Abstract
Continuous drug monitoring is a promising alternative to current therapeutic drug monitoring strategies and has a strong potential to reshape our understanding of pharmacokinetic variability and to improve individualised therapy. This review highlights recent advances in biosensing technologies that support continuous drug monitoring in real time. We focus primarily on aptamer-based biosensors, wearable and implantable devices. Emphasis is given to the approaches employed in constructing biosensors. We pay attention to sensors’ biocompatibility, calibration performance, long-term characteristics stability and measurement quality. Last, we discuss the current challenges and issues to be addressed in continuous drug monitoring to make it a promising, future tool for individualised therapy. The ongoing efforts are expected to result in fully integrated implantable drug biosensing technology. Thus, we may anticipate an era of advanced healthcare in which wearable and implantable biochips will automatically adjust drug dosing in response to patient health conditions, thus enabling the management of diseases and enhancing individualised therapy.
Keywords: Continuous drug monitoring, Wearable biosensors, Implantable biosensors, In vivo pharmacokinetics, Electrochemical aptamer-based sensors, Individualised therapy
Graphical abstract
Highlights
-
•
The review summarizes biosensing technologies in real-time continuous drug monitoring.
-
•
The review focuses on aptamer-based sensors, wearables and implantable sensors.
-
•
The review summaries challenges and issues to address in continuous drug monitoring.
1. Introduction
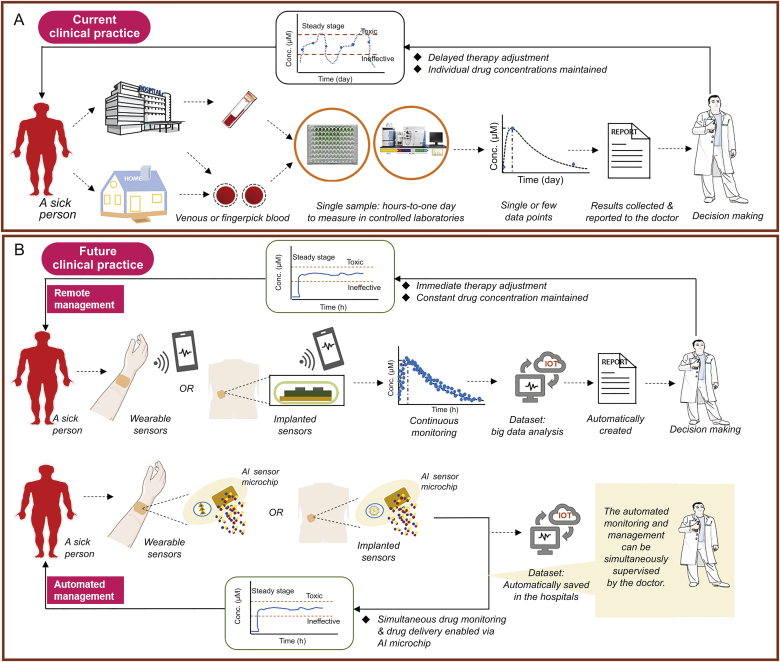
Precision medicine promises the right drug, at the right dose, for the right patient, at the right time [1]. One of the effective strategies for ensuring the right dosage for a patient and individualised care is therapeutic drug monitoring [2,3]. Therapeutic drug monitoring is the clinical practice of detecting given drugs at fixed intervals to maintain the drug concentration within a narrow therapeutic window in the patient’s bloodstream. It is adapted primarily for small-molecule drugs but has recently shown promise for optimising monoclonal antibodies [[4], [5], [6], [7]]. Therapeutic drug monitoring is typically performed regularly at single or fixed time points [8]. High performance liquid chromatography-tandem mass spectrometry (HPLC-MS) and enzyme-linked immunosorbent assays (ELISAs) are the two most commonly used methods for regular monitoring, with advantages of high sensitivity, selectivity and the capability for multiple simultaneous measurements [9,10]. The disadvantages are strong dependence on the lab environment, a multistep batch process, hours-to-days measurements, and slow communication of results to physicians (Fig. 1A). The entire process often results in delayed patient treatment [11], which can be especially problematic in acute clinical settings [12], such as emergency medicine, surgery, and treatment of infectious diseases (e.g., coronavirus disease [13,14]). Moreover, the measurements only produce single or little data per patient [15]. This provides clinicians with only fragmented information on how a drug reacts inside the body, and can also lead to less precise pharmacokinetic (PK)/pharmacodynamic (PD) modelling.
Fig. 1.
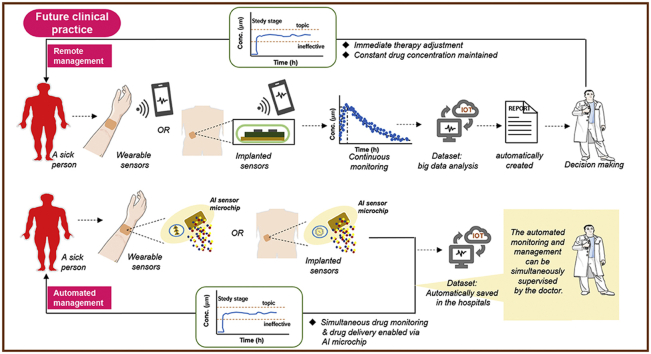
(A) Schematic representation of current clinical practice based on single (or few) datapoint therapeutic drug monitoring, and (B) futuristic clinical practice based on any-time datapoint continuous drug monitoring. The current practice is complicated and time consuming, involving the drawing of venous blood in a hospital or fingerpick blood collection at home, blood delivery to testing centres, and sample measurement (washing, separation, and sequential addition of reagents). The outcome is a single result or a few parameter values per patient, and the long process hinders the clinician from delivering timely therapy adjustment to the patient. Moreover, the limited drug dosing adjustments fail to ensure that drug concentrations are always within the proposed therapeutic window, which either causes toxicity or renders the treatment ineffective. In ideal clinical practice, drug dosing can be adjusted immediately, continuously, or even automatically in response to drug concentrations measured in real time. This would contribute to maximal drug efficacy and minimal adverse effects through the maintenance of constant therapeutic drug concentrations. Such advanced healthcare can be achieved via wearable and implantable biosensors, especially with the aid of the recent advances in artificial intelligence. AI: artificial intelligence; IOT: Internet of Things.
Individualised medicine would benefit immensely from real-time, continuous drug monitoring (CDM) given the multiple benefits it holds. First, CDM provides clinicians with an invaluable direct window into an individual’s response to therapeutics in real time, and into this patient’s health status over time. This provides an in-the-moment opportunity to adjust the therapy as needed. Furthermore, for clinical trials, CDM will, to a great extent, enhance drug development by making the process more intelligent and faster [16]. Second, CDM offers complete rather than fragmented information about the drug’s interaction inside an individual body over a prolonged period. Some key information can be missed in unstable patients by discrete measurements [17]. This is the case in intensive care units during surgery. Third, CDM enables researchers to glean deeper insights into drug intra-individual and inter-individual PK variability, which is the result of complex factors, including genetic differences, renal function, gender, age, pregnancy, co-morbidities, and food [18]. Undoubtedly, this aids clinicians and researchers in identifying factors that make a therapy ineffective in some patients. Fourth, complete information captured helps to establish a more accurate PK/PD model, with which reliable treatment decision algorithms can be created. Accurate decision-making is the key to an optimised therapy [19]. Furthermore, CDM makes an integrated closed-loop drug system possible (preferably in wearable or fully implantable format) [20], in which real-time drug sensing triggers immediate drug dosing that is further adjusted via a feedback controller. This will certainly improve the automated control of current drug administration and disease management (Fig. 1B) [21]. On the whole, CDM can effectively minimise drug toxicity and reduce the burden on the patient and the hospital staff, and potentially, the national healthcare systems, while enhancing patient trust and satisfaction in the treatment [22].
To achieve CDM, biosensing technologies should meet multiple requirements. First, the transducer, including the target identification, requires handling the drugs in matrices such as sweat and fresh bloodstream. Also, it should be delivered continuously to the sensor surface for detection. The latter may require either a sample collection interface or a microfluidic channel. The transducer includes also the biorecognition phase which needs elements with high-specificity and capability to capture the target in real time. Next, a data convertor and a signal processing core are required to handle the interaction of drug with biorecognition elements which provoke changes in electrical properties. The latter changes should be sensitively sensed out in real time. Besides, the sensor should be utilized in a simple label-free, reagent-free, and wash-free process. The final step is signal processing of resulting electrical signals, which must be done in real time too to enable feedback for immediate decision making.
The electrochemical biosensor is a promising tool for achieving CDM. Analytically, electrochemical biosensors allow real-time detection, have a wide linear range of detection, low limits of detection, reproducibility, and optimal stability [23,24]. Technologically, electrochemical biosensors have extensive applicability, are compatible with advanced microfabrication technologies and state-of-the-art nanotechnologies, and have low cost and minimal power requirements. These made electrochemical biosensors attract the largest market share in the global biosensors market in 2018, with broad applications in continuously monitoring of targets: from plant signalling pathways and metabolism to physiological parameters in biological systems, and in vivo imaging and sensing in diagnostic medicine [[25], [26], [27], [28]]. Continuous monitoring of glucose, lactate, biomarkers, and neurotransmitters are the most investigated topics in diagnostic medicine [29,30].
CDM is a relatively new research topic. Limited, though exciting, biosensing technologies have been established in the past to support CDM using a range of emerging nanomaterials, a variety of aptamers, and integration with novel micro/nano systems. In this review, we focus on micro-nano biosensors developed for CDM and operated via electronic and electrochemical signals. Based on the function of the biosensor interface with the living body, the micro/nanoscale CDM techniques can be divided into three main categories: in vitro, ex vivo, and in vivo based solutions. In vitro biosensing technologies involve performing measurements in pure buffers or pre-collected blood. Ex vivo biosensing involves validation performed in an artificial environment outside the body. In vivo validation involves emplaced or implanted devices inside the body.
In the subsequent sections of this paper, we will describe achievements and undergoing research in biosensing technologies for CDM. Emphasis is given to design approaches to biosensors and the corresponding performance.
2. In vitro biosensing technologies for CDM
Electrochemical sensors are the commonly-used devices for in vitro continuous measurements in the sample matrices including buffer, serum, plasma, and whole blood. Electrochemical biosensors analyse the content of a biological sample through the direct conversion of a biological event into an electronical signal via an electrochemical transductor [31,32]. An electrochemical system comprises a three-electrode sensing cell in direct contact with the target solution, and a potentiostat to drive the measurements and read out the signal [33]. To enhance the biosensing performance, electrode surfaces are either biofunctionalised with various nanomaterials to increase the active sensing areas, or immobilised antibodies or aptamers for specific target capture. Research efforts are subsequently divided into two categories based on the surface modification strategies.
2.1. Nanomaterial-based electrochemical sensors
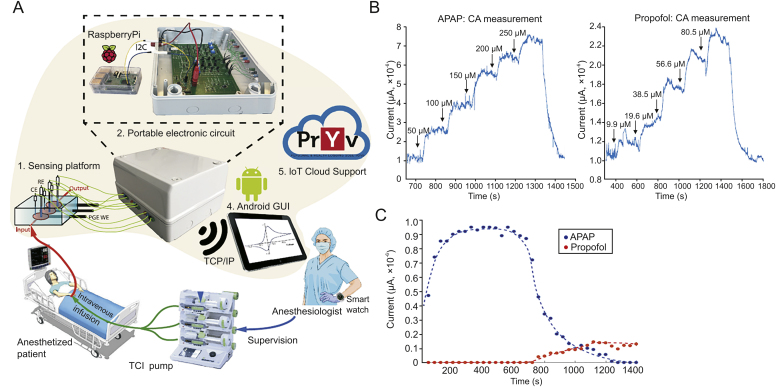
Surface modification via functional nanomaterials, such as carbon nanotubes, graphite, or graphene, yields high sensitivity and selectivity, rapid response, and excellent durability in biological media [34]. Baj-Rossi et al. [35] developed an electrochemical biosensor via cyclic voltammetry for continuous monitoring of naproxen, a standard anti-inflammatory compound. The biosensor was developed based on multi-walled carbon nanotubes (MWCNTs) and microsomal cytochrome P4501A2 on a graphite screen-printed electrode, constructed by printing conductive inks on insulating plastic or ceramic substrates. The strategy brought the limit of detection (LOD) down to 16 μM, and enabled real-time monitoring of naproxen for 16 h with high specificity and greatly simplified sensor preparation. Subsequently, Stradolini et al. [36] presented a pencil graphite electrode (PGE) based electrochemical sensor for long-term cyclic voltammetric monitoring of propofol, a benchmark anaesthetic. Continuous tracking of anaesthetic plasma concentrations is of critical importance to achieving and maintaining a constant level of sedation during surgery. In this study, a clay to graphite ratio of 0.4 was tested and verified to be the most suitable surface modification strategy: the clay content provides a protective shield against fouling while the graphite content supports electrochemical activity. The sensor monitored propofol for up to 4 h without any intermediate cleaning. Following this study, the group developed a drug monitoring system with an Internet of Things (IoT) link for continuous and simultaneous online detection of propofol and paracetamol, the two main anaesthetics often administered in combination [37]. The system comprises a microcontroller (Raspberry Pi) used to control and read out the signal from a PGE-based electrochemical sensor. The device consists of an IoT network including a cloud-based system that enables physicians to control and share all the patient’s data via an Android app and a smart watch (Fig. 2). A full capacity test run of the system performed real-time measurement of propofol (60 μM) and paracetamol (300 μM) in undiluted human serum for over 24 min.
Fig. 2.
Schematic representation of an electrochemical sensor for in vitro continuous drug monitoring (CDM). (A) An IoT system for continuous and simultaneous online monitoring of two anaesthetics: paracetamol (APAP) and propofol, proposed for integration into clinical practice; (B) chronoamperometry measurements of APAP and propofol; (C) real-time monitoring of the two drugs in undiluted human serum for over 24 min [37]. TCP/IP: Transmission Control Protocol/Internet Protocol; GUI: graphical user interface; TCI: target controlled infusion; CA: chronoamperometry.
In addition, Sweilam et al. [38] developed a miniaturised, flexible fibre-based sensor for constant detection of lithium, the main antipsychotic drug for treating mood disorders. Functionalised with single-walled carbon nanotubes (SWCNT) and an ion-selective membrane cocktail, the lithium sensor detected lithium in aqueous LiCl solutions without the preconditioning steps required for traditional ion-selective electrodes. Furthermore, the sensors were stable for six months at 4 °C. The sensor not only continuously monitored lithium over a wide range for up to 26 min in solution, but also produced a linear detection curve for human plasma. This study represents an initial step towards the design of a wearable sensor for lithium drug monitoring.
2.2. Aptamer-based electrochemical and electronic sensors
Aptamers (chemical antibodies) are single-stranded DNA or RNA molecules that can bind to a wide range of targets with high specificity and affinity. They are generated in the systematic evolution of ligands via an exponential enrichment (SELEX) process, which was first reported thirty years ago by Ellington and Szostak [39] and Tuerk and Gold [40]. Compared to antibodies, aptamers are smaller in size, cheaper to produce, and are more easily modified and generated against a wide array of target molecules. Aptamers are highly promising for use in CDM due to their capacity for regeneration (i.e., to be denatured and refolded) multiple times without loss of activity, as well as a long shelf life and good stability [41,42].
Electrochemical aptamer-based (E-AB) sensors can achieve high selectivity towards a given target because the induced reversible conformational change in aptamer probes occurs only during recognition of the specific target. Furthermore, it is signalled electrochemically, and electroactive impurities within the potential range of the adapted redox tags are rare [43]. In principle, reversible conformational change of aptamer enhances the rate of electron (e−) transfer between the redox reporter and the electrode, read out as an electric current. The use of aptamer for real-time, continuous monitoring of drug concentrations was first demonstrated in vitro by Swensen et al. [44] for drug cocaine in human serum, and subsequently by Li et al. [45,46] against multiple drugs in undiluted whole blood over multiple hours with good accuracy and precision. Similarly, in two recent studies, an aptamer probe was used in a label-free field-effect transistor-based biosensor and memristive biosensor to monitor tenofovir, an antiretroviral drug for HIV treatment to keep the concentration within its therapeutic range [47,48]. Both studies have so far achieved real-time detection of tenofovir (< 2 min) in human serum with an approximately 1 nM detection limit and good reproducibility after regeneration, which is promising for application in continuous monitoring.
3. Ex vivo biosensing technologies for CDM
Following proven in vitro performance, more research efforts are required to investigate the potential use of the sensor in ex vivo conditions. An ex vivo environment indicates either body biofluids (e.g., sweat and interstitial fluids) collected from the human body or their artificial environment, or fresh blood drawn directly from the body via catheters.
3.1. Microfluidic electrochemical aptamer-based sensors
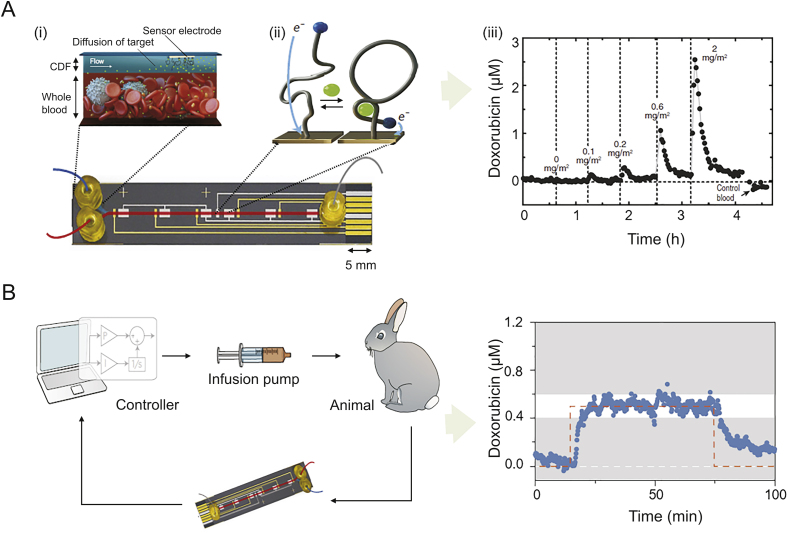
The first microchip to detect drug concentrations directly in the bloodstream of living subjects is an ex vivo microchip that accesses the bloodstream via catheters and makes continuous, sensitive and specific detection feasible by fabricating the E-AB sensor (Fig. 3Ai), along with the insertion of a continuous-flow diffusion filter (CDF) [49]. The filter prevents biofouling by excluding blood-borne interferents (e.g., albumin, immunoglobulin, and fibrinogen). The sensor achieved a LOD to doxorubicin (a chemotherapy drug) of 10 nM in buffer over a dynamic range of 0.01–10 mM. Within 45 s, the probe reached 90% saturation and then returned to within 10% of the baseline by 100 s. After intravenous injection of doxorubicin into anesthetised Sprague-Dawley rats, the sensor dosed increasing concentrations of doxorubicin (0–2 mg/m2) over a period of 4.5 h (Fig. 3Aiii). In a follow-up study, the microchip was integrated into a closed-loop system for precise control of drug concentrations in vivo [50,51]. This was fabricated by equipping the sensor with a controller and an infusion pump (Fig. 3B, left). Within 5–10 min, the system reached 95% of the concentration set point (Fig. 3B, right, dashed orange line) in live conscious rabbits, and this level could be maintained within 20% over an infusion period of approximately 2 h (Fig. 3B, right). The system automatically compensated for animal-to-animal PK variability, corrected for acute drug–drug interaction, and was easily adapted for rats with minimal modification.
Fig. 3.
A microfluidic electrochemical detector chip for ex vivo application. (A) The microchip incorporated (i) the electrochemical aptamer-based (E-AB) sensor with (ii) continuous-flow diffusion filter (CDF); (iii) real-time measurement of doxorubicin in the blood of live rats [49]. (B) Close-loop infusion control of in vivo doxorubicin concentrations by E-AB sensor. Following infusion and sampling, doxorubicin concentrations were sensed in real time and analyzed by the feedback controller. The controller automatically modulated the drug infusion rate to maintain in vivo drug levels at a target value [50].
3.2. Wearable sweat sensors
Wearable biosensors have been used for a long time for physical sensing and are attracting attention for continuous non-invasive capture of molecular data to retrieve more insightful physiological information from biofluids (e.g., sweat, tears, saliva, and interstitial fluid) [52,53]. Human sweat is perhaps the most attractive carrier of biochemical biomarkers of potential diagnostic value [54]. Many research groups across the world are actively researching sweat biosensing [[55], [56], [57], [58]]. The recent advance by Gao et al. [58] put forward a wearable, flexible sensing platform for real-time capture of multiplexed sweat metabolites, electrolytes as well as skin temperature. The utility of sweat as a base medium for drug PK studies has been well proven in previous studies, making continuous detection of drug concentrations via wearable sweat sensors feasible [59,60].
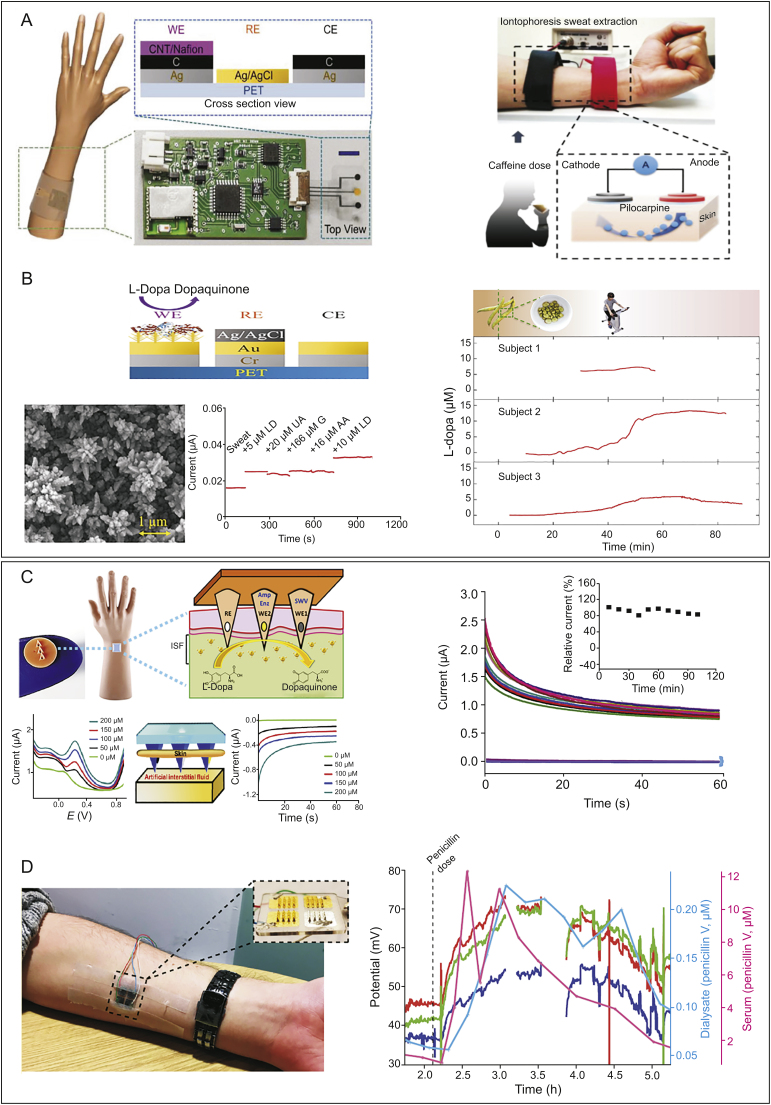
Real-time monitoring of drug levels via wearable sweat sensors was achieved in 2018 for the methylxanthine drug caffeine [61]. Caffeine has been adopted as the standard drug of choice for apnoea of prematurity [62,63]. The sensor (Fig. 4A, left) employed a triple-electrode array patterned on a flexible polyethylene terephthalate substrate interfaced with a printed circuit board, while carbon nanotube (CNTs)/nafion films were adapted on the electrode surface to prevent biofouling and simultaneously improve sensitivity. Detected via differential pulse voltammetry, the sensor recorded real-time caffeine dynamics during exercise or iontophoresis, a sweat inducing technique (Fig. 4A, right). Through a sweatband worn around a healthy volunteer’s wrist, confirmable caffeine physiological trends could be observed during exercise. This concept was very recently expanded to continuously collect levodopa in sweat to enable personalised management of Parkinson’s disease [64]. This research applied gold dendritic nanostructures onto an evaporated Au/Cr conductive layer to increase the surface sensing area (Fig. 4B, left). Levodopa was oxidised via tyrosinase enzyme and immobilised on the surface via crosslinker glutaraldehyde into dopaquinone during amperometric response (Fig. 4B, left). The sensor reached an ultralow LOD of 1.25 μM in sweat within a 0–20 μM linear range and with minimal interference from uric acid, glucose, and ascorbic acid, all within their physiologically relevant ranges (Fig. 4B, left). During prolonged exercise of around 90 min, sweat levodopa was continuously captured via the sweatband in three different human subjects after consuming 450 g of fava beans (Fig. 4B, right). Such flexible bands show promise as an effective pathway toward accurate optimisation of some other drugs in the treatment of chronic diseases.
Fig. 4.
Wearable sensors for non-invasive or minimally invasive monitoring of drugs. (A) Sweat sensors for caffeine detection: schematic of the sweat band worn on a subject’s wrist and cross-sectional view of the flexible triple-electrode sensor (left), and schematic of iontophoresis sweat extraction, in which a 5 min 1 mA current is applied to induce sweating after the subject consumed a certain amount of caffeine (right) [61]. (B) Sweat sensors for levodopa detection: cross-section view (left, upper), scanning electron microscope of the gold nanodendritic. Cross-reactivity study (levodopa (LD), uric acid (UA), glucose (G), and ascorbic acid (AA)) (left, down), and dynamic sweat levodopa concentrations for three human subjects after levodopa intake (right) [64]. (C) Microneedle sensor for levodopa detection: schematic of the sensor with two working electrodes (WE1 for non-enzymatic SWV reaction and WE2 for enzymatic amperometric reaction) (left, upper), and ex vivo evaluation in mice skin (left, down). Profile of the detection of 100 μM levodopa in artificial ISF over 2 h at 10 min intervals (right) [72]. (D) Microneedle sensor towards penicillin: photo of the microneedle arrays on a subject’s inner forearm (left). In vivo tracking of three β-lactamase sensors in a volunteer (red, green, and dark blue traces) compared to the measurements obtained in blood (taken every 15–30 min, in pink) and dialysates (taken every 15 min, in light blue) in vivo (right). Gaps in the profiles refer to times when the arrays were temporarily disconnected so that the participant could move around [73]. WE: working electrode; RE: reference electrode; CE: counter electrode; CNT: carbon nanotube; PET: poly(ethylene terephthalate); SWV: square-wave voltametric; ISF: interstitial fluid.
3.3. Wearable microneedle sensors
Interstitial fluid (ISF) is another class of biofluids that can be collected continuously and analyzed via body-interfacing devices [65]. ISF-based sensors, such as the microneedle sensor, are garnering substantial interest because of their simple, rapid, continuous, minimally invasive sensing of chemical biomarkers of the body. Microneedle arrays only penetrate the stratum corneum layer of the skin and do not cause discomfort or draw blood as they do not reach the nerve endings or capillary blood vessels in the dermis [66]. Their application is primarily in diabetes management via continuous monitoring of glucose and transdermal drug delivery [[67], [68], [69]]. ISF-based sensors are rarely used in drug monitoring. However, the ISF microenvironment provides a compartment suitable for capturing the dynamics of therapeutic drugs as well as biomarkers. The feasibility of conducting therapeutic drug monitoring (TDM) in ISF was confirmed by Kiang et al. [70] with a comprehensive panel of 13 drugs, including antibiotics, immunosuppressant drugs, anticonvulsants, and chemotherapeutic drugs. This was determined using traditional techniques.
An early attempt to design a microneedle array prototype for drug monitoring was made in 2017, for theophylline, a methylxanthine drug used as a medicine for respiratory diseases [71]. The transdermal devices, coated with an epoxy polyurethane membrane for electrode functionalisation and xanthine oxidase for oxidisation, enabled real-time tracking of theophylline in an in vitro solution condition, but were incapable of continuous operation. It was not until the year 2019 that the first microneedle sensor for real CDM was developed by Goud et al. [72]. The wearable microneedle sensor allowed parallel independent enzymatic amperometric detection and non-enzymatic square-wave voltammetric (SWV) detection of levodopa using different microneedles on the same sensor array patch (Fig. 4C, left, upper) [72]. The two microneedles, labelled WE1 and WE2, contained an unmodified and a tyrosinase-modified carbon paste electrode, respectively, upon which levodopa was oxidised to dopaquinone via redox and biocatalytic reaction. The sensor achieved a LOD of 0.5 μM for SWV and 0.25 μM for amperometry in artificial ISF. This dual-mode platform performed well whether penetrating mice skin placed on top of an artificial ISF (Fig. 4C, left, down) or a phantom gel mimicked skin, displaying high sensitivity and selectivity along with a linear current response within 0–200 μM levodopa. The sensors allowed continuous detection of levodopa in the ISF over a 2 h period with a stable response at 10 min intervals (Fig. 4C, right).
In the same year, an intradermal microneedle biosensor, highly promising as wearable, was developed for continuous tracking of penicillin (a β-lactam antibiotic) in vivo [73]. The microneedle arrays comprising four metallised sets of microneedles, three coated with gold and one with silver (Fig. 4D, left), with the gold WEs fabricated in five different layers [74].
Detection was facilitated by a pH-sensitive iridium oxide (IrOx) layer, which measured local changes in pH arising from β-lactam hydrolysis by β-lactamase, contained in an optimised enzyme hydrogel. The sensor had a LOD of 6.8 μM in 10 mM PBS, remained stable for 2 weeks at −20 °C, and withstood sterilisation (preventing potential infection in critically ill patients). During an approximately 5-h in vivo test, a healthy volunteer was asked to take five doses of penicillin (500 mg every 4–6 h), with the last dose taken just over 2 h into the study (indicated by dotted line in Fig. 4D, right).
The three sensors (marked as red, green, and dark blue traces) responded similarly, with the potential of rising after the final dose, plateauing after an hour, and gradually reducing afterwards. Penicillin levels measured by the microneedle sensors were well tracked; the levels were measured using both discrete blood and microdialysis sampling in vivo. The study still needs to establish the in vivo calibration curve, and thus, currently fails to quantify the magnitude of these changes.
The performance of some typical in vitro and ex vivo sensors is summarised in Table 1.
Table 1.
Representative in vitro and ex vivo biosensing technologies for CDM.
| Type | Sensing platforms | Surface modification | Drug | Testing medium | LOD (Linear range) | Assay time (Duration) | Comments | Refs. |
|---|---|---|---|---|---|---|---|---|
| In vitro | E-NB sensor: Graphite SPE | MWCNTs + Cytochrome P450 | Naproxen | Mouse serum | Buffer: 16 μM, serum: 33 μM (50–300 μM) | N/A (16 h) | High sensitivity, extreme simplicity, and low cost | [35] |
| E-NB sensor: PGE |
58% graphite +36% clay +5% wax |
Propofol | Buffer | 7.79 μM (1–60 μM) | N/A (4 h) | High sensitivity and prevents fouling | [36] | |
| E-NB sensor: PGE-based IoT system | 58% graphite +36% clay +5% wax |
Propofol Paracetamol |
Full human serum | N/A for both (APAP: 10–80 μM, Para: 50–250 μM) | 30 s (24 min) | Includes a PCB, a PDMS fluidic device, and an IoT network | [37] | |
| E-NB sensor: Conductive cotton fibre |
SWCNT + ion-selective membrane | Lithium | Solution; human plasma | N/A (0.40–8.00 μM) | Solution: <20 s (26 min) |
High potential for use as a wearable sensor | [38] | |
| Ex-vivo | Ex vivo E-AB sensor |
MB-modified aptamer | Doxorubicin | Humans; rats |
Buffer: 0.01 μM (nonlinear: 0.01–10.00 μM) |
45 s (4.5 h) | Antifouling continuous-flow diffusion filter used | [49] |
| Closed-loop infusion control system | MB-modified aptamer | Doxorubicin | Rabbits; rats |
N/A | 11 s (2 h) | PK variability and drug–drug interaction compensation | [50] | |
| Wearable sweat sensor | CNTs/nafion film modified carbon WE | Caffeine | Humans | 3 μM (0–40 μM) | N/A (N/A) | Dynamic drug tracking every 10 min | [61] | |
| Gold nano-dendrites, tyrosinase coated Au/Cr WE | Levodopa | Humans | 1.25 μM (0–20 μM) | N/A (∼90 min) | Surface area is increased by gold nanodendrites | [64] | ||
| Wearable microneedle sensor | Carbon paste, tyrosinase, Nafion coated WE | Levodopa | Mimicked skin | SWV: 0.50 μM Amperometry: 0.25 μM (50–250 μM) |
60 s (100 min) | SWV/chronoamperometry dual-sensing platform | [72] | |
| Iridium oxide, β-lactamase coated gold WE | Penicillin | Humans | Buffer: 6.80 μM (nonlinear: 0–5000 μM) |
N/A (>5 h) | Failed to quantify the concentrations | [73] |
APAP: paracetamol; E-NB: electrochemical nanomaterial-based; IoT: Internet of Thing; CNT: carbon nanotubes; LOD: limit of detection; MB: methylene blue; MWCNTs: multi-walled CNTs; N/A: not applied; Para: paracetamol; PCB: printed circuit board; PDMS: polydimethylsiloxane; PGE: pencil graphite electrode; PK: pharmacokinetic; SPE: screen-printed electrode; SWV: square-wave voltammetry; WE: working electrode.
4. In vivo biosensing technologies for CDM
In vivo sensors provide powerful tools for improving our understanding of inter-individual or intra-individual drug PK fluctuations through continuous physiologic monitoring, and could facilitate future advances in personalised therapy [75]. In vivo sensing can be effectively achieved via electrochemical measurement as electroactive contaminants are rare in vivo [76]. E-AB sensors perform well when challenged (in vitro and ex vivo) for hours in continuously flowing whole blood, making them a valuable anti-fouling single-step biosensor platform. Implantable sensors can measure one analyte continuously without the need for patient intervention, regardless of the patient’s physiological state. The most popular clinical application for implantable sensors is glucose sensors for patients with diabetes [[77], [78], [79]].
In vivo continuous monitoring is informative but challenging to achieve. An in vivo sensor must have a robust design to perform reliably given the harsh environment, and should meet several key parameters. These parameters include reversibility (must be responsive within relevant time intervals), sensitivity (must be within an analyte’s physiological range), selectivity (must specifically target the desired drug), and response time (must respond much faster than the analyte’s metabolising rate). Regarding development of implantable sensors, biocompatibility, implantation, and removal are the additional key issues to consider. Biocompatibility determines the functional lifetime of a sensor, which partially depends on the shape and size of a sensor and the nanomaterials used. Implantation and removal are closely related to biocompatibility and also depend on whether the implanted device is intended to circulate in the blood or to be stationary at an anatomic site [80]. Advances in nanotechnology have contributed significantly to the miniaturisation and enhanced biocompatibility of sensors and driven the development of in vivo drug sensors [81,82]. The next section illustrates recent advances in in vivo sensors for continuous study of drug PK in a live body, with special focus on how the microsystems have overcome the hurdles to biocompatibility, stability, and calibration issues.
4.1. In vivo E-AB biosensors
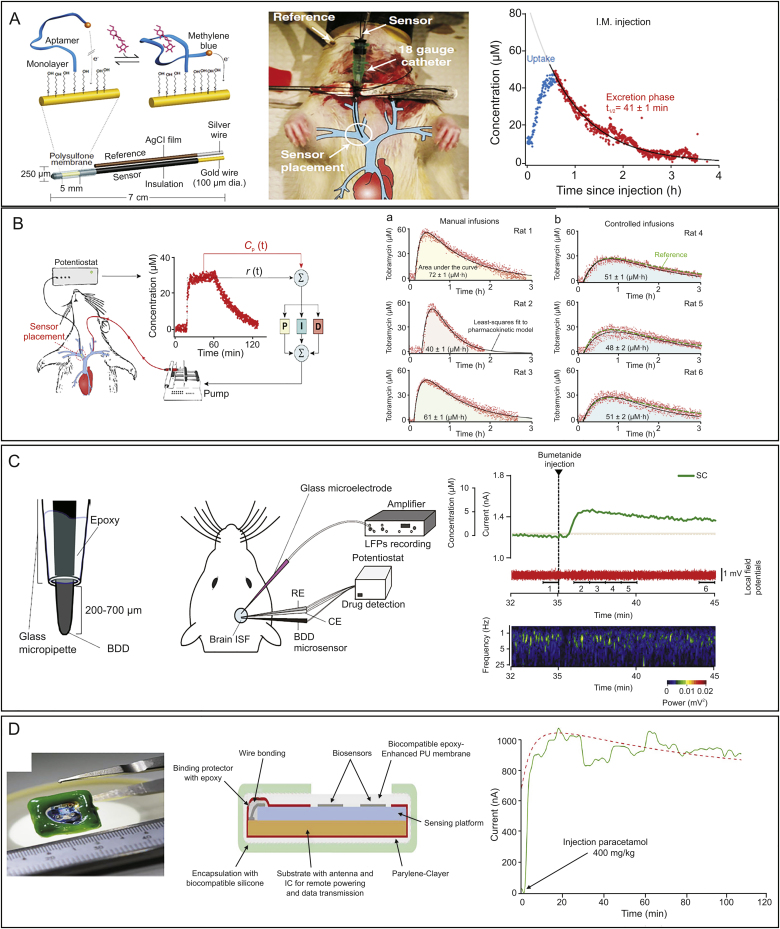
E-AB sensors have recently been critical tools in the precise investigation of in vivo drug metabolic and PK data in real time. In early 2017, the use of E-AB sensor for real-time, multi-hour detection of small-molecule drugs in awake bodies was achieved by Arroyo-Currás et al. [83]. The E-AB sensors employed a methylene blue modified freshly roughened gold electrode-bound aptamer as the sensing element to induce conformational change upon binding the analytes when interrogated via SWV (Fig. 5A, left). Furthermore, the system employed a microporous polysulfone membrane to prevent fouling from blood cells, and a kinetic differential measurements approach to correcting baseline drift. Emplaced in the external jugular vein of anesthetised rats (Fig. 5A, middle), the E-AB sensor achieved a 3 s temporal resolution for real time sensing of cancer chemotherapeutic doxorubicin. Interestingly, the in vivo sensors were able to collect precise drug (tobramycin) metabolic and PK data within the circulatory system in real time in awake, ambulatory rats while they were feeding, drinking, and exploring the environment for up to 12 h upon single (Fig. 5A, right) and sequential injections. The upper part of Table 2 summarizes the recent advances in E-AB sensors in vivo and the strategies adapted to address the key issues, such as increasing the active surface area, preventing signal gain fluctuations due to sensor-to-sensor fabrication variation, and preventing biofouling [[83], [84], [85], [86], [87]].
Fig. 5.
Continuous in vivo measurement of drug concentrations. (A) In vivo E-AB sensor for monitoring tobramycin in ambulatory animals. Mechanism (left), construction and implantation of the sensor in the external jugular vein of rats (middle), and a high-precision PK profile directly in awake, ambulatory animals while undertaking daily routine upon single I.M. injection of 25 mg/kg tobramycin (right) [83]. (B) Precise control of tobramycin in vivo by a feedback-controller dosing system. The system included an E-AB sensor (diameter, ∼300 nm) emplaced inside the right vein of a rat, a PID controller, and an infusion pump delivering drug into the left vein (left). Following a programmed PK function, the system eliminated variability between subjects in (a) drug exposure that often occurred in empirically determined doses following (b) the calculation against body surface area (right) [88]. (C) Real-time tracking of local kinetics of bumetanide and its actions in organs. Schematic of the BDD microsensor (middle), experimental setup in the rat brain (middle). and simultaneous recording of the BDD-sensor subtraction current (SC) and the brain local field potentials (LFP). 50 mg/kg bumetanide was injected into the lateral tail vein 35 min following the onset of recording. The subtraction current began to rise around 40 s after the injection (right, top). LFP data were analyzed by fast Fourier transform and shown as a dynamic spectrum, indicating the suppression of neural activity (right, bottom) [91]. (D) A fully implantable multi-panel device for remote monitoring of paracetamol in vivo in a living animal. Photo (left) and schematic of the implantable device in detail with biocompatible packaging (middle), and the in-vivo monitoring of paracetamol in the peritoneum (right) [94]. I.M.: intramuscular injection; Cp(t): measured drug concentration; r(t): time-varying reference concentration; ISF: interstitial fluid; BDD: boron-doped diamond; RE: reference electrode; CE: counter electrode; SC: subcutaneous injection; PU: polyurethane; IC: integrated circuits.
Table 2.
Representative in vivo biosensing technologies that support continuous monitoring of drug concentrations and PK profile.
| Sensing platforms | Sensing element | Target drug | Subject | Resolution time# (Duration) | Comments | Refs. |
|---|---|---|---|---|---|---|
| In vivo E-AB sensors | MB-modified aptamer | Doxorubicin Kanamycin Gentamicin Tobramycin |
Awake, ambulatory rats | 3 s (12 h) | Polysulfone membrane to prevent biofouling and kinetic differential measurement to correct drift | [83] |
| MB-modified aptamer | Tobramycin | Anesthetised rats | 7 s (3 h) | Twelve individual animals in vivo PKs (following I.M. and I.V.) were studied, with disposition variability strictly controlled | [84] | |
| MB-modified aptamer | Tobramycin | Anesthetised rats | 6 s (3 h) | Nanostructure architectures significantly increased the surface electrode area | [85] | |
| MB-modified aptamer | Tobramycin | Anesthetised rats | 300 ms (100 min) | The sensor distinguished not only the injected drug, but also the time required for the drug to mix with the blood before reaching homogeneity | [86] | |
| MB-modified aptamer | Doxorubicin | Anesthetised rats | 10 s (3 h) | The phosphatidylcholine modified monolayer effectively resisted biofouling by blood cells | [87] | |
| Closed-loop infusion control system | MB-modified aptamer | Tobramycin | Anesthetised rats | 7 s dosing rate (5 h∗) | Real-time drug biosensing activated immediate drug dosing adjustment | [88] |
| MB-modified aptamer | Vancomycin | Anesthetised rats | 9 s (5 h∗) | The first reported aptamer used against vancomycin | [89] | |
| Micro-sensing system | Boron-doped diamond | Bumetanide Lamotrigine Doxorubicin |
Guinea pigs, rats | 5 s (60 min) | Parallel tracking of drug and its effects (ototoxicity, neuronal activity) in organs | [91] |
| Implantable biochip | WE with no modification | Paracetamol | Anesthetised mice | N/A (103 min) | Multiplexing monitoring of pH, temperature, metabolite, and drug concentration | [94] |
Note: # Resolution time refers to the discrete resolution of a measurement with respect to time, indicating how often a sensor measures the drug; response time refers to how long a sensor takes to measure the drug. ∗ The duration in the feedback-controlled delivery systems refers to the period during which the drug concentrations are constantly monitored while being kept stable as adjusted by the controller. E-AB: electrochemical aptamer-based; MB: methylene blue; I.M.: intramuscular injection; I.V.: intravenous injection; PK: pharmacokinetic; WE: working electrode.
The significant advantage of E-AB sensors being an in vivo tool is that they allow precise identification of the inter and intra-animal PK variability over a multi-hour course that would otherwise be undetectable via conventional HPLC-MS methods. An in vivo study conducted on 12 individual rats revealed that there was a > 50% animal-to-animal variance in the PK parameters of distribution, elimination, and maximum plasma concentration; and none of the parameters showed a correlation with body surface area [84]. These findings may explain why in general the current body-based approaches are inaccurate and fail to achieve effective drug efficacy.
4.2. In vivo feedback-controlled dosing system
The advent of in vivo E-AB sensors empowered the development of in vivo feedback-controlled drug delivery systems [88]. The system (Fig. 5B, left) integrates selective E-AB sensors inserted into the right vein of anesthetised rats while an infusion line is inserted into the opposite vein, with a proportional-integral-derivative (PID) controller for computation of an appropriate dosing rate, and a syringe pump for delivery of the drug via intravenous infusion. Dosing is adjusted based on the difference between the drug concentration measured (Cp(t)) and a user-defined fixed or time-varying reference concentration (r(t)). By adjusting dosing every 7 s, the controller maintains precise tobramycin levels (±10%) over ∼4 h even in the face of an ∼3-fold fluctuation in the drug’s elimination rate. This also enables predefined, near-identical PK profiles from animal to animal (Fig. 5B, right). Interestingly, the system can accurately emulate the human drug exposure profiles in a rat model, which is achieved by generating a humanised rat profile using an average human PK profile, extracted from clinically-published PK data, to serve as a reference for guiding the infusion of tobramycin into a rat. This study opened up the possibility of investigating interspecies metabolic differences and evaluating the effects of human-mimicked PK profiles on human diseases being studied in animal models. High-precision feedback control of vancomycin concentration in vivo has also been achieved very recently, with defined plasma vancomycin rapidly achieved (∼30 min) and maintained at precise levels for over 5 h regardless of significant hour-to-hour changes in drug metabolism [89].
4.3. Boron-doped diamond drug microsensor system
Evaluation of drug kinetics in organs is essential. The reason is that differential spatial and temporal drug distribution occurs through not only the human body but also the cellular microenvironments of each organ following systemic administration [90].
Ogata et al. [91] developed a drug-sensing system supporting real-time monitoring of both drugs in in vivo and the respective organ functions over time. The microsystem comprises a conductive boron-doped diamond (BDD) with an ∼40 μm tip diameter sensor for drug detection, a double-barrelled glass microcapillary glass microelectrode that monitors extra or intracellular electrical parameters resulting from ion-channel or ion-transporter activity (Fig. 5C, left). In the live rat brain (Fig. 5C, middle), the BDD microsensor accurately tracked the kinetics of bumetanide (a diuretic that can be ototoxic) over approximately 10 min, with the local field potentials representing neuronal activity being monitored via the microelectrode in parallel (Fig. 5C, right). Bumetanide concentrations within 0.6 and 5.7 μM could be sensitively detected. The system revealed a considerable difference between the local peak bumetanide concentrations in brains (measured via the BDD sensor) and systemic peak bumetanide plasma concentrations (measured via HPLC-MS/MS). The microsystem can equally be used to simultaneously monitor bumetanide concentrations in the guinea pig cochlea and its impact on hearing, with a 5 s time resolution, and similarly, to monitor the kinetics and actions of anti-epileptic lamotrigine and anticancer doxorubicin in organs. A significant advantage of this microsystem lies in its capability to track pharmacological and physiological responses that otherwise might remain undetected by conventional low-precision techniques. If used in earlier clinical trials for testing a drug candidate, the microsystem would facilitate the precise investigation of the maximal drug efficacy while trying to maintain minimal adverse events [92].
4.4. Fully implantable biochip
Increasing attention in healthcare is focused on multiplexing as disease occurrence and the therapeutic effects of drugs are often a result of an interplay between a variety of complex biological networks, including a large set of biological molecules [93]. Fully integrated biochip platforms are a promising tool for achieving multiplexing.
Baj-Rossi et al. [94] developed a fully implantable biosensor array equipped for continuous monitoring of glucose and drug paracetamol (a pain reliever and fever reducer) in freely moving animals. The 1-cm square device (Fig. 5D, left) is composed of three main components: a sensing platform containing four individual electrochemical sensors, three integrated circuits (the sensor’s front-end circuit, a microcontroller, and the power/data manager), and a powering antenna [95]. Proper integration with the surrounding tissue was achieved by encapsulating the sensor within an external biocompatible packaging (Fig. 5D, middle). In vitro evaluation revealed a LOD of (34 ± 11) μM within 0–300 mΜ of paracetamol. Implantation of this device in the peritoneum of live mice, unfortunately, caused inflammation after 30 days due to a foreign body response, represented by relatively high levels of ATP and neutrophils, both serving as inflammatory mediators. In vivo evaluation was performed in the peritoneum of mice by injecting 400 mg/kg of paracetamol and the time/current profile obtained (Fig. 5D, right) showed that the signal rose after the injection and slowly reduced after around 30 min of measurements, with the trend roughly highlighted in the red dashed line. During the test, the mice could move freely without a wire tracker getting in the way, which caused noise. Because paracetamol was determined in the peritoneal fluid rather than in bloodstream, the research obtained only a nontypical PK profile at that time.
Similarly, Hammoud et al. [96] designed an implantable biosensor platform prototype in an attempt to achieve continuous monitoring of anti-epileptic drugs in real time by integrating: (i) the front-end circuit, hosting an inductive coil for absorbing energy from an external base station and communicating data to the base station; (ii) the power management block; (iii) the read-out and control block; and (iv) the biosensor array. This implantable microsystem was intended for real-time monitoring of carbamazepine concentrations in vitro at the time, and its CDM capability remains to be proved.
The performance of some typical in vivo sensors is summarised in Table 2.
5. Discussion and conclusions
This study highlights the importance of continuous monitoring of therapeutic drugs in real time and reviews recent advances in biosensing technologies, from in vitro, ex vivo to in vivo, towards achieving this goal. Wearable sensors and in vivo sensors support direct, dynamic, and continuous biosensing of drug concentrations in vivo, and nanomaterial-based electrochemical biosensors are currently used only in an in vitro environment. In particular, wearable sensors evidently present a promising and exciting tool for drug detection as the entire process is executed automatically, continuously, and non-invasively or is at least minimally invasive. Previously, wearable sensors were also used in point-of-care and in situ detection of suspected toxicity or drug abuse via sensing at the fingertips in a wearable glove [[97], [98], [99]]. In contrast, E-AB sensors are being proven as a universal approach by which an arbitrary molecular analyte can be monitored continuously. This capability is enabled by a high temporal resolution and independence of chemical reactivity towards the target. The seconds-resolved measurement frequency of E-AB sensors yields many hundreds of data points collected per PK profile, in contrast to the merely several time points PK produced via HPLC-MS/MS [84] and ELISAs. Table 3 outlines the key performance and functions of currently-available CDM technologies against therapeutic drug monitoring technologies. Clearly, traditional methods are inadequate for continuous sensing due to their being cumbersome and painful, and because of the need for recurring sampling, which may cause infection, hematoma, bruising, and in rare cases wounds may bleed excessively (this also applies to fingerpicked blood spots) [100,101].
Table 3.
Comparison of currently available CDM technologies with therapeutic drug monitoring (TDM) technologies.
| Items | TDM | CDM |
|---|---|---|
| Representative technologies | Classic tests: HPLC-MS, ELISA Rapid tests: lateral flow assay, fiber optic SPR |
E-AB sensors, wearable sensors, implantable sensors |
| Sensor size | Bulky or portable | Micro or nanoscale |
| Detection time | Classic tests: hours to a day Rapid tests: 10–20 min |
Seconds |
| Detection matrix | Pre-prediluted blood, serum, or plasma | Bloodstream, sweat, or interstitial fluid |
| Detection environment | In vitro | In vitro, ex vivo, and in vivo |
| Detection process | Multistep batch process | Label-free, reagent-free, wash-free |
| Development criteria to consider | Specificity, sensitivity, repeatability, etc. | Specificity, sensitivity, reproducibility, reusability, long-term stability against biofouling, compatibility, integration, etc. |
| Availability | Commercially available | In proof-of-concept stage |
| roles in individualised therapy |
|
|
| Challenges |
|
|
HPLC-MS: high performance liquid chromatography-mass spectrometry; ELISA: enzyme linked immunosorbent assay; E-AB: electrochemical aptamer-based; SPR: surface plasma resonance; PK: pharmacokinetic.
This review illustrates the technologies that have shown clear evidence of realising CDM via electronic and electrochemical signals. There are other alternative technologies that have great potential to achieve CDM in the near future. In a very recent research from KU Leuven, the researchers carefully discuss the strategies for using label-free surface plasma resonance (SPR) based biosensors to achieve continuous or in vivo monitoring of therapeutic drugs [102]. Another study on a multisensor-organs-on-chips platform demonstrates its capacity to constantly determine drug concentration and the drug’s effect on human organs (e.g., liver, heart or brain) along with a simultaneous evaluation of microenvironment parameters (pH, temperature) [103]. Nanoscale field-effect transistor-based biosensors are also promising because the primary challenge, Debye length limitations, has been adequately addressed in several recent studies via the use of polymer polyethylene glycol [104], or cross-linked polymer networked hydrogel [105] or aptamers [106].
The development of wearable and in vivo sensors to realise CDM is challenging. Three main issues should be taken into account when developing an in vivo sensing system: (i) quality (depending on the biocompatibility or inflammation due to the presence of the sensor inside the body, often a result of the sensor shape, size, and the nanomaterials used); (ii) calibration performance (including the specificity, sensitivity, and time resolution of the sensor); and (iii) stability of the sensor (depending on the fouling effects and the sufficiency of the supply power). In the preceding studies, devices were designed in small size, e.g., the < 300 μm diameter electrodes in E-AB sensors and the 1-cm square implantable chip. An external biocompatible integrating package guarantees proper integration of the sensor with its surrounding environment. Biofouling, the result of the accumulation of proteins, cells, or macromolecules on the sensors’ surface through non-specific binding, was addressed using polysulfone membranes, a phosphatidylcholine modified monolayer, or miniaturised diffusion filters. These prevent blood cells from approaching the sensor surface while enabling rapid transport of target molecules. Strategies for reducing biofouling can be learned from an earlier review and two very recent studies [[107], [108], [109]]. The key issues and strategies for developing fully integrated biochips platform for advanced healthcare have been carefully discussed in an earlier study by Carrara et al. [110], which covers nanomaterials for improving device sensitivity, selecting and immobilising bioreceptors, integration of bio and nanomaterials onto micro-arrays, electronics front-end, remote powering, device biocompatibility, and data security.
6. Future perspectives
CDM is promising for considerably improving TDM and reshaping our understanding of individual PK variation. It helps us understand how real drug concentrations change inside the body with maximum precision and to evaluate the potential effects of a given drug on the organs. Furthermore, when integrated with a feedback controller, it empowers us to adjust and maintain optimised drug dosing within its therapeutic window in response to minute-to-minute concentration changes in vivo. Given ongoing efforts in miniaturisation, in vivo sensors will evolve, to their full capacities, into implantable sensors in subsequent years. Together with wearable sensors, they will serve as critical tools for the continuous biosensing of drug concentrations in real time, with the final healthcare goal being to achieve remote, or even automated disease monitoring and management. As thus, we believe that CDM may become a future tool for achieving enhanced individualised therapy. However, wearable, implantable sensors and E-AB sensors are still in their proof-of-concept stages for application to drug determination. Effort is required to make the sensors more robust for use in complex biomatrices and further integrated for insertion into live bodies. Key parameters to improve the sensors’ robustness include specificity, sensitivity, reproducibility, reusability, long-term stability against biofouling and biocompatibility. Furthermore, several key issues should be addressed to make the sensors more applicable and the idea of CDM as a future tool for individualised therapy more promising and feasible.
The first issue is to enlarge the application of wearable and E-AB sensors into more drugs that have clearly shown their clinical needs of therapeutic drug monitoring to optimise dosing. This can be very beneficial to management of more types of diseases. Strategies may include the production of a variety of high-quality aptamers for use in E-AB sensors. The second related issue is clinical validity. The clinical validity of in vivo drug sensors has only recently been evaluated in animals. The next step is clinically validation on humans, during which one thing to consider is specie-to-specie metabolism variation towards a given drug. At the very later stage, extensive clinical trials are required if a drug sensor, being in vivo or wearable, is to enter the market. The successful entry of continuous glucose sensors into clinical practice is an excellent precedent for learning to transfer state-of-the-art CDM technologies. The last key issue is the vast amount of data captured during continuous drug sensing. This clearly requires attention to private data confidentiality, and big data processing and management. In recent years we have witnessed explosive developments in artificial intelligence (AI) and its applications in drug discovery and development. The significance of AI in making CDM a promising tool is twofold: (i) AI facilitates the analysis of the signals mass-produced during continuous operation at an utmost speed while automatedly reducing background noise signals; (ii) AI is good at predicting and analyzing complicated relationships, and thus, pharmaceutical scientists will be able to precisely mine the collected data to create accurate decision-making algorithms that can effectively guide clinically oriented investigations and deliver personalised therapy.
The development of CDM technologies requires the combination of much multidisciplinary knowledge and expertise from areas such as the pharmaceutical sciences, electrical engineering, surface biochemistry, nanotechnology, medicine, and AI. With joint efforts, we believe that CDM will usher in an era of smart, automated, real-time, continuous management of the state of patients with a more precise personalised therapy.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work was supported by the start-up funds from Westlake University to CenBRAIN lab and Bright Dream Joint Institute for Intelligent Robotics.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
References
- 1.Hodson R. Precision medicine. Nature. 2016;537:S49. doi: 10.1038/537S49a. [DOI] [PubMed] [Google Scholar]
- 2.Birkett D.J. Therapeutic drug monitoring. Aust. Prescr. 1977;20:9–11. [Google Scholar]
- 3.Gross A.S. Best practice in therapeutic drug monitoring. Br. J. Clin. Pharmacol. 2002;46:95–99. doi: 10.1046/j.1365-2125.1998.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Figueras A. Fundació Institut Català de Farmacologia; Barcelona, Spain: 2019. Review of the evidence to include TDM in the Essential in vitro Diagnostics List and prioritization of medicines to be monitored.https://www.who.int/medical_devices/diagnostics/selection_in-vitro/selection_in-vitro-meetings/sage-ivd-2nd-meeting/Report-on-TherapeuticDrugMonitoring-tests.pdf (accessed on 25 September, 2020) [Google Scholar]
- 5.Feuerstein J.D., Nguyen G.C., Kupfer S.S. American Gastroenterological Association Institute guideline on therapeutic drug monitoring in inflammatory bowel disease. Gastroenterology. 2017;153:827–834. doi: 10.1053/j.gastro.2017.07.032. [DOI] [PubMed] [Google Scholar]
- 6.Vande Casteele N., Herfarth H., Katz J. American Gastroenterological Association Institute technical review on the role of therapeutic drug monitoring in the management of inflammatory bowel diseases. Gastroenterology. 2017;153:835–857. doi: 10.1053/j.gastro.2017.07.031. e6. [DOI] [PubMed] [Google Scholar]
- 7.Eliasson E., Lindh J.D., Malmström R.E. Therapeutic drug monitoring for tomorrow. Eur. J. Clin. Pharmacol. 2013;69(Suppl 1):25–32. doi: 10.1007/s00228-013-1504-x. [DOI] [PubMed] [Google Scholar]
- 8.Hiemke C. Clinical utility of drug measurement and pharmacokinetics: therapeutic drug monitoring in psychiatry. Eur. J. Clin. Pharmacol. 2008;64:159–166. doi: 10.1007/s00228-007-0430-1. [DOI] [PubMed] [Google Scholar]
- 9.Adaway J.E., Keevil B.G. Therapeutic drug monitoring and LC–MS/MS. J. Chromatogr. B. 2012;883–884:33–49. doi: 10.1016/j.jchromb.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 10.Bian S., Van Stappen T., Baert F. Generation and characterization of a unique panel of anti-adalimumab specific antibodies and their application in therapeutic drug monitoring assays. J. Pharmaceut. Biomed. Anal. 2016;125:62–67. doi: 10.1016/j.jpba.2016.03.029. [DOI] [PubMed] [Google Scholar]
- 11.Verstockt B., Moors G., Bian S. Influence of early adalimumab serum levels on immunogenicity and long-term outcome of anti-TNF naïve Crohn’s disease patients: the usefulness of rapid testing. Aliment. Pharmacol. Ther. 2018;48:731–739. doi: 10.1111/apt.14943. [DOI] [PubMed] [Google Scholar]
- 12.Boyer A., Gruson D., Bouchet S. Aminoglycosides in septic shock: an overview, with specific consideration given to their nephrotoxic risk. Drug Saf. 2013;36:217–230. doi: 10.1007/s40264-013-0031-0. [DOI] [PubMed] [Google Scholar]
- 13.Li X., Geng M., Peng Y. Molecular immune pathogenesis and diagnosis of COVID-19. J. Pharm. Anal. 2020;10:102–108. doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen M.Z., Zhou Y., Ye J.W. Recent advances and perspectives of nucleic acid detection for coronavirus. J. Pharm. Anal. 2020;10:97–101. doi: 10.1016/j.jpha.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bian S., Lu J., Delport F. Development and validation of a device for rapid monitoring of adalimumab in serum of patients with Crohn’s disease. Drug Test. Anal. 2018;10:592–596. doi: 10.1002/dta.2250. [DOI] [PubMed] [Google Scholar]
- 16.Aspinall M.G., Hamermesh R.G. Realizing the promise of personalized medicine. Harv. Bus. Rev. 2007;85:108–117. 165. [PubMed] [Google Scholar]
- 17.Wang J. Amperometric biosensors for clinical and therapeutic drug monitoring: a review. J. Pharmaceut. Biomed. Anal. 1999;19:47–53. doi: 10.1016/s0731-7085(98)00056-9. [DOI] [PubMed] [Google Scholar]
- 18.Landmark C.J., Johannessen S.I., Tomson T. Dosing strategies for antiepileptic drugs: from a standard dose for all to individualised treatment by implementation of therapeutic drug monitoring. Epileptic Disord. 2016;18:367–383. doi: 10.1684/epd.2016.0880. [DOI] [PubMed] [Google Scholar]
- 19.Fantana A.L., Cella G.M., Benson C.T. The future of drug trials Is better data and continuous monitoring. Harv. Bus. Rev. 2019 https://hbr.org/2019/05/the-future-of-drug-trials-is-better-data-and-continuous-monitoring [Google Scholar]
- 20.Ates H.C., Roberts J.A., Lipman J. On-Site Therapeutic drug monitoring. Trends Biotechnol. 2020;38:1262–1277. doi: 10.1016/j.tibtech.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Vozeh S., Steimer J.L. Feedback control methods for drug dosage optimisation. Concepts, classification and clinical application. Clin. Pharmacokinet. 1985;10:457–476. doi: 10.2165/00003088-198510060-00001. [DOI] [PubMed] [Google Scholar]
- 22.Jakka S., Rossbach M. An economic perspective on personalized medicine. HUGO J. 2013;7:1. doi: 10.1186/1877-6566-7-1. [DOI] [Google Scholar]
- 23.Hammoud A., Khoa Nguyen D.K., Sawan M. Detection methods and tools of administered anti-epileptic drugs - a review. Biosens. Bioelectron. Open Acc. 2019 doi: 10.29011/2577-2260.100046. [DOI] [Google Scholar]
- 24.Ronkainen N.J., Halsall H.B., Heineman W.R. Electrochemical biosensors. Chem. Soc. Rev. 2010;39:1747–1763. doi: 10.1039/b714449k. [DOI] [PubMed] [Google Scholar]
- 25.Database of Market Research Report: "Biosensors market size, share & trends analysis report by application by technology by end use and segment forecasts, 2019 - 2026". Grand View Research. https://www.reportlinker.com/p05807287/Biosensors-Market-Size-Share-Trends-Analysis-Report-By-Application-By-Technology-By-End-Use-And-Segment-Forecasts.html (accessed on 25 September, 2020)
- 26.Kwak S.Y., Wong M.H., Lew T.T.S. Nanosensor technology applied to living plant systems. Annu. Rev. Anal. Chem. (Palo Alto Calif) 2017;10:113–140. doi: 10.1146/annurev-anchem-061516-045310. [DOI] [PubMed] [Google Scholar]
- 27.Rong G., Tuttle E.E., Neal Reilly A. Recent developments in nanosensors for imaging applications in biological systems. Annu. Rev. Anal. Chem. (Palo Alto Calif) 2019;12:109–128. doi: 10.1146/annurev-anchem-061417-125747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rong G., Corrie S.R., Clark H.A. In vivo biosensing: progress and perspectives. ACS Sens. 2017;2:327–338. doi: 10.1021/acssensors.6b00834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yokus M.A., Songkakul T., Pozdin V.A. Wearable multiplexed biosensor system toward continuous monitoring of metabolites. Biosens. Bioelectron. 2020;153:112038. doi: 10.1016/j.bios.2020.112038. [DOI] [PubMed] [Google Scholar]
- 30.Lee H., Song C., Hong Y. Wearable/disposable sweat-based glucose monitoring device with multistage transdermal drug delivery module. Sci. Adv. 2017;3 doi: 10.1126/sciadv.1601314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Majdinasab M., Mitsubayashi K., Marty J.L. Optical and electrochemical sensors and biosensors for the detection of quinolones. Trends Biotechnol. 2019;37:898–915. doi: 10.1016/j.tibtech.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Grieshaber D., MacKenzie R., Vörös J. Electrochemical biosensors - sensor principles and architectures. Sensors. 2008;8:1400–1458. doi: 10.3390/s80314000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carrara S. Springer; New York: 2013. Bio/CMOS Interfaces and Co-design; pp. 185–205. [Google Scholar]
- 34.Maduraiveeran G., Sasidharan M., Ganesan V. Electrochemical sensor and biosensor platforms based on advanced nanomaterials for biological and biomedical applications. Biosens. Bioelectron. 2018;103:113–129. doi: 10.1016/j.bios.2017.12.031. [DOI] [PubMed] [Google Scholar]
- 35.Baj-Rossi C., Rezzonico Jost T., Cavallini A. Continuous monitoring of naproxen by a cytochrome P450-based electrochemical sensor. Biosens. Bioelectron. 2014;53:283–287. doi: 10.1016/j.bios.2013.09.058. [DOI] [PubMed] [Google Scholar]
- 36.Stradolini F., Kilic T., Di Consiglio A. Long-term monitoring of propofol and fouling effect on pencil graphite electrodes. Electroanalysis. 2018;30:1363–1369. [Google Scholar]
- 37.Stradolini F., Tuoheti A., Kilic T. An IoT solution for online monitoring of anesthetics in human serum based on an integrated fluidic bioelectronic system. IEEE Trans. Biomed. Circuits Syst. 2018;12:1056–1064. doi: 10.1109/TBCAS.2018.2855048. [DOI] [PubMed] [Google Scholar]
- 38.Sweilam M.N., Varcoe J.R., Crean C. Fabrication and optimization of fiber-based lithium sensor: a step toward wearable sensors for lithium drug monitoring in interstitial fluid. ACS Sens. 2018;3:1802–1810. doi: 10.1021/acssensors.8b00528. [DOI] [PubMed] [Google Scholar]
- 39.Ellington A.D., Szostak J.W. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990;346:818–822. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]
- 40.Tuerk C., Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990;249:505–510. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- 41.Xiao Y., Lai R.Y., Plaxco K.W. Preparation of electrode-immobilized, redox-modified oligonucleotides for electrochemical DNA and aptamer-based sensing. Nat. Protoc. 2007;2:2875–2880. doi: 10.1038/nprot.2007.413. [DOI] [PubMed] [Google Scholar]
- 42.Nimjee S.M., White R.R., Becker R.C. Aptamers as therapeutics. Annu. Rev. Pharmacol. Toxicol. 2017;57:61–79. doi: 10.1146/annurev-pharmtox-010716-104558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dehghani S., Nosrati R., Yousefi M. Aptamer-based biosensors and nanosensors for the detection of vascular endothelial growth factor (VEGF): a review. Biosens. Bioelectron. 2018;110:23–37. doi: 10.1016/j.bios.2018.03.037. [DOI] [PubMed] [Google Scholar]
- 44.Swensen J.S., Xiao Y., Ferguson B.S. Continuous, real-time monitoring of cocaine in undiluted blood serum via a microfluidic, electrochemical aptamer-based sensor. J. Am. Chem. Soc. 2009;131:4262–4266. doi: 10.1021/ja806531z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li H., Arroyo-Currás N., Kang D. Dual-reporter drift correction to enhance the performance of electrochemical aptamer-based sensors in whole blood. J. Am. Chem. Soc. 2016;138:15809–15812. doi: 10.1021/jacs.6b08671. [DOI] [PubMed] [Google Scholar]
- 46.Li H., Dauphin-Ducharme P., Ortega G. Calibration-free electrochemical biosensors supporting accurate molecular measurements directly in undiluted whole blood. J. Am. Chem. Soc. 2017;139:11207–11213. doi: 10.1021/jacs.7b05412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aliakbarinodehi N., Jolly P., Bhalla N. Aptamer-based field-effect biosensor for tenofovir detection. Sci. Rep. 2017;7:44409. doi: 10.1038/srep44409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tzouvadaki I., Aliakbarinodehi N., De Micheli G. The memristive effect as a novelty in drug monitoring. Nanoscale. 2017;9:9676–9684. doi: 10.1039/c7nr01297g. [DOI] [PubMed] [Google Scholar]
- 49.Ferguson B.S., Hoggarth D.A., Maliniak D. Real-time, aptamer-based tracking of circulating therapeutic agents in living animals, Sci. Transl. Med. 2013;5:213ra165. doi: 10.1126/scitranslmed.3007095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mage P.L., Ferguson B.S., Malinia D. Closed-loop control of circulating drug levels in live animals. Nat. Biomed. Eng. 2017;1 0070. [Google Scholar]
- 51.Karnik R. Drug delivery: closed-loop dynamic dosing. Nat. Biomed. Eng. 2017;1 [Google Scholar]
- 52.Kim J., Campbell A.S., de Ávila B.E. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019;37:389–406. doi: 10.1038/s41587-019-0045-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yetisen A.K., Martinez-Hurtado J.L., Ünal B. Wearables in medicine. Adv. Mater. 2018;30:e1706910. doi: 10.1002/adma.201706910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mena-Bravo A., Luque de Castro M.D. Sweat: a sample with limited present applications and promising future in metabolomics. J. Pharmaceut. Biomed. Anal. 2014;90:139–147. doi: 10.1016/j.jpba.2013.10.048. [DOI] [PubMed] [Google Scholar]
- 55.Kim J., Sempionatto J.R., Imani S. Simultaneous monitoring of sweat and interstitial fluid using a single wearable biosensor platform. Adv. Sci. (Weinh). 2018;5:1800880. doi: 10.1002/advs.201800880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brothers M.C., DeBrosse M., Grigsby C.C. Achievements and challenges for real-Time sensing of analytes in sweat within wearable platforms. Acc. Chem. Res. 2019;52:297–306. doi: 10.1021/acs.accounts.8b00555. [DOI] [PubMed] [Google Scholar]
- 57.Bandodkar A., Jeang W.J., Ghaffari R. Wearable sensors for biochemical sweat analysis. Annu. Rev. Anal. Chem. (Palo Alto Calif) 2019;12:1–22. doi: 10.1146/annurev-anchem-061318-114910. [DOI] [PubMed] [Google Scholar]
- 58.Gao W., Emaminejad S., Nyein H.Y.Y. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature. 2016;529:509–514. doi: 10.1038/nature16521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Raju K.S., Taneja I., Singh S.P. Utility of noninvasive biomatrices in pharmacokinetic studies. Biomed. Chromatogr. 2013;27:1354–1366. doi: 10.1002/bmc.2996. [DOI] [PubMed] [Google Scholar]
- 60.Tsunoda M., Hirayama M., Tsuda T. Noninvasive monitoring of plasma L-dopa concentrations using sweat samples in Parkinson’s disease. Clin. Chim. Acta. 2015;442:52–55. doi: 10.1016/j.cca.2014.12.032. [DOI] [PubMed] [Google Scholar]
- 61.Tai L.C., Gao W., Chao M. Methylxanthine drug monitoring with wearable sweat sensors. Adv. Mater. 2018;30 doi: 10.1002/adma.201707442. [DOI] [PubMed] [Google Scholar]
- 62.Gal P. Caffeine therapeutic drug monitoring is necessary and cost-effective. J. Pediatr. Pharmacol. Therapeut. 2007;12:212–215. doi: 10.5863/1551-6776-12.4.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Comer A.M., Perry C.M., Figgitt D.P. Caffeine citrate: a review of its use in apnoea of prematurity. Paediatr. Drugs. 2001;3:61–79. doi: 10.2165/00128072-200103010-00005. [DOI] [PubMed] [Google Scholar]
- 64.Tai L.C., Liaw T.S., Lin Y. Wearable sweat band for noninvasive levodopa monitoring. Nano Lett. 2019;9:6346–6351. doi: 10.1021/acs.nanolett.9b02478. [DOI] [PubMed] [Google Scholar]
- 65.Zhao J., Guo H.X., Li J.H. Body-interfaced chemical sensors for noninvasive monitoring and analysis of biofluids. Trends Chem. 2019;1:559–571. [Google Scholar]
- 66.Lee K.J., Jeong S.S., Roh D.H. A practical guide to the development of microneedle systems - in clinical trials or on the market. Int. J. Pharm. 2020;573:118778. doi: 10.1016/j.ijpharm.2019.118778. [DOI] [PubMed] [Google Scholar]
- 67.Lee H., Choi T.K., Lee Y.B. A graphene-based electrochemical device with thermo-responsive microneedles for diabetes monitoring and therapy. Nat. Nanotechnol. 2016;11:566–572. doi: 10.1038/nnano.2016.38. [DOI] [PubMed] [Google Scholar]
- 68.Wang H., Pastorin G., Lee C.K. Toward self-powered wearable adhesive skin patch with bendable microneedle array for transdermal drug delivery. Adv. Sci. (Weinh) 2016;3:1500441. doi: 10.1002/advs.201500441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim Y.C., Park J.H., Prausnitz M.R. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 2012;64:1547–1568. doi: 10.1016/j.addr.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kiang T.K., Schmitt V., Ensom M.H. Therapeutic drug monitoring in interstitial fluid: a feasibility study using a comprehensive panel of drugs. J. Pharm. Sci. 2012;101:4642–4652. doi: 10.1002/jps.23309. [DOI] [PubMed] [Google Scholar]
- 71.Sharma S., Saeed A., Johnson C. Rapid, low cost prototyping of transdermal devices for personal healthcare monitoring. Sens. Biosensing Res. 2017;13:104–108. doi: 10.1016/j.sbsr.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goud K.Y., Moonla C., Mishra R.K. Wearable electrochemical microneedle sensor for continuous monitoring of levodopa: toward Parkinson management. ACS Sens. 2019;4:2196–2204. doi: 10.1021/acssensors.9b01127. [DOI] [PubMed] [Google Scholar]
- 73.Gowers S.A.N., Freeman D.M.E., Rawson T.M. Development of a minimally invasive microneedle-based sensor for continuous monitoring of β-lactam antibiotic concentrations in vivo. ACS Sens. 2019;4:1072–1080. doi: 10.1021/acssensors.9b00288. [DOI] [PubMed] [Google Scholar]
- 74.Sharma S., Ahmed El-Laboudi A., Reddy M. A pilot study in humans of microneedle sensor arrays for continuous glucose monitoring. Anal. Methods. 2018;10:2088–2095. [Google Scholar]
- 75.Eckert M.A., Vu P.Q., Zhang K. Novel molecular and nanosensors for in vivo sensing. Theranostics. 2013;3:583–594. doi: 10.7150/thno.6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Plaxco K.W., Soh H.T. Switch-based biosensors: a new approach towards real-time, in vivo molecular detection. Trends Biotechnol. 2011;29:1–5. doi: 10.1016/j.tibtech.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wilson G.S., Gifford R. Biosensors for real-time in vivo measurements. Biosens. Bioelectron. 2005;20:2388–2403. doi: 10.1016/j.bios.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 78.Vaddiraju S., Tomazos I., Burgess D.J. Emerging synergy between nanotechnology and implantable biosensors: a review. Biosens. Bioelectron. 2010;25:1553–1565. doi: 10.1016/j.bios.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Deiss D., Irace C., Carlson G. Real-world safety of an implantable continuous glucose sensor over multiple cycles of use: a post-market registry study. Diabetes Technol. Therapeut. 2020;22:48–52. doi: 10.1089/dia.2019.0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ruckh T.T., Clark H.A. Implantable nanosensors: toward continuous physiologic monitoring. Anal. Chem. 2014;86:1314–1323. doi: 10.1021/ac402688k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nichols S.P., Koh A., Storm W.L. Biocompatible materials for continuous glucose monitoring devices. Chem. Rev. 2013;113:2528–2549. doi: 10.1021/cr300387j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sanati A., Jalali M., Raeissi K. A review on recent advancements in electrochemical biosensing using carbonaceous nanomaterials. Mikrochim. Acta. 2019;186:773. doi: 10.1007/s00604-019-3854-2. [DOI] [PubMed] [Google Scholar]
- 83.Arroyo-Currás N., Somerson J., Vieira P.A. Real-time measurement of small molecules directly in awake, ambulatory animals. Proc. Natl. Acad. Sci. U.S.A. 2017;114:645–650. doi: 10.1073/pnas.1613458114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vieira P.A., Shin C.B., Arroyo-Currás N. Ultra-high-precision, in-vivo pharmacokinetic measurements highlight the need for and a route toward more highly personalized medicine. Front. Mol. Biosci. 2019;6:69. doi: 10.3389/fmolb.2019.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Arroyo-Currás N., Scida K., Ploense K.L. High surface area electrodes generated via electrochemical roughening improve the signaling of electrochemical aptamer-based biosensors. Anal. Chem. 2017;89:12185–12191. doi: 10.1021/acs.analchem.7b02830. [DOI] [PubMed] [Google Scholar]
- 86.Arroyo-Currás N., Dauphin-Ducharme P., Ortega G. Subsecond-resolved molecular measurements in the living body using chronoamperometrically interrogated aptamer-based sensors. ACS Sens. 2018;3:360–366. doi: 10.1021/acssensors.7b00787. [DOI] [PubMed] [Google Scholar]
- 87.Li H., Dauphin-Ducharme P., Arroyo-Currás N. A biomimetic phosphatidylcholine-terminated monolayer greatly improves the in vivo performance of electrochemical aptamer-based sensors. Angew. Chem., Int. Ed. Engl. 2017;56:7492–7495. doi: 10.1002/anie.201700748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Arroyo-Currás N., Ortega G., Copp D.A. High-precision control of plasma drug levels using feedback-controlled dosing. ACS Pharmacol. Transl. Sci. 2018;1:110–118. doi: 10.1021/acsptsci.8b00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dauphin-Ducharme P., Yang K., Arroyo-Currás N. Electrochemical aptamer-based sensors for improved therapeutic drug monitoring and high-precision, feedback-controlled drug delivery. ACS Sens. 2019;4:2832–2837. doi: 10.1021/acssensors.9b01616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Weiss M. Pharmacokinetics in organs and the intact body: model validation and reduction. Eur. J. Pharm. Sci. 1999;7:119–127. doi: 10.1016/s0928-0987(98)00014-1. [DOI] [PubMed] [Google Scholar]
- 91.Ogata G., Ishii Y., Asai K. A microsensing system for the in vivo real-time detection of local drug kinetics. Nat. Biomed. Eng. 2017;1:654–666. doi: 10.1038/s41551-017-0118-5. [DOI] [PubMed] [Google Scholar]
- 92.Li C.Y., Narayan R.K. Real-time drug pharmacokinetics. Nat. Biomed. Eng. 2017;1:627–628. doi: 10.1038/s41551-017-0122-9. [DOI] [PubMed] [Google Scholar]
- 93.Spindel S., Sapsford K.E. Evaluation of optical detection platforms for multiplexed detection of proteins and the need for point-of-care biosensors for clinical use. Sensors (Basel) 2014;14:22313–22341. doi: 10.3390/s141222313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Baj-Rossi C., Cavallini A., Kilinc E.G. In-vivo validation of fully implantable multi-panel devices for remote monitoring of metabolism. IEEE Trans. Biomed. Circuits Syst. 2016;10:955–962. doi: 10.1109/TBCAS.2016.2584239. [DOI] [PubMed] [Google Scholar]
- 95.Baj-Rossi C., Kilinc E.G., Ghoreishizadeh S.S. Full fabrication and packaging of an implantable multi-panel device for monitoring of metabolites in small animals. IEEE Trans. Biomed. Circuits Syst. 2014;8:636–647. doi: 10.1109/TBCAS.2014.2359094. [DOI] [PubMed] [Google Scholar]
- 96.Hammoud A., Chamseddine A., Nguyen D.K. Towards an implantable bio-sensor platform for continuous real-time monitoring of anti-epileptic drugs. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2016;2016:2982–2985. doi: 10.1109/EMBC.2016.7591356. [DOI] [PubMed] [Google Scholar]
- 97.Hubble L.J., Wang J. Sensing at your fingertips: glove-based wearable chemical sensors. Electroanalysis. 2019;31:428–436. [Google Scholar]
- 98.Ferreira P.C., Ataíde V.N., Chagas C.L.S. Wearable electrochemical sensors for forensic and clinical applications. Trends Anal. Chem. 2019;119:115622. [Google Scholar]
- 99.Barfidokhta A., Mishraa R.K., Seenivasanb R. Wearable electrochemical glove-based sensor for rapid and on-site detection of fentanyl. Sens. Actuators B Chem. 2019;296:126422. doi: 10.1016/j.snb.2019.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li W., Tse F.L. Dried blood spot sampling in combination with LC-MS/MS for quantitative analysis of small molecules. Biomed. Chromatogr. 2010;24:49–65. doi: 10.1002/bmc.1367. [DOI] [PubMed] [Google Scholar]
- 101.Bian S., Van den Berghe N., Vandersmissen L. Evaluating an easy sampling method using dried blood spots to determine vedolizumab concentrations. J. Pharmaceut. Biomed. Anal. 2020;185:113224. doi: 10.1016/j.jpba.2020.113224. [DOI] [PubMed] [Google Scholar]
- 102.Qu J.H., Dillen A., Saeys W. Advancements in SPR biosensing technology: an overview of recent trends in smart layers design, multiplexing concepts, continuous monitoring and in vivo sensing. Anal. Chim. Acta. 2020;1104:10–27. doi: 10.1016/j.aca.2019.12.067. [DOI] [PubMed] [Google Scholar]
- 103.Zhang Y.S., Aleman J., Shin S.R. Multisensor-integrated organs-on-chips platform for automated and continual in situ monitoring of organoid behaviors. Proc. Natl. Acad. Sci. U.S.A. 2017;114:E2293–E2302. doi: 10.1073/pnas.1612906114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gao N., Gao T., Yang X. Specific detection of biomolecules in physiological solutions using graphene transistor biosensors. Proc. Natl. Acad. Sci. U.S.A. 2016;113:14633–14638. doi: 10.1073/pnas.1625010114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bay H.H., Vo R., Dai X. Hydrogel gate graphene field-effect transistors as multiplexed biosensors. Nano Lett. 2019;19:2620–2626. doi: 10.1021/acs.nanolett.9b00431. [DOI] [PubMed] [Google Scholar]
- 106.Nakatsuka N., Yang K.A., Abendroth J.M. Aptamer–field-effect transistors overcome Debye length limitations for small-molecule sensing. Science. 2018;362:319–324. doi: 10.1126/science.aao6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wisniewski N., Reichert M. Methods for reducing biosensor membrane biofouling. Colloids Surf. B Biointerfaces. 2000;18:197–219. doi: 10.1016/s0927-7765(99)00148-4. [DOI] [PubMed] [Google Scholar]
- 108.Li Y., Xu Y., Fleischer C.C. Impact of anti-biofouling surface coatings on the properties of nanomaterials and their biomedical applications. J. Mater. Chem. B. 2018;6:9–24. doi: 10.1039/C7TB01695F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sabaté Del Río J., Henry O.Y., Jolly P. An antifouling coating that enables affinity-based electrochemical biosensing in complex biological fluids. Nat. Nanotechnol. 2019;14:1143–1149. doi: 10.1038/s41565-019-0566-z. [DOI] [PubMed] [Google Scholar]
- 110.Carrara S., Ghoreishizadeh S., Olivo J. Fully integrated biochip platforms for advanced healthcare. Sensors (Basel) 2012;12:11013–11060. doi: 10.3390/s120811013. [DOI] [PMC free article] [PubMed] [Google Scholar]