Abstract
Background
Studies have shown that cardiac arrhythmias may occur in up to 44% of patients with severe coronavirus disease 2019 (COVID-19) and has been associated with an increased risk of death. This systematic review and meta-analysis aimed to evaluate the incidence of cardiac arrhythmias in patients with COVID-19 and their implications on patient prognosis.
Methods
We performed a systematic literature search from PubMed, SCOPUS, Europe PMC, Cochrane Central Databases, and Google Scholar + Preprint Servers. The primary endpoint of the study was poor outcomes including mortality, severe COVID-19, and the need for ICU care.
Results
A total of 4 studies including 784 patients were analyzed. The incidence of arrhythmia in patients with COVID-19 was 19% (9–28%; I2: 91.45). Arrhythmia occurred in 48% (38–57%; I2: 48.08) of patients with poor outcome and 6% (1–12%; I2: 85.33%) of patients without poor outcome. Patients with COVID-19 experiencing arrhythmia had an increased risk of poor outcome (RR 7.96 [3.77, 16.81], p < 0.001; I2: 71.1%). The funnel-plot analysis showed an asymmetrical funnel plot with most of the studies on the right side of the effect estimate. The regression-based Egger’s test showed indication of small-study effects (p = 0.001).
Conclusion
Cardiac arrhythmias were significantly associated with an increased risk of poor outcome in COVID-19. Arrhythmias were observed in 19% of patients with COVID-19 and in 48% of patients with COVID-19 and poor outcomes.
Keywords: Arrhythmia, Cardiovascular, Coronavirus, COVID-19, Rhythm, SARS-CoV-2
1. Introduction
Coronavirus disease 2019 (COVID-19) was declared pandemic, and at the time this paper was written, it has infected more than 4.7 million people and caused more than 300.000 deaths [1]. Even though most of the infected patients have mild or no symptoms, some of them develop severe pneumonia, acute respiratory distress syndrome, multi-organ failure, and death. Factors such as age, smoking, diabetes, hypertension, and cardiovascular diseases are associated with severity and mortality in patients with COVID-19 [[2], [3], [4], [5], [6]]. Previously, influenza epidemics have been shown to be associated with an increased risk of ventricular arrhythmia in patients with implantable cardiac defibrillators [8], indicating that respiratory-borne infections might also be related to cardiovascular manifestations.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been shown to cause cardiac injury and cytokine storm, which may lead to cardiac arrhythmias [9,10]. A study has shown that cardiac arrhythmias may occur in up to 44% of patients with severe COVID-19 [11], and has been associated with an increased risk of death [12]. This systematic review and meta-analysis aimed to evaluate the incidence of cardiac arrhythmias in patients with COVID-19 and their implications on patient prognosis.
2. Methods
2.1. Eligibility criteria
This systematic review and meta-analysis included research articles on adult COVID-19 patients that contain information on arrhythmia as a complication and contain a clinically validated definition of severe COVID-19, death, or intensive care unit (ICU) care. We excluded articles other than original research articles (e.g., review articles, non-research letters, or editorial/commentaries), studies with a sample of less than 20 or case reports, non-English language articles, and pediatric populations (age of 17 years old or younger).
2.2. Search strategy and study selection
We systematically searched PubMed, SCOPUS, Europe PMC, Cochrane Central Databases, and Google Scholar + Preprint Servers with the search terms 1) “COVID-19″ OR “SARS-CoV-2″ AND “Characteristics” 2) “COVID-19″ OR “SARS-CoV-2″ AND “Arrhythmia”. Duplicate results were removed. The remaining articles were independently screened by two authors (I. H. and R. P.) for the relevance of their abstracts. The full text of the remaining articles was assessed applying the inclusion and exclusion criteria. The search was finalized on April 14, 2020. The study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
2.3. Data extraction
Data extraction was performed independently by two of the authors (I. H. and R. P.). We used standardized extraction forms that included author, year, study design, age, sex, cardiovascular diseases, history of hypertension and diabetes mellitus, respiratory comorbidities, antibiotics and antiviral use, glucocorticoid use, lymphopenia, follow-up duration, arrhythmia, mortality, ICU care, and severe COVID-19. The quality and risk of bias assessment of the included studies will be performed using the Newcastle-Ottawa Scale.
The primary endpoint of the study was poor outcome, including mortality, severe COVID-19, and the need for ICU care. Severe COVID-19 was defined as patients who had any of the following features at the time of admission or after admission: (1) respiratory distress (≥30 breaths per min); (2) oxygen saturation at rest ≤ 93%; (3) ratio of partial pressure of arterial oxygen (PaO2) to fractional concentration of oxygen inspired air (fiO2) ≤ 300 mmHg; or (4) critical complication (respiratory failure, septic shock, and multiple organ dysfunction/failure) [13].
2.4. Statistical analysis
The meta-analysis of the included studies was performed using STATA 16.0 (StataCorp LLC, Texas, US). Meta-analysis of proportions was used to pool the incidence of arrhythmia in the groups. Dichotomous variables were calculated to obtain risk ratios (RRs) along with their 95% confidence intervals (CIs). Random effect models were used regardless of heterogeneity. The p-values were two-tailed and statistical significance was set at ≤ 0.05. A sensitivity analysis by leave-one-out was performed to test the robustness of the findings. To assess the small-study effect, we performed a regression-based Egger’s test. Random-effects meta-regression was performed for gender, hypertension, diabetes, cardiovascular diseases, cerebrovascular diseases, respiratory comorbidities, and lymphopenia.
3. Results
3.1. Study selection
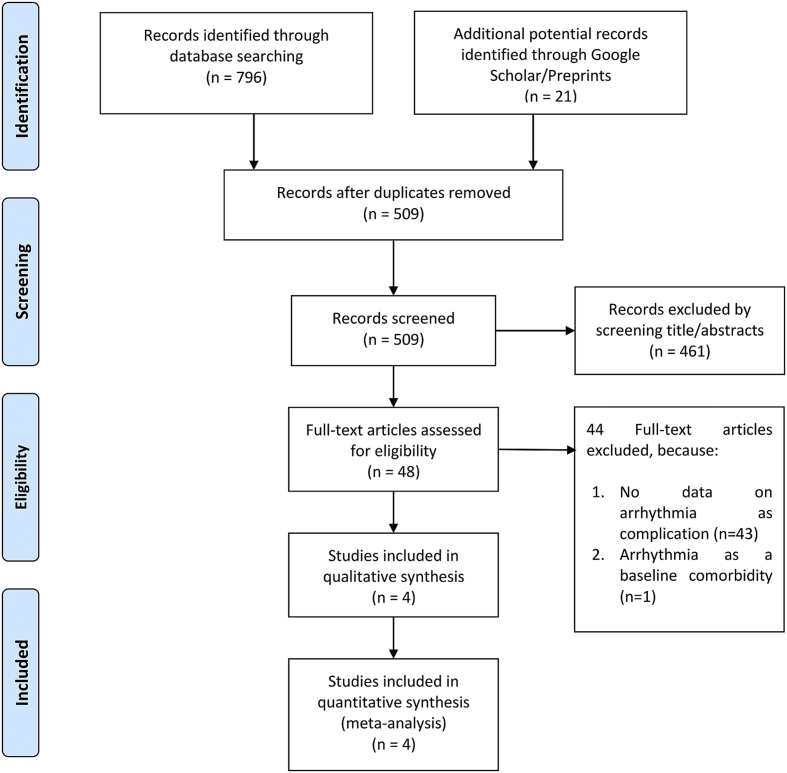
We found 817 records and 509 remained after the removal of duplicates. A total of 461 records were excluded after screening the titles and abstracts. After assessing 48 articles for eligibility, we excluded 44 because of the following conditions: 1) there were no data on arrhythmia as complication (n = 43), and 2) arrhythmia was the baseline comorbidity, not an outcome (n = 1). As a result, 4 studies remained for qualitative synthesis and meta-analysis (Fig. 1). There were 784 patients from the 4 studies [11,12,14,15].
Fig. 1.
Prisma flowchart.
3.2. Baseline characteristics
Baseline characteristics is displayed in Table 1, Table 2. Meta-analysis of baseline characteristics showed that male (RR 1.28 [1.06, 1.56], p = 0.01; I2: 49%, p = 0.12), hypertension (RR 2.38 [1.61, 3.51], p < 0.001; I2: 69%, p = 0.02), diabetes (RR 2.60 [1.47, 4.61], p = 0.001; I2: 45%, p = 0.14), cardiovascular diseases (RR 3.51 [2.28, 5.42], p < 0.001; I2: 0%, p = 0.55), and respiratory comorbidities (RR 4.90 [2.12, 11.29], p < 0.001; I2: 0%, p = 0.75) were associated with poor outcome in this pooled analysis. Cerebrovascular diseases (RR 3.78 [0.72, 19.88], p = 0.12; I2: 81%, p = 0.001) was not associated with poor outcome.
Table 1.
Characteristics of the included studies. Data in the table were arranged as total patients/prevalence (poor outcome group vs. without poor outcome group).
| Authors | Study Design | Samples | Overall Age (Mean/Median) (years) | Male (%) | Hypertension (%) | Cardiovascular Comorbidities (%) | Diabetes (%) | Respiratory Comorbidities (%) | Hs-troponin I on Admission |
|---|---|---|---|---|---|---|---|---|---|
| Cao J 2020 | Observational Retrospective | 102 (17/85) | 54 (72 vs. 53) | 52 (76.5 vs. 47.1) | 27.5 (64.7 vs. 20) | 4.9 (17.6 vs. 2.4) | 10.8 (35.3 vs. 5.9) | 9.8 (23.5 vs. 7.1) | 7.6 (Overall) |
| Hu L 2020 | Observational Retrospective | 323 (172/151) | 61 (65 vs. 56) | 51.4 (52.9 vs. 49.7) | 32.5 (38.3 vs. 25.8) | 12.7 (19.2 vs. 5.3) | 14.6 (19.2 vs. 9.3) | 1.9 (3.5 vs. 0) | Mean/Median was not reported |
| Zhang Guqin 2020 | Observational Retrospective | 221 (55/166) | 55 (62 vs. 51) | 48.9 (63.6 vs. 44.0) | 24.4 (47.3 vs. 16.9) | 10 (23.6 vs. 5.4) | 10 (12.7 vs. 9.0) | 2.7 (7.3 vs. 1.2) | Mean/Median was not reported |
| Wang Dawei 2020 | Observational Retrospective | 138 (36 vs. 102) | 56 (66 vs. 51) | 54.3 (61.1 vs. 52.0) | 31.2 (58.3 vs. 21.6) | 14.5 (25 vs. 10.8) | 10.1 (22.2 vs. 5.9) | 2.9 (8.3 vs. 1.0) | 6.4 (11 vs. 5.1) |
Table 2.
Characteristics of the included studies. Data in the table were arranged as total patients/prevalence (poor outcome group vs. without poor outcome group).
| Authors | Outcome | NOS | Mean Follow-up Duration | Antiviral Use (%) | Antibiotics Use (%) | Glucocorticoid Use (%) |
|---|---|---|---|---|---|---|
| Cao J 2020 | Mortality | 7 | 28 days Mean Duration: Not Reported Sample: January 3, 2020 to February 15, 2020 Followed up to: February 15, 2020 |
100 vs 97.6 Arbidol: 5.9 vs. 37.6 Oseltamivir: 82.4 vs. 61.2 Lopinavir + Ritonavir: 23.5 vs. 28.2 |
100 vs. 98.8 | Methylprednisolone: 64.7 vs 47.1 |
| Hu L 2020 | Severe COVID-19 | 7 | 28 days Mean Duration: Not Reported Sample: January 8, 2020 to February 20, 2020 Followed up to: March 10, 2020 |
Oseltamivir: 67.4 vs 72.2 Ganciclovir: 68.0 vs 74.8 Arbidol: 63.4 vs 65.6 Kaletra: 13.4 vs 3.3 Interferon-alpha: 8.1 vs 5.3 |
93.6 vs. 94.7 | 63.4 vs. 57.6 |
| Zhang Guqin 2020 | Severe COVID-19 | 7 | Mean Duration: Not Reported Sample: January 2, 2020 to February 10, 2020 Followed up to: February 15, 2020 |
90.9 vs 88 | Not Reported | 72.7 vs 45.2 |
| Wang Dawei 2020 | Intensive Care Unit | 7 | Mean Duration: Not Reported Sample: January 1, 2020 to January 28, 2020 Followed up to: February 3, 2020 |
90 (Overall) | 100 (Overall) | Methylprednisolone: 45 (Overall) |
NOS: Newcastle-Ottawa Scale.
3.3. Arrhythmia incidence
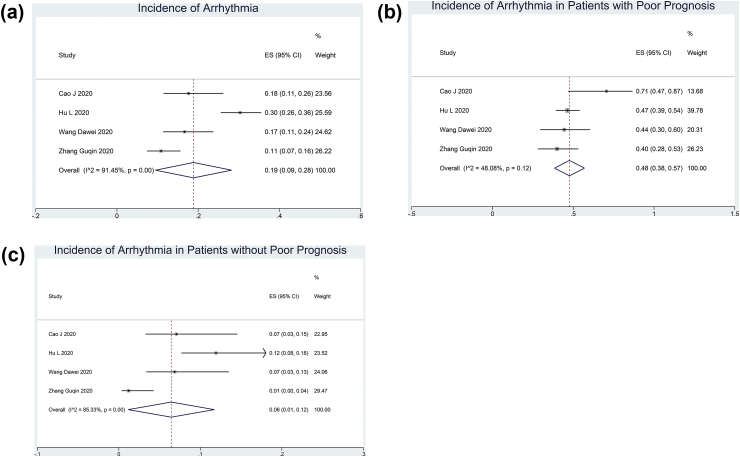
The incidence of arrhythmia in patients with COVID-19 was 19% (9–28%; I2: 91.45, p < 0.001) (Fig. 2A). Arrhythmia occurred in 48% (38–57%; I2: 48.08, p = 0.12) of patients with poor outcome (Figs. 2B) and 6% (1–12%; I2: 85.33%) of patients without poor outcome (Fig. 2C).
Fig. 2.
Incidence of arrhythmia in COVID-19 patients with COVID 19. The incidence of arrhythmia is 19% (A). Arrhythmia occurs in 48% (B) of the patients with poor outcomes and 6% (C) of the patients without poor outcomes.
3.4. Arrhythmia and outcome of the COVID-19
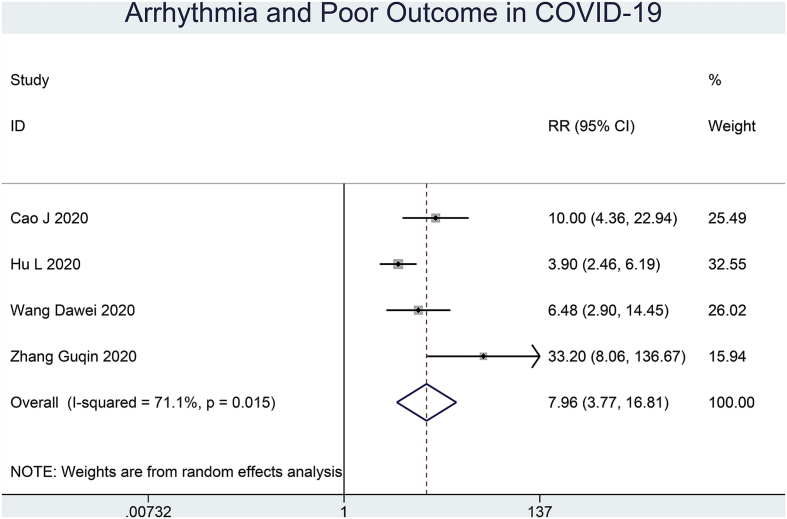
Patients with COVID-19 experiencing arrhythmia had an increased risk of poor outcome (RR 7.96 [3.77, 16.81], p < 0.001; I2: 71.1%, p = 0.02) (Fig. 3). Sensitivity analysis removing the study of Zhang Guqin et al. showed the statistical robustness of the effect estimate (RR 5.83 [3.26, 10.42], p < 0.001; I2: 54%, p = 0.11).
Fig. 3.
Arrhythmia and outcome of COVID-19. Patients with COVID-19 experiencing arrhythmia have an increased risk of poor outcome.
3.5. Meta-regression
The association between arrhythmia and outcome in patients with COVID-19 did not vary significantly with age (p = 0.840), male (p = 0.220), hypertension (p = 0.977), cardiovascular diseases (p = 0.848), diabetes (p = 0.139), respiratory comorbidities (p = 0.729), and lymphopenia (p = 0.084).
3.6. Publication bias
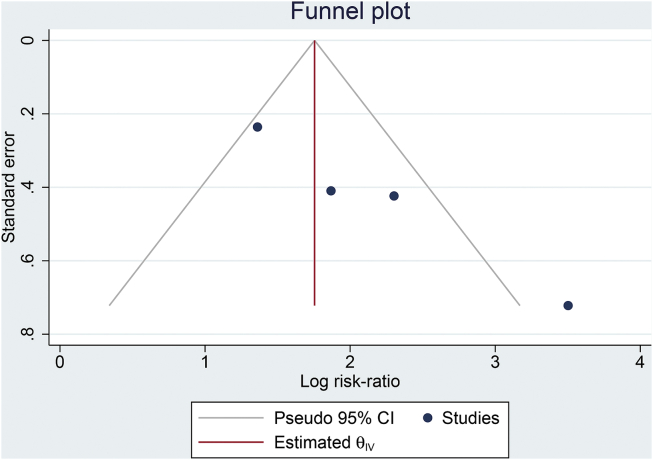
The funnel-plot analysis showed an asymmetrical funnel plot with most of the studies on the right side of the effect estimate (Fig. 4). Regression-based Egger’s test showed indication of small-study effects (p = 0.001).
Fig. 4.
Publication bias. The funnel-plot analysis shows an asymmetrical funnel plot with most of the studies on the right side of the effect estimate.
4. Discussion
This meta-analysis showed that the incidence of arrhythmia was significantly higher in patients with poor outcomes, hence a marker of poor prognosis.
Cardiac arrhythmias occurred frequently in patients with COVID-19, and the percentage was even higher in those with poor outcomes. However, this topic remains to be explored and currently, the included studies did not specify the types of arrhythmia. Hence, the specific types of arrhythmia that increase mortality and severity of COVID-19 remain inconclusive.
Defining the types of arrhythmia in patients with COVID-19 is important because of the frequent incidence and is strongly associated with poor prognosis. Providing details on the type of arrhythmia may help clinicians to discriminate the prognostic significance of the arrhythmia, allowing more precise risk stratification. Several baseline characteristics are also associated with poor outcome in this pooled studies. These factors include gender, hypertension, diabetes, cardiovascular diseases, and respiratory comorbidities which is accordance to our previous meta-analyses assessing these factors [[2], [3], [4], [5], [6], [7]]. We performed meta-regression and found that the association between arrhythmia and poor outcome did not varied with the gender and other comorbidities, nevertheless the number of studies <10.
Arrhythmia may be attributed to metabolic disarray, hypoxia, inflammation, or neurohormonal stress associated with SARS-CoV-2 infection [16]. Furthermore, COVID-19 induced coagulopathy may cause thrombosis leading to hypoxia [17,18]. Additionally, which patients often present electrolyte abnormalities that may precipitate/exacerbate cardiac arrhythmias [19]. SARS-CoV-2 itself has been shown to cause cardiac injury signified by increase in cardiac biomarkers [20,21]. After activation of the viral surface spike (S) protein of SARS-CoV-2 by the transmembrane protease serine 2 (TMPRSS2), the virus binds to angiotensin-converting enzyme 2 (ACE2) [22,23], which has high affinity to the human receptor [24,25]. The aforementioned enzyme is highly expressed in cardiac pericytes [26], infection, and subsequent immune response may cause damage to the heart. Myocardial inflammation, ion channel dysfunction, electrophysiological and structural remodeling associated with viral myocarditis has been shown to cause life-threatening arrhythmias [27]. Evidence shows that the rise of troponin is accompanied by increased inflammatory biomarkers, indicating the possible role of cytokine storm in cardiac injury [28]. Systemic inflammation has been shown to prolong ventricular action potential duration through disturbance of the potassium channel [29]. Interleukin-6, tumor necrosis factor alpha, and interleukin-1 may prolong the action potential duration through interplay with cardiomyocytes ion channels [30]. Elevated interleukin-6 during systemic inflammation may cause QT prolongation, which increases the risk for torsades de pointes [31].
Furthermore, QT-prolonging drugs are frequently used in the management of COVID-19, synergistically amplifying the risk of cardiac arrhythmias. Drugs such as azithromycin and hydroxychloroquine are associated with QT prolongation [32,33]. Azithromycin is known to prolong the QT interval [34] and increase the risk of cardiovascular death [35]. The anti-malarial chloroquine is associated with prolonged QT interval with or without azithryomycin according to a randomized controlled trial from the Pfizer Labs [36]. Moreover, lopinavir-ritonavir, a combination of drugs commonly known as protease inhibitors (PI) and usually used as 2nd-line drugs in treating HIV infection, is also recognized as arrhythmogenic and can prolong the QT interval [37,38]. Interestingly, Hu et al. reported that these drugs are associated with severe COVID-19, thus potentially affecting the association between arrhythmia and severe COVID-19 [15]. A risk score developed by Tisdale et al. may be used to predict patients at risk for QTc interval prolongation [39]. This score may be used to guide treatment choices for patients with COVID-19.
4.1. Limitations
This systematic review and meta-analysis has several limitations. First, the types of cardiac arrhythmias were not described in the included studies. Second, the risk of publication bias was high, as indicated by the asymmetrical funnel plot analysis and statistically significant Egger’s test. Third, most of the studies are from China and may not be generalizable to other populations. Fourth, the number of studies were limited and not divided into arrhythmias versus non-arrhythmias; hence, we were not able to assess the risk factors for cardiac arrhythmias in these patients. Fifth, the studies did not report the level of electrolytes. Sixth, the studies were mostly retrospective. The predictive power of meta-regression analysis is weaker if the number of studies are <10. Nevertheless, this study emphasizes the clinical importance of arrhythmia in patients with COVID-19, and further studies should explore cardiac arrhythmias in these patients.
5. Conclusion
Cardiac arrhythmias were significantly associated with an increased risk of poor outcome in COVID-19. Arrhythmias were observed in 19% of patients with COVID-19 and in 48% of patients with poor outcomes.
Authors contribution
R. P. conceived and designed the study and drafted the manuscript. R. P. and I. H. acquired the data and drafted the manuscript. R. P. and I. H. performed the data extraction. R. P., I. H., and S. B. R. interpreted the data and performed extensive research on the topic. S. B. R. reviewed and performed extensive editing of the manuscript. All authors contributed to the writing of the manuscript. R. P. performed the statistical analysis.
Funding
None.
Declaration of competing interest
Authors declare no Conflict of Interests for this article.
Acknowledgement
None.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
Contributor Information
Raymond Pranata, Email: raymond_pranata@hotmail.com, raymond_pranata@hotmail.com.
Ian Huang, Email: ianhuang2108@gmail.com.
Sunu Budhi Raharjo, Email: sunu.b.raharjo@gmail.com.
References
- 1.World Health Organization . 2020. Coronavirus disease 2019 (COVID-19) Situation Report – 95. [Google Scholar]
- 2.Huang I., Lim M.A., Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia – a systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr Clin Res Rev. 2020;14:395–403. doi: 10.1016/j.dsx.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pranata R., Huang I., Lim M.A., Wahjoepramono E.J., July J. Impact of cerebrovascular and cardiovascular diseases on mortality and severity of COVID-19 – systematic review, meta-analysis, and meta-regression. J Stroke Cerebrovasc Dis. 2020 doi: 10.1016/j.jstrokecerebrovasdis.2020.104949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pranata R., Lim M.A., Huang I., Raharjo S.B., Lukito A.A. Hypertension is associated with increased mortality and severity of disease in COVID-19 pneumonia: a systematic review, meta-analysis and meta-regression. J Renin-Angiotensin-Aldosterone Syst JRAAS. 2020;21 doi: 10.1177/1470320320926899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pranata R., Soeroto A.Y., Ian H., Lim M.A., Santoso P., Permana H. Effect of chronic obstructive pulmonary disease and smoking on the outcome of COVID-19. Int J Tubercul Lung Dis. 2020 doi: 10.5588/ijtld.20.0278. [DOI] [PubMed] [Google Scholar]
- 6.Huang I., Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. J Intensive Care. 2020;8:36. doi: 10.1186/s40560-020-00453-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pranata Raymond, Lim Michael A., Yonas Emir, Vania Rachel, Lukito Antonia A., Siswanto Bambang B. Body mass index and outcome in patients with COVID-19: a dose-response meta-analysis. Diabetes Metabol. 2020 doi: 10.1016/j.diabet.2020.07.005. S1262-3636(20)30097-5, In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madjid M., Connolly A.T., Nabutovsky Y., Safavi-Naeini P., Miller C.C. Influenza epidemics are associated with an increased risk of ventricular arrythmia therapies in patients with implantable cardiac defibrillators. J Am Coll Cardiol. 2017;69:1736. doi: 10.1016/S0735-1097(17)35125-2. [DOI] [Google Scholar]
- 9.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in wuhan, China. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lazzerini P.E., Boutjdir M., Capecchi P.L. COVID-19, arrhythmic risk and inflammation: mind the gap! Circulation. CIRCULATIONAHA. 2020;120 doi: 10.1161/CIRCULATIONAHA.120.047293. [DOI] [PubMed] [Google Scholar]
- 11.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in wuhan, China. JAMA, J Am Med Assoc. 2020:1–9. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao J., Tu W.-J., Cheng W., Yu L., Liu Y.-K., Hu X. Clinical features and short-term outcomes of 102 patients with corona virus disease 2019 in wuhan, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization . 2019. Report of the WHO-China Joint Mission on Coronavirus Disease. ( COVID-19 ). vol. 2019. n.d. [Google Scholar]
- 14.Zhang G., Hu C., Luo L., Fang F., Chen Y., Li J. Clinical features and outcomes of 221 patients with COVID-19 in Wuhan, China. MedRxiv. 2020 doi: 10.1101/2020.03.02.20030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu L., Chen S., Fu Y. Risk factors associated with clinical outcomes in 323 COVID-19 patients in wuhan , China. MedRxiv. 2020 doi: 10.1101/2020.03.25.20037721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Driggin E., Madhavan M.V., Bikdeli B., Chuich T., Laracy J., Bondi-Zoccai G. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID-19) pandemic. J Am Coll Cardiol. 2020 doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang I., Pranata R., Lim M.A., Oehadian A., Alisjahbana B. C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis. Ther Adv Respir Dis. 2020;14 doi: 10.1177/1753466620937175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim M.A., Pranata R., Huang I., Yonas E., Soeroto A.Y., Supriyadi R. Multiorgan failure with emphasis on acute kidney injury and severity of COVID-19: systematic review and meta-analysis. Can J Kidney Heal Dis. 2020;7 doi: 10.1177/2054358120938573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lakkireddy D.R., Chung M.K., Gopinathannair R., Patton K.K., Gluckman T.J., Turagam M. Guidance for cardiac electrophysiology during the coronavirus (COVID-19) pandemic from the heart rhythm society COVID-19 task force; electrophysiology section of the American college of cardiology; and the electrocardiography and arrhythmias committee of. Heart Rhythm. 2020 doi: 10.1016/j.hrthm.2020.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santoso A., Pranata R., Wibowo A., Al-Farabi M.J., Huang I., Antariksa B. Cardiac injury is associated with mortality and critically ill pneumonia in COVID-19: a meta-analysis. Am J Emerg Med. 2020 doi: 10.1016/j.ajem.2020.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pranata R., Huang I., Lukito A.A., Raharjo S.B. Elevated N-terminal pro-brain natriuretic peptide is associated with increased mortality in patients with COVID-19: systematic review and meta-analysis. Postgrad Med. 2020 doi: 10.1136/postgradmedj-2020-137884. postgradmedj-2020-137884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pranata R., Permana H., Huang I., Lim M.A., Soetedjo N.N.M., Supriyadi R. The use of renin angiotensin system inhibitor on mortality in patients with coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Diabetes Metab Syndr Clin Res Rev. 2020 doi: 10.1016/j.dsx.2020.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tai W., He L., Zhang X., Pu J., Voronin D., Jiang S. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol. 2020 doi: 10.1038/s41423-020-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li W., Moore M.J., Vasllieva N., Sui J., Wong S.K., Berne M.A. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen L., Li X., Chen M., Feng Y., Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. 2020 doi: 10.1093/cvr/cvaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tse G., Yeo J.M., Chan Y.W., Lai E.T.H.L., Yan B.P. What is the arrhythmic substrate in viral myocarditis? Insights from clinical and animal studies. Front Physiol. 2016;7 doi: 10.3389/fphys.2016.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clerkin K.J., Fried J.A., Raikhelkar J., Sayer G., Griffin J.M., Masoumi A. Coronavirus disease 2019 (COVID-19) and cardiovascular disease. Circulation. 2020;120 doi: 10.1161/circulationaha.120.046941. CIRCULATIONAHA. [DOI] [PubMed] [Google Scholar]
- 29.Lazzerini P.E., Capecchi P.L., Laghi-Pasini F. Systemic inflammation and arrhythmic risk: lessons from rheumatoid arthritis. Eur Heart J. 2016 doi: 10.1093/eurheartj/ehw208. [DOI] [PubMed] [Google Scholar]
- 30.Lazzerini P.E., Laghi-Pasini F., Boutjdir M., Capecchi P.L. Cardioimmunology of arrhythmias: the role of autoimmune and inflammatory cardiac channelopathies. Nat Rev Immunol. 2019;19:63–64. doi: 10.1038/s41577-018-0098-z. [DOI] [PubMed] [Google Scholar]
- 31.Lazzerini P.E., Laghi-Pasini F., Bertolozzi I., Morozzi G., Lorenzini S., Simpatico A. Systemic inflammation as a novel QT-prolonging risk factor in patients with torsades de pointes. Heart. 2017;103:1821–1829. doi: 10.1136/heartjnl-2016-311079. [DOI] [PubMed] [Google Scholar]
- 32.Hancox J.C., Hasnain M., Vieweg W.V.R., Crouse E.L.B., Baranchuk A. 2013. Azithromycin , cardiovascular risks , QTc interval prolongation , torsade de pointes , and regulatory issues : a narrative review based on the study of case reports; pp. 155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kapoor A., Pandurangi U., Arora V., Gupta A., Jaswal A., Nabar A. Cardiovascular risks of hydroxychloroquine in treatment and prophylaxis of COVID-19 patients: a scientific statement from the Indian Heart Rhythm Society. Indian Pacing Electrophysiol J. 2020;20:117–120. doi: 10.1016/j.ipej.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mosholder A.D., Mathew J., Alexander J.J., Smith H., Nambiar S. Cardiovascular risks with azithromycin and other antibacterial drugs. N Engl J Med. 2013;368:1665–1668. doi: 10.1056/NEJMp1302726. [DOI] [PubMed] [Google Scholar]
- 35.Ray W.A., Murray K.T., Hall K., Arbogast P.G., Stein C.M. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881–1890. doi: 10.1056/NEJMoa1003833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pfizer Labs . 2013. Zithromax (azithromycin tablets) and azithromycin for oral suspension. [Google Scholar]
- 37.Reyskens K.M.S.E., Fisher T.L., Schisler J.C., O’Connor W.G., Rogers A.B., Willis M.S. The maladaptive effects of HIV protease inhibitors (Lopinavir/Ritonavir) on the rat heart. PLoS One. 2013;8 doi: 10.1371/journal.pone.0073347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu J., Shah S.K., Basu-Ray I., Garcia-Diaz J., Khalid K., Saeed M. QT prolongation in HIV-positive patients: review article. Indian Heart J. 2019;71:434–439. doi: 10.1016/j.ihj.2019.11.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tisdale J.E., Jaynes H.A., Kingery J.R., Mourad N.A., Trujillo T.N., Overholser B.R. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes. 2013;6:479–487. doi: 10.1161/CIRCOUTCOMES.113.000152. [DOI] [PMC free article] [PubMed] [Google Scholar]