Abstract
Respiratory and fecal aerosols play confirmed and suspected roles, respectively, in transmitting severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). An extensive environmental sampling campaign of both toilet and non-toilet environments was performed in a dedicated hospital building for patients with coronavirus disease 2019 (COVID-19), and the associated environmental factors were analyzed. In total, 107 surface samples, 46 air samples, two exhaled condensate samples, and two expired air samples were collected within and beyond four three-bed isolation rooms. The data of the COVID-19 patients were collected. The building environmental design and the cleaning routines were reviewed. Field measurements of airflow and CO2 concentrations were conducted. The 107 surface samples comprised 37 from toilets, 34 from other surfaces in isolation rooms, and 36 from other surfaces outside the isolation rooms in the hospital. Four of these samples were positive, namely two ward door handles, one bathroom toilet seat cover, and one bathroom door handle. Three were weakly positive, namely one bathroom toilet seat, one bathroom washbasin tap lever, and one bathroom ceiling exhaust louver. Of the 46 air samples, one collected from a corridor was weakly positive. The two exhaled condensate samples and the two expired air samples were negative. The fecal-derived aerosols in patients' toilets contained most of the detected SARS-CoV-2 in the hospital, highlighting the importance of surface and hand hygiene for intervention.
Keywords: COVID-19, SARS-CoV-2, Fecal aerosols, Aerosol transmission, Environment samples, Hospital
Graphical abstract
1. Introduction
The coronavirus disease 2019 (COVID-19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has spread rapidly around the globe. More than five million confirmed cases and more than 500,000 deaths were recorded by the end of June 2020 (WHO, 2020). The characteristics of this epidemic suggest that droplets exhaled during close contact and fomites may mediate transmission of SARS-CoV-2 (WHO-China, 2020). Potential airborne spread due to certain aerosol-generating procedures in healthcare facilities has also been envisaged. The role of the fecal–oral route in indoor environments remains to be determined after detection of the virus in stools (Zhang et al., 2020a; Zhang et al., 2020b; Zhang et al., 2020c). Crucially, however, the relative importance of transmission by fomites, close contact, and the suspected fecal–oral route remains unknown. Significant infection has also occurred in hospitals. According to the China CDC Weekly 2020 (Novel Coronavirus Pneumonia Emergency Response Epidemiology Team, 2020), 1716 of 44,672 COVID-19 cases confirmed in China by February 11, 2020 affected healthcare workers. Thus, understanding the infection risk in a hospital environment is essential to protecting healthcare workers.
An environmental sampling campaign was performed in four occupied isolation rooms housing 10 COVID-19 patients in The Second Hospital of Nanjing, China, and the association between the sampling results and the environment and the risk of SARS-CoV-2 transmission were analyzed. The studied infectious disease hospital was built in 2015 and is now a designated hospital for receiving COVID-19 patients during the epidemic. The aim of this study was to identify the high-risk areas in a hospital containing COVID-19 patients and thus enable the adoption of adequate and effective interventions.
2. Materials and methods
2.1. Patient data
Basic data of the COVID-19 patients in the sampled isolation rooms were first collected; these included the date of symptom onset, throat-sample polymerase chain reaction (PCR) results, CT scan findings, symptoms, and mask-wearing behavior. The data of these patients are summarized in Table S1. On each date, patients and rooms were randomly chosen for sampling.
2.2. Environmental sampling
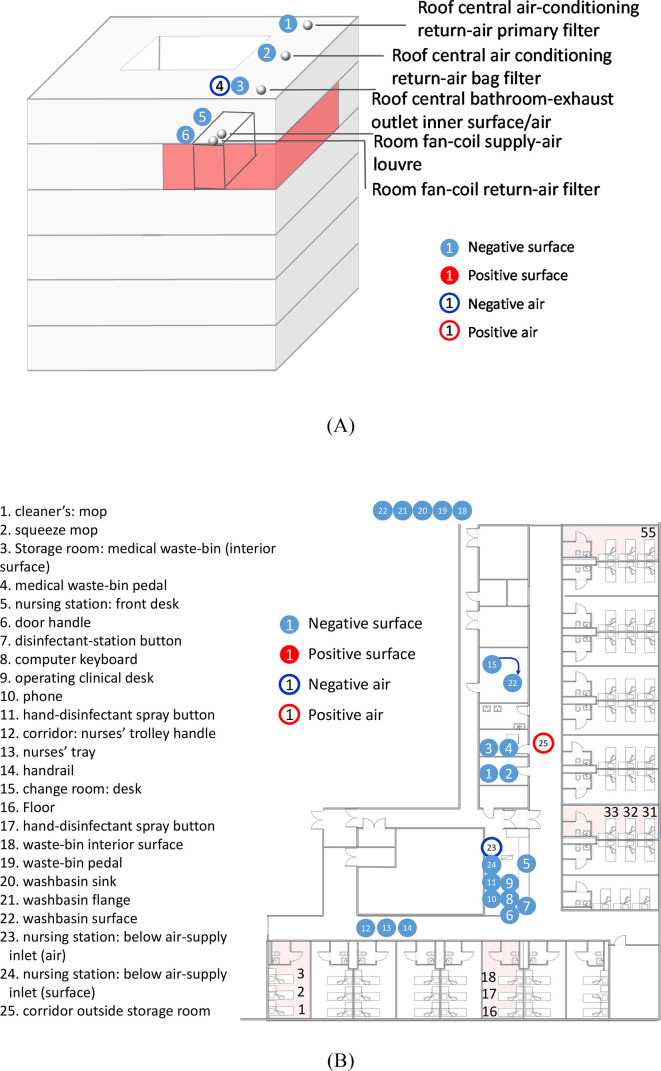
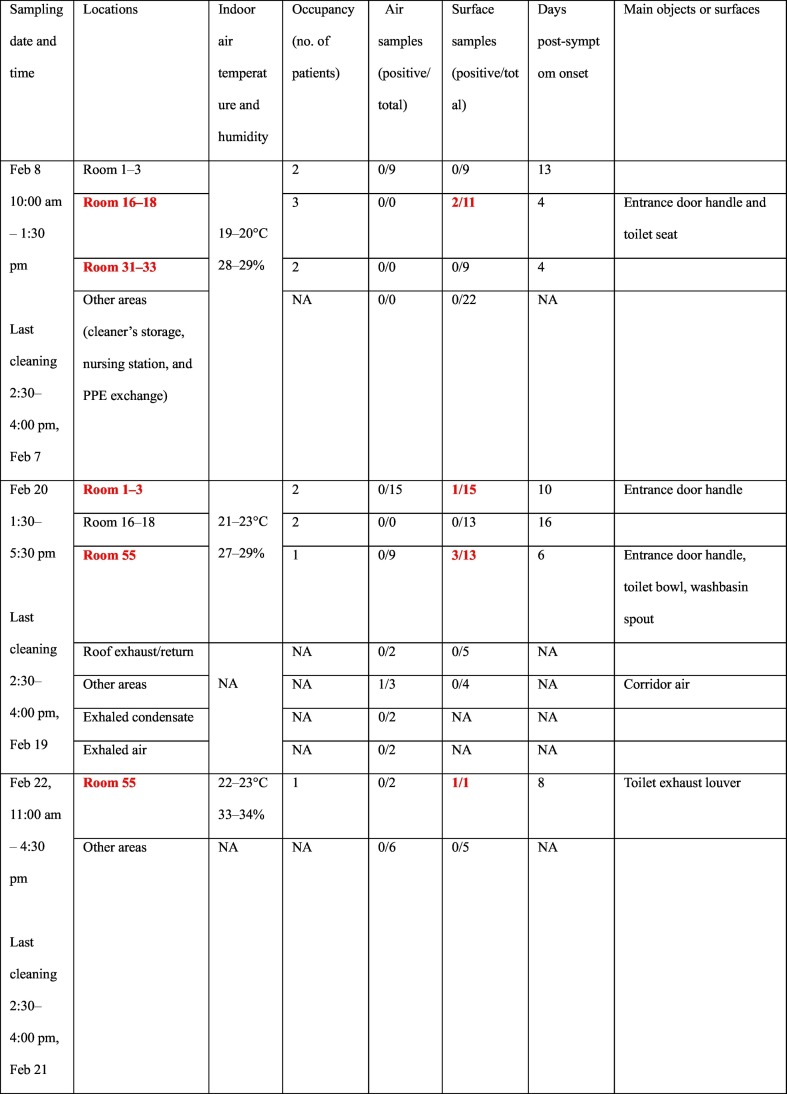
Environmental sampling was conducted in four isolation rooms, a nursing station, a corridor, an air-conditioning system, and other spaces in the airborne infectious-disease zone on the fifth floor of the hospital (Fig. 1A). The hospital is a six-story building with a courtyard. The sampling was conducted on February 8, 20, and 22, 2020. At the time of sampling, only some of the 19 isolation rooms in the studied zone were occupied by 34 patients on February 8, 21 patients on February 20, and 34 patients on February 22. Each isolation room contained three beds and measured 7.9 m × 3.9 m × 8.2 m but was not necessarily fully occupied at the time of sampling.
Fig. 1.
Summary of hospital sites where air and surface samples were collected. (A) Locations of the sampling points in the air-conditioning systems and on the hospital building roof. The building zone highlighted in red is the airborne infectious-disease zone on the fifth floor of the hospital. (B) Locations of the sampling points at the nursing station, storage/cleaner's rooms, healthcare workers' PPE changing room, and corridor of the airborne infectious-disease zone. (C) Locations of the sampling points in a typical isolation room. Positive samples are indicated by either empty or filled red circles. Negative samples are indicated by either empty or filled blue circles. In Panel B, the four sampled rooms are highlighted in light red, namely the isolation rooms containing beds 2 and 3, beds 16–18, beds 31 and 32, and bed 55. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The sites of air and surface sample collection are shown in Fig. 1. A detailed list of air and surface samples is given in Table S2. All samples were collected by a trained CDC officer who had a medical background, with assistance from two trained nurses from the hospital. The CDC officer wore full personal protective equipment (PPE), including an N95 respirator, goggles, face shield, gloves, shoe covers, cap, and gown. The CDC officer was quarantined for 12 days after the collection on February 8 and resumed sampling on February 20 and 22. Thereafter, he was quarantined again for another 14 days and again disinfected.
For airborne-aerosol sampling, four bioaerosol samplers were used: an Andersen one-stage viable impactor (QuickTake-30, SKC, USA; sampled at 10 L/min for 30 min, Gibco cell-culture medium, 10 mL, only on February 8), an AirPort MD8 (Sartorius, Germany; 50 L/min for 20 min, water-soluble gel film), an ASE-100 (Langsi Medical Technology, Shenzhen, China; 500 L/min for 2 min, biological aerosol special-collection liquid on February 8 and 500 L/min for 20 min, biological aerosol special-collection liquid on February 20 and 22), and a WA-15 (Dinglan Technology, Beijing, China; sampled at 14 L/min for 30 min on February 8; Youkang virus-sampling kit, Youkang Hengye Biotechnology, Beijing, China). To collect exhaled breath condensates, an AT-150 (Dingblue Technology, Beijing, China) was used to obtain samples from respiratory fluids, which aggregated on the hydrophobic film surfaces after freezing. To collect surface-wiped samples, a Youkang virus-sampling kit (Youkang Hengye Biotechnology, Beijing, China) was used. A cotton swab was moistened with the collection liquid and then used to wipe the surface of the object once.
The sampling sites were extended to other areas in the hospital and its roof air-exhausts on February 20 and 22. The sampling duration of the ASE-100 was also increased from 2 min to 20 min. All sampling was conducted from 9:00 am to 12:30 pm, and the morning surface-cleaning in the hospital was arranged to be suspended. For the general routine, cleaning and disinfection of these rooms was conducted twice daily. The frequently touched surfaces were cleaned using a sodium dichloroisocyanurate solution containing 500 mg/L chlorine (disinfectant tablets, Lvshaxin Aiershi, Shanghai). During the morning round, cleaners wearing PPE entered at 8:30 am, emptied the waste bins from 8:30 to 9:00 am, cleaned environmental surfaces from 9:00 to 10:30 am, and exited at 11:00 am. During the afternoon round, cleaners entered at 2:00 pm and cleaned the same surfaces from 2:30 to 4:00 pm.
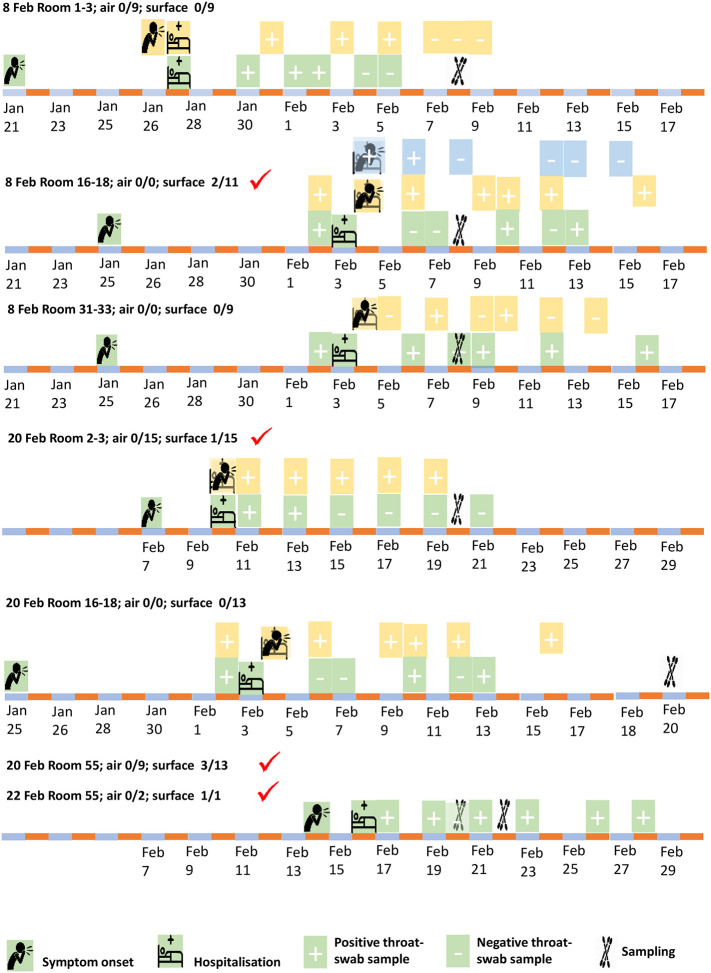
In total, 107 surface samples, 46 air samples, two exhaled condensate samples, and two exhaled air samples were collected. On February 8, 60 samples were collected, including nine air samples (with the QuickTake30, MD-8, and ASE-100) and 51 surface samples. On February 20, 29 air samples (with the MD-8, ASE-100, and WA-15), two exhaled condensate samples, two exhaled air samples, and 50 surface samples were collected. On February 22, eight air samples (using the MD-8 and ASE-100) and six surface samples were collected. The sampling dates in each studied isolation room are summarized in Fig. 2 , which also includes the patients' information.
Fig. 2.
Summary of the isolation rooms containing beds 2 and 3, 16–18, 31 and 32, and 55, the 10 patients housed in these rooms (onset and hospitalization dates), and the sampling dates. The sampling dates on which positive samples were detected are highlighted by red ticks. In each room, a patient and his/her bed are shown in the same color. When events (e.g., symptom onset and hospitalization) occurred on the same day, the symbols overlap and are shown in a transparent format. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.3. Method of analysis
The environmental sample detection methods included nucleic acid extraction (NP968, Tianlong Science & Technology, Xi'an, China) and amplification by real-time quantitative reverse-transcription (RT)-PCR (Applied Biosystems QuantStudio Dx, Thermo Fisher Scientific, Waltham, MA, USA). Detection of SARS-CoV-2 RNA was performed using a real-time RT-PCR kit from Shanghai Chromysky Medical Research Co. Ltd. (Shanghai, China). Each sample for RT-PCR was run in duplicate according to the manufacturer's instructions. The patients' samples were analyzed by The Second Hospital of Nanjing using an Applied Biosystems 7500 Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA, USA). The nucleic acid detection reagents were obtained from Huada Biotechnology (Wuhan) Co., Ltd. A sample was defined as positive at a Ct ≤38, and weakly positive at a Ct of 37–38. A standard curve generated from the RNA standards was previously published by this institution for validation of the RT-PCR method used to quantify SARS-CoV-2 (Shi et al., 2020).
2.4. Statistical analysis
The chi-square test was used to determine whether there were statistically significant differences in the percentages of positive samples among different groups of surfaces. Specifically, the chi-square test statistic, χ2, was calculated and compared to a chi-square distribution to acquire the p-value. In this study, a p-value less than 0.05 was considered statistically significant.
2.5. Building and its ventilation data
The studied hospital was a dedicated COVID-19 patient hospital in Nanjing. The hospital is a six-story building, and the sampled area, patient zone no. 5, occupies part of the fifth floor (Fig. 1A). Four patient rooms were monitored, and sampling was also conducted in the corridor and elsewhere on the floor and on the hospital roof. Unlike the sixth-floor wards, the fifth-floor wards, where environmental sampling was conducted, do not have anterooms.
The CO2 concentrations were measured in the rooms using an indoor air-quality meter (IAQ-Calc 7515, TSI, USA) for 24 min with four people present. Based on the CO2 data, the outdoor air-supply rate was calculated to be 64 L/s (2.5 air-changes per hour, ACH). Using incense smoke, the airflow pattern was determined to lead from the corridor to each isolation room, and to subsequently exhaust via the bathroom.
3. Results and discussion
3.1. Possible surface samples associated with toilets in patients' rooms
Of the 107 surface samples taken throughout and beyond the infectious disease zone, seven were positive (four positive and three weakly positive). These samples were collected from the inside door handle of the isolation room containing beds 16, 17, and 18 (Ct = 36.8, 407 RNA copies, February 8), the toilet seat in the same isolation room (Ct = 38.0, February 8), the inside door handle of the isolation room containing beds 2 and 3 (Ct = 36.2, 666 RNA copies, February 20), the toilet seat cover (lower surface) in the isolation room containing bed 55 (Ct = 36.1, 723 RNA copies or 29 copies/cm2, February 20), the bathroom tap lever of the same room (Ct = 37.7, February 20), the bathroom door handle of the same room (Ct = 36.8, 407 RNA copies, February 20), and the exhaust air grille surface in the bathroom of the same room (Ct = 37.9, February 22). Note that we did not calculate the RNA copy numbers for the three weakly positive samples.
Note also that five of seven positive/weakly positive samples were obtained from two bathrooms used by patients, and all of the throat swabs of at least one of these patients had been positive for two days before sampling. Among all other surface areas both within and beyond the isolation rooms, only two surface samples were positive, namely the door handles of Rooms 16–18 and 2–3. Fig. 1A and B demonstrate that all surface samples outside of the patients' rooms were negative, as shown by a lack of solid red circles, while only the toilet area in one patient room was found to be contaminated, as shown by solid red circles in Fig. 1C.
Table 1 shows that positive surface samples were only detected in the rooms containing a patient whose throat swabs were all positive on a given day. The numbers of positive air samples and surface samples and the corresponding shortest symptom onsets in the sampling period are also shown.
Table 1.
Summary of samples collected on the three sampling dates in the four isolation rooms and other areas in the airborne infectious-disease zone. Rooms containing a patient whose throat swabs were all positive on a given day (Table S1) are shown in bold red font.
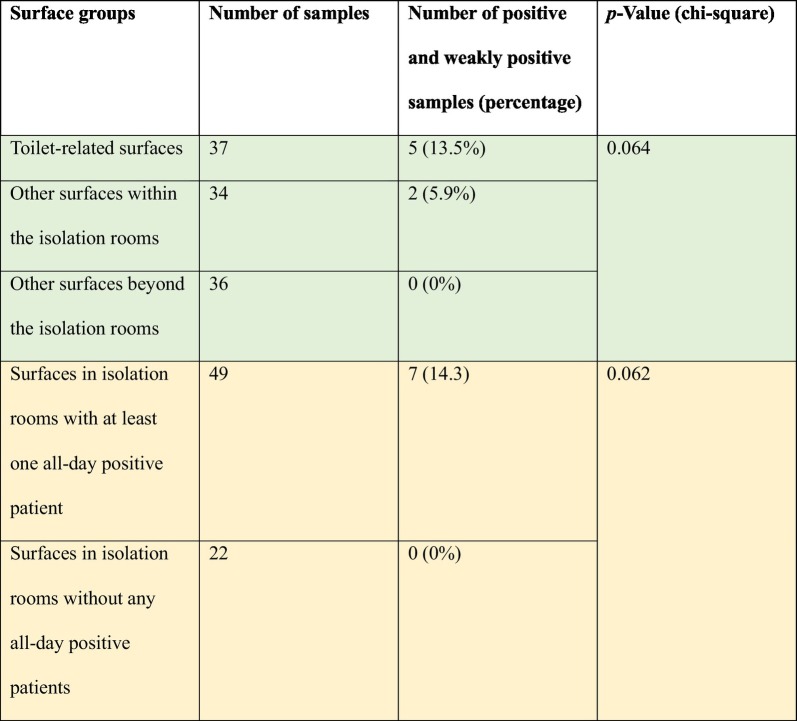
These results suggest that for the COVID-19 patients studied here, the toilet was the most contaminated environment, although the chi-square p-value is only 0.064 (Table 2 ). The detection of more positive surface samples in the bathrooms than elsewhere suggests that these samples may be fecal in origin. The previous detection of the virus in stools3–5,16,18 supports this interpretation, as does the fact that stools obtained from the first COVID-19 patient in the United States also tested positive.7 Zhang et al.5 also observed more anal swab positives than oral swab positives in the later stage of infection, and approximately 10% of the patients in Wuhan presented with diarrhea and nausea prior to fever and dyspnea (Wang et al., 2020). In other studies, diarrhea and nausea have been observed in one-third of patients (Foladori et al., 2020). High levels of viral load were also detected in the stools of SARS-CoV patients (Peiris et al., 2003).
Table 2.
Statistical significance of the positive results for toilet-related or other surfaces in isolation rooms containing at least one patient with all-positive throat swabs before and/or after the sampling.
3.2. One positive surface sample at a toilet ceiling exhaust grille
The detection of positive surface samples on one toilet ceiling exhaust grille in a bathroom suggests that fine virus particles existed in that bathroom. Deposition on exhaust grille surfaces can result from either the long-term deposition of low-concentration particles in the air or the rapid deposition of high-concentration particles. Three possible sources of fine airborne aerosols exist in the bathroom: exhaled release from patients when using the bathroom, toilet-generated aerosols from feces and urine when the toilets are flushed (Gerba et al., 1975), and import of airborne particles from the cubicles where the patients spend most of their time. Ong et al. (2020) also detected SARS-CoV-2 in an air-outlet fan. Among surfaces in a patient ward, including a bench, bedside rail, locker, bedside table, alcohol dispenser, and windowsill, Cheng et al. (2020) found that only the windowsill sample (1/13) was positive for SARS-CoV-2 even when the viral loads of the patients were 3.3 × 106 copies per mL in the pooled nasopharyngeal sample and throat swab and 5.9 × 106 copies per mL in saliva. A patient's hands can be contaminated during toilet usage. Consistent with this observation, the toilet bowl and sink surface samples in a Singaporean hospital also tested positive for SARS-CoV-2 (Ong et al., 2020). In addition to aerosols generated by toilet flushing, Yu (2004) found that during the 2003 Amoy Garden outbreak, SARS-CoV bio-aerosols were generated in drainage stacks after patients flushed the toilets.
A toilet is housed in a small area that is commonly shared by patients in relatively large isolation rooms. The personal spaces of these patients overlap in the toilet area and thus are likely to generate an additive contamination effect. This effect may explain why the toilet surfaces were particularly likely to be positive for SARS-CoV-2. Nevertheless, the duration of a patient's visit to the toilet would have been brief, as only mildly symptomatic patients were housed on the tested floor. Additionally, the surface material or finish inside a toilet may allow a better transfer during swab-sampling than the other sampled surfaces. In this context, there is generally a higher surface-touch transfer rate from smooth to rough surfaces, and most toilet surfaces are made to be smooth (Zhao et al., 2019). No virus was detected on non-toilet object surfaces in the patients' rooms, in contrast to a 2020 study by Ong et al. (2020) wherein 13 of 15 (87%) samples in one patient's room and three of five (60%) toilet sites were positive, although anteroom and corridor samples were negative. Interestingly, the patient in that earlier study had no pneumonia or diarrhea but provided stool samples that were positive for SARS-CoV-2.
3.3. One corridor air sample was weakly positive for SARS-CoV-2
Forty-five of the 46 air samples were negative. The only weakly positive air sample was obtained from the corridor close to the patients' isolation rooms and was collected on February 20 using the ASE-100 with a total air-volume of 10 m3. The exact location of the weakly positive sample is shown in Fig. 1B (sample location no. 25). Of the two exhaled condensate samples, both were negative. We characterized the airflow pattern in the hospital zone using incense smoke, which revealed a weak flow from all isolation rooms to their shared corridor, as shown in Fig. S1. In other words, none of these rooms had negative pressure, despite the continuous operation of a relatively strong exhaust fan in the bathrooms of all isolation rooms. Therefore, aerosols may have been leaked from the isolation rooms to the corridor. Airborne aerosols may have also been released from the PPE worn by healthcare workers into the environment in the multi-function room. We were not able to quantitatively measure the airflow in that zone.
The above described detection of the virus in the corridor air (only weakly positive, and lower than the limit of quantification) and also on the surfaces of exhaust grilles in the bathrooms suggests the possible existence of airborne virus particles. Previous evidence suggested the airborne transmission of SARS-CoV in hospital wards (Li et al., 2005). Both Ong et al. (2020) and Cheng et al. (2020) failed to detect any SARS-CoV-2-positive air samples, although this may have been due to the fact that the former group sampled only 1.2 m3 or 1.5 m3 air, depending on the sampler used, while the latter collected only 1 m3 of air. Our single weakly positive result was obtained from an air volume of 10 m3 while using absorption solution as the collection medium, and no positive samples were detected when the air volume was less than 10 m3. Therefore, it appears that the airborne concentration of SARS-CoV-2 was very low in our studied hospital.
In summary, the study data strongly imply that toilets may be high-risk areas in hospitals with COVID-19 patients and emphasize the importance of hygiene in both private and public toilets. The strong need for hand and environmental hygiene as an intervention for COVID-19 transmission is also indicated.
Our study had some limitations. Due to the possibly strong infectivity of this new virus, the sampling operative was subject to the requirements for quarantine and the use of inconvenient full PPE, which might have affected the sampling operation. Moreover, access was provided only to the rooms of patients with mild symptoms. Furthermore, the number of collected air samples was small.
This study was part of the epidemiological studies conducted by the Jiangsu CDC on the COVID-19 outbreak. The requirement for ethical approval was waived.
CRediT authorship contribution statement
Z. Ding, H. Qian, and Y. Li contributed equally. Y. Li, Z. Ding, H. Qian, and B. Zhu contributed to the study design, hypothesis formulation, and coordination. Z. Ding and H. Qian contributed to the field investigation, data analyses, and reporting. B. Xu contributed to sampling. Y. Huang contributed to patient data collection. S. Xiao contributed to the statistical analyses. X. Wu, L. Zhou, and Y. Xu contributed to the sampling design. W. Shao, Y. Song, and S. Li contributed to sampling and patient surveys in the hospital. Y. Li and Z. Ding wrote the first draft of the paper, and H. Qian, H. Yen, and T. Miao contributed to the major revision. All the other authors contributed to the revision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
All the authors approved the submitted version and have agreed to be personally accountable for their own contributions.
The corresponding authors also confirm that they had full access to all the data in the study and hold the final responsibility for the decision to submit for publication.
Acknowledgments
The authors are grateful to the many field workers and hospital staff who helped to collect data and provided support for our field measurements in the hospital during the most challenging period of their work. This work was partially supported by the National Natural Science Foundation of China (no. 41977370), the Research Grants Council of Hong Kong (no. 17202719, no. C7025-16G), and the Scientific Research Fund of Jiangsu Provincial Department of Health (no. S21017002).
The funding bodies had no involvement in the study design; the collection, analysis, and interpretation of data; the writing of the report; and the decision to submit the paper for publication.
Editor: Damia Barcelo
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2020.141710.
Appendix A. Supplementary data
Supplementary material
References
- Cheng V.C., Wong S.C., Chen J.H., Yip C.C., Chuang V.W., Tsang O.T., Sridhar S., Chan J.F.W., Ho P.-L., Yuen K.Y. Escalating infection control response to the rapidly evolving epidemiology of the coronavirus disease 2019 (COVID-19) due to SARS-CoV-2 in Hong Kong. Infect. Control Hosp. Epidemiol. 2020;41(5):493–498. doi: 10.1017/ice.2020.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foladori P., Cutrupi F., Segata N., Manara S., Pinto F., Malpei F., Bruni L., La Rosa G. SARS-CoV-2 from faeces to wastewater treatment: what do we know? A review. Sci. Total Environ. 2020;140444 doi: 10.1016/j.scitotenv.2020.140444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerba C.P., Wallis C., Melnick J.L. Microbiological hazards of household toilets: droplet production and the fate of residual organisms. Appl. Environ. Microbiol. 1975;30(2):229–237. doi: 10.1128/am.30.2.229-237.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Huang X., Yu I.T.S., Wong T.W., Qian H. Role of air distribution in SARS transmission during the largest nosocomial outbreak in Hong Kong. Indoor Air. 2005;15:83–95. doi: 10.1111/j.1600-0668.2004.00317.x. [DOI] [PubMed] [Google Scholar]
- Novel Coronavirus Pneumonia Emergency Response Epidemiology Team Vital Surveillances: The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19)—China, 2020. 2020. http://weekly.chinacdc.cn/en/article/id/e53946e2-c6c4-41e9-9a9b-fea8db1a8f51 China CDC Weekly. [PMC free article] [PubMed]
- Ong S.W.X., Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Wong M.S.Y., Marimuthu K. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. J. Am. Med. Assoc. 2020;323(16):1610–1612. doi: 10.1001/jama.2020.3227. (Published online March 4, 2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris J.S.M., Chu C.M., Cheng V.C.C., Chan K.S., Hung I.F.N., Poon L.L. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361(9371):1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F., Wu T., Zhu X., Ge Y., Zeng X., Chi Y. Association of viral load with serum biomarkers among COVID-19 cases. Virology. 2020;546:122–126. doi: 10.1016/j.virol.2020.04.011. https://www.sciencedirect.com/science/article/pii/S0042682220300787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. J. Am. Med. Assoc. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Coronavirus Disease 2019 (COVID-19) Situation Report – 162. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/
- WHO-China . 2020. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19) pp. 16–24.https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19COVID-19-final-report.pdf February 2020. Accessed on March 8, 2020. [Google Scholar]
- Yu ITS. vidence of airborne transmission of the severe acute respiratory syndrome virus. N. Engl. J. Med. 2004;350:1731–1739. doi: 10.1056/NEJMoa032867. [DOI] [PubMed] [Google Scholar]
- Zhang J., Wang S., Xue Y. Fecal specimen diagnosis 2019 novel coronavirus–infected pneumonia. J. Med. Virol. 2020;92:680–682. doi: 10.1002/jmv.25742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Du R.H., Li B., Zheng X.S., Yang X.L., Hu B. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerging Microbes & Infections. 2020;9(1):386–389. doi: 10.1080/22221751.2020.1729071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Chen C., Zhu S., Shu C., Wang D., Song J. Isolation of 2019-nCoV from a stool specimen of a laboratory-confirmed case of the coronavirus disease 2019 (COVID-19) China CDC Weekly. 2020;2(8):123–124. [PMC free article] [PubMed] [Google Scholar]
- Zhao P., Chan P.T., Gao Y., Lai H.W., Zhang T., Li Y. Physical factors that affect microbial transfer during surface touch. Build. Environ. 2019;158:28–38. doi: 10.1016/j.buildenv.2019.05.005. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material