et al. [17] developed superhydrophobic LDPE with lotus-leaf-like characteristics and contact angle in the range of 156°. A vast array of inorganic fillers could be employed for designing hybrid superhydrophobic nanocoatings. Qing et al. [19] developed ZnO/polystyrene nano composite coatings having a WCA of 158°.
Graphene, a younger cohort material, is nothing but the allotrope of carbon isolated by basic mechanical exfoliation in the earliest years of 21st century [20]. It is a mono-atomic thin sheet of carbon arranged in a hexagonal honeycomb like crystalline lattice and it has attracted worldwide interest within no time because of fascinating properties arising from the two-dimensional structure and existence of sp2 bonded carbon atoms [21]. Graphene is believed to have a better future for the fabrication of various carbonaceous technical devices, owing to its superior conductivities at room temperature and inertness towards corrosion [20]. Graphene was isolated in 2004 and prior to that intercalated clays and fullerenes were used as coating materials for corrosion resistance [22,23].
For the last few years, application of graphene and graphene-related materials are topics of increasing research interests due to many outstanding features which make them suitable for a passive layer formation that protects metals from oxidation and corrosion [[24], [25], [26], [27]]. The surface wettability of graphene has been discussed a lot and many efforts have been taken to control the same by modifying the surface of graphene [24]. In order to reduce surface energy and increase surface roughness, a lot of methods have been employed by means of layer-by-layer assemblies [[28], [29], [30]], spraying [31], electrospinning [32,33], spin coating [34], electrochemical reaction and deposition [[35], [36], [37], [38]], chemical vapor deposition [39] etc.
Though application of superhydrophobic nanocoatings is the main destination of all researches, the theory, materials and moulding has also fascinated the research community [9]. Self-cleaning is the most promising property of graphene coated superhydrophobic surfaces as water droplets can roll on the surface and take away the dirt sticking on the surface effectively. Numerous artificial superhydrophobic nanocoatings with self-cleaning traits have been synthesized by different methods and are applied in diverse domains based on their applications [[40], [41], [42], [43], [44], [45]]. Graphene coated nano-composite materials can be effectively used in anti-icing and de-icing methods [[46], [47], [48], [49]] and used in biosensors, biomedical implants and devices, food packaging, industrial and marine equipment [[50], [51], [52]].
Graphene is an oil repellent material and hence could be used for effective separation of oil and water [53]. It also imparts anti-corrosion property to a coating system [54]. Diverse varieties of graphene, mainly single layer/bilayer graphene films, graphene oxide (GO), graphene nanoplatelets and graphene nanoribbons can be synthesized by modern techniques [55,56]. Now a days, nanopellets of graphene are extensively used for the synthesis of composite materials via mixing with metals, ceramics and polymers to tailor the properties for various applications [57].
2. Fabrication of superhydrophobic graphene based composites
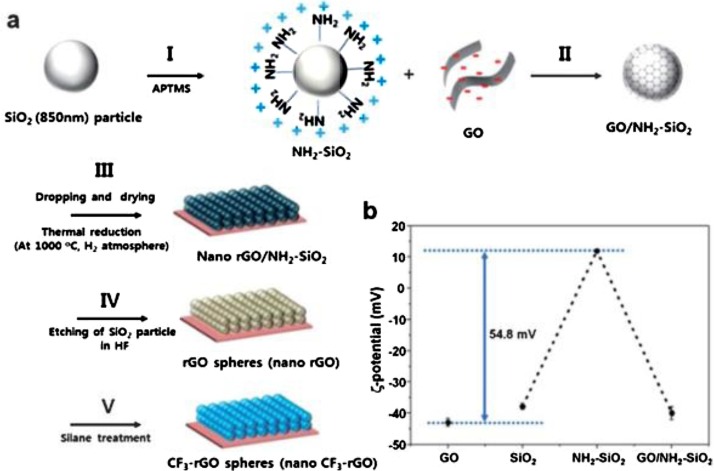
Graphene coatings are generally synthesized by chemical vapour deposition. Nilsson and his co-workers studied the drawbacks regarding the corrosion inhibition properties of graphene coatings on metal surfaces and concluded that graphene coated by CVD acts as corrosion inhibitor only at low gas pressures [58]. Choi and co-workers [59] synthesized graphene/nafion nanohybrids with hierarchical petal-like, porous structure and superhydrophobicity (WCA = 161°) by regulating the structures with respect to the chemical composition. Lee et al. [60] presented another simple route towards the fabrication of superhydrophobic graphene surface via thermal reduction method and they obtained a transparent nano-sphere structure with excellent WCA as shown in Fig. 1 .
Fig. 1.
(a) Schematic illustration of the fabrication of the nano-structure of graphene spheres (nano-rGO). (I) Surface modification of silica particles into a cationic surface by APTMS. (II) Surface coating of surface-modified silica by GO. (III) Self-assembly of GO-coated silica particles followed by reduction. (IV) Removal of particles. (V) Surface-treatment of silane. (b) Zeta-potential of aqueous solution of SiO2,GO,NH2–SiO2, and GO-coated NH2–SiO2measured at pH 7 [60]. Reproduced with permission from Royal society of Chemistry.
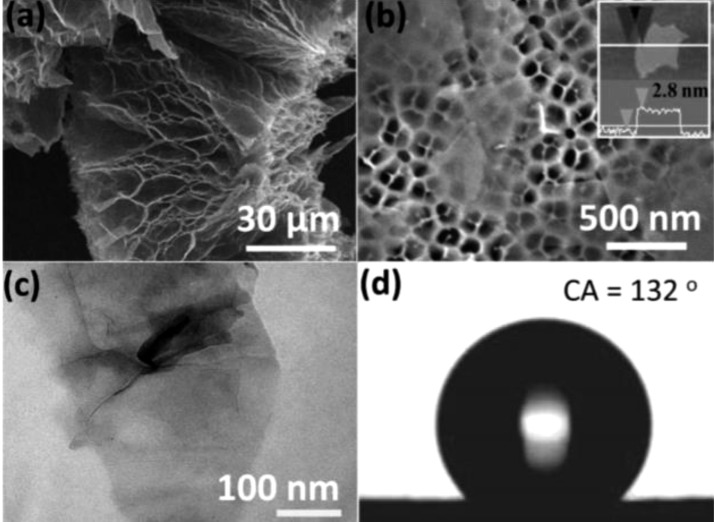
Ngyuen and co-workers synthesized graphene-based sponges with enormous absorption capacities (165 times their own weight) there by making them apt for large-scale application [61]. Li et al. [62] made novel rGO/polypyrrole composite via voltammetry reduction method which proved as an effective protective coating for corrosion prevention of mild steel substrates. The corrosion protection is due to the combined effect of generation of passive layer of rGO/PPy hybrid and oxide film. Dip coating is another possible method for fabrication of graphene based superhydrophobic surface. Nguyen et al. [61] fashioned graphene exfoliated graphite by ultrasonic stripping method, and then obtained the superhydrophobic graphene sponge by dip coating method. The superhydrophic and superoleophilic sponges were used as an adsorbent for the removal of broad range of solvents and oils. The sponges of poly-dimethylsiloxane (PDMS) were having a three-dimensional pore like structure with a pore size of 50−130 μm. For enhancing the superhydrophobicity, the sponges were coated with graphene. Cementation of PDMS ensured the stability of CA of the sponge. The FESEM images of the graphitic film and the observed contact angle is shown in Fig. 2 . They observed that as the graphene loading increased, the contact angle also increased to 162°.
Fig. 2.
Typical FESEM images of expanded graphite (a) and graphene nanosheets (b), (the inset in (b) is an AFM image of a graphene nanosheet with the thickness of 2.8 nm). TEM image of graphene nanosheets (c) and optical image of a water droplet on a graphene film (c) and optical image of a water droplet on a graphene film at a CA of 132 (d) [61]. Reproduced with permission from Royal society of chemistry.
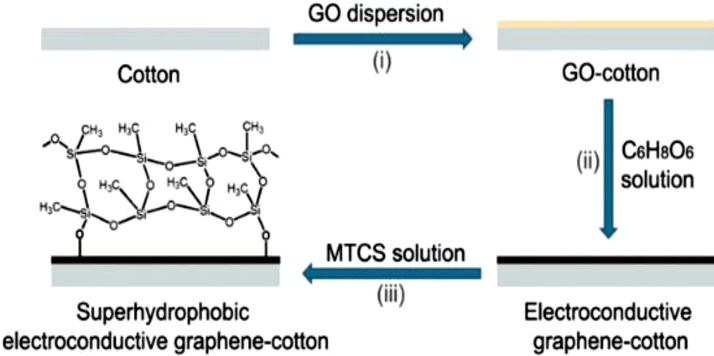
In another study, Zhang et al. [63] made superhydrophobic sponge using thiolated graphene by following the same dip coating method. First they synthesized thiolated graphene using thiourea and then the sponge was dipped in it. Wang et al. [64] developed superhydrophobic graphene coating using the electrically exfoliated graphene by painting it on a steel substrate using disposable pasture pipette. Khalilabad et al. [65] made superhydrophobic graphene based cotton fabric by dipping it in GO solution followed by the surface modification using methyltrichlorosilane (MTCS) (Fig. 3 ). Ji et al. [66] followed the same dip coating method for the fabrication of melamine/graphene sponge for oil separation.
Fig. 3.
Schematic representation of fabrication of superhydrophobic graphene fabric [65]. Reprduced with permission from springer.
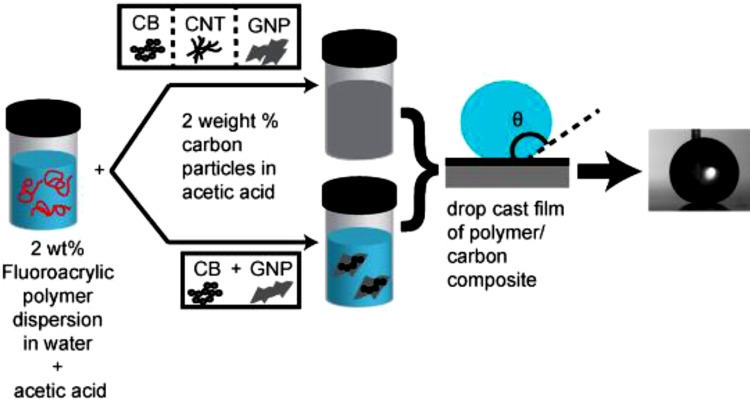
Asthana et al. [67] developed a method for making carbon based films using Fluoropolymer dispersions. In this study they have made superhydrophobic nanocomposites by mixing fluroacrylic acid based polymer dispersion and carbon black, graphene and carbon nanotube as shown in Fig. 4 . Many researchers have followed the same method for the fabrication of carbon based superhydrophobic nanocomposite coatings.
Fig. 4.
Schematic of the superhydrophobic nanocomposite synthesis using CB, CNT, and GNP nanofillers or their combinations [67]. Reproduced with permission from American Chemical Society.
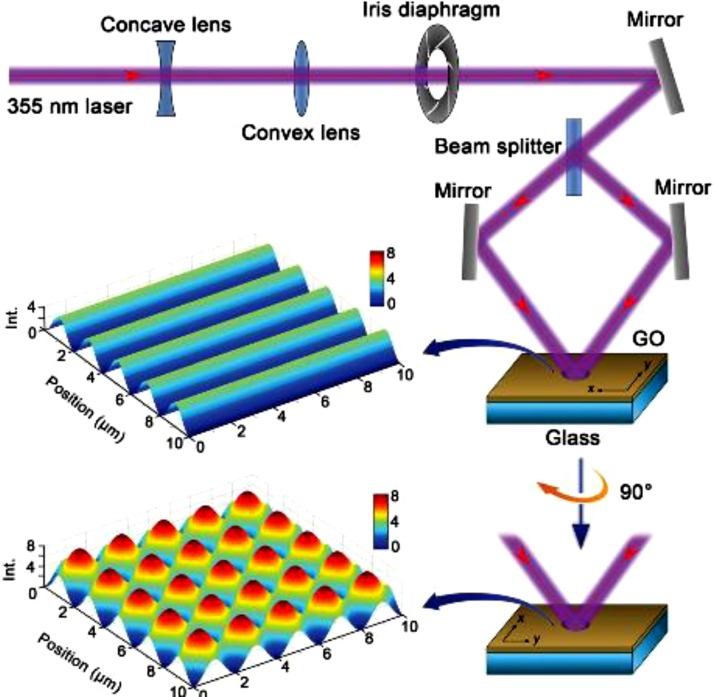
Jiang et al. [68] developed an optical system for the fabrication of superhydrophobic graphene films by a two-beam laser interference (TBLI) treatment. They fabricated graphene films by the method of TBLI from graphene oxide (GO) and the arrangement of the optical system is shown in Fig. 5 . This technique helped in the development of hierarchical roughness and nano fragments which led to superhydrophobicity. The laser treatment removed the hydrophilic functionalities over the surface and thus enabled a CA higher than 150°. Wang et al. [69] prepared a rainbow colored superhydrophobic surface of biomimetic graphene through interference technology. They also utilized the same method of two laser interference for the design of this graphene based biomimetic superhydrophobic surface. Li et al. [70] also utilized the laser induced method for fabricating superhydrophobic graphene based surface.
Fig. 5.
Schematic representation of developed optical system [68]. Reproduced with permission from wiley.
Muthu et al. [71] demonstrated the preparation of hydrophobic coatings of graphene using recycled pencil stubs as the source material combined with a sonication exfoliation methodology and the coatings were able to repel bacterial adhesion. Liu et al. [26] followed the spin coating technique for making superhydrophobic surface over aluminium alloy. The graphene suspension was coated over the alloy by spin coating and then it was dried in an oven. In another study, Bai et al. [72] used electrodeposition method for making superhydrophobic coating over Ni. In another study, Jin et al. [73] deposited graphene over glass substrate by simple casting process followed by thermal treatment. Freeze drying is another method for developing graphene based superhydrophobic coatings, since it ensures the required surface roughness which is a necessary condition for superhydrophobicity. Wang et al. [74] utilized the effect of freeze drying for preparing graphene based superhydrophobic coating by strengthening it with p-p-phenylene diamine (pPDA). Lotus leaf like superhydrophobic surfaces were made recently by the method of soft lithography by utilizing replica molding of PDMS to obtain the lotus leaf like structure. The increased surface roughness in nanoscale dimension was responsible for the superhydrophobicity with a CA of 161° [75]. Table 1 tabulates general methods of synthesis of graphene based superhydrophobic materials.
Table 1.
General fabrication methods of graphene based superhydrophobic materials.
| Synthesis methods | Characteristics of the coatings | Reference |
|---|---|---|
| Chemical Vapour Deposition | Corrosion Inhibition | [58] |
| Solution chemistry and self-assembly | Hierarchical roughness and superhydrophobicity | [59] |
| Thermal reduction | Flexible and transparent superhydrophobic electrodes | [60] |
| Ultrasonic stripping and Dip-Coating method | Selective oil-water and organic solvents separation ability | [61] |
| Voltammetry reduction method | High electrochemical stability and anti-corrosion ability | [62] |
| Dipping drying method | Flame retardant sponge with oil-water separation ability | [63] |
| Painting using disposable pasture pipette | Self-cleaning superhydrophobic surface | [64] |
| Dip Drying method | Electrical conductive superhydrophobic surface | [65] |
| Dip-coating method | Oil-water separating sponge | [66] |
| Drop casting | High conductive superhydrophobic surface | [67] |
| Two-beam laser interference | Hierarchical roughness and nano fragments | [68] |
| Interference Technology | superhydrophobicity and iridescence surface | [69] |
| Laser induced fabrication | Surface with different wetting property | [70] |
| Sonication followed dip coating | Anti-fouling coating | [71] |
| Spin Coating | Abrasion and corrosion resistant surface | [26] |
| Casting followed thermal treatment | Conductive and superhydrophobic surface | [73] |
| Freeze drying | Micro and nano hierarchical structured superhydrophobic surface | [74] |
| Soft lithography | Chemically stable superhydrophobic surface | [75] |
3. Characterization of superhydrophobic coatings
The superhydrophobicity of a surface is purely dependent on the functionalities present on the surface as well as the morphology and contact angle and hence characterization of the coating is highly essential for determining the end performance [76]. Graphene and modified graphene are widely used for the fabrication of superhydrophobic materials. Hence the characterization of these graphene based materials is very important in terms of surface modification, roughness and water contact angle measurements.
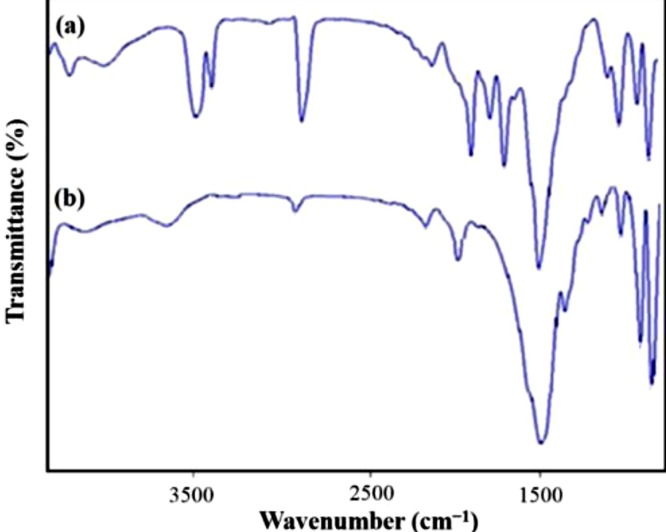
Wang et al. [74] modified graphene oxide using pPDA for making it more superhydrophobic in nature by surface polymerization and thus it got self-assembled over the surface of GO. The surface modification made GO rougher and thus it became superhydrophobic. In order to confirm the modification, they have used basic spectroscopic techniques. The shift in the vibrational frequency and development of additional peaks help in confirming the surface modification. They also sought the help of X-ray photo electron spectroscopic technique in order to confirm the same. Shanmugharaj et al. [77] confirmed the amine functionalization of GO which is highly responsible for the enhanced superhydrophobicity with the help of FTIR spectroscopy. Moradi et al. [78] made polyvinylidene fluoride (PVDF) based graphene nanofibrous films by the method of electrospinning. The incorporation of graphene into the PVDF membrane was confirmed with the help of FTIR as shown in Fig. 6 . It compares the FTIR peak of PVDF and that of the graphene incorporated PVDF membranes. Peaks at 1700, 2000 and 3000 cm−1 confirm the presence of graphene in the polymer.
Fig. 6.
FT-IR spectra of (a) PVDF/G nanofibers (M4) and (b) pristine PVDF nanofibers (M0) [78]. Reprduced with permission from MDPI.
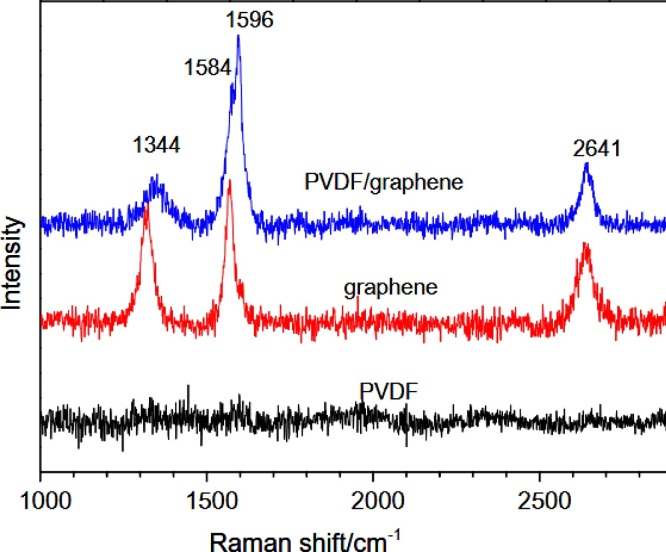
Zha et al. [79] made graphene based superhydrophobic gels by mixing it with PVDF. The modification of PVDSF gels using graphene was confirmed with the help of Raman spectra. Raman spectroscopy is a powerful tool in analyzing the modification of carbonaceous materials like graphene. The presence of D and G band can explain the surface functionalization in detail, as D band corresponds to the SP3 hybridized and G band corresponds to SP2 hybridized carbon. Functionalization of graphene leads to an improvement in intensity of D band and thus the ratio of intensities of D and G band(ID/IG) varies. This help in confirming the surface functionalization of graphene as we have reported previously in one of our works [80]. The existence of D, G and the 2D bands in the PVDF gel confirmed the presence of graphene in the gel (Fig. 7 ). The intensity changes associated with these bands also helps in confirming the defects and modifications made.
Fig. 7.
Raman spectra of PVDF, graphene and PVDF/graphene hybrid [79]. Reproduced with permission from Elsevier.
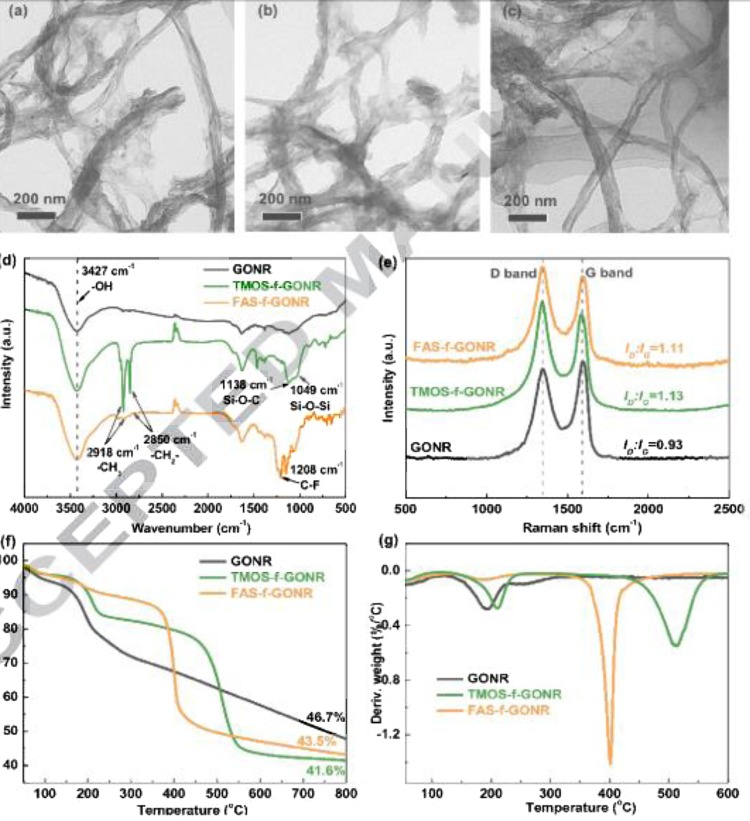
Qiang et al. [81] confirmed the silane modification of graphene nano ribbons with the help of Raman spectra by evaluating the ratio of intensities of D and G band. The trimethoxyoctadecylsilane (TMOS) and (heptadecafluoro-1, 1, 2, 2-tetradecyl) trimethoxysilane (FAS) modifications of graphene nano ribbons made from multiwalled carbon nanotubes(MWCNT) were confirmed with the help of FTIR, Raman analysis and thermal stability was analysed with the help of thermo-gravimetric analysis (TGA) and Differential Thermogravimetry (TGA). Mostly, TGA and DTG are used to analyze the thermal stability of graphene and modified graphene materials. The morphological analysis is usually carried out with the help of microscopic techniques like High Resolution Transmission Electron Microscopy (HRTEM) and Scanning Electron Microscopy (SEM) as shown in Fig. 8 .
Fig. 8.
Structural characterizations of GONR and f-GONR: TEM images of (a) GONR, (b) TMOS-f-GONR and (c) FAS-f-GONR, and (d) FTIR, (e) Raman spectra, (f) TGA and (g) DTG curves [81]. Reproduced with permission from Elsevier.
The major characterization of superhydrophobicity can be carried out by the analysis of contact angle (CA). The measurement of contact angle is a key parameter; the value of CA determines the nature of the coating. Water contact angles are of two types ; static and dynamic which can be measured by sessile drop method and Wenzel method [76,82]. Nine et al. [83] used the sessile drop method for the determination of CA of a graphene based coating. The study shows that the rGO/TiO2/PDMS based Diatomaceous Earth (DE) composites show superhydrophobicity mainly due to the bouncing effect of TiO2. But rGO slightly reduces the CA from 170° to 154°. It is due to the presence of hydrophilic functionalities present on GO even after the reduction. Khalilabad et al. [65] made electro-conductive as well as superhydrophobic cotton fabrics using graphene coating by immersing the cloth in the GO solution and subsequently modifying with silane. The study showed that the water droplets were able to stay on the cotton surface for a long time without changing the spherical shape. Compared to the graphene coated cotton, the silane modified cotton fabrics show improvement in CA. The graphene-coated fabrics were hydrophilic but the silane modification makes it hydrophobic. The shape of water droplets are shown in Fig. 9 . Hence it is evident that surface modification is an apt method for the enhancement of superhydrophobicity of GO.
Fig. 9.
Red-dyed water droplets sitting on a) the original cotton; b) the graphene-cotton; c) the PMS-graphene-cotton. The images on the right show corresponding goniometer images for 5ll droplets [65]. Reproduced with permission from springer.
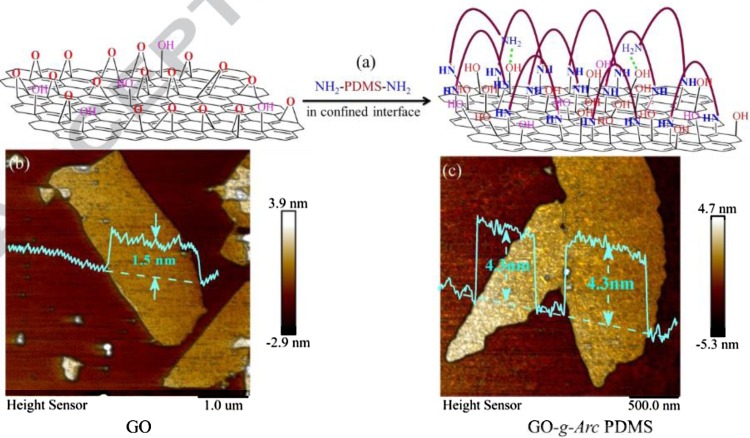
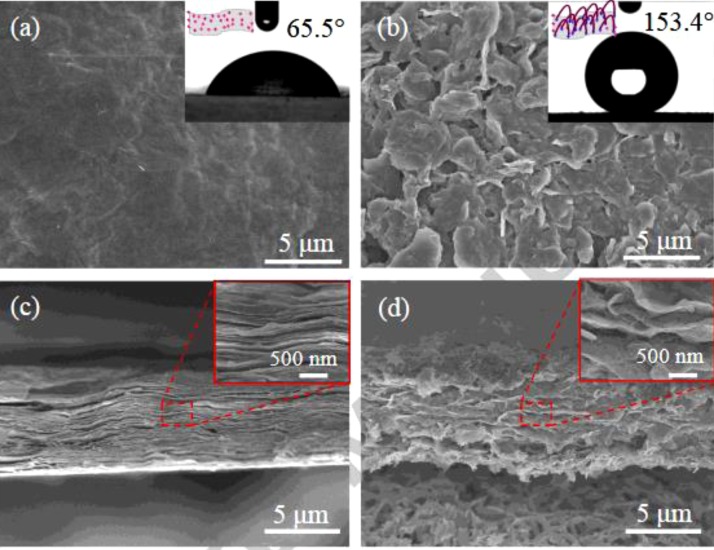
As the surface modification of GO is very important, Mo et al. [84] grafted arc like macromolecules over the surface of GO by following ‘grafting onto’ technique using diamino-terminated PDMS. The interface interaction leads to the formation of arc like GO-g-PDMS as shown in Fig. 10 . The AFM analysis confirms the surface roughness enhancement after the grafting of arc like macromolecules. This is an important parameter in determining the superhydrophobicity. The grafting of PDMS enhances the WCA of GO from 30.1 to 65.5° and the membranes made of GO-g-PDMS showed WCA of 153.4°. SEM images of the surface shows the lamellar structure and improved roughness which is the reason behind the enhancement in WCA as in Fig. 11 .
Fig. 10.
(a) Reaction between GO and NH2-PDMS-NH2 macromolecular chains to form arc-like PDMS bridge architecture surface; AFM height images for (b) GO and (c) GO-g-Arc PDMS [84]. Reproduced with permission from Elsevier.
Fig. 11.
SEM images and WCA photos (insert of SEM image) for (a) GO membrane and (b) GO-g-Arc PDMS-0.80 hybrid membrane; the cross-sectional SEM images of (c) GO and (d) GO-g-Arc PDMS-0.80 hybrid membrane [84]. Reproduced with permission from Elsevier.
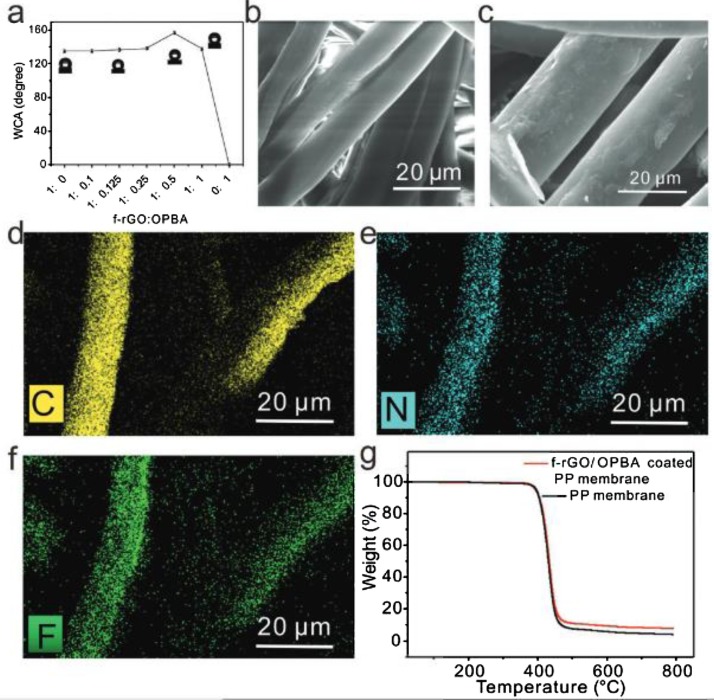
Guo and co-workers [85] utilized fluorine grafted GO (f-GO) for making functional superhydrophobic g-oxo-1-pyrenebutyric acid (OPBA) membranes by following the method of dip coating. The f-GO/OPBA coated fabrics were prepared and the CA was measured by varying fGO and OPBA concentration and optimized the concentration corresponding to maximum CA. For 1:0.5 concentration, the polypropylene (PP) membranes show a CA of 163.7°. The SEM images of the uncoated and coated PP membranes show that the coating makes the f-GO/OPBA to glue over it and enhance the roughness. They have carried out Energy dispersive X-ray (EDX) to account the elemental change as shown in Fig. 12 . In one of our studies, we have utilized the technique of EDAX to analyze the elements present in the self-assembled nano porous core-shell TiO2-GO hybrid material [86].
Fig. 12.
(a) The variation of water contact angle (WCA) of f-rGO/OPBAcoating with different mass ratios. SEM images of the uncoated PPmembrane (b) and f-rGO/OPBA coated PP membrane (c). (d–f) Elementalmapping of f-rGO/OPBA coated PP membrane for C, N, and F, respec-tively. (g) TGA results of uncoated PP membrane and f-rGO/OPBA coatedPP membrane, respectively [85]. Reproduced with permission from Royal Society of Chemistry.
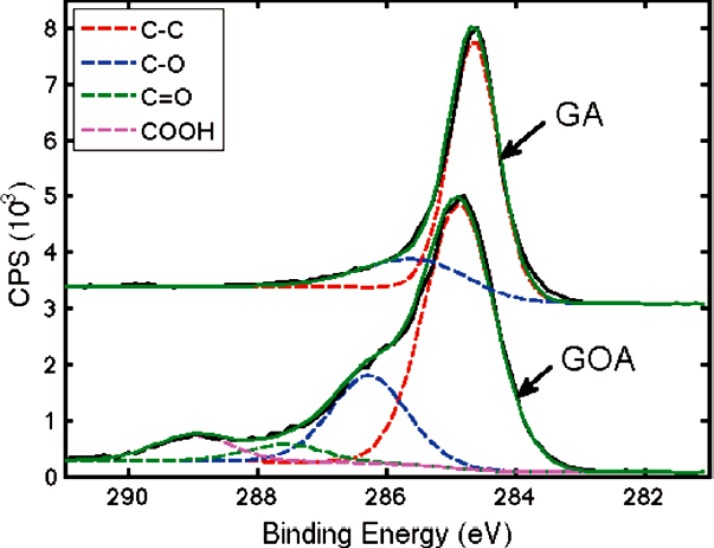
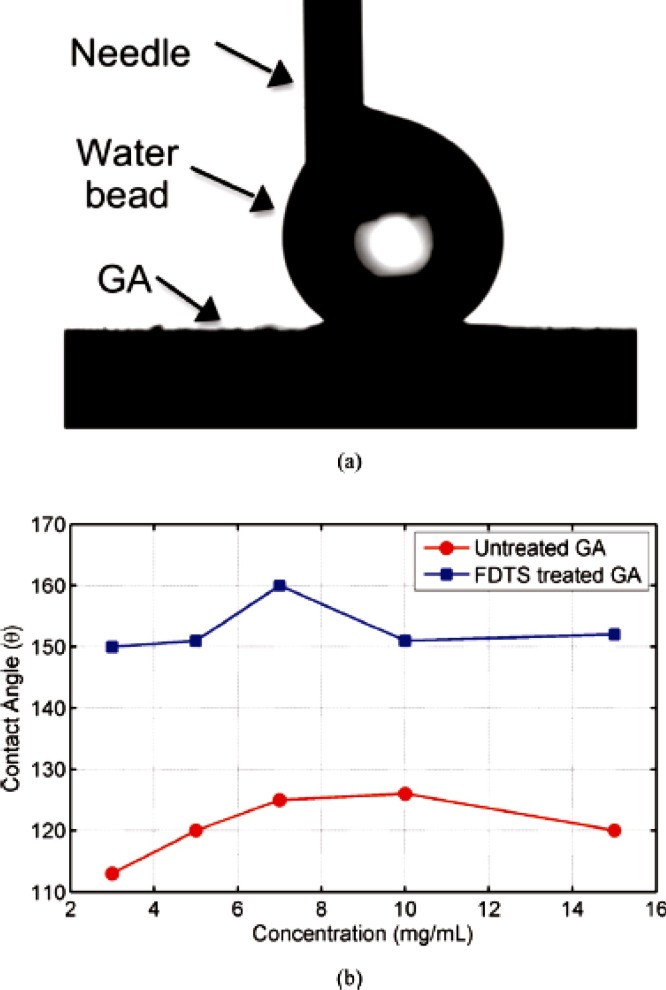
Zhang et al. [87] made Poly (vinylidene fluoridehexafluoropropylene)/Graphene superhydrophobic composites by mixing followed by freeze drying. The nano scaled surfaces were capable of enhancing the superhydrophobicity. They studied the crystallization behavior with the help of X-ray diffraction methods. Lin et al. [88] used XPS technique for the characterization of superhydrophobic graphene aerogels(GA) and graphene oxide aerogel(GOA). C1s spectra of GOA confirm the presence of oxidation moieties and the shift in the C—C bond was associated with the reduction as shown in Fig. 13 . The study revealed that an enhancement in hydrophobicity was attained by silane modification. It is observed that a slight modification of the surface leads to a change in surface energy of coating and thus a CA of 160° was obtained for GA. From Fig. 14 , it can be understood that the effect of silane modification of GA changes the CA because of the reduction in surface energy. Density of the aerogel is one of the factors which determine the pore size and surface roughness. Hence it is a very important parameter in determining the superhydrophobicity. Lin et al. [89] modified GO using octadecyl amine and the modified GO films over silicon were able to show a CA of 163.2° due to the enhanced surface roughness. Recently, in one of our study we have utilized the same technique of XPS in monitoring the surface decoration of GO by a triblock copolymer in order to obtain a micellar nanostructure. Further the AFM and HRTEM images were taken to understand the micellar arrangement of the modified GO in the nanostructures [90].
Fig. 13.
XPS spectra of graphene oxide aerogel and graphene aerogel [88]. Reproduced with permission from American Chemical Society.
Fig. 14.
Water contact angle testing: (a) photograph of water contact angle of the surface of the chemically treated graphene aerogel, (b) contact angles of treat and untreated graphene aerogel surface with varies concentration [88]. Reproduced with permission from American Chemical Society.
4. Durability of superhydrophobic surfaces
Durability analysis of superhydrophobic surface is very important as it has an immense effect in determining the application. Mechanical durability is one of the crucial components in meeting the real-world application. The fabrication of a durable superhydrophobic material as a coating is an important aspect as it easily gets tampered by mechanical and chemical action [91]. Mechanical abrasion tests were carried out in graphene oxide and nickel coated superhydrophobic surfaces. The study reveals that after the 100-cycle, the CA changed from 162.7° to 155.8° with slight improvement in SA. Thus the mechanical abrasion test shows that the water repellency of these composites are robust even after 100 cycles. The pinecone like structures were capable of attributing mechanical durability as well as superhydrophobicity [92]. But in another study, the rGO containing superhydrophobic surface showed less durability to abrasion test. After continuous abrasion of 1520 cycles, the surface lost its superhydrophobicity compared to TiO2 based system [83]. But the fluorine grafted rGO based superhydrophobic surfaces were capable of showing enhanced mechanical, chemical and hot water resistance. It can tolerate harsh environment even after the continuous exposure for 1 week [85]. Later it was observed that octadecylamine grafted rGO based superhydrophobic foams enhance the chemical and PH stability to a greater extent [93]. Thus the functionalization of rGO can tune the superhydrophobic nature. rGO modified polydimethylsiloxane (PDMS) were used as superhydrophobic fabric coating and these materials were capable of showing excellent PH and thermal stability. These materials were also capable of showing excellent durability even after 200 cycles of laundry. Hierarchical nanostructures were behind this excellent superhydrophobicity [94]. In another study, it was observed that the graphene/polyurethane sponges made by dip coating method were capable of showing enhanced mechanical durability and thermal and chemical stability. The sponges also showed good elastic recovery and the test using lubricating oil confirmed the recyclability as well [95]. Superhydrophobic surfaces made by laser induction process for desalination application were capable of showing enhanced mechanical durability as well as thermal stability. Even after water jet treatment and scotch tape test, the surface maintained the superhydrophobic character. Moreover, the surfaces were capable of retaining the nature even after seven cycles of scotch test ensuring the desalination application. The surface maintained the same pattern even after the removal of the tape, but later the degradation occurred due to the failure of adhesion and it is not associated with the failure in superhydrophobicity [96]. It is observed that the topology of graphene/polypropylene surface is highly efficient to keep the water away and it forms a perfect Cassie-Baxter state. Interestingly, after abrasion, the new surfaces were having increased roughness and maintains the hydrophobicity [97]. In another study, a stretchable, robust and superhydrophobic graphene based thermoplastic polyurethane (TPU) composite with excellent mechanical durability was prepared. Knife scratch and hand rub test were carried out in order to understand the durability. The composites were capable of exhibiting superhydrophobicity even after these harsh tests and water easily rolled off from the surface. Sand paper abrasion test revealed that the surfaces were capable of maintaining the superhydrophobic behavior even after five cycles of 32.5 KPa of abrasion. Moreover, at a low load of 20 KPa, the surface maintained the behavior even after 450 cycles due to the tensile strength of pristine graphene, physical support of TPU to the partially embedded nanoplatelets of graphene and the elastic nature of TPU [98]. Graphene based superhydrophobic surfaces tend to show excellent mechanical durability than rGO based systems. Hence modification of rGO is usually done to increase the durability. Recently, superhydrophobic hexadecyltrimethoxysilane-grafted rGO based melamine sponges having excellent mechanical and chemical stabilities were made. These materials maintained the mechanical stability even after 40 cycles. Due to the interaction between rGO and the grafted material, it shows chemical inertness as well [99]. Nanofibers of thermoplastic polyurethane (TPU) prepared via electrospinning were decorated using graphene for imparting hydrophobicity. Even after 20, 50 and 80 % stretching, the fibers retained the superhydrophobicity due to the surface modification using graphene and they also maintained the superhydrophobicity in harsh chemical environment [100].
5. Applications
5.1. Corrosion resistance
Corrosion generally describes the damage to metal or its alloy by means of chemical or electrochemical interactive reactions that occur on the surface of the metal due to the environmental exposure. This serious problem leading to the loss of metal as scrap is a major economic issue faced throughout the world. Oiling, painting, electroplating etc. are the common methods followed to avoid this damage [101]. Protective coatings are the viable practical solutions for corrosion and hence superhydrophobic surfaces are being used effectively [102]. Hammer et al. [103] developed anticorrosive hybrid surfaces of carbon nanotube incorporated siloxane-PMMA aiming at the protection of metals. From literature, it is understood that superhydrophobic nanocoatings are very effective in forming anticorrosive surfaces and the major suggested corrosion mechanism is the presence of air pockets. Being chemically inert, graphene has the potential to function as an effective coating material due to its possible superhydrophobicity [27]. The presence of lattice structure and its inherent ability to form single as well as multilayer coatings enable graphene to function as a potential anticorrosive coating material. Yu et al. [104] used vinyl-polystyrene/GO (V-PS/GO) based hybrid coatings for imparting anticorrosive properties. The use of graphene sheets in the polymer matrix enabled an extra corrosion protection to metals. This happens because it is extremely difficult for the molecules of the electrolyte to travel into the defective sites of graphene since it becomes immobilized in the polymer matrix [23].
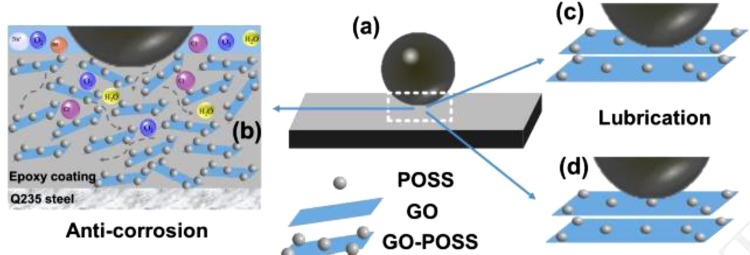
Ye et al. [105] modified GO using Polyhedral Oligomeric Silsesquioxane(POSS and incorporated it in the epoxy resin. This composite material was then coated over mild steel. The hydrophilic characteristics of GO was overcome using POSS and a CA of 153.8° was observed. The coatings were able to avoid the penetration of the corrosive liquid, and the incorporated POSS was capable of enhancing the spacing and flatness of GO causing complications in the penetrating path as shown in Fig. 15 .
Fig. 15.
Corrosion protection and lubrication mechanism of 0.5 wt% GP/EP specimen [105]. Reproduced with permission from Elsevier.
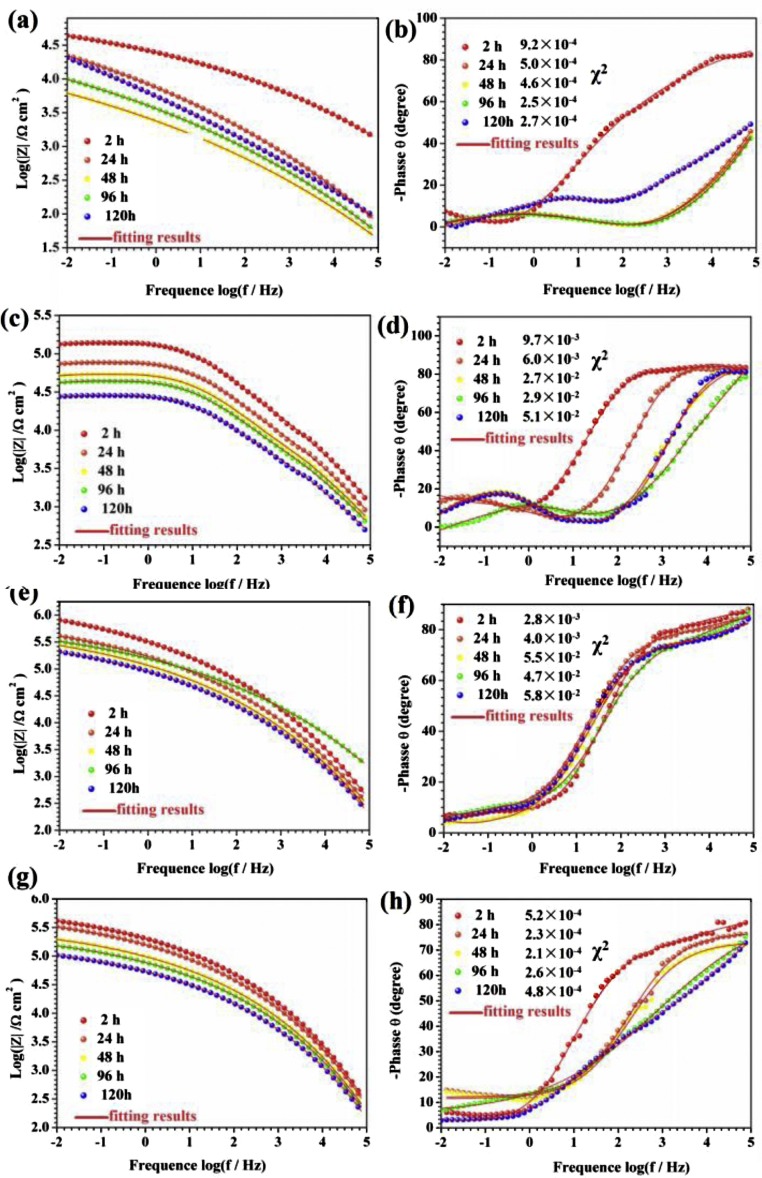
Yang et al. [106] used flurographene(FG) incorporated epoxy for coating applications. The superhydrophobic coatings show excellent anticorrosion property as revealed from Electrochemical impedance spectra (EIS) and Potentiodynamic polarization. They compared the effectiveness of the coating by comparing the polarization curves of blank, GO and FG incorporated epoxy coatings in NaCl solution. Positive shifts were obtained for GO and FG incorporated epoxy coatings over blank coating. From the EIS spectra, it can be seen that the effectiveness of FG incorporated epoxy is due to the air trapped between the solid surface and the liquid medium. The entrapped air is capable of acting like a barrier which restricts the penetration of aggressive reactive species to the surface. In another study, Ding et al. [107] used novel hydroxyl epoxy phosphate monomer (PGHEP) for dispersing graphene in epoxy resin. Using the dispersed graphene/waterborne epoxy, they have developed microstructures containing PGHEP. The functionalized graphene/epoxy showed superhydrophobicity with excellent anti-corrosion properties as evident from salt spray test and EIS. The EIS spectra shows that PGHEP based coatings have highest modulus due to the enhanced anti-corrosion ability. In the case of water born epoxy coating, after an immersion of 120 h in NaCl, the modulus and phase angle decreased due to the approach of corrosive ions to the surface. But the PGHEP incorporated coating showed constant phase angle throughout the immersion time due to excellent corrosion resistance (Fig. 16 ).
Fig. 16.
EIS curves of of (a) WEP, (a-b) PGHEP/WEP(c-d) PGHEP-G0.5 %/WEPand (e-f) PGHEP-G1%/WEP(g-h) coatingsimmersed in3.5 % NaCl solution during 120 hof immersion [107]. Reproduced with permission from Elsevier.
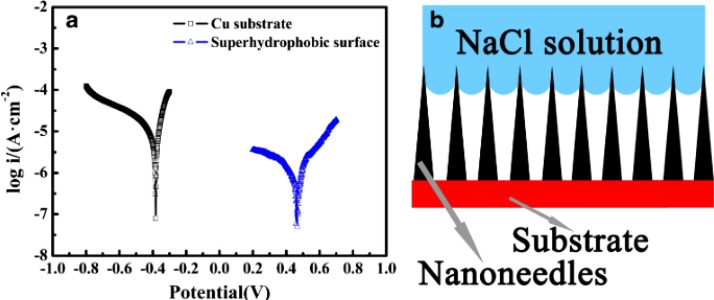
Ye et al. [108] made POSS-GO based epoxy superhydrophobic coatings with excellent anti-corrosive properties for advanced application in marine environment. Open Circuit potential (OCP) revealed the excellent anti-corrosive nature of the epoxy coating. High OCP is due to high stability against harsh environment and as the immersion time increases, the bare epoxy coating shows a reduction in OCP values. But the POSS-GO/epoxy coating showed the lowest corrosion trend. Fan et al. [109] made a hybrid material of GO@CU silicate with a nano hierarchal structure for coating purpose with higher surface roughness. This hybrid coating material displays a CA of 152°. To reduce the surface energy, a silane modification was carried out and coatings were made by following the drop-coating method. As the loadings of the coating increased, the WCA also showed an enhancement. Tafel plots (Fig. 17 ) show the enhanced anti-corrosive nature of the super-hydrophobic surface from the lower corrosion current. This enhanced anti-corrosiveness is due to the nano-needle shaped hierarchical structure.
Fig. 17.
Tafel plots of bare copper and superhydrophobic samples (a) and schematic views of corrosive liquid on superhydrophobic surfaces (b) [109]. Reproduced with permission from springer.
5.2. Oil–water separation
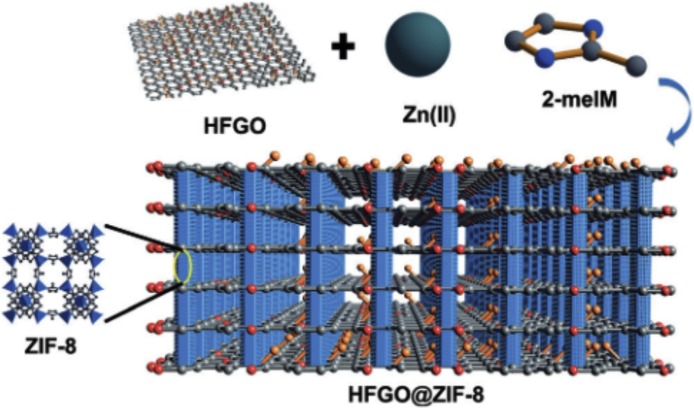
The presence of organic pollutants in water is a major threat to the ecosystem. Hence oil –water separation is considered as a major solution for environmental problems. Oil water separation is a problem that is gaining momentum in the present scenario. Hence the development of oil water separating materials from superhydrophobic graphene coatings is considered very important. Conventional materials used for this process include zeolites, activated carbon, natural clays, straw, wool fibers etc. These materials exhibit low efficiency in the separation process due to their hydrophilic nature. Hence the fabrication of novel superhydrophobic materials is the need of the hour. Superhydrophobic surfaces that are oleophilic or super oleophilic can be employed to separate oil and water by concentrating oil into a semi solid phase. Tai and co-workers employed sponges for effective oil water separation [61]. GO sponges devoid of any positively charged groups exhibit the capability to adsorb anionic dyes via π–π interactions [110]. Jayaramalu et al. [111] made a biomimetic composite for oil-water separation using highly fluorinated GO and nanocrystalline zeolite imidazole framework ZIF-8 (Fig. 18 ). This oil water separation is necessary to avoid the spilled oil in oceans and other water sources. Due to the self-assembly of the micro-mesoporous structure, the material was able to show a WCA of 162° with an oil CA of 0°.
Fig. 18.
Illustration showing the concept of the formation and structure of HFGO@ZIFZIF-8:Zn(meIM)2,meIM = 2-methylimidazole,HFGO = highlyfluorinated graphene oxide [111]. Reproduced with permission from Wiley.
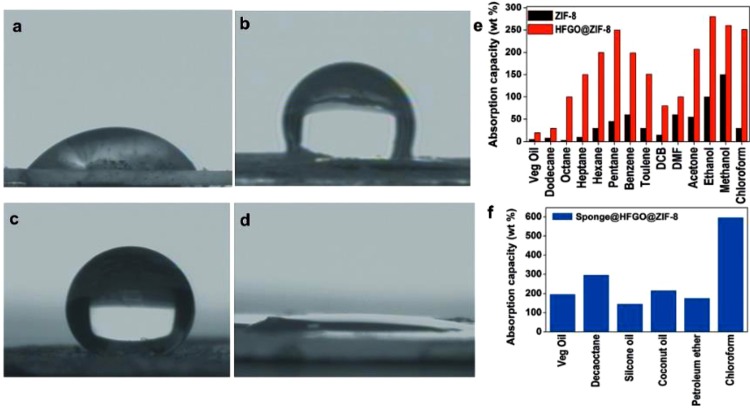
The sponges incorporated with these hybrid materials were able to show high absorption capacity to oils, polar and non-polar solvents. Since these sponges float over water, they can easily extract oil. Oil absorption capacity of the sponge and the hybrids are shown in Fig. 19 .
Fig. 19.
PhotographsdemonstratingthehydrophobicityofZIF-8withawatercontactangleof5688(a)andHFGOwithawatercontactangleof12588(b),andthesuperhydrophobicityoftheHFGO@ZIF-8hybridshowingwatercontactangleof16288(c)andanoilcontactangleof088(d).e)AbsorptionofoilandorganicsolventswithZIF-8andtheHFGO@ZIF-8composite(DCB:dichlorobenzene)andf) oilabsorptionwithSponge@HFGO@ZIF-8(111). Reproduced with permission from Wiley.
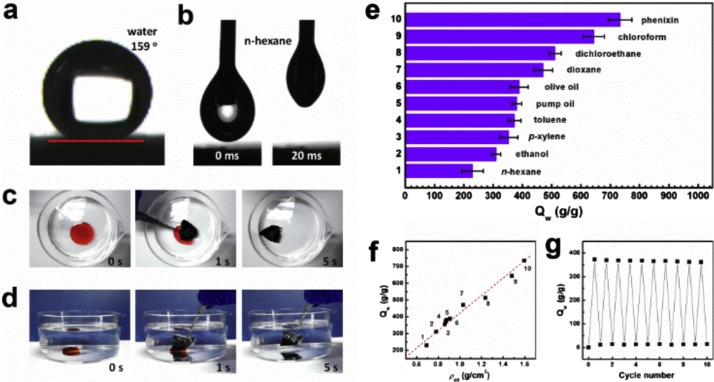
Dashairya et al. [112] made superolieophilic and superhydrophobic rGO/Cotton fabrics for oil-water separation. The fabrics showed a WCA of 162.9° and oil retention of about 35–50 %. Moreover the reusability of the fabrics was confirmed by the sorption-mechanical squeezing test. The absorption capacity was determined by simply immersing the cotton/rGO fabric in inorganic solvents and oil. The soaked fabric absorbed the oil and the absorbed oil was separated by simple squeezing and are suitable for the reuse. It was also suitable for removing oil/solvents about 40–60 times of its weight. Cao et al. [113] made anticorrosive and superhydrophobic coatings using fluorinated polydopamine/chitosan/rGO composite. By following the self-polymerization, polydopamine was anchored onto the surface of rGO. 3D structured chitosan/rGO hybrid hydrogels had oil absorption capacity 12–21 times higher than their weight. Nguyen et al. [61] used the superhydrophobic PDMS/GO sponges for oil water separation. These materials were able to absorb the spilled oil to about 54–165 times higher than their weight. But the absorption capacity was found to be reduced after repeated use in the case of oil. Whereas, the sponges were able to retain the absorption capacity to organic solvents even after 5 repeated cycles. Xiao et al. [114] fabricated GO/nanofiber gels for oil-water separation. Higher WCA ensures easy transport of oil into these aerogels. This can be monitored by directly immersing the material into the oil-water medium as shown in Fig. 20 and absorption capacity can be evaluated from its weight. Yang et al. [115] synthesized silica incorporated GO by sol-gel process, for separating oil from water.
Fig. 20.
a) Superhydrophobic surface of GNA-2 (ρ =1.8 mg/cm3, f = 0.5) with a water contact angle 159°. (b) Fast permeation of n-hexane on the surface of GNA-2. c-d) Absorption process of toluene (stained with OR) spreading on water and 1,2-dichloroethane by GNA-2. e) Qw of GNA-2 for various oils and organic solvents. f) Qw as a function of ρfor various organic liquids identified by numbers being the same with those marked in e). g) Recyclability of GNA-2 for the adsorption of toluene within 10 cycles [114]. Reproduced with permission from Elsevier.
Zhou et al. [116] used graphene/polyurethane based superhydrophobic sponge for oil-water separation. It showed an absorption efficiency of 53,000 times higher than its weight due to the superhydrophobic network structure (Fig. 21 ).
Fig. 21.
Photographs of (a) the continuous oil-water separation system, (b) contrast images before and after continuous adsorption, (c) diesel labelled by SudanⅠcollected in conical flask [116]. Reproduced with permission from Elsevier.
Liu et al. [117] fabricated multifunctional mesh films containing GO–SiO2 nanohybrids with underwater oleophobicity and antibacterial property which could be employed for effective oil water separation. Synthesis of graphene/melamine composite sponge with enhanced diesel absorption [99.0 g/g] was carried out by Liu et al. [118] It was also found that the sponge had excellent mechanical and environmental stability. Similar works on graphene sponge was carried out by Zhao et al. [119].
5.3. Self-cleaning
Self-cleaning can be achieved by following judicious surface engineering techniques to enhance the surface roughness as well as superhydrophobicity. The most important parameter that is to be taken care of while designing self-cleaning surfaces is the adhesion of material onto the surface and its durability. Literature shows that the hierarchical structures as well as epicuticular wax on the leaves are the main factors responsible for the hydrophobicity and self-cleaning ability. Due to the high contact angle and low surface area, dirt can be easily removed by the rolling water droplets. Bai et al. [72] evaluated the self-cleaning ability of rGO/Ni surfaces by spreading graphite powder over it. Due to the higher contact angle of 162.7°, the graphite powder was washed out easily by water. Karimi et al. [126] included graphene oxide/titanium oxide hybrids on fabric via a simple dip coating method to fabricate self-cleaning fabrics. Nine et al. [83] made superhydrophobic GO based self-cleaning surfaces. The self-cleaning properties were analysed by coating DE, DE/TiO2 and DE/TiO2/rGO on glass substrates. In one of our works, we have made porous titnia-GO hybrids [86], whose self-cleaning properties are under evaluation. Fan et al. [109] utilized hierarchical nano-needle like arrangements of GO@Cu Silicate for self-cleaning applications. The dust particles over the GO@Cu Silicate surfaces were effectively removed by the water droplets as shown in Fig. 22 . Yang et al. [106] also utilized the superhydrophobic nature of graphene in making self-cleaning coatings. Wang et al. [64] prepared a superhydrophobic surface via painting a mixture of electrochemically exfoliated graphene and polydimethylsiloxane. The as-framed superhydrophobic surface exhibited excellent self-cleaning and smaller corrosion current density. Ding et al. [102] coated nickel/graphene hybrid on mild steel via electrodeposition method and then modified it by mystiric acid. It exhibited anticorrosion performance, self -cleaning and excellent superhydrophobicity with static water contact angle 160.4 + 1.5 and sliding angle 4.0 + 0.9. Self-cleaning and anti-corrosive superhydrophobic surface with excellent mechanical durability even after 100 cycles of abrasion can be made by the rGO/Ni composite material [92]. General properties and applications of graphene based superhydrophobic surfaces are tabulated in Table 2 .
Fig. 22.
Spread dust particles (left) and self-cleaned superhydrophobic coating (right)(102). Reproduced with permission from Elsevier.
Table 2.
Properties and applications of graphene based superhydrophobic materials.
| Graphene based system | Properties | Applications | Reference |
|---|---|---|---|
| POSS-GO Nanosheets/Epoxy | WCA of 153.8°, Strong physical barrier property towards NaCl with 0.5 wt% of POSS-GO, Improved water repellency of epoxy by POSS-GO | Anticorrosive coatings | [105] |
| Flurographene(FG)/Epoxy | WCA>155°, Excellent anticorrosive activity 3.5 wt% of NaCl due to trapped air in the interface and maintenance of superhydrophobicity even after immersing in solutions of different pH. Anti-corrosive activities were confirmed from EIS and potentiodynamic polarization, Improved water repellency | Excellent self-cleaning and pollution protection ability | [106] |
| Waterborne Graphene/Epoxy Coating |
WCA of 152°, Act as anodic inhibitor for steel protection in 3.5 wt% NaCl and showed higher impedance modulus, Improved repellency than waterborne epoxy | Corrosion resistance coating | [107] |
| GO@CU silicate | WCA of 152°, Air protection shield and oxygen diffusion resistance shows enhanced anticorrosive property, higher corrosion potential and lower corrosion current, Enhanced water repellency | Higher CA and low SA showing enhanced self-cleaning | [109] |
| Flurinated GO/ ZIF-8 Composites | WCA of 162° and oil contact angle 0°, | Oil-water separation, self-cleaning ability, Excellent absorption to all polar and non-polar organic solvents | [111] |
| rGO/Cotton fabric | WCA of 162°, oil-water separation behavior, selectivity, absorption efficiency and reusability | Oil-water separation, Reusable after squeezing | [112] |
| polydopamine/chitosan/rGO aerogel (fCGA) and chitosan/rGO aerogel (CGA) | CGA have superamphiphilicity in air and superoleophobicity in water, fCGA have superhydrophobicity, excellent performance, high oil-water separation efficiency, mechanically robust, high thermal and chemical stability, absorbs oil 21 times higher than its weight | Utilized for oil-water separation from oil waste water and oil-spill accidents, fabrication of anti-corrosive materials | [113] |
| PDMS/GO sponges | WCA>160°, Selective separation of oil, recyclability, absorbs oil of about 165 times higher than its weight. | Removal of organic pollutants and oil spill accident treatment | [61] |
| GO/nanofiber aerogels | WCA of 159°, oil-water separation ability, high oil-adsorption capacity, reusability, squeezing removes secondary pollutants, low cost, compressive elasticity | Absorption of organic solvents, water treatment | [114] |
| silica incorporated GO foam | WCA of 158°, increased surface roughness, higher absorption capacity and recyclable, anti-corrosive | Purification of contaminated water, removal of organic solvents | [115] |
| graphene/polyurethane sponge | Adsorption of oil 53,000 times more than its weight, WCA>160°, chemically robust, | Water purification | [116] |
| Graphene coating | WCA>153°, Excellent Abrasion resistance, Corrosion resistance, | Anticorrosive coating | [26] |
| rGO/Ni | WCA>162.7°, Durable even after 100 cycles of abrasion, Better anti-corrosive character, Chemical resistance | Fabrication of Self-cleaning surface, | [72] |
| DE/TiO2/rGO composite | WCA of 170°, 96.78 % corrosion resistance, excellent abrasion resistance, Bouncing and rolling removed dirt | Self-cleaning surface | [83] |
| nickel/graphene hybrid | WCA of 160°, long term durable, mechanical and chemical resistance | Self-cleaning | [102] |
| rGO/Ni composite | WCA of 155.8°, retains superhydrophobicity even 100 cycles of mechanical abrasion, 99.98 % chemical inhibition | Self-cleaning, anti-corrosive surface | [92] |
| closed shell rGO | WCA 152.7°, fast response to external damage, switchable hydrophobic character | Self-sensing smart materials | [120] |
| rGO | WCA of 154°, fragility, selective and sensitive sensing of NO2 | Superhydrophobic sensing materials | [121] |
| fluorine-based graphene oxide (GO)-DE/epoxy composite | WCA of 155.6°, mechanical robustness, durability | Anti-icing applications | [122] |
| rGO/polyimide | WCA of 141°, High self-rejection performance, long term use, self-cleaning | Reusable and recyclable mask | [123] |
| Graphene/silica/MWCNT | WCA of 167°, superhydrophobicity combined joule heating, icephobicity, abrasion resistance, chemical resistance | Anti-icing applications | [124] |
| Silica nanoparticles functionalized GO | WCA of 173°, Chemical stability, mechanical durability | Superhydrophobic antibacterial coatings | [125] |
5.4. Other applications
Anti-bacterial property is very effective for biosensing and biomedical applications, food packaging and for industrial as well as marine equipment manufacturing. Amine functionalized GO was used recently by Zarafu et al. [127] for fabricating antimicrobial and antibiofilms. These materials are very effective against Gram negative as well as Gram-positive bacteria. Liu et al. [117] made antibacterial mesh films by coating it with GO-silica superhydrophobic nanohybrids. The antibacterial activities were evaluated using E-Coli bacteria before and after coating (Fig. 23 ). The coated mesh films showed better antibacterial efficiency due to the damage of the cell membrane by the direct contact with the hybrid. This further leads to the physical disruption and extraction of lipid membranes of the bacterial cells.
Fig. 23.
a) Escherichia coli (E. coli) were cultured with the bare stainless steel mesh.
(b) Bacteriostatic rings of coated stainless steel mesh [120]. Reproduced with permission from [117]. Reproduced with permission from IWA publishing
Ding et al. [120] used superhydrophobic surface of closed shell rGO for sensing applications. They have utilized the sensing properties of GO and used the closed shell for microdroplet transportation. For this first they placed a water droplet on the CNT/octadecylamine superhydrophobic surface and observed that the droplet moved towards the closed shell rGO which picked up the droplet and transferred it towards the hydrophilic glass surface (Fig. 24 ).
Fig. 24.
Transportation of a water droplet from a superhydrophobic surface to a hydrophilic one using CCGF-20(120).Reproduced with permission from Royal Society of Chemistry.
This mechanism of transportation is associated with the sensing action of rGO by the change in resistance of the closed shell with the approach of water behind it. This is due to the charge transfer between the water droplet and rGO in the closed shell. Wu et al. [121] used rGO based 3D superhydrophobic surface for NO2 sensing application. rGO shows better sensing properties over GO and utilizing this property of rGO they have fabricated a sensor. Superhydrophobic nature reduces the response to humidity and it can be made selective to NO2 gas. In another study, Wu et al. [128] fabricated superhydrophobic graphene sensors that could respond to very low concentrations of NO2 gas (5 ppb).
Graphene coatings were effectively used as an anti-/de-icing composite material for improving the anti-icing and de-icing efficiency of the helicopter rotor. Wang et al. [129] also developed perfluorododecyl modified graphene nanoribbon films for the fabrication of anti-icing surfaces. In another study, Shateri-Khalilabad and Yazdanshenas [65] invented electro conductive superhydrophobic coating from graphene/polymethylsiloxane composite. A superhydrophobic surface should be tolerant to repeated icing and freezing for better applications. Hence Bai et al. [122] made mechanically stable GO/DE/epoxy coating with enhanced anti-icing properties. In order to analyse this property, they have frozen water droplet over the coating to −10 °C for 2760s. When compared with other results, (Table 3 ) this coating was able to show high freezing delay time (FDT). Recently, graphene based superhydrophobic surgical mask having self-cleaning activity and recycled by direct solar driven desalination process have been developed. This mask made by laser fabrication is reported as capable of blocking the respiration droplets [123]. Such innovative ideas are especially relevant in the scenario of the outbreak of pandemic. It is observed that the superhydrophobic surface made of titania can be made antibacterial by incorporating GO [125].Even though graphene shows excellent optical absorption, adsorption is not that much efficient, hence the incorporation of plasmonic nanoparticles together with graphene could enhance the adsorption behavior of the mask. Superhydrophobicity together with electrothermal efficiency helps the surface to remain dry and clean in the glaze ice condition [124]. The removal of surface ice can be easily done in graphene due to its highly hierarchical structure and moreover the partially embedded structure shows excellent durability against sand paper abrasion [124].
Table 3.
FDT of GO-DE/EP specimens and comparison with reports in literatures [122]. Reproduced with permission from Elsevier.
6. Conclusions and future perspectives
The review gives an overall idea about the fabrication and application of graphene based superhydrophobic surfaces. The effectiveness of superhydrophobic coatings and the advantages of graphene and its derivatives in the enhancement of superhydrophobicity of various metals as well as alloy substrates are depicted in a comprehensive manner. The possibility of surface engineering of graphene and its different forms for superhydrophobic coatings is in its preliminary stage and is a rapidly developing field of research. Even though the research on superhydrophobic materials is attaining wider attention, only limited works on graphene based superhydrophobic coatings are reported. Thus the functionalization of graphene and its derivatives can bring about a tremendous advancement in the area of superhydrophobic materials and consequently tune their properties for tailor made applications. Superhydrophobic reusable and recyclable facial masks having excellent antibacterial and virucidal properties are of top priority due to the outbreak of pandemic Covid-19. Excellent superhydrophobic masks capable of blocking respiration droplets and viruses can bring about a tremendous change in health care. The antibacterial, and mechanical robustness of functionalized and hybrid graphene based superhydrophobic materials can be exploited for making cost effective facial masks and PP kits. Apart from medical applications, graphene based superhydrophobic materials can also be utilized for anti-icing, sensing, pollution control and self-cleaning applications. Switchable superhydrophobic graphene based surfaces can also be utilized in the development of smart materials. Thus there lies a vast prospect for graphene based superhydrophobic surfaces in the development of value added products in future.
Declaration of Competing Interest
The authors declare that they have no known competing financial interestsor personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors would like to thank Amrita Vishwa Vidyapeetham for the financial support.
References
- 1.Sun T., Wang G., Feng L., Liu B., Ma Y., Jiang L., et al. Reversible switching between superhydrophilicity and superhydrophobicity. Angew. Chem. Int. Ed. 2004;43(3):357–360. doi: 10.1002/anie.200352565. [DOI] [PubMed] [Google Scholar]
- 2.Nosonovsky M., Hejazi V. Why superhydrophobic surfaces are not always icephobic. ACS Nano. 2012;6(10):8488–8491. doi: 10.1021/nn302138r. [DOI] [PubMed] [Google Scholar]
- 3.Enríquez E., Fernández J.F., De Frutos J., De la Rubia M.A. Tailoring of the electrical properties of carbon black–silica coatings for de-icing applications. Ceram. Int. 2015;41(2):2735–2743. [Google Scholar]
- 4.Tee B.C.K., Wang C., Allen R., Bao Z. An electrically and mechanically self-healing composite with pressure-and flexion-sensitive properties for electronic skin applications. Nat. Nanotechnol. 2012;7(12):825. doi: 10.1038/nnano.2012.192. [DOI] [PubMed] [Google Scholar]
- 5.Chen F., Song J., Lu Y., Huang S., Liu X., Sun J., et al. Creating robust superamphiphobic coatings for both hard and soft materials. J. Mater. Chem. A Mater. Energy Sustain. 2015;3(42):20999–21008. [Google Scholar]
- 6.Ma M., Hill R.M. Superhydrophobic surfaces. Curr. Opin. Colloid Interface Sci. 2006;11(4):193–202. [Google Scholar]
- 7.Hwang G.B., Patir A., Page K., Lu Y., Allan E., Parkin I.P. Buoyancy increase and drag-reduction through a simple superhydrophobic coating. Nanoscale. 2017;9(22):7588–7594. doi: 10.1039/c7nr00950j. [DOI] [PubMed] [Google Scholar]
- 8.Adams J., King C., Ma N. 2010. Global Research Report. Africa Leeds, UK Evidence, A Thomson Reuters Bus. [Google Scholar]
- 9.Si Y., Guo Z. Superhydrophobic nanocoatings: from materials to fabrications and to applications. Nanoscale. 2015;7(14):5922–5946. doi: 10.1039/c4nr07554d. [DOI] [PubMed] [Google Scholar]
- 10.Guo Z.-G., Zhou F., Hao J.-C., Liang Y.-M., Liu W.-M., Huck W.T.S. “Stick and slide” ferrofluidic droplets on superhydrophobic surfaces. Appl. Phys. Lett. 2006;89(8):81911. [Google Scholar]
- 11.Li H., Wang X., Song Y., Liu Y., Li Q., Jiang L., et al. Super‐“amphiphobic” aligned carbon nanotube films. Angew. Chem. Int Ed. 2001;40(9):1743–1746. doi: 10.1002/1521-3773(20010504)40:9<1743::aid-anie17430>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 12.Wenzel R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936;28(8):988–994. [Google Scholar]
- 13.Cassie A.B.D., Baxter S. Wettability of porous surfaces. Trans Faraday Soc. 1944;40:546–551. [Google Scholar]
- 14.Yazdanshenas M.E., Shateri-Khalilabad M. One-step synthesis of superhydrophobic coating on cotton fabric by ultrasound irradiation. Ind. Eng. Chem. Res. 2013;52(36):12846–12854. [Google Scholar]
- 15.Seyedmehdi S.A., Zhang H., Zhu J. Superhydrophobic RTV silicone rubber insulator coatings. Appl. Surf. Sci. 2012;258(7):2972–2976. [Google Scholar]
- 16.Hong D., Ryu I., Kwon H., Lee J.-J., Yim S. Preparation of superhydrophobic, long-neck vase-like polymer surfaces. Phys. Chem. Chem. Phys. 2013;15(28):11862–11867. doi: 10.1039/c3cp51833g. [DOI] [PubMed] [Google Scholar]
- 17.Yuan Z., Bin J., Wang X., Liu Q., Zhao D., Chen H., et al. Preparation and anti‐icing property of a lotus‐leaf‐like superhydrophobic low‐density polyethylene coating with low sliding angle. Polym. Eng. Sci. 2012;52(11):2310–2315. [Google Scholar]
- 18.Huovinen E., Takkunen L., Korpela T., Suvanto M., Pakkanen T.T., Pakkanen T.A. Mechanically robust superhydrophobic polymer surfaces based on protective micropillars. Langmuir. 2014;30(5):1435–1443. doi: 10.1021/la404248d. [DOI] [PubMed] [Google Scholar]
- 19.Qing Y., Zheng Y., Hu C., Wang Y., He Y., Gong Y., et al. Facile approach in fabricating superhydrophobic ZnO/polystyrene nanocomposite coating. Appl. Surf. Sci. 2013;285:583–587. [Google Scholar]
- 20.Novoselov K.S., Geim A.K., Morozov S.V., Jiang D., Zhang Y., Dubonos S.V., et al. Electric field effect in atomically thin carbon films. Science (80-) 2004;306(5696):666–669. doi: 10.1126/science.1102896. [DOI] [PubMed] [Google Scholar]
- 21.Yang S.-T., Chen S., Chang Y., Cao A., Liu Y., Wang H. Removal of methylene blue from aqueous solution by graphene oxide. J. Colloid Interface Sci. 2011;359(1):24–29. doi: 10.1016/j.jcis.2011.02.064. [DOI] [PubMed] [Google Scholar]
- 22.Yeh J.-M., Liou S.-J., Lin C.-Y., Cheng C.-Y., Chang Y.-W., Lee K.-R. Anticorrosively enhanced PMMA− clay nanocomposite materials with quaternary alkylphosphonium salt as an intercalating agent. Chem. Mater. 2002;14(1):154–161. [Google Scholar]
- 23.Jayatissa A.H., Nadarajah A., Dutta A.K. Nanofabrication: Technologies, Devices, and Applications II. International Society for Optics and Photonics; 2005. Investigation of C 60 films for surface finishing applications. p. 60021A. [Google Scholar]
- 24.Wang J.-N., Zhang Y.-L., Liu Y., Zheng W., Lee L.P., Sun H.-B. Recent developments in superhydrophobic graphene and graphene-related materials: from preparation to potential applications. Nanoscale. 2015;7(16):7101–7114. doi: 10.1039/c5nr00719d. [DOI] [PubMed] [Google Scholar]
- 25.Singh B.P., Nayak S., Nanda K.K., Jena B.K., Bhattacharjee S., Besra L. The production of a corrosion resistant graphene reinforced composite coating on copper by electrophoretic deposition. Carbon. 2013;61:47–56. [Google Scholar]
- 26.Liu Y., Zhang J., Li S., Wang Y., Han Z., Ren L. Fabrication of a superhydrophobic graphene surface with excellent mechanical abrasion and corrosion resistance on an aluminum alloy substrate. RSC Adv. 2014;4(85):45389–45396. [Google Scholar]
- 27.Bunch J.S., Verbridge S.S., Alden J.S., Van Der Zande A.M., Parpia J.M., Craighead H.G., et al. Impermeable atomic membranes from graphene sheets. Nano Lett. 2008;8(8):2458–2462. doi: 10.1021/nl801457b. [DOI] [PubMed] [Google Scholar]
- 28.Li X., Du X., He J. Self-cleaning antireflective coatings assembled from peculiar mesoporous silica nanoparticles. Langmuir. 2010;26(16):13528–13534. doi: 10.1021/la1016824. [DOI] [PubMed] [Google Scholar]
- 29.Li Y., Liu F., Sun J. A facile layer-by-layer deposition process for the fabrication of highly transparent superhydrophobic coatings. Chem. Commun. (Camb.) 2009;(19):2730–2732. doi: 10.1039/b900804g. [DOI] [PubMed] [Google Scholar]
- 30.Bravo J., Zhai L., Wu Z., Cohen R.E., Rubner M.F. Transparent superhydrophobic films based on silica nanoparticles. Langmuir. 2007;23(13):7293–7298. doi: 10.1021/la070159q. [DOI] [PubMed] [Google Scholar]
- 31.Jung Y.C., Bhushan B. Mechanically durable carbon nanotube− composite hierarchical structures with superhydrophobicity, self-cleaning, and low-drag. ACS Nano. 2009;3(12):4155–4163. doi: 10.1021/nn901509r. [DOI] [PubMed] [Google Scholar]
- 32.Li F., Li Q., Kim H. Spray deposition of electrospun TiO2 nanoparticles with self-cleaning and transparent properties onto glass. Appl. Surf. Sci. 2013;276:390–396. [Google Scholar]
- 33.Cengiz U., Avci M.Z., Erbil H.Y., Sarac A.S. Superhydrophobic terpolymer nanofibers containing perfluoroethyl alkyl methacrylate by electrospinning. Appl. Surf. Sci. 2012;258(15):5815–5821. [Google Scholar]
- 34.Söz C.K., Yilgör E., Yilgör I. Influence of the average surface roughness on the formation of superhydrophobic polymer surfaces through spin-coating with hydrophobic fumed silica. Polymer (Guildf) 2015;62:118–128. [Google Scholar]
- 35.Liu Q., Chen D., Kang Z. One-step electrodeposition process to fabricate corrosion-resistant superhydrophobic surface on magnesium alloy. ACS Appl. Mater. Interfaces. 2015;7(3):1859–1867. doi: 10.1021/am507586u. [DOI] [PubMed] [Google Scholar]
- 36.Wang P., Zhang D., Lu Z. Advantage of super-hydrophobic surface as a barrier against atmospheric corrosion induced by salt deliquescence. Corros. Sci. 2015;90:23–32. [Google Scholar]
- 37.Khorsand S., Raeissi K., Ashrafizadeh F. Corrosion resistance and long-term durability of super-hydrophobic nickel film prepared by electrodeposition process. Appl. Surf. Sci. 2014;305:498–505. [Google Scholar]
- 38.Chen Z., Hao L., Chen C. Simultaneous fabrication of superhydrophobic coatings on cathodic and anodic copper surfaces with micro/nano-structures. ECS Electrochem. Lett. 2012;1(4):D21–3. [Google Scholar]
- 39.Rezaei S., Manoucheri I., Moradian R., Pourabbas B. One-step chemical vapor deposition and modification of silica nanoparticles at the lowest possible temperature and superhydrophobic surface fabrication. Chem. Eng. J. 2014;252:11–16. [Google Scholar]
- 40.Tung W.S., Daoud W.A. Self-cleaning fibers via nanotechnology: a virtual reality. J. Mater. Chem. 2011;21(22):7858–7869. [Google Scholar]
- 41.Bhushan B., Jung Y.C., Koch K. Micro-, nano-and hierarchical structures for superhydrophobicity, self-cleaning and low adhesion. Philos. Trans. R. Soc. A Math Phys. Eng. Sci. 2009;367(1894):1631–1672. doi: 10.1098/rsta.2009.0014. [DOI] [PubMed] [Google Scholar]
- 42.Gao L., McCarthy T.J., Zhang X. Wetting and superhydrophobicity. Langmuir. 2009;25(24):14100–14104. doi: 10.1021/la903043a. [DOI] [PubMed] [Google Scholar]
- 43.Ganesh V.A., Raut H.K., Nair A.S., Ramakrishna S. A review on self-cleaning coatings. J. Mater. Chem. 2011;21(41):16304–16322. [Google Scholar]
- 44.Liu K., Li Z., Wang W., Jiang L. Facile creation of bio-inspired superhydrophobic Ce-based metallic glass surfaces. Appl. Phys. Lett. 2011;99(26) [Google Scholar]
- 45.Liu K., Jiang L. Bio-inspired self-cleaning surfaces. Annu. Rev. Mater. Res. 2012;42:231–263. [Google Scholar]
- 46.Peng C., Xing S., Yuan Z., Xiao J., Wang C., Zeng J. Preparation and anti-icing of superhydrophobic PVDF coating on a wind turbine blade. Appl. Surf. Sci. 2012;259:764–768. [Google Scholar]
- 47.Boinovich L., Emelyanenko A.M. Role of water vapor desublimation in the adhesion of an iced droplet to a superhydrophobic surface. Langmuir. 2014;30(42):12596–12601. doi: 10.1021/la503447f. [DOI] [PubMed] [Google Scholar]
- 48.Yang J., Li W. Preparation of superhydrophobic surfaces on Al substrates and the anti-icing behavior. J. Alloys. Compd. 2013;576:215–219. [Google Scholar]
- 49.Cao L., Jones A.K., Sikka V.K., Wu J., Gao D. Anti-icing superhydrophobic coatings. Langmuir. 2009;25(21):12444–12448. doi: 10.1021/la902882b. [DOI] [PubMed] [Google Scholar]
- 50.Privett B.J., Youn J., Hong S.A., Lee J., Han J., Shin J.H., et al. Antibacterial fluorinated silica colloid superhydrophobic surfaces. Langmuir. 2011;27(15):9597–9601. doi: 10.1021/la201801e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Banerjee I., Pangule R.C., Kane R.S. Antifouling coatings: recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms. Adv Mater. 2011;23(6):690–718. doi: 10.1002/adma.201001215. [DOI] [PubMed] [Google Scholar]
- 52.Mi H.-Y., Jing X., Huang H.-X., Peng X.-F., Turng L.-S. Superhydrophobic graphene/cellulose/silica aerogel with hierarchical structure as superabsorbers for high efficiency selective oil absorption and recovery. Ind. Eng. Chem. Res. 2018;57(5):1745–1755. [Google Scholar]
- 53.Hsieh C.-T., Chen W.-Y. Water/oil repellency and work of adhesion of liquid droplets on graphene oxide and graphene surfaces. Surf Coat. Technol. 2011;205(19):4554–4561. [Google Scholar]
- 54.Kirkland N.T., Schiller T., Medhekar N., Birbilis N. Exploring graphene as a corrosion protection barrier. Corros. Sci. 2012;56:1–4. [Google Scholar]
- 55.Varela-Rizo H., Rodriguez-Pastor I., Merino C., Martin-Gullon I. Highly crystalline graphene oxide nano-platelets produced from helical-ribbon carbon nanofibers. Carbon. 2010;48(12):3640–3643. [Google Scholar]
- 56.Hansora D.P., Mishra S. CRC Press; 2017. Graphene Nanomaterials: Fabrication, Properties, and Applications. [Google Scholar]
- 57.Hussain A.K., Sudin I., Basheer U.M., Yusop M.Z.M. A review on graphene-based polymer composite coatings for the corrosion protection of metals. Corros Rev. 2019;37(4):343–363. [Google Scholar]
- 58.Nilsson L., Andersen M., Balog R., Lægsgaard E., Hofmann P., Besenbacher F., et al. Graphene coatings: probing the limits of the one atom thick protection layer. ACS Nano. 2012;6(11):10258–10266. doi: 10.1021/nn3040588. [DOI] [PubMed] [Google Scholar]
- 59.Choi B.G., Park H.S. Superhydrophobic graphene/nafion nanohybrid films with hierarchical roughness. J. Phys. Chem. C. 2012;116(5):3207–3211. [Google Scholar]
- 60.Lee J.-S., Yoon J.-C., Jang J.-H. A route towards superhydrophobic graphene surfaces: surface-treated reduced graphene oxide spheres. J. Mater. Chem. A Mater. Energy Sustain. 2013;1(25):7312–7315. [Google Scholar]
- 61.Nguyen D.D., Tai N.-H., Lee S.-B., Kuo W.-S. Superhydrophobic and superoleophilic properties of graphene-based sponges fabricated using a facile dip coating method. Energy Environ. Sci. 2012;5(7):7908–7912. [Google Scholar]
- 62.Li M., Ji X., Cui L., Liu J. In situ preparation of graphene/polypyrrole nanocomposite via electrochemical co-deposition methodology for anti-corrosion application. J. Mater. Sci. 2017;52(20):12251–12265. [Google Scholar]
- 63.Zhang L., Li H., Lai X., Su X., Liang T., Zeng X. Thiolated graphene-based superhydrophobic sponges for oil-water separation. Chem. Eng. J. 2017;316:736–743. [Google Scholar]
- 64.Wang P., Yao T., Sun B., Fan X., Dong S., Bai Y., et al. A cost-effective method for preparing mechanically stable anti-corrosive superhydrophobic coating based on electrochemically exfoliated graphene. Colloids Surf. A Physicochem. Eng. Asp. 2017;513:396–401. [Google Scholar]
- 65.Shateri-Khalilabad M., Yazdanshenas M.E. Preparation of superhydrophobic electroconductive graphene-coated cotton cellulose. Cellulose. 2013;20(2):963–972. [Google Scholar]
- 66.Ji C., Zhang K., Li L., Chen X., Hu J., Yan D., et al. High performance graphene-based foam fabricated by a facile approach for oil absorption. J. Mater. Chem. A Mater. Energy Sustain. 2017;5(22):11263–11270. [Google Scholar]
- 67.Asthana A., Maitra T., Büchel R., Tiwari M.K., Poulikakos D. Multifunctional superhydrophobic polymer/carbon nanocomposites: graphene, carbon nanotubes, or carbon black? ACS Appl. Mater. Interfaces. 2014;6(11):8859–8867. doi: 10.1021/am501649w. [DOI] [PubMed] [Google Scholar]
- 68.Jiang H., Zhang Y., Han D., Xia H., Feng J., Chen Q., et al. Bioinspired fabrication of superhydrophobic graphene films by two‐beam laser interference. Adv. Funct. Mater. 2014;24(29):4595–4602. [Google Scholar]
- 69.Wang J., Shao R., Zhang Y., Guo L., Jiang H., Lu D., et al. Biomimetic graphene surfaces with superhydrophobicity and iridescence. Chem. Asian J. 2012;7(2):301–304. doi: 10.1002/asia.201100882. [DOI] [PubMed] [Google Scholar]
- 70.Li Y., Luong D.X., Zhang J., Tarkunde Y.R., Kittrell C., Sargunaraj F., et al. Laser‐Induced Graphene in Controlled Atmospheres: From Superhydrophilic to Superhydrophobic Surfaces. Adv. Mater. 2017;29(27) doi: 10.1002/adma.201700496. [DOI] [PubMed] [Google Scholar]
- 71.Muthu M., Gopal J., Chun S., Lee S.K. Hydrophobic bacteria-repellant graphene coatings from recycled pencil stubs. Arab. J. Sci. Eng. 2018;43(1):241–249. [Google Scholar]
- 72.Bai Z., Zhang B. Fabrication of superhydrophobic reduced-graphene oxide/nickel coating with mechanical durability, self-cleaning and anticorrosion performance. Int. J. Green Nanotechnol. Mater. Sci. Eng. 2019 [Google Scholar]
- 73.Jin J., Wang X., Song M. Graphene-based nanostructured hybrid materials for conductive and superhydrophobic functional coatings. J. Nanosci. Nanotechnol. 2011;11(9):7715–7722. doi: 10.1166/jnn.2011.4730. [DOI] [PubMed] [Google Scholar]
- 74.Wang Y., Yu Y., Hu X., Feng A., Jiang F., Song L. p-Phenylenediamine strengthened graphene oxide for the fabrication of superhydrophobic surface. Mater. Des. 2017;127:22–29. [Google Scholar]
- 75.Yun X., Xiong Z., He Y., Wang X. Superhydrophobic lotus-leaf-like surface made from reduced graphene oxide through soft-lithographic duplication. RSC Adv. 2020;10(9):5478–5486. doi: 10.1039/c9ra10373b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jayadev D., Jayan J.S., Pillai Z.S., Joseph K., Saritha A. Superhydrophobic Polymer Coatings. Elsevier; 2019. Characterization of superhydrophobic polymer coating; pp. 91–121. [Google Scholar]
- 77.Shanmugharaj A.M., Yoon J.H., Yang W.J., Ryu S.H. Synthesis, characterization, and surface wettability properties of amine functionalized graphene oxide films with varying amine chain lengths. J. Colloid Interface Sci. 2013;401:148–154. doi: 10.1016/j.jcis.2013.02.054. [DOI] [PubMed] [Google Scholar]
- 78.Moradi R., Karimi-Sabet J., Shariaty-Niassar M., Koochaki M.A. Preparation and characterization of polyvinylidene fluoride/graphene superhydrophobic fibrous films. Polymers (Basel) 2015;7(8):1444–1463. [Google Scholar]
- 79.Zha D., Mei S., Wang Z., Li H., Shi Z., Jin Z. Superhydrophobic polyvinylidene fluoride/graphene porous materials. Carbon. 2011;49(15):5166–5172. [Google Scholar]
- 80.Jayan J.S., Saritha A., Deeraj B.D.S., Joseph K. Graphene oxide as a prospective graft in polyethylene glycol for enhancing the toughness of epoxy nanocomposites. Polym. Eng. Sci. 2020 [Google Scholar]
- 81.Qiang F., Hu L.-L., Gong L.-X., Zhao L., Li S.-N., Tang L.-C. Facile synthesis of super-hydrophobic, electrically conductive and mechanically flexible functionalized graphene nanoribbon/polyurethane sponge for efficient oil/water separation at static and dynamic states. Chem. Eng. J. 2018;334:2154–2166. [Google Scholar]
- 82.Jayan J.S., Jayadev D., Pillai Z.S., Joseph K., Saritha A. Superhydrophobic Polymer Coatings. Elsevier; 2019. The stability of the superhydrophobic surfaces; pp. 123–159. [Google Scholar]
- 83.Nine M.J., Cole M.A., Johnson L., Tran D.N.H., Losic D. Robust superhydrophobic graphene-based composite coatings with self-cleaning and corrosion barrier properties. ACS Appl. Mater. Interfaces. 2015;7(51):28482–28493. doi: 10.1021/acsami.5b09611. [DOI] [PubMed] [Google Scholar]
- 84.Mo Z.-H., Luo Z., Huang Q., Deng J.-P., Wu Y.-X. Superhydrophobic hybrid membranes by grafting arc-like macromolecular bridges on graphene sheets: synthesis, characterization and properties. Appl. Surf. Sci. 2018;440:359–368. [Google Scholar]
- 85.Guo G., Liu L., Zhang Q., Pan C., Zou Q. Solution-processable, durable, scalable, fluorine-grafted graphene-based superhydrophobic coating for highly efficient oil/water separation under harsh environment. New J. Chem. 2018;42(5):3819–3827. [Google Scholar]
- 86.Jayan J.S., Saritha A., Deeraj B.D.S., Joseph K. Synthesis of self-assembled and porous nano titania-graphene oxide hybrids for toughening the epoxy. Polym. Compos. 2020 doi: 10.1002/pc.25696. First published: 10 July 2020. [DOI] [Google Scholar]
- 87.Zhang L., Zha D., Du T., Mei S., Shi Z., Jin Z. Formation of superhydrophobic microspheres of poly (vinylidene fluoride–hexafluoropropylene)/graphene composite via gelation. Langmuir. 2011;27(14):8943–8949. doi: 10.1021/la200982n. [DOI] [PubMed] [Google Scholar]
- 88.Lin Y., Ehlert G.J., Bukowsky C., Sodano H.A. Superhydrophobic functionalized graphene aerogels. ACS Appl. Mater. Interfaces. 2011;3(7):2200–2203. doi: 10.1021/am200527j. [DOI] [PubMed] [Google Scholar]
- 89.Lin Z., Liu Y., Wong C. Facile fabrication of superhydrophobic octadecylamine-functionalized graphite oxide film. Langmuir. 2010;26(20):16110–16114. doi: 10.1021/la102619n. [DOI] [PubMed] [Google Scholar]
- 90.Jayan J.S., Saritha A., Deeraj B.D.S., Joseph K. Triblock copolymer grafted Graphene oxide as nanofiller for toughening of epoxy resin. Mater. Chem. Phys. 2020 [Google Scholar]
- 91.Zhang C., Liang F., Zhang W., Liu H., Ge M., Zhang Y., et al. Constructing mechanochemical durable and self-healing superhydrophobic surfaces. ACS Omega. 2020;5(2):986–994. doi: 10.1021/acsomega.9b03912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bai Z., Zhang B. Fabrication of superhydrophobic reduced-graphene oxide/nickel coating with mechanical durability, self-cleaning and anticorrosion performance. Int. J. Green Nanotechnol. Mater. Sci. Eng. 2020;2(2):151–158. [Google Scholar]
- 93.Chen C., Zhu X., Chen B. Durable superhydrophobic/superoleophilic graphene-based foam for high-efficiency oil spill cleanups and recovery. Environ. Sci. Technol. 2019;53(3):1509–1517. doi: 10.1021/acs.est.8b04642. [DOI] [PubMed] [Google Scholar]
- 94.Yan H., Zhou H., Ye Q., Wang X., Cho C.M., Tan A.Y.X., et al. Engineering polydimethylsiloxane with two-dimensional graphene oxide for an extremely durable superhydrophobic fabric coating. RSC Adv. 2016;6(71):66834–66840. [Google Scholar]
- 95.Zhang X., Liu D., Ma Y., Nie J., Sui G. Super-hydrophobic graphene coated polyurethane (GN@ PU) sponge with great oil-water separation performance. Appl. Surf. Sci. 2017;422:116–124. [Google Scholar]
- 96.Tittle C.M., Yilman D., Pope M.A., Backhouse C.J. Robust superhydrophobic laser‐induced graphene for desalination applications. Int. J. Adv. Mater. Technol. 2018;3(2) [Google Scholar]
- 97.Chen A., Ding S., Huang J., Zhang J., Dong Y., Fu X., et al. Fabrication of superrepellent microstructured polypropylene/graphene surfaces with enhanced wear resistance. J. Mater. Sci. 2019;54(5):3914–3926. [Google Scholar]
- 98.Wang P., Sun B., Liang Y., Han H., Fan X., Wang W., et al. A stretchable and super-robust graphene superhydrophobic composite for electromechanical sensor application. J. Mater. Chem. A Mater. Energy Sustain. 2018;6(22):10404–10410. [Google Scholar]
- 99.Zhang Z., Liu H., Qiao W. Reduced graphene-based superhydrophobic sponges modified by hexadecyltrimethoxysilane for oil adsorption. Colloids Surf. A Physicochem. Eng. Asp. 2020;589 [Google Scholar]
- 100.Li B., Luo J., Huang X., Lin L., Wang L., Hu M., et al. A highly stretchable, super-hydrophobic strain sensor based on polydopamine and graphene reinforced nanofiber composite for human motion monitoring. Compos. B Eng. 2020;181 [Google Scholar]
- 101.Mohamed A.M.A., Abdullah A.M., Younan N.A. Corrosion behavior of superhydrophobic surfaces: a review. Arab J. Chem. 2015;8(6):749–765. [Google Scholar]
- 102.Ding S., Xiang T., Li C., Zheng S., Wang J., Zhang M., et al. Fabrication of self-cleaning super-hydrophobic nickel/graphene hybrid film with improved corrosion resistance on mild steel. Mater. Des. 2017;117:280–288. [Google Scholar]
- 103.Hammer P., Dos Santos F.C., Cerrutti B.M., Pulcinelli S.H., Santilli C.V. Carbon nanotube-reinforced siloxane-PMMA hybrid coatings with high corrosion resistance. Prog. Org. Coat. 2013;76(4):601–608. [Google Scholar]
- 104.Yu Y.-H., Lin Y.-Y., Lin C.-H., Chan C.-C., Huang Y.-C. High-performance polystyrene/graphene-based nanocomposites with excellent anti-corrosion properties. Polym. Chem. 2014;5(2):535–550. [Google Scholar]
- 105.Ye Y., Zhang D., Liu T., Liu Z., Liu W., Pu J., et al. Improvement of anticorrosion ability of epoxy matrix in simulate marine environment by filled with superhydrophobic POSS-GO nanosheets. J. Hazard. Mater. 2019;364:244–255. doi: 10.1016/j.jhazmat.2018.10.040. [DOI] [PubMed] [Google Scholar]
- 106.Yang Z., Wang L., Sun W., Li S., Zhu T., Liu W., et al. Superhydrophobic epoxy coating modified by fluorographene used for anti-corrosion and self-cleaning. Appl. Surf. Sci. 2017;401:146–155. [Google Scholar]
- 107.Ding J., ur Rahman O., Peng W., Dou H., Yu H. A novel hydroxyl epoxy phosphate monomer enhancing the anticorrosive performance of waterborne graphene/epoxy coatings. Appl. Surf. Sci. 2018;427:981–991. [Google Scholar]
- 108.Ye Y., Zhang D., Li J., Liu T., Pu J., Zhao H., et al. One-step synthesis of superhydrophobic polyhedral oligomeric silsesquioxane-graphene oxide and its application in anti-corrosion and anti-wear fields. Corros. Sci. 2019;147:9–21. [Google Scholar]
- 109.Fan P., Chen J., Yang J., Chen F., Zhong M. GO@ CuSilicate nano-needle arrays hierarchical structure: a new route to prepare high optical transparent, excellent self-cleaning and anticorrosion superhydrophobic surface. J. Nanopart. Res. 2017;19(2):36. [Google Scholar]
- 110.Yousefi N., Lu X., Elimelech M., Tufenkji N. Environmental performance of graphene-based 3D macrostructures. Nat. Nanotechnol. 2019;14(2):107–119. doi: 10.1038/s41565-018-0325-6. [DOI] [PubMed] [Google Scholar]
- 111.Jayaramulu K., Datta K.K.R., Rösler C., Petr M., Otyepka M., Zboril R., et al. Biomimetic superhydrophobic/superoleophilic highly fluorinated graphene oxide and ZIF‐8 composites for oil–water separation. Angew. Chem. Int. Ed. 2016;55(3):1178–1182. doi: 10.1002/anie.201507692. [DOI] [PubMed] [Google Scholar]
- 112.Dashairya L., Rout M., Saha P. Reduced graphene oxide-coated cotton as an efficient absorbent in oil-water separation. Adv. Compos. Hybrid Mater. 2018;1(1):135–148. [Google Scholar]
- 113.Cao N., Lyu Q., Li J., Wang Y., Yang B., Szunerits S., et al. Facile synthesis of fluorinated polydopamine/chitosan/reduced graphene oxide composite aerogel for efficient oil/water separation. Chem. Eng. J. 2017;326:17–28. [Google Scholar]
- 114.Xiao J., Lv W., Song Y., Zheng Q. Graphene/nanofiber aerogels: performance regulation towards multiple applications in dye adsorption and oil/water separation. Chem. Eng. J. 2018;338:202–210. [Google Scholar]
- 115.Yang S., Chen L., Wang C., Rana M., Ma P.-C. Surface roughness induced superhydrophobicity of graphene foam for oil-water separation. J. Colloid Interface Sci. 2017;508:254–262. doi: 10.1016/j.jcis.2017.08.061. [DOI] [PubMed] [Google Scholar]
- 116.Zhou S., Hao G., Zhou X., Jiang W., Wang T., Zhang N., et al. One-pot synthesis of robust superhydrophobic, functionalized graphene/polyurethane sponge for effective continuous oil–water separation. Chem. Eng. J. 2016;302:155–162. [Google Scholar]
- 117.Liu J., He W., Li P., Xia S., Lü X., Liu Z., et al. Synthesis of graphene oxide–SiO2 coated mesh film and its properties on oil–water separation and antibacterial activity. Water Sci. Technol. 2016;73(5):1098–1103. doi: 10.2166/wst.2015.584. [DOI] [PubMed] [Google Scholar]
- 118.Liu T., Zhao G., Zhang W., Chi H., Hou C., Sun Y. The preparation of superhydrophobic graphene/melamine composite sponge applied in treatment of oil pollution. J. Porous Mater. 2015;22(6):1573–1580. [Google Scholar]
- 119.Zhao J., Ren W., Cheng H.-M. Graphene sponge for efficient and repeatable adsorption and desorption of water contaminations. J. Mater. Chem. 2012;22(38):20197–20202. [Google Scholar]
- 120.Ding G., Jiao W., Wang R., Niu Y., Hao L., Yang F., et al. A biomimetic, multifunctional, superhydrophobic graphene film with self-sensing and fast recovery properties for microdroplet transportation. J. Mater. Chem. A Mater. Energy Sustain. 2017;5(33):17325–17334. [Google Scholar]
- 121.Wu J., Li Z., Xie X., Tao K., Liu C., Khor K.A., et al. 3D superhydrophobic reduced graphene oxide for activated NO 2 sensing with enhanced immunity to humidity. J. Mater. Chem. A Mater. Energy Sustain. 2018;6(2):478–488. [Google Scholar]
- 122.Bai Z.-G., Zhang B. Fabrication of a mechanically-stable anti-icing graphene oxide-diatomaceous earth/epoxy coating. Mater. Res. Express. 2019;6(8):85090. [Google Scholar]
- 123.Zhong H., Zhu Z., Lin J., Cheung C.F., Lu V.L., Yan F., et al. Reusable and recyclable graphene masks with outstanding superhydrophobic and photothermal performances. ACS Nano. 2020;14(5):6213–6221. doi: 10.1021/acsnano.0c02250. [DOI] [PubMed] [Google Scholar]
- 124.Wang P., Yao T., Li Z., Wei W., Xie Q., Duan W., et al. A superhydrophobic/electrothermal synergistically anti-icing strategy based on graphene composite. Compos. Sci. Technol. 2020 [Google Scholar]
- 125.Vanithakumari S.C., Jena G., Sofia S., Thinaharan C., George R.P., Philip J. Fabrication of superhydrophobic titanium surfaces with superior antibacterial properties using graphene oxide and silanized silica nanoparticles. Surf. Coat. Technol. 2020 [Google Scholar]
- 126.Karimi L., Yazdanshenas M.E., Khajavi R., Rashidi A., Mirjalili M. Using graphene/TiO 2 nanocomposite as a new route for preparation of electroconductive, self-cleaning, antibacterial and antifungal cotton fabric without toxicity. Cellulose. 2014;21(5):3813–3827. [Google Scholar]
- 127.Zarafu I., Turcu I., Culiță D.C., Petrescu S., Popa M., Chifiriuc M.C., et al. Antimicrobial features of organic functionalized graphene-oxide with selected amines. Materials (Basel) 2018;11(9):1704. doi: 10.3390/ma11091704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wu J., Tao K., Miao J., Norford L.K. 2018 IEEE Micro Electro Mechanical Systems (MEMS) IEEE; 2018. Three-dimensional hierarchical and superhydrophobic graphene gas sensor with good immunity to humidity; pp. 901–904. [Google Scholar]
- 129.Wang T., Zheng Y., Raji A.-R.O., Li Y., Sikkema W.K.A., Tour J.M. Passive anti-icing and active deicing films. ACS Appl. Mater. Interfaces. 2016;8(22):14169–14173. doi: 10.1021/acsami.6b03060. [DOI] [PubMed] [Google Scholar]