Abstract
Chronic lung allograft dysfunction (CLAD) following lung transplantation limits long-term survival considerably. The main reason for this is a lack of knowledge regarding the pathological condition and the establishment of treatment. The consensus statement from the International Society for Heart and Lung Transplantation on CLAD in 2019 classified CLAD into two main phenotypes: Bronchiolitis obliterans syndrome and restrictive allograft syndrome. Along with this clear classification, further exploration of the mechanisms and the development of appropriate prevention and treatment strategies for each phenotype are desired. In this review, we summarize the new definition of CLAD and update and summarize the existing knowledge on the underlying mechanisms of bronchiolitis obliterans syndrome and restrictive allograft syndrome, which have been elucidated from clinicopathological observations and animal experiments worldwide.
Keywords: Lung transplantation, Chronic lung allograft dysfunction, Bronchiolitis obliterans syndrome, Restrictive allograft syndrome, Interaction of immune cells, Anatomical changes in transplanted lungs
Core tip: Long-term prognosis following lung transplantation has not improved due to chronic lung allograft dysfunction (CLAD). Although a decade has passed since restrictive allograft syndrome with poor outcome was proposed, which was subsequently included as a new CLAD phenotype in the consensus report from International Society for Heart and Lung Transplantation in 2019, detailed mechanisms involved remain largely unknown. Here, we discuss the mechanisms of CLAD from an immunological point of view.
INTRODUCTION
Lung transplantation is an established surgical treatment for end-stage respiratory failure. Since the first successful case of clinical lung transplantation in 1983[1], the short-term survival within 1 year is over 80% because of better surgical techniques and perioperative management[2]. However, the overall survival at 5 years is still approximately 50%–70% without much improvement[3], and is worse than that of other solid-organ transplantations[4]. Chronic lung allograft dysfunction (CLAD) has been the most common cause of poor survival for the past 35 years. Half of recipients receiving lung transplantation develop CLAD within 5 years[5,6], which aggravates their respiratory condition and worsens prognosis. The pathological condition of CLAD is thought not to be associated with chronic rejection alone, but rather as a multifactorial syndrome[7-12]. The lung is a unique organ that is consistently exposed to the external environment and is easily affected by external stimuli including infection[8-11], air pollution, and aspiration[12,13]. Until recently, CLAD was recognized as bronchiolitis obliterans syndrome (BOS) when it was first introduced in 1993[14]. The main histologic findings of BOS include obliterative bronchiolitis (OB) accompanying chronic inflammation and fibrosis in the respiratory tract[15,16]. In 2010, the concept of restrictive allograft syndrome (RAS), which accompanies restrictive ventilatory impairment and a poor prognosis, was proposed as a new entity of CLAD[17]. Interestingly, in pathological specimens of RAS patients, inflammation and fibrotic lesions were observed in the respiratory tract as well as the visceral pleura and peripheral lung tissues. RAS accounts for one-third to one-fourth of all cases of CLAD. A lower survival rate after RAS onset was shown in multiple lung transplant centers (survival period: 1 year for RAS vs 2.5 years for BOS)[18,19]. In response, the pulmonary council of International Society for Heart and Lung Transplantation (ISHLT) formulated the international diagnostic criteria for RAS in 2019[20]. Accordingly, the new definitions and diagnostic criteria for CLAD and BOS were also published in another consensus report[21]. The framework of CLAD has greatly changed, and further study is now needed to elucidate the mechanisms involved for each phenotype. This review summarizes the new definition of CLAD and provides some mechanistic insights obtained from clinicopathological observations and animal experiments.
NEW DEFINITION OF CLAD
CLAD is defined as a substantial and persistent decline in the measured forced expiratory volume in 1 second (FEV1) with ≥ 20% from the baseline value. The baseline value was set as the mean value of the two best FEV1 values following lung transplantation, which were measured at least 3 wk apart[21].
Three steps to diagnose CLAD according to its progress
Possible CLAD: FEV1 declined by ≥ 20% from the reference value but the duration of functional decline is still within 3 wk, irrespective of any forced vital capacity (FVC) changes.
Probable CLAD: FEV1 is still < 80% of the reference value after more than 3 wk but less than 3 months, despite appropriate treatment for secondary causes such as infection, acute rejection [cellular- or antibody-mediated rejection (AMR)], aspiration, and airway stenosis. In this case, new CLAD staging (Figure 1) and phenotypic clinical subtyping (Table 1) should be temporarily decided.
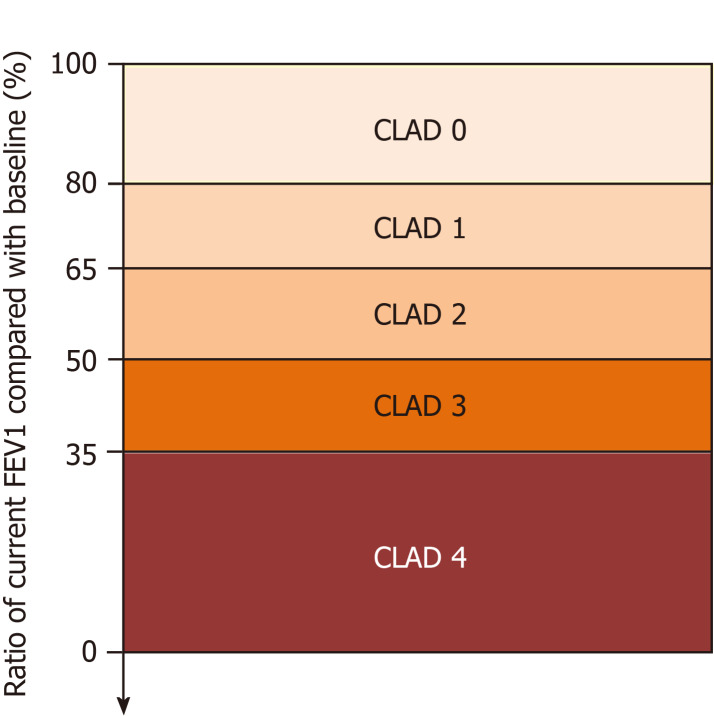
Figure 1.
Chronic lung allograft dysfunction staging. CLAD: Chronic lung allograft dysfunction; FEV1: Forced expiratory volume in 1 second.
Table 1.
Phenotypes of chronic lung allograft dysfunction
| Obstruction findings | Restriction findings | CT findings | |
| FEV1/FVC < 0.7 | TLC decline ≥ 10% from baseline | Parenchymal opacities and/or pleural thickening | |
| BOS | √ | ||
| RAS | √ | √ | |
| Mixed | √ | √ | √ |
| Undefined (2 types) | √ | √ | |
| √ | √ |
BOS: Bronchiolitis obliterans syndrome; CLAD: Chronic lung allograft dysfunction; CT: Computed tomography; FEV1: Forced expiratory volume in 1 second; FVC: Forced vital capacity; RAS: Restrictive allograft syndrome; TLC: Total lung capacity; √: Yes; Black box: No.
Definite CLAD: FEV1 has a persistent decline of ≥ 20% from the reference value after more than 3 months. Finally, a definite CLAD phenotype should be determined. Based on respiratory function examined by spirometry and computed tomography (CT) findings, CLAD is classified into four groups: BOS, RAS, mixed (BOS and RAS), and undefined.
If patients meet the CLAD criteria, their stage is determined by the new CLAD staging based on the decline rate of FEV1 during the disease course (Figure 1).
CLAD 0: Current FEV1 > 80% FEV1 baseline.
CLAD 1: Current FEV1 > 65%-80% FEV1 baseline.
CLAD 2: Current FEV1 > 50%-65% FEV1 baseline.
CLAD 3: Current FEV1 > 35%-50% FEV1 baseline.
CLAD 4: Current FEV1 ≤ 35% FEV1 baseline.
The old BOS staging[22] will no longer be used because RAS is included in this CLAD criterion. The four phenotypes (Table 1) represented mainly by BOS and RAS, and CLAD staging (5 stages: from 0 to 4) will be used to describe each disease state.
RISKS AND MECHANISM
Risk factors for CLAD include alloantigen-dependent (cellular and antibody-mediated rejection) and alloantigen-independent factors (infection, aspiration, ischemia, and autoimmunity). However, it is difficult to categorize them clearly into risk factors of BOS or of RAS because of the small amount of pooled evidence for RAS at present. For patients who underwent lung transplantation, the risk of developing BOS increased because of primary graft dysfunction (PGD), acute rejection, infections such as cytomegalovirus (CMV) pneumonitis or colonization by Pseudomonas aeruginosa or Aspergillus, and gastroesophageal reflux[5,23]. In contrast, RAS is also associated with acute cellular rejection (ACR), lymphocytic bronchiolitis, chronic lung infection caused by Pseudomonas aeruginosa, and neutrophil increase caused by bronchoalveolar lavage (BAL)[23]. However, among these risk factors, only severe lymphocytic bronchiolitis was thought to be associated with the onset of RAS compared with BOS. As described above, some risk factors overlapped between the two phenotypes. According to further clinical data, early-onset diffuse alveolar damage (DAD) (within 3 months after lung transplantation)[24] was considered a risk for BOS development. However, the following factors might increase the risk of RAS relative to BOS: elevated eosinophils in blood and BAL[25], preoperative lung diseases in recipients (not cystic fibrosis but interstitial lung disease/idiopathic pulmonary fibrosis (ILD/IPF) or chronic obstructive pulmonary disease (COPD)[26], antibody-mediated rejection (AMR)[27], HLA mismatching at the eplet level (HLA-DRB1/3/4/5 + DQA/B)[28], late-onset DAD (more than 3 months after lung transplantation)[24] and acute fibrinoid organizing pneumonia (AFOP)[29] (Table 2). In our recent animal experiments, airway stimulation of rats with lipopolysaccharide (LPS) induced airway-centered inflammation similar to BOS in humans[30]. We think BOS is caused by inflammation in the local respiratory tract, whereas RAS may be caused by fulminant rejection.
Table 2.
Possible risk factors of bronchiolitis obliterans syndrome and restrictive allograft syndrome based on the clinical evidence
| BOS | Undetermined[5,23] | RAS |
| Early-onset DAD[24] | ACR | AFOP[29] |
| Air pollution[37] | AMR1[27] | |
| Aspiration/GERD | Elevated eosinophils in blood and BAL[25] | |
| CMV pneumonitis | HLA-DRB1/3/4/5 + DQA/B[28] | |
| Colonization of Pseudomonas aeruginosa and Aspergillus | Late-onset DAD[24] | |
| PGD | LB1[23] | |
| Specific recipients’ lung disease (COPD/ ILD/IPF)[26] |
AMR and LB might be associated with the development of RAS compared with BOS. ACR: Acute cellular rejection; AFOP: Acute fibrinoid organizing pneumonia; AMR: Acute antibody-mediated rejection; BAL: Bronchoalveolar lavage; BOS: Bronchiolitis obliterans syndrome; COPD: Chronic obstructive pulmonary disease; CLAD: Chronic lung allograft dysfunction; CMV: Cytomegalovirus; GERD: Gastro esophageal reflux disease; LB: Lymphocytic bronchiolitis; RAS: Restrictive allograft syndrome.
Although RAS has been clearly categorized in the ISHLT CLAD consensus report, most previous studies used the term, BOS, which may also include RAS. Therefore, it is still unclear why CLAD following lung transplantation can take the form of one of the two phenotypes: BOS or RAS. The mechanism that differentiates these two phenotypes is unknown. Thus, based on the findings of previous research, we can start by considering the common pathways involved including innate immunity, cellular rejection, antibody-mediated rejection (humoral immunity), and autoimmunity.
INTERACTION OF IMMUNE CELLS
Innate immunity
Innate immunity refers to a nonspecific biological defense mechanism that plays an essential role in the initial recognition of pathogens such as bacteria and viruses, the initiation of subsequent inflammatory reactions, and the establishment of adaptive immunity. Inflammatory cell groups are generated in secondary lymphoid organs such as the bone marrow, spleen, and lymph nodes[31]. These cells infiltrate damaged tissues through blood vessels and defend and repair the tissues. In acute inflammation, edema is caused by the action of vasoactive mediators such as histamine and leukotriene immediately after injury[32]. Subsequently, tissues are infiltrated by cells, mainly consisting of neutrophils, and tissue damage is repaired by infiltrating monocytes or macrophages[33]. In general, the innate immune system detects pathogen invasion through pattern-recognition receptors (PRRs) that recognize pathogen-associated molecular patterns[34]. PRRs including Toll-like receptor (TLR), RIG-I-like receptor, NOD-like receptor, C-type lectin receptor, and intracellular DNA sensors have been identified[35].
PRRs are mainly expressed by dendritic cells and macrophages. Dendritic cells serve as a bridge between innate and adaptive immunity because they induce T cell responses after being activated and matured via innate immune receptors. TLR recognizes microbial components and autologous molecules. They have attracted increasing attention because of their involvement in autoimmune diseases and lifestyle-related diseases[36]. After lung transplantation, alloantigen-independent factors such as air pollution or bacterial infection may act directly on patient airways to induce the release of endogenous danger signals, such as alarmins from injured cells[37]. Dendritic cells and macrophages are activated through PRRs to promote the innate immune system and inflammatory responses, which is followed by activation of the adaptive immune system. This serial activation of the immune system might trigger CLAD. Indeed, TLR signaling was reported to activate alloimmune responses after lung transplantation. TLR2, TLR4, and TLR9 polymorphisms, which are involved in bacterial and viral recognition, were associated with CLAD development in humans[38]. Rat studies showed that activation of TLR4 resulted in obstructive bronchiolitis induced by the administration of synthetic double-stranded DNA or repeated doses of aerosolized lipopolysaccharide (LPS)[39,40].
We established a BOS model by inducing airway inflammation with LPS[30,42] and demonstrated the mechanism of fibrosis by direct TLR4-mediated stimulation of fibroblasts[46]. In an animal model of BOS where LPS was transtracheally administered, increased levels of Th1-type transcription factors and cytokines were only present in the grafts, but not in secondary lymphoid organs[43]. Although LPS induced macrophage infiltration, effector molecules of innate immunity, the expression of proinflammatory cytokine mRNAs, and T cell reactivity were not enhanced. Furthermore, we found that TLR4 signaling contributed to the activation of fibroblasts in coordination with transforming growth factor (TGF)-β1 in vitro. TLR4 signaling may play an important role in allograft fibrosis in addition to activation of alloimmune responses.
Alarmin might be a factor that helps us understand the mechanisms of CLAD and distinguish between RAS and BOS. When blood flow to the respiratory tract is impaired by primary graft dysfunction or gastroesophageal reflux disease, intracellular molecules are released from damaged cells. Within a few hours after transplantation, high-mobility group box 1 (HMGB1) is released, and innate immunity is mobilized. HMGB1 is secreted from necrotic cells under ischemic conditions, by TLR-mediated signals, and receptor for advanced glycation endproducts (RAGE) signals[44]. HMGB1 induced by RAGE signals is involved in early lung dysfunction, but it was also shown to be involved in the development of CLAD through the activation of innate immunity. In patients with RAS, various alarmins, such as S100A9 and HMGB1, were increased in alveolar lavage fluid at disease onset compared with BOS patients[45], suggesting a more intense inflammatory process in RAS than BOS. The analysis of specific alarmins may promote a better understanding of the clinical conditions of the two phenotypes.
Cellular immunity
The two main modes of cellular immunity (i.e., T cell responses to alloantigens in transplanted organs) are direct and indirect recognition. Direct recognition is associated with rejection that occurs immediately after transplantation[46]. In this recognition pathway, the recipient T cell recognizes donor cell molecules (major histocompatibility complex) via its T cell receptor[47]. In the early stage of transplantation, donor-derived antigen-presenting cells interact with and activate recipient CD4+ T cells. For indirect recognition, allo-MHC and other antigens are phagocytosed by recipient antigen-presenting cells, which are then presented to recipient T cells as MHC-peptide complexes. This sequence continues for the duration of the existence of the transplanted organ.
In recent years, exosomes have begun to attract attention as factors that trigger a common immunological mechanism of rejection[48]. Exosomes contain self-antigens, costimulatory molecules, MHC class II, transcription factors, and the 20S proteasome[49]. Cellular immunity is activated after exposure to these molecules. When a donor lung sustains an injury caused by PGD, viral infection, or acute rejection, stress-induced exosomes are released from the donor lung tissue[50]. The antigenicity of the donor lung is enhanced, leading to more intense immune responses against alloantigens and autoantigens (as discussed later), finally resulting in CLAD.
Humoral immunity
Acute phase humoral immunity typically involves AMR mediated by donor-specific antibodies (DSA). Clinical AMR is sub-categorized into three categories (Definite, Probable or Possible) according to (1) the presence of allograft dysfunction; (2) presence of DSA; (3) positive histology suggestive of AMR; and (4) positive C4d staining[51]. A representative DSA is the anti-human leukocyte antigen (HLA) antibody, which is involved in alloimmunity and might be associated with the development of CLAD[52,53]. The emergence of anti-HLA antibodies might induce alloimmune responses and graft injury through various pathways, including complement-dependent mechanisms (classical, alternative and lectin pathways) and complement-independent mechanisms that induce intracellular signaling in endothelial cells, and finally cause vasculopathy by MHC ligation[54]. After binding to airway epithelial cells, anti-HLA class I induces cell death and the release of fibrogenic growth factors such as platelet-derived growth factor, insulin-like growth factor-1, and TGF-β[55]. These events activate fibroblasts and myofibroblasts, and induce inflammatory cascades and extracellular matrix regeneration.
As reported for other organs, DSA-positive patients against HLA have a higher incidence of CLAD and a lower survival rate[56]. In addition, patients with anti-HLA antibodies prior to transplant determined by a panel reactive antibody test were found to have poor survival[57]. Furthermore, the development of de novo DSA was associated with CLAD and graft failure[58]. In a prospective study, preformed and de novo anti-HLA antibodies were monitored regularly every 3 months for 1 year after transplantation using the LABScreen® Single Antigen assay (One Lambda Inc., Canoga Park, CA)[59]. The incidence of CLAD did not change between the de novo DSA positive group who received antibody-directed therapy and the negative group. However, if the DSA did not disappear after treatment, chronic rejection developed resulting in poor survival. Therefore, the continuous monitoring of de novo DSA after lung transplantation using a highly sensitive solid-phase antibody detection immunoassay is considered important for early disease detection and treatment[60]. There have been few studies on differentiating between the development of BOS and RAS by DSA or AMR. Long-term continuous AMR might be associated with CLAD[51]. It was reported that patients with persistent DSA were more likely to develop RAS than BOS[56].
However, it has been recognized that during AMR, DSA can disappear in the serum of the recipient. An explanation might be that DSA are absorbed by the graft. In a study that examined human CLAD lungs, levels of tissue-bound graft DSA and serum DSA were measured and showed differences between serological and pathological findings[61]. In patients with RAS, the level of anti-HLA antibody as DSA in grafts was higher than in BOS, which indicated a strong relationship with fibrosis[61]. Furthermore, our laboratory reported that local anti-donor antibody production occurred in tertiary lymphoid structures of the donor lung in a rat lung transplant model[62]. Thus, the humoral immune response may occur locally in rejected lung allografts as well as in the spleen, a secondary lymphoid tissue.
Autoimmunity
Researchers have reported a relationship between CLAD and immune responses to lung-associated self-antigens[63]. The immunogenic antigens identified included collagen type I, collagen type V (Col-V), and k-alpha 1 tubulin. Col-V is a heterotrimer consisting of two-fragment α1 (V) and one-fragment α2 (V). Many patients without anti-HLA antibodies at transplantation harbored autoantibodies predisposing to chronic rejection. Another study reported that in cases where autoantibodies might exist before transplantation, the incidence of DSA and BOS was increased[64]. The development of autoimmune responses is promoted by interleukin-17 (IL-17) among many other factors[65]. IL-17-dependent cellular immunity to Col-V predisposes human lung transplants to obliterative bronchiolitis. While alloimmunity initiates lung transplant rejection, de novo autoimmunity for Col-V mediated by specific Th17 cells and monocytes or macrophages as accessory cells may ultimately contribute to progressive airway obstruction[66]. At BOS onset, the number of IL-10 secreting T cells was decreased and the numbers of CD4+ T cells secreting interferon-γ and IL-17 were significantly increased[67]. Some researchers have reported a loss of peripheral tolerance mechanisms after transplantation, mainly mediated by a decrease in Treg and loss of IL-10 response to self-antigens[63,67]. This Th phenotype switch may lead to an autoimmune response that predisposes towards chronic rejection. However, there is no evidence for differences in autoantibodies between BOS or RAS.
ANATOMICAL CHANGES IN TRANSPLANTED LUNGS
Lymphoid neogenesis
At sites of chronic inflammation in lungs, tertiary lymphoid structures (TLS) or lymph node-like cell aggregates are formed by lymphoid neogenesis, which are considered to play an immunoregulatory role[68]. They have been observed in transplanted organs with chronic rejection as well as at sites of chronic inflammation caused by viruses, bacterial infections, autoimmune diseases, and chronic obstructive pulmonary disease[69]. Bronchus-associated lymphoid tissue (BALT) is a representative TLS in the lungs[70], and might actively promote local immune responses and cause rejection by triggers including infection or GERD.
Chronic inflammation occurs when there is insufficient repair of tissue damage in acute inflammation. It can also be observed when mild tissue damage persists without significant acute inflammatory responses as seen in patients with collagen diseases such as rheumatoid arthritis[71]. During this chronic inflammation phase, tissue remodeling may occur with the replacement of blood vessels and connective tissues. Although this remodeling was also reported in donor lungs affected by CLAD, the initiation of lung-specific lymphoid structures such as BALT further complicate the elucidation of the mechanism of CLAD[68,72]. During acute rejection, de novo lymphatic vessels are formed within 2 wk after transplantation[73]. This constructed lymphatic network further promotes the immune response and might be associated with the eventual formation of TLS in CLAD.
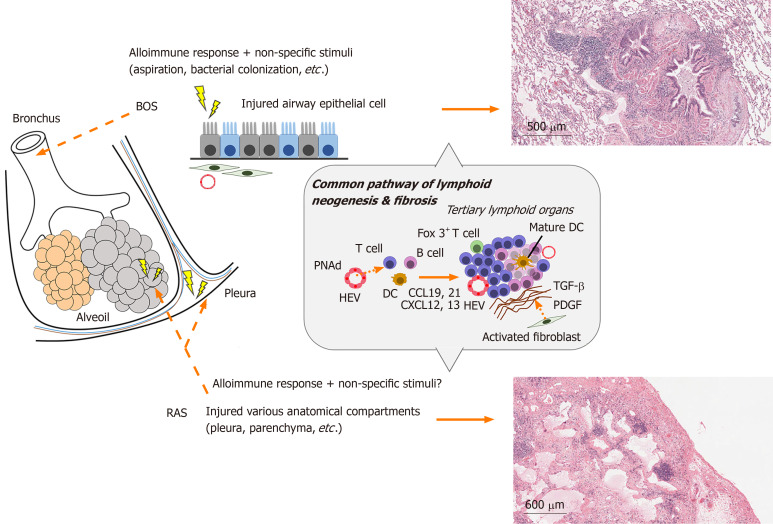
When inflammation persists chronically, the activation of resident stromal cells such as epithelial and endothelial cells plays an important role in immune responses. The resident stromal cells induce immune cells in transplanted lungs by expressing ectopic lymphoid chemokines and adhesion molecules (Figure 2). CCL21 attracts DCs and T cells, CXCL12 attracts immature DCs, and CXCL13 attracts B cells[74-76]. Furthermore, the peripheral lymph node address (PNAd) of adhesion molecules is expressed by endothelial cells and induces lymphocyte extravasation from the blood circulation[77]. Indeed, among lung transplant recipients that developed OB, BALT contained effector memory T cells and high endothelial venules (HEV) characterized by the expression of PNAd[68]. Previous reports provided evidence showing ectopic lymphoid tissues in chronically rejected grafts in the heart and kidney play a role in generating local humoral alloimmune responses[72]. Moreover, in a recent study of mouse orthotopic lung transplantation, chronic rejection after ischemia-reperfusion injury showed an increase in B cells in TLS of the graft[78]. Thus, BALT may be associated with rejection after lung transplantation by inducing immune cells. In our investigation on the development of OB after human lung transplantation, we showed that lymphoid tissue was generated around small airways[68], which was thought to be a pathological feature of BOS. Furthermore, in another animal model of OB, lymphoid neogenesis in the lung contributed to allograft airway rejection[68]. However, the association between BALT and CLAD (BOS or RAS) remains controversial because another group confirmed lymphoid follicles in transplanted lungs affected by RAS but not BOS[79]. However, in transplanted lungs, which maintained allograft tolerance, the induction of BALT was related to the local immune static state[80]. Of note, the existence of Foxp3+ T (Regulatory T; Treg) cells in BALT was considered a key factor to prevent CLAD after lung transplantation. Mouse lung retransplant studies showed that untreated mice with reimplanted lung allografts survived for a long time after the first 72 hours of engraftment to immunosuppressed recipients[80]. This suggests that immunoregulatory pathways are established within lung allografts after a short period in immunosuppressed hosts. B cells and Treg cells are abundant in BALT. Recently, the development of HEV with the expression of PNAd, and mobilization of B cells was shown to be dependent on IL-22 but not on Treg cells in intra-graft BALT[81]. Treg cells maintain tolerance of the autoimmune system by controlling the activity of effector T cells. Furthermore, they inhibited Th1 autoimmunity by inducing IL-10-producing T cells following human lung transplantation[82] and prevented AMR by inhibiting the local activation of B cells. In a study of the long-term peripheral blood dynamics of CD4+ CD25high CD127− Treg cells in lung recipients with CLAD, the number of Treg cells gradually decreased with the higher severity of CLAD. High numbers of Treg cells was associated with a low risk of CLAD development[83]. Thus, Treg cells are considered to play a major role in maintaining “calm” status after transplantation[84]. However, there have been no studies on the association of Treg cells and the two phenotypes in CLAD.
Figure 2.
Anatomical and histopathological differences between bronchiolitis obliterans syndrome and restrictive allograft syndrome based on immune responses in transplanted lungs. BOS: Bronchiolitis obliterans syndrome; CCL: CC chemokine ligand; CXCL: CXC chemokine ligand; DC: Dendritic cell; HEV: High endothelial venules; PDGF: Platelet-derived growth factor; PNAd: Peripheral node addressin; RAS: Restrictive allograft syndrome; TGF-β: Transforming growth factor beta.
Fibrosis
The representative pathological hallmark of CLAD is obliterans bronchiolitis (OB). Although it is unclear how the two phenotypes of CLAD differentially develop in a patient, it is likely that these factors cause a fibroproliferative response in a transplanted donor lung. BOS is histologically correlated with OB of the terminal bronchioles and results in abnormal remodeling of the airway epithelium, vasculature, stroma, and lymphatic system[85]. The histological findings of RAS include OB and peripheral lesions such as pleural and interlobular hypertrophy. The final morphology includes pleuroparenchymal fibroelastosis, which is frequently dominant in the upper lobe, and this finding was confirmed in half of RAS patients[86]. The different fibrotic sites in the BOS and RAS phenotypes might be explained by the lymphangitic distribution of lymphoid neogenesis.
A potentially important mechanism that promotes the progressive and treatment-resistant nature of BOS and probably that of RAS is a cycle of continuous damage and abnormal fibrotic remodeling[87]. The mechanism of inflammation and tissue remodeling is likely to be multifactorial and complex. Particularly, myofibroblasts play a central role in fibroproliferative airway remodeling by producing large amounts of extracellular matrix in OB after lung transplantation[88], which is probably related to the group of enzymes termed matrix metalloproteinases (MMPs). Immune cells promote airway remodeling through the production of MMP-9[89] or MMP-2, which are expressed by myofibroblasts[90]. The potential origins of myofibroblasts are considered to be (1) tissue-resident fibroblasts; (2) peripheral blood mononuclear cells (PBMC) including bone marrow-derived fibrocytes[88,91]; (3) donor-derived multipotent mesenchymal stem cells (MSC)[92], or (4) the epithelial-to-mesenchymal transition (EMT) of donor cells in transplanted lungs[93]. According to a study analyzing the origin of myofibroblasts in OB lesions after lung transplantation, myofibroblasts were derived from recipient and donor fibroblasts, indicating microchimerism[94]. These results suggest the complexity of fibrosis after lung transplantation. Further research is required to clarify the fibrotic mechanism of these two phenotypes.
ARTICLE HIGHLIGHTS
Currently, CLAD is mainly classified into two clinical phenotypes, BOS and RAS. These mechanisms are not clear but considered to involve complex immune-mediated mechanisms such as innate immunity, cellular immunity, humoral immunity and autoimmunity. Finally, tissue remodeling takes place, resulting in irreversible fibrosis. An apparent histological difference between BOS and RAS is the anatomical locations involved: namely, BOS mainly involves small airways while peripheral lung tissue remains relatively intact, while RAS involves multiple anatomical compartments including airways, pleura, interlobular septum, alveoli, and vasculature. Such difference in the distribution of fibrosis may be associated with different magnitude and quality of immune mechanisms including lymphoid neogenesis.
CONCLUSION
Consensus reports on the international classification of CLAD and the definition of the BOS and RAS subtypes were published in 2019. Although the associated mechanisms are largely unknown, multiple complex immunological pathways including innate immunity and cellular and humoral adaptive immune responses are likely to be involved. Based on these new insights into the refined classification system and recent basic research, strategies for individualized diagnosis and treatment need to be explored further.
Footnotes
Conflict-of-interest statement: Authors declare no conflict of interests for this article.
Manuscript source: Invited manuscript
Peer-review started: February 24, 2020
First decision: April 25, 2020
Article in press: May 12, 2020
Specialty type: Transplantation
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen JY S-Editor: Gong ZM L-Editor: A E-Editor: Liu MY
Contributor Information
Nobuyuki Yoshiyasu, Department of Thoracic Surgery, The University of Tokyo Hospital, Tokyo 113-8655, Japan.
Masaaki Sato, Department of Thoracic Surgery, The University of Tokyo Hospital, Tokyo 113-8655, Japan. satom-sur@h.u-tokyo.ac.jp.
References
- 1.Toronto Lung Transplant Group. Unilateral lung transplantation for pulmonary fibrosis. N Engl J Med. 1986;314:1140–1145. doi: 10.1056/NEJM198605013141802. [DOI] [PubMed] [Google Scholar]
- 2.Yusen RD, Edwards LB, Dipchand AI, Goldfarb SB, Kucheryavaya AY, Levvey BJ, Lund LH, Meiser B, Rossano JW, Stehlik J International Society for Heart and Lung Transplantation. The Registry of the International Society for Heart and Lung Transplantation: Thirty-third Adult Lung and Heart-Lung Transplant Report-2016; Focus Theme: Primary Diagnostic Indications for Transplant. J Heart Lung Transplant. 2016;35:1170–1184. doi: 10.1016/j.healun.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Thabut G, Christie JD, Kremers WK, Fournier M, Halpern SD. Survival differences following lung transplantation among US transplant centers. JAMA. 2010;304:53–60. doi: 10.1001/jama.2010.885. [DOI] [PubMed] [Google Scholar]
- 4.2003 annual report of the US Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients. Rockville, MD, and Richmond, VA: U.S. Department of Health and Human Services/Health Resources and Services Administration and United Network for Organ Sharing; 2004.
- 5.Heng D, Sharples LD, McNeil K, Stewart S, Wreghitt T, Wallwork J. Bronchiolitis obliterans syndrome: incidence, natural history, prognosis, and risk factors. J Heart Lung Transplant. 1998;17:1255–1263. [PubMed] [Google Scholar]
- 6.Verleden GM, Vos R, Verleden SE, De Wever W, De Vleeschauwer SI, Willems-Widyastuti A, Scheers H, Dupont LJ, Van Raemdonck DE, Vanaudenaerde BM. Survival determinants in lung transplant patients with chronic allograft dysfunction. Transplantation. 2011;92:703–708. doi: 10.1097/TP.0b013e31822bf790. [DOI] [PubMed] [Google Scholar]
- 7.Daud SA, Yusen RD, Meyers BF, Chakinala MM, Walter MJ, Aloush AA, Patterson GA, Trulock EP, Hachem RR. Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2007;175:507–513. doi: 10.1164/rccm.200608-1079OC. [DOI] [PubMed] [Google Scholar]
- 8.Keenan RJ, Lega ME, Dummer JS, Paradis IL, Dauber JH, Rabinowich H, Yousem SA, Hardesty RL, Griffith BP, Duquesnoy RJ. Cytomegalovirus serologic status and postoperative infection correlated with risk of developing chronic rejection after pulmonary transplantation. Transplantation. 1991;51:433–438. doi: 10.1097/00007890-199102000-00032. [DOI] [PubMed] [Google Scholar]
- 9.Smith MA, Sundaresan S, Mohanakumar T, Trulock EP, Lynch JP, Phelan DL, Cooper JD, Patterson GA. Effect of development of antibodies to HLA and cytomegalovirus mismatch on lung transplantation survival and development of bronchiolitis obliterans syndrome. J Thorac Cardiovasc Surg. 1998;116:812–820. doi: 10.1016/S0022-5223(98)00444-9. [DOI] [PubMed] [Google Scholar]
- 10.Vilchez RA, Dauber J, Kusne S. Infectious etiology of bronchiolitis obliterans: the respiratory viruses connection - myth or reality? Am J Transplant. 2003;3:245–249. doi: 10.1034/j.1600-6143.2003.00056.x. [DOI] [PubMed] [Google Scholar]
- 11.Kumar D, Erdman D, Keshavjee S, Peret T, Tellier R, Hadjiliadis D, Johnson G, Ayers M, Siegal D, Humar A. Clinical impact of community-acquired respiratory viruses on bronchiolitis obliterans after lung transplant. Am J Transplant. 2005;5:2031–2036. doi: 10.1111/j.1600-6143.2005.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D'Ovidio F, Mura M, Ridsdale R, Takahashi H, Waddell TK, Hutcheon M, Hadjiliadis D, Singer LG, Pierre A, Chaparro C, Gutierrez C, Miller L, Darling G, Liu M, Post M, Keshavjee S. The effect of reflux and bile acid aspiration on the lung allograft and its surfactant and innate immunity molecules SP-A and SP-D. Am J Transplant. 2006;6:1930–1938. doi: 10.1111/j.1600-6143.2006.01357.x. [DOI] [PubMed] [Google Scholar]
- 13.Blondeau K, Mertens V, Vanaudenaerde BA, Verleden GM, Van Raemdonck DE, Sifrim D, Dupont LJ. Gastro-oesophageal reflux and gastric aspiration in lung transplant patients with or without chronic rejection. Eur Respir J. 2008;31:707–713. doi: 10.1183/09031936.00064807. [DOI] [PubMed] [Google Scholar]
- 14.Cooper JD, Billingham M, Egan T, Hertz MI, Higenbottam T, Lynch J, Mauer J, Paradis I, Patterson GA, Smith C. A working formulation for the standardization of nomenclature and for clinical staging of chronic dysfunction in lung allografts. International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 1993;12:713–716. [PubMed] [Google Scholar]
- 15.Martinu T, Howell DN, Davis RD, Steele MP, Palmer SM. Pathologic correlates of bronchiolitis obliterans syndrome in pulmonary retransplant recipients. Chest. 2006;129:1016–1023. doi: 10.1378/chest.129.4.1016. [DOI] [PubMed] [Google Scholar]
- 16.Stewart S, Fishbein MC, Snell GI, Berry GJ, Boehler A, Burke MM, Glanville A, Gould FK, Magro C, Marboe CC, McNeil KD, Reed EF, Reinsmoen NL, Scott JP, Studer SM, Tazelaar HD, Wallwork JL, Westall G, Zamora MR, Zeevi A, Yousem SA. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26:1229–1242. doi: 10.1016/j.healun.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 17.Sato M, Waddell TK, Wagnetz U, Roberts HC, Hwang DM, Haroon A, Wagnetz D, Chaparro C, Singer LG, Hutcheon MA, Keshavjee S. Restrictive allograft syndrome (RAS): a novel form of chronic lung allograft dysfunction. J Heart Lung Transplant. 2011;30:735–742. doi: 10.1016/j.healun.2011.01.712. [DOI] [PubMed] [Google Scholar]
- 18.Sato M, Ohmori-Matsuda K, Saito T, Matsuda Y, Hwang DM, Waddell TK, Singer LG, Keshavjee S. Time-dependent changes in the risk of death in pure bronchiolitis obliterans syndrome (BOS) J Heart Lung Transplant. 2013;32:484–491. doi: 10.1016/j.healun.2013.01.1054. [DOI] [PubMed] [Google Scholar]
- 19.Kulkarni HS, Cherikh WS, Chambers DC, Garcia VC, Hachem RR, Kreisel D, Puri V, Kozower BD, Byers DE, Witt CA, Alexander-Brett J, Aguilar PR, Tague LK, Furuya Y, Patterson GA, Trulock EP, 3rd, Yusen RD. Bronchiolitis obliterans syndrome-free survival after lung transplantation: An International Society for Heart and Lung Transplantation Thoracic Transplant Registry analysis. J Heart Lung Transplant. 2019;38:5–16. doi: 10.1016/j.healun.2018.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glanville AR, Verleden GM, Todd JL, Benden C, Calabrese F, Gottlieb J, Hachem RR, Levine D, Meloni F, Palmer SM, Roman A, Sato M, Singer LG, Tokman S, Verleden SE, von der Thüsen J, Vos R, Snell G. Chronic lung allograft dysfunction: Definition and update of restrictive allograft syndrome-A consensus report from the Pulmonary Council of the ISHLT. J Heart Lung Transplant. 2019;38:483–492. doi: 10.1016/j.healun.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Verleden GM, Glanville AR, Lease ED, Fisher AJ, Calabrese F, Corris PA, Ensor CR, Gottlieb J, Hachem RR, Lama V, Martinu T, Neil DAH, Singer LG, Snell G, Vos R. Chronic lung allograft dysfunction: Definition, diagnostic criteria, and approaches to treatment-A consensus report from the Pulmonary Council of the ISHLT. J Heart Lung Transplant. 2019;38:493–503. doi: 10.1016/j.healun.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 22.Estenne M, Maurer JR, Boehler A, Egan JJ, Frost A, Hertz M, Mallory GB, Snell GI, Yousem S. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21:297–310. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 23.Verleden SE, Ruttens D, Vandermeulen E, Vaneylen A, Dupont LJ, Van Raemdonck DE, Verleden GM, Vanaudenaerde BM, Vos R. Bronchiolitis obliterans syndrome and restrictive allograft syndrome: do risk factors differ? Transplantation. 2013;95:1167–1172. doi: 10.1097/TP.0b013e318286e076. [DOI] [PubMed] [Google Scholar]
- 24.Sato M, Hwang DM, Ohmori-Matsuda K, Chaparro C, Waddell TK, Singer LG, Hutcheon MA, Keshavjee S. Revisiting the pathologic finding of diffuse alveolar damage after lung transplantation. J Heart Lung Transplant. 2012;31:354–363. doi: 10.1016/j.healun.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 25.Vandermeulen E, Verleden SE, Bellon H, Ruttens D, Lammertyn E, Claes S, Vandooren J, Ugarte-Berzal E, Schols D, Emonds MP, Van Raemdonck DE, Opdenakker G, Verleden GM, Vos R, Vanaudenaerde BM. Humoral immunity in phenotypes of chronic lung allograft dysfunction: A broncho-alveolar lavage fluid analysis. Transpl Immunol. 2016;38:27–32. doi: 10.1016/j.trim.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Koutsokera A, Royer PJ, Antonietti JP, Fritz A, Benden C, Aubert JD, Tissot A, Botturi K, Roux A, Reynaud-Gaubert ML, Kessler R, Dromer C, Mussot S, Mal H, Mornex JF, Guillemain R, Knoop C, Dahan M, Soccal PM, Claustre J, Sage E, Gomez C, Magnan A, Pison C, Nicod LP SysCLAD Consortium. Development of a Multivariate Prediction Model for Early-Onset Bronchiolitis Obliterans Syndrome and Restrictive Allograft Syndrome in Lung Transplantation. Front Med (Lausanne) 2017;4:109. doi: 10.3389/fmed.2017.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Todd JL, Jain R, Pavlisko EN, Finlen Copeland CA, Reynolds JM, Snyder LD, Palmer SM. Impact of forced vital capacity loss on survival after the onset of chronic lung allograft dysfunction. Am J Respir Crit Care Med. 2014;189:159–166. doi: 10.1164/rccm.201306-1155OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walton DC, Hiho SJ, Cantwell LS, Diviney MB, Wright ST, Snell GI, Paraskeva MA, Westall GP. HLA Matching at the Eplet Level Protects Against Chronic Lung Allograft Dysfunction. Am J Transplant. 2016;16:2695–2703. doi: 10.1111/ajt.13798. [DOI] [PubMed] [Google Scholar]
- 29.Verleden SE, Gottlieb J, Dubbeldam A, Verleden GM, Suhling H, Welte T, Vos R, Greer M. "White-Out" After Lung Transplantation: A Multicenter Cohort Description of Late Acute Graft Failure. Am J Transplant. 2017;17:1905–1911. doi: 10.1111/ajt.14268. [DOI] [PubMed] [Google Scholar]
- 30.Takahagi A, Sato M, Chen-Yoshikawa TF, Miyamoto E, Saito M, Gochi F, Hamaji M, Yoshizawa A, Terasaki Y, Urushiyama H, Aoyama A, Sonobe M, Date H. LPS-induced airway-centered inflammation leading to BOS-like airway remodeling distinct from RAS-like fibrosis in rat lung transplantation. Transplantation. 2020:Online ahead of print. doi: 10.1097/TP.0000000000003097. [DOI] [PubMed] [Google Scholar]
- 31.Ruddle NH, Akirav EM. Secondary lymphoid organs: responding to genetic and environmental cues in ontogeny and the immune response. J Immunol. 2009;183:2205–2212. doi: 10.4049/jimmunol.0804324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7:803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 33.Ingersoll MA, Platt AM, Potteaux S, Randolph GJ. Monocyte trafficking in acute and chronic inflammation. Trends Immunol. 2011;32:470–477. doi: 10.1016/j.it.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawai T, Akira S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int Immunol. 2009;21:317–337. doi: 10.1093/intimm/dxp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 36.Baccala R, Hoebe K, Kono DH, Beutler B, Theofilopoulos AN. TLR-dependent and TLR-independent pathways of type I interferon induction in systemic autoimmunity. Nat Med. 2007;13:543–551. doi: 10.1038/nm1590. [DOI] [PubMed] [Google Scholar]
- 37.Nawrot TS, Vos R, Jacobs L, Verleden SE, Wauters S, Mertens V, Dooms C, Hoet PH, Van Raemdonck DE, Faes C, Dupont LJ, Nemery B, Verleden GM, Vanaudenaerde BM. The impact of traffic air pollution on bronchiolitis obliterans syndrome and mortality after lung transplantation. Thorax. 2011;66:748–754. doi: 10.1136/thx.2010.155192. [DOI] [PubMed] [Google Scholar]
- 38.Kastelijn EA, van Moorsel CH, Ruven HJ, Lammers JW, Grutters JC. Genetic polymorphisms and bronchiolitis obliterans syndrome after lung transplantation: promising results and recommendations for the future. Transplantation. 2012;93:127–135. doi: 10.1097/TP.0b013e31823915d5. [DOI] [PubMed] [Google Scholar]
- 39.Ding X, Jin S, Tong Y, Jiang X, Chen Z, Mei S, Zhang L, Billiar TR, Li Q. TLR4 signaling induces TLR3 up-regulation in alveolar macrophages during acute lung injury. Sci Rep. 2017;7:34278. doi: 10.1038/srep34278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Atanasova S, Hirschburger M, Jonigk D, Obert M, Petri K, Evers A, Hecker A, Schmitz J, Kaufmann A, Wilhelm J, Chakraborty T, Warnecke G, Gottlieb J, Padberg W, Grau V. A relevant experimental model for human bronchiolitis obliterans syndrome. J Heart Lung Transplant. 2013;32:1131–1139. doi: 10.1016/j.healun.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 41.Takahagi A, Sato M, Chen-Yoshikawa T, Yoshizawa A, Ohata K, Saito M, Okabe R, Gochi F, Yamagishi H, Hamaji M, Motoyama H, Hijiya K, Aoyama A, Date H. Airway-centered inflammation induced by lipopolysaccharide (LPS) leads to BOS-like phenotype of CLAD in rat lung transplantation. J Heart Lung Transplant. 2017;38:48–49. [Google Scholar]
- 42.Kawashima M, Sato M, Murakawa T, Anraku M, Konoeda C, Hosoi A, Kakimi K, Nakajima J. Role of Toll-like Receptor 4 Expressed by Fibroblasts in Allograft Fibrosis in Mouse Orthotopic Tracheal Transplantation. Transplant Proc. 2018;50:3863–3872. doi: 10.1016/j.transproceed.2018.06.023. [DOI] [PubMed] [Google Scholar]
- 43.Evers A, Atanasova S, Fuchs-Moll G, Petri K, Wilker S, Zakrzewicz A, Hirschburger M, Padberg W, Grau V. Adaptive and innate immune responses in a rat orthotopic lung transplant model of chronic lung allograft dysfunction. Transpl Int. 2015;28:95–107. doi: 10.1111/tri.12444. [DOI] [PubMed] [Google Scholar]
- 44.He L, Sun F, Wang Y, Zhu J, Fang J, Zhang S, Yu Q, Gong Q, Ren B, Xiang X, Chen Z, Ning Q, Hu J, Yang P, Wang CY. HMGB1 exacerbates bronchiolitis obliterans syndrome via RAGE/NF-κB/HPSE signaling to enhance latent TGF-β release from ECM. Am J Transl Res. 2016;8:1971–1984. [PMC free article] [PubMed] [Google Scholar]
- 45.Saito T, Liu M, Binnie M, Sato M, Hwang D, Azad S, Machuca TN, Zamel R, Waddell TK, Cypel M, Keshavjee S. Distinct expression patterns of alveolar "alarmins" in subtypes of chronic lung allograft dysfunction. Am J Transplant. 2014;14:1425–1432. doi: 10.1111/ajt.12718. [DOI] [PubMed] [Google Scholar]
- 46.Shastri N, Yewdell JW. Editorial overview: Antigen processing and presentation: Where cellular immunity begins. Curr Opin Immunol. 2015;34:v–vii. doi: 10.1016/j.coi.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 47.Bentzen AK, Hadrup SR. Evolution of MHC-based technologies used for detection of antigen-responsive T cells. Cancer Immunol Immunother. 2017;66:657–666. doi: 10.1007/s00262-017-1971-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gregson AL, Hoji A, Injean P, Poynter ST, Briones C, Palchevskiy V, Weigt SS, Shino MY, Derhovanessian A, Sayah D, Saggar R, Ross D, Ardehali A, Lynch JP, 3rd, Belperio JA. Altered Exosomal RNA Profiles in Bronchoalveolar Lavage from Lung Transplants with Acute Rejection. Am J Respir Crit Care Med. 2015;192:1490–1503. doi: 10.1164/rccm.201503-0558OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gunasekaran M, Sharma M, Hachem R, Bremner R, Smith MA, Mohanakumar T. Circulating Exosomes with Distinct Properties during Chronic Lung Allograft Rejection. J Immunol. 2018;200:2535–2541. doi: 10.4049/jimmunol.1701587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mohanakumar T, Sharma M, Bansal S, Ravichandran R, Smith MA, Bremner RM. A novel mechanism for immune regulation after human lung transplantation. J Thorac Cardiovasc Surg. 2019;157:2096–2106. doi: 10.1016/j.jtcvs.2018.12.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Levine DJ, Glanville AR, Aboyoun C, Belperio J, Benden C, Berry GJ, Hachem R, Hayes D, Jr, Neil D, Reinsmoen NL, Snyder LD, Sweet S, Tyan D, Verleden G, Westall G, Yusen RD, Zamora M, Zeevi A. Antibody-mediated rejection of the lung: A consensus report of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2016;35:397–406. doi: 10.1016/j.healun.2016.01.1223. [DOI] [PubMed] [Google Scholar]
- 52.Palmer SM, Davis RD, Hadjiliadis D, Hertz MI, Howell DN, Ward FE, Savik K, Reinsmoen NL. Development of an antibody specific to major histocompatibility antigens detectable by flow cytometry after lung transplant is associated with bronchiolitis obliterans syndrome. Transplantation. 2002;74:799–804. doi: 10.1097/00007890-200209270-00011. [DOI] [PubMed] [Google Scholar]
- 53.Brugière O, Suberbielle C, Thabut G, Lhuillier E, Dauriat G, Metivier AC, Gautreau C, Charron D, Mal H, Parquin F, Stern M. Lung transplantation in patients with pretransplantation donor-specific antibodies detected by Luminex assay. Transplantation. 2013;95:761–765. doi: 10.1097/TP.0b013e31827afb0f. [DOI] [PubMed] [Google Scholar]
- 54.Valenzuela NM, McNamara JT, Reed EF. Antibody-mediated graft injury: complement-dependent and complement-independent mechanisms. Curr Opin Organ Transplant. 2014;19:33–40. doi: 10.1097/MOT.0000000000000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jaramillo A, Smith CR, Maruyama T, Zhang L, Patterson GA, Mohanakumar T. Anti-HLA class I antibody binding to airway epithelial cells induces production of fibrogenic growth factors and apoptotic cell death: a possible mechanism for bronchiolitis obliterans syndrome. Hum Immunol. 2003;64:521–529. doi: 10.1016/s0198-8859(03)00038-7. [DOI] [PubMed] [Google Scholar]
- 56.Verleden SE, Vanaudenaerde BM, Emonds MP, Van Raemdonck DE, Neyrinck AP, Verleden GM, Vos R. Donor-specific and -nonspecific HLA antibodies and outcome post lung transplantation. Eur Respir J. 2017;50 doi: 10.1183/13993003.01248-2017. [DOI] [PubMed] [Google Scholar]
- 57.Hadjiliadis D, Chaparro C, Reinsmoen NL, Gutierrez C, Singer LG, Steele MP, Waddell TK, Davis RD, Hutcheon MA, Palmer SM, Keshavjee S. Pre-transplant panel reactive antibody in lung transplant recipients is associated with significantly worse post-transplant survival in a multicenter study. J Heart Lung Transplant. 2005;24:S249–S254. doi: 10.1016/j.healun.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 58.Le Pavec J, Suberbielle C, Lamrani L, Feuillet S, Savale L, Dorfmüller P, Stephan F, Mussot S, Mercier O, Fadel E. De-novo donor-specific anti-HLA antibodies 30 days after lung transplantation are associated with a worse outcome. J Heart Lung Transplant. 2016;35:1067–1077. doi: 10.1016/j.healun.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 59.Hachem RR, Yusen RD, Meyers BF, Aloush AA, Mohanakumar T, Patterson GA, Trulock EP. Anti-human leukocyte antigen antibodies and preemptive antibody-directed therapy after lung transplantation. J Heart Lung Transplant. 2010;29:973–980. doi: 10.1016/j.healun.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, Castro MC, David DS, David-Neto E, Bagnasco SM, Cendales LC, Cornell LD, Demetris AJ, Drachenberg CB, Farver CF, Farris AB, 3rd, Gibson IW, Kraus E, Liapis H, Loupy A, Nickeleit V, Randhawa P, Rodriguez ER, Rush D, Smith RN, Tan CD, Wallace WD, Mengel M Banff meeting report writing committee. Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014;14:272–283. doi: 10.1111/ajt.12590. [DOI] [PubMed] [Google Scholar]
- 61.Sacreas A, Taupin JL, Emonds MP, Daniëls L, Van Raemdonck DE, Vos R, Verleden GM, Vanaudenaerde BM, Roux A, Verleden SE and the Leuven Lung Transplant Group. Intragraft donor-specific anti-HLA antibodies in phenotypes of chronic lung allograft dysfunction. Eur Respir J. 2019;54 doi: 10.1183/13993003.00847-2019. [DOI] [PubMed] [Google Scholar]
- 62.Miyamoto E, Motoyama H, Sato M, Aoyama A, Menju T, Shikuma K, Sowa T, Yoshizawa A, Saito M, Takahagi A, Tanaka S, Takahashi M, Ohata K, Kondo T, Hijiya K, Chen-Yoshikawa TF, Date H. Association of Local Intrapulmonary Production of Antibodies Specific to Donor Major Histocompatibility Complex Class I With the Progression of Chronic Rejection of Lung Allografts. Transplantation. 2017;101:e156–e165. doi: 10.1097/TP.0000000000001665. [DOI] [PubMed] [Google Scholar]
- 63.Haque MA, Mizobuchi T, Yasufuku K, Fujisawa T, Brutkiewicz RR, Zheng Y, Woods K, Smith GN, Cummings OW, Heidler KM, Blum JS, Wilkes DS. Evidence for immune responses to a self-antigen in lung transplantation: role of type V collagen-specific T cells in the pathogenesis of lung allograft rejection. J Immunol. 2002;169:1542–1549. doi: 10.4049/jimmunol.169.3.1542. [DOI] [PubMed] [Google Scholar]
- 64.Tiriveedhi V, Gautam B, Sarma NJ, Askar M, Budev M, Aloush A, Hachem R, Trulock E, Myers B, Patterson AG, Mohanakumar T. Pre-transplant antibodies to Kα1 tubulin and collagen-V in lung transplantation: clinical correlations. J Heart Lung Transplant. 2013;32:807–814. doi: 10.1016/j.healun.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shilling RA, Wilkes DS. Role of Th17 cells and IL-17 in lung transplant rejection. Semin Immunopathol. 2011;33:129–134. doi: 10.1007/s00281-011-0257-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Burlingham WJ, Love RB, Jankowska-Gan E, Haynes LD, Xu Q, Bobadilla JL, Meyer KC, Hayney MS, Braun RK, Greenspan DS, Gopalakrishnan B, Cai J, Brand DD, Yoshida S, Cummings OW, Wilkes DS. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest. 2007;117:3498–3506. doi: 10.1172/JCI28031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tiriveedhi V, Angaswamy N, Brand D, Weber J, Gelman AG, Hachem R, Trulock EP, Meyers B, Patterson G, Mohanakumar T. A shift in the collagen V antigenic epitope leads to T helper phenotype switch and immune response to self-antigen leading to chronic lung allograft rejection. Clin Exp Immunol. 2012;167:158–168. doi: 10.1111/j.1365-2249.2011.04486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sato M, Hirayama S, Hwang DM, Lara-Guerra H, Wagnetz D, Waddell TK, Liu M, Keshavjee S. The role of intrapulmonary de novo lymphoid tissue in obliterative bronchiolitis after lung transplantation. J Immunol. 2009;182:7307–7316. doi: 10.4049/jimmunol.0803606. [DOI] [PubMed] [Google Scholar]
- 69.Yadava K, Bollyky P, Lawson MA. The formation and function of tertiary lymphoid follicles in chronic pulmonary inflammation. Immunology. 2016;149:262–269. doi: 10.1111/imm.12649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Silva-Sanchez A, Randall TD. Role of iBALT in Respiratory Immunity. Curr Top Microbiol Immunol. 2020:Online ahead of print. doi: 10.1007/82_2019_191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Araki Y, Mimura T. The Mechanisms Underlying Chronic Inflammation in Rheumatoid Arthritis from the Perspective of the Epigenetic Landscape. J Immunol Res. 2016;2016:6290682. doi: 10.1155/2016/6290682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thaunat O, Field AC, Dai J, Louedec L, Patey N, Bloch MF, Mandet C, Belair MF, Bruneval P, Meilhac O, Bellon B, Joly E, Michel JB, Nicoletti A. Lymphoid neogenesis in chronic rejection: evidence for a local humoral alloimmune response. Proc Natl Acad Sci USA. 2005;102:14723–14728. doi: 10.1073/pnas.0507223102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dashkevich A, Heilmann C, Kayser G, Germann M, Beyersdorf F, Passlick B, Geissler HJ. Lymph angiogenesis after lung transplantation and relation to acute organ rejection in humans. Ann Thorac Surg. 2010;90:406–411. doi: 10.1016/j.athoracsur.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 74.Parsonage G, Filer AD, Haworth O, Nash GB, Rainger GE, Salmon M, Buckley CD. A stromal address code defined by fibroblasts. Trends Immunol. 2005;26:150–156. doi: 10.1016/j.it.2004.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sato M, Hirayama S, Matsuda Y, Wagnetz D, Hwang DM, Guan Z, Liu M, Keshavjee S. Stromal activation and formation of lymphoid-like stroma in chronic lung allograft dysfunction. Transplantation. 2011;91:1398–1405. doi: 10.1097/TP.0b013e31821b2f7a. [DOI] [PubMed] [Google Scholar]
- 76.Manzo A, Bugatti S, Caporali R, Prevo R, Jackson DG, Uguccioni M, Buckley CD, Montecucco C, Pitzalis C. CCL21 expression pattern of human secondary lymphoid organ stroma is conserved in inflammatory lesions with lymphoid neogenesis. Am J Pathol. 2007;171:1549–1562. doi: 10.2353/ajpath.2007.061275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Girard JP, Springer TA. High endothelial venules (HEVs): specialized endothelium for lymphocyte migration. Immunol Today. 1995;16:449–457. doi: 10.1016/0167-5699(95)80023-9. [DOI] [PubMed] [Google Scholar]
- 78.Watanabe T, Martinu T, Chruscinski A, Boonstra K, Joe B, Horie M, Guan Z, Bei KF, Hwang DM, Liu M, Keshavjee S, Juvet SC. A B cell-dependent pathway drives chronic lung allograft rejection after ischemia-reperfusion injury in mice. Am J Transplant. 2019;19:3377–3389. doi: 10.1111/ajt.15550. [DOI] [PubMed] [Google Scholar]
- 79.Vandermeulen E, Lammertyn E, Verleden SE, Ruttens D, Bellon H, Ricciardi M, Somers J, Bracke KR, Van Den Eynde K, Tousseyn T, Brusselle GG, Verbeken EK, Verschakelen J, Emonds MP, Van Raemdonck DE, Verleden GM, Vos R, Vanaudenaerde BM. Immunological diversity in phenotypes of chronic lung allograft dysfunction: a comprehensive immunohistochemical analysis. Transpl Int. 2017;30:134–143. doi: 10.1111/tri.12882. [DOI] [PubMed] [Google Scholar]
- 80.Li W, Bribriesco AC, Nava RG, Brescia AA, Ibricevic A, Spahn JH, Brody SL, Ritter JH, Gelman AE, Krupnick AS, Miller MJ, Kreisel D. Lung transplant acceptance is facilitated by early events in the graft and is associated with lymphoid neogenesis. Mucosal Immunol. 2012;5:544–554. doi: 10.1038/mi.2012.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tanaka S, Gauthier JM, Fuchs A, Li W, Tong AY, Harrison MS, Higashikubo R, Terada Y, Hachem RR, Ruiz-Perez D, Ritter JH, Cella M, Colonna M, Turnbull IR, Krupnick AS, Gelman AE, Kreisel D. IL-22 is required for the induction of bronchus-associated lymphoid tissue in tolerant lung allografts. Am J Transplant. 2020;20:1251–1261. doi: 10.1111/ajt.15701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bharat A, Fields RC, Steward N, Trulock EP, Patterson GA, Mohanakumar T. CD4+25+ regulatory T cells limit Th1-autoimmunity by inducing IL-10 producing T cells following human lung transplantation. Am J Transplant. 2006;6:1799–1808. doi: 10.1111/j.1600-6143.2006.01383.x. [DOI] [PubMed] [Google Scholar]
- 83.Piloni D, Morosini M, Magni S, Balderacchi A, Scudeller L, Cova E, Oggionni T, Stella G, Tinelli C, Antonacci F, D'Armini AM, Meloni F. Analysis of long term CD4+CD25highCD127- T-reg cells kinetics in peripheral blood of lung transplant recipients. BMC Pulm Med. 2017;17:102. doi: 10.1186/s12890-017-0446-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li W, Gauthier JM, Higashikubo R, Hsiao HM, Tanaka S, Vuong L, Ritter JH, Tong AY, Wong BW, Hachem RR, Puri V, Bharat A, Krupnick AS, Hsieh CS, Baldwin WM, 3rd, Kelly FL, Palmer SM, Gelman AE, Kreisel D. Bronchus-associated lymphoid tissue-resident Foxp3+ T lymphocytes prevent antibody-mediated lung rejection. J Clin Invest. 2019;129:556–568. doi: 10.1172/JCI122083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sato M, Keshavjee S. Bronchiolitis obliterans syndrome: alloimmune-dependent and -independent injury with aberrant tissue remodeling. Semin Thorac Cardiovasc Surg. 2008;20:173–182. doi: 10.1053/j.semtcvs.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 86.Ofek E, Sato M, Saito T, Wagnetz U, Roberts HC, Chaparro C, Waddell TK, Singer LG, Hutcheon MA, Keshavjee S, Hwang DM. Restrictive allograft syndrome post lung transplantation is characterized by pleuroparenchymal fibroelastosis. Mod Pathol. 2013;26:350–356. doi: 10.1038/modpathol.2012.171. [DOI] [PubMed] [Google Scholar]
- 87.Belperio JA, Keane MP, Burdick MD, Gomperts B, Xue YY, Hong K, Mestas J, Ardehali A, Mehrad B, Saggar R, Lynch JP, Ross DJ, Strieter RM. Role of CXCR2/CXCR2 ligands in vascular remodeling during bronchiolitis obliterans syndrome. J Clin Invest. 2005;115:1150–1162. doi: 10.1172/JCI24233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sato M, Hirayama S, Lara-Guerra H, Anraku M, Waddell TK, Liu M, Keshavjee S. MMP-dependent migration of extrapulmonary myofibroblast progenitors contributing to posttransplant airway fibrosis in the lung. Am J Transplant. 2009;9:1027–1036. doi: 10.1111/j.1600-6143.2009.02605.x. [DOI] [PubMed] [Google Scholar]
- 89.Pain M, Royer PJ, Loy J, Girardeau A, Tissot A, Lacoste P, Roux A, Reynaud-Gaubert M, Kessler R, Mussot S, Dromer C, Brugière O, Mornex JF, Guillemain R, Dahan M, Knoop C, Botturi K, Pison C, Danger R, Brouard S, Magnan A COLT Consortium. T Cells Promote Bronchial Epithelial Cell Secretion of Matrix Metalloproteinase-9 via a C-C Chemokine Receptor Type 2 Pathway: Implications for Chronic Lung Allograft Dysfunction. Am J Transplant. 2017;17:1502–1514. doi: 10.1111/ajt.14166. [DOI] [PubMed] [Google Scholar]
- 90.Sato M, Hwang DM, Guan Z, Yeung JC, Anraku M, Wagnetz D, Hirayama S, Waddell TK, Liu M, Keshavjee S. Regression of allograft airway fibrosis: the role of MMP-dependent tissue remodeling in obliterative bronchiolitis after lung transplantation. Am J Pathol. 2011;179:1287–1300. doi: 10.1016/j.ajpath.2011.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.LaPar DJ, Burdick MD, Emaminia A, Harris DA, Strieter BA, Liu L, Robbins M, Kron IL, Strieter RM, Lau CL. Circulating fibrocytes correlate with bronchiolitis obliterans syndrome development after lung transplantation: a novel clinical biomarker. Ann Thorac Surg. 2011;92:470–7; discussion 477. doi: 10.1016/j.athoracsur.2011.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Walker N, Badri L, Wettlaufer S, Flint A, Sajjan U, Krebsbach PH, Keshamouni VG, Peters-Golden M, Lama VN. Resident tissue-specific mesenchymal progenitor cells contribute to fibrogenesis in human lung allografts. Am J Pathol. 2011;178:2461–2469. doi: 10.1016/j.ajpath.2011.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Borthwick LA, Parker SM, Brougham KA, Johnson GE, Gorowiec MR, Ward C, Lordan JL, Corris PA, Kirby JA, Fisher AJ. Epithelial to mesenchymal transition (EMT) and airway remodelling after human lung transplantation. Thorax. 2009;64:770–777. doi: 10.1136/thx.2008.104133. [DOI] [PubMed] [Google Scholar]
- 94.Bröcker V, Länger F, Fellous TG, Mengel M, Brittan M, Bredt M, Milde S, Welte T, Eder M, Haverich A, Alison MR, Kreipe H, Lehmann U. Fibroblasts of recipient origin contribute to bronchiolitis obliterans in human lung transplants. Am J Respir Crit Care Med. 2006;173:1276–1282. doi: 10.1164/rccm.200509-1381OC. [DOI] [PubMed] [Google Scholar]