Abstract
The SARS-CoV-2 is the causative agent of the COVID-19 disease, a severe acute respiratory syndrome-coronavirus (SARS-CoV). Its main transmission pathway is through large respiratory droplets, as well as direct and indirect contact. Copper in different formats has been used in research and clinical settings to reduce the risk of bacterial and viral contamination. Therefore, this review aims to search for evidence about the biocidal properties of copper over the Coronaviridae family. A literature review was performed using PubMed and Ovid servers without date or language restrictions. The search was carried out on March 7, 2020, using the following search terms: [Copper] Coronavirus OR CoV OR SARS OR MERS OR Influenza. Copper destroys the replication and propagation abilities of SARS-CoV, influenza, and other respiratory viruses, having high potential disinfection in hospitals, communities, and households. Copper can eliminate pathogenic organisms such as coronavirus bacterial strains, influenza virus, HIV, and fungi after a short period of exposure. Copper seems to be an effective and low-cost complementary strategy to help reduce the transmission of several infectious diseases by limiting nosocomial infectious transmission. Copper oxide or nanocompounds may be used as filters, face masks, clothing, and hospital common surfaces to reduce viruses and bacterial incubation.
Keywords: Nanoparticles, Coronavirus, Copper, Severe acute respiratory syndrome, Influenza
Graphical abstract
1. Introduction
The SARS-CoV-2 is the causative agent of the COVID-19 disease, a Coronaviridae enveloped positive-sense single-stranded RNA virus. Its main transmission pathway is through large respiratory droplets and direct and indirect contact. Phylogenetic analyses indicate a close relation to bat SARS-like and MERS-like coronavirus (Tang et al., 2020a). The first 9 cases of SARS-CoV-2 were analyzed, and sequences of the virus were more than 99.98% similar between them. SARS-CoV-2 shares at least 88% genetic sequence similarities with SARS-like coronaviruses and shares 79% similar sequence identity with SARS-CoV and about 50% with MERS-COV, additionally, SARS-CoV-2 has similar receptor binding domain structure compared with SARS-CoV (Lu et al., 2020; Wang et al., 2020c). Therefore, although survival time and conditions affecting SARS-CoV-2 remain unknown, it is plausible to extrapolate knowledge from SARS-CoV family; it is a zoonotic bat-originated virus, from the family of severe acute respiratory syndrome-coronavirus (SARS-CoV) and Middle East respiratory syndrome-coronavirus (MERS-CoV) (Song et al., 2019), and capable of surviving for several days in the environment (European Centre for Disease Prevention and Control, 2020; Otter et al., 2016).
The emergence of this novel coronavirus (SARS-CoV-2) on December 2019 in Wuhan, Hubei province of China, is having a significant impact over the world, with cases rapidly increasing in every continent in only a few months (Tang et al., 2020a; Wang et al., 2020a). According to the World Health Organization (WHO) situation report 179 (WHO, n.d.-a), up to 17 July 2020, the pandemic has killed 585,727 people with cases reported in 216 countries. Due to its significant impact, the emergence of this novel coronavirus has been declared a global health emergency. The fast and worldwide spreading of the virus points at a human-to-human transmission.
SARS-CoV gained attention after the 2003 pandemic (Drosten et al., 2003; Ksiazek et al., 2003) that killed 774 individuals in 24 countries, and later in 2012, MERS-CoV killed 858 across 25 countries. Since then, some studies have described virus characteristics (Yip et al., 2007). Its influenza-like symptoms are nonspecific and may include cough, breathing difficulties, runny nose, fever, and headache. Laboratory exam shows lymphopenia and bilateral ground-glass opacity or consolidation CT scans, making difficult differential diagnostic against influenza or other respiratory viruses (Wang et al., 2020a). Unlike influenza, these symptoms seem to last longer in SARS-CoV-2 infection (Tang et al., 2020a), becoming a life-threatening condition for specific risk groups, such as immune-compromised and older patients. Several treatments are currently under study, the most promising being the use of remdesivir and chloroquine (Wang et al., 2020b). Other medications are also under study with fewer positive results, such as hydroxychloroquine (Gao et al., 2020; Tang et al., 2020b) and azithromycin (Gautret et al., 2020) in combination with hydroxychloroquine.
Since the 2019-nCoV-2 outbreak, extraordinary public health measures have been implemented around the globe to help reduce virus spreading. These measures mainly point at people’s transport restriction including quarantine of suspicious cases, airport and public transport closure, massive events cancellation, and social distancing and isolation. Preventing campaign has been launched by several world-leading agencies (Public Health England, n.d.; WHO, n.d.-b; CDC, 2020) and from almost every governmental Health Department. Preventive actions, such as frequent hand-washing, correct mouth-covering when coughing, and use of masks, are common indications that have given way to increasingly restrictive measures aiming to social distancing and isolation.
Recent investigations have found novel and effective ways of decreasing the risk of transmission of several respiratory viruses from the Coronaviridae and influenza family, such as the use of copper (Borkow et al., 2010, Borkow et al., 2011; Fujimori et al., 2012; Imai et al., 2012; Ito et al., 2016; Majbauddin et al., 2015; Minoshima et al., 2016; Warnes et al., 2015; Zerbib et al., 2020). Copper has been used in clinical settings to reduce the risk of bacterial and viral contamination, complementing traditional protocols. Furthermore, the addition of copper nanoparticles to polymer/plastic matrices can also produce highly effective antimicrobial materials. This review highlights the results of the research studying the effect of copper over the coronaviruses and influenza, summarizing the state-of-the-art related to its antiviral properties.
2. Methods
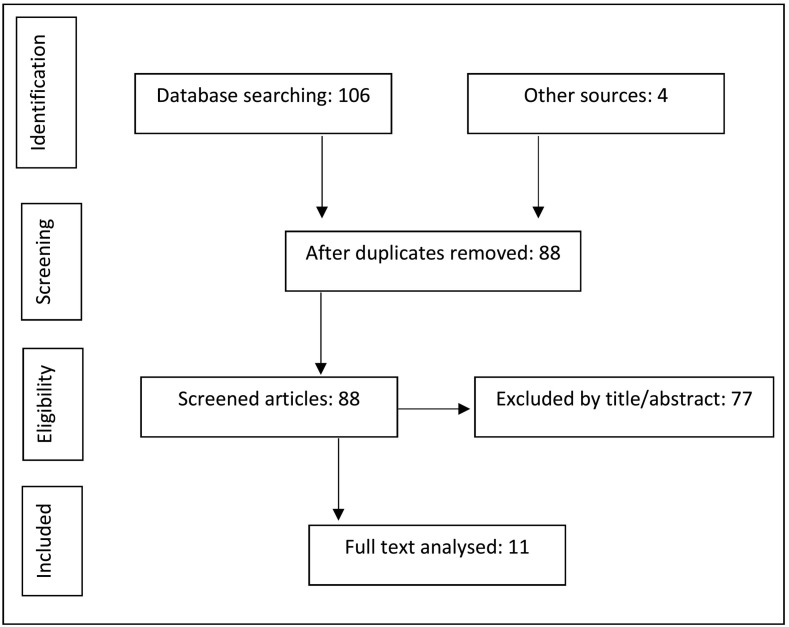
PubMed and Ovid servers were used without date or language restrictions. The PICOS method was followed to identify suitable terms. Table 1 describes the PICOS analysis. The search was carried out on March 7, 2020, using the following search terms: [Copper] Coronavirus OR CoV OR SARS OR MERS OR Influenza. The search yielded a total of 110 articles. Duplicate items were eliminated, leaving 88 studies. Title and abstract elimination left 11 suitable articles with the defined outcome and study design for full analyses. Figure 1 describes the search phases.
Table 1.
PICOS description for key terms identification.
| Description | Identifier | |
|---|---|---|
| P | Patient, population, or disease | SARS-Cov, coronavirus |
| I | Intervention | Copper |
| C | Comparison group | MERS-CoV; influenza |
| O | Outcome | Inactivation, exposition |
| S | Study type, design | Clinical and laboratory trials, prospective |
Figure 1.
Phases of studies selection.
3. Results and discussion
Excluded titles included the following: 32 that did not refer to copper properties, 25 that referred to other viruses or bacteria, 7 epidemiological/demographic studies, and 13 other different criteria. Several studies have described the antimicrobial and antiviral properties of copper over a variety of pathogens. Table 2 summarizes the identified studies related to copper and respiratory viruses.
Table 2.
Studies about the copper effect on respiratory viruses.
| N | Author | Year | Virus | Substrate | Media | Results |
|---|---|---|---|---|---|---|
| 1 | Miyamoto | 1998 | Influenza (H1N1, H2N2, H3N2) | Copper chelates | Madin–Darby canine kidney (MDCK) | 5 μM copper inhibits apoptosis of the 98% to 100% influenza virus, independent of the influenza type. Inhibited viruses release during apoptosis |
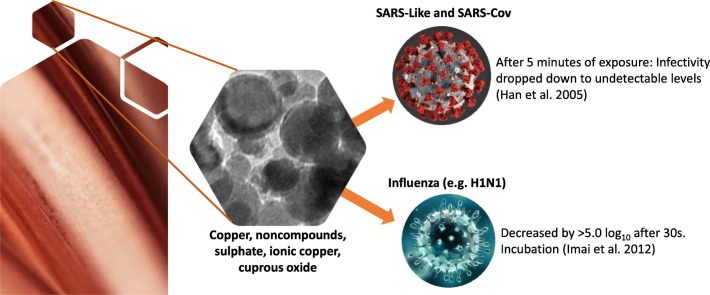
| 2 | Han | 2005 | SARS-Cov, Escherichia coli | Cu/AI203 | Metal catalyst surface | After 5 min of exposure, the infectivity dropped down to undetectable levels. |
| 3 | Noyce | 2007 | Influenza (H1N1) | Stainless steel and copper | Metal surface | 500,000 virus particles remain infectious after 24 h of incubation on stainless steel; 500 particles were active after 6 h on copper incubation. |
| 4 | Horie | 2008 | Influenza (H9N2) | Cu2+ at 2.5- to 250-μM concentrations | MDCK | Titer reduction of 3 and 4 log within 3 and 6 h of exposure |
| 5 | Borkow | 2010 | Influenza (H1N1, H9N2) | Copper oxide | Impregnated cotton textile | After 30 min of simulated breathing, 5.03±0.54 log10TCID50 virus titers were recovered from control masks. No influenza titers were found on copper oxide–containing masks (≤0.88 log10TCID50). |
| 6 | Imai | 2012 | Influenza (H5N1, H5N3) | Cu2+ (0.1 mL) | Impregnated cotton textile | Titers of H5N1 decreased by >5.0 log10 and 5.0 log10, respectively, after 30-s incubation on the textile. H5N3 decreased at similar rates. |
| 7 | Fujimori | 2012 | Influenza (H1N1) | Nanosized copper iodide (CuI) | Aqueous solution concentration dose. 17 μ/mL | One-hour exposure resulted on the 50% effective concentration dose |
| 8 | Warnes | 2015 | SARS-Cov (HuCoV-229E) | Copper alloy surfaces | Different dry surface | Complete and irreversible destruction of the coronavirus. Rapid damage to the surface proteins and membrane, braking the envelop. Coronavirus genomic RNA revealed nonspecific fragmentation |
| 9 | Minoshima | 2016 | Influenza (H1N1) and bacteriophage Qβ. | Ionic copper and silver cuprous oxide (Cu2O) | Solid-state and soluble compounds | Solid state of cuprous oxide (Cu2O) inactivates influenza A virus and bacteriophage Qβ; however, solid-state cupric oxide (CuO) and silver sulfide had little antiviral effect. |
| 10 | Ito | 2016 | Influenza (H1N1) and HIV (type 1) | Sodium copper chlorophyllin | MDCK | Sodium copper chlorophyllin inhibited HIV adsorption at 2.5 mM (P < 0.05) and inhibited the influenza virus adsorption at 200 μM (P < 0.05). |
| 11 | Zerbib | 2020 | Hand-transmitted healthcare-associated infection | Copper alloy surfaces | Different dry surface | The relative risk of hand-transmitted healthcare-associated infection was significantly lower in the copper-equipped surface (RR 0.3, 95% CI 0.1–0.5). |
A prospective observational pilot study (Zerbib et al., 2020) in a nursing home for older adults setting studied the antimicrobial properties of copper, comparing copper-equipped versus not equipped surfaces. Researchers randomly equipped with copper alloy (containing 90% copper) door handles, handrails, and grab-bars of 2 out of 4 areas of the building; all areas were equal in terms of the type of patients. Flu caused by influenza A, gastroenteritis by norovirus, and keratoconjunctivitis and gastroenteritis by unidentified pathogens (possibly adenovirus and norovirus, respectively) were the outbreaks occurring the study period. The outbreaks of keratoconjunctivitis and gastroenteritis were significantly lower in the copper-equipped areas. The relative risk of hand-transmitted healthcare-associated infection (adenovirus and norovirus) was significantly lower in the copper-equipped surface (RR 0.3, 95% CI 0.1–0.5). This indicates that copper reduces the incidence of infection in the healthcare context. This effect related to surface contact transmission. Airborne transmission, to some extent, may be affected by equipping common-use areas with copper surfaces.
Most of the research about the use of copper or copper alloy is related to influenza virus. Noyce et al. (2007) studied the effect of copper over influenza virus: 20 μL of virus (108 particles) was inoculated into copper or stainless steel; about 500,000 virus particles remained infectious after 24-h incubation on stainless steel. On the other hand, after 6 h on copper incubation, only 500 particles remained viable. Similarly to the study of Zerbib et al. (2020), influenza may not be prevented by replacing steel fittings with copper; however, copper may reduce transmission. Miyamoto et al. (1998), in a laboratory model, demonstrated that copper chelates inhibit apoptosis of the influenza virus, and such effect is independent of the influenza type. Additionally, copper chelates inhibited viruses’ release during apoptosis. Minoshima et al. (2016) compared the effect of solid-state and soluble ionic copper and silver compounds against influenza A (A/PR8/H1N1) and bacteriophage Qβ. Solid state of cuprous oxide (Cu2O) inactivates influenza A virus and bacteriophage Qβ. Hemagglutinin (HA) and neuraminidase (NA) are proteins highly involved in the influenza infection process by binding with the host cell and allowing the release of viruses. These proteins are expressed by a hundred on the surface of the virus envelope; CU2O decreased the HA titer below the detection limit within 30 min, and NA activity was significantly reduced after 10 min. Higher activity against viruses was reported for solid-state Cu2O compared to silver compounds, meaning a low-cost and widely available product usable in public places and health facilities. Imai et al. (2012) studied the effect of cotton textiles containing Cu2+ (CuZeo textile) over a highly pathogenic H5N1 and the low-pathogenic H5N3 viruses; titers of H5N1 decreased by >5.0 log10 and 5.0 log10, respectively, after 30-s incubation on the textile. Horie et al. (2008) demonstrated H9N2 virus titer reduction of 3 and 4 logs within 3 and 6 h of exposition to Cu2+ at concentrations of 2.5–250 μM. Fujimori et al. (2012) examined the antiviral properties of nanosized copper iodide (CuI) in aqueous solution against influenza A virus (H1N1). Virus titers decreased depending on concentration dose from 0 to 1000 μ/mL, resulting in a 50% effective concentration dose at 17 μ/mL after 1-h exposure. The authors hypothesize that Cu+ may inactivate viruses by oxidizing lipids; lipids are the main components of enveloped influenza viruses. The authors claimed that these properties can be applied to filters, masks, and clothing by mixing or coating polymers base materials. Ito et al. (2016) in a laboratory model showed that sodium copper chlorophyllin had antiviral effect against influenza and HIV. Copper chlorophyllin inhibits the virus-to-cell interaction on the cell surface, and this mechanism has been described for several microbes (Benati et al., 2009; Majbauddin et al., 2015).
Few but conclusive studies have focused on the antiviral properties of copper against coronavirus. Han et al. (2005) studied the effect of metal catalysts of Cu/Al203 against SARS-CoV, baculovirus, and E. coli. After 5 min of exposure, the infectivity dropped down to undetectable levels. SARS-CoV inactivation was achieved only when the loading viruses on the surfaces were exposed to air. Considering that central air-conditioning (AC) systems are known pathways for infectious agents, the author concludes that the capacity to inactivate viruses or bacteria in the air filtered by AC systems depends on the efficient usage of oxygen, having the potential to disinfect the air in community settings and health services.
Human SARS and MERS coronaviruses, as well as H1N1, H5N1, and H5N7 influenza viruses, share an important number of characteristics. These viruses are RNA respiratory viruses from zoonotic origins that have mutated for human-to-human transmission by droplets of about 5 μm in diameter. These droplets may directly contact the nose, mouth, or eyes of the recipient. Indirect contact involving contaminated surfaces (Boone and Gerba, 2007; Bridges et al., 2003) is also a predominant transmission route (Brankston et al., 2007; Spicknall et al., 2010). These respiratory viruses are the main cause of deaths among any other infectious agent (Warnes et al., 2015).
One of the causes of the high rate of transmission of respiratory viruses relates with it capability to survive on dry surfaces for more than 5 days on a variety of daily-use surfaces, such as ceramic tiles, glass, rubber, and stainless steel (Warnes et al., 2015). Nevertheless, it has also been reported that, depending on the surface, the SARS coronavirus, MERS coronavirus, and HCoV can remain infectious for up to 9 days, and for up to 28 days at 4 °C (Kampf et al., 2020). Thus, the virus can survive for a considerably long time until a person touches a surface contaminated by respiratory droplets from an infected individual. In 2015, the study by Warnes et al. (2015) showed conclusive results related to the effect of copper on HCoV 229E. HCoV 229E remains infectious for at least 5 days on nonmetal surfaces such as Teflon, PVC, ceramic tiles, glass, silicone rubber, and stainless steel. On the other hand, when exposed to copper (metal surface) and copper alloy (Cu/Zn brasses), after 10 min of exposition, viral genomes and morphology were irreversibly affected. Warnes described rapid damage to the surface proteins and membrane, breaking the envelope or losing its capacity of self-containing folding upon itself. Coronavirus genomic RNA after copper exposure revealed nonspecific fragmentation, and this pattern of damage increased as exposure time increased.
Some application designs of this knowledge have been successfully tested; Borkow et al. (2010) developed a face mask impregnated with copper oxide and tested its antiviral effects against influenza (H1N1 and H9N2). Above 99.85% of aerosolized viruses were filtered by both control and copper oxide–impregnated masks. Nevertheless, after 30 min of simulated breathing, 5.03±0.54 log10TCID50 was recovered from control masks. No infectious influenza was found on copper oxide–containing masks (≤0.88 log10TCID50).
Borkow et al. (2011) used powder copper oxide (of about 18 μm) to inactivate HIV in 5 min and, following a mathematical model, estimated that using nanoparticles (of about 1 μm) would reduce that time to only 14 s. Copper nanoparticles have been preliminarily tested against HIV, and as predicted by Borkow, the needed time to inactivate the virus was about 15 s (unpublished data; Copper3D, n.d.).
4. Conclusion
A variety of respiratory pathogenic agents such as influenza, SARS-CoV, MERS-CoV, and HCoV have been exposed to a variety of copper forms (copper alloy dry surface, sodium copper, ionic copper oxide, copper iodide, Cu2+, and lay copper) in several cultivating media (MDCK, metal, textile, aqueous solution, dry surfaces), having similar results and arriving at the same conclusion: copper is capable to inhibit, inactivate, reduce, and irreversibly destroy coronavirus, influenza virus, and other pathogenic agents in a matter of minutes. A recent study has evaluated and compared SARS-CoV-1 and SARS-CoV-2 stability and decay rates in aerosols, copper (metallic plate at 99% of copper), cardboard, stainless steel, and plastic. Despite significant reduction on infectivity of SARS-CoV 1 and SARS-CoV 2 after 3 h in aerosols, 72 h on plastic, and 48 h on stainless steel, the virus remained infectious. On the other hand, after exposition on copper, no viable virus was observed after 8 h and after 4 h for SARS-CoV-1 and SARS-CoV-2, respectively (van Doremalen et al., 2020). Perhaps, these data suggest a higher effect of copper on SARS-CoV-2. Based on the reviewed literature, copper nanoparticles together with efficient usage of oxygen appears to be the most effective formulation against coronaviruses.
The described data appear to support the use of copper in different presentations to actively inactivate viruses (and a wide range of microorganisms), and it seems to be an effective and low-cost complementary strategy to help reducing transmission of several infectious diseases such as the coronavirus. Nevertheless, and despite the genetic similarities, more research would be beneficial to support its usage with the new SARS-Cov-2.
However, the described data support the incorporation of copper alloys or impregnated copper materials on health services as a complementary strategy that may help reduce bacterial and viral load, therefore limiting nosocomial infections and reducing transmission from touching surfaces in the communal city or home areas (Prado et al., 2012; Warnes et al., 2015).
Additionally, copper oxide or nanocompounds may be used as filters, face masks, clothing, and hospital common-use devices to reduce viruses and bacterial incubation (Fujimori et al., 2012). Some copper product application has been tested with conclusive results, describing copper’s potent antiviral properties, reducing disease spreading by limiting environmental contamination and subsequent infections (Borkow et al., 2010).
References
- Benati F.J., Lauretti F., Faccin L.C., Nodari B., Ferri D.V., Mantovani M.S., et al. Effects of chlorophyllin on replication of poliovirus and bovine herpesvirus in vitro. Lett Appl Microbiol. 2009;49(6):791–795. doi: 10.1111/j.1472-765X.2009.02744.x. [DOI] [PubMed] [Google Scholar]
- Boone S.A., Gerba C.P. Significance of fomites in the spread of respiratory and enteric viral disease. Appl Environ Microbiol. 2007;73(6):1687–1696. doi: 10.1128/AEM.02051-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkow G., Zhou S.S., Page T. Gabbay J. A novel anti-influenza copper oxide containing respiratory face mask. PLoS One. 2010;5(6) doi: 10.1371/journal.pone.0011295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkow G., Covington C.Y., Gautam B., Anzala O., Oyugi J., Juma M., et al. Prevention of human immunodeficiency virus breastmilk transmission with copper oxide: proof-of-concept study. Breastfeed Med. 2011;6(4):165–170. doi: 10.1089/bfm.2010.0090. [DOI] [PubMed] [Google Scholar]
- Brankston G., Gitterman L., Hirji Z., Lemieux C., Gardam M. Transmission of influenza A in human beings. Lancet Infect Dis. 2007;7(4):257–265. doi: 10.1016/S1473-3099(07)70029-4. [DOI] [PubMed] [Google Scholar]
- Bridges C.B., Kuehnert M.J., Hall C.B. Transmission of influenza: implications for control in health care settings. Clin Infect Dis Off Publ Infect Dis Soc Am. 2003;37(8):1094–1101. doi: 10.1086/378292. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control & Prevention (CDC) Centers for Disease Control and Prevention; 2020. Coronavirus disease 2019 (COVID-19) [Internet] https://www.cdc.gov/coronavirus/2019-ncov/community/index.html [cited 2020 Mar 7]. Available from.
- Copper3D Copper3D desarrolla inactivador viral de HIV.pdf(Shared)-Adobe Document Cloud [Internet] [cited 2020 Mar 8] https://documentcloud.adobe.com/link/track?uri=urn%3Aaaid%3Ascds%3AUS%3A686c3152-5162-4471-974f-19bcb8e8f5df Available from:
- Drosten C., Günther S., Preiser W., van der Werf S., Brodt H.-R., Becker S., et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348(20):1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- European Centre for Disease Prevention and Control European Centre for Disease Prevention and Control; 2020. Interim guidance for environmental cleaning in non-healthcare facilities exposed to SARS-CoV-2 [Internet] https://www.ecdc.europa.eu/en/publications-data/interim-guidance-environmental-cleaning-non-healthcare-facilities-exposed-2019 [cited 2020 Mar 8]. Available from.
- Fujimori Y., Sato T., Hayata T., Nagao T., Nakayama M., Nakayama T., et al. Novel antiviral characteristics of nanosized copper(I) iodide particles showing inactivation activity against 2009 pandemic H1N1 influenza virus. Appl Environ Microbiol. 2012;78(4):951–955. doi: 10.1128/AEM.06284-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14(1):72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- Gautret P., Lagier J.-C., Parola P., Hoang V.T., Meddeb L., Sevestre J., et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: a pilot observational study. Travel Med Infect Dis. 2020;34:101663. doi: 10.1016/j.tmaid.2020.101663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J., Chen L., Duan S., Yang Q., Yang M., Gao C., et al. Efficient and quick inactivation of SARS coronavirus and other microbes exposed to the surfaces of some metal catalysts. Biomed Environ Sci. 2005;18(3):176–180. [PubMed] [Google Scholar]
- Horie M., Ogawa H., Yoshida Y., Yamada K., Hara A., Ozawa K., et al. Inactivation and morphological changes of avian influenza virus by copper ions. Arch Virol. 2008;153(8):1467–1472. doi: 10.1007/s00705-008-0154-2. [DOI] [PubMed] [Google Scholar]
- Imai K., Ogawa H., Bui V.N., Inoue H., Fukuda J., Ohba M., et al. Inactivation of high and low pathogenic avian influenza virus H5 subtypes by copper ions incorporated in zeolite-textile materials. Antivir Res. 2012;93(2):225–233. doi: 10.1016/j.antiviral.2011.11.017. [DOI] [PubMed] [Google Scholar]
- Ito A., Tsuneki A., Yoshida Y., Ryoke K., Kaidoh T., Kageyama S. In vitro inhibition of cytopathic effect of influenza virus and human immunodeficiency virus by bamboo leaf extract solution and sodium copper chlorophyllin. Yonago Acta Med. 2016;59(1):61–65. [PMC free article] [PubMed] [Google Scholar]
- Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. 2020;104(3):246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., et al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348(20):1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majbauddin A., Kodani I., Ryoke K. The effect of bamboo leaf extract solution and sodium copper chlorophyllin solution on growth and volatile sulfur compounds production of oral malodor associated some anaerobic periodontal bacteria. Yonago Acta Med. 2015;58(3):129–136. [PMC free article] [PubMed] [Google Scholar]
- Minoshima M., Lu Y., Kimura T., Nakano R., Ishiguro H., Kubota Y., et al. Comparison of the antiviral effect of solid-state copper and silver compounds. J Hazard Mater. 2016;312:1–7. doi: 10.1016/j.jhazmat.2016.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto D., Kusagaya Y., Endo N., Sometani A., Takeo S., Suzuki T., et al. Thujaplicin-copper chelates inhibit replication of human influenza viruses. Antivir Res. 1998;39(2):89–100. doi: 10.1016/s0166-3542(98)00034-5. [DOI] [PubMed] [Google Scholar]
- Noyce J.O., Michels H., Keevil C.W. Inactivation of influenza A virus on copper versus stainless steel surfaces. Appl Environ Microbiol. 2007;73(8):2748–2750. doi: 10.1128/AEM.01139-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otter J.A., Donskey C., Yezli S., Douthwaite S., Goldenberg S.D., Weber D.J. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. J Hosp Infect. 2016;92(3):235–250. doi: 10.1016/j.jhin.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado J.V., Vidal A.R., Duran T.C. Application of copper bactericidal properties in medical practice. Rev Med Chil. 2012;140(10):1325–1332. doi: 10.4067/S0034-98872012001000014. [DOI] [PubMed] [Google Scholar]
- Public Health England Coronavirus (COVID-19) [Internet] [cited 2020 Mar 7] https://campaignresources.phe.gov.uk/resources/campaigns/101-coronavirus-/resources Available from.
- Song Z., Xu Y., Bao L., Zhang L., Yu P., Qu Y., et al. From SARS to MERS, thrusting coronaviruses into the spotlight. Viruses. 2019;11(1):59. doi: 10.3390/v11010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spicknall IH, Koopman JS, Nicas M, Pujol JM, Li S, Eisenberg JNS. Informing optimal environmental influenza interventions: how the host, agent, and environment alter dominant routes of transmission. PLoS Comput Biol [Internet]. 2010 Oct 28 [cited 2020 Mar 7];6(10). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2965740/ [DOI] [PMC free article] [PubMed]
- Tang J.W., Tambyah P.A., Hui D.S.C. Emergence of a novel coronavirus causing respiratory illness from Wuhan, China. J Infect. 2020;80(3):350–371. doi: 10.1016/j.jinf.2020.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W., Cao Z., Han M., Wang Z., Chen J., Sun W., et al. Public and Global Health; 2020. Hydroxychloroquine in patients mainly with mild to moderate COVID-19: an open-label, randomized, controlled trial [Internet] http://medrxiv.org/lookup/doi/10.1101/2020.04.10.20060558 Available from:
- van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;0(0) doi: 10.1056/NEJMc2004973. null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Zhang X., Irwin D.M., Shen Y. Emergence of SARS-like coronavirus poses new challenge in China. J Infect. 2020;80(3):350–371. doi: 10.1016/j.jinf.2020.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnes S.L., Little Z.R., Keevil C.W. Human coronavirus 229E remains infectious on common touch surface materials. MBio. 2015;6(6) doi: 10.1128/mBio.01697-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organisation (WHO) Coronavirus disease (COVID-19) situation reports — 179 [Internet] [cited 2020a Jul 17] https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports Available from:
- World Health Organisation (WHO) Infection prevention and control [Internet] [cited 2020b Mar 7] https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/infection-prevention-and-control Available from:
- Yip T.T.C., Cho W.C.S., Cheng W.W., Chan J.W.M., Ma V.W.S., Yip T.-T., et al. In: Microarrays: volume 2: applications and data analysis [Internet] Rampal J.B., editor. Humana Press; Totowa, NJ: 2007. Application of ProteinChip array profiling in serum biomarker discovery for patients suffering from severe acute respiratory syndrome. [cited 2020 Mar 7]. p. 313–31. (Methods in Molecular Biology). Available from: doi: 10.1007/978-1-59745-304-2_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbib S., Vallet L., Muggeo A., de Champs C., Lefebvre A., Jolly D., et al. Copper for the prevention of outbreaks of health care-associated infections in a long-term care facility for older adults. J Am Med Dir Assoc. 2020;21(1):68–71.e1. doi: 10.1016/j.jamda.2019.02.003. [DOI] [PubMed] [Google Scholar]