Graphical abstract
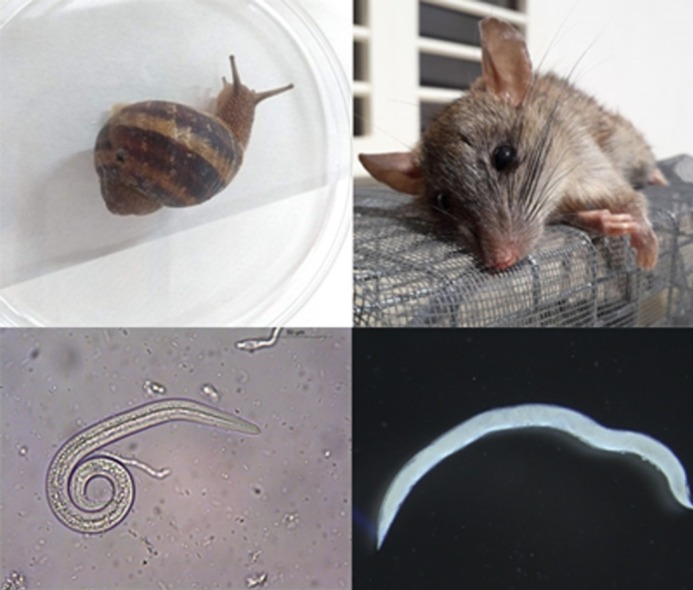
Keywords: Aelurostrongylus abstrusus, Feline lungworms, Intermediate hosts, Lizards, Paratenic hosts, Snails, Transmammary transmission, Troglostrongylus brevior
Highlights
-
•
Transmammary transmission of Troglostrongylus brevior in domestic cats is suggested.
-
•
Snails, rats and lizards play a role in the epidemiology of this feline lungworm.
-
•
Observational parasitology is important in studying events that may occur in small ecological niches around us.
Abstract
Feline lungworms such as Aerulostrongylus abstrusus and Troglostrongylus brevior are snail-borne pathogens causing respiratory disease in domestic cats. Paratenic hosts such as rodents and reptiles have also been implicated in the epidemiology of these parasites. Although A. abstrusus has been recognized for a long time as the most prevalent lungworm among cats worldwide, T. brevior is of major concern in kittens. Bearing in mind that disease due to T. brevior occurs mainly in pediatric patients younger than 6 months of age, the diagnosis of this parasite in two kittens presenting severe respiratory disease from the garden of one of the authors inspired us to investigate the potential routes of transmission for T. brevior in domestic cats. Of the three queens (A, B and C) that delivered kittens (n = 8), only cat A was positive for T. brevior, presenting her two kittens severe respiratory clinical signs, which lead to the exitus in one of them, 18 days of age. In addition, three kittens, the offspring of queen B, turned to be positive at the coprological examination after suckling from queen A, whereas those from queen C (that suckled only on their own mother) remained negative. A series of coprological, histological and molecular tests were conducted to confirm the presence of T. brevior in the patients as well as in the other cats cohabiting the same garden. Adult nematodes were retrieved from the trachea and bronchi of the dead kitten (kitten 1A), and larvae at the histology of the lung and liver parenchyma associated with bronco pneumonitis and lymphocytic pericholangitis, respectively. Cornu aspersum (n = 60), Eobania vermiculata (n = 30) snails (intermediate hosts) as well as lizards and rats (potential paratenic hosts) were collected from the same garden and processed through tissue digestion and molecular detection. Troglostrongylus brevior larvae were recovered through tissue digestion from two C. aspersum (3.33 %) and it was confirmed by PCR-sequencing approach, which also detected T. brevior DNA in the liver and lungs of one rat and in the coelomatic cavity of one gecko lizard. During the COVID-19 lockdown, when scientists spent more time at home, we grasp the opportunity to decipher T. brevior biology and ecology starting in a small ecological niche, such as the garden of our house. Data herein presented led us to suggest: i) the transmammary transmission of T. brevior in domestic cats; ii) the role of intermediate and paratenic hosts (including reptiles) in the epidemiology of the infection which they transmit; as well as iii) the importance of observational parasitology in studying any event that certainly occurs in small ecological niches, as it could be in our home gardens.
1. Introduction
Amongst snail-borne parasites, feline lungworms (superfamily Metastrongyloidea) cause major respiratory diseases in domestic and wild felids, with Aerulostrongylus abstrusus being the most prevalent worldwide (Elsheikha et al., 2016). Another species of feline lungworm, Troglostrongylus brevior (Gerichter, 1949) has for long time been regarded as of minor importance, most likely because it was overlooked by parasitologists (Brianti et al., 2012) and eventually considered typical of wild cats (Traversa and Di Cesare, 2013; Diakou et al., 2015). However, in the last decade, T. brevior has gained substantial attention of the scientific community after being identified as causative agent of severe broncho-pulmonary disease in a large number of cat population, mostly in kittens and young cats (Brianti et al., 2012, 2013; Di Cesare et al., 2014; Cavalera et al., 2018; Salant et al., 2020). For instance, a study performed in some European countries recorded that 19.5 % of the cats that scored positive for lungworms were infected by T. brevior (Giannelli et al., 2017).
Though a comprehensive picture of the distribution of this parasite is still far from being achieved, it has been reported in domestic cats from many countries in Europe, including Spain (Jefferies et al., 2010; Giannelli et al., 2017), Italy (Brianti et al., 2012, 2013; Tamponi et al., 2014, 2017; Giannelli et al., 2017; Cavalera et al., 2018; Traversa et al., 2019), Greece (Diakou et al., 2014, 2015), Cyprus (Diakou et al., 2017), Bulgaria (Giannelli et al., 2017) and Romania (Deak et al., 2017). At the same time, T. brevior has been recorded in wild felids (Felis silvestris silvestris) from Italy (Falsone et al., 2014; Napoli et al., 2016; Veronesi et al., 2016) and Greece (Liatis et al., 2017), and in Lynx lynx from Bosnia and Herzegovina (Alić et al., 2015) suggesting the circulation of this parasite in endemic areas. The clinical relevance of this lungworm species is mainly observed in cats younger than 12 months, in which it frequently causes severe illness of the lower respiratory tract, with the animals presenting cough, dyspnea and tachypnea, as well as nonspecific clinical signs (e.g. partial or complete anorexia, hyperthermia or hypothermia, dehydration, ocular and/or nasal discharge, sneezing, pulmonary hypertension, poor body conditions, and lethargy) (Crisi et al., 2015, 2018). Conversely, adult individuals commonly present subclinical infection (Crisi et al., 2018).
Aerulostrongylus abstrusus and T. brevior present similar biological cycles, with mollusks (i.e. snails and slugs) of various species (e.g. Chondrula septemdentata, Helicella barbesiana, Helicella ustalis, Limax flavus, Monaca syriaca, Retinella nitellina, Theba pisana, and Cornu aspersum) regarded as intermediate hosts, and rodents, birds and amphibians acting as paratenic hosts (Gerichter, 1949; Giannelli et al., 2014). While the role of reptiles as paratenic host has been demonstrated for A. abstrusus (Hobmaier, 1937; Anderson, 2000), no information is available for T. brevior. This could be due to the scant number of parasitological studies available for reptiles, in spite of their role as source of many zoonotic agents (Mendoza-Roldan et al., 2020). Paratenic hosts have a relevant role in the epidemiology of lungworm disease, as these small vertebrates are commonly preyed upon by domestic cats, and act as a source of infection (Crisi et al., 2018; Colella et al., 2019). Alternative transmission pathways for A. abstrusus and T. brevior have been suggested. For example, the shedding of infective third stage larvae in the environment via the mucus, or contamination of water by larvae released by dead snails have been demonstrated (Giannelli et al., 2015), and snail to snail transmission of infective larval stage has been described as intermediesis (Colella et al., 2015).
While A. abstrusus affects primarily adult cats, T. brevior is of pediatric concern, as most studies reporting these lungworm species found kittens to be mostly affected by severe respiratory disease (Salant et al., 2020), which in many cases is fatal (Brianti et al., 2013; Di Cesare et al., 2014). This is further confirmed by an epidemiological study performed in cats from different age groups in Italy, which demonstrated that in young cats and kittens (i.e., ≤ 6 months) T. brevior is the most frequent lungworm detected (i.e. 89.8 %; 44/49), whereas in individuals aging 6–24 months, a low prevalence was observed (Cavalera et al., 2018).
Transmammary transmission of nematodes has been previously described for Toxocara cati in experimentally and naturally infected cats (Swerczek et al., 1971; Coati et al., 2004). For T. brevior, it has been hypothesized that lactational transmission occurs soon after delivery through the ingestion of colostrum/milk (Brianti et al., 2013); nevertheless, reliable data about the transmission of this lungworm in kittens is still unknown. Parasitology is based on the observation and interpretation of occasional findings. During the COVID-19 lockdown, we profited of the time spent at home to examine and decipher the epidemiological chain of troglostrongylosis occurring in a small garden. Based on the report of a sudden death of a kitten suffering from severe respiratory distress in one of the authors’ garden (Fifth author), we further investigated the potential routes of transmission of T. brevior in domestic cats and the epidemiologic chain of the infection in the paratenic and intermediate hosts in that garden.
2. Material and methods
2.1. Sampling procedures
Two 18 days old kittens (1 female, 1 male, herein identified as 1A and 2A) presenting severe respiratory disease were observed in one of the authors’ garden. A rectal swab was used to sample both kittens (1A and 2A) and metastrongylid larvae were detected. On the following day, the female kitten (1A) was found dead, while the male (2A) was taken away by its mother to an unknown location, therefore the outcome for this individual was not known to the authors. A new rectal swab and a pharyngeal swab were taken from the dead kitten for viral (Feline Herpes Virus 1 – FHV1, Feline Calicivirus – FCV) and bacterial tests, and a full necropsy was then performed on the animal.
Liver, spleen, lungs, heart, stomach, intestine and mesenteric lymph node samples were fixed in 10 % buffered formalin, embedded in paraffin, and cut at 5 μm sections (Canene-Adams, 2013). Thereafter, sections were deparaffinized, stained with hematoxilin and eosin and observed under a light microscope. Additionally, fragments of the same organs (except lungs) were submitted to artificial peptic digestion (Giannelli et al., 2015) and PCR screening (see molecular analysis heading).
Aiming to perform an epidemiological investigation on these clinical cases (two kittens), all cats inhabiting the house, including the mother (queen A) of the pediatric patients, two other queens (B and C), one adult male, and the offspring of the female queen B (3 kittens identified as 1B, 2B and 3B) and C (3 kittens identified as 1C, 2C and 3C) were tested for the presence of lungworms in their feces by the Baermann technique (MAFF, 1986).
2.2. Investigation on intermediate and paratenic hosts
Intermediate (C. aspersum and E. vermiculata) and paratenic hosts (rats and lizards) were collected in the garden where the study was performed. Cornu aspersum (n = 60) and E. vermiculata (n = 30) snails were artificially digested for the retrieval of larvae as previously described (Colella et al., 2015). Two rats (Rattus norvegicus) found dead were collected in the garden. In addition, lizards (Podarcis siculus, n = 1; Tarentola mauritanica, n = 2) were captured and humanely euthanized by cervical dislocation. These paratenic hosts were dissected and their organs (i.e. brain, diaphragm, heart, kidneys, liver, lungs, spleen and skeletal muscle) were individually digested. PCR for the detection of nematode DNA was performed in snails from positive pools and in the organs of paratenic hosts (See molecular analysis heading).
2.3. Molecular analysis
Genomic DNA extraction of the dead kitten organs, snails, and paratenic hosts organs was performed using a commercial kit (QIAamp DNA Micro Kit; Qiagen, Hilden, Germany) following the manufacturer’s instructions. Thereafter, samples were screened by employing a duplex PCR using the forward primers TrogloF (5′-GCACTTGAAATCTTCGACA-3′), AeluroF (5′-GCATTTATGCTAGTGATATC-3′) and the single reverse primer MetR (5′-TAAGCATATCATTTAGCGG-3′), which amplify the ITS-2 region of different sizes (i.e. 220 bp for A. abstrusus; 370 bp for T. brevior) (Annoscia et al., 2014). The same primers were used to purify amplicons, which were sequenced in both directions, using the Taq Dye Deoxy Terminator Cycle Sequencing Kit v.2 (Applied Biosystems, Foster City, California, USA) in an automated sequencer (ABI-PRISM 377; Applied Biosystems, Foster City, California, USA). The obtained sequences were edited and aligned using the Mega7 software and compared with those available in the GenBank database, using the Basic Local Alignment Search Tool (BLAST, http://blast.ncbi.nlm.nih.gov/Blast.cgi).
3. Results
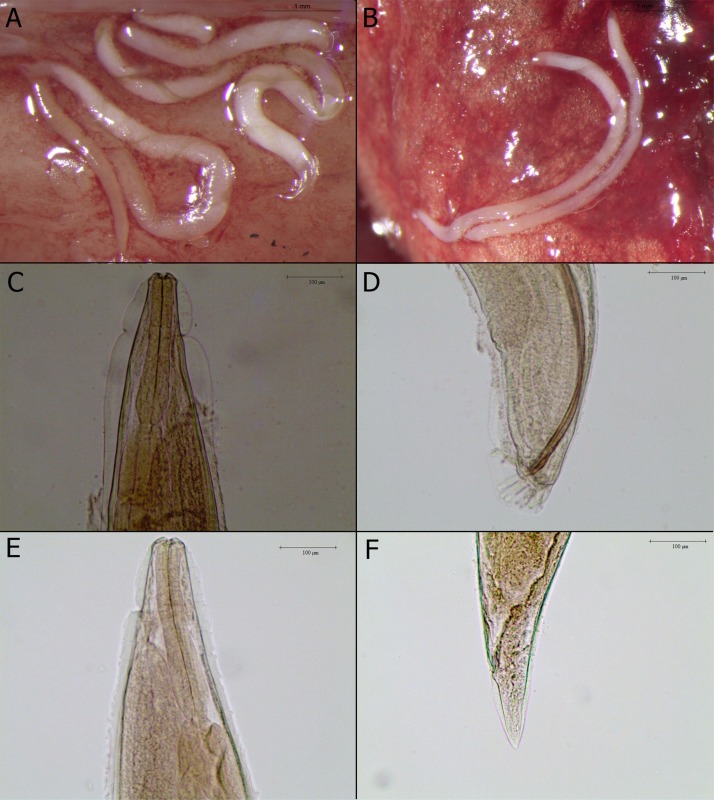
Rectal and pharyngeal swabs of dead kitten scored negative for both viruses (FHV1, FCV) at real time PCR. Microbiological tests were negative for Chlamydia spp. and Bordetella spp., but positive for Pasteurella spp. At necropsy, adult lungworms (n = 35) morphologically identified as T. brevior (Gerichter, 1949; Brianti et al., 2012) were recovered from the trachea and bronchi. Briefly, parasites were cylindrical, with an inflated cuticle thrown in folds, a club-shaped and short esophagus, a weakly developed small stoma, and a large excretory gland following the first third of the esophagus (Fig. 1 ). Female worms (n = 19) presented an average length of 9.55 mm and an average width of 0.43 mm, while male parasites (n = 16) were ∼5.97 mm in length and ∼0.32 mm in width. Additionally, larvae of the same lungworm species were isolated from the content of the stomach, esophagus and intestine, as well as in the feces through Baermann’s technique. Diffuse bronchopneumonia was the only gross abnormality observed, all the other organs were unremarkable.
Fig. 1.
A and B - adults in the lungs of kitten 1B at necropsy (Scale bar: 1 mm); C and D - anterior and posterior end of male adult (Scale bar: 100 μm); E and F - anterior and posterior end of female adult (Scale bar: 100 μm).
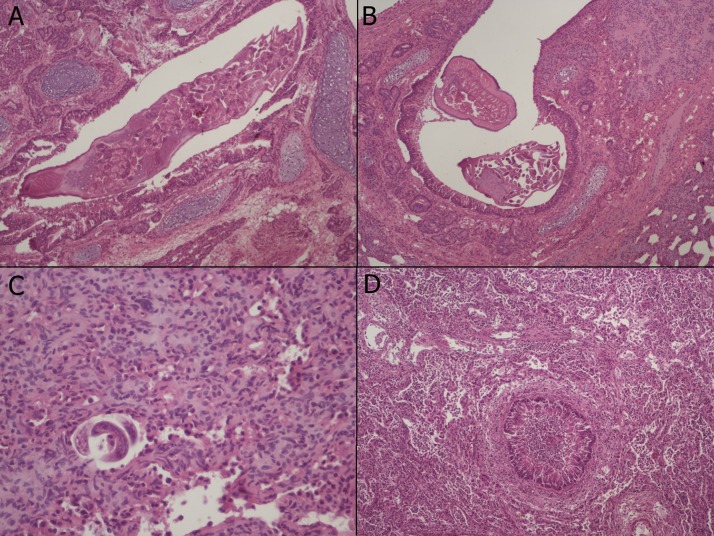
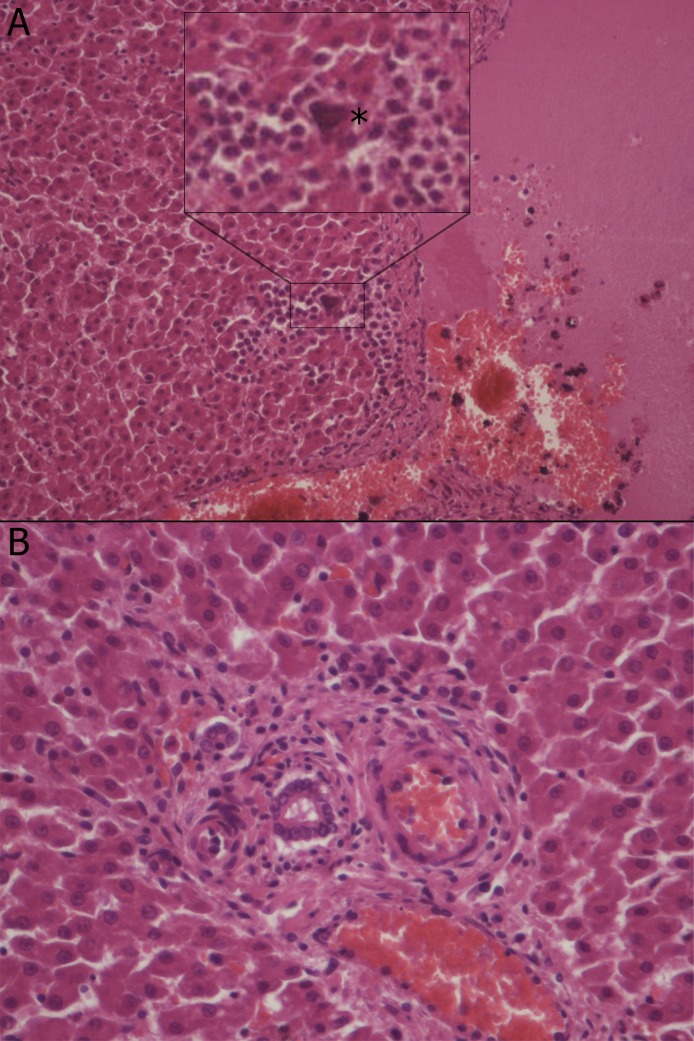
Lung histopathology revealed the presence of numerous adult lungworms in the bronchi (Fig. 2 A, B) and a few larvae in the parenchyma (Fig. 2C); entire lobes were affected by severe catarrhal-purulent bronchopneumonia (Fig. 2D). Lymphocytic pericholangitis was seen in the liver and a coiled larva was found within a neutrophilic focal infiltrate in the liver parenchyma (Fig. 3 ). No histopathological alteration neither parasites/larvae were observed from the other organs examined.
Fig. 2.
A) Main bronchus with longitudinal section of an adult of T. brevior (100X magnification). B) Main bronchus with transverse sections of two T. brevior adult parasites (100X magnification). C) Lung pneumonia with larvae embedded within collapsed alveolar spaces (400X magnification). D) Bronchopneumonia, accumulation of neutrophils within a main bronchus and alveolar spaces (100X magnification).
Fig. 3.
A) Coiled larva (*) within a focal accumulation of neutrophils in a liver lobule around a centrilobular vein (200X magnification). B) Portal tract, mild lymphocytic pericholangitis (400X magnification).
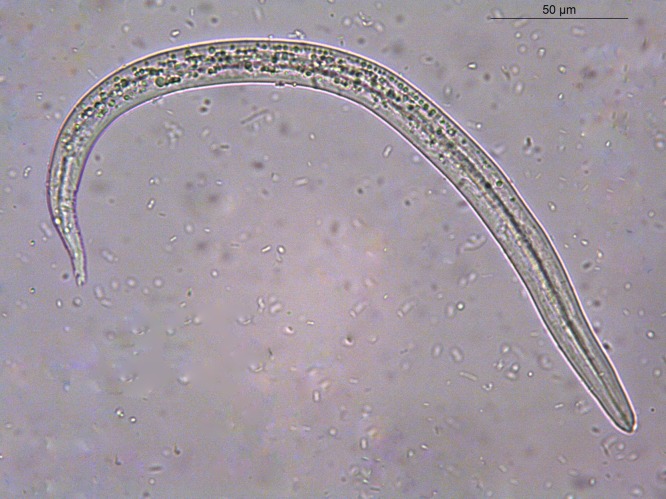
First-stage larvae (L1) of T. brevior were retrieved from the feces of queen cat A, and from 1 month-old kittens 1B, 2B and 3B (i.e. the offspring of queen B), which fed on milk from queen A, being all asymptomatic. Queens B and C, as well as the male cat and the kittens of queen C (1C, 2C, 3C) scored negative for nematode larvae in the feces (Table 1 ). Retrieved L1 (average length = 326.8 μm ± 14.8 μm; average width 16.6 μm ± 0.5 μm) presented rhabditoid esophagus, various granulated cells composing the intestine, and a pointed tail with cuticular dorsal and ventral spines (Fig. 4 ).
Table 1.
Fecal test results of adult cats and kittens (marked with the same letters of the queen) tested for Troglostrongylus brevior every week, for 3 weeks.
| Identification | Sex | Age class | Baermann test | N. of larvae (week/mL) |
|---|---|---|---|---|
| Adults | ||||
| A | Female | Adult | Positive | w1/350; w2/500; w3/300 |
| B | Female | Adult | Negative | w1-w2-w3 |
| C | Female | Adult | Negative | w1-w2-w3 |
| D | Male | Adult | Negative | w1-w2-w3 |
| Kittens | ||||
| 1A | Female | Kitten | Positive | * |
| 2A | Male | Kitten | Positive | ** |
| 1B | Male | Kitten | Positive | w2/1550; w3/400 |
| 2B | Male | Kitten | Positive | w1/1150; w2/50 |
| 3B | Male | Kitten | Positive | w2/650 |
| 1C | Female | Kitten | Negative | w1-w2-w3 |
| 2C | Female | Kitten | Negative | w1-w2-w3 |
| 3C | Male | Kitten | Negative | w1-w2-w3 |
w – week.
* Positive at rectal swab and at necropsy examination and histopathology.
** Positive at rectal swab.
Fig. 4.
First-stage larva recovered from the feces of the cats herein evaluated.
Larvae were not detected at artificial digestion of the organs; however, molecular analysis confirmed the histological findings of T. brevior in the liver and lungs. At artificial digestion, nematode larvae were recovered from two out of 60 (3.33 %) C. aspersum, which was confirmed as T. brevior by PCR-sequencing approach. In paratenic hosts, larvae of this lungworm were not detected through artificial digestion, however, PCR analysis detected T. brevior DNA in the liver and lungs in one rat (R. norvegicus), and in the coelomatic cavity of one gecko lizard (T. mauritanica). PCR yielded amplicons of the expected size (i.e. 370bp) and sequence obtained showed a high homology (100 %) at BLAST analysis, with T. brevior DNA sequences available in Genbank (accession number: KF241978.1, KT818789.1, MH537789.1, MH780055.1). Obtained T. brevior sequences were submitted to GenBank under the accession number: MT772032 to MT772037.
4. Discussion
This study assessed the transmammary transmission of T. brevior in kittens, as well as the epidemiologic chain of the infection in paratenic (rats, lizards) and intermediate hosts (C. aspersum snails) that may occur in a garden. In addition, this is the first report of lizards as potential paratenic hosts for this parasite. Severe respiratory disease associated to T. brevior adults in two 18-day-old kittens from the infected cat (queen A), as well as the positive coprological tests in the kittens born from queen B, negative for T. brevior, following suckling milk from queen A, strongly support that the infection occurred by feeding of milk from the positive cat. This transmission pathway was previously suggested in a kitten that was found positive to adult nematodes in the trachea at necroscopy examination and that died 25 days after it was born from a queen positive by fecal tests for T. brevior (Brianti et al., 2013). Indeed, the presence of T. brevior larvae in the fecal samples of both kittens (1A and 2A) and of adult worms in the trachea and bronchi of one of them (1A) supports the hypothesis of transmammary transmission soon after birth via ingestion of colostrum and/or milk containing infective L3.
This finding is further suggested by the retrieval of larvae at the histopathology of the liver and lungs of kitten 1A and of their molecular identification at species level, which indicates a larval migratory pattern through entero-hepato-pneumo-trachea-enteral cycle. Though the presence of T. brevior larvae in the liver is a new finding for this lungworm species, this location has been previously reported in cats for T. cati second stage larvae 3 days post-infection with eggs (Sprent, 1956). In the animal herein examined, lymphocytic pericholangitis observed within the liver, could be the consequence of the migration of larvae through the hepatic tissue as suggested in previous studies where hepatic congestion, lipidosis and increased levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) have been associated with mixed infection by T. brevior and A. abstrusus (Crisi et al., 2017).
This finding advocates for the need of suitable age-related therapeutic protocols against T. brevior in kittens and pregnant females, since it is suggested that adult cats present less risk to developing clinical disease by T. brevior as compared to kittens (Cavalera et al., 2018), facilitating its transmission from asymptomatic pregnant queens that are not treated before delivering their kittens, and ultimately enabling the infection during nursing. Some treatment (i.e. emodepside/praziquantel spot-on solution) of pregnant queens to prevent lactogenic transmission of nematodes (i.e. T. cati) has been effectively demonstrated (Böhm et al., 2015). Likewise, the same treatment has also been effective against T. brevior and A. abstrusus mixed infection in a ∼2-month-old kitten (Di Cesare et al., 2015). However, the safety of most chemicals against feline lungworms has not been guaranteed for kittens or pregnant female (Cavalera et al., 2018).
The presence of T. brevior DNA in the liver and lungs of a rat, as well as in the coelomatic cavity of a gecko lizard, confirms the participation of these small vertebrates in the transmission cycle of this lungworm. It is worth noticing that the positive lizard was captured in a box full of snails, which suggests that this paratenic host could have become infected through the ingestion of snails or, merely, by contact with L3 shed in the mucus of those snails (Giannelli et al., 2015). Despite being known as paratenic hosts for lungworms, no records of rats infected with T. brevior has been reported. However, an encysted larva of this nematode was retrieved from the lung surface of one mouse experimentally infected (Gerichter, 1949). Additionally, mice have been previously proven to be paratenic hosts with an important role in the transmission of A. abstrusus, as cats acquired this nematode after feeding on experimentally infected mice (Colella et al., 2019). In the same study, third stage larvae of A. abstrusus were recovered from the liver, spleen, brain, skeletal muscle and gastrointestinal tissues of mice, demonstrating that this nematode migrates to various anatomical organs in the host (Colella et al., 2019). Likewise, our findings suggest the same pattern of migration for T. brevior in rats and lizards, demonstrating that these hosts have a relevant role in the epidemiology of feline lungworm disease, as they may be the main source of T. brevior infection to adult cats. In particular, cats present an increased hunting activity of mice and lizards during pregnancy or lactation due to the increased need for protein intake, therefore resulting in a greater risk of infection to lungworms through predation (Brianti et al., 2013).
The presence of infected intermediate hosts (e.g. C. aspersum snails) in the garden, highlights the importance of these mollusks in the epidemiology of the disease, although cats usually do not feed on snails, but rather on paratenic hosts, such as rats and lizards (Gerichter, 1949; Woods et al., 2003; Giannelli et al., 2014). Despite not being considered the main direct source of infection to domestic cats, intermediate hosts have an essential role in the epidemiological chain of T. brevior, as they are predated upon by paratenic hosts, such as rats (Hadfield and Saufler, 2009). Under the above circumstances, infective third stage larvae released from snails in the environment, or in water soon after they drown, may enhance risk of T. brevior transmission to cats and paratenic hosts (Giannelli et al., 2015). This is further suggested in our study, as the presence of T. brevior was confirmed in a rat and a lizard collected at the same site where the clinical cases occurred and a high population of snails was present.
In parasitology, constant interactions among hosts, parasites and their vectors occur in small ecological niches such as a home garden. Observational approaches should be prompted to further investigate scientific events that would be further confirmed under experimental conditions. As new scientific discoveries require time and reproducible, confirmatory, results, very often we rely much more on laboratory-based observations than on events that occur in nature. Without any doubts, during the COVID-19 lockdown, scientists spent more time at home, which might have allowed to decipher observational events, such as the one herein described. This provides a good lesson for planning future research in many fields of science, including parasitology.
5. Conclusions
The findings herein reported support: i) the hypothesis that T. brevior could be vertically transmitted through the transmammary route; ii) the role of intermediate and paratenic hosts in the epidemiology of the disease in cats (including the first evidence of reptiles as paratenic hosts); and iii) the importance of observational parasitology in studying any event that occurs in small ecological niches, such as our home gardens. Moreover, this study brings important knowledge on the epidemiological chain of T. brevior transmission in domestic cats, drawing attention for the need of preventive measures such as early diagnosis and suitable age-related treatment of pregnant queens and kittens.
Ethical standards
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
CRediT authorship contribution statement
Marcos Antônio Bezerra-Santos: Data curation, Formal analysis, Investigation, Methodology, Writing - original draft, Writing - review & editing. Jairo Alfonso Mendoza-Roldan: Formal analysis, Investigation, Methodology, Writing - review & editing. Francesca Abramo: Formal analysis, Methodology, Writing - review & editing. Riccardo Paolo Lia: Methodology, Writing - review & editing. Viviana Domenica Tarallo: Methodology, Writing - review & editing. Harold Salant: Formal analysis, Writing - review & editing. Emanuele Brianti: Formal analysis, Writing - review & editing. Gad Baneth: Formal analysis, Writing - review & editing. Domenico Otranto: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing - review & editing.
Declaration of Competing Interest
The authors declare no competing interest.
Acknowledgements
Authors would like to thank Professor Nicola Decaro and his team of infectious disease unit (Department of Veterinary Medicine/University of Bari) for the support in the microbiological and virologic tests. Additionally, authors thank the co-owners of the garden where this study was performed for giving us full access to the place to perform such a scientific investigation.
References
- Alić A., Traversa D., Duscher G.G., Kadrić M., Di Cesare A., Hodžić A. Troglostrongylus brevior in an Eurasian lynx (Lynx lynx) from Bosnia and Herzegovina. Parasit. Vectors. 2015;8:13–15. doi: 10.1186/s13071-015-1272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R.C. 2nd ed. CABI Publishing, Wallingford; UK: 2000. Nematode Parasites of Vertebrates. Their Development and Transmission. [Google Scholar]
- Annoscia G., Latrofa M.S., Campbell B.E., Giannelli A., Ramos R.A.N., Dantas-Torres F., Brianti E., Otranto D. Simultaneous detection of the feline lungworms Troglostrongylus brevior and Aelurostrongylus abstrusus by a newly developed duplex-PCR. Vet. Parasitol. 2014;199:172–178. doi: 10.1016/j.vetpar.2013.10.015. [DOI] [PubMed] [Google Scholar]
- Böhm C., Petry G., Schaper R., Wolken S., Strube C. Prevention of lactogenic Toxocara cati infections in kittens by application of an Emodepside/Praziquantel spot-on (Profender®) to the pregnant queen. Parasitol. Res. 2015;114:175–184. doi: 10.1007/s00436-015-4523-y. [DOI] [PubMed] [Google Scholar]
- Brianti E., Gaglio G., Giannetto S., Annoscia G., Latrofa M.S., Dantas-Torres F., Traversa D., Otranto D. Troglostrongylus brevior and Troglostrongylus subcrenatus (Strongylida: Crenosomatidae) as agents of broncho-pulmonary infestation in domestic cats. Parasit. Vectors. 2012;5:1–12. doi: 10.1186/1756-3305-5-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brianti E., Gaglio G., Napoli E., Falsone L., Giannetto S., Latrofa M.S., Giannelli A., Dantas-Torres F., Otranto D. Evidence for direct transmission of the cat lungworm Troglostrongylus brevior (Strongylida: Crenosomatidae) Parasitology. 2013;140:821–824. doi: 10.1017/S0031182013000188. [DOI] [PubMed] [Google Scholar]
- Canene-Adams K. Methods in Enzymology. Academic Press Inc; 2013. Preparation of formalin-fixed paraffin-embedded tissue for immunohistochemistry; pp. 225–233. [DOI] [PubMed] [Google Scholar]
- Cavalera M.A., Iatta R., Colella V., Dantas-Torres F., Corsaro A., Brianti E., Otranto D. Troglostrongylus brevior: a feline lungworm of paediatric concern. Vet. Parasitol. 2018;253:8–11. doi: 10.1016/j.vetpar.2018.02.017. [DOI] [PubMed] [Google Scholar]
- Coati N., Schnieder T., Epe C. Vertical transmission of Toxocara cati Schrank 1788 (Anisakidae) in the cat. Parasitol. Res. 2004;92:142–146. doi: 10.1007/s00436-003-1019-y. [DOI] [PubMed] [Google Scholar]
- Colella V., Giannelli A., Brianti E., Ramos R.A.N., Cantacessi C., Dantas-Torres F., Otranto D. Feline lungworms unlock a novel mode of parasite transmission. Sci. Rep. 2015;5:1–6. doi: 10.1038/srep13105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colella V., Knaus M., Lai O., Cantile C., Abramo F., Rehbein S., Otranto D. Mice as paratenic hosts of Aelurostrongylus abstrusus. Parasit. Vectors. 2019;12:1–8. doi: 10.1186/s13071-019-3293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisi P.E., Traversa D., Di Cesare A., Luciani A., Civitella C., Santori D., Boari A. Irreversible pulmonary hypertension associated with Troglostrongylus brevior infection in a kitten. Res. Vet. Sci. 2015;102:223–227. doi: 10.1016/j.rvsc.2015.08.019. [DOI] [PubMed] [Google Scholar]
- Crisi P.E., Aste G., Traversa D., Di Cesare A., Febo E., Vignoli M., Santori D., Luciani A., Boari A. Single and mixed feline lungworm infections: clinical, radiographic and therapeutic features of 26 cases (2013–2015) J. Feline Med. Surg. 2017;19:1017–1029. doi: 10.1177/1098612X16670563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisi P.E., Di Cesare A., Boari A. Feline troglostrongylosis: current epizootiology, clinical features, and therapeutic options. Front. Vet. Sci. 2018;5:1–7. doi: 10.3389/fvets.2018.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deak G., Ionicǎ A.M., Mihalca A.D., Gherman C.M. Troglostrongylus brevior: a new parasite for Romania. Parasit. Vectors. 2017;10:10–13. doi: 10.1186/s13071-017-2551-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cesare A., Di Regalbono A.F., Tessarin C., Seghetti M., Iorio R., Simonato G., Traversa D. Mixed infection by Aelurostrongylus abstrusus and Troglostrongylus brevior in kittens from the same litter in Italy. Parasitol. Res. 2014;113:613–618. doi: 10.1007/s00436-013-3690-y. [DOI] [PubMed] [Google Scholar]
- Di Cesare A., Iorio R., Crisi P., Paoletti B., Di Costanzo R., Dimitri C.F., Traversa D. Treatment of Troglostrongylus brevior (Metastrongyloidea, Crenosomatidae) in mixed lungworm infections using spot-on emodepside. J. Feline Med. Surg. 2015;17:181–185. doi: 10.1177/1098612X14533552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diakou A., Di Cesare A., Aeriniotaki T., Traversa D. First report of Troglostrongylus brevior in a kitten in Greece. Parasitol. Res. 2014;113:3895–3898. doi: 10.1007/s00436-014-4122-3. [DOI] [PubMed] [Google Scholar]
- Diakou A., Di Cesare A., Barros L.A., Morelli S., Halos L., Beugnet F., Traversa D. Occurrence of Aelurostrongylus abstrusus and Troglostrongylus brevior in domestic cats in Greece. Parasit. Vectors. 2015;8:4–9. doi: 10.1186/s13071-015-1200-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diakou A., Sofroniou D., Di Cesare A., Kokkinos P., Traversa D. Occurrence and zoonotic potential of endoparasites in cats of Cyprus and a new distribution area for Troglostrongylus brevior. Parasitol. Res. 2017;116:3429–3435. doi: 10.1007/s00436-017-5651-3. [DOI] [PubMed] [Google Scholar]
- Elsheikha H.M., Schnyder M., Traversa D., Di Cesare A., Wright I., Lacher D.W. Updates on feline aelurostrongylosis and research priorities for the next decade. Parasit. Vectors. 2016;9:1–15. doi: 10.1186/s13071-016-1671-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsone L., Brianti E., Gaglio G., Napoli E., Anile S., Mallia E., Giannelli A., Poglayen G., Giannetto S., Otranto D. The European wildcats (Felis silvestris silvestris) as reservoir hosts of Troglostrongylus brevior (Strongylida: Crenosomatidae) lungworms. Vet. Parasitol. 2014;205:193–198. doi: 10.1016/j.vetpar.2014.06.024. [DOI] [PubMed] [Google Scholar]
- Gerichter C.B. Studies on the nematodes parasitic in the lungs of felidae in palestine. Parasitology. 1949;39:251–262. doi: 10.1017/S0031182000083827. [DOI] [PubMed] [Google Scholar]
- Giannelli A., Ramos R.A.N., Annoscia G., Di Cesare A., Colella V., Brianti E., Dantas-Torres F., Mutafchiev Y., Otranto D. Development of the feline lungworms Aelurostrongylus abstrusus and Troglostrongylus brevior in Helix aspersa snails. Parasitology. 2014;141:563–569. doi: 10.1017/S003118201300187X. [DOI] [PubMed] [Google Scholar]
- Giannelli A., Colella V., Abramo F., Ramos R.A.N., Falsone L., Brianti E., Varcasia A., Dantas-Torres F., Knaus M., Fox M.T., Otranto D. Release of lungworm larvae from snails in the environment: potential for alternative transmission pathways. PLoS Negl. Trop. Dis. 2015;9:1–12. doi: 10.1371/journal.pntd.0003722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannelli A., Capelli G., Joachim A., Hinney B., Losson B., Kirkova Z., René-Martellet M., Papadopoulos E., Farkas R., Napoli E., Brianti E., Tamponi C., Varcasia A., Margarida Alho A., Madeira de Carvalho L., Cardoso L., Maia C., Mircean V., Mihalca A.D., Miró G., Schnyder M., Cantacessi C., Colella V., Cavalera M.A., Latrofa M.S., Annoscia G., Knaus M., Halos L., Beugnet F., Otranto D. Lungworms and gastrointestinal parasites of domestic cats: a European perspective. Int. J. Parasitol. 2017;47:517–528. doi: 10.1016/j.ijpara.2017.02.003. [DOI] [PubMed] [Google Scholar]
- Hadfield M.G., Saufler J.E. The demographics of destruction: isolated populations of arboreal snails and sustained predation by rats on the island of Moloka’i 1982-2006. Biol. Invasions. 2009;11:1595–1609. doi: 10.1007/s10530-008-9409-9. [DOI] [Google Scholar]
- Hobmaier A. PAPERS ON HELMINTHOLOGY Published in Commemoration of the 30 Year Jubileum of K. J. Skrjabin and of 15th Anniversary of the All-union Institute of Helminthology. 1937. Auxiliary hosts in life cycle of lungworm in cat Aelurostrongylus abstrusus; pp. 231–233. Moscow. [Google Scholar]
- Jefferies R., Vrhovec M.G., Wallner N., Catalan D.R. Aelurostrongylus abstrusus and Troglostrongylus sp. (Nematoda: Metastrongyloidea) infections in cats inhabiting Ibiza, Spain. Vet. Parasitol. 2010;173:344–348. doi: 10.1016/j.vetpar.2010.06.032. [DOI] [PubMed] [Google Scholar]
- Liatis T.K., Monastiridis A.A., Birlis P., Prousali S., Diakou A. Endoparasites of wild mammals sheltered in wildlife hospitals and rehabilitation centres in Greece. Front. Vet. Sci. 2017;4:1–8. doi: 10.3389/fvets.2017.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAFF . 3rd ed. HMSO; London, UK: 1986. Manual of Veterinary Parasitological Laboratory Techniques. [Google Scholar]
- Mendoza-Roldan J.A., Modry D., Otranto D. Zoonotic parasites of reptiles: a crawling threat. Trends Parasitol. 2020;36:677–687. doi: 10.1016/j.pt.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli E., Anile S., Arrabito C., Scornavacca D., Mazzamuto M.V., Gaglio G., Otranto D., Giannetto S., Brianti E. Survey on parasitic infections in wildcat (Felis silvestris silvestris Schreber, 1777) by scat collection. Parasitol. Res. 2016;115:255–261. doi: 10.1007/s00436-015-4742-2. [DOI] [PubMed] [Google Scholar]
- Salant H., Yasur-Landau D., Rojas A., Otranto D., Mazuz M.L., Baneth G. Troglostrongylus brevior is the dominant lungworm infecting feral cats in Jerusalem. Parasitol Res. 2020 doi: 10.1007/s00436-020-06852-8. (in press) [DOI] [PubMed] [Google Scholar]
- Sprent J.F.A. The life history and development of Toxocara cati (Schrank 1788) in the domestic cat. Parasitology. 1956;46:54–78. doi: 10.1017/S0031182000026342. [DOI] [PubMed] [Google Scholar]
- Swerczek T.W., Nielsen S.W., Helmboldt C.F. Transmammary passage of Toxocara cati in the cat. Am. J. Vet. Res. 1971;32:89–92. [PubMed] [Google Scholar]
- Tamponi C., Varcasia A., Brianti E., Pipia A.P., Frau V., Pinna Parpaglia M.L., Sanna G., Garippa G., Otranto D., Scala A. New insights on metastrongyloid lungworms infecting cats of Sardinia, Italy. Vet. Parasitol. 2014;203:222–226. doi: 10.1016/j.vetpar.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Tamponi C., Varcasia A., Pinna S., Melis E., Melosu V., Zidda A., Sanna G., Pipia A.P., Zedda M.T., Pau S., Brianti E., Scala A. Endoparasites detected in faecal samples from dogs and cats referred for routine clinical visit in Sardinia, Italy. Vet. Parasitol. Reg. Stud. Reports. 2017;10:13–17. doi: 10.1016/j.vprsr.2017.07.001. [DOI] [PubMed] [Google Scholar]
- Traversa D., Di Cesare A. Feline lungworms: what a dilemma. Trends Parasitol. 2013;29:423–430. doi: 10.1016/j.pt.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Traversa D., Morelli S., Cassini R., Crisi P.E., Russi I., Grillotti E., Manzocchi S., Simonato G., Beraldo P., Viglietti A., De Tommaso C., Pezzuto C., Pampurini F., Schaper R., Di Regalbono A.F. Occurrence of canine and feline extra-intestinal nematodes in key endemic regions of Italy. Acta Trop. 2019;193:227–235. doi: 10.1016/j.actatropica.2019.03.009. [DOI] [PubMed] [Google Scholar]
- Veronesi F., Traversa D., Lepri E., Morganti G., Vercillo F., Grelli D., Cassini R., Marangi M., Iorio R., Ragni B., Di Cesare A. Occurrence of lungworms in European wildcats (Felis silvestris silvestris) of central Italy. J. Wildl. Dis. 2016;52:270–278. doi: 10.7589/2015-07-187. [DOI] [PubMed] [Google Scholar]
- Woods M., McDonald R.A., Harris S. Predation of wildlife by domestic cats Felis catus in Great Britain. Mamm. Rev. 2003;33:174–188. doi: 10.1046/j.1365-2907.2003.00017.x. [DOI] [Google Scholar]