Abstract
Purpose
Our objective was to evaluate the influence of pretreatment with tocilizumab (TCZ) in bone healing after tooth extraction in rats.
Methods
Wistar male rats were equally divided into sham (ie, nonoperated), saline (both treated with 0.1 ml/kg saline), and six TCZ groups treated with 1, 2, 4, 8, 16, and 32 mg/kg TCZ (TCZ1 to TCZ32, respectively). Twenty-four hours after administration of vehicle or TCZ, exodontia of the first lower left molar was performed, and the animals were euthanized three days later for hematological analysis and organ (liver, spleen, and kidney mass indexes, and histological evaluation), gingiva (myeloperoxidase [MPO] assay), and mandible (radiographic, histomorphometric analysis, and IL-6 immunostaining) evaluation. Analysis of variance/Bonferroni test (statistical significance, P < .05) was performed using GraphPad Prism version 5.0 (GraphPad Inc, San Diego, CA, USA).
Results
There was no difference in radiographic results; however, leukopenia (P = .039) and neutropenia (P < .001) were statistically significant in the TCZ16 and TCZ32 groups. Weight loss (P < .001) and reduced liver index (P = .001) were significantly dose-dependent; however, no histological alterations were observed in the other organs. Osteoclast counts were reduced in groups TCZ4 to TCZ32 (P < .001), and IL-6 immunostaining increased in the TCZ8 to TCZ32 groups (P < .001). Alveolar infection rates increased in groups TCZ4 to TCZ32 (P < .001), and MPO had a biphasic response, exhibiting a reduction in groups TCZ2 and TCZ4, and an increase in group TCZ32 (P = .004).
Conclusion
TCZ-induced immunosuppression led to a reduction in osteoclast function, an increase in alveolar infection, and compensatory neutrophil infiltration.
Introduction
Monoclonal antibodies are a class of drugs widely used to treat autoimmune diseases and some cancers, as well as for immune control of infections occurring as side effects. The mechanism involves the blockage of cytokines or receptors that inhibit the natural course of some diseases.1 This therapeutic approach has improved the health of some patients with chronic local and systemic diseases.2
A humanized anti-interleukin (IL)-6 receptor monoclonal antibody, tocilizumab (TCZ), has recently been authorized for use in humans. TCZ binds reversibly to the IL-6 receptor (IL-6R), blocking Janus kinase/signal transducers and activators of transcription (JAK/STAT) activation and the subsequent overproduction of cytokines.3 Due to its ability to inhibit inflammation, TCZ is being used in the treatment of rheumatoid arthritis, including as monotherapy, and Crohn disease, asthma, and Coleman disease, and as an adjuvant for multiple myeloma, some lymphomas, and solid-organ cancers, including colorectal, breast, prostate, ovarium, pancreas, lung, and kidney cancers.4 Recently, TCZ has been used as palliative treatment for oral squamous cell carcinomas5 , 6 and multi-drug-resistant anemia7 , 8; thus, its range of therapeutic applications has increased.
Furthermore, TCZ has been used, with good results, in the treatment of patients with coronavirus disease 2019 (COVID-19), caused by the novel severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2).9 In these conditions, a large number of critically ill patients experience a “cytokine storm” caused by the overproduction of proinflammatory cytokines, especially IL-6. This storm contributes to increased disease severity and poorer prognosis, and TCZ contributes to the improvement of symptoms by reducing the inflammation associated with this IL-6 storm.10 , 11
The blockage of IL-6R reduces the overexpression of several inflammatory chemical mediators that are important in osteoclastogenesis,4 interfering with bone remodeling.12 After exodontia, there is a rapid and natural increase in the levels of proinflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α), IL-1β, and IL-6, leading to the production of receptor activator of nuclear factor kappa-B ligand (RANKL) and bone loss.4 , 13
Despite pathological bone loss being attenuated,14 , 15 the interruption of inflammatory pathways can delay bone remodeling.16 After exodontia in the dental-exposed alveolus, delayed bone remodeling can heighten infection risk due to the rich oral microbiota17 and the blockage of neutrophil migration and macrophage activation by the blockage of IL-6 activity.18 Based on the wide therapeutic potential of TCZ and its relevance in clinical dental procedures, the objective of the present study was to evaluate the effect of pretreatment with TCZ on bone remodeling in rats subjected to tooth extraction.
Materials and Methods
Experimental Groups and Doses
This research was approved by the Animal Use Ethics Committee of the Federal University of Ceara (UFC) under protocol number 23/2015 (May 23, 2016) in compliance with Federal Law 11.794 (October 8, 2008) and Decree 6.689 (July 15, 2009) regulating law 11,794 (www.planalto.gov.brccivil03Ato2007-20102008LeiL11794.htm).
The present experimental study included 48 male Wistar rats (weight, 270–310 g) supplied by the experimental animal facility of UFC. The animals were acclimated for 1 week under controlled laboratory conditions (temperature, 24°C; relative humidity 40 to 60%; 12-h light-dark cycle, and ad libitum access to food and water) and then randomly assigned to 8 experimental groups, with 6 animals per group.
The sham and saline groups were treated with 0.1 mL/kg of saline solution (0.9% sodium chloride [NaCl]). The experimental groups received TCZ at doses of 1 (TCZ1), 2 (TCZ2), 4 (TCZ4), 8 (TCZ8), 16 (TCZ16), or 32 (TCZ32) mg/kg in equal volumes of 0.1 mL/kg saline solution (0.9% NaCl). All solutions were intravenously administered (penile access) 24 h before the extraction of the first lower left molar.
The dose-response curve was constructed based on the therapeutic doses of 4 and 8 mg/kg proposed by Guptarak et al. (2013).3 Two lower and 2 upper doses (ie, 1 and 2 mg/kg, and 16 and 32 mg/kg, respectively) were added, respecting a scale of “multiples of 2”, as previously described.19 Exodontia was not performed in the sham group, and rats in both the saline and TCZ groups underwent extraction of the first left lower molar.
Experimental Protocol
Twenty-four hours after saline or TCZ injection (day 0), the animals were weighed (initial weight), anesthetized with an intraperitoneal injection of a freshly prepared mixture of ketamine (90 mg/kg) and xylazine (10 mg/kg), and subjected to tooth extraction (Hollemback 3s spatula) or sham surgery in a blinded manner. On the day exodontia was performed, 2 mL of blood (retro-orbital plexus) was collected to count leukocytes (myeloid and lymphoid cells [initial leukocyte count]).
After exodontia, the teeth were dried on absorbent paper and weighed using a precision balance. Additionally, an operator blinded to the group assignments recorded the number of root fractures.20
Three days after surgery (day 3), the animals were weighed and anesthetized, and 2 mL of blood (retro-orbital plexus) was collected to count leukocytes (myeloid and lymphoid cells) (Sysmex KX 21N, Roche). The animals were euthanized using ketamine (270 mg/kg) and xylazine (30 mg/kg) overdose, and alveolar mucosa, alveolar bone, kidneys, spleen, and liver samples were collected.
Weight and leukocyte count evaluations were performed by dividing the final value by the initial value multiplied by 100%.
Histological Analysis of Organs
On the day the animals were euthanized, the liver, spleen, and kidneys were removed and weighed to verify the organ index. The organ index was calculated by dividing the weight of the organ by the final weight of the rat multiplied by 100%. These organs and alveolar bone were then fixed in 10% neutral formalin solution for macroscopic and microscopic evaluation.
After the organs were subjected to macroscopic analysis, they were histologically processed and embedded in paraffin blocks. Sections (4-μm thick) were cut using a semiautomatic microtome and stained with hematoxylin and eosin for examination under a conventional light microscope (magnification 40 × , 100 × , and 400 × ) and histological analysis of the organs was based on the presence and extension of lesions.20
Radiographic Analysis
The mandibles of all groups underwent radiography using a conventional X-ray machine (63 Kvp, 8 mA; Dabi Atlante Ribeirão Preto, SP) coupled with a Digora digital image capture system (parallelism radiographic technique). The mandibles were positioned parallel to the radiographic digital film, with a focal distance of 10 cm. The exposure time established by the manufacturer for upper anterior dental (0.18 s) was used for all samples. Radiographs were quantitatively analyzed using ImageJ software. After randomization, an operator blinded to the group assignments manually measured the radiolucent area in triplicate using a freehand selection approach. The area of the demarcated radiolucent region was measured by using the measure command. The average value of three measurements was designated as the sample unit.20
Histological Processing
The mandibles were fixed in a 10% neutral formalin solution and decalcified (10% EDTA, pH 7.3, NaOH, PA) for 30 days and maintained in suspension. After the hemimandibles were histologically processed and embedded in paraffin blocks, sections (4-μm thick) were cut using a semiautomatic microtome and stained with hematoxylin and eosin for examination under a conventional light microscope (magnification 400 × ).
Ten micro-fields were selected for micrographs using a Leica DFC295 microscope coupled to a Leica DM 2000 microscope with LAS software (Leica, Heerbrugg, Switzerland). The images were exported and analyzed using ImageJ software. The cell counter command was used to count osteoclasts. The slides were randomized to minimize observation bias, and the investigator was blinded to the group assignments. The sum of 10 micrographs was considered the sample unit for statistical analysis.21 , 22
Tissue Microarray Procedures and Immunohistochemical Assay
The referent area of the alveolus was marked on a slide with a permanent brush to select the area for the tissue microarray procedure. The slide was paired with a paraffin block and a tissue microarrayer (Quick-Ray Unitma Co. Ltd., Seoul, Korea) was used to punch a 2-mm diameter sample of material from the paraffin block. The sample was transferred to 2 receptor paraffin blocks with capacity for 36 samples each. For the immunohistochemical assay, sections (4-μm thick) were cut using a semiautomatic microtome and mounted on silanized slides.
After deparaffinization and rehydration, antigen retrieval was performed by heating in citrate solution (pH 6.0). After reaching room temperature, the slides were blocked in peroxidase with 3% hydrogen peroxide (H2O2) and diluted in phosphate-buffered saline for 30 min. After being blocked with albumin for 30 min, the slides were incubated with IL-6 antibody (1:300 dilution; Abcam, Cambridge, United Kingdom).
Universal Immune-peroxidase Polymer (Histofine, Nicherei Biosciences Inc., Tokyo, Japan) for 30 min was used as the secondary antibody, and 5,5-diaminobenzidine tetrahydrochloride (DAB; Dako, Dopenhage, Denmark) was used to identify positive cells. Harris hematoxylin was used for counterstaining.
The same method of osteoclast counting was performed to obtain photomicrographs of IL-6 reactions. The cell counter command was used to count IL-6 cytoplasm-positive cells. For minimizing observation bias, the slides were randomized, and the investigator was blinded to the group assignments. The sum of 10 micrographs was considered a sample unit for statistical analysis.21 , 22
Determination of Myeloperoxidase in the Alveolar Mucosa
A myeloperoxidase (MPO) quantification assay is broadly used as a marker for myeloid cell infiltration into tissues, mainly neutrophils. Following euthanasia, a specimen of the alveolar mucosa was collected, weighed, and freeze-dried at -80°C until required for the assay. Briefly, 15–20 mg of oral mucosa was homogenized in hexadecyltrimethylammonium bromide (HTAB) buffer (Sigma-Aldrich, St Louis, MO, USA). The homogenates were centrifuged at 2,000 × g for 15 min at 4°C. MPO activity in the resuspended pellet was assayed by measuring the change in absorbance at 450 nm using o-dianisidine dihydrochloride (Sigma) and 1% H2O2 (Merck, Whitehouse Station, NJ, USA). The results are reported as MPO units per mg of tissue.23
Statistical Analysis
The Shapiro-Wilk test was used to assess the normality of data distribution. Data are expressed as mean ± standard error of the mean (SEM) or absolute or relative frequency. Statistical analysis was performed using GraphPad Prism version 5.0 (GraphPad Software, Inc., San Diego, CA, USA). One-way ANOVA/Bonferroni or chi-squared tests were used to compare the groups. The level of statistical significance was set at 5% (ie, P < .05).
Results
Surgical Difficulty
When the weight of each tooth was analyzed, we verified that there was no statistically significant difference between the nonoperated sham group and the other groups (P = .602). The number of radicular fractures did not differ between the control and TCZ groups (P = .910) in the experimental groups (Table 1 ).
Table 1.
Surgical Difficulty and Systemic Parameters of Toxicity in rats Submitted to Exodontia of First Lower Molar and Treated With Different Doses of TCZ
| Sham | Saline | TCZ (mg/kg) |
P-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 4 | 8 | 16 | 32 | ||||
| Surgical Difficulty | |||||||||
| Tooth Mass (mg) | 0.0 ± 0.0 | 16.0 ± 0.6∗ | 16.1 ± 0.9 | 15.9 ± 0.8 | 14.7 ± 0.9 | 14.5 ± 0.1 | 15.6 ± 0.8 | 16.9 ± 1.0 | <.001 |
| Radicular Fractures (n) | 0.0 ± 0.0 | 0.6 ± 0.2∗ | 1.0 ± 0.3 | 0.8 ± 0.4 | 1.0 ± 0.4 | 1.0 ± 0.0 | 0.7 ± 0.2 | 0.8 ± 0.3 | .043 |
| Ratio final/initial blood cells counting (%) | |||||||||
| Withe Blood Cells | 124.9 ± 15.0 | 122.1 ± 6.6 | 125.9 ± 7.7 | 109.2 ± 14.6 | 102.5 ± 4.0 | 89.2 ± 3.3∗,† | 88.7 ± 12.1∗,† | 87.7 ± 11.9∗,† | .039 |
| Lymphocytes | 124.5 ± 16.8 | 121.6 ± 6.5 | 129.7 ± 12.0 | 110.5 ± 14.8 | 103.8 ± 3.3 | 94.0 ± 3.8 | 98.6 ± 17.1 | 87.2 ± 15.6 | .310 |
| Myeloid cells | 125.1 ± 14.0 | 98.1 ± 12.2 | 61.6 ± 14.6 | 74.3 ± 5.1 | 79.9 ± 10.5 | 66.9 ± 9.2 | 34.0 ± 7.1∗,† | 29.1 ± 4.9∗,† | <.001 |
| Systemic parameters of toxicity | |||||||||
| Body mass (%) | 102.7 ± 1.9 | 105.2 ± 1.1 | 104.6 ± 1.8 | 106.7 ± 1.6 | 105.8 ± 0.8 | 100.2 ± 1.2 | 96.60 ± 1.3∗,† | 96.40 ± 3.2∗,† | <.001 |
| Liver Index (%) | 4.96 ± 0.39 | 5.34 ± 0.27 | 5.01 ± 0.23 | 5.22 ± 0.27 | 4.81 ± 0.31 | 4.23 ± 0.22 | 4.01 ± 0.11† | 3.92 ± 0.15∗,† | .001 |
| Spleen Index (%) | 0.29 ± 0.05 | 0.30 ± 0.05 | 0.29 ± 0.03 | 0.28 ± 0.03 | 0.28 ± 0.03 | 0.28 ± 0.04 | 0.29 ± 0.06 | 0.27 ± 0.08 | .972 |
| Renal Index (%) | 0.47 ± 0.04 | 0.47 ± 0.01 | 0.45 ± 0.01 | 0.49 ± 0.02 | 0.44 ± 0.02 | 0.42 ± 0.01 | 0.48 ± 0.03 | 0.45 ± 0.02 | .420 |
Organs mass calculated by the ratio between the weight of each organ and weight of rat on euthanasia day.
Body mass variation calculated by the ratio between final/initial weight. Bold values indicate significant P-values.
Abbreviation: TCZ, Tocilizumab.
P < .5 versus Sham group.
P < .5 versus saline group.
Systemic Evaluation: TCZ Led to Weight Loss, Leukopenia, and Hepatic Toxicity
There were no significant differences in the variation in leukocyte count between the sham group, control group, and rats in groups TCZ1, TCZ2, and TCZ4. However, rats in groups TCZ8, TCZ16, and TCZ32 exhibited a significant decrease in the total number of total leukocytes (P = .039) from day 0 to day 3 (Table 1).
Although there were no differences in variation of lymphoid cell counts among the groups (P = .310), the TCZ16 and TCZ32 groups exhibited significant decreases in the variation of myeloid cell counts without differences among the sham, control, TCZ1, TCZ2, TCZ4, and TCZ8 groups (Table 1).
Weight loss was greater in the TCZ16 and TCZ32 groups than in the other groups (P < .001) (Table 1). The spleen (P = .972) and kidney (P = .420) indexes did not exhibit significant differences; however, there was a significant reduction in the liver index in animals in the TCZ16 and TCZ32 groups compared with the other groups (P = .001) (Table 1).
Histological parameters, including renal, splenic, and hepatic toxicity, did not demonstrate significant variation among the 8 experimental groups (Supplementary Tables 1 to 3).
Radiolucent Area and Histological Evaluation
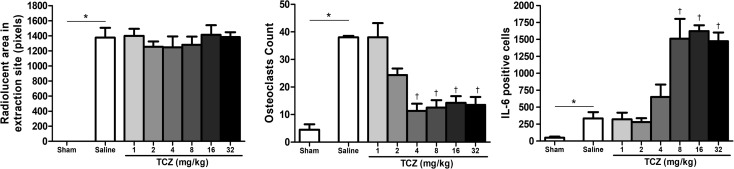
All animals that underwent exodontia of the first left inferior molar exhibited a radiolucent dental alveolus surrounded by a thin radiopaque line. The radiolucent area of tooth extractions did not differ among the saline (1,376 ± 130 pixels), TCZ1 (1,401 ± 92 pixels), TCZ2 (1,255 ± 71 pixels), TCZ4 (1,247 ± 146 pixels), TCZ8 (1,282 ± 107 pixels), TCZ16 (1,413 ± 128 pixels), and TCZ32 (pixels) groups (P = .867) (Fig 1 ).
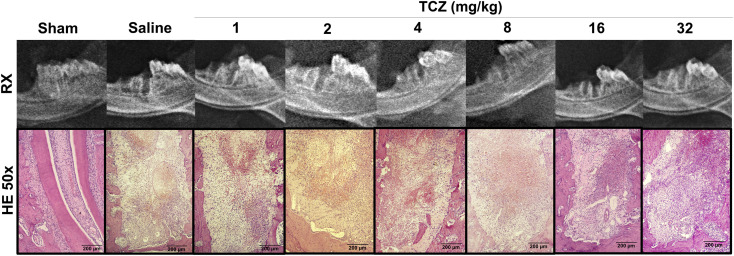
Figure 1.
Radiographic and histological profile of dental alveoli three days after exodontia and treatment with varying doses of tocilizumab (TCZ) showing no significant alterations in a macroscopic and low magnification qualitative analysis. Histologic magnification: 50x (H&E).
The sham group exhibited root surfaces and periodontal ligaments without morphological alterations. The control group exhibited an exposed dental alveolus with a high number of multinucleated osteoclasts around the bone associated with Howship lacunae. In the TCZ1 and TCZ2 groups, the number of multinucleated osteoclasts associated with Howship lacunae exhibited a slight reduction. The TCZ4 and TCZ8 groups exhibited a significant reduction in osteoclasts and lack of Howship lacunae, as well as in the TCZ16 and TCZ32 groups. Additionally, intense inflammatory infiltrates composed of neutrophil cells was observed in the TCZ32 group (Fig 1).
Rats in the control group exhibited a greater mean number of osteoclasts in the alveolus (38.0 ± 0.6) compared with the sham group (4.5 ± 2.0). Although there were no differences in the control, TCZ1 (38.0 ± 5.2) and TCZ2 (24.3 ± 2.4) groups, the TCZ4 (11.3 ± 2.6), TCZ8 (12.5 ± 2.7), TCZ16 (14.3 ± 2.4), and TCZ32 (13.5 ± 2.9) groups exhibited a significant reduction in osteoclast count (P < .001) (Figs 2 and 3 ).
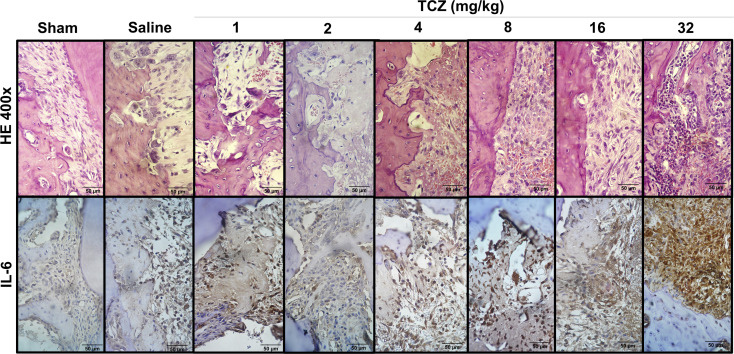
Figure 2.
Histomorphometric and immunostaining profile of interleukin (IL)-6 in dental alveoli three days after exodontia and treatment with varying doses of tocilizumab (TCZ) showing a reduction in Howship lacunae and osteoclasts close to the bone and an increase in polymorphonuclear cells in TCZ32 group. Histologic magnification: 400x (H&E); IHC magnification: 400x (Dab/Harris hematoxylin).
Figure 3.
Radiographic analysis, osteoclast counting, and the number of interleukin (IL)-6 positive cells in dental alveoli three days after exodontia and treatment with varying doses of tocilizumab (TCZ) showing no radiographic alterations, reduction in osteoclasts counting, and increase in the number of IL-6 immunostained cells. ∗P < .05 versus sham group; †P < .05 versus saline group; (ANOVA/Bonferroni, data presented as mean ± standard error; n = 6/group). Original magnification × 400.
The number of rats exhibiting bacterial colonies suggestive of Actinomyces was significantly higher in the TCZ4, TCZ8, TCZ16, and TCZ32 (100%) groups than in the other groups (sham, 0%; control, 25%; TCZ1, 17%; and TCZ2, 40%) (P < .001). These data suggest that the increase in the number of bacterial colonies was dose-dependent.
MPO Assay
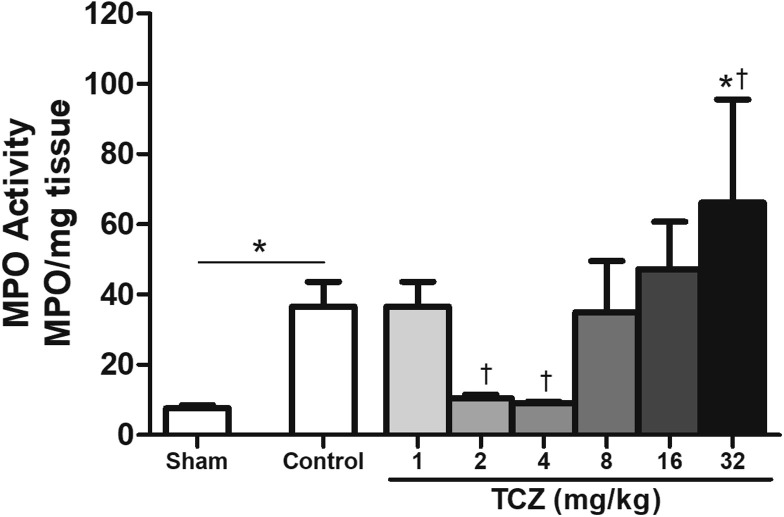
MPO activity in the sham group (7.6 ± 0.9) was significantly lower than that of the control group (36.5 ± 7.1). There was no difference in MPO activity between the control group and TCZ1 (36.5 ± 7.1). The TCZ2 (10.4 ± 0.1) and TCZ4 (9.0 ± 0.5) groups exhibited a significant decrease in MPO activity, and the TCZ32 group (66.2 ± 29.3) exhibited a significant increase (66.2 ± 29.3) (P = .004) (Fig 4 ).
Figure 4.
Myeloperoxidase assay of dental alveolus post-exodontia gingiva three days after tooth extraction in rats treated with varying doses of tocilizumab (TCZ) showing a biphasic behavior of neutrophils by TCZ dose. ∗P < .05 versus sham group; †P < .05 versus saline group; (ANOVA/Bonferroni, data presented as mean ± standard error; n = 6/group). Original magnification × 400.
IL-6 Immunostaining
The mean number of IL-6-positive cells in the sham group (48.5 ± 17.0) was significantly lower than that of the control group (333.5 ± 93.8). There was no difference in MPO activity between the control group and TCZ1 (320.5 ± 98.3), TCZ2 (280.3 ± 56.8), and TCZ4 (650.7 ± 182.9) groups; however, rats in the TCZ8 (1,510.0 ± 294.2), TCZ16 (1,623.0 ± 85.9), and TCZ32 (1,474 ± 128.7) groups exhibited a higher number of IL-6-positive cells than the saline group (P < .001) (Figs 2 and 3).
Discussion
TCZ is a monoclonal antibody that binds to the IL-6R, inhibiting its activation and blocking the activation of IL-6. Thus, this mechanism is vital in controlling diseases characterized by the overproduction of IL-6.24 Because this agent has a wide range of therapeutic applications, we studied initial bone remodeling post-tooth extraction in rats pretreated with TCZ. We evaluated the tooth alveolus 3 days postexodontia because this is the day with the highest number of inflammatory cells.23 So, the maximum impact of IL-6R blockage in inflammatory cell migration would be observed in tooth alveolus at this time.
IL-6 is an important cytokine related to some physiological processes. Although controlling IL-6 is considered to be indispensable in the treatment of some diseases, including periodontitis,25 its total or partial blockage can impact physiological functions.26
Leukocyte counts in the groups treated with high doses of TCZ exhibited significant leukopenia that was myeloid cell-dependent. IL-6 is indispensable in the late phase of myeloid differentiation27; however, its effect on lymphoid cells appears to be more qualitative (modulation of the T-helper cell immune profile response) than quantitative.28 This explains the more intense effect of TCZ in cells with a myeloid than lymphoid lineage. Additionally, this could explain the weight loss in the 2 high-dose groups because suppression of the medulla is often accompanied by intense cachexia.29
Histological analysis revealed that TCZ reduced osteoclast counts in the therapeutic doses (ie, TCZ4 and TCZ8) and overdoses (ie, TCZ16 and TCZ32) in the alveolar bone. Immediately after exodontia, there is an increase in the levels of some proinflammatory cytokines, such as TNF-α, IL-1β, and IL-6, which lead to monocyte recruitment and osteoclast differentiation.30 IL-6 blockage directly interferes with monocyte recruitment and activation and osteoclast differentiation independent of the RANKL axis.31 Furthermore, IL-6 blockage inhibits the classical activation pathway of macrophages and other monocyte lineage cells,32 decreasing phagocytic potential, and contributing as a risk factor to infection. In this study, we found a high prevalence of Actinomyces in the first 3 days of post-tooth extraction, probably due to the lack of phagocytic potential.
Parallel to osteoclast reduction and increase in infection rates, neutrophils exhibited a biphasic response to TCZ treatment: in low doses, neutrophil infiltration of the alveolar mucosa was reduced; at high doses, MPO activity demonstrated a significant increase accompanied by intense polymorphonuclear cell staining in the postexodontia alveolus. IL-6 is essential for the migration and activation of macrophages in bone (osteoclast differentiation),33 directly leading to IL-8 (chemotactic to neutrophils) as monocyte chemoattractant protein-1 (MCP-1).34 When IL-6 is blocked, there is a decrease in neutrophil infiltration and osteoclasts35 , 36; however, with increased doses of TCZ, there was also an increase in infection rates and neutrophil infiltration.
IL-8 production in response to bacterial byproducts is independent of TNF-α, IL-1β, and IL-6. In the severe absence of IL-6 (ie, TCZ32 group), macrophage activity was engaged, such as osteoclast function; therefore, compensatory IL-8 overexpression can lead to a high level of neutrophil infiltration.34 Neutrophils produce high levels of prostaglandin E2,37 which inhibit MCP-1 production by reducing infiltration of progenitor osteoclast cells38 and lead to tissue damage39 such as osteomyelitis.40
There were no significant alterations to the kidneys or spleen; however, TCZ caused significant hepatic damage. Hepatotoxicity is a limiting factor to TCZ treatment in patients with rheumatoid arthritis,41 which has been described in case reports of severe hepatitis after TCZ use.42 It is probable that the indispensable axis involving IL-6 and hepatocyte growth factor is partly responsible for this toxicity.42 , 43
Although TCZ does not directly inhibit the IL-6R in rodents,44 some experimental models have demonstrated its benefits in rats.19 , 45 In addition, IL-6R is mainly found on hepatocytes, neutrophils, monocytes, and LTCD4,46 all of which were altered in this experimental model.
Inevitably, there will be an increase in the use of TCZ due to the current COVID-19 pandemic. A clinical human dose of TCZ (8 mg/kg) has a long half-life (>4 weeks).9 In patients with COVID-19, the administration of TCZ quickly improves respiratory symptoms, and further doses are necessary for disease control.11 The results of our study can help in understanding why some COVID-19 patients worsen after TCZ treatment. With high suppression of the IL-6 pathway, secondary bacterial infections can activate noncanonical pathways, leading to the overexpression of both IL-6 and other cytokines.10 Although this was not the focus of our study, indications for TCZ use to control inflammatory processes will increase significantly in the future, and dentistry will need to prepare for the adverse effects of this monoclonal antibody even in a short period of time. In rats IL-6-/-, there is an increase in inflammatory alveolar bone destruction because other cytokines like IL-1α increase compensatively.47 So, the suppression of IL-6 is likely, even at low doses, to have significant adverse effects on the alveolar bone.
In conclusion, the results of the present study demonstrated that pretreatment with TCZ reduced osteoclast count, increased histological signs of infection, caused myeloid suppression and cachexia in a dose-dependent manner, and induced a biphasic response in neutrophil infiltration of the alveolar mucosa. With the promising future perspectives of TCZ use in immunoinflammatory diseases and in COVID-19 treatment, this research showed that in the clinical setting, surgeons need to pay attention to side effects of TCZ, especially due to increased risk of postsurgical infection. Further studies aiming to understand the mechanisms involved in the modulation of bone metabolism by TCZ and whether TCZ treatment leads to delayed bone remodeling in the late phases are warranted.
Footnotes
Conflict of Interest Disclosures: None of the authors have any relevant financial relationship(s) with a commercial interest
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.joms.2020.08.012.
Supplementary Data
References
- 1.Kim D.Y., Hussack G., Kandalaft H., Tanha J. Mutational approaches to improve the biophysical properties of human single-domain antibodies. Biochim Biophys Acta. 2014;1844:1983. doi: 10.1016/j.bbapap.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Miyazaki T., Tokimura F., Tanaka S. A review of denosumab for the treatment of osteoporosis. Patient Prefer Adherence. 2014;8:463. doi: 10.2147/PPA.S46192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guptarak J., Wanchoo S., Durham-lee J. Inhibition of IL-6 signaling: A novel therapeutic approach to treating spinal cord injury pain. Pain. 2013;154:1115. doi: 10.1016/j.pain.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 4.Yao X., Huang J., Zhong H. Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol Ther. 2014;141:125. doi: 10.1016/j.pharmthera.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Ando K., Takahashi F., Motojima S. Possible role for tocilizumab, an anti-interleukin-6 receptor antibody, in treating cancer cachexia. J Clin Oncol. 2013;31:69. doi: 10.1200/JCO.2012.44.2020. [DOI] [PubMed] [Google Scholar]
- 6.Mallery S.R., Wang D., Santiago B. Benefits of Multifaceted Chemopreventives in the suppression of the oral squamous cell carcinoma (OSCC) Tumorigenic Phenotype. Cancer Prev Res. 2017;10:76. doi: 10.1158/1940-6207.CAPR-16-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hashizume M., Uchiyama Y., Horai N. Tocilizumab, a humanized anti-interleukin-6 receptor antibody, improved anemia in monkey arthritis by suppressing IL-6-induced hepcidin production. Rheumatol Int. 2010;30:917. doi: 10.1007/s00296-009-1075-4. [DOI] [PubMed] [Google Scholar]
- 8.Song S.N., Iwahashi M., Tomosugi N. Comparative evaluation of the effects of treatment with tocilizumab and TNF-α inhibitors on serum hepcidin, anemia response and disease activity in rheumatoid arthritis patients. Arthritis Res Ther. 2013;15:R141. doi: 10.1186/ar4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang C., Wu Z., Li J.W. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020;15:105954. doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo P., Liu Y., Qiu L. Tocilizumab treatment in COVID-19: A single center experience. J Med Virol. 2020;92:814. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu X., Han M., Li T. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci. 2020;117:10970. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nibali L., Fedele S., D'aiuto F., Donos N. Interleukin-6 in oral diseases: A review. Oral Dis. 2012;18:236. doi: 10.1111/j.1601-0825.2011.01867.x. [DOI] [PubMed] [Google Scholar]
- 13.Kimachi K., Kajiya H., Nakayama S. Zoledronic acid inhibits RANK expression and migration of osteoclast precursors during osteoclastogenesis. Naunyn-Schmied Arch Pharmacol. 2011;383:297. doi: 10.1007/s00210-010-0596-4. [DOI] [PubMed] [Google Scholar]
- 14.Araújo A.A., Pereira A., Medeiros C. Effects of metformin on inflammation, oxidative stress, and bone loss in a rat model of periodontitis. PLoS one. 2017;12:e0183506. doi: 10.1371/journal.pone.0183506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonçalves D.C., Evangelista R.C., Silva R.R. Infliximab attenuates inflammatory osteolysis in a model of periodontitis in Wistar rats. Exp Biol Med. 2014;239:442. doi: 10.1177/1535370213520114. [DOI] [PubMed] [Google Scholar]
- 16.Prystaz K., Kaiser K., Kovtun A. Distinct effects of IL-6 classic and Trans-signaling in bone fracture healing. The Am J Pathol. 2018;188:474. doi: 10.1016/j.ajpath.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 17.Jabbour Z., Nascimento C., Kotake B.G. Assessing the oral microbiota of healthy and alcohol-treated rats using whole-genome DNA probes from human bacteria. Arch Oral Biol. 2013;58:317. doi: 10.1016/j.archoralbio.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka T., Narazaki M., Kishimoto T. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy. 2016;8:959. doi: 10.2217/imt-2016-0020. [DOI] [PubMed] [Google Scholar]
- 19.Chen K.L., Lv Z.Y., Yang H.W. Effects of tocilizumab on experimental severe acute Pancreatitis and associated acute lung injury. Crit Care Med. 2016;44:e664. doi: 10.1097/CCM.0000000000001639. [DOI] [PubMed] [Google Scholar]
- 20.Silva P.G.B., Ferreira Junior A.E.C., Teófilo C.R. Effect of different doses of zoledronic acid in establishing of bisphosphonate-related osteonecrosis. Arch Oral Biol. 2015;60:1237. doi: 10.1016/j.archoralbio.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 21.Melo R.B., de Barros Silva P.G., Oriá R.B. Anti-inflammatory effect of a fatty acid mixture with high ω-9:ω-6 ratio and low ω-6:ω-3 ratio on rats submitted to dental extraction. Arch Oral Biol. 2017;74:63. doi: 10.1016/j.archoralbio.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Silva P.G.B., de Oliveira C., Brizeno L. Immune cellular profile of bisphosphonate-related osteonecrosis of the jaw. Oral Dis. 2016;22:649. doi: 10.1111/odi.12513. [DOI] [PubMed] [Google Scholar]
- 23.Ferreira Júnior A.E.C., de Barros Silva P.G., de Oliveira C.C. Influence of infliximab therapy on bone healing post-dental extraction in rats. Arch Oral Biol. 2020;112:104680. doi: 10.1016/j.archoralbio.2020.104680. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y., Van boxel-dezaire A.H.H., Cheon H. STAT3 activation in response to IL-6 is prolonged by the binding of IL-6 receptor to EGF receptor. Proc The Natl Acad Of Sci. 2013;110:16975. doi: 10.1073/pnas.1315862110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Otenio C.C., Fonseca I., Martins M.F. Expression of IL-1β, IL-6, TNF-α, and iNOS in pregnant women with periodontal disease. Genet Mol Res. 2012;11:4468. doi: 10.4238/2012.September.20.3. [DOI] [PubMed] [Google Scholar]
- 26.Calabrese L.H., Rose-John S. IL-6 biology: Implications for clinical targeting in rheumatic disease. Nat Rev Rheumatol. 2014;10:720. doi: 10.1038/nrrheum.2014.127. [DOI] [PubMed] [Google Scholar]
- 27.Zhang T.Y., Dutta R., Benard B. IL-6 blockade reverses bone marrow failure induced by human acute myeloid leukemia. Sci Translational Med. 2020;12:eaax5104. doi: 10.1126/scitranslmed.aax5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slaats J., ten Oever J., van de Veerdonk F.L., Netea M.G. IL-1β/IL-6/CRP and IL-18/ferritin: Distinct inflammatory Programs in infections. PLOS Pathog. 2016;12:e1005973. doi: 10.1371/journal.ppat.1005973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberts E.W., Deonarine A., Jones J.O. Depletion of stromal cells expressing fibroblast activation protein-α from skeletal muscle and bone marrow results in cachexia and anemia. The J Exp Med. 2013;210:1137. doi: 10.1084/jem.20122344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Komori T. Functions of the osteocyte network in the regulation of bone mass. Cell Tissue Res. 2013;352:191. doi: 10.1007/s00441-012-1546-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim H.J., Kim H.J., Choi Y. Zoledronate Enhances osteocyte-Mediated osteoclast differentiation by IL-6/RANKL Axis. Int J Mol Sci. 2019;20:1467. doi: 10.3390/ijms20061467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guerrero A.R., Uchida K., Nakajima H. Blockade of interleukin-6 signaling inhibits the classic pathway and promotes an alternative pathway of macrophage activation after spinal cord injury in mice. J Neuroinflammation. 2012;9:40. doi: 10.1186/1742-2094-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang C., Li Y., Wu Y. Interleukin-6/Signal transducer and activator of transcription 3 (STAT3) pathway is essential for macrophage infiltration and Myoblast Proliferation during muscle Regeneration. J Biol Chem. 2012;288:1489. doi: 10.1074/jbc.M112.419788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta (Bba) - Mol Cell Res. 2011;1813:878. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y., Wang K., Han G.C. Neutrophil infiltration favors colitis-associated tumorigenesis by activating the interleukin-1 (IL-1)/IL-6 axis. Mucosal Immunol. 2014;7:1106. doi: 10.1038/mi.2013.126. [DOI] [PubMed] [Google Scholar]
- 36.Harre U., Keppeler H., Ipseiz N. Moonlighting osteoclasts as undertakers of apoptotic cells. Autoimmunity. 2012;45:612. doi: 10.3109/08916934.2012.719950. [DOI] [PubMed] [Google Scholar]
- 37.Balderramas H.A., Penitenti M., Rodrigues D.R. Human neutrophils produce IL-12, IL-10, PGE2 and LTB4 in response to Paracoccidioides brasiliensis. Involvement Tlr2, Mannose Receptor Dectin-1 Cytokine. 2014;67:36. doi: 10.1016/j.cyto.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 38.Harmer D., Falank C., Reagan M.R. Interleukin-6 Interweaves the bone marrow Microenvironment, bone loss, and multiple myeloma. Front Endocrinol. 2019;9:788. doi: 10.3389/fendo.2018.00788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kruger P., Saffarzadeh M., Weber A.N.R. Neutrophils: Between Host Defence, immune modulation, and tissue injury. PLOS Pathog. 2015;11:e1004651. doi: 10.1371/journal.ppat.1004651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang N., Qin C., Hou Y. Serum TNF-α, erythrocyte sedimentation rate and IL-6 are more valuable biomarkers for assisted diagnosis of extremity chronic osteomyelitis. Biomarkers Med. 2017;11:597. doi: 10.2217/bmm-2017-0082. [DOI] [PubMed] [Google Scholar]
- 41.Mahamid M., Mader R., Safadi R. Hepatotoxicity of tocilizumab and anakinra in rheumatoid arthritis: Management decisions. Clin Pharmacol Adv Appl. 2011;3:39. doi: 10.2147/CPAA.S24004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drepper M., Rubbia-Brandt L., Spahr L. Tocilizumab-induced acute liver injury in Adult Onset Still’s disease. Case Rep Hepatol. 2013;1–3:2013. doi: 10.1155/2013/964828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Norris C.A., He M., Kang L.I. Synthesis of IL-6 by hepatocytes is a normal response to Common hepatic Stimuli. PLoS One. 2014;9:e96053. doi: 10.1371/journal.pone.0096053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bugelski P.J., Martin P.L. Concordance of preclinical and clinical pharmacology and toxicology of therapeutic monoclonal antibodies and fusion proteins: Cell surface targets. Br J Pharmacol. 2012;166:823. doi: 10.1111/j.1476-5381.2011.01811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taskin M.I., Gungor A.C., Adali E. A humanized anti-interleukin 6 receptor monoclonal antibody, tocilizumab, for the treatment of Endometriosis in a rat model. Reprod Sci. 2016;23:662. doi: 10.1177/1933719115612134. [DOI] [PubMed] [Google Scholar]
- 46.Schaper F., Rose-John S. Interleukin-6: Biology, signaling and strategies of blockade. Cytokine Growth Factor Rev. 2015;26:475. doi: 10.1016/j.cytogfr.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 47.Baldo K., Sasaki H., Stashenko P. Interleukin-6 Deficiency increases inflammatory bone destruction. Infect Immun. 2001;69:744. doi: 10.1128/IAI.69.2.744-750.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.