Abstract
The COVID-19 pandemic has led to twin public health and economic crises around the world. Not only has it cost hundreds of thousands of lives but also severely impacted livelihoods and placed enormous strain on community healthcare and welfare services. In this review, we explore the events associated with SARS-CoV-2 pathogenesis and host immunopathological reactivity due to the clinical manifestations of this coronavirus infection. We discuss that the metallopeptidase enzyme ADAM17, also known as tumor necrosis factor-α-converting enzyme, TACE, is responsible for shedding of angiotensin-converting enzyme 2 and membrane-bound interleukin (IL)-6 receptor. This leads to elevated pro-inflammatory responses that result in cytokine storm syndrome. We argue that cytokine balance may be restored by recovering an IL-6 trans-signaling neutralizing buffer system through the mediation of recombinant soluble glycoprotein 130 and recombinant ADAM17/TACE prodomain inhibitor. This cytokine restoration, possibly combined with inhibition of SARS-CoV-2 entry as well as replication and coagulopathy, could be introduced as a novel approach to treat patients with severe COVID-19. In cases of co-morbidity, therapies related to the management of associated disease conditions could ameliorate those clinical manifestations.
Keywords: COVID-19, SARS-CoV-2, IL-6, ADAM17, TACE, ADAM17/TACE inhibitor, sgp130Fc, Therapy
1. Introduction
The healthcare services of nations the world over were caught unprepared for the recent emergence of the coronavirus disease (COVID)-19 pandemic caused by infection with a novel human pathogen, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Scientists are now urgently seeking possible therapeutic interventions. Currently, proposals are made daily, and, with no confirmed drugs available yet, several of these are being considered for trial [1], [2]. The global biomedical research community is sharing ideas and data on various open access platforms at a time when it is important to consider the validity of all possible approaches, reports, case studies and hypotheses. We appear to still be near the bottom of a steep learning curve to understand the complex interactions in COVID-19 immunopathogenesis [3]. It is therefore imperative to act fast, while adhering to approved regulatory guidelines, in order to not miss any opportunity to advance knowledge of the disease process and ways in which it can be prevented.
As treatment options, there is a focus on viral pathogenesis and management of either cytokines or clinical symptoms [4], [5]. We argue that a multi-pronged strategy could prove effective as the clinical manifestations of COVID-19 are shown to be interconnected. Very recently, the antiviral remdesivir passed through a randomized, double-blinded, placebo-controlled trial and the US Food and Drug Administration has issued an authorization for emergency use of this drug for hospitalized COVID-19 patients [6], [7], [8]. Dr. Anthony Fauci, Director of the National Institutes of Allergy and Infectious Diseases, was reported as saying that the upcoming second Adaptive COVID-19 Treatment Trial “will examine if adding an anti-inflammatory agent to the remdesivir regimen can provide additional benefit for patients, including improving mortality outcomes” [7].
We consider that a combined approach should be evaluated in which antiviral and immune modulatory drugs or inhibitors may show better prospects for reducing cytokine storm severity. Interleukin (IL)-6 is an inflammatory cytokine that has been demonstrated to play a leading role in cytokine storm syndrome. Studies have shown that an imbalance in molar concentrations of IL-6, membrane-bound IL-6 receptor (IL-6R) and its soluble form (sIL-6R) can lead to up-regulation of IL-6-mediated trans-signaling, thereby initiating formation of a cytokine storm [9]. Together with sIL-6R, a soluble form of glycoprotein 130 (sgp130) constitutes an IL-6 neutralizing buffer system [10]. ADAM Metallopeptidase Domain 17 (ADAM17), also known as tumor necrosis factor-α converting enzyme (TACE), is a membrane-bound enzyme that cleaves IL-6R to produce sIL-6R and which is responsible for disrupting fine tuning of the neutralizing buffer system [11]. In order to develop an effective therapeutic strategy for COVID-19 new investigations should consider approaches that can provide control over both viral replication and cytokine imbalance. We have analyzed the different factors involved in these complex clinical manifestations as an interactive model. In light of the insights gained we propose that if administered along with antivirals a combination of ADAM17/TACE prodomain inhibitor (TPD), sgp130Fc protein (a recombinant form of sgp130) and anti-coagulant may produce enhanced outcomes in COVID-19 patient recovery.
2. Explaining cytokine storm and associated complications in COVID-19
Acute respiratory distress syndrome (ARDS) is identified as the cause of death in COVID-19 cases [12]. Typical manifestations of ARDS include inflammation, barrier disruption, airspace edema, cell injury and cell death [13]. In COVID-19 patients, all these events are consequences of hyperinflammation induced by cytokine storm in which IL-6 plays a major role [14], [15]. Uncontrolled production of pro-inflammatory cytokines (principally IFN-α, IFN-γ, TNF-α, IL-1β, IL-6, IL-2, IL-12 and TGF-β) is a commonly reported feature of cytokine storm syndromes in severe COVID-19 patients [16], [17]. Of these cytokines, IL-6 is distinguishably notable because of its function in initiating pro-inflammatory responses via the IL-6 trans-signaling pathway. IL-6 presents a dual face in inflammatory responses [15]. Depending on its signaling cascade it can be either pro-inflammatory or anti-inflammatory. Complete IL-6 signaling requires binding of IL-6 to IL-6R and subsequent association of IL-6/IL-6R complex with gp130 receptor. IL-6R is expressed on hepatocytes and leukocytes while gp130 is expressed ubiquitously by all cells in the body. IL-6R is distributed in two distinct forms, sIL-6R and IL-6R. When IL-6 binds to sIL-6R and IL-6/sIL-6R complex associates with gp130, this initiates trans-signaling, which is pro-inflammatory in function. In contrast, in the event of IL-6 interacting with IL-6R, IL-6/IL-6R complex associates with gp130 to trigger classical (cis)-signaling, which is anti-inflammatory [18]. Trans-signaling involves activation of endothelial cells and smooth muscle cells, mononuclear cell recruitment and apoptosis of neutrophils, chemokine expression, and T cell expression and recruitment [19]. Conversely, classical-signaling plays protective roles via stimulation of intestinal regeneration, induction of hepatic acute phase responses and inhibition of epithelial cell apoptosis [20]. During infection, IL-6 trans-signaling is first to appear, classical-signaling activating later to regulate the inflammation caused by trans-signaling-induced pro-inflammatory responses [18], [21].
Found in the peripheral circulation, sgp130, a naturally soluble form of gp130, is generated by alternatively spliced mRNA rather than by proteolytic cleavage [9]. There are three different forms of sgp130. Although the individual function of different forms is not yet known but they have been shown to bind to IL-6R with varying efficacy [22]. The sgp130 is the natural inhibitor of IL-6 trans-signaling [18], [23]. sgp130 shows high specific affinity for IL-6/sIL-6R complex but not for either IL-6 or sIL-6R alone. It also confers protection from trans-signaling-mediated inflammation by forming a neutralizing buffer with IL-6/sIL-6R complex. The polarization from trans- to cis-signaling is fully dependent on the relative concentrations of IL-6, sIL-6R and sgp130. In healthy individuals, IL-6 is barely detectable in plasma, ranging between 2 and 6 pg/ml. Yet, during inflammation IL-6 concentrations increase massively and can reach µg/ml levels. At steady state, sIL-6R levels are in the range of 75 ng/ml and those of sgp130 are approximately 250–400 ng/ml. During acute inflammation, concentrations of IL-6 can increase up to a million-fold, but sIL-6R rises only 2- to 5-fold while sgp130 remains constant. As soon as antigen-presenting cells (APC) trigger the pro-inflammatory cascade, IL-6 binds to sIL-6R and the resultant IL-6/sIL-6R complex is neutralized by the action of sgp130 inhibiting trans-signaling [24]. Trans-signaling occurs if the molar concentration of sIL-6R becomes higher than that of both IL-6 and sgp130. The binding of sgp130 is highly specific but not sufficient to block IL-6 trans-signaling [23]. The neutralizing buffer complex of IL-6/sIL-6R/sgp130 will be formed until sIL-6R exceeds the molar concentration of IL-6. When this occurs, sgp130 cannot trap all available sIL-6R and trans-signaling starts, whereby IL-6/sIL-6R complex binds to membrane-bound gp130 available on any cells [23], [24]. However, when IL-6 exceeds the molar concentration of sIL-6R, sgp130 traps all available IL-6/sIL-6R complex, then the circulating free IL-6 binds to IL-6R and thereby initiates cis-signaling to maintain cytokine homeostasis [18], [25].
In the event of ADAM17 activation, IL-6 fails to switch from trans- to cis-signaling, uncontrolled release of pro-inflammatory cytokines occurs which precipitates a cytokine storm [11], [26]. Activated ADAM17 sheds IL-6R to become sIL-6R [11], [27]. Continuous shedding raises the concentration of sIL-6R above normal and soluble receptors form IL-6/sIL-6R complexes. Protective sgp130 fails to trap these abundant IL-6/sIL-6R complexes, which are able to bind to gp130 receptors on endothelial cells and fibroblasts [23]. As these cells possess gp130 receptors, a prerequisite for trans-signaling, activation via trans-signaling triggers endothelial cells to express intercellular adhesion molecule-1, vascular cell adhesion molecule-1, IL-8, macrophage chemoattractant protein-1 and IL-6. It is IL-6 that thereby attracts more leukocytes to the infection site [28]. By means of this ADAM17-mediated shedding event the pro-inflammatory cascade skips IL-6 classical-signaling and thus prevents establishment of cytokine homeostasis. Trans-signaling can activate all cells of the human body to release pro-inflammatory cytokines [23]. Therefore, as an unavoidable consequence, cytokine storm is initiated (Fig. 1 ) [18], [26]. Macrophage activation and polarization are also found to be correlated with IL-6 and its receptor. M1 macrophages are responsible for amplifying the inflammatory feedback loop whereas M2 macrophages are anti-inflammatory and therefore critical to establishing metabolic control and tissue homeostasis [29], [30]. Studies have shown that blockade of IL-6R function positively correlates with activation of M1 macrophages as well as with IL-6 trans-signaling-mediated pro-inflammatory responses. IL-6 classical-signaling directly induces IL-4 expression, which is a potent inducer of M2 macrophage polarization [31], [32].
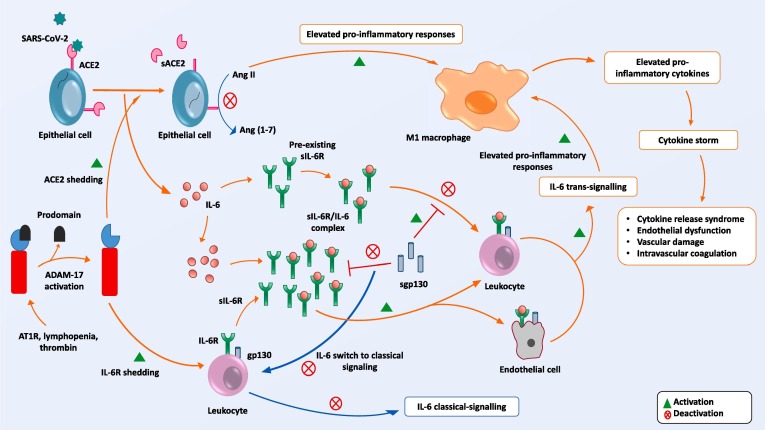
Fig. 1.
Establishment of cytokine storm in COVID-19. From upper left to right: SARS-CoV-2 binds with ACE2, thereby facilitating entry of viral RNA into the host cell; ACE2 is shed by ADAM17; Ang II triggers pro-inflammatory responses via M1 macrophages. Centre: IL-6/sIL-6R complexes are neutralized by sgp130 until the concentration of sIL-6R is lower than that of IL-6. Lower left: When infection is activated ADAM17 triggers IL-6R shedding. Once the concentration of sIL-6R is higher than that of IL-6, sgp130 cannot neutralize increased levels of IL-6/sIL-6R complexes. IL-6 trans-signaling is triggered and, as a consequence, cytokine storm is initiated.
Trans-signaling by endothelial cells may result in increased vasculopathy, fibrosis, endothelial dysfunction, vascular permeability and disseminated intravascular coagulation [28], [33]. Histopathological examination of fatal cases of COVID-19 has revealed evidence of a disturbed vascular equilibrium due to vasoconstriction with organ ischemia, tissue edema and a pro-coagulation state [34], [35]. Exceptions have been observed in patients with an Ala358 variant as well as patients with an rs2228145 single nucleotide polymorphism in multiple inflammatory disease models [36], [37]. Patients with these variants show greater shedding of IL-6R than is normal, resulting in significantly higher levels of sIL-6R than detected in healthy individuals. Interestingly, these patients appear to have a lower risk of acquiring multiple metabolic diseases like congestive heart disease, abdominal aneurism, rheumatoid arthritis and Crohn’s disease [38], [39]. This protection may be explained by increased action of a neutralizing buffer system in response to elevated sIL-6R [40].
Lungs are ‘ground zero’ for SARS-CoV-2 pathogenesis. The virus gains entry by binding to the angiotensin-converting enzyme 2 (ACE2) receptor that is expressed on the membrane of respiratory tract epithelial cells [41], [42]. In response to this event APC rapidly activate a pro-inflammatory cascade, and hence SARS-CoV-2 entry and replication are linked directly to virus killing of lymphocytes as well as to the aberrant release of cytokines [43], [44]. The ensuing massive loss of lymphocytes contributes to the phenomenon known as lymphopenia that is commonly observed in COVID-19 [44], [45], [46]. ACE2, an important regulator of the renin-angiotensin system (RAS), plays a major role in blood pressure homeostasis and prevention of lung injury by converting angiotensin (Ang) II to Ang 1-7 [47]. Ang II, a vasoconstrictor hormone, directly triggers pro-inflammatory responses as well as stimulating the release of IL-6, IL-8 and TNF-ɑ by M1 macrophages via up-regulation of NF-κB. Ang II is also responsible for fibrosis, vascular damage and hypoxia [48], [49]. Ang 1-7 is a vasodilation hormone that is responsible for anti-inflammatory responses and which thus acts to prevent lung injury. Ang 1-7 up-regulates M2 macrophage-mediated anti-inflammatory cytokines and reduces circulating levels of IL-6 and TNF-ɑ as well as of other pro-inflammatory responses by down-regulating NF-κB [50], [51]. Therefore, during COVID-19 pathogenesis ACE2-mediated conversion of Ang II to Ang 1-7 is required to maintain cytokine homeostasis as well as to offer protection from cytokine storm.
Interestingly, once SARS-CoV-2 enters host cells, ACE2 becomes down-regulated. Down-regulation of ACE2 is achieved by ADAM17-mediated shedding, whereby membrane-bound ACE2 is shed to a soluble form of ACE2 (sACE2) [52].
Another shedding protease of ACE2, named transmembrane protease, serine 2 (TMPRSS2), has a differential cleavage site to ADAM17 and consequently the two are compared. ACE2 cleavage by TMPRSS2 requires arginine and lysine residues within ACE2 amino acids 697 to 716 whereas in the case of ADAM17 arginine and lysine residues within ACE2 amino acids 652 to 659 are critical [53], [54]. Studies of both SARS-CoV and SARS-CoV-2 have shown that TMPRSS2-mediated ACE2 cleavage is involved in spike protein-mediated coronavirus entry and promotes its uptake by the host cell [55], [56]. ADAM17-mediated shedding events were not found to modulate virus entry but instead are associated with ACE2 ectodomain shedding [55]. As ADAM17-mediated multiple shedding events have a profound effect on cytokine balance our discussion is focused on ADAM17 activation and regulation.
ADAM17 is expressed by almost all types of tissues but its cell surface activity is tightly regulated. In the presence of inactive rhomboid-like protein (iRhom) 1 and 2, crucial upstream regulators of ADAM17-dependent epidermal growth factor receptor signaling, ADAM17 is transferred from the Golgi apparatus to the plasma membrane. In the absence of iRhom1 or iRhom2, ADAM17 mRNA is still expressed but trafficking to the cell membrane does not take place [40], [57]. The prodomain of ADAM17 inhibits its proteinase and shedding activities while its release results in ADAM17 activation [23], [40]. The process by which ADAM17 is stimulated to initiate shedding events is not fully elucidated. However, it is apparent that the early stages of lymphopenia and the presence of Ang II type 1 receptor (AT1R) are potent activators of ADAM17-mediated shedding activity [58], [59]. By shedding ACE2, ADAM17 both inhibits the production of Ang 1-7 and increases Ang II levels in COVID-19 patients [60]. Down-regulation of ACE2 is thus related to RAS imbalance, an elevated pro-inflammatory response and multi-organ damage from SARS-CoV-2 infection [61].
Pro-inflammatory cytokines can up-regulate the coagulation system while down-regulating important physiological anticoagulant pathways [62], [63]. Thrombin, a key component of the coagulation cascade, is known to stimulate ADAM17-mediated shedding events [57]. Platelets also express gp130 on their membrane, so therefore are capable of IL-6 trans-signaling. Platelet-derived IL-6 trans-signaling is associated with inflammation of damaged blood vessels and thrombogenicity [64].
Thrombin converts fibrinogen into fibrin in order to stabilize a blood clot. Tissue plasminogen activator (tPA) and urokinase plasminogen activator (uPA) trigger the formation of plasmin, the presence of which breaks down clots by a process called fibrinolysis. Both tPA and uPA can be inhibited by plasminogen activator inhibitor-1 (PAI-1). The balance between tPA/uPA and PAI-1 ensures homeostasis of the blood circulation system, disturbance of which is observed during COVID-19 ARDS [65]. Non-specific ARDS patients have significantly higher plasma levels of PAI-1 compared to non-ARDS patients [65], [66]. There is strong evidence to suggest that IL-6 and sIL-6R are responsible for increased expression of PAI-1 and for decreased expression of tPA and uPA. This clearly indicates the involvement of IL-6 trans-signaling in thrombosis [67], [68]. Moreover, Ang II also stimulates PAI-1 expression by endothelial cells, contributing to disruption of the balance between PAI-1 and tPA/uPA to establish a hypercoagulable state [65].
Pulmonary thrombosis may result in retraining of the compensatory ventilation response, as well as in vascular leakage, alveolar edema, severe hypoxia and multi-organ failure [35], [63]. This phenomenon could be linked to the multi-organ dysfunction and blood clotting that are observed in fatal cases of COVID-19 [69]. Endothelial dysfunction and damaged vessels could be linked to SARS-CoV-2 spread in multiple organs. We believe our proposed model also explains COVID-19 severity with comorbidities like hypertension, diabetes mellitus, coronary heart disease and cerebrovascular disease. Of note, serum concentrations of sIL-6R and sgp130 are significantly lower in type 2 diabetes patients compared to healthy individuals [37]. Similar findings were confirmed for patients awaiting coronary artery surgery [70], [71]. These data firmly demonstrate that comorbid patients already have lower levels of IL-6 trans-signaling protective buffer, which renders them more prone to induction of IL-6 trans-signaling within a short timeframe [37]. Individuals with pre-existing metabolic disease conditions already possess inflammation and consequent pro-inflammatory responses [72]. SARS-COV-2-mediated ADAM17 activation therefore facilitates disruption of cytokine equilibrium. Hence, such patients develop cytokine storm syndrome as an unavoidable outcome of comorbidity [15].
3. Prospects of therapeutic combinations
Many therapeutic strategies against SARS-CoV-2 are already proposed and undergoing testing. Some of these approaches target virus replication while others relate to blockade of cytokines [73], [74]. Unfortunately, none is yet proven to show fully protective effects and thus to promote patient recovery from disease. We believe that there is unlikely to be a ‘magic bullet’ for COVID-19 treatment, at least not anytime soon. Rather, a combinational drug strategy should be advocated as a realistic alternative treatment regimen. Clinical manifestations of disease should be observed from a holistic perspective as viral replication, cytokine storm, coagulation and other events occur simultaneously or in very rapid succession. In addition, at the time they are admitted to critical care facilities the extent of disease progression is specific to each patient, so it becomes extremely challenging for healthcare providers to address the therapeutic needs of the individual. Therefore, based on this complex scenario, in aiming to reduce the severity of disease for as many critical care patients as possible a guideline combinational regimen of treatment with several therapeutics is preferred.
Since patients with comorbidity are likely to experience cytokine imbalance, we consider the first priority to be rescue from cytokine storm. In order to restore the cytokine balance efforts should be taken to switch IL-6 trans-signaling to classical-signaling and to curtail ADAM17-mediated shedding events. In this regard, we propose the use of sgp130 and ADAM17 inhibitor with high specificity, which may show considerably more therapeutic potential than either a global cytokine inhibitor or IL-6R inhibitor [75], [76]. We have already discussed how sgp130 forms a protective neutralizing buffer against IL-6 trans-signaling. Multiple studies have confirmed that sgp130 is highly specific to IL-6 trans-signaling [19], [77]. It inhibits IL-6 trans-signaling by trapping IL-6/sIL-6R without influencing the protective roles of IL-6 classical-signaling. In the event of cytokine storm, it is necessary to neutralize the sIL-6R function without any compromise to broader IL-6 activity [24]. Anti-inflammatory drugs often inhibit global IL-6 function or global immune suppression, whereas it is evident that selective inhibition of IL-6 trans-signaling is greatly superior in this regard to global suppression of IL-6 [78]. Immunosuppressive drugs can inadvertently prove fatal to patients with comorbidity by increasing their susceptibility to new infection [79]. A recombinant protein, sgp130Fc, was developed in which the extracellular portion of gp130 is fused to the Fc portion of human IgG1 antibody [10], [20]. Using sgp130Fc, successful inhibition of IL-6 trans-signaling can be achieved both in vitro and in vivo without affecting IL-6 classical-signaling at low concentrations [19], [24]. Moreover, it has been reported that sgp130Fc achieves more effective inhibition (10- to 50-fold) of IL-6 trans-signaling than does sgp130 monomer itself [80]. This protein also showed superior results over the anti-IL-6-R antibody tocilizumab and proved that inhibition of trans-signaling is more desirable to global blockade of IL-6 signaling [24].
By the time sgp130Fc forms neutralizing buffer to trap excess sIL-6R complex it is also necessary to inhibit ADAM17-mediated IL-6R and ACE2 shedding events [20]. We should bear in mind that the entire IL-6 signaling pathway is concentration-dependent. Under normal circumstances upon activation IL-6 can increase in level many folds higher than sIL-6R whereas sIL-6R increases only modestly. It is worth noting that at steady state the concentration of sIL-6R is not related to ADAM17 shedding but instead depends on differential splicing of IL-6R mRNA [9]. ADAM17-mediated shedding events act to increase sIL-6R concentration continuously. Therefore, we also need to block proteolytic cleavage of IL-6R in order to reduce the sIL-6R molar concentration lower than that of IL-6. If and when the concentration of IL-6 exceeds that of sIL-6R sgp130Fc neutralizes trans-signaling and IL-6 classical-signaling commences.
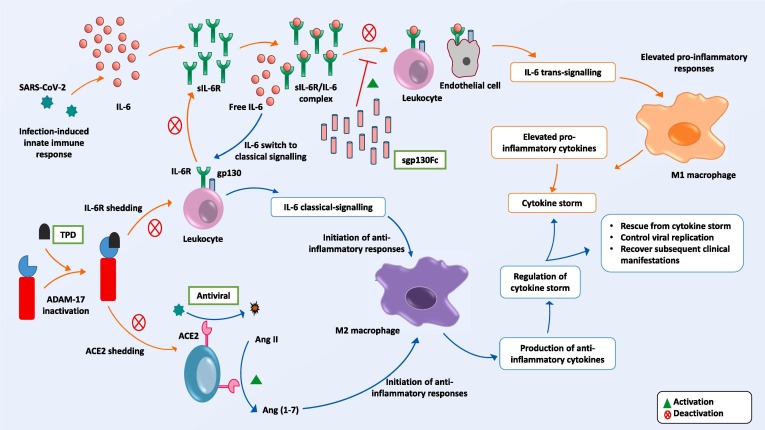
Inhibition of ADAM17 will suppress ACE2 shedding [76]. Through this mechanism Ang II concentration is reduced and M2 macrophage-mediated anti-inflammatory responses are triggered [50]. For this purpose, ADAM17 inhibition may be considered as the preferred and most targeted approach [81]. Therapeutic targeting of ADAM17 inhibitor has necessarily to be highly specific and non-toxic. Several ADAM17-based drug candidates have been tested in the past, of which a number were discontinued due to their lesser efficacy and higher toxicity [82]. Recently, a recombinant ADAM17/TACE prodomain (TPD) was developed, which is reported to be stable, highly specific and auto-inhibitory [83]. This inhibitory prodomain binds to human TACE in a precise manner and successfully inhibits shedding events. Most importantly, this inhibitor is noted not to share any sequence homology with any other related proteins from the ADAM family, to fold correctly and to remain stable in vivo, properties that collectively make it a prime candidate as an ADAM17 prodomain inhibitor [83], [84]. The therapeutic potential of TPD was illustrated by its successful blocking of IL-6 trans-signaling involved in severe disease pathology [85]. A combination of both sgp130Fc and TPD may rescue the balance between cytokines (Fig. 2 ).
Fig. 2.
Proposed therapeutic model. Administration of sgp130Fc suppresses IL-6 trans-signaling by trapping available IL-6/sIL-6R complexes (upper section). TPD inhibits ADAM17 activation and stops shedding of ACE2 and IL-6 receptors. As a consequence, the concentration of sIL-6R becomes lower than that of IL-6, whereupon IL-6 switches from trans- to classical-signaling. By this means, regulation of cytokine storm may be achieved, while antiviral therapy will restrict viral host cell entry.
While protecting ACE2 receptor from shedding will protect from Ang II- and AT1R-mediated ADAM17 activation and pro-inflammatory responses, paradoxically it also offers an opportunity for virus entry by binding of SARS-CoV-2 spike protein to ACE2 receptor [86]. ACE2 receptor is distributed across a wide range of cell surfaces that may provide potential targets for virus spread if replication is not controlled [87], [88], [89]. Hence, use of an antagonist to SARS-CoV-2 entry or replication should additionally be investigated. The mechanisms by which virus entry is inhibited have been explained in detail in current works [90], [91], and therefore are not part of this discussion.
Lastly, the coagulation observed in some COVID-19 cases should not be overlooked [69]. Patients could be admitted to hospital at any stage of disease progression and those with comorbidity who may already have higher sIL-6R are susceptible to developing trans-signaling-associated intravascular coagulation. Therefore, anticoagulant administration may have a beneficial effect if used in combination with TPD, sgp130Fc and a candidate antiviral. Already, use of anticoagulants has shown a degree of therapeutic value [92].
4. Future challenges
Recently, an open-label, randomized, phase 2 trial of a triple drug combination was announced. This reported a better outcome by using two antiviral drugs, lopinavir-ritonavir and ribavirin, in combination with immunomodulatory cytokine IFN-β-1b [93]. The findings of this study support our notion to develop a combinatorial therapeutic approach with emphasis on IL-6 signaling modulation. However, we prefer to consider immune-modulation by inhibiting IL-6 trans-signaling specifically rather than by a global means. A complex and interconnected picture of the immunopathogenesis observed in COVID-19 is described herein. Following consideration of these networked events we propose a combinatorial drug-based approach to therapy that includes TPD in order to inhibit shedding of ACE2 and IL-6 that ultimately cascades to cytokine storm, sgp130Fc in order to restore cytokine balance, an antiviral in order to reduce viral load, and an anticoagulant in order to avoid inflammation-associated coagulopathy. Theoretically at least, we consider that this drug combination may well promote enhanced recovery from COVID-19, yet its safety and efficacy first needs to be explored fully by pre-clinical testing and in clinical trials.
To our knowledge, sgp130Fc has passed successfully toxicology tests and phase I clinical trial, although confirmatory data await publication. Based on this premise, multiple phase II trials are ongoing in China, Taiwan and South Korea [94]. Another potential candidate, TPD protein, has not yet undergone clinical trial. A group from The Weizmann Institute of Science in Israel is researching the development of TPD for clinical use [personal communication].
To date, there are still no regulatory approved therapeutic approaches with which to fight this pandemic. However, the US National Institutes of Health have announced a keenly anticipated clinical trial of the anti-inflammatory drug baricitinib plus the antiviral remdesivir co-administered for treatment of COVID-19 patients [7]. From prior experience, we prefer selective inhibition to global suppression of inflammatory cytokines as it is proven to be beneficial in pathophysiological states and to curb disease progression [40], [95].
5. Concluding remarks
With a view to developing a promising drug candidate against COVID-19 there is a pressing need right now to bridge existing knowledge gaps and to use the lessons learned from previous trials. In order to address the treatment plan for COVID-19 it should be remembered that the disease manifestations are not caused simply by an imbalance of a single biomolecule. Rather, they reflect an overall dysfunction of the convoluted and intricate immune system due to cytokine imbalance and other associated complex events that are triggered by SARS-CoV-2 infection. Therefore, future development of a therapeutic strategy should recognize the advantages of a combinatorial approach over a reductionist, single drug-based therapy. However, careful evaluation of any clinical trial based on our proposition is recommended to determine the therapeutic potential. Until such drugs are available we should continue to promote best hygiene practices and social distancing measures, to seek early and accurate diagnosis and to explore mechanisms for disease management and prevention that take into consideration as much as possible the economic burden of this pandemic [96], [97]. This is particularly crucial during the ongoing perilous period of undetermined length while an efficacious prophylactic vaccine against SARS-CoV-2 remains in development.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors wish to thank Prof. Dr. Stefan Rose-John, Department of Biochemistry Christian-Albrechts-Universität zu Kiel, Germany, and Prof. Irit Sagi, Department of Biological Regulation, The Weizmann Institute of Science, Israel, for their valuable support and constructive comments during preparation of this paper.
References
- 1.Lurie N., Saville M., Hatchett R., Halton J. Developing Covid-19 vaccines at pandemic speed. N. Engl. J. Med. 2020 doi: 10.1056/nejmp2005630. [DOI] [PubMed] [Google Scholar]
- 2.Mullard A. Flooded by the torrent: the COVID-19 drug pipeline. Lancet. 2020;395:1245–1246. doi: 10.1016/S0140-6736(20)30894-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.C. Wadman, Meredith, Couzin-Frankel, Jennifer, Kaiser, Jocelyn, Matacic, How does coronavirus kill? Clinicians trace a ferocious rampage through the body, from brain to toes, Science (80-.). (2020). doi:10.1126/science.abc3208. [DOI] [PubMed]
- 4.Thorlund K., Dron L., Park J., Hsu G., Forrest J.I., Mills E.J. A real-time dashboard of clinical trials for COVID-19. Lancet Digit. Heal. 2020 doi: 10.1016/S2589-7500(20)30086-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitjà O., Clotet B. Use of antiviral drugs to reduce COVID-19 transmission. Lancet Glob. Heal. 2020 doi: 10.1016/S2214-109X(20)30114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y., Fu S., Gao L., Cheng Z., Lu Q., Hu Y., Luo G., Wang K., Lu Y., Li H., Wang S., Ruan S., Yang C., Mei C., Wang Y., Ding D., Wu F., Tang X., Ye X., Ye Y., Liu B., Yang J., Yin W., Wang A., Fan G., Zhou F., Liu Z., Gu X., Xu J., Shang L., Zhang Y., Cao L., Guo T., Wan Y., Qin H., Jiang Y., Jaki T., Hayden F.G., Horby P.W., Cao B., Wang C. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020 doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Press Release: NIH clinical trial testing antiviral remdesivir plus anti-inflammatory drug baricitinib for COVID-19 begins | National Institutes of Health (NIH), NIH News Release. (2020). https://www.nih.gov/news-events/news-releases/nih-clinical-trial-testing-antiviral-remdesivir-plus-anti-inflammatory-drug-baricitinib-covid-19-begins (accessed May 9, 2020).
- 8.J. Grein, N. Ohmagari, D. Shin, G. Diaz, E. Asperges, A. Castagna, T. Feldt, G. Green, M.L. Green, F.-X. Lescure, E. Nicastri, R. Oda, K. Yo, E. Quiros-Roldan, A. Studemeister, J. Redinski, S. Ahmed, J. Bernett, D. Chelliah, D. Chen, S. Chihara, S.H. Cohen, J. Cunningham, A. D’Arminio Monforte, S. Ismail, H. Kato, G. Lapadula, E. L’Her, T. Maeno, S. Majumder, M. Massari, M. Mora-Rillo, Y. Mutoh, D. Nguyen, E. Verweij, A. Zoufaly, A.O. Osinusi, A. DeZure, Y. Zhao, L. Zhong, A. Chokkalingam, E. Elboudwarej, L. Telep, L. Timbs, I. Henne, S. Sellers, H. Cao, S.K. Tan, L. Winterbourne, P. Desai, R. Mera, A. Gaggar, R.P. Myers, D.M. Brainard, R. Childs, T. Flanigan, Compassionate use of remdesivir for patients with severe Covid-19, N. Engl. J. Med. (2020). 10.1056/nejmoa2007016. [DOI] [PMC free article] [PubMed]
- 9.Rose-John S. IL-6 trans-signaling via the soluble IL-6 receptor: importance for the pro-inflammatory activities of IL-6. Int. J. Biol. Sci. 2012;8:1237–1247. doi: 10.7150/ijbs.4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jostock T., Müllberg J., Özbek S., Atreya R., Blinn G., Voltz N., Fischer M., Neurath M.F., Rose-John S. Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. Eur. J. Biochem. 2001;268:160–167. doi: 10.1046/j.1432-1327.2001.01867.x. [DOI] [PubMed] [Google Scholar]
- 11.Scheller J., Chalaris A., Garbers C., Rose-John S. ADAM17: a molecular switch to control inflammation and tissue regeneration. Trends Immunol. 2011;32:380–387. doi: 10.1016/j.it.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 12.C. Huang, Y. Wang, X. Li, L. Ren, J. Zhao, Y. Hu, L. Zhang, G. Fan, J. Xu, X. Gu, Z. Cheng, T. Yu, J. Xia, Y. Wei, W. Wu, X. Xie, W. Yin, H. Li, M. Liu, Y. Xiao, H. Gao, L. Guo, J. Xie, G. Wang, R. Jiang, Z. Gao, Q. Jin, J. Wang, B. Cao, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet. 395 (2020) 497–506. Doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed]
- 13.Villar J., Zhang H., Slutsky A.S. Lung repair and regeneration in ARDS: role of PECAM1 and Wnt Signaling. Chest. 2019;155:587–594. doi: 10.1016/j.chest.2018.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi Y., Wang Y., Shao C., Huang J., Gan J., Huang X., Bucci E., Piacentini M., Ippolito G., Melino G. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020 doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu Y., Cheng Y., Wu Y. Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol. Sin. 2020 doi: 10.1007/s12250-020-00207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X., Geng M., Peng Y., Meng L., Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J. Pharm. Anal. 2020 doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scheller J., Chalaris A., Schmidt-Arras D., Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. BBA - Mol. Cell Res. 1813;2011:878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 19.Barkhausen T., Tschernig T., Rosenstiel P., van Griensven M., Vonberg R.-P., Dorsch M., Mueller-Heine A., Chalaris A., Scheller J., Rose-John S., Seegert D., Krettek C., Waetzig G.H. Selective blockade of interleukin-6 trans-signaling improves survival in a murine polymicrobial sepsis model. Crit. Care Med. 2011;39:1407–1413. doi: 10.1097/CCM.0b013e318211ff56. [DOI] [PubMed] [Google Scholar]
- 20.Rose-John S. The soluble interleukin 6 receptor: advanced therapeutic options in inflammation. Clin. Pharmacol. Ther. 2017;102:591–598. doi: 10.1002/cpt.782. [DOI] [PubMed] [Google Scholar]
- 21.Hunter C.A., Jones S.A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2015;16:448–457. doi: 10.1038/ni.3153. [DOI] [PubMed] [Google Scholar]
- 22.Wolf J., Waetzig G.H., Chalaris A., Reinheimer T.M., Wege H., Rose-John S., Garbers C. Different soluble forms of the interleukin-6 family signal transducer gp130 fine-tune the blockade of interleukin-6 trans-signaling. J. Biol. Chem. 2016;291:16186–16196. doi: 10.1074/jbc.M116.718551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baran P., Hansen S., Waetzig G.H., Akbarzadeh M., Lamertz L., Huber H.J., Reza Ahmadian M., Moll J.M., Scheller J. The balance of interleukin (IL)-6, IL-6soluble IL-6 receptor (sIL-6R), and IL-6sIL-6Rsgp130 complexes allows simultaneous classic and trans-signaling. J. Biol. Chem. 2018;293:6762–6775. doi: 10.1074/jbc.RA117.001163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garbers C., Thaiss W., Jones G.W., Waetzig G.H., Lorenzen I., Guilhot F., Lissilaa R., Ferlin W.G., Grötzinger J., Jones S.A., Rose-John S., Scheller J. Inhibition of classic signaling is a novel function of soluble glycoprotein 130 (sgp130), which is controlled by the ratio of interleukin 6 and soluble interleukin 6 receptor. J. Biol. Chem. 2011;286:42959–42970. doi: 10.1074/jbc.M111.295758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garbers C., Heink S., Korn T., Rose-John S. Interleukin-6: designing specific therapeutics for a complex cytokine. Nat. Rev. Drug Discov. 2018;17:395–412. doi: 10.1038/nrd.2018.45. [DOI] [PubMed] [Google Scholar]
- 26.Mahmud-Al-Rafat A., Majumder A., Taufiqur Rahman K.M., Mahedi Hasan A.M., Didarul Islam K.M., Taylor-Robinson A.W., Billah M.M. Decoding the enigma of antiviral crisis: does one target molecule regulate all? Cytokine. 2019;115:13–23. doi: 10.1016/j.cyto.2018.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riethmueller S., Ehlers J.C., Lokau J., Düsterhöft S., Knittler K., Dombrowsky G., Grötzinger J., Rabe B., Rose-John S., Garbers C. cleavage site localization differentially controls interleukin-6 receptor proteolysis by ADAM10 and ADAM17. Sci. Rep. 2016;6:25550. doi: 10.1038/srep25550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barnes T.C., Anderson M.E., Moots R.J. The many faces of interleukin-6: the role of IL-6 in inflammation, vasculopathy, and fibrosis in systemic sclerosis. Int. J. Rheumatol. 2011;2011 doi: 10.1155/2011/721608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luig M., Kluger M.A., Goerke B., Meyer M., Nosko A., Yan I., Scheller J., Mittrücker H.W., Rose-John S., Stahl R.A.K., Panzer U., Steinmetz O.M. Inflammation-induced IL-6 functions as a natural brake on macrophages and limits GN. J. Am. Soc. Nephrol. 2015;26:1597–1607. doi: 10.1681/ASN.2014060620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salama A.D., Little M.A. The janus faces of IL-6 in GN. J. Am. Soc. Nephrol. 2015;26:1480–1482. doi: 10.1681/ASN.2014111141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mauer J., Denson J.L., Brüning J.C. Versatile functions for IL-6 in metabolism and cancer. Trends Immunol. 2015 doi: 10.1016/j.it.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 32.Mauer J., Chaurasia B., Goldau J., Vogt M.C., Ruud J., Nguyen K.D., Theurich S., Hausen A.C., Schmitz J., Brönneke H.S., Estevez E., Allen T.L., Mesaros A., Partridge L., Febbraio M.A., Chawla A., Wunderlich F.T., Brüning J.C. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat. Immunol. 2014;15:423–430. doi: 10.1038/ni.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hou T., Tieu B., Ray S., Recinos A., III, Cui R., Tilton R., Brasier A. Roles of IL-6-gp130 signaling in vascular inflammation. Curr. Cardiol. Rev. 2008;4:179–192. doi: 10.2174/157340308785160570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020 doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garbers C., Monhasery N., Aparicio-Siegmund S., Lokau J., Baran P., Nowell M.A., Jones S.A., Rose-John S., Scheller J. The interleukin-6 receptor Asp358Ala single nucleotide polymorphism rs2228145 confers increased proteolytic conversion rates by ADAM proteases. Biochim. Biophys. Acta - Mol. Basis Dis. 1842;2014:1485–1494. doi: 10.1016/j.bbadis.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 37.Aparicio-Siegmund S., Garbers Y., Flynn C.M., Waetzig G.H., Gouni-Berthold I., Krone W., Berthold H.K., Laudes M., Rose-John S., Garbers C. The IL-6-neutralizing sIL-6R-sgp130 buffer system is disturbed in patients with type 2 diabetes. Am. J. Physiol. - Endocrinol. Metab. 2019;317:E411–E420. doi: 10.1152/ajpendo.00166.2019. [DOI] [PubMed] [Google Scholar]
- 38.Swerdlow D.I., Holmes M.V., Kuchenbaecker K.B., Engmann J.E.L., Shah T., Sofat R., Guo Y., Chung C., Peasey A., Pfister R., Mooijaart S.P., Ireland H.A., Leusink M., Langenberg C., Li K., Palmen J., Howard P., Cooper J.A., Drenos F., Hardy J., Nalls M.A., Li Y.R., Lowe G., Stewart M., Bielinski S.J., Peto J., Timpson N.J., Gallacher J., Dunlop M., Houlston R., Tomlinson I., Tzoulaki I., Luan J., Boer J.M.A., Forouhi N.G., Onland-Moret N.C., Van Der Schouw Y.T., Schnabel R., Hubacek J.A., Kubinova R., Baceviciene M., Tamosiunas A., Pajak A., Topor-Madry R., Malyutina S., Baldassarre D., Sennblad B., Tremoli E., De Faire U., Ferrucci L., Bandenelli S., Tanaka T., Meschia J.F., Singleton A., Navis G., Leach I.M., Bakker S.J.L., Gansevoort R.T., Ford I., Epstein S.E., Burnett M.S., Devaney J.M., Jukema J.W., Westendorp R.G.J., De Borst G.J., Van Der Graaf Y., De Jong P.A., Maitland-Van Der Zee A.H., Klungel O.H., De Boer A., Doevendans P.A., Stephens J.W., Eaton C.B., Robinson J.G., Manson J.E., Fowkes F.G.R., Frayling T.M., Price J., Whincup P.H., Morris R.W., Lawlor D.A., Smith G.D., Ben-Shlomo Y., Redline S., Lange L.A., Kumari M., Wareham N.J., Verschuren W.M.M., Benjamin E.J., Whittaker J.C., Hamsten A., Dudbridge F., Delaney J.A.C., Wong A., Kuh D., Hardy R., Castillo B.A., Connolly J.J., Van Der Harst P., Brunner E.J., Marmot M.G., Wassel C.L., Humphries S.E., Talmud P.J., Kivimaki M., Asselbergs F.W., Voevoda M., Bobak M., Pikhart H., Wilson J.G., Hakonarson H., Reiner A.P., Keating B.J., Sattar N., Hingorani A.D., Casas J.P. The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisation analysis. Lancet. 2012;379:1214–1224. doi: 10.1016/S0140-6736(12)60110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferreira R.C., Freitag D.F., Cutler A.J., Howson J.M.M., Rainbow D.B., Smyth D.J., Kaptoge S., Clarke P., Boreham C., Coulson R.M., Pekalski M.L., Chen W.-M., Onengut-Gumuscu S., Rich S.S., Butterworth A.S., Malarstig A., Danesh J., Todd J.A. Functional IL6R 358Ala Allele Impairs Classical IL-6 receptor signaling and influences risk of diverse inflammatory diseases. PLoS Genet. 2013;9:e1003444. doi: 10.1371/journal.pgen.1003444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schumacher N., Rose-John S. Adam17 activity and il-6 trans-signaling in inflammation and cancer. Cancers (Basel) 2019;11:1736. doi: 10.3390/cancers11111736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., Guo L., Guo R., Chen T., Hu J., Xiang Z., Mu Z., Chen X., Chen J., Hu K., Jin Q., Wang J., Qian Z. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11:1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.F.A. Rabi, M.S. Al Zoubi, A.D. Al-Nasser, G.A. Kasasbeh, D.M. Salameh, Sars-cov-2 and coronavirus disease 2019: What we know so far, Pathogens. 9 (2020) pii: E231. Doi: 10.3390/pathogens9030231. [DOI] [PMC free article] [PubMed]
- 44.Yang M. Cell pyroptosis, a potential pathogenic mechanism of 2019-nCoV infection. SSRN Electron. J. 2020 doi: 10.2139/ssrn.3527420. [DOI] [Google Scholar]
- 45.Xu X., Han M., Li T., Sun W., Wang D., Fu B., Zhou Y., Zheng X., Yang Y., Li X., Zhang X., Pan A., Wei H. Effective treatment of severe COVID-19 patients with tocilizumab. ChinaXiv. 2020:1–12. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020:1–12. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin–angiotensin–aldosterone system inhibitors in patients with Covid-19. N. Engl. J. Med. 2020;382:1653–1659. doi: 10.1056/nejmsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuba K., Imai Y., Ohto-Nakanishi T., Penninger J.M. Trilogy of ACE2: a peptidase in the renin-angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacol. Ther. 2010;128:119–128. doi: 10.1016/j.pharmthera.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Skurk T., Van Harmelen V., Hauner H. Angiotensin II stimulates the release of interleukin-6 and interleukin-8 from cultured human adipocytes by activation of NF-κB. Arterioscler. Thromb. Vasc. Biol. 2004;24:1199–1203. doi: 10.1161/01.ATV.0000131266.38312.2e. [DOI] [PubMed] [Google Scholar]
- 50.M. de Carvalho Santuchi, M.F. Dutra, J.P. Vago, K.M. Lima, I. Galvão, F.P. de Souza-Neto, M. Morais E Silva, A.C. Oliveira, F.C.B. de Oliveira, R. Gonçalves, M.M. Teixeira, L.P. Sousa, R.A.S. Dos Santos, R.F. da Silva, Angiotensin-(1-7) and alamandine promote anti-inflammatory response in macrophages in vitro and in vivo., Mediators Inflamm. 2019 (2019) 2401081. Doi: 10.1155/2019/2401081. [DOI] [PMC free article] [PubMed]
- 51.Souza L.L., Costa-Neto C.M. Angiotensin-(1–7) decreases LPS-induced inflammatory response in macrophages. J. Cell. Physiol. 2012;227:2117–2122. doi: 10.1002/jcp.22940. [DOI] [PubMed] [Google Scholar]
- 52.Lambert D.W., Yarski M., Warner F.J., Thornhill P., Parkin E.T., Smith A.I., Hooper N.M., Turner A.J. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the SARS-CoV receptor, angiotensin-converting enzyme-2 (ACE2) J. Biol. Chem. 2005;280:30113–30119. doi: 10.1074/jbc.M505111200. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15983030 (accessed April 17, 2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Glowacka I., Bertram S., Muller M.A., Allen P., Soilleux E., Pfefferle S., Steffen I., Tsegaye T.S., He Y., Gnirss K., Niemeyer D., Schneider H., Drosten C., Pohlmann S. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J. Virol. 2011;85:4122–4134. doi: 10.1128/jvi.02232-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shulla A., Heald-Sargent T., Subramanya G., Zhao J., Perlman S., Gallagher T. A transmembrane serine protease is linked to the severe acute respiratory syndrome coronavirus receptor and activates virus entry. J. Virol. 2011;85:873–882. doi: 10.1128/jvi.02062-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heurich A., Hofmann-Winkler H., Gierer S., Liepold T., Jahn O., Pohlmann S. TMPRSS2 and ADAM17 Cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J. Virol. 2014;88:1293–1307. doi: 10.1128/jvi.02202-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lorenzen I., Lokau J., Korpys Y., Oldefest M., Flynn C.M., Künzel U., Garbers C., Freeman M., Grötzinger J., Düsterhöft S. Control of ADAM17 activity by regulation of its cellular localisation. Sci. Rep. 2016;6:35067. doi: 10.1038/srep35067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chalaris A., Rabe B., Paliga K., Lange H., Laskay T., Fielding C.A., Jones S.A., Rose-John S., Scheller J. Apoptosis is a natural stimulus of IL6R shedding and contributes to the proinflammatory trans-signaling function of neutrophils. Blood. 2007;110:1748–1755. doi: 10.1182/blood-2007-01-067918. [DOI] [PubMed] [Google Scholar]
- 59.Li G., He X., Zhang L., Ran Q., Wang J., Xiong A., Wu D., Chen F., Sun J., Chang C. Assessing ACE2 expression patterns in lung tissues in the pathogenesis of COVID-19. J. Autoimmun. 2020 doi: 10.1016/j.jaut.2020.102463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gheblawi M., Wang K., Viveiros A., Nguyen Q., Zhong J.-C., Turner A.J., Raizada M.K., Grant M.B., Oudit G.Y. Angiotensin converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system. Circ. Res. 2020 doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Esmon C.T. Does inflammation contribute to thrombotic events? Haemostasis. 2000;30:34–40. doi: 10.1159/000054161. [DOI] [PubMed] [Google Scholar]
- 63.Yang Y., Tang H. Aberrant coagulation causes a hyper-inflammatory response in severe influenza pneumonia. Cell. Mol. Immunol. 2016;13:432–442. doi: 10.1038/cmi.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bester J., Pretorius E. Effects of IL-1β, IL-6 and IL-8 on erythrocytes, platelets and clot viscoelasticity. Sci. Rep. 2016;6:1–10. doi: 10.1038/srep32188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Henry B.M., Vikse J., Benoit S., Favaloro E.J., Lippi G. Hyperinflammation and derangement of renin-angiotensin-aldosterone system in COVID-19: a novel hypothesis for clinically suspected hypercoagulopathy and microvascular immunothrombosis. Clin. Chim. Acta. 2020;507:167–173. doi: 10.1016/j.cca.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ozolina A., Sarkele M., Sabelnikovs O., Skesters A., Jaunalksne I., Serova J., Ievins T., Bjertnaes L.J., Vanags I. Activation of coagulation and fibrinolysis in acute respiratory distress syndrome: a prospective pilot study. Front. Med. 2016;3 doi: 10.3389/fmed.2016.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aida Y., Honda K., Tanigawa S., Nakayama G., Matsumura H., Suzuki N., Shimizu O., Takeichi O., Makimura M., Maeno M. IL-6 and soluble IL-6 receptor stimulate the production of MMPs and their inhibitors via JAK–STAT and ERK–MAPK signalling in human chondrocytes. Cell Biol. Int. 2012;36:367–376. doi: 10.1042/cbi20110150. [DOI] [PubMed] [Google Scholar]
- 68.Bouwman J.J.M., Diepersloot R.J.A., Visseren F.L.J. Intracellular infections enhance interleukin-6 and plasminogen activator inhibitor 1 production by cocultivated human adipocytes and THP-1 monocytes. Clin. Vaccine Immunol. 2009;16:1222–1227. doi: 10.1128/CVI.00166-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spyropoulos A.C., Ageno W., Barnathan E.S. Hospital-based use of thromboprophylaxis in patients with COVID-19. Lancet. 2020 doi: 10.1016/S0140-6736(20)30926-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schuett H., Oestreich R., Waetzig G.H., Annema W., Luchtefeld M., Hillmer A., Bavendiek U., von Felden J., Divchev D., Kempf T., Wollert K.C., Seegert D., Rose-John S., Tietge U.J.F., Schieffer B., Grote K. Transsignaling of Interleukin-6 crucially contributes to atherosclerosis in mice. Arterioscler. Thromb. Vasc. Biol. 2012;32 doi: 10.1161/ATVBAHA.111.229435. http://atvb.ahajournals.org/content/32/2/281.long (accessed August 28, 2017) [DOI] [PubMed] [Google Scholar]
- 71.P.A.Z.G. Morieri ML, Interleukin-6 “Trans-Signaling” and Ischemic Vascular Disease: The Important Role of Soluble gp130., Mediat. Inflamm. 2017 (2017) 1396398. [DOI] [PMC free article] [PubMed]
- 72.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020;8:e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li L., Li R., Wu Z., Yang X., Zhao M., Liu J., Chen D. Therapeutic strategies for critically ill patients with COVID-19. Ann. Intensive Care. 2020;10:45. doi: 10.1186/s13613-020-00661-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Monteleone G., Sarzi-Puttini P., Ardizzone S. Preventing COVID-19-induced pneumonia with anti-cytokine therapy. Lancet Rheumatol Press. 2020:483–504. doi: 10.1016/S2665-9913(20)30092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gooz M. ADAM-17: the enzyme that does it all. Crit. Rev. Biochem. Mol. Biol. 2010;45:146–169. doi: 10.3109/10409231003628015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lambert D.W., Yarski M., Warner F.J., Thornhill P., Parkin E.T., Smith A.I., Hooper N.M., Turner A.J. Tumor necrosis factor-α convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2) J. Biol. Chem. 2005;280:30113–30119. doi: 10.1074/jbc.M505111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rose-John S. New insights into the role and signalling processes of gp130. Arthritis Res. Ther. 2011;13:O10. doi: 10.1186/ar3414. [DOI] [Google Scholar]
- 78.Guo C., Li B., Ma H., Wang X., Cai P., Yu Q., Zhu L., Jin L., Jiang C., Fang J., Liu Q., Zong D., Zhang W., Lu Y., Li K., Gao X., Fu B., Liu L., Ma X., Weng J., Wei H., Jin T., Lin J., Qu K. Tocilizumab treatment in severe COVID-19 patients attenuates the inflammatory storm incited by monocyte centric immune interactions revealed by single-cell analysis. BioRxiv. 2020;2020(04) doi: 10.1101/2020.04.08.029769. [DOI] [Google Scholar]
- 79.Favalli E.G., Ingegnoli F., De Lucia O., Cincinelli G., Cimaz R., Caporali R. COVID-19 infection and rheumatoid arthritis: Faraway, so close! Autoimmun. Rev. 2020;19:102523. doi: 10.1016/j.autrev.2020.102523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schumacher N., Schmidt S., Schwarz J., Dohr D., Lokau J., Scheller J., Garbers C., Chalaris A., Rose-John S., Rabe B. Circulating soluble IL-6R but not ADAM17 activation drives mononuclear cell migration in tissue inflammation. J. Immunol. 2016;197:3705–3715. doi: 10.4049/jimmunol.1600909. [DOI] [PubMed] [Google Scholar]
- 81.Palau V., Riera M., Soler M.J. ADAM17 inhibition may exert a protective effect on COVID-19. Nephrol. Dial. Transplant. 2020 doi: 10.1093/ndt/gfaa093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Arribas J., Esselens C. ADAM17 as a therapeutic target in multiple diseases. Curr. Pharm. Des. 2009;15:2319–2335. doi: 10.2174/138161209788682398. [DOI] [PubMed] [Google Scholar]
- 83.Wong E., Cohen T., Romi E., Levin M., Peleg Y., Arad U., Yaron A., Milla M.E., Sagi I. Harnessing the natural inhibitory domain to control TNFα Converting Enzyme (TACE) activity in vivo. Sci. Rep. 2016;6:1–12. doi: 10.1038/srep35598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Weizman T., Levin I., Zaretsky M., Sagi I., Aharoni A. Increased potency of a bi-specific TL1A-ADAM17 (TACE) inhibitor by cell surface targeting. Front. Mol. Biosci. 2017;4:61. doi: 10.3389/fmolb.2017.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Saad M.I., Alhayyani S., McLeod L., Yu L., Alanazi M., Deswaerte V., Tang K., Jarde T., Smith J.A., Prodanovic Z., Tate M.D., Balic J.J., Watkins D.N., Cain J.E., Bozinovski S., Algar E., Kohmoto T., Ebi H., Ferlin W., Garbers C., Ruwanpura S., Sagi I., Rose-John S., Jenkins B.J. ADAM 17 selectively activates the IL -6 trans-signaling/ERK MAPK axis in KRAS -addicted lung cancer. EMBO Mol. Med. 2019;11:e9976. doi: 10.15252/emmm.201809976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mourad J.J., Levy B.I. Interaction between RAAS inhibitors and ACE2 in the context of COVID-19. Nat. Rev. Cardiol. 2020;17:313. doi: 10.1038/s41569-020-0368-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H., Li Y., Hu Z., Zhong W., Wang M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6 doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. The FDA-approved Drug Ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;178 doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huang J., Song W., Huang H., Sun Q. Pharmacological therapeutics targeting RNA-dependent RNA polymerase, proteinase and spike protein: from mechanistic studies to clinical trials for COVID-19. J. Clin. Med. 2020;9:1131. doi: 10.3390/jcm9041131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jiang S., Hillyer C., Du L. Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses. Trends Immunol. 2020;41:355–359. doi: 10.1016/j.it.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xia S., Liu M., Wang C., Xu W., Lan Q., Feng S., Qi F., Bao L., Du L., Liu S., Qin C., Sun F., Shi Z., Zhu Y., Jiang S., Lu L. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 2020;30:343–355. doi: 10.1038/s41422-020-0305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kollias A., Kyriakoulis K.G., Dimakakos E., Poulakou G., Stergiou G.S., Syrigos K. Thromboembolic risk and anticoagulant therapy in COVID-19 patients: Emerging evidence and call for action. Br. J. Haematol. 2020 doi: 10.1111/bjh.16727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hung I.F.-N., Lung K.-C., Tso E.Y.-K., Liu R., Chung T.W.-H., Chu M.-Y., Ng Y.-Y., Lo J., Chan J., Tam A.R., Shum H.-P., Chan V., Wu A.K.-L., Sin K.-M., Leung W.-S., Law W.-L., Lung D.C., Sin S., Yeung P., Yip C.C.-Y., Zhang R.R., Fung A.Y.-F., Yan E.Y.-W., Leung K.-H., Ip J.D., Chu A.W.-H., Chan W.-M., Ng A.C.-K., Lee R., Fung K., Yeung A., Wu T.-C., Chan J.W.-M., Yan W.-W., Chan W.-M., Chan J.F.-W., Lie A.K.-W., Tsang O.T.-Y., Cheng V.C.-C., Que T.-L., Lau C.-S., Chan K.-H., To K.K.-W., Yuen K.-Y. Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020 doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Clinical Trial Protocol, A Phase II, Randomized, Double-blind, Placebo-controlled Study to Evaluate the Safety and Efficacy of TJ301 (FE 999301) Administered Intravenously in Patients With Active Ulcerative Colitis, (n.d.). https://clinicaltrials.gov/ProvidedDocs/52/NCT03235752/Prot_000.pdf (accessed July 9, 2020).
- 95.Bennett J.M., Reeves G., Billman G.E., Sturmberg J.P. Inflammation-nature’s way to efficiently respond to all types of challenges: implications for understanding and managing “the epidemic” of chronic diseases. Front. Med. 2018;5:316. doi: 10.3389/fmed.2018.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ozili P.K., Arun T. Spillover of COVID-19: impact on the global economy. SSRN Electron. J. 2020 doi: 10.2139/ssrn.3562570. [DOI] [Google Scholar]
- 97.Oxley T.J., Mocco J., Majidi S., Kellner C.P., Shoirah H., Singh I.P., De Leacy R.A., Shigematsu T., Ladner T.R., Yaeger K.A., Skliut M., Weinberger J., Dangayach N.S., Bederson J.B., Tuhrim S., Fifi J.T. Large-vessel stroke as a presenting feature of Covid-19 in the young. N. Engl. J. Med. 2020:e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]