Abstract
Cooling or the application of mentholated liniments to the skin has been used to treat itch for centuries, yet remarkably little is known about how counter-stimuli such as these induce itch relief. Indeed, there is no clear consensus in the scientific literature as to whether or not cooling does in fact block the transduction of itch signals or if it is simply a placebo effect. This gap in our understanding led us to hypothesize that cooling is antipruritic and, like cooling analgesia, requires function of the cold-gated ion channel TRPM8, a receptor for menthol expressed on peripheral afferent nerve endings. Using a combination of pharmacologic, genetic, and mouse behavioral assays, we find that cooling inhibits both histaminergic and non-histaminergic itch pathways, and that inhibition of itch by cooling requires TRPM8 channels or intact and functional TRPM8-expressing afferent neurons. The cold mimetic menthol is also effective in ameliorating itch in a TRPM8-dependent manner. Moreover, we find that chronic itch can be ameliorated by cooling, demonstrating that this counter-stimulus activates a specific neural circuit that leads to broad itch relief and a potential cellular mechanism for treatment of chronic itch.
INTRODUCTION
Itch (pruritus) management is an important aspect of the health care system. The etiology of itch involves the immune system, skin cells, and sensory neurons, with the latter divided into sub-populations based on the different molecular mediators of pruritogens (Bautista et al., 2014). The best-understood molecular mechanism of itch is the histaminergic pathway, yet antihistamines are only marginally effective in resolving chronic forms of itch (Liu and Ji, 2013). The definition of itch as an “unpleasant sensation evoking the desire to scratch” may hold the key to the problem of itch therapy, as it implies that mechanical (scratching) and thermal counter-stimuli ameliorates all types of itch (Schmelz et al., 1997, 2003). These stimuli do not act directly on pruriceptors, but rather through inhibitory pathways in the spinal cord dorsal horn (Ma, 2012).
Cold has been used traditionally to relieve itch and pain, but the mechanisms underlying its actions are not well understood. Cooling diminishes nerve conduction velocity, vesicle release, and general protein function (Chung and Wang, 2011; Kichko and Reeh, 2004, Rutkove, 2001), yet this does not account for the efficacy of cold mimetics like menthol. Cold and menthol activate the ion channel TRPM8 (McKemy et al., 2002;Peier et al., 2002), a molecule that has been shown to be the principle mediator of cold stimuli in the mammalian peripheral nervous system (Bautista et al., 2007;Colburn et al., 2007;Dhaka et al., 2007;Knowlton et al., 2013). In addition to its role as a thermosensor, activation of TRPM8 also underlies cooling-induced pain relief (analgesia). For example, mild cooling, between 17°C and 20°C, of rodent hindpaws has been shown to inhibit mechanical and thermal (heat) hyperalgesia in a TRPM8-dependent manner (Knowlton et al., 2013;Proudfoot et al., 2006). Furthermore, cooling-induced relief from neurogenic inflammation also requires TRPM8 (Dhaka et al., 2007). Chemical cooling with TRPM8 agonists also leads to analgesia in inflammatory and neuropathic conditions (Liu et al., 2013; Proudfoot et al., 2006). Remarkably, these studies find that only mild cooling or low doses of TRPM8 agonists lead to analgesia, with more robust stimulation leading to cold pain in the injured states (Fleetwood-Walker et al., 2007; Proudfoot et al., 2006). Lastly, we have shown that in addition to TRPM8 channels, this process is also dependent on TRPM8 neurons (Knowlton et al., 2013), yet the role of TRPM8 and TRPM8 afferents in cooling-induced antipruritus is unclear.
Here we test the hypotheses that cooling suppresses acute itch in a process that is TRPM8-dependent. Our results show that cold reduces both histamine-dependent and histamine-independent itch, a process that only partially requires TRPM8 channels, but is dependent on intact TRPM8-positive neurons. These data show that cooling alleviates itch by activating specific afferents that are likely part of a larger counter-stimulus pathway that attenuates pruritic signaling in the spinal cord dorsal horn.
RESULTS
Pruritogen-induced behaviors in the mouse hindpaw
To test cooling on itch, we assessed behaviors directed toward a pruritogen-injected mouse hind paw in animals standing on a thermally controlled surface. This approach was used in lieu of the traditional cheek or nape assays, as applying a cold stimulus to these regions requires excessive animal handling, which strongly alters itch behaviors, and because human studies show itch relief by cooling requires continual stimulation (Fruhstorfer et al., 1986;Shimada and LaMotte, 2008). Hind-paw analysis has been used previously as a reliable measure of itch by several investigators (Akiyama et al., 2010b;Nojima et al., 2004). Moreover, we and others have used these models to measure thermal discrimination and cooling analgesia (Knowlton et al., 2013; Pogorzala et al., 2013;Proudfoot et al., 2006), suggesting that a similar approach can test whether cooling alleviates itchlike behaviors.
First, to define behaviors induced by intradermal hind-paw pruritogen injections, we compared the algogen capsaicin to the pruritogens histamine and chloroquine (Cqx), finding that, in addition to significant time spent flinching and lifting the injected paw, capsaicin (5 μg) also evoked robust biting and licking (Figure 1a). Histamine (100 μg) produced similar behaviors, consistent with reports of histamine evoking both pain and itch under certain conditions (LaMotte et al., 2011; Snyder and Ross, 2015). Cqx (200 μg), a substance that induces itch but not pain (Bautista et al., 2014;LaMotte et al., 2011;Snyder and Ross, 2015), also evoked robust licking and biting behaviors. However, unlike with capsaicin and histamine, we observed no flinching or lifting, demonstrating differences in the responses to an algogen versus a pruritogen. We then tested lower doses of histamine, finding that amounts down to 0.5 μg produced licking and biting behaviors similar to those at 100 μg (P > 0.05), but with minimal flinching and lifting (Figure 1a; P < 0.001). Similar behaviors were observed with endogenous histamine release induced by 0.5 μg of the mast cell degranulator compound 48/80 and 30 μg of the serotonin receptor agonist α-methyl 5-HT (αMe5-HT, Figure 1a), and all flinch/lift behaviors of the latter were not significantly different than that observed with injection of vehicle (P > 0.05). Lastly, analysis of all behaviors (flinches, lifts, licks, bites) showed that licking and biting dominated the duration of animals’ responses.
Figure 1. Hind paw pruritogen injections promote itch-like behaviors in mice.
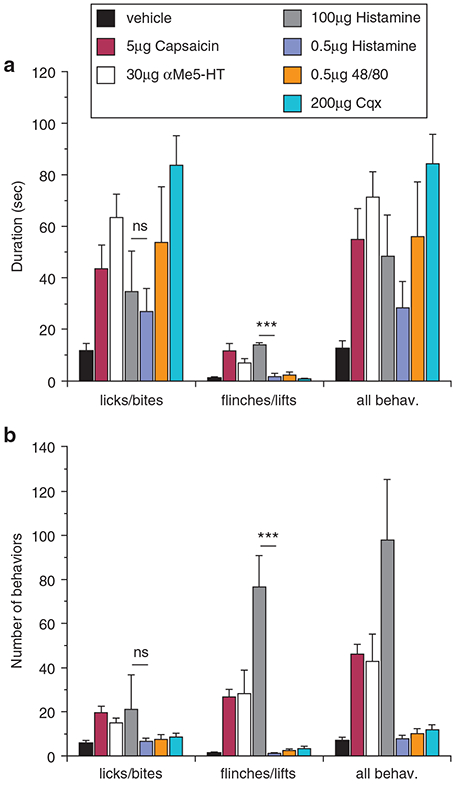
(a) In wild-type mice, unilateral intradermal hind paw injections of both pruritogens and algogens induce robust lick and biting behaviors (P < 0.001 compared to vehicle, one-way ANOVA followed by Neuman-Keuls post-test) measured as duration of behaviors in a 30-minute period post-injection, with no differences between injections of 100 or 0.5 μg histamine (P > 0.05, one-way ANOVA, Neuman-Keuls post-test). Capsaicin, α-methyl 5-HT (αMe5-HT), and a high dose of histamine (100 μg, n = 8) produced flinching and hind paw lifts (P < 0.01 compared to vehicle, one-way ANOVA, Neuman-Keuls post-test), whereas chloroquine (Cqx; 200 μg, n = 8), 48/80 (0.5 μg, n = 6), and a low dose of histamine (0.5 μg, n = 6) did not induce these behaviors (P > 0.05 compared to vehicle, one-way ANOVA, Neuman-Keuls post-test). The duration of flinches/lifts between histamine doses was significantly different (***P < 0.001, one-way ANOVA, Neuman-Keuls post-test). All behaviors were combined (flinches/lifts/bites/licks) for comparisons. (b) The number of behavioral bouts produced similar results with no significant difference in the number of licks/bites between the different stimuli (nsP < 0.05 compared to vehicle, one-way ANOVA, Neuman-Keuls post-test). The number of flinches/lifts between histamine doses of was significantly different (***p < 0.001, one-way ANOVA, Neuman-Keuls post-test). All behaviors were combined (flinches/lifts/bites/licks) for comparisons.
Lifts/flinches are unlike licks/bites in that they are short-lasting behaviors of similar duration and better represented by the number of bouts. Thus, we also examined bouts, finding that capsaicin, 100 μg histamine, and αMe5-HT evoked significant flinches/lifts compared to vehicle (P < 0.01), whereas Cqx, compound 48/80, and 0.5 μg histamine-induced flinches/lifts were negligible (P > 0.05 compared to vehicle, Figure 1b). As with duration, there was no difference in lick/bite bouts at the two histamine doses (P > 0.05), but 0.5 μg histamine and compound 48/80 produced fewer flinches/lifts (P < 0.001). While our observations do not eliminate the possible pain component inherent in licking/biting behaviors, it suggests that Cqx, compound 48/80, and 0.5 μg histamine minimized pain and the behaviors produced likely represent itch. Moreover, the mechanical stimulation of the injection site due to the animals walking and standing on the hind paw is a potential confound of this approach. Nonetheless, in the absence of a viable method to stimulate other classical itch sites (cheek or nape) with cold, we contend that the hind paw is a viable anatomical site for these studies, as shown previously (Akiyama et al., 2010a, 2010b, 2014).
Cooling inhibits pruritogen-induced behaviors
Next, we asked whether cooling inhibits pruritogen-evoked behaviors by examining licks/bites induced by either Cqx (Figure 2a), compound 48/80 (Figure 2b), or 100 μg histamine (Figure 2c) in mice placed on a surface set to a range of temperatures. At the preferred temperature of 30°C (Lee et al., 2005; Moqrich et al., 2005), robust licking/biting was observed with each pruritogen, responses that were not statistically different at 24°C (Figure 2a-2c;P > 0.05). However, at 20°C and below, licking/biting evoked by Cqx was essentially abolished (Figure 2a, P < 0.001 compared to 30°C). Similarly, compound 48/80 responses were significantly reduced at 20°C (Figure 2b, P < 0.01) with a further reduction as the surface was lowered into the noxious cold range (10°C, P < 0.001). Flinches and lifts were not observed at any temperature tested with Cqx (Figure 2d) or compound 48/80 (Figure 2e), demonstrating that licking/biting is the predominant behavior to these stimuli.
Figure 2. Cooling inhibits acute itch.
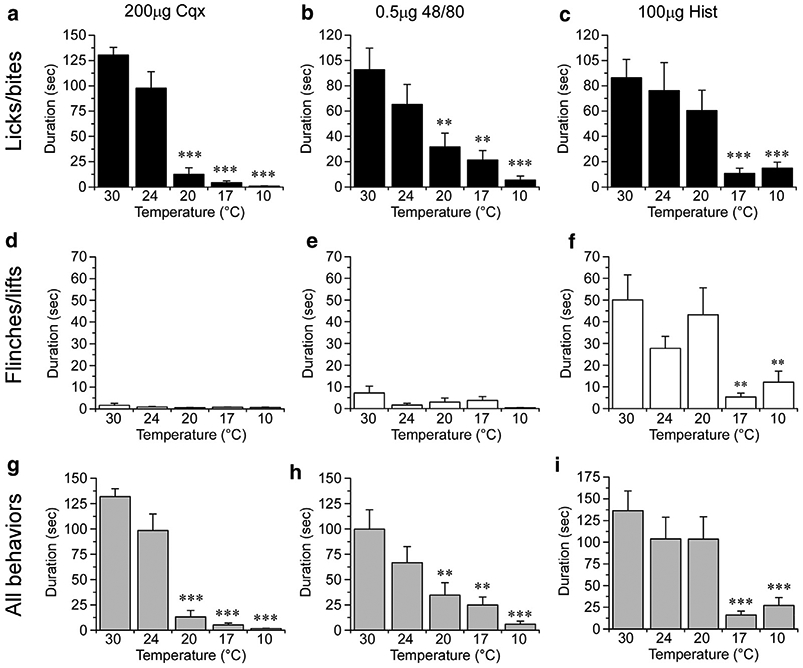
The duration of licking and biting behaviors at different plate temperatures (30, 24, 20, 17, and 10°C) in mice injected with chloroquine (Cqx) (a, n = 8–10), 48/80 (b, n = 8), or histamine (c, n = 9). Neither Cqx (d) or 48/80 (e) induced flinches or lifts, whereas 100 μg histamine (f) induced robust pain-like behaviors that were inhibited at plate temperatures of 17°C and 10°C. (g–i) Duration of all behaviors showed cooling inhibition to all three substances tested. ***P < 0.001, **P < 0.01 and all data compared to 30°C via one-way ANOVA, Neuman-Keuls post-test.
Histamine (100 μg) also evoked robust licking/biting (Figure 2c) at control temperatures, behaviors that, unlike Cqx and compound 48/80, were not reduced at 20°C (P > 0.05). However, at 17°C and below, these behaviors were reduced significantly (Figure 2c, P < 0.001). Of note, significant flinching/lifting was observed at this dose of histamine, yet these responses were also reduced at 17°C and 10°C (Figure 2f; P < 0.01). Thus, even as this dose of histamine is likely producing pain, cooling is inhibitory, results consistent with our prior analysis of cooling analgesia (Knowlton et al., 2013).
Lastly, analysis of all behaviors (flinches, lifts, licks, bites) showed that cooling can block these responses (Figure 2g-2i), data that largely mirror the findings when just licking and biting was quantified (Figure 2a-2c). Taken together, these data show that cooling, in addition to being analgesic, also alleviates pruritic behaviors, suggesting that regardless of whether these behaviors are itch or pain, the use of hind paw is a suitable method to determine the underlying mechanisms of antipruritic cooling.
Cooling delays pruritogen-induced itch
Pruritogen-evoked behaviors resolve within 30 minutes post-injection in the cheek and nape assays (LaMotte et al., 2011), and we found this to be true in the hind paw of mice injected with Cqx or the serotonin receptor agonist αMe5-HT (30 μg) at 24°C (Figure 3a, 3c). Human studies show that cooling-induced itch relief requires constant cooling and that itch sensations will return upon rewarming of the skin (Fruhstorfer et al., 1986). This is also the case in mice, as Cqx-behaviors inhibited at 20°C (Figure 3b; P < 0.001 compared to mice at 24°C after 15 minutes) return when the surface was warmed to 24°C (Figure 3a). In vehicle-injected mice exposed to the 20°C to 24°C temperature shift, a slight increase in attention to the hind paw was observed upon warming, but clearly not to the extent observed if Cqx-injected animals. Moreover, total behaviors were similar in mice at 24°C for the entirety of the recording period compared to those placed at 20°C initially, then raised to 24°C after 15 minutes (Figure 3b, P > 0.05).
Figure 3. Antipruritic cooling requires constant stimulation.
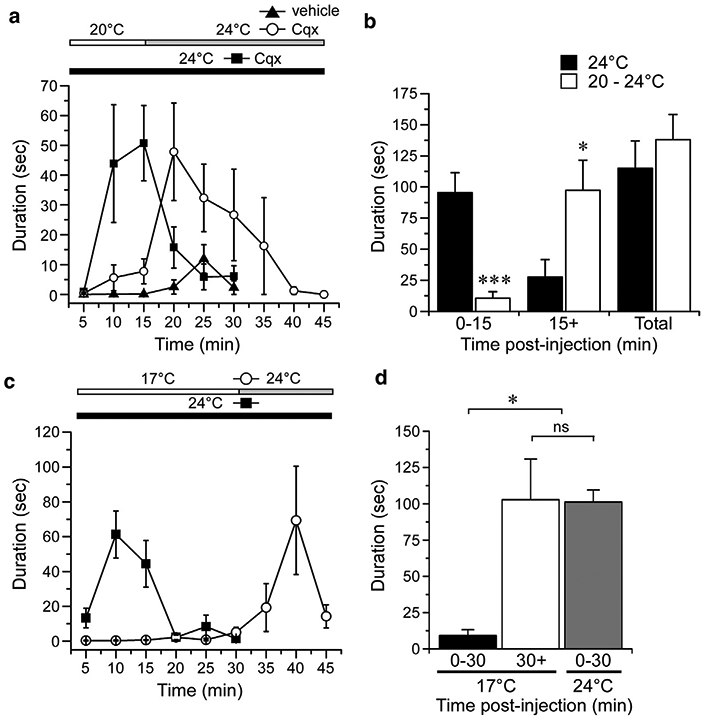
(a) Chloroquine (Cqx; 200 μg) induced licking/biting behaviors resolved within 25 minutes post injection when the test plated was held at 24°C (n = 7). A plate temperature of 20°C showed inhibition with robust behaviors produced when the plate was warmed to 24°C 15 minutes after Cqx injections, responses not observed in vehicle-injected mice. (b) Summary of the data in (a) shows inhibition in the first 15-minute period post-injection when the plate is at 20°C (***P < 0.001 compared to 24°C, one-way ANOVA, Neuman-Keuls post-test), with behaviors uncovered in the second 15–45 minute period when the plate was warmed to 24°C (*P < 0.05 compared to when the plate was held constant at 24°C, one-way ANOVA, Neuman-Keuls post-test). Summary of the total behaviors showed no difference between the two stimulation protocols (P>0.05, one-way ANOVA, Neuman-Keuls post-test). (c) In a test of the duration in which cooling inhibits itch, α-methyl 5-HT (aMe5-HT; 30 μg) behaviors were blocked by cooling to 17°C, but were observed when the plate was warmed to 24°C after 30 minutes. (d) Summary of the αMe5-HT responses in mice on a 17°C plate for 30 minutes followed by warming to 24°C compared to mice placed at 24°C only. *P < 0.05, nsP> 0.05, n = 6–8, one-way ANOVA, Neuman-Keuls post-test.
Next, we determined whether increasing the duration of cooling was more effective in inhibiting pruritogen-induced behaviors and whether such delay in behaviors occurs with other pruritogens, in this case with aMe5-HT. As with Cqx, animals robustly attend to their injected hind paw when the plate was at 24°C, behaviors which resolved by 20 minutes post-injection (Figure 3c). When μMe5-HT–injected animals were placed on a 17°C plate, these behaviors were inhibited for the 30-minute test duration, but were observed immediately after the plate was warmed to 24°C (Figure 3c). Furthermore, the summed responses of these mice were not significantly different from αMe5-HT–injected mice placed at 24°C immediately after injection (Figure 3d; P > 0.05). Thus, consistent with human studies, antipruritic cooling requires constant stimulation, and pruritogen-evoked behaviors return when the skin is warmed to ambient temperatures (Fruhstorfer et al., 1986).
Cooling antipruritus is TRPM8-channel and TRPM8-neuron – dependent
Next, we examined the molecular and cellular basis for the effect of cooling on itch. TRPM8 is the principal detector of cold temperatures in the mammalian peripheral nervous system (McKemy et al., 2002;Peier et al., 2002). With the knowledge that cooling can alleviate pruritogen-induced behaviors in wild-type mice, we next asked whether this effect of cooling was dependent on functioning TRPM8 ion channels. Wild-type and TRPM8 nulls (Trpm8−/−) (Bautista et al., 2007; Knowlton et al., 2013) were injected with Cqx and then subjected to a range of cold temperatures (Figure 4). As mentioned, wild-type mice showed robust responses at 24°C, behaviors that were significantly reduced at 20°C and 17°C (Figure 4a; P < 0.001). Similarly, Cqx evoked robust behaviors in Trpm8−/− mice at 24°C, responses that were not different than wild-types (P > 0.05 wild-type vs. Trpm8−/− at 24°C). However, unlike wild-type animals, Cqx-evoked behaviors were not attenuated at 20°C in Trpm8−/− mice (Figure 4b; P > 0.05), demonstrating that TRPM8 channels are required at this temperature. However, these behaviors were reduced in Trpm8−/− mice when the plate was cooled to near the noxious cold threshold (17°C, P < 0.001 compared to Trpm8−/− at 24°C and P > 0.05 compared to wild-type at 17°C). These data suggest that TRPM8 is required for inhibition of Cqx-induced behaviors with mild cooling, but that the channel is not needed at near-noxious temperatures.
Figure 4. Antipruritic cooling requires TRPM8 channels and neurons.
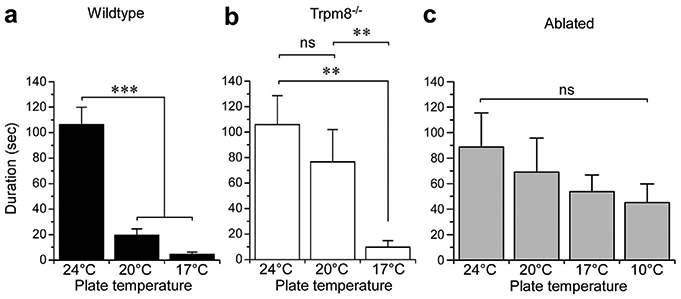
(a) Chloroquine (Cqx)-induced licking/biting behaviors were inhibited in wild-type mice (n = 8–10) at 20°C and 17°C (***p < 0.001 compared to 24°C, one-way ANOVA, Neuman-Keuls post-test). (b) In mice lacking functional TRPM8 channels (Trpm8−/−, n = 8–9), 20°C was ineffective in blocking these behaviors (nsP > 0.05, one-way ANOVA, Neuman-Keuls post-test), while a colder temperature of 17°C did inhibit (**P < 0.01 compare to both 24° and 20°C, one-way ANOVA, Neuman-Keuls post-test). (c) In comparison, mice in which TRPM8-expressing afferent neurons are ablated, cooling was unable to inhibit Cqx-induce behaviors, even at 10°C (nsP > 0.05 compared to 24°C for all temperatures tested, one-way ANOVA, Neuman-Keuls post-test, n = 8).
These results raise several hypotheses, including that cold is inhibiting nerve conduction in pruriceptive afferents (unlikely at 20°C), or that other cold transduction mechanisms are involved in antipruritic cooling at noxious cold temperatures. We and others have shown that TRPM8 neurons represent a labeled line for cold and mediate all aspects of cold perception, even in the absence of TRPM8 channels (Knowlton et al., 2013; Pogorzala et al., 2013). Therefore, to test whether block by noxious cold requires TRPM8 neurons, we repeated the assays described here using our Trpm8DTR mouse line in which TRPM8+ neurons are ablated in the adult mouse via genetically targeted expression of the cell-death–promoting human diphtheria toxin receptor (Knowlton et al., 2013). As with Trpm8−/− mice, we observed robust behaviors at 24°C and 20°C in TRPM8-neuron–ablated animals, but in contrast Cqx still evoked behaviors at 17°C (Figure 4c, P > 0.05 compared to 24°C). As 17°C is a suprathreshold noxious cold temperature, we tested Cqx responses at subthreshold temperature of 10°C, also observing robust behaviors that were not significantly different than those at 24°C (P > 0.05 compared to 24°C). While there was a downward trend as temperatures decreased, there was no significant difference between all four temperatures tested (P > 0.05, repeated-measures of ANOVA), showing that TRPM8 neurons are critical for antipruritic cooling.
Chemical cooling requires TRPM8 channels
TRPM8 channels are also activated by the cold mimetic menthol, which is also a TRPM8-dependent analgesic (Proudfoot et al., 2006). Menthol and other TRPM8 agonists have been reported to alleviate severe pruritus in several studies (Andersen et al., 2017; Frolich et al., 2009; Stander et al., 2017), with recent evidence that an over-the-counter topical menthol solution inhibits Cqx-induced itch in mice (Kardon et al., 2014). However, the TRPM8-dependence of this inhibition has not been established. Thus, to confirm that its effects are mediated by TRPM8, we used the cheek assay in wild-type and Trpm8−/− mice (Shimada and LaMotte, 2008). First, to establish that the topical menthol product (8% solution, Stopain; Troy Healthcare LLC, Hazelton, PA) used before to block itch (Kardon et al., 2014) stimulates sensory afferents in the cheek in a TRPM8-dependent manner, we applied it to the cheek and measured wipes, an indicator of irritation and not itch. As expected, robust wiping was induced by this solution in wild-type mice (P < 0.001 vs. control, Figure 5a), responses that were significantly reduced in Trpm8−/− animals (P < 0.001). However, there was a slight increase in Stopain responses in Trpm8−/− mice (P < 0.05 compared to vehicle), consistent with non-TRPM8–dependent effects that have been attributed to menthol when used at high concentrations in vivo (Liu et al., 2013). Thus, while TRPM8 channels are the predominant mediator of responses to menthol, additional factors lead to behaviors induced by high-dose menthol in vivo.
Figure 5. Menthol inhibits Cqx-induced itch in a TRPM8-dependent manner.
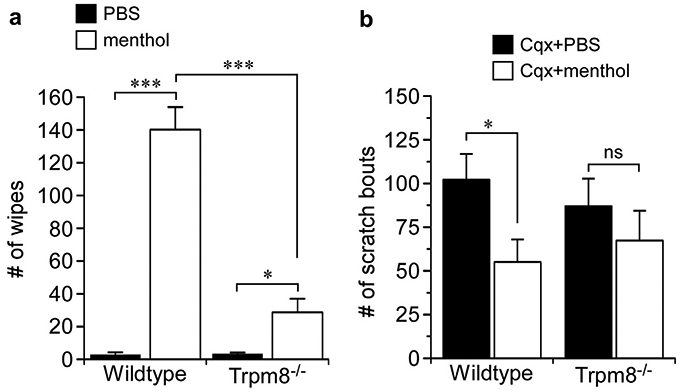
(a) Topical menthol (8%) produced robust cheek wiping behaviors in wild-type mice (***P < 0.001 compared to vehicle, one-way ANOVA, Neuman-Keuls post-test, n = 8) that were significantly reduced in Trpm8−/− animals (***P < 0.001 compared to vehicle, one-way ANOVA, Neuman-Keuls post-test, n = 6–8). Menthol did evoke significant wiping compared to vehicle (*P < 0.05, one-way ANOVA, Neuman-Keuls post-test, n = 6). (b) Topical menthol (8%) significantly inhibited Cqx-induced cheek scratches in wild-type (*P < 0.05, one-way ANOVA, Neuman-Keuls post-test, n = 8), an effect that was not observed in Trpm8−/− animals (nsP > 0.05, one-way ANOVA, Neuman-Keuls post-test, n = 6–8). Cqx, chloroquine; PBS, phosphate buffered saline.
Next, to determine whether the effects of topical menthol on itch reported previously were TRPM8-dependent, we applied Stopain 10 minutes before an intradermal injection of Cqx (50 μg) into the cheek and measured the number of hind-paw scratches directed at the injected cheek in wild-type and Trpm8−/− mice (Figure 5b). As predicted, Cqx induced a significant number of scratches, responses that were significantly reduced with prior application of the menthol solution (P < 0.05);results similar to those reported previously (Kardon et al., 2014). Cqx also induced scratching in Trpm8−/− mice, but these responses were not significantly affected by treatment with the menthol solution (Figure 5b, P > 0.05). Thus, similar to cooling, cold mimetics can alleviate itch and do so in a manner that is largely dependent on TRPM8 channels.
Cooling inhibits chronic itch
While pruritogen-induced itch is an important diagnostic for understanding the cellular and molecular basis of itch, the real unmet clinical need are remedies for chronic itch. To determine whether cooling also alleviates chronic itch, we employed a dry skin model (xerosis) in which mice are treated unilaterally with a mixture of acetone, ether, and water for several days (AEW model) (Akiyama et al., 2010a, 2010b). In this model, at 30°C we observed increased licking/biting behaviors in the treated, ipsilateral paw compared to the contralateral, untreated control paw (Figure 6a; P < 0.001 ipsilateral vs. contralateral), behaviors that were abolished when the surface was subsequently cooled to 20°C (P > 0.05). Moreover, as expected, control mice treated with water alone showed no evidence of licking/biting behaviors (Figure 6b). Thus, as with behaviors evoked by intradermal pruritogen injection, these findings show that cooling alleviates chronic itch.
Figure 6. Cooling inhibits chronic itch.
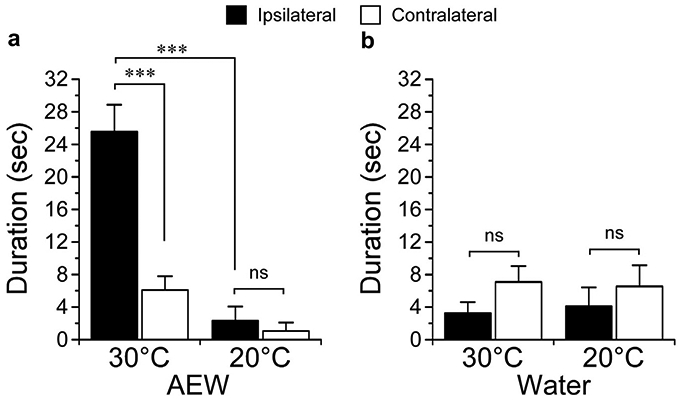
(a) A chronic itch model of dry skin (acetone, ether, and water [AEW]) was induced by twice daily treatments with an acetone/ether/water treatment protocol for 15 days. At 30°C, spontaneous hind-paw behaviors observed in the treated hind paw (ipsilateral) were significantly different than those in the untreated paw (contralateral, ***P < 0.001, one-way ANOVA, Neuman-Keuls post-test, n = 10), and inhibited when the plate was cooled to 20°C (***P < 0.001, one-way ANOVA, Neuman-Keuls post-test, n = 10). At 20°C, there was no significant difference between the ipsilateral and contralateral paws (nsP > 0.05, one-way ANOVA, Neuman-Keuls post-test, n = 10). (b) Control, water alone–treated hind paws displayed no significant difference in spontaneous behaviors at either temperature (nsP > 0.05, one-way ANOVA, Neuman-Keuls post-test, n = 6).
DISCUSSION
Chronic itch is common in many skin diseases (atopic dermatitis, psoriasis, xerosis), nervous system disorders (multiple sclerosis, shingles), a side effect of drugs (antima-larials, antibiotics, morphine), and associated with diseases such as HIV and acquired immune-deficiency syndrome (Huesmann et al., 2013; Sommer et al., 2007). Itch is mediated by several endogenous and environmental chemicals acting on a multitude of distinct itch receptors and divergent transduction pathways distributed among a range of sensory neurons (Jeffry et al., 2011; McNeil and Dong, 2012). This diversity hinders therapeutic interventions to prevent itch via receptor antagonism by making a “one-size fits all” approach unlikely, but counter-stimuli are effective, potentially highlighting a mechanism for general itch relief.
With regard to temperature, cold has been shown to shorten itch duration and intensity (Melton and Shelley, 1950) and increase histamine-itch thresholds (Cormia and Kuykendall, 1953). In patients with atopic dermatitis, cooling abolished spontaneous and histamine-induced itch (Fruhstorfer et al., 1986), and menthol was the only effective treatment for a patient with itch associated with lichen amyloidosis (Frolich et al., 2009). Lastly, in the remarkably few studies in animal models, cooling of histamine-stimulated rat skin reduced evoked electrical activity in the spinal cold dorsal horn (Carstens and Jinks, 1998), and menthol diminished Cqx-induced itch in the mouse (Kardon et al., 2014). Conversely, other studies dispute the antipruritic properties of cooling and menthol, reporting that menthol does not alleviate histamine-evoked itch (Melton and Shelley, 1950, Yosipovitch et al., 1996). Thus, even though menthol and cold stimuli are generally considered effective in blocking itch (Eccles, 1994), their efficacy, as well as mode of action, are still debated.
Pain and itch activate partially overlapping afferent populations of nociceptors and pruriceptors, respectively, with both sending axonal projections from the skin to the spinal cord dorsal horn, the first relay point for these and other modes of somatosensory information (Ma, 2010). We find that cooling reduces the effect of pruritogens in a manner that is partially dependent on TRPM8 channels, but clearly requires TRPM8 neurons, thereby highlighting a previously unrecognized inhibitory role for these afferents in itch. Moreover, both menthol and mildly cool temperatures require functional TRPM8 to block Cqx itch, indicating that TRPM8 activation is able to promote this inhibitory pathway. Intense cooling blocked itch in Trpm8−/− mice, but was poorly effective in TRPM8-neuron–ablated animals, suggesting that TRPM8-independent mechanism mediate antipruritic effects at noxious cold temperatures, consistent with prior findings (Knowlton et al., 2013; Pogorzala et al., 2013). Prolonged exposure to noxious cold temperatures clearly alters a number of TRPM8-independent processes, such as vascular tone, slowing nerve conduction, or enzymatically inhibiting pruritic signal transduction pathways. Nonetheless, the absence of robust antipruritic cooling in TRPM8-ablated animals shows that these afferents comprise a counter-stimulus afferent circuit that leads to itch inhibition even in absence of TRPM8 channels.
Most notable was that the effects of cooling were short lasting and only blocked itch as long as the cold stimulus was applied. Itch responses returned when the plate was warmed, as if they were simply being masked by cooling. This result is consistent with human studies (Fruhstorfer et al., 1986), but it is unknown why mechanical scratching (licking and biting in this case) provides lasting relief, while cold only blocks itch sensation temporarily. Perhaps scratching clears the pruritogen by dispersion or activates an immune response that clears it from the skin. The latter is unlikely, as skin can retain Cqx 48 to 72 hours after injection (Olatunde, 1971). Alternatively, cold-mediated blocking of signal transmission via TRPM8 neurons may lead to wind-up in the spinal cord. These are hypotheses that warrant further study and will help highlight important aspects of itch relief.
In the spinal cord dorsal horn, ablation of gastrin-related peptide receptor–expressing projection neurons or a reduction in transcription factor testicular orphan factor 4–dependent excitatory interneurons significantly diminish itch (Sun and Chen, 2007; Wang et al., 2013), suggesting these cells are part of an excitatory itch neurocircuit (Bautista et al., 2014). Similarly, spinal inhibitory interneurons that are dependent on the transcription factor bHLHb5 are essential for itch inhibition (Ross et al., 2010), and have been shown to receive direct synaptic inputs from capsaicin-, mustard oil-, and menthol-sensitive afferent neurons (Kardon et al., 2014). These results have led to the hypothesis that glutamate release from select afferents activates inhibitory neurons within the dorsal horn to suppress itch (Ma, 2012). However, the specific afferent pathways that mediate this inhibitory signal are unknown, and there is not sufficient information about the exact neurocircuitry underlying the inhibitory effects in the spinal cord (Bautista et al., 2014; Kardon et al., 2014; Snyder and Ross, 2015).
Based on the studies presented here, we propose that TRPM8-expressing afferents are part of this circuit. In addition to providing an understanding of somatosensory biology, these data also suggest that TRPM8 is a good target for developing itch treatments. Several studies using potent TRPM8 agonists to improve treatment of chronic itch in patients (Stander et al., 2017) are already under way. The success of these trials can result in marked improvement in the management of pruritic diseases.
MATERIALS AND METHODS
Statistical analysis
Values are reported as mean ± standard error of the mean. Statistical analyses were performed using Prism 5, version 5.0e (GraphPad, San Diego, CA). For statistical comparisons, one-way ANOVA followed by Neuman-Keuls post-hoc test were used. P < 0.05 was considered statistically significant.
Animals
All experiments were performed with adult C57BL6/J mice between the ages of 8 and 14 weeks. Wild-type, TRPM8-knockout mice, and TRPM8-DTR mice were on the same C57Bl/6 genetic background, and all animals were provided standard mouse chow and water ad libitum. All procedures and tests were approved by the University of Southern California Institutional Animal Care and Use Committee and conducted in accordance with the recommendations of the International Association for the Study of Pain and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Ablation of TRPM8 neurons was performed and validated as described previously (Knowlton et al., 2013).
Behavior tests
Cold plate.
Animals were habituated to a cold plate (IITC Part PE34) for 15 minutes at 24°C on the day of the experiment. They were briefly anesthetized with isoflurane (Risling et al., 2012) and injected with the desired vehicle or pruritogen in one hind paw in a volume of 20 μl. Animals regained consciousness within 30 seconds of injection and were fully awake and active (as judged by their grooming and exploratory behaviors) within the first 2 minutes of the video. Mice were recorded for 30 minutes post injection with subsequent analysis of the number and duration of flinching, licking, lifting, or guarding of the injected paw were performed by two individuals who were blind to the experimental set-up.
Dynamic cold plate.
The set-up was very similar to the cold plate, except that animals in the experimental group were exposed to a different temperature 15 minutes into the experiment. Also, results were broken down into 5-minute bins.
Cheek assay.
This assay was performed as described in Shimada and Lamotte (2008). Cheeks were shaved (4 mm2) at least 2 days before the experiment and animals were habituated to the recording chamber for half an hour each day before the experiment. Experiment was performed in triangular, mirrored chambers with high walls so that the mice would not jump over. For topical application of menthol compared to vehicle, 10 ul of either menthol solution (Stopain) or phosphate buffered saline were dropped onto the shaved area. Stopain was used for these studies as this is a prevalent formulation used in over-the-counter itch remedies and was previously shown to block itch in mice (Kardon et al., 2014). Of note, menthol (8%) is the active ingredient in this product, which also contains the inactive ingredients Boswellia serrata extract, carbomer, dimethylsulfone, eucalyptus oil, glucosamine sulfate, glycerin, peppermint oil, SD alcohol 39-C, and triethanolamine. As the concentrations of these substance is not available, phosphate buffered saline was used for vehicle controls. Mice were placed in the recording chamber immediately and responses recorded for 10–12 minutes, after which they were briefly anesthetized (see description for cold plate) and 10 ul of pruritogen or vehicle solution was injected into their cheek. Animals were then recorded for 30 minutes after the injection. Insulin syringes (0.3 ml) were used to inject solutions into the cheek.
Dry skin model of chronic itch
Animals were habituated to the cold plate 1 h/day for 2 days before recording at both the beginning and end of the experiment. Behavior was recorded on day 0 and day 15 for 30 minutes at each of the following temperatures: 30°C, 20°C, and 30°C again. From day 1 to day 15, mice were treated with 1:1 acetone-ether for 30 seconds, followed by water for 15 seconds on one hind paw. Mice in the control group were treated only with water. Treatments were administered twice daily 8 hours apart.
Chemicals used
Histamine (H7125; Sigma, St Louis, MO), Cqx diphosphate salt solid (C6628 ≥98%; Sigma), compound 48/80 (C2313; Sigma), and menthol (M2772; Sigma) were used.
ACKNOWLEDGMENTS
The authors wish to thank members of the McKemy Laboratory for their assistance in the completion of this work. We thank Diana Bautista, Qiufu Ma, and Wendy Knowlton for their guidance and advice in establishing the itch behavioral paradigms in this study. The present study was funded by grant NS087542 from the National Institutes of Health.
Abbreviations:
- Cqx
chloroquine
- α-Me5-HT
α-methyl 5-HT
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
REFERENCES
- Akiyama T, Carstens MI, Carstens E. Enhanced scratching evoked by PAR-2 agonist and 5-HT but not histamine in a mouse model of chronic dry skin itch. Pain 2010a;151:378–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Carstens MI, Carstens E. Spontaneous itch in the absence of hyperalgesia in a mouse hindpaw dry skin model. Neurosci Lett 2010b;484:62–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Nagamine M, Carstens MI, Carstens E. Behavioral model of itch, alloknesis, pain and allodynia in the lower hindlimb and correlative responses of lumbar dorsal horn neurons in the mouse. Neuroscience 2014;266:38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen HH, Melholt C, Hilborg SD, Jerwiarz A, Randers A, Simoni A, et al. Antipruritic effect of cold-induced and transient receptor potential-agonist-induced counter-irritation on histaminergic itch in humans. Acta Derm Venereol 2017;97:63–7. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, et al. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 2007;448(7150):204–8. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Wilson SR, Hoon MA. Why we scratch an itch: the molecules, cells and circuits of itch. Nat Neurosci 2014;17:175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstens E, Jinks SL. Skin cooling attenuates rat dorsal horn neuronal responses to intracutaneous histamine. Neuroreport 1998;9:4145–9. [DOI] [PubMed] [Google Scholar]
- Chung MK, Wang S. Cold suppresses agonist-induced activation of TRPV1. J Dent Res 2011;90:1098–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colburn RW, Lubin ML, Stone DJ Jr, Wang Y, Lawrence D, D’Andrea MR, et al. Attenuated cold sensitivity in TRPM8 null mice. Neuron 2007;54: 379–86. [DOI] [PubMed] [Google Scholar]
- Cormia FE, Kuykendall V. Experimental histamine pruritus. II. Nature; physical and environmental factors influencing development and severity. J Invest Dermatol 1953;20:429–46. [PubMed] [Google Scholar]
- Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. TRPM8 is required for cold sensation in mice. Neuron 2007;54:371–8. [DOI] [PubMed] [Google Scholar]
- Eccles R Menthol and related cooling compounds. J Pharm Pharmacol 1994;46:618–30. [DOI] [PubMed] [Google Scholar]
- Fleetwood-Walker SM, Proudfoot CW, Garry EM, Allchorne A, Vinuela-Fernandez I, Mitchell R. Cold comfort pharm. Trends Pharmacol Sci 2007;28:621–8. [DOI] [PubMed] [Google Scholar]
- Frolich M, Enk A, Diepgen TL, Weisshaar E. Successful treatment of therapy-resistant pruritus in lichen amyloidosis with menthol. Acta Derm Venereol 2009;89:524–6. [DOI] [PubMed] [Google Scholar]
- Fruhstorfer H, Hermanns M, Latzke L. The effects of thermal stimulation on clinical and experimental itch. Pain 1986;24:259–69. [DOI] [PubMed] [Google Scholar]
- Huesmann M, Huesmann T, Osada N, Phan NQ, Kremer AE, Stander S. Cholestatic pruritus: a retrospective analysis on clinical characteristics and treatment response. J Dtsch Dermatol Ges 2013;11:158–68. [DOI] [PubMed] [Google Scholar]
- Jeffry J, Kim S, Chen ZF. Itch signaling in the nervous system. Physiology (Bethesda) 2011;26:286–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardon AP, Polgar E, Hachisuka J, Snyder LM, Cameron D, Savage S, et al. Dynorphin acts as a neuromodulator to inhibit itch in the dorsal horn of the spinal cord. Neuron 2014;82:573–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kichko TI, Reeh PW. Why cooling is beneficial: non-linear temperature-dependency of stimulated iCGRP release from isolated rat skin. Pain 2004;110:215–9. [DOI] [PubMed] [Google Scholar]
- Knowlton WM, Palkar R, Lippoldt EK, McCoy DD, Baluch F, Chen J, et al. A sensory-labeled line for cold: TRPM8-expressing sensory neurons define the cellular basis for cold, cold pain, and cooling-mediated analgesia. J Neurosci 2013;33:2837–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMotte RH, Shimada SG, Sikand P. Mouse models of acute, chemical itch and pain in humans. Exp Dermatol 2011;20:778–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Iida T, Mizuno A, Suzuki M, Caterina MJ. Altered thermal selection behavior in mice lacking transient receptor potential vanilloid 4. J Neurosci 2005;25:1304–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Fan L, Balakrishna S, Sui A, Morris JB, Jordt SE. TRPM8 is the principal mediator of menthol-induced analgesia of acute and inflammatory pain. Pain 2013;154:2169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Ji RR. New insights into the mechanisms of itch: are pain and itch controlled by distinct mechanisms? Pflugers Arch 2013;465: 1671–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q Labeled lines meet and talk: population coding of somatic sensations. J Clin Invest 2010;120:3773–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q Population coding of somatic sensations. Neurosci Bull 2012;28: 91–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 2002;416(6876):52–8. [DOI] [PubMed] [Google Scholar]
- McNeil B, Dong X. Peripheral mechanisms of itch. Neurosci Bull 2012;28: 100–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton FM, Shelley WB. The effect of topical antipruritic therapy on experimentally induced pruritus in man. J Invest Dermatol 1950;15:325–32. [DOI] [PubMed] [Google Scholar]
- Moqrich A, Hwang SW, Earley TJ, Petrus MJ, Murray AN, Spencer KS, et al. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science 2005;307(5714):1468–72. [DOI] [PubMed] [Google Scholar]
- Nojima H, Cuellar JM, Simons CT, Carstens MI, Carstens E. Spinal c-fos expression associated with spontaneous biting in a mouse model of dry skin pruritus. Neurosci Lett 2004;361(1–3):79–82. [DOI] [PubMed] [Google Scholar]
- Olatunde IA. Chloroquine concentrations in the skin of rabbits and man. Br J Pharmacol 1971;43:335–40. [PMC free article] [PubMed] [Google Scholar]
- Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, et al. ATRP channel that senses cold stimuli and menthol.Cell 2002;108:705–15. [DOI] [PubMed] [Google Scholar]
- Pogorzala LA, Mishra SK, Hoon MA. The cellular code for mammalian thermosensation. J Neurosci 2013;33:5533–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot CJ, Garry EM, Cottrell DF, Rosie R, Anderson H, Robertson DC, et al. Analgesia mediated by the TRPM8 cold receptor in chronic neuropathic pain. Curr Biol 2006;16:1591–605. [DOI] [PubMed] [Google Scholar]
- Risling TE, Caulkett NA, Florence D. Open-drop anesthesia for small laboratory animals. Can Vet J 2012;53:299–302. [PMC free article] [PubMed] [Google Scholar]
- Ross SE, Mardinly AR, McCord AE, Zurawski J, Cohen S, Jung C, et al. Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron 2010;65:886–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkove SB. Effects of temperature on neuromuscular electrophysiology. Muscle Nerve 2001;24:867–82. [DOI] [PubMed] [Google Scholar]
- Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjork HE. Specific C-receptors for itch in human skin. J Neurosci 1997;17:8003–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz M, Schmidt R, Weidner C, Hilliges M, Torebjork HE, Handwerker HO. Chemical response pattern of different classes of C-nociceptors to pruritogens and algogens. J Neurophysiol 2003;89: 2441–8. [DOI] [PubMed] [Google Scholar]
- Shimada SG, LaMotte RH. Behavioral differentiation between itch and pain in mouse. Pain 2008;139:681–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder LM, Ross SE. Itch and its inhibition by counter stimuli. Handb Exp Pharmacol 2015;226:191–206. [DOI] [PubMed] [Google Scholar]
- Sommer F, Hensen P, Bockenholt B, Metze D, Luger TA, Stander S. Underlying diseases and co-factors in patients with severe chronic pruritus: a 3-year retrospective study. Acta Derm Venereol 2007;87:510–6. [DOI] [PubMed] [Google Scholar]
- Stander S, Augustin M, Roggenkamp D, Blome C, Heitkemper T, Worthmann AC, et al. Novel TRPM8 agonist cooling compound against chronic itch: results from a randomized, double-blind, controlled, pilot study in dry skin. J Eur Acad Dermatol Venereol 2017;31:1064–8. [DOI] [PubMed] [Google Scholar]
- Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature 2007;448(7154):700–3. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang J, Eberhart D, Urban R, Meda K, Solorzano C, et al. Excitatory superficial dorsal horn interneurons are functionally heterogeneous and required for the full behavioral expression of pain and itch. Neuron 2013;78:312–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yosipovitch G, Szolar C, Hui XY, Maibach H. Effect of topically applied menthol on thermal, pain and itch sensations and biophysical properties of the skin. Arch Dermatol Res 1996;288(5–6):245–8. [DOI] [PubMed] [Google Scholar]


