Abstract
Objectives
Occupational exposure to trichloroethylene (TCE) induces trichloroethylene hypersensitivity syndrome (TCEHS), which causes hypersensitivity dermatitis and hepatitis. However, whether TCE itself or its two metabolites, trichloroethanol (TCEOH) and trichloroacetic acid (TCA), are involved in TCEHS remains unclear. Therefore, in this study we explored the allergens causing TCEHS and characterized TCEHS‐related liver injury in guinea pigs.
Method
The guinea pig maximization test was performed using TCE, TCEOH, and TCA as candidate allergens. Skin inflammation was scored, and liver function and histopathological changes were evaluated by biochemical tests and hematoxylin and eosin staining, respectively.
Results
The sensitization rates for TCE, TCEOH, and TCA were 90.0%, 50.0%, and 0.0%, respectively. In the TCE and TCEOH experimental groups, the skin showed varying degrees of erythema with eosinophil granulocyte infiltration in the dermis. Additionally, serum alanine aminotransferase and γ‐glutamyl transpeptidase levels increased significantly, and histological analysis revealed focal hepatocellular necrosis with inflammatory cell infiltration in the liver.
Conclusions
TCE is the main cause of allergy and TCEOH is a secondary factor for allergy in guinea pigs. TCE and TCEOH can cause immune‐mediated skin sensitization complicated by focal hepatic necrosis.
Keywords: guinea pig maximization test, hepatic necrosis, skin sensitization, trichloroethylene
1. INTRODUCTION
Trichloroethylene (TCE) is widely used to clean metal parts and electronic components used in industrial processes, mainly in Asian countries because it is an incombustible, cheap, and powerful cleaning agent; however, its use has been decreasing in more developed countries. In the past 30 years, a hypersensitivity syndrome induced by TCE has gradually become one of the most serious occupational diseases in Guangdong, China. This hypersensitivity syndrome was named “Occupational Dermatitis Medicamentosa‐like of TCE” in China, which was also called occupational TCE hypersensitivity syndrome (TCEHS). TCEHS is characterized by fever, a generalized rash, liver dysfunction (indicated by alanine aminotransferase [ALT] and γ‐glutamyl transpeptidase [γ‐GTP] activities), and superficial lymphadenopathy. 1 According to the Chinese National Diagnostic Criteria, TCEHS has four dermatological manifestations: exfoliative dermatitis, erythema multiforme, Stevens‐Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN). 2 , 3
Studies have shown that TCEHS is an immunological disease. Clinical data suggest that type IV allergic reactions or delayed‐type hypersensitivity reactions play an important role in TCEHS. 4 In addition to severe hypersensitivity skin disorders, liver immune damage is also reported in TCEHS. 5 However, whether TCEHS is triggered by TCE or by its metabolites is still debatable. Trichloroethanol (TCEOH) and trichloroacetic acid (TCA) are the two main metabolites of TCE, which is metabolized by cytochrome P450 2E1 (CYP2E1) in the liver. 6 Recent studies on animal exposure to TCE and its immune‐related effects have suggested that TCE is a potent immunomodulator that accelerates allergic disease development. The guinea pig maximization test (GPMT) is widely used as a national standard test in many countries. 7 Using the GPMT, Tang et al 8 reported that TCE appears to be a strong allergen, whereas TCA is a moderate allergen. 8 They also showed that TCE induces liver injury with diffuse ballooning changes without lymphocyte infiltration and necrosis. 9 However, this liver damage was histopathologically different from that of TCEHS patients, who showed hepatic necrosis with inflammatory cell infiltration. 10 To further confirm the allergens of TCEHS and explore the skin and liver damage caused, GPMT was used in this study to assess the hypersensitivity induced by TCE, TCEOH, and TCA.
2. MATERIALS AND METHODS
2.1. Animals
Specific‐pathogen‐free female Hartley guinea pigs, weighing 300‐400 g, were obtained from the Guangdong Medical Laboratory Animal Center (No. 2008A027). All guinea pigs were healthy, nulliparous, and nonpregnant. They were housed in cages (five guinea pigs per cage) at a constant temperature of 20°C‐25°C in a 12/12 h light/dark cycle. Food and water were provided ad libitum. After 1 week of adaptive feeding in the laboratory, the guinea pigs were randomly divided into eight groups: TCE, TCEOH, and TCA experimental groups (n = 10 each); TCE, TCEOH, and TCA solvent control groups (n = 5 each); one positive control group (2,4‐dinitrochlorobenzene [DNCB]) (n = 5); and a blank group (n = 5).
The use of guinea pigs and the experimental protocol were approved by the Animal Care and Use Committee of the Guangdong Province Hospital for Occupational Disease Prevention and Treatment (approval no. GDHOD MEC 2015020).
2.2. Preparation of chemicals
TCE (Sigma‐Aldrich, St Louis, MO, USA) and TCEOH (Fluka, Sigma‐Aldrich) were mixed in olive oil (Sigma‐Aldrich), and TCA (Tianjin Chemical Reagent Second Factory, Tianjin, China) was mixed with saline to prepare different concentrations of the test substance. On the basis of studies by other investigators, 8 , 11 a pilot study was carried out using three animals for dose selection. The concentration of test substance used for each induction exposure was well‐tolerated, and the highest concentration caused mild‐to‐moderate skin irritation, while the concentration used for the challenge exposure was the highest nonirritant dose. Thus, 5%, 20%, and 10% TCE (v/v) in olive oil were administered to the TCE experimental group via intradermal injection, topical induction, and challenge, respectively; 1.25%, 20%, and 10% TCEOH (v/v) in olive oil were administered to the TCEOH experimental group via intradermal injection, topical induction, and challenge, respectively; and 0.25%, 5%, and 2.5% TCA (m/v) in physiological saline were administered to the TCA experimental group via intradermal injection, topical induction, and challenge, respectively. Freund's complete adjuvant (FCA) was purchased from Sigma‐Aldrich, and DNCB was obtained from the Tokyo Chemical Industry (Tokyo, Japan).
2.3. Guinea pigs maximization test
The GPMT (Figure 1) was performed in accordance with the Organisation for Economic Co‐operation and Development guideline for testing of chemicals (no. 406). According to this guideline, DNCB is recommended to be used as a positive control for the GPMT. Before intradermal injection, a 4 cm × 6 cm area on both sides of the scapular region was shaved using an electric clipper. Then, 24 hours after shaving, each guinea pig was administered three pairs of 0.1 mL intradermal injections: (a) FCA, (b) the test substance (5% TCE, 1.25% TCEOH, or 0.25% TCA), and (c) the test substance at the selected concentration formulated in a 1:1 mixture (v/v) FCA. All control groups were injected with olive oil or physiological saline instead of the test substance.
FIGURE 1.
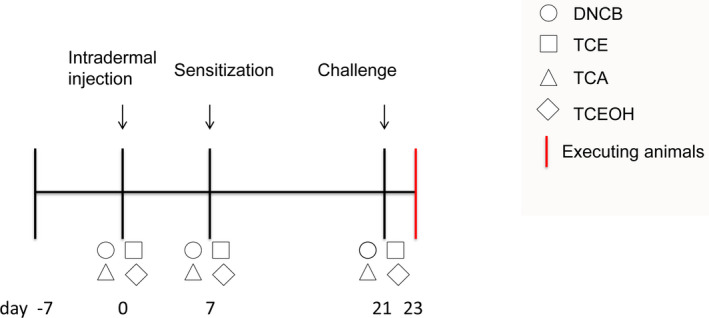
Experimental schedule for GPMT induction. After 7 days of adaptive feeding, DNCB, TCE, TCEOH, and TCA were administered to the back of guinea pigs via intradermal injection. After 7 days, the four test substances were applied on the back of guinea pigs for sensitization. After 21 days, the guinea pigs were challenged with DNCB, TCE, TCEOH, and TCA and then euthanized on day 23. GPMT, guinea pig maximization test; DNCB, 2,4‐dinitrochlorobenzene; TCE, trichloroethylene; TCEOH, trichloroethanol; TCA, trichloroacetic acid
Next, 7 days after injection, 0.5 mL of 20% TCE, 20% TCEOH, and 5% TCA for the TCE, TCEOH, and TCA experimental groups, respectively, were patched using nonirritant tape on the backs of all guinea pigs for 48 hours in the second induction stage. The solvent control groups were administered only olive oil or physiological saline. In the challenge patch test stage, 21 days after the first injection, all guinea pigs were challenged with 0.5 mL of the test substance (10% TCE, 10% TCEOH, or 2.5% TCA) patched on their shaved flanks for 24 hours. Patch test responses were evaluated 24 hours after removing the patches. Allergenic reactions were scored using the Magnusson‐Kligman grading scale to evaluate challenge patch test reactions as follows: 0, no visible change; 1, discrete or patchy erythema; 2, moderate, confluent erythema; and 3, intense erythema and swelling. The responses were read again 48 hours after removing the patches, and the frequency of sensitization was calculated by blind reading of the experimental and control groups.
2.4. Histopathological examination of the skin and liver
Each experimental group was further subdivided into dermatitis‐positive and dermatitis‐negative groups on the basis of skin allergenic reactions. After the challenge patch test stage, all guinea pigs were weighed and then euthanized using diethyl ether. Blood was collected via an abdominal aortic puncture and then centrifuged at 3000 rpm for 15 minutes at 4°C to obtain a serum fraction. The livers were quickly removed and weighed. Skin from the test and control sites as well as a small portion of the liver were soaked in 10% formalin (v/v) for fixation. The tissues were embedded in paraffin, then cut into 3 μm sections, and stained with hematoxylin and eosin (H&E). Three slices from all guinea pigs in each group were observed under an Axioskop 40 microscope (Zeiss, Germany).
2.5. Evaluation of liver function
Serum ALT and γ‐GTP levels were analyzed by the SRL Inc Company (Tokyo, Japan).
2.6. Statistical analysis
All data were expressed as the mean ± standard deviation. One‐way analysis of variance was used to compare multiple groups, followed by the Bonferroni test if variances showed homogeneity or Tamhane's method if they did not. All statistical analyses were based on two‐tailed hypothesis tests and performed using SPSS 13.0 (IBM Corporation, Armonk, NY, USA). P < .05 indicated statistical significance.
3. RESULTS
3.1. Sensitization of the GPMT
Table 1 shows the skin inflammation scores of the guinea pigs. The sensitization rate was 90.0% in the TCE experimental group, 50.0% in the TCEOH experimental group, 0% in the TCA experimental group, and 100% in the DNCB group. Of 10 guinea pigs, six showed patchy erythema, while three showed confluent erythema after TCE challenge. In addition, 5 of the 10 guinea pigs showed patchy erythema after TCEOH challenge, whose reaction was slightly mild compared to the TCE challenge. In contrast, the skin patch test was negative in all solvent control groups (Figure 2A). Compared to the solvent control groups (2‐4 layers), the epidermal stratum spinosum was significantly thickened (6‐10 layers) in TCE and TCEOH dermatitis‐positive groups. Eosinophil granulocyte infiltration in the dermis was observed in the TCE and TCEOH experimental groups, while changes in the TCA experimental and solvent control groups were negative (Figure 2B). These results indicated that both TCE and TCEOH cause hypersensitivity skin reaction in guinea pigs.
TABLE 1.
Sensitization rates and skin inflammation scores in guinea pigs after the GPMT with TCE, TCA, and TCEOH
| Group | Number of guinea pigs (n) | Number of guinea pigs with different skin inflammation scores | Sensitization rate (%) | |||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |||
| Blank | 5 | 5 | 0 | 0 | 0 | 0.0 |
| DNCB | 5 | 0 | 0 | 3 | 2 | 100.0 |
| TCE solvent control | 5 | 5 | 0 | 0 | 0 | 0.0 |
| TCEOH solvent control | 5 | 5 | 0 | 0 | 0 | 0.0 |
| TCA solvent control | 5 | 5 | 0 | 0 | 0 | 0.0 |
| TCE | 10 | 1 | 6 | 3 | 0 | 90.0 |
| TCEOH | 10 | 5 | 5 | 0 | 0 | 50.0 |
| TCA | 10 | 10 | 0 | 0 | 0 | 0.0 |
TCE solvent control: induced with olive and challenged with TCE.
TCEOH solvent control: induced with olive and challenged with TCEOH.
TCA solvent control: induced with saline and challenged with TCA.
Abbreviations: DNCB, 2,4‐dinitrochlorobenzene; GPMT, guinea pig maximization test; TCA, trichloroacetic acid; TCE, trichloroethylene; TCEOH; trichloroethanol.
FIGURE 2.
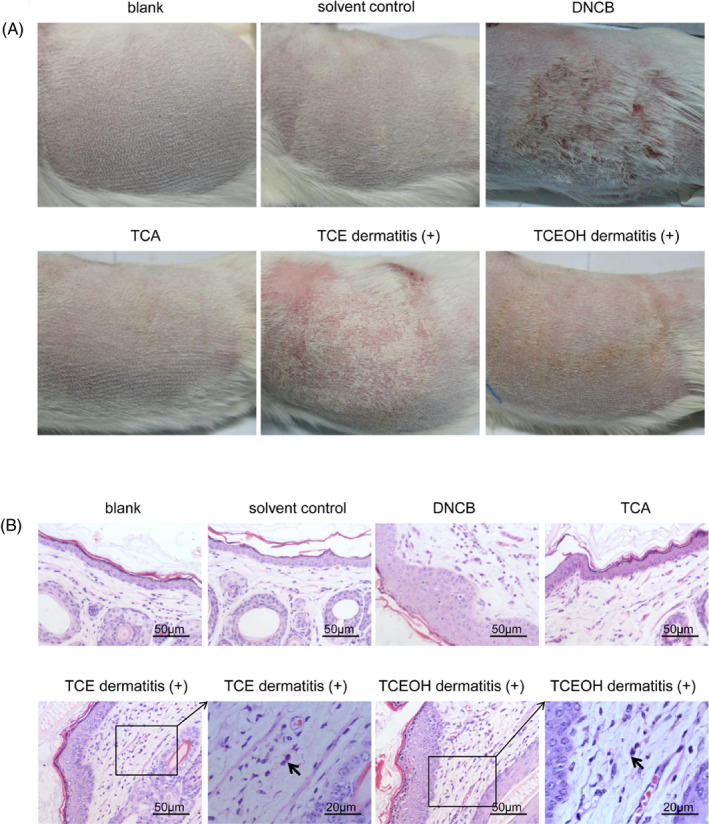
Typical skin changes in guinea pigs after the challenge. A, Images showing typical skin erythema and swelling. B, Histological analysis of the skin by H&E staining. The black arrow indicates an eosinophil granulocyte in the dermis. H&E, hematoxylin and eosin
3.2. Liver‐to‐body‐weight ratio
The guinea pigs were weighted before being euthanized. The absolute weights of the livers removed were measured, and the liver‐to‐body‐weight ratio (%) was calculated as follows:
TCE and TCEOH dermatitis‐positive groups showed no significant difference in the relative liver weight compared to the solvent control groups. The relative liver weight did not differ between the TCEOH dermatitis‐positive and dermatitis‐negative groups. Since there was only one guinea pig in the TCE dermatitis‐negative group, comparison of value between the TCE dermatitis‐positive and dermatitis‐negative groups was not possible (Table 2).
TABLE 2.
Relative liver weight (%) and liver function in guinea pigs after the GPMT challenged with TCE, TCA, and TCEOH
| Group | Relative Liver weight (%) | Liver functions | |||||
|---|---|---|---|---|---|---|---|
| Number of guinea pigs (n) | Body weight (g) | Liver (g) | Ratio % | Number of guinea pigs (n) | ALT | γ‐GTP | |
| Blank | 5 | 421.17 ± 27.69 | 15.86 ± 1.57 | 3.78 ± 0.56 | — | — | |
| DNCB | 5 | 370.96 ± 26.37 | 13.74 ± 2.09 | 3.69 ± 0.31 | — | — | — |
| TCE solvent control | 5 | 402.04 ± 27.01 | 14.37 ± 1.10 | 3.58 ± 0.31 | 5 | 38.78 ± 4.21 | 12.80 ± 3.42 |
| TCEOH solvent control | 5 | 340.56 ± 40.12 | 11.96 ± 1.67 | 3.51 ± 0.29 | 5 | 40.04 ± 12.87 | 13.20 ± 6.10 |
| TCA solvent control | 5 | 319.88 ± 27.31 | 15.53 ± 1.70 | 3.91 ± 0.29 | 5 | 41.60 ± 11.97 | 14.25 ± 2.06 |
| TCE | |||||||
| dermatitis (‐) | 1 | 352.8 | 14.07 | 3.99 | 1 | 41 | 27 |
| dermatitis (+) | 9 | 378.72 ± 17.66 | 13.90 ± 2.18 | 3.66 ± 0.53 | 9 | 56.40 ± 14.52 a | 26.00 ± 7.14 a |
| TCEOH | |||||||
| dermatitis (‐) | 5 | 288.46 ± 20.33 | 10.32 ± 0.91 | 3.58 ± 0.24 | 4 | 41.50 ± 12.87 | 16.00 ± 4.08 |
| dermatitis (+) | 5 | 336.90 ± 35.61 | 11.64 ± 2.24 | 3.47 ± 0.61 | 4 | 52.75 ± 1.50 b , c | 21.17 ± 4.50 b , c |
| TCA | |||||||
| dermatitis (‐) | 10 | 344.31 ± 25.06 | 11.01 ± 0.87 | 3.56 ± 0.41 | 8 | 45.13 ± 9.72 | 14.13 ± 5.33 |
| dermatitis (+) | 0 | — | — | — | — | — | — |
DNCB, 2,4‐dinitrochlorobenzene; GPMT, guinea pig maximization test; TCA, trichloroacetic acid; TCE, trichloroethylene; TCEOH; trichloroethanol.
P < .05, compared to the TCE solvent control group.
P < .05, compared to the TCEOH solvent control group.
P < .05, compared to the TCEOH dermatitis‐negative group.
3.3. TCE‐ and TCEOH‐induced liver damage
Most TCEHS patients are found to suffer from hepatitis. 12 Therefore, investigation was carried out into whether dermatitis‐positive guinea pigs have liver damage. The serum ALT and γ‐GTP levels were measured to evaluate liver function. Compared to the solvent control groups, the TCE and TCEOH dermatitis‐positive groups showed significantly higher serum ALT and γ‐GTP levels. The TCEOH dermatitis‐positive group also showed higher serum ALT and γ‐GTP levels compared to the TCEOH dermatitis‐negative group (Table 2).
Microscopic examination showed no changes in the liver histological structure in the blank, DNCB, solvent control, TCE, TCA, and TCEOH dermatitis‐negative groups. However, focal hepatocyte necrosis was clearly observed in the hepatic lobule, alongside inflammatory cell infiltration (lymphocytes and eosinophils) around the necrosis area in the livers of the TCE and TCEOH experimental groups (Figure 3) that were similar to drug‐induced liver damage in humans. 10
FIGURE 3.
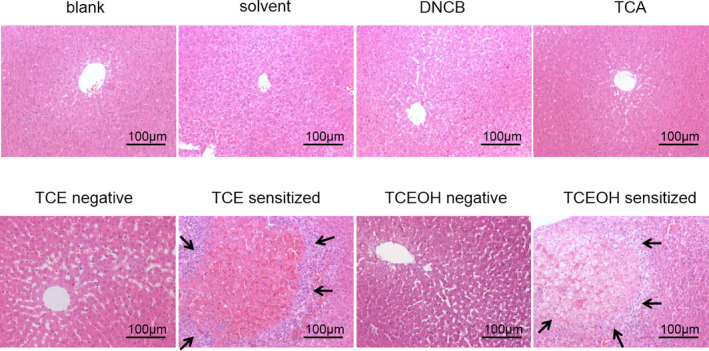
Histological analysis of guinea pig livers by H&E staining. Bottom panels show higher‐magnification views of the necrotic area. Black arrows indicate lymphocyte infiltration in the liver. H&E, hematoxylin and eosin
4. DISCUSSION
Hypersensitivity dermatitis occurring in workers exposed to TCE is a severe skin disorder resembling drug‐induced hypersensitivity syndrome. 13 , 14 Although their pathogenesis is still unclear, these dermatoses are presumably determined by dysregulation of T cell‐mediated immune mechanisms. In this study, TCE and its metabolite TCEOH were highlighted as likely allergens of TCEHS. In addition, both TCE and TCEOH induced skin sensitization in guinea pigs along with focal hepatic cell necrosis surrounded by lymphocyte infiltration.
In mammals, TCE is metabolized mainly by CYP2E1 to chloral hydrate (CH) and then converted to TCEOH and TCA by alcohol dehydrogenase and aldehyde dehydrogenase, respectively. In addition, part of TCEOH is converted by CYP2E1 to TCA via CH, 15 indicating that CYP2E1 might play an important role in TCEHS onset. A previous study has shown that constitutive expression of CYP2E1 in the liver of female guinea pigs is greater compared to male guinea pigs. 7 Furthermore, the sensitization rates of TCE and TCEOH are higher in female (90% and 50%, respectively) than in male guinea pigs (70% and 20%, respectively). 7 Accordingly, female guinea pigs were the ideal candidates for this study. Previous GPMT studies have shown TCE to be a strong allergen, 7 , 8 , 9 , 11 , 16 , 17 , 18 , 19 , 20 , 21 and existence of other strong allergens is still debatable. Tang et al 8 reported that the sensitization rate of TCA in albino guinea pigs is 58.3% (gender not specified). 8 This difference might be due to the different guinea pig strains used by Tang et al and this study. A few skin patch test studies were performed on TCEHS patients and TCE‐exposed workers (exposed to TCE for >12 weeks but did not develop TCEHS). Nakayama et al 22 used TCE and its metabolites to perform a patch test on a TCEHS patient who was sensitive to TCE and TCEOH, 22 while Watanabe et al 23 reported a male patient who had a positive reaction to TCEOH, TCA, and 15% CH. 22 Recently, Huang et al 24 showed that TCEOH and CH have a positive reaction in workers who had recovered from TCEHS. 24 These findings suggested that TCEOH is one of the allergens in humans, which was consistent with our results.
Liver injury occurs in the vast majority (86%–100%) of TCEHS patients, but TCE‐tolerant workers under the same exposure conditions do not develop TCEHS. 25 Acute liver failure is the primary cause of death in TCEHS patients. 26 Ikeoka et al 15 reported a male TCEHS patient whose skin histopathology showed lymphocyte and eosinophil infiltration around the small vein in the corium layer, which was similar to the skin damage in TCE and TCEOH experimental groups in this study. 15 In addition, the epidermal stratum spinosum was thickened in the TCE and TCEOH experimental groups. Furthermore, the liver histopathology of this patient 15 showed lymphocyte and eosinophil infiltration around the portal area along with hepatocyte abscise in the hepatic lobule that was consistent with liver injury in TCE and TCEOH experimental groups in this study. Tang et al 9 reported that hepatocytes show diffused ballooning changes without lymphocyte infiltration and necrotic hepatocytes in TCE dermatitis‐positive guinea pigs. In this study, hepatocyte necrosis with lymphocyte infiltration was observed in the TCE experimental group, which involved immune cells. The different liver pathological manifestation might be due to the slightly different TCE dose: 5%, 20%, and 10% TCE were selected for intradermal injection, topical induction, and challenge, respectively, while Tang et al selected 10%, 20%, and 10% TCE, respectively. Lower intradermal injection doses were used to avoid the potential direct chemical damage. In addition, serum ALT and γ‐GTP levels significantly increased in TCE and TCEOH experimental groups, clearly indicating that the dermatitis‐positive groups suffer from hepatic injury, which is consistent with TCEHS patients. 26 While the workers are directly exposed to TCE in their workshop, internal exposure to TCEOH as a major metabolite of TCE may have enhanced TCEHS development, which is a focus for future research. Although, no data on liver function were generated in the DNCB skin sensitization group; little histological change in liver slides from this group suggests that significant liver damage is unlikely. Therefore, chemical skin sensitization does not necessarily cause liver damage: the immunological mechanism of DNCB‐induced skin sensitization may be different from that induced by TCE and TCEOH. It is difficult to determine whether damage is caused first to the liver or skin by these chemicals in the present study. We hope to be able to report an answer regarding this in the near future.
Recently, many studies focused on mice exposure to TCE and its immune‐related effects have explored the immunological pathogenesis of TCEHS. Cellular immunity and humoral immunity are also involved in TCEHS. A Japanese team reported that TCE enhances TCR‐CD3‐induced proliferation of CD8+ rather than CD4+ cells and disrupts various activities of peripheral T cells in male Balb/c mice. 27 Li et al 28 showed that TCE‐exposed mice show a shift in Th1/Th2 and Th17/Treg ratios. 28 In addition, liver injury caused by immune sensitization through TCE is also mediated by activation of the complement system. Zhang et al provided evidence for upregulation of C3 messenger RNA expression in the liver of TCE‐sensitized Balb/c mice. 29 Blocking complement C3a binding to C3aR and its negative regulation of Th2 cell response leads to liver damage. 30
Clinical data suggest that skin lesions and organ injury caused by TCE belong to the type IV hypersensitivity reaction. 2 , 3 , 4 , 24 , 31 Yang et al 31 showed that CD4+ T cells and the CD4+/CD8+ ratio in peripheral blood decreased significantly, while CD8+ T cells increased significantly in the TCEHS acute period compared to volunteer controls. CD4+ T cells and the CD4+/CD8+ ratio increased in the treatment period but are still low compared to control groups. 31 Jia et al 32 identified that TCEHS patients have higher serum interleukin (IL)‐1β, IL‐6, IL‐8, and tumor necrosis factor alpha (TNF‐α) levels than TCE‐exposed workers and nonexposed controls. 32 In this study, inflammatory cell infiltration was found including lymphocytes and eosinophils around focal hepatocyte necrosis in the hepatic lobule, supporting the immunological effects of TCE. Further studies on immune cells and related cytokines will be performed in the future.
This study has several limitations. First, this is an animal experimental study and the findings of TCE and TCEOH as potential skin sensitizators does not necessarily mean they are TCEHS‐developing allergens for workers. Secondly, no biochemical data were available on liver function for the DNCB group. Thirdly, this study does not test the CH, another metabolite of TCE. Further studies involving CH need to determine the molecular mechanism of TCEHS and identify the culprit compound which propagates TCEHS.
In summary, TCE and TCEOH are specific allergens that cause a hypersensitivity skin reaction along with liver dysfunction and hepatocyte necrosis in guinea pigs. A better understanding of the allergens in TCEHS will create new opportunities for prevention and therapeutic intervention for TCEHS.
DISCLOSURES
Approval of the research protocol: Animal Care and Use Committee of Guangdong Province Hospital for Occupational Disease Prevention and Treatment (Approval number GDHOD MEC 2015020). Informed consent: N/A. Registry and the registration no. of the study/trial: N/A. Animal studies: License of Laboratory Animals Use: SYXK (Guangdong) 2014‐0048. Conflict of interest: All authors have no competing financial interests.
AUTHOR CONTRIBUTIONS
Hanlin Huang, Tamie Nakajima, and Hailan Wang designed the research; Na Zhao, Xiangrong Song, Hongling Li, Yongshun Huang, Lili Liu, Fengrong Lu, and Tingfeng Cai performed the research; Na Zhao, Hisao Naito, and Yuki Ito analyzed the data; and Na Zhao, Tamie Nakajima, and Michihiro Kamijima wrote the paper.
ACKNOWLEDGMENTS
This study was supported, in part, by grants from National Natural Science Foundation of China (81202246, 81502769, 81903269), Natural Science Foundation of Guangdong (2018A030313569), Science and Technology Planning Project of Guangdong Province (2010B050700026, 2014A020212551, 2016A020215131), Grants‐in‐Aid for Challenging Exploratory Research (16K15383 and 18K19691) and Guangdong Provincial Key Laboratory of Occupational Disease Prevention and Treatment (2017B030314152).
Zhao N, Song X, Naito H, et al. Trichloroethylene and trichloroethanol induce skin sensitization with focal hepatic necrosis in guinea pigs. J Occup Health. 2020;62:e12142 10.1002/1348-9585.12142
Na Zhao and Xiangrong Song contributed equally to this work.
Contributor Information
Tamie Nakajima, Email: wanghl@gdoh.org, Email: tnasu23@med.nagoya-u.ac.jp.
Hailan Wang, Email: wanghl@gdoh.org.
REFERENCES
- 1. Ministry of Health of the People's Republic of China . Diagnostic criteria of occupational medicamentosa‐like dermatitis due to trichloroethylene. National Occupational Health Standards. 2007; GBZ185‐2006 of P.R. of China: (In Chinese).
- 2. Kamijima M, Wang H, Yamanoshita O, et al. Occupational trichloroethylene hypersensitivity syndrome: human herpesvirus 6 reactivation and rash phenotypes. J Dermatol Sci. 2013;72(3):218‐224. [DOI] [PubMed] [Google Scholar]
- 3. Nakajima T, Yamanoshita O, Kamijima M, et al. Generalized skin reactions in relation to trichloroethylene exposure: a review from the viewpoint of drug‐metabolizing enzymes. J Occup Health. 2003;45(1):8‐14. [DOI] [PubMed] [Google Scholar]
- 4. Xia LH, Huang HL, Kuang SR, et al. A clinical analysis of 50 cases of medicament‐like dermatitis due to trichloroethylene. Chin J Ind Hyg Occup Dis. 2004;22(3):207‐210. (in Chinese). [PubMed] [Google Scholar]
- 5. Wang X, Yu Y, Xie H‐B, et al. Complement regulatory protein CD59a plays a protective role in immune liver injury of trichloroethylene‐sensitized BALB/c mice. Ecotoxicol Environ Safety. 2019;172:105‐113. [DOI] [PubMed] [Google Scholar]
- 6. Ramdhan D, Kamijima M, Yamada N, et al. Molecular mechanism of trichloroethylene‐induced hepatotoxicity mediated by CYP2E1. Toxicol Appl Pharmacol. 2008;231(3):300‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hibino Y, Wang H, Naito H, et al. Sex differences in metabolism of trichloroethylene and trichloroethanol in guinea pigs. J Occup Health. 2013;55(6):443‐449. [DOI] [PubMed] [Google Scholar]
- 8. Tang XJ, Li LY, Huang JX, et al. Guinea pig maximization test for trichloroethylene and its metabolites. Biomed Environ Sci. 2002;15(2):113‐118. [PubMed] [Google Scholar]
- 9. Tang X, Que B, Song X, et al. Characterization of liver injury associated with hypersensitive skin reactions induced by trichloroethylene in the guinea pig maximization test. J Occup Health. 2008;50(2):114‐121. [DOI] [PubMed] [Google Scholar]
- 10. Ikeoka T, Saito T, Hosaka Y, et al. Drug‐induced hypersensitivity syndrome caused by trichloroethylene exposure. Nihon Naika Gakkai Zasshi. 2009;98(5):1120‐1123. (in Japanese). [Google Scholar]
- 11. Yu J‐F, Leng J, Shen T, et al. Possible role of complement activation in renal impairment in trichloroethylene‐sensitized guinea pigs. Toxicology. 2012;302(2–3):172‐178. [DOI] [PubMed] [Google Scholar]
- 12. Xu X, Yang R, Wu N, et al. Severe hypersensitivity dermatitis and liver dysfunction induced by occupational exposure to trichloroethylene. Ind Health. 2009;47(2):107‐112. [DOI] [PubMed] [Google Scholar]
- 13. Fukuchi T, Takizawa D, Adachi T, et al. A case of drug‐induced hypersensitivity syndrome due to trichloroethylene. Nihon Naika Gakkai Zasshi. 2017;106(3):598‐604. (in Japanese). [PubMed] [Google Scholar]
- 14. Watanabe H. Hypersensitivity syndrome due to trichloroethylene exposure: a severe generalized skin reaction resembling drug‐induced hypersensitivity syndrome. J Dermatol. 2011;38(3):229‐235. [DOI] [PubMed] [Google Scholar]
- 15. Ikeda M, Miyake Y, Ogata M, Ohmori S. Metabolism of trichloroethylene. Biochem Pharmacol. 1980;29(21):2983‐2992. [DOI] [PubMed] [Google Scholar]
- 16. Bogen KT, Colston BW Jr, Machicao LK. Dermal absorption of dilute aqueous chloroform, trichloroethylene, and tetrachloroethylene in hairless guinea pigs. Fundam Appl Toxicol. 1992;18(1):30‐39. [DOI] [PubMed] [Google Scholar]
- 17. Dai YF, Niu Y, Cheng J, Leng SG, Zheng YX. Immunological mechanism in development of allergic dermatitis in guinea pig induced by trichloroethylene in vitro. Chin J Ind Hyg Occup Dis. 2005;23(2):129‐131. (in Chinese). [PubMed] [Google Scholar]
- 18. Dai YF, Nui Y, Cheng J, Leng SU. The immunological effects in guinea pig sensitized by trichloroethylene. Wei Sheng Yan Jiu. 2005;34(1):16‐18. (in Chinese). [PubMed] [Google Scholar]
- 19. Wang LJ, Guo RJ, Shen T, Zhu QX. Beta‐arrestin and NF‐kappaB, AP‐1 activity in peripheral blood mononuclear cells of guinea pigs sensitized by trichloroethylene. Chin J Ind Hyg Occup Dis. 2010;28(7):494‐497. (in Chinese). [PubMed] [Google Scholar]
- 20. Xu XY, Li XY, Liu YF, et al. Study on skin sensitization as well as liver and kidney impairment in guinea pigs treated with trichloroethylene. Chin J Ind Hyg Occup Dis. 2010;28(2):81‐83. (in Chinese). [PubMed] [Google Scholar]
- 21. Zhu QX, Xu H, Leng J, Shen T. Changes in humoral immunity in sensitized guinea pigs exposed to trichloroethylene. Chin J Ind Hyg Occup Dis. 2012;30:641‐644. (in Chinese). [PubMed] [Google Scholar]
- 22. Nakayama H, Kobayashi M, Takahashi M, et al. Generalized eruption with severe liver dysfunction associated with occupational exposure to trichloroethylene. Contact Derm. 1988;19(1):48‐51. [DOI] [PubMed] [Google Scholar]
- 23. Watanabe H, Tohyama M, Kamijima M, et al. Occupational trichloroethylene hypersensitivity syndrome with human herpesvirus‐6 and cytomegalovirus reactivation. Dermatol Basel Switzerland. 2010;221(1):17‐22. [DOI] [PubMed] [Google Scholar]
- 24. Huang Y, Xia L, Wu Q, et al. Trichloroethylene hypersensitivity syndrome is potentially mediated through its metabolite chloral hydrate. PLoS One. 2015;10(5):e0127101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kamijima M, Wang H, Huang H, et al. Trichloroethylene causes generalized hypersensitivity skin disorders complicated by hepatitis. J Occup Health. 2008;50(4):328‐338. [DOI] [PubMed] [Google Scholar]
- 26. Huang H, Kamijima M, Wang H, et al. Human herpesvirus 6 reactivation in trichloroethylene‐exposed workers suffering from generalized skin disorders accompanied by hepatic dysfunction. J Occup Health. 2006;48(6):417‐423. [DOI] [PubMed] [Google Scholar]
- 27. Kobayashi R, Nakanishi T, Nagase H. Trichloroethylene enhances TCR‐CD3‐induced proliferation of CD8(+) rather than CD4(+) T cells. J Toxicol Sci. 2012;37(2):381‐387. [DOI] [PubMed] [Google Scholar]
- 28. Li S‐L, Yu Y, Yang P, et al. Trichloroethylene alters Th1/Th2/Th17/Treg paradigm in mice: a novel mechanism for chemically induced autoimmunity. Int J Toxicol. 2018;37(2):155‐163. [DOI] [PubMed] [Google Scholar]
- 29. Zhang J, Zha W, Wang F, et al. Complement activation and liver impairment in trichloroethylene‐sensitized BALB/c mice. Int J Toxicol. 2013;32(6):431‐441. [DOI] [PubMed] [Google Scholar]
- 30. Wang F, Zha W‐S, Zhang J‐X, et al. Complement C3a binding to its receptor as a negative modulator of Th2 response in liver injury in trichloroethylene‐sensitized mice. Toxicol Lett. 2014;229(1):229‐239. [DOI] [PubMed] [Google Scholar]
- 31. Yang ZQ, Liu WW, Yu W. Characteristics of change of T‐cell subgroup in patients with trichloroethylene‐induced medicamentosa‐like dermatitis. Chin J Ind Hyg Occup Dis. 2008;26(8):482‐483. (in Chinese). [PubMed] [Google Scholar]
- 32. Jia Q, Zang D, Yi J, et al. Cytokine expression in trichloroethylene‐induced hypersensitivity dermatitis: an in vivo and in vitro study. Toxicol Lett. 2012;215(1):31‐39. [DOI] [PubMed] [Google Scholar]


