Abstract
Background:
Across major races in the United States (U.S.), ovarian cancer incidence is low among Asian American women. However, this observation aggregates Asian Americans as a single group despite their heterogeneity. Disaggregating the ethnic Asian population will produce more useful information to better understand ovarian cancer incidence among Asian women in the U.S.
Methods:
Data from the Surveillance, Epidemiology, and End Results Program from 1990 to 2014 were used to compare age-adjusted incidence rates (AAIRs, per 100,000 women) for ovarian cancer for the six largest U.S. Asian ethnicities (Asian Indian/Pakistani, Chinese, Filipino, Japanese, Korean, Vietnamese) to non-Hispanic whites (NHWs). The race/ethnicity-specific AAIRs were calculated by time period and histotype. We examined the magnitude and direction of AAIR trends using average annual percent change (AAPC) statistics.
Results:
All Asian ethnicities had significantly lower ovarian cancer incidence rates than NHWs. However, among Asian ethnicities, Asian Indians/Pakistanis had the highest rate of ovarian cancer (AAIR = 10.51, 95% CI: 9.65–11.42) while Koreans had the lowest (AAIR = 7.23, 95% CI: 6.62–7.88). Clear cell ovarian cancer had significantly higher incidence rates among Chinese, Filipino, and Japanese women than NHW women (incidence rate ratio (IRR) = 1.49, 95% CI: 1.29–1.72, IRR = 1.30, 95% CI: 1.12–1.51, IRR = 1.64, 95% CI: 1.36–1.97, respectively). Incidence trends also differed by Asian ethnicity with significant decreases only observed for Chinese (AAPC = −1.49, 95% CI: −2.22 to −0.74) and Japanese (AAPC = −1.75, 95% CI: −2.57 to −0.92).
Conclusions:
Examining Asian Americans as a single group results in missed ethnic-specific disparities in ovarian cancer, hence disaggregating this heterogeneous population in future research is warranted.
Keywords: Ovarian cancer, Racial/ethnic disparities, Asians, Incidence
1. Introduction
Ovarian cancer is the most fatal cancer of the female reproductive system, but its etiology is still poorly understood. Worldwide, ovarian cancer incidence rates are higher in Europe and North America than in Asia and Africa [1]. This may be attributable to distinct differences in ovarian cancer histotype, risk factors, and biomarkers, which have been observed between Asian women and women of European descent [2,3]. Variation in ovarian cancer incidence rates by country has also been noted within regions of the world; for example, the incidence rate in Japan is more than twice that of China [1,4,5].
There has been a growing body of literature highlighting the heterogeneity in the Asian population and the need to disaggregate the Asian ethnic groups to better understand cancer burden and develop more targeted and effective cancer control measures [6–12]. However, thus far, ovarian cancer research in the United States (U.S.) has regarded Asian Americans as a single aggregated group, which may have likely masked important ethnic-specific differences for generating new research hypotheses and identifying high-risk groups.
Hence, in the following, we present our study of subgroup differences in ovarian cancer incidence rates among the six largest Asian American ethnic groups: Asian Indian/Pakistani, Chinese, Filipino, Japanese, Korean, and Vietnamese. While it has been well-established that Asian American women as a single group have a lower rate of ovarian cancer incidence relative to non-Hispanic white (NHW) women based on findings from previous studies, we hypothesize that the rate of incidence may vary when each Asian American ethnicity is considered separately.
2. Methods
2.1. Cancer case identification
Data from the National Cancer Institute’s SEER database November 2016 submission [13] were used in this analysis. All data were de-identified and coded for public use, thus this study was exempt from Institutional Review Board review. A total of 13 population-based cancer registries were included (Atlanta (metropolitan), Connecticut, Detroit (metropolitan), Hawaii, Iowa, New Mexico, Utah, New Jersey, Seattle-Puget Sound, San Francisco/Oakland, San Jose/Monterey, Los Angeles, and all remaining areas in California) representing 54% of the U.S. Asian and Pacific Islander population [14].
Ovarian cancer cases diagnosed between 1990 and 2014 were identified using International Classification of Diseases for Oncology, Third Edition (ICD-O-3), primary site: C56.9 ovary [15]. Only malignant cases with a known age at diagnosis and race/ethnicity were included in the study. These tumors were categorized by SEER historic stage (local, regional, distant, unknown), grade (low = well-differentiated, high = moderately differentiated + poorly differentiated + undifferentiated, unknown), and histotype including serous (8050, 8120, 8122, 8130, 8140, 8201, 8260, 8440–8442, 8450, 8452, 8460–8463, 9014), mucinous (8144, 8384, 8470–8472, 8480–8482, 9015), endometrioid (8290, 8380–8383), and clear cell (8005, 8310, 8313, 8443, 8444); the remaining cases that were not classified as one of these four primary histotypes (including carcinosarcomas as well as mixed, other, undifferentiated, and unspecified carcinomas) were grouped together as “other/not otherwise specified (NOS)”. Nonepithelial tumors, such as germ cell and sex cord-stromal tumors, were excluded.
Cases classified as Asian Indian/Pakistani, Chinese, Filipino, Japanese, Korean, and Vietnamese were included, along with the NHW cases for comparison purposes. Asian Indians and Pakistanis were combined based on SEER coding rules for race.
2.2. Population estimates
To calculate the incidence rates, the annual at-risk population by age, sex, and ethnicity was estimated by the SEER program as described in previous publications [6,11]. Briefly, the population distributions by age, sex, and detailed/specific Asian ethnicity within the total Asian population from 1990, 2000, and 2010 Censuses of a given registry catchment area were used to disaggregate the Census Bureau’s annual population estimates of the total Asian American group for the same geographic area. Due to the multiracial identification method used in the 2000 and 2010 Censuses, estimates were based on the averages of the single-race and multi-race counts. The 1991 to 1999 and the 2001 to 2009 population estimates were developed from a linear interpolation between the 1990 and 2000 estimates and the 2000 and 2010 estimates, respectively. The 2011 to 2014 population estimates were developed from a linear extrapolation of the 2000–2010 growth trends. The annual population estimates for the NHW group came directly from the same Census Bureau estimation series.
2.3. Statistical analysis
Ovarian cancer patient demographic and tumor characteristics were compared across NHWs and all six Asian American ethnicities using Chi-square tests. Chi-square tests were also performed excluding NHWs to determine if such characteristics differed across the six Asian American ethnicities only. These analyses were performed using SAS software, release 9.4 (SAS Institute, Inc., Cary, North Carolina).
Age-adjusted incidence rates (AAIRs, per 100,000 women) for overall ovarian cancer with 95% confidence intervals (CIs) were calculated for each race/ethnicity. AAIRs were also calculated for 5-year time periods (i.e. 1990–1994, 1995–1999, 2000–2004, 2005–2009, 2010–2014) as well as for each histotype (i.e. serous, mucinous, endometrioid, clear cell, other/NOS). All rates were age-adjusted to the 2000 U.S. Standard Population. In addition, overall ovarian cancer age-specific incidence rates (ASIRs, per 100,000 women) with 95% CIs were calculated by race/ethnicity for the age groups of < 40, 40–49, 50–59, 60–69, 70–79, and 80+ years. Age-adjusted incidence rate ratios (IRRs) with 95% CIs comparing each Asian ethnic group to NHW, based on the method described by Tiwari et al. [16], were calculated for the AAIRs by histotype and for the ASIRs. All AAIRs and ASIRs were calculated using SEER*Stat software, version 8.3.5 (http://seer.cancer.gov/seerstat/) [17].
To evaluate trends in overall ovarian cancer AAIRs from 1990 to 2014, average annual percent change (AAPC) statistics were calculated using joinpoint regression models for each race/ethnicity [18,19]. AAPC is a summary measure of trend that weights the average of the annual percent change over multiple time intervals. Trends for each tumor histotype or stage were not examined due to limited numbers of cases.
All tests of statistical significance were two-sided.
3. Results
A total of 90,854 malignant, epithelial ovarian cancer cases diagnosed between 1990 and 2014 was identified across the 13 SEER registries. The majority of cases were NHW (N = 84,416). Among the Asian American ethnic subgroups, most cases were Filipino (N = 1,978) followed by Chinese (N = 1,559), Japanese (N = 1,091), Asian Indian/Pakistani (N = 680), Vietnamese (N = 578), and Korean (N = 552).
Table 1 shows the percentage of ovarian cancer cases by age at diagnosis and tumor characteristics for each race/ethnicity. There was a significant difference in histotype distribution when comparing across NHWs and all six Asian American ethnicities together (p < 0.0001) (Table 1). Although serous tumors constituted the majority of ovarian cancer diagnoses for all women, clear cell tumors accounted for less than 5% of all NHW cases in comparison to 13.66% and 11.76% of all Chinese and Vietnamese cases, respectively; however, they only accounted for 7.35% of Asian Indian/Pakistani cases, which was lowest among the Asian subgroups (Table 1). In addition, there were fewer low-grade ovarian cancers regardless of race/ethnicity although the tumor grade distribution varied with NHWs having the lowest percent of low-grade tumors (6.89%) and Koreans having the highest percent (9.24%) (Table 1). We also noted a significant difference in the distribution of tumor staging when we compared across NHWs and the six Asian American ethnicities together (p < 0.0001) and across the six Asian subgroups only (p = 0.0002); localized and regional tumors constituted 21.31% of NHW ovarian cancer cases and 26.18% of Asian Indian/Pakistani ovarian cancer cases, yet they accounted for over 31% for the other five Asian subgroups (Table 1). Lastly, all Asian American ovarian cancer cases were diagnosed at younger ages than NHW cases; 65.40% of ovarian cancer diagnoses among Vietnamese were under the age of 60 versus 35.64% among NHWs (Table 1). There was also significant variation in the age distribution of ovarian cancer cases across the six Asian American subgroups (p < 0.0001) (Table 1).
Table 1.
Distribution of demographic and tumor characteristics of ovarian cancer cases by race/ethnicity, 1990–2014.
| Characteristic | Non-Hispanic White* | Asian Indian/Pakistani* | Chinese* | Filipino* | Japanese* | Korean* | Vietnamese* | Chi-square P-value† | Chi-square P-value‡ |
|---|---|---|---|---|---|---|---|---|---|
| Total: | 84,416 | 680 | 1,559 | 1,978 | 1,091 | 552 | 578 | ||
| Age at diagnosis | < 0.0001 | < 0.0001 | |||||||
| < 40 years | 3,340 (3.96) | 91 (13.38) | 134 (8.60) | 168 (8.49) | 61 (5.59) | 47 (8.51) | 68 (11.76) | ||
| 40–49 years | 9,665 (11.45) | 151 (22.21) | 348 (22.32) | 418 (21.13) | 149 (13.66) | 120 (21.74) | 146 (25.26) | ||
| 50–59 years | 17,077 (20.23) | 177 (26.03) | 405 (25.98) | 562 (28.41) | 237 (21.72) | 169 (30.62) | 164 (28.37) | ||
| 60–69 years | 19,803 (23.46) | 132 (19.41) | 265 (17.00) | 408 (20.63) | 251 (23.01) | 111 (20.11) | 112 (19.38) | ||
| 70–79 years | 19,572 (23.19) | 91 (13.38) | 231 (14.82) | 294 (14.86) | 240 (22.00) | 60 (10.87) | 54 (9.34) | ||
| 80+ years | 14,959 (17.72) | 38 (5.59) | 176 (11.29) | 128 (6.47) | 153 (14.02) | 45 (8.15) | 34 (5.88) | ||
| Tumor Histotype | < 0.0001 | < 0.0001 | |||||||
| Serous | 50,732 (60.10) | 394 (57.94) | 698 (44.77) | 948 (47.93) | 514 (47.11) | 306 (55.43) | 285 (49.31) | ||
| Mutinous | 5,546 (6.57) | 43 (6.32) | 144 (9.24) | 196 (9.91) | 107 (9.81) | 51 (9.24) | 64 (11.07) | ||
| Endometrioid | 8,886 (10.53) | 83 (12.21) | 210 (13.47) | 296 (14.96) | 156 (14.30) | 59 (10.69) | 74 (12.80) | ||
| Clear cell | 3,846 (4.56) | 50 (7.35) | 213 (13.66) | 194 (9.81) | 128 (11.73) | 47 (8.51) | 68 (11.76) | ||
| Other/NOS** | 15,406 (18.25) | 110 (16.18) | 294 (18.86) | 344 (17.39) | 186 (17.05) | 89 (16.12) | 87 (15.05) | ||
| Tumor Grade*** | < 0.0001 | 0.14 | |||||||
| Low | 5,819 (6.89) | 49 (7.21) | 139 (8.92) | 156 (7.89) | 80 (7.33) | 51 (9.24) | 53 (9.17) | ||
| High | 48,643 (57.62) | 408 (60.00) | 898 (57.60) | 1162 (58.75) | 693 (63.52) | 312 (56.52) | 335 (57.96) | ||
| Unknown | 29,954 (35.48) | 223 (32.79) | 522 (33.48) | 660 (33.37) | 318 (29.15) | 189 (34.24) | 190 (32.87) | ||
| Tumor stage | < 0.0001 | 0.0002 | |||||||
| Localized | 12,297 (14.57) | 126 (18.53) | 407 (26.11) | 434 (21.94) | 274 (25.11) | 123 (22.28) | 149 (25.78) | ||
| Regional | 5,686 (6.74) | 52 (7.65) | 129 (8.27) | 219 (11.07) | 87 (7.97) | 50 (9.06) | 60 (10.38) | ||
| Distant | 49,882 (59.09) | 407 (59.85) | 863 (55.36) | 1,118 (56.52) | 616 (55.46) | 318 (57.61) | 321 (55.54) | ||
| Unknown | 16,551 (19.61) | 95 (13.97) | 160 (10.26) | 207 (10.47) | 114 (10.45) | 61 (11.05) | 48 (8.30) |
Abbreviation: NOS = not otherwise specified.
Number of ovarian cancer cases (percent of ovarian cancer cases).
Across non-Hispanic whites and all six Asian ethnicities.
Across the six Asian ethnicities only.
Includes carcinosarcomas as well as mixed, other, undifferentiated, and unspecified tumors.
Low grade includes well-differentiated tumors only. High grade includes moderately differentiated, poorly differentiated, and undifferentiated tumors.
Incidence of ovarian cancer was significantly lower in all Asian American ethnicities relative to NHWs (p < 0.0001) (Table 2, Fig. 1). However, among the Asian ethnicities, Asian Indians/Pakistanis had the highest incidence rate (10.51, 95% CI: 9.65–11.42) and Koreans had the lowest incidence rate (7.23, 95% CI: 6.62–7.88) (Fig. 1). When we examined incidence trends, we observed a significant decrease only among NHW (AAPC = −1.52, 95% CI: −2.09 to −0.95), Chinese (AAPC = −1.49, 95% CI: −2.22 to −0.74), and Japanese (AAPC = −1.75, 95% CI: −2.57 to −0.92) women (Table 2). Decreasing trends were observed for Filipino and Vietnamese women as well, but neither AAPC was statistically significant (AAPC = −0.59, 95% CI: −1.33 to 0.16 and AAPC = −1.02, 95% CI: −2.15 to 0.13, respectively) (Table 2, Fig. 2). Koreans and Asian Indians/Pakistanis, on the other hand, showed a non-significant increase in ovarian cancer incidence (AAPC = 0.94, 95% CI: −0.31 to 2.20 and AAPC = 0.95, 95% CI: −0.55 to 2.47, respectively) (Table 2, Fig. 2).
Table 2.
Age-adjusted incidence rates by race/ethnicity and year of diagnosis, 1990–2014.
| 1990–1994 | 1995–1999 | 2000–2004 | 2005–2009 | 2010–2014 | 1990–2014 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Race/Ethnicity | N | AAIR (95% CI)* | N | AAIR (95% CI)* | N | AAIR (95% CI)* | N | AAIR (95% CI)* | N | AAIR (95% CI)* | AAIR (95% CI)* | AAPC (95% CI) | AAPC P-value |
| Non-Hispanic White | 17,594 | 15.99 (15.75–16.23) | 17,360 | 15.18 (14.95–15.41) | 17,318 | 14.50 (14.28–14.72) | 16.565 | 13.37 (13.16–13.58) | 15,579 | 12.08 (11.88–12.28) | 14.15 (14.06–14.25) | −1.52 (−2.09 to −0.95) | < 0.0001 |
| Asian Indian/Pakistani | 56 | 9.31 (6.75–12.59) | 84 | 9.23 (7.20–11.67) | 127 | 10.90 (8.86–13.26) | 166 | 9.71 (8.15–11.47) | 247 | 11.34 (9.85–12.98) | 10.51 (9.65–11.42) | 0.95 (−0.55 to 2.47) | 0.21 |
| Chinese | 196 | 8.49 (7.32–9.80) | 274 | 8.83 (7.80–9.95) | 348 | 8.90 (7.98–9.89) | 344 | 7.09 (6.35–7.89) | 397 | 6.95 (6.27–7.68) | 7.87 (7.48–8.27) | −1.49 (−2.22 to −0.74) | 0.0004 |
| Filipino | 249 | 9.99 (8.73–11.38) | 329 | 10.39 (9.27–11.62) | 403 | 10.02 (9.05–11.07) | 481 | 9.79 (8.92–10.72) | 516 | 8.98 (8.21–9.81) | 9.73 (9.30–10.17) | −0.59 (−1.33 to 0.16) | 0.12 |
| Japanese | 215 | 9.70 (8.40–11.16) | 229 | 9.54 (8.31–10.91) | 231 | 9.25 (8.06–10.58) | 229 | 8.58 (7.45–9.84) | 187 | 6.38 (5.44–7.46) | 8.75 (8.22–9.30) | −1.75 (−2.57 to −0.92) | 0.0002 |
| Korean | 61 | 7.02 (5.24–9.20) | 78 | 6.28 (4.91–7.91) | 103 | 6.80 (5.52–8.30) | 138 | 7.46 (6.24–8.85) | 172 | 7.91 (6.75–9.22) | 7.23 (6.62–7.88) | 0.94 (−0.31 to 2.20) | 0.13 |
| Vietnamese | 59 | 10.29 (7.61–13.58) | 86 | 8.91 (6.99–11.20) | 120 | 9.10 (7.46–10.98) | 135 | 7.87 (6.55–9.39) | 178 | 8.42 (7.19–9.81) | 8.64 (7.91–9.41) | −1.02 (−2.15 to 0.13) | 0.079 |
Abbreviation: AAIR=age-adjusted incidence rate, CI=confidence interval, AAPC=average annual percent change.
Units of per 100,000 women.
Fig. 1.
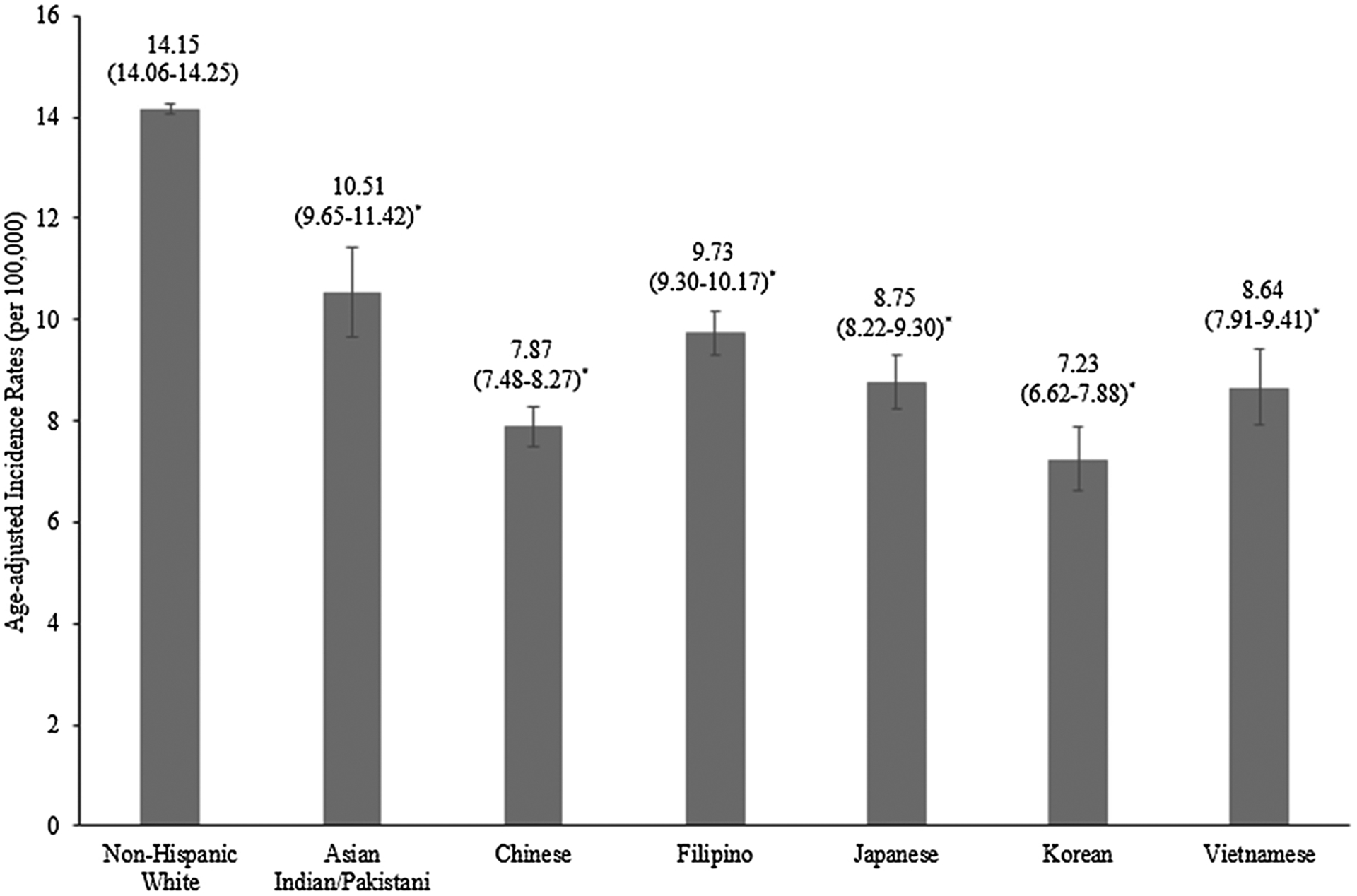
Age-adjusted incidence rates for ovarian cancer by race/ethnicity, 1990–2014. The error bars represent 95% confidence intervals. Note: * indicates statistical significance at a p < 0.0001 level with non-Hispanic Whites as the reference.
Fig. 2.
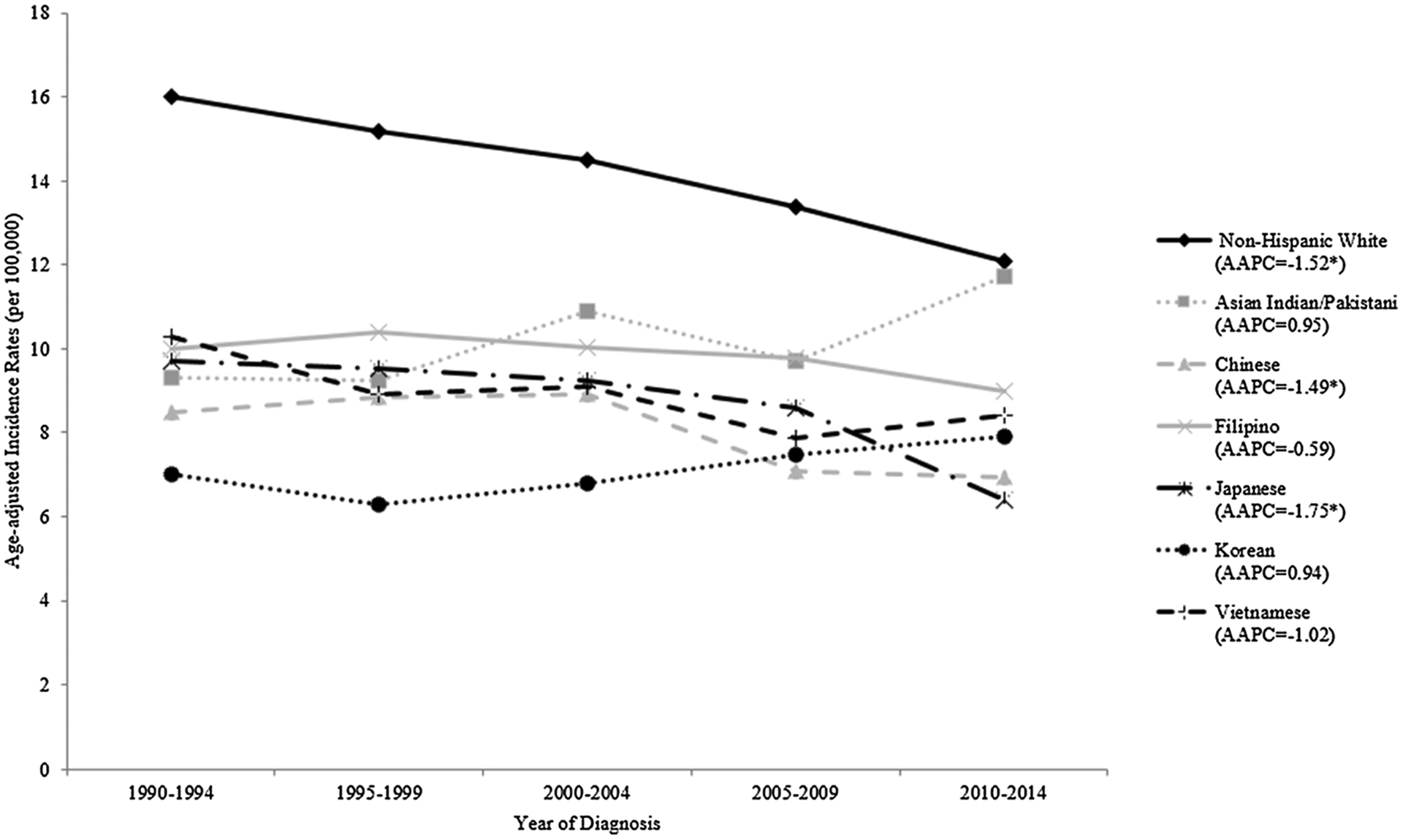
Age-adjusted incidence rates for ovarian cancer by race/ethnicity and time period, 1990–2014. Note: AAPC = average annual percent change; * indicates statistical significance at a p < 0.05 level.
Table 3 presents ovarian cancer AAIRs by histotype and race/ethnicity. All six Asian American ethnic groups showed a significant lower incidence of serous and endometrioid ovarian cancer relative to NHWs (Table 3). However, incidence of the clear cell histotype was statistically significantly higher in Chinese, Filipino, and Japanese women when compared to NHW women (IRR = 1.49, 95% CI: 1.29–1.72, IRR = 1.30, 95% CI: 1.12–1.51, IRR = 1.64, 95% CI: 1.36–1.97, respectively) (Table 3). This increased clear cell incidence when compared to the NHWs was not observed among Asian Indian/Pakistani (IRR = 0.92, 95% CI: 0.67–1.24) and Korean (IRR = 0.81, 95% CI: 0.59–1.09) women.
Table 3.
Age-adjusted incidence rates of ovarian cancer by histotype and race/ethnicity, 1990–2014.
| Histotype | Race/Ethnicity | N | AAIR (95% CI)* | IRR (95% CI) | IRR P-value |
|---|---|---|---|---|---|
| Serous | Non-Hispanic White | 50,732 | 8.45 (8.37–8.52) | 1.00 | ‒ |
| Asian Indian/Pakistani | 394 | 6.24 (5.57–6.95) | 0.74 (0.66–0.82) | < 0.0001 | |
| Chinese | 698 | 3.57 (3.31–3.85) | 0.42 (0.39–0.46) | < 0.0001 | |
| Filipino | 948 | 4.71 (4.41–5.03) | 0.56 (0.52–0.60) | < 0.0001 | |
| Japanese | 514 | 3.96 (3.62–4.33) | 0.47 (0.43–0.51) | < 0.0001 | |
| Korean | 306 | 4.05 (3.60–4.54) | 0.48 (0.43–0.54) | < 0.0001 | |
| Vietnamese | 285 | 4.37 (3.85–4.93) | 0.52 (0.46–0.58) | < 0.0001 | |
| Mutinous | Non-Hispanic White | 5,546 | 1.00 (0.97–1.03) | 1.00 | ‒ |
| Asian Indian/Pakistani | 43 | 0.57 (0.39–0.79) | 0.57 (0.39–0.79) | 0.0004 | |
| Chinese | 144 | 0.72 (0.60–0.85) | 0.72 (0.60–0.85) | 0.0001 | |
| Filipino | 196 | 0.96 (0.83–1.10) | 0.96 (0.83–1.11) | 0.59 | |
| Japanese | 107 | 0.94 (0.76–1.14) | 0.94 (0.76–1.14) | 0.58 | |
| Korean | 51 | 0.61 (0.46–0.81) | 0.62 (0.46–0.82) | 0.0003 | |
| Vietnamese | 64 | 0.87 (0.67–1.13) | 0.88 (0.67–1.13) | 0.34 | |
| Endometrioid | Non-Hispanic White | 8,886 | 1.60 (1.57–1.63) | 1.00 | ‒ |
| Asian Indian/Pakistani | 83 | 1.00 (0.79–1.26) | 0.63 (0.49–0.79) | < 0.0001 | |
| Chinese | 210 | 1.02 (0.89–1.17) | 0.64 (0.55–0.73) | < 0.0001 | |
| Filipino | 296 | 1.40 (1.24–1.57) | 0.87 (0.77–0.98) | 0.022 | |
| Japanese | 156 | 1.35 (1.15–1.59) | 0.85 (0.72–1.00) | 0.047 | |
| Korean | 59 | 0.71 (0.54–0.92) | 0.45 (0.34–0.58) | < 0.0001 | |
| Vietnamese | 74 | 1.01 (0.79–1.27) | 0.63 (0.49–0.80) | < 0.0001 | |
| Clear cell | Non-Hispanic White | 3,846 | 0.69 (0.67–0.71) | 1.00 | ‒ |
| Asian Indian/Pakistani | 50 | 0.64 (0.47–0.85) | 0.92 (0.67–1.24) | 0.66 | |
| Chinese | 213 | 1.03 (0.90–1.18) | 1.49 (1.29–1.72) | < 0.0001 | |
| Filipino | 194 | 0.90 (0.78–1.04) | 1.30 (1.12–1.51) | 0.0007 | |
| Japanese | 128 | 1.13 (0.94–1.35) | 1.64 (1.36–1.97) | < 0.0001 | |
| Korean | 47 | 0.56 (0.41–0.75) | 0.81 (0.59–1.09) | 0.18 | |
| Vietnamese | 68 | 0.89 (0.69–1.13) | 1.29 (0.99–1.65) | 0.052 | |
| Other/NOS† | Non-Hispanic White | 15,406 | 2.42 (2.38–2.46) | 1.00 | ‒ |
| Asian Indian/Pakistani | 110 | 2.07 (1.66–2.55) | 0.86 (0.68–1.05) | 0.15 | |
| Chinese | 294 | 1.53 (1.36–1.72) | 0.63 (0.56–0.71) | < 0.0001 | |
| Filipino | 344 | 1.76 (1.58–1.97) | 0.73 (0.65–0.81) | < 0.0001 | |
| Japanese | 186 | 1.36 (1.17–1.58) | 0.56 (0.48–0.65) | < 0.0001 | |
| Korean | 89 | 1.30 (1.03–1.61) | 0.54 (0.43–0.66) | < 0.0001 | |
| Vietnamese | 87 | 1.50 (1.18–1.87) | 0.62 (0.49–0.77) | < 0.0001 |
Abbreviation: AAIR = age-adjusted incidence rate, IRR = incidence rate ratio, CI = confidence interval, NOS = not otherwise specified.
Units of per 100,000 women.
Includes carcinosarcomas as well as mixed, other, undifferentiated, and unspecified tumors.
Lastly, the ASIRs for NHWs were statistically significantly higher than the ASIRs for all Asian American ethnic groups after age 50 with the exception of Asian Indians/Pakistanis in the 80+ years group (Table 4). Asian Indian/Pakistani women also constituted the only Asian American ethnic group whose age-specific incidence patterns notably increased after age 50 (Fig. 3). Prior to age 50, the ASIRs across all Asian American ethnicities were comparable to NHWs with the exception of Koreans who had statistically significantly lower ASIRs for the < 40 years and 40–49 years groups (IRR = 0.68, 95% CI: 0.50–0.91 and IRR = 0.69, 95% CI: 0.57–0.83, respectively) (Table 2, Fig. 3).
Table 4.
Age-specific incidence rates of ovarian cancer by race/ethnicity, 1990–2014.
| Age Group | Race/Ethnicity | N | ASIR (95% CI)* | IRR (95% CI) | IRR P–value |
|---|---|---|---|---|---|
| < 40 years | Non–Hispanic White | 3,340 | 1.33 (1.28–1.38) | 1.00 | ‒ |
| Asian Indian/Pakistani | 91 | 1.26 (1.01–1.56) | 0.95 (0.76–1.18) | 0.69 | |
| Chinese | 134 | 1.13 (0.95–1.34) | 0.85 (0.71–1.01) | 0.066 | |
| Filipino | 168 | 1.40 (1.20–1.63) | 1.05 (0.90–1.23) | 0.54 | |
| Japanese | 61 | 1.22 (0.93–1.57) | 0.92 (0.70–1.18) | 0.57 | |
| Korean | 47 | 0.91 (0.67–1.21) | 0.68 (0.50–0.91) | 0.008 | |
| Vietnamese | 68 | 1.32 (1.03–1.68) | 1.00 (0.77–1.27) | 0.99 | |
| 40–49 years | Non–Hispanic White | 9,665 | 13.26 (13.00–13.53) | 1.00 | ‒ |
| Asian Indian/Pakistani | 151 | 12.05 (10.20–14.14) | 0.91 (0.77–1.07) | 0.26 | |
| Chinese | 348 | 10.92 (9.80–12.13) | 0.82 (0.74–0.92) | 0.0003 | |
| Filipino | 418 | 13.33 (12.08–14.67) | 1.01 (0.91–1.11) | 0.94 | |
| Japanese | 149 | 10.60 (8.97–12.45) | 0.80 (0.68–0.94) | 0.006 | |
| Korean | 120 | 9.18 (7.61–10.98) | 0.69 (0.57–0.83) | < 0.0001 | |
| Vietnamese | 146 | 12.58 (10.62–14.80) | 0.95 (0.80–1.12) | 0.56 | |
| 50–59 years | Non–Hispanic White | 17,077 | 27.42 (27.01–27.84) | 1.00 | ‒ |
| Asian Indian/Pakistani | 177 | 21.00 (18.02–24.33) | 0.77 (0.66–0.89) | 0.0003 | |
| Chinese | 405 | 16.98 (15.37–18.72) | 0.62 (0.56–0.68) | < 0.0001 | |
| Filipino | 562 | 22.04 (20.25–23.94) | 0.80 (0.74–0.87) | < 0.0001 | |
| Japanese | 237 | 19.25 (16.86–21.87) | 0.70 (0.61–0.80) | < 0.0001 | |
| Korean | 169 | 17.51 (14.97–20.35) | 0.64 (0.55–0.74) | < 0.0001 | |
| Vietnamese | 164 | 18.91 (16.12–22.04) | 0.69 (0.59–0.80) | < 0.0001 | |
| 60–69 years | Non–Hispanic White | 19,803 | 43.03 (42.43–43.63) | 1.00 | ‒ |
| Asian Indian/Pakistani | 132 | 25.62 (21.42–30.42) | 0.60 (0.50–0.71) | < 0.0001 | |
| Chinese | 265 | 17.44 (15.40–19.69) | 0.41 (0.36–0.46) | < 0.0001 | |
| Filipino | 408 | 24.06 (21.78–26.53) | 0.56 (0.51–0.62) | < 0.0001 | |
| Japanese | 251 | 24.23 (21.32–27.42) | 0.56 (0.50–0.64) | < 0.0001 | |
| Korean | 111 | 18.31 (15.06–22.07) | 0.43 (0.35–0.51) | < 0.0001 | |
| Vietnamese | 112 | 22.15 (18.22–26.67) | 0.51 (0.42–0.62) | < 0.0001 | |
| 70–79 years | Non–Hispanic White | 19,572 | 55.55 (54.78–56.34) | 1.00 | ‒ |
| Asian Indian/Pakistani | 91 | 38.20 (30.70–46.97) | 0.69 (0.55–0.85) | 0.0002 | |
| Chinese | 231 | 23.19 (20.29–26.38) | 0.42 (0.37–0.48) | < 0.0001 | |
| Filipino | 294 | 29.69 (26.38–33.30) | 0.53 (0.47–0.60) | < 0.0001 | |
| Japanese | 240 | 27.66 (24.27–31.40) | 0.50 (0.44–0.57) | < 0.0001 | |
| Korean | 60 | 17.74 (13.52–22.87) | 0.32 (0.24–0.41) | < 0.0001 | |
| Vietnamese | 54 | 21.01 (15.75–27.45) | 0.38 (0.28–0.49) | < 0.0001 | |
| 80+ years | Non–Hispanic White | 14,959 | 56.87 (55.96–57.80) | 1.00 | ‒ |
| Asian Indian/Pakistani | 38 | 44.42 (31.35–61.09) | 0.78 (0.55–1.07) | 0.14 | |
| Chinese | 176 | 30.58 (26.23–35.45) | 0.54 (0.46–0.62) | < 0.0001 | |
| Filipino | 128 | 27.75 (23.14–33.02) | 0.49 (0.41–0.58) | < 0.0001 | |
| Japanese | 153 | 26.60 (22.55–31.16) | 0.47 (0.40–0.55) | < 0.0001 | |
| Korean | 45 | 29.20 (21.29–39.08) | 0.51 (0.37–0.69) | < 0.0001 | |
| Vietnamese | 34 | 29.69 (20.55–41.50) | 0.52 (0.36–0.73) | < 0.0001 |
Abbreviation: ASIR = age-specific incidence rate; IRR = incidence rate ratio, CI = confidence interval.
Units of per 100,000 women.
Fig. 3.
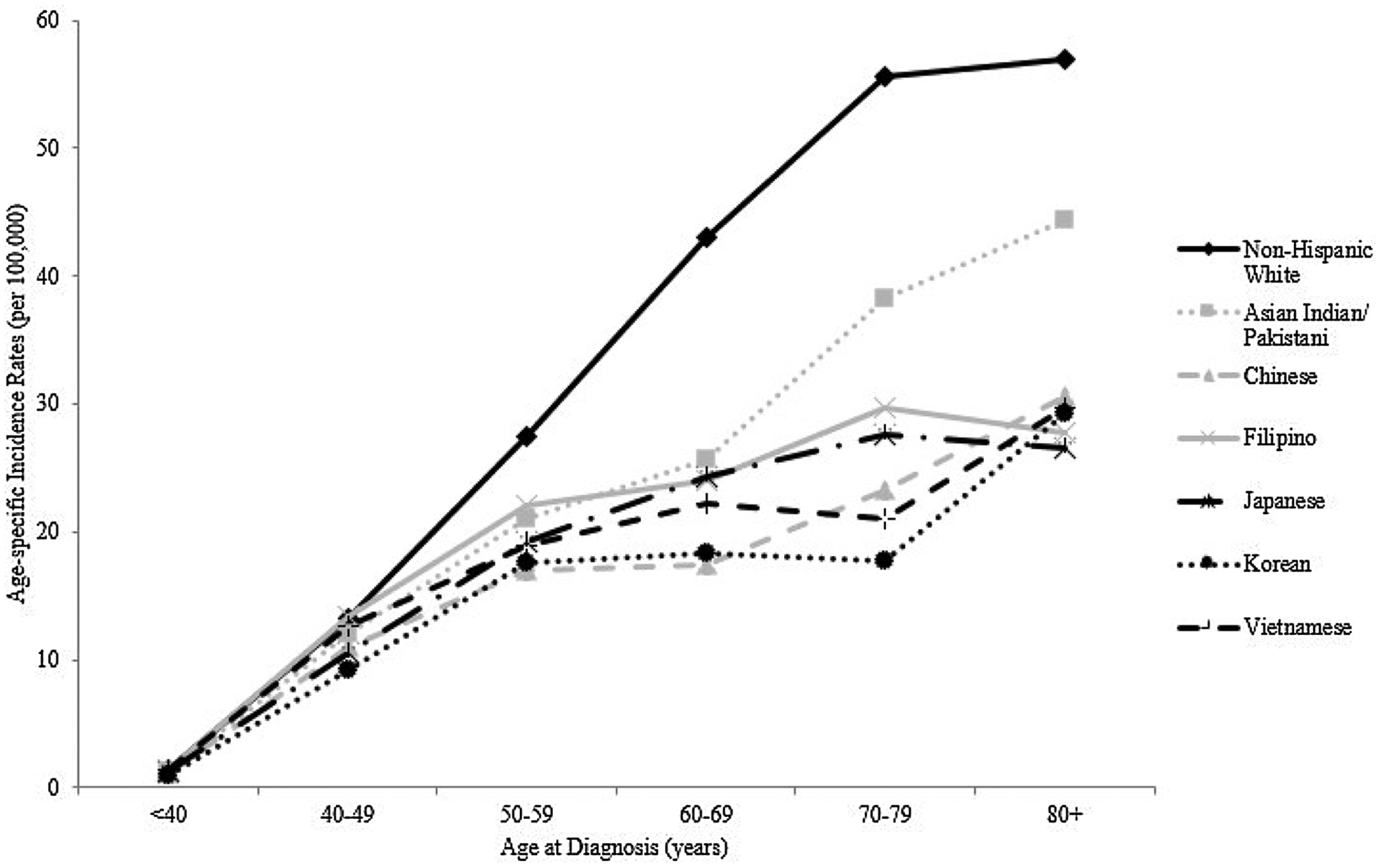
Age-specific incidence rates for ovarian cancer by race/ethnicity, 1990–2014.
4. Discussion
Our analysis comprehensively examines ovarian cancer among the six largest ethnic groups of the Asian American population. We observed significant differences in ovarian cancer tumor characteristics, incidence rates and trends not only between Asian subgroups and NHWs, but also across the various Asian ethnicities, highlighting the heterogeneity of ovarian cancer incidence rates among Asian Americans, as well as the need for ethnic-specific cancer statistics and research for Asian Americans, as previously noted by previous studies for other cancer sites [6,11].
Although all Asian American ethnic groups had a lower incidence rate of ovarian cancer relative to NHWs, there are some very interesting findings of unique incidence patterns associated with certain ethnic groups. For example, in our analysis, Asian Indian/Pakistani women showed a higher ovarian cancer incidence rate than other Asians and had a lower percentage of the clear cell tumor that is known to be more common among Asians [3,20,21]. Genetics may play a role in such observations, since previous phylogenetic work has presented Asian Indians/Pakistanis as distinct from other Asian ethnicities and more closely related to Caucasians [22]. This could explain why Asian Indian/Pakistani women’s ovarian cancer incidence rates and percent distributions of tumor characteristics, such as stage and histotype, were more comparable to those for NHWs.
In agreement with existing literature, we found that Chinese, Filipino, and Japanese women indeed had higher incidence of clear cell ovarian cancer. Endometriosis could partly explain this given that it is a strong risk factor for the clear cell histotype [23]. Miyazawa reported the highest hospital admission rates for endometriosis among Japanese women in comparison to non-Japanese Asian ethnicities and non-Asian races [24]. Similarly, a retrospective cohort study based on electronic medical records of infertility clinic patients observed women of Filipino and Japanese origin to be more likely to have endometriosis than NHW women [25]. Given that endometriosis is also a risk factor for endometrioid ovarian cancer, we would expect to see a higher incidence of the endometrioid histotype among these Asian American ethnic groups in our results. However, this was not observed, which may be due to other ovarian cancer etiologic factors with histotype-specific effects.
The ASIRs for all Asian American ethnic groups were statistically significantly lower than those of NHWs after age 50, which has been observed in previous work [26]. Again, the age-specific incidence pattern for Asian Indians/Pakistanis appeared to diverge from the other Asian subgroups since their ASIRs continued to increase after age 50 mimicking that of NHWs (Fig. 3). Given the timing of these observations, the racial/ethnic differences in these patterns may be related to menopause given that menopausal symptoms, lifestyles, and behaviors during and after the menopause transition have been shown to differ by race/ethnicity [27,28], and such factors could differentially impact disease incidence. For example, differences in the prevalence of post-menopausal hormone therapy use by race has been noted, with White women being more commonly prescribed hormone therapy than other races [29]. Also, the specific effect of hormone therapy and other factors on ovarian cancer risk could vary by race; Peres et al. found significant heterogeneity in the association between reproductive, hormonal, and lifestyle factors and ovarian cancer by race, such as parity being more protective for Asian women [30]. Such variation across the Asian ethnic groups is likely, but there is limited reporting on this, highlighting the need for greater diversity in clinical and epidemiologic studies.
It has been noted that incidence rates of ovarian cancer has either remained unchanged or only slightly decreased among the aggregated Asian American women, contrary to the significantly greater decline that has been observed among NHW women [31–34]. However, when we disaggregate the Asian American population, we observed incidence to be significantly decreasing for Chinese and Japanese women as well. Interestingly, increases in ovarian cancer incidence have been reported in both China [35,36] and Japan [37–39]. However, those living in the U.S. are likely to be different from those living in their native countries with respect to socioeconomic and lifestyle factors [26], as the ovarian cancer incidence differential between Japanese American and Chinese American women is much less than the reported two-fold between Japan and China [1,4,5]. Redaniel et al. also found significant ovarian cancer survival differences between Philippine residents and Filipino-Americans [40], underlying possible environmental mediations.
The lack of readily available cancer data, as well as population data, by detailed Asian ethnicity coupled with the rareness of ovarian cancer are often why most ovarian cancer research evaluates Asians in the aggregate. By leveraging the population-based SEER registry database, we are able to provide valuable insights into ovarian cancer incidence patterns and trends among the heterogeneous Asian American population with disaggregated ethnic groups. A limitation of such population-based studies is possible misclassification of race/ethnicity given that this information is primarily based on medical records [41]. However, studies comparing administrative databases to self-report have shown low misclassification even when specific Asian ethnicities were considered [42,43]. The agreement between our findings and supporting literature adds to our confidence in the quality of data used in the analysis. In addition, we did not correct for salpingo-oophorectomy in our rate calculations, which underestimates the true incidence of ovarian cancer. However, prior incidence correction by Merrill that took into account the impact of this surgical procedure on ovarian cancer rates showed the biggest impact on NHWs and the smallest impact on Asians, suggesting an even greater disparity between the two [44]. Ethnic-specific salpingo-oophorectomy rates would be useful given that they may differ by Asian American subgroup, which could explain some of our findings despite salpingo-oophorectomy’s small impact on Asians as a whole. However, current data on this is sparse, hence future work in this area could refine our understanding of ovarian cancer incidence rates among the Asian American ethnic populations.
The ovarian cancer disparities revealed by our analysis further underscore the need for disaggregated Asian American cancer studies to more accurately understand disease burden and generate etiologic hypotheses. They also provide needed information for developing more targeted ovarian cancer educational outreach and intervention strategies. Future research should take advantage of the ethnic diversity in the Asian American community to advance cancer research that can benefit public health in not only the U.S., but also the world.
Acknowledgements
This work was supported by an intramural award from the California State University, Fullerton’s Health Promotion Research Institute. The funding source had no involvement in the analysis and interpretation of the data, the writing of the report, and the decision to submit the article for publication.
Abbreviations:
- U.S.
United States
- NHW
non-Hispanic white
- NOS
not otherwise specified
- AAIR
age-adjusted incidence rate
- IRR
incidence rate ratio
- AAPC
average annual percent change
- SEER
Surveillance, Epidemiology, and End Results
- ASIR
age-specific incidence rate
- CI
confidence interval
Footnotes
Declaration of competing interest
The authors have no conflicts of interest to disclose.
References
- [1].Chornokur G, Amankwah EK, Schildkraut JM, Phelan CM, Global ovarian cancer health disparities, Gynecol. Oncol 129 (1) (2013) 258–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sung PL, Chang YH, Chao KC, Chuang CM, Task Force on Systematic Review, Meta-analysis of Ovarian Cancer, Global distribution pattern of histological subtypes of epithelial ovarian cancer: a database analysis and systematic review, Gynecol. Oncol 133 (2) (2014) 147–154. [DOI] [PubMed] [Google Scholar]
- [3].Ugai T, Kelemen LE, Mizuno M, Ong JS, Webb PM, Chenevix-Trench G, et al. , Ovarian cancer risk, ALDH2 polymorphism and alcohol drinking: Asian data from the Ovarian Cancer Association Consortium, Cancer Sci. 109 (2) (2018) 435–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ferlay J, Colombet M, Soerjomataram I, Dyba T, Randi G, Bettio M, et al. , Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018, Eur. J. Cancer 103 (2018) 356–387. [DOI] [PubMed] [Google Scholar]
- [5].Parkin DM, Bray F, Ferlay J, Pisani P, Global cancer statistics, 2002, CA Cancer J. Clin 55 (2) (2005) 74–108. [DOI] [PubMed] [Google Scholar]
- [6].Gomez SL, Noone AM, Lichtensztajn DY, Scoppa S, Gibson JT, Liu L, et al. , Cancer incidence trends among Asian American populations in the United States, 1990–2008, J. Natl. Cancer Inst 105 (15) (2013) 1096–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Miller BA, Chu KC, Hankey BF, Ries LA, Cancer incidence and mortality patterns among specific Asian and Pacific Islander populations in the U.S, Cancer Causes Control 19 (3) (2008) 227–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].McCracken M, Olsen M, Chen MS Jr., Jemal A, Thun M, Cokkinides V, et al. , Cancer incidence, mortality, and associated risk factors among Asian Americans of Chinese, Filipino, Vietnamese, Korean, and Japanese ethnicities, CA Cancer J. Clin 57 (4) (2007) 190–205. [DOI] [PubMed] [Google Scholar]
- [9].Wang SS, Carreon JD, Gomez SL, Devesa SS, Cervical cancer incidence among 6 asian ethnic groups in the United States, 1996 through 2004, Cancer 116 (4) (2010) 949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Liu L, Zhang J, Wu AH, Pike MC, Deapen D, Invasive breast cancer incidence trends by detailed race/ethnicity and age, Int. J. Cancer 130 (2) (2012) 395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cheng I, Le GM, Noone AM, Gali K, Patel M, Haile RW, et al. , Lung cancer incidence trends by histology type among Asian American, Native Hawaiian, and Pacific Islander populations in the United States, 1990–2010, Cancer Epidemiol. Biomarkers Prev 23 (11) (2014) 2250–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pham C, Fong TL, Zhang J, Liu L, Striking racial/ethnic disparities in liver cancer incidence rates and temporal trends in California, 1988–2012, J. Natl. Cancer Inst 110 (11) (2018) 1259–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Surveillance, Epidemiology, and End Results (SEER) Program, SEER*Stat Database: Incidence - SEER 9, Plus Remainder of CA and NJ, Nov 2016 Sub (1990–2014) Detailed API Plus White Non-hispanic - Pops Projected From Populations, Based on the November 2016 Submission, National Cancer Institute, DCCPS, Surveillance Research Program, (https://www.seer.cancer.gov), released May 2017.
- [14].National Cancer Institute, Surveillance Epidemiology and End Results, (https://www.seer.cancer.gov) (Accessed 2 October 2018).
- [15].Fritz ACP, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, Whelan S, International Classification of Diseases Oncology, 3rd ed, World Health Organization, Geneva, 2000. [Google Scholar]
- [16].Tiwari RC, Clegg LX, Zou Z, Efficient interval estimation for age-adjusted cancer rates, Stat. Methods Med. Res 15 (6) (2006) 547–569. [DOI] [PubMed] [Google Scholar]
- [17].Surveillance Research Program, National Cancer Institute SEER*Stat software (https://www.seer.cancer.gov/seerstat) version 8.3.5.
- [18]. Joinpoint Regression Program, Version 4.6.0.0 - April 2018, Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute.
- [19].Kim HJ, Fay MP, Feuer EJ, Midthune DN, Permutation tests for joinpoint regression with applications to cancer rates, Stat. Med 19 (3) (2000) 335–351. [DOI] [PubMed] [Google Scholar]
- [20].Mabuchi S, Sugiyama T, Kimura T, Clear cell carcinoma of the ovary: molecular insights and future therapeutic perspectives, J. Gynecol. Oncol 27 (3) (2016) e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chan JK, Teoh D, Hu JM, Shin JY, Osann K, Kapp DS, Do clear cell ovarian carcinomas have poorer prognosis compared to other epithelial cell types? A study of 1411 clear cell ovarian cancers, Gynecol. Oncol 109 (3) (2008) 370–376. [DOI] [PubMed] [Google Scholar]
- [22].Nei M, Roychoudhury AK, Evolutionary relationships of human populations on a global scale, Mol. Biol. Evol 10 (5) (1993) 927–943. [DOI] [PubMed] [Google Scholar]
- [23].Pearce CL, Templeman C, Rossing MA, Lee A, Near AM, Webb PM, et al. , Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case-control studies, Lancet Oncol. 13 (4) (2012) 385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Miyazawa K, Incidence of endometriosis among Japanese women, Obstet. Gynecol 48 (4) (1976) 407–409. [PubMed] [Google Scholar]
- [25].Yamamoto A, Johnstone EB, Bloom MS, Huddleston HG, Fujimoto VY, A higher prevalence of endometriosis among Asian women does not contribute to poorer IVF outcomes, J. Assist. Reprod. Genet 34 (6) (2017) 765–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Herrinton LJ, Stanford JL, Schwartz SM, Weiss NS, Ovarian cancer incidence among Asian migrants to the United States and their descendants, J. Natl. Cancer Inst 86 (17) (1994) 1336–1339. [DOI] [PubMed] [Google Scholar]
- [27].Im EO, Lee S, Chee W, Subethnic differences in the menopausal symptom experience of Asian American midlife women, J. Transcult. Nurs 21 (2) (2010) 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].van Noord PA, Dubas JS, Dorland M, Boersma H, te Velde E, Age at natural menopause in a population-based screening cohort: the role of menarche, fecundity, and lifestyle factors, Fertil. Steril 68 (1) (1997) 95–102. [DOI] [PubMed] [Google Scholar]
- [29].Brown AF, Perez-Stable EJ, Whitaker EE, Posner SF, Alexander M, Gathe J, et al. , Ethnic differences in hormone replacement prescribing patterns, J. Gen. Intern. Med 14 (11) (1999) 663–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Peres LC, Risch H, Terry KL, Webb PM, Goodman MT, Wu AH, et al. , Racial/ethnic differences in the epidemiology of ovarian cancer: a pooled analysis of 12 case-control studies, Int. J. Epidemiol 47 (2) (2018) 460–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Park HK, Ruterbusch JJ, Cote ML, Recent trends in ovarian cancer incidence and relative survival in the United States by race/ethnicity and histologic subtypes, Cancer Epidemiol. Biomarkers Prev 26 (10) (2017) 1511–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Torre LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, et al. , Ovarian cancer incidence, 2018, CA Cancer J. Clin 68 (2018) 284–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sopik V, Iqbal J, Rosen B, Narod SA, Why have ovarian cancer mortality rates declined? Part I. Incidence, Gynecol. Oncol 138 (3) (2015) 741–749. [DOI] [PubMed] [Google Scholar]
- [34].Liu L, Wang Y, Sherman RL, Cockburn M, Deapen D (), Cancer in Los Angeles County: Trends by Race/Ethnicity, 1976–2012, Los Angeles Cancer Surveillance Program, University of Southern California, 2016, 2012. [Google Scholar]
- [35].Teng Z, Han R, Huang X, Zhou J, Yang J, Luo P, et al. , Increase of incidence and mortality of ovarian cancer during 2003–2012 in Jiangsu Province, China, Front. Public Health 4 (2016) 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Jiang X, Tang H, Chen T, Epidemiology of gynecologic cancers in China, J. Gynecol. Oncol 29 (1) (2018) e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Coburn SB, Sherman ME, Trabert B, International patterns and trends in ovarian cancer incidence, overall and by histologic subtype, Int. J. Cancer 140 (11) (2017) 2451–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Yamagami W, Nagase S, Takahashi F, Ino K, Hachisuga T, Aoki D, et al. , Clinical statistics of gynecologic cancers in Japan, J. Gynecol. Oncol 28 (2) (2017) e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Cabasag CJ, Arnold M, Butler J, Inoue M, Trabert B, Webb PM, et al. , The influence of birth cohort and calendar period on global trends in ovarian cancer incidence, Int. J. Cancer (2019) Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Redaniel MT, Laudico A, Mirasol-Lumague MR, Gondos A, Uy GL, Toral JA, et al. , Ovarian cancer survival population differences: a “high resolution study” comparing Philippine residents, and Filipino-Americans and Caucasians living in the US, BMC Cancer 9 (2009) 340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gomez SL, West DW Le GM, Satariano WA, O’Connor L, Hospital policy and practice regarding the collection of data on race, ethnicity, and birthplace, Am. J. Public Health 93 (10) (2003) 1685–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Gomez SL, Kelsey JL, Glaser SL, Lee MM, Sidney S, Inconsistencies between self-reported ethnicity and ethnicity recorded in a health maintenance organization, Ann. Epidemiol 15 (1) (2005) 71–79. [DOI] [PubMed] [Google Scholar]
- [43].Moscou S, Anderson MR, Kaplan JB, Valencia L, Validity of racial/ethnic classifications in medical records data: an exploratory study, Am. J. Public Health 93 (7) (2003) 1084–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Merrill RM, Impact of hysterectomy and bilateral oophorectomy on race-specific rates of corpus, cervical, and ovarian cancers in the United States, Ann. Epidemiol 16 (12) (2006) 880–887. [DOI] [PubMed] [Google Scholar]


