Abstract
Alginate is one of the natural polymers that are often used in drug- and protein-delivery systems. The use of alginate can provide several advantages including ease of preparation, biocompatibility, biodegradability, and nontoxicity. It can be applied to various routes of drug administration including targeted or localized drug-delivery systems. The development of alginates as a selected polymer in various delivery systems can be adjusted depending on the challenges that must be overcome by drug or proteins or the system itself. The increased effectiveness and safety of sodium alginate in the drug- or protein-delivery system are evidenced by changing the physicochemical characteristics of the drug or proteins. In this review, various routes of alginate-based drug or protein delivery, the effectivity of alginate in the stem cells, and cell encapsulation have been discussed. The recent advances in the in vivo alginate-based drug-delivery systems as well as their toxicities have also been reviewed.
1. Introduction
1.1. Chemistry and Physicochemical Properties of Alginate
Alginate is a polysaccharide extracted from brown seaweeds, including Laminaria hyperborea, Laminaria digitata, Laminaria japonica, Ascophyllum nodosum, and Macrocystis pyrifera [1, 2]. It is composed by a sequence of two (1Ñ4)-linked α-L-guluronate (G) and β-D-mannuronate (M) monomers. The proportion of M and G blocks may vary with the type of seaweed from where it is extracted (Figure 1). For example, alginate extracted from Laminaria digitata and Ascophyllum nodosum has been shown to have M/G ratios of 1.16 and 1.82, respectively. Alginate is a biocompatible polymer with very low toxicity [3]. These are the main advantages that make alginate one of the biopolymers with the widest biomedical applicability [4, 5]. One of the most common applications of alginate is their use as an excipient in drug-delivery systems, namely, acting as a stabilizer agent in various pharmaceutical formulations [6, 7].
Figure 1.
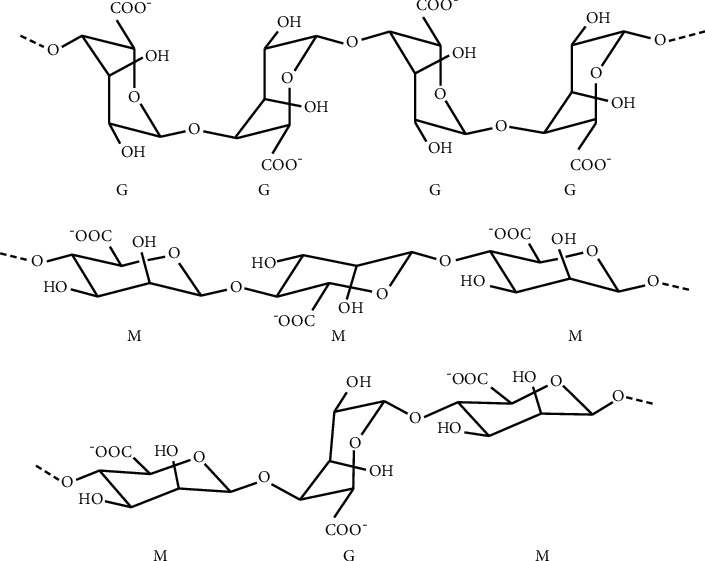
Chemical structures of G-block, M-block, and alternating block in alginate [1].
Alginate has carboxyl groups which are charged at pH values higher than 3-4, making alginate soluble at neutral and alkaline conditions to promote the widespread use of alginates. For some drugs which require greater protection with preferential absorption in the intestinal tract or other conditions such as modified drug release, alginate is a preferable polymer. Thus, solubility and pH sensitivity make alginate a good biomaterial for drug-delivery systems [8]. Sodium alginate is the type of alginate mainly used in the pharmaceutical industry and may be used for the purpose of extending the drug release. Using sodium alginate with different chemical features and degree of viscosities, the slow release of ibuprofen from press-coated tablets was reported [8]. In acidic environments, alginate carboxyl groups are protonated, thereby limiting drug release. Alginate has the ability to crosslink with Ca2+ ions through an ionotropic gelation process, usually above pH 6. Ba2+ or Zn2+ ions are also used as crosslinkers [9–11].
Alginate hydrogels are applied in wound healing treatments through the construction of wound dressings [12–15]. Several studies showed that the bioavailability of drugs encapsulated in alginate hydrogels is greater than that of the free drug applied directly at the lesion site, thus increasing the efficacy of healing. Alginate hydrogels are also used widely in tissue regeneration treatments and cell encapsulation [16–22]. Alginate may be used in the construction of capsules for cell encapsulation often associated with cytotherapy treatments or simply the creation of cellular microcultures in more complex systems. A new approach to the construction of alginate-based capsules for the incorporation of different types of cells has been demonstrated [23]. Cells were encapsulated in alginate liquefied particles, followed by coating it with chitosan and alginate. Poly(lactic acid) microparticles along with the cells were coencapsulated to protect cell survival with high viability of the encapsulated cells. Hydrogels obtained from alginate nowadays present some advantages of being appropriate materials to be used in tissue engineering and regenerative medicine applications [23–31].
Some important uses of alginates in nanomedicines in the forms of dendrimers, nanocrystals, emulsions, liposomes, solid lipid nanoparticles, micelles, and polymeric nanoparticles have provided advantages over conventional medicines including efficacy, safety, physicochemical properties, and pharmacokinetic/pharmacodynamic profiles [32].
1.2. Crosslinker for Alginate Micro/Nanoparticles to Encapsulate Drugs
Typical shapes of alginate are processing through several different techniques, including emulsion, multiple-phase emulsion, and cation crosslinked encapsulation (Ca2+, Ba2+, or Cu2+) [33–37]. The ability of alginate to create complexes with other biomaterials by electrostatic interactions, chemical modification, or crosslinking can be exploited for building hybrid and more versatile DDSs. Capsules constructed from chitosan/alginate-PEG complexes are reliable models for encapsulating proteins, such as albumin, one of the most common model proteins used in controlled release studies [38–43]. This approach can promote higher control release of drugs, proteins, and other biomolecules.
1.2.1. Effect of Different Classes of Crosslinkers on Alginate Polyelectrolyte Nanoparticle
Mirtic et al. [10] investigated the preparation of alginate nanoparticles using complexation of different classes of crosslinkers (divalent cations, polycations, and positively charged surfactants) and found that alginate nanoparticles were formed across a limited range of molar ratios that were specific for each crosslinker and had different size and stability. Additionally, the ionic strengths of the media influenced the characteristics and stabilities of the polyelectrolyte nanoparticles.
1.2.2. Effect of Divalent Cation on Morphology and Drug-Delivery Efficiency
A study by Deepika et al. [44] was about the formation of levofloxacin in chitosan-alginate hybrid gel for controlled release and effect of divalent alkaline ions (Mg2+, Ca2+, Sr2+, and Ba2+) on encapsulation efficiency and drug release kinetics from chitosan-alginate nanostructure was investigated. The particle size increases and encapsulation efficiency decreases with the size of the divalent ions. Spherical shaped particles were formed by Mg2+ and Ca2+, whereas Sr2+ and Ba2+ produced nonspherical particles. Transformation of microspheres is shown by SEM as truncated tetrahedron by Sr2+ and clear rod shape by Ba2+ was identified. This suggested that metal ions have a significant influence on the morphology, drug encapsulation, and release profile of the chitosan-alginate hybrid polymer nanoparticles.
1.2.3. Effect of Zinc-Ion Complex with Alginates
Kotagale et al. [45] complexed alginates with zinc metal ion to improve beads' physicochemical and biological properties for controlling the drug release. They found that the atenolol-zinc polymeric beads exhibited pulsed release with increased half-life. Moreover, no significant differences in in vitro and in vivo atenolol release behavior among the N,O-dimethyl, N-methyl, or N-benzyl hydroxylamine derivatives of sodium alginate were observed.
1.2.4. Effect of Ferric Ion Crosslinker on Alginates
Microspheres of acrylamide- (AAm-) grafted poly(vinyl alcohol) (PVA)/sodium alginate (NaAlg) were prepared by crosslinking with FeCl3 and 5-fluorouracil (5-FU) [46]. Microspheres were characterized by particle diameter, equilibrium swelling values and morphology, elemental analysis, and release profiles. This group studied the effects of PVA-g-PAAm/NaAlg ratio, drug/polymer ratio, crosslinker concentration, and exposure time to FeCl3 on the release of 5-FU. The highest 5-FU release was found to be as 99.57% after 6 h for PVA-g-PAAm/NaAlg and release kinetics was described by Fickian and non-Fickian approaches.
1.3. Purposes of Encapsulation of Drugs Using Alginates
Alginate can also undergo complexation with natural polymers, like chitosan, to enhance the absorption and cargo protection in oral delivery, for example, for the administration of insulin [47, 48]. Alginate was also combined with pectin polymer which has a similar mechanism. This research also showed successfully encapsulated drugs [49–52]. Alginate-based drugs encapsulated into nanoparticles/microparticles with various purposes are presented in Table 1.
Table 1.
Drugs or substances encapsulating in alginate nanoparticles/microparticles.
| Drug/protein/substances | Polymer | Aims of encapsulation | References |
|---|---|---|---|
| Nanoparticles | |||
| Indomethacin | Alginate-mesoporous silica | Sustained drug-delivery system for poorly water-soluble drug | [53, 54] |
| Bacteriophages | Alginate-nanohydroxyapatite | Delivery system to prevent orthopedic implant-associated infections | [55] |
| Bacteriophage | Alginate-CaCO3 | Encapsulation of bacteriophages | [56] |
| VEGF | Alginate | Injectable hydrogels for implant | [57] |
| Prednisolone and inulin | Alginate-chitosan | Nanoparticles for colon delivery | [58] |
| Amphotericin B | Sodium alginate glycol chitosan stearate | Nanoparticles for better chemotherapy in visceral leishmaniasis | [59] |
| R6G | Sodium alginate and hydroxyapatite (HAP) | The HAP@Alg nanoparticles show significant potential for the intracellular controlled release of cell-membrane-impermeable drugs | [60] |
| Dasatinib and zein-lactoferrin | Sodium alginate | Nano-in-micro drug-delivery system for anticancer | [61] |
| Curcumin and resveratrol | Alginate | Evaluation against DU145 prostate cancer cell line | [62] |
| Amygdalin | Alginate-chitosan | Biocompatible drug-delivery carriers for anticancer | [63] |
| 5-Fluorouracil | Alginate | Treatment for colon cancer liver metastasis | [64, 65] |
| Doxorubicin hydrochloride | Alginate/CaCO3/DNA | Mediate gene transfection and deliver drug to the cells for cancer treatments | [66] |
| Tilmicosin | Sodium alginate and carboxymethyl chitosan (CMCS) | The novel TIL-nanogel for treatment of Staphylococcus aureus (S. aureus) cow mastitis | [67] |
|
| |||
| Microparticles | |||
| Bismuth sulfide | Alginate | Microfluidic alginate microspheres and photothermal effect | [41] |
| Polystyrene | Sodium alginate | Microspheres of 400 µm to 900 µm produced pH-responsive smart drug-delivery systems | [68] |
| Gold NPs | Sodium alginate | Alginate hydrogels of higher than 10 nm released PEG-AuNPs for diagnostic and therapeutic purposes | [69] |
| D-Mannitol | Sodium alginate, sodium cellulose sulfate (SCS), and poly(methylene-co-cyanoguanidine) hydrochloride (PMCG) | Alginate microbeads of 600 to 800 μm stabilized by two coexisting networks for the treatment of diabetes or others | [70] |
| Sorbitan ester-based organogels | Alginate | Organogels in alginate microparticles | [71] |
| Corticosteroids | Alginate | Microparticles for colon delivery | [72] |
| Vancomycin | Chitosan-alginate polyelectrolyte | Vancomycin-chitosan-alginate polyelectrolyte microparticles as the controlled drug-delivery system | [73] |
|
| |||
| Other substances | |||
| Allogeneic pancreatic islet | Alginate | Long-term immune protection of allogeneic pancreatic islet cells | [74] |
| Lactoferrin | Alginate | Target Clostridioides difficile infection | [75] |
| Probiotic bacteria | Alginate and silica | Freeze-dried microparticles | [76] |
| Micronutrient | Alginate and chitosan | Functionalization for micronutrient | [77] |
| E. coli Nissle (EcN) | Sodium alginate and chitosan | Alginate-chitosan microcapsule enhanced the survival of EcN | [78] |
| Cefdinir | Alginate | Floating system and Box–Behnken design | [79] |
| MICP bacterial spores | Alginate | Self-healing concrete | [80] |
| SiRNA | Alginate | Vaginal delivery using the scaffold system | [81, 82] |
| Bacillus subtilis | Alginate-chitosan | Alginate microcapsule for uranium ion absorption | [83] |
| Hyaluronate | Alginate | Regenerating cartilage | [84] |
1.4. Use of Alginates in the Pharmaceutical Industry
Many application areas of sodium alginate-based drug-delivery systems, and these systems can be formulated as gels, matrices, membranes, nanospheres, microspheres, and others [2, 81]. Researchers are exploring possible applications of alginates as a coating material and preparation of controlled release drug-delivery systems.
1.4.1. Alginate for Protein Delivery and Cell Encapsulation
Alginate microparticles as a carrier for protein delivery prepared by spray-drying processes have been studied for their application in nasal and pulmonary drug delivery [85–87] prepared inhalable alginate particles (of an average diameter 3.23 ± 0.25 μm) with a high encapsulation efficiency of 97% with the preserved structure and bioactivity of BSA. The alginate particles released approximately 20% of the loaded BSA over 24 h and then a slow release occurred, reaching a cumulative release of only 35% after 180 h. Möbus et al. [88] prepared Zn2+-crosslinked alginate microparticles containing the model protein BSA via a simple one-step spray-drying process to produce microparticles of 2–4 µm size. They found BSA release into the simulated lung fluid increased with an increasing content of protein in the alginate microparticles. Alginate hydrogels have also been studied for oral delivery of proteins [89, 90]. Hariyadi et al. [91] prepared alginate microspheres containing lysozyme and insulin resulting in 30 to 60 μm in size with high protein loadings. Moreover, it was found to retain 75% activity using the ARCHITECT® assay and exhibit at least 80% bioactivity using the Micrococcus lysodeikticus assay. Another study using BSA demonstrated that the BSA release from the hydrated microparticles reached less than 7% in the simulated gastric fluid over 2 h, whereas 90% of the protein load was gradually released in the simulated intestinal fluid over 10 h. Another cell viability study was also conducted by Morachis et al. [92]; Severino et al. [93]; Joddar et al. [94]; Ciriza et al. [95]; Yoncheva et al. [96]; and Gurruchaga [18]. Applications of alginates for protein delivery and cell encapsulation are presented in Tables 2 and 3.
Table 2.
Alginate nano/microparticles with protein content.
| Protein types | Polymer | Method for encapsulation | Significant findings | References |
|---|---|---|---|---|
| Salmonella effector enzyme (AvrA) | Alginate-chitosan | Microfluidics | Capable of releasing AvrA NPs in the small intestine and colon | [97] |
| Silk fibroin | Alginate and PLGA | Layer-by-layer deposition | Silk coatings provide stable-encapsulated protein | [98] |
| Bovine serum albumin | Alginate-poloxamer | Spray drying | Spherical in shape with a size range of 4–6 μm and faster protein release | [99] |
| Bovine serum albumin | Alginate | Microemulsions-based reactors | Microemulsions of 6 nm stabilized the protein | [100] |
| Dextran-HEMA | Alginate | Partial oxidation | Good gelling ability | [101] |
Table 3.
Cell studies using alginate nano/microparticles.
| Cell types | Polymer | Parameter study | Significant findings | References |
|---|---|---|---|---|
| Tumor therapeutic cells | Alginate | Encapsulation of cytotoxic compounds encapsulated into liposomes, micelles, and nanoparticles | Long-time release of nanoparticles in the brain parenchyma | [16] |
| Epithelial cells | Alginate | Physicochemical characteristics and biological properties of the airways | Solubility, lipophilicity, and therapeutic efficacy of microparticles Shape, size, and density have an impact on the microparticles |
[19] |
| Cell-dispersed collagen | Alginate | Microfluidic-based by anisotropic gelation of the capillary | Magnetic-responsive nanoparticles or cell-dispersed collagen for tissue scaffold was functionalized microsprings | [21] |
| Pancreatic rat islets | Alginates | Cell encapsulation by zwitterionic group | Alginates improved outcome of islet encapsulation in a chemically induced diabetic mouse model | [22] |
| Riboflavin | Sodium alginate and furfurylamine | Coupling and photo-crosslinked method | Photo-crosslinked F-alginate resulted in slow release and potential for cell growth enhancement for medical application, biomaterials, soft and hard tissue applications, and tissue interfaces | [102] |
1.4.2. Alginate Particles with Ovalbumin (OVA)
(1) Peptide as a Carrier and Adjuvant. Ovalbumin (OVA) peptide 323–339 encapsulated in alginate has been reported to be involved in immune response as carrier and adjuvant for the immune therapy of cancer [53]. A tumor model was established in C57BL/6J mice via subcutaneous injection of 3 × 105 B16-OVA tumor cells. Alginate/OVA peptide inhibited tumor progression more effectively than using the peptide alone. The viability and uptake study illustrated that this particle is safe and nontoxic. Furthermore, alginate particles can promote the activation of surface markers on macrophages. ELISA assay showed that the particles with peptide can promote the secretion of inflammatory and effector cytokines from macrophages.
1.4.3. Liposomal Alginate for Bupivacaine Delivery and MSC Function
Mesenchymal stromal cell (MSC) therapies have become potential treatment options for multiple ailments and traumatic injuries. Davis et al. [103] developed and characterized a sustained release delivery formulation comprised of alginate-encapsulated liposomal bupivacaine to evaluate the effect of this formulation on the secretion of three key MSC regulatory molecules, interleukin 6 (IL-6), prostaglandin E2 (PGE2), and transforming growth factor-beta 1 (TGF-β1). Bupivacaine release profile analyses indicated that the mode of drug delivery controlled the liposomal-alginate (LA) concentration over time and pathway analysis identified several shared and cytokine-specific molecular mediators for IL-6, PGE2, and TGF-β1. These studies support the potential utility of LA for anti-inflammatory cell therapy coadministration.
1.4.4. Curcumin-Alginate-Based Composite Sponges
Alginate-based composite sponges were developed as carriers to prolong the gastric retention time and controlled release of curcumin-loaded self-microemulsifying drug-delivery systems (Cur-SMEDDS) [104]. Researchers used adsorbent (colloidal silicon dioxide) and additional polymers such as sodium carboxymethyl cellulose (SCMC) and hydroxypropyl methylcellulose (HPMC) to form composite sponges. The formulation exhibited a droplet size of approximately 30 nm and provided a sustained release.
2. Application of Alginates in Context of the Routes of Drug Administration
Alginates have been extensively investigated for delivering drugs via oral, parenteral, pulmonary, and transdermal routes (Table 4). Using alginate as a single polymer or the combined polymer, controlled or sustained release delivery of quercetin, isoniazid, rifampicin, ciprofloxacin, bovine insulin, and lentivectors has been investigated. All formulations showed increased entrapment efficiency of drugs, increased dissolution and bioavailability, and reduced degradation of drugs [105–107, 109–112, 130–132]. Some chemotherapeutic agents encapsulated in alginate polymer showed enhanced penetration in the target cells. Antigen-encapsulated alginate showed enhanced immune response [8, 115, 116, 133, 134]. Alginates have been also widely investigated for pulmonary drug delivery [99, 117, 119–128]. Alipour et al. developed paclitaxel-alginate microparticles which increased the site-specific efficacy of drugs with reduced toxicity [117]. Using alginate and PLGA polymers, Abdelaziz et al. studied inhalable particulate delivery of cisplatin and doxorubicin for lung cancer therapy [120]. The alginate-based BSA and BCG vaccines have been used to study the efficacy of smaller inhalable vaccines, which provided better protection and more immunogenic effect [99, 124, 125]. Applications of alginate in transdermal delivery for wound dressing or wound healing were shown to be effective to produce a high porosity and sustained release and able to inhibit preinfection [126–128, 135].
Table 4.
Route of administration of drug delivery.
| Drugs | Polymer | Route | Formulation/design approach | References |
|---|---|---|---|---|
| Quercetin | Na alginate and chitosan | Oral | Ionic crosslinking method for oral controlled release | [105] |
| Isoniazid and rifampicin | Sodium alginate | Oral | Drop technique for oral sustained delivery carriers | [106, 107] |
| 4-(2-Aminoethyl) benzoic acid | Sodium alginate | Oral | Chemically modified (amidation and reductive amination) | [108] |
| Ciprofloxacin | Alginate-gelatin | Oral | Crosslinked method | [109, 110] |
| Bovine insulin | Sodium alginate | Oral | Ionotropic gelation using calcium chloride dihydrate | [111] |
| Lentivectors | Alginate | Oral | Polymers were ionically crosslinked to create bimodal hydrogel | [112] |
| Resveratrol | Alginate | Oral | Ionic and shelled with soy protein isolate (SPI) | [5] |
| Metformin | Alginate | Oral | DDS for oral antidiabetic | [113] |
| Metronidazole | Alginate | Oral | Matrix for oral DDS | [114] |
| Recombinant hepatitis B surface antigen (rHBsAg) | Alginate | Parenteral | Antigen delivery system for intramuscular administration by mild ionic crosslinking technique | [8] |
| Furosemide | Alginate-chitosan | Parenteral | Mucopenetrating nanoparticles for enhancement of oral bioavailability | [115] |
| Exemestane | Sodium alginate | Parenteral | Simple controlled gelation method for oral chemotherapeutic drug | [116] |
| Paclitaxel | Alginate | Pulmonary | Emulsification technique | [117] |
| Isoniazid rifampicin, pyrazinamide, and paclitaxel | Chitosan, alginate, PLGA, and polysaccharides | Pulmonary | Emulsification and complexation | [118] |
| Amikacin, ciprofloxacin, and polymyxin | PLGA and alginate | Pulmonary | Spray drying | [119] |
| Cisplatin and doxorubicin | Alginate, HAS, chitosan, and PLGA | Pulmonary | Emulsification/gelation and spray drying | [120] |
| Ciprofloxacin | Polyethylene glycol, phthaloyl chitosan, and sodium alginate | Pulmonary | Grafted and spray drying | [121] |
| BCG vaccine | Alginate | Pulmonary | Emulsification | [122] |
| Tobramycin | Alginate and chitosan | Pulmonary | Precipitation | [123] |
| BCG vaccine | Alginate | Pulmonary | Aerosol liquid encapsulation | [124] |
| BSA | Alginate | Pulmonary | Spray drying | [99] |
| BSA | Alginate, chitosan, and trimethyl chitosan | Pulmonary | Liposomal formulation | [125] |
| Ciprofloxacin | Calcium alginate | Transdermal | Lyophilized hydrogels for wound dressing | [126] |
| Resveratrol | Chitosan, alginate, and poly(d,l-lactide-co-glycolide) | Transdermal | Nanoprecipitation | [127] |
| Metronidazole | Alginate | Transdermal | Ionotropic gelation combination with freeze-thawing cycle | [128, 129] |
2.1. Alginate-Based Hybrid Aerogel Microparticles for Mucosal Drug Delivery
Some polysaccharides (e.g., alginate, chitosan, and pectin) have been applied as biopolymer aerogels to have mucoadhesive properties for mucosal drug delivery [136] Alginate-based hybrid aerogels of microparticles (<50 μm) were produced. Low methoxyl pectin and κ-carrageenan were also cogelled with alginate and further dried with supercritical CO2 (sc-CO2). Spherical mesoporous aerogel microparticles were obtained for alginate, hybrid alginate/pectin, and alginate/κ-carrageenan aerogels, presenting high specific surface area and mucoadhesive properties. The microparticles were loaded with ketoprofen and quercetin. Release of both drugs from alginate/κ-carrageenan aerogel was slightly faster compared to alginate/pectin indicating that alginate-based aerogel microparticles are potential for mucosal drug-delivery applications.
2.2. Alginates for Ocular Drug Delivery
To develop potential ocular drug delivery, mucoadhesive microspheres is one of the best approaches to prolong the drug residence inside the cul-de-sac, consequently increasing the bioavailability. Thus, some researchers worked to overcome the limitations of ocular drug delivery [137–139]. The chitosan-sodium alginate microspheres or other polymers encapsulating of ocular drugs have been investigated widely. Sodium alginate microspheres prepared were in particle size range suitable for ocular purpose and were able to improve the therapeutic efficacy.
2.3. Alginates for Stem Cell Purposes
Alginates as polymer have been used for stem cell studies. For example, Leslie et al. studied the controlled release of rat adipose-derived stem cells from alginate microbead [140]. Maia et al. formed hydrogel depots for local codelivery of osteoinductive peptides and mesenchymal stem cells [141]. Another study used cartilage cells in a combination of alginate and hyaluronate hydrogels for cartilage regeneration [37, 84, 142, 143]. Ulker and Erkey studied spermatogonial stem cells and evaluated alginate hydrogel cytotoxicity on three-dimensional culture [144].
3. Various Techniques to Produce Alginate Micro/Nanoparticles for Drug Delivery
Over the years, various methods have been developed to fabricate drug-delivery particles of bioactive substances. Using superhydrophobic surfaces, it is possible to produce polymer particles suitable as DDSs. This method allowed loading drugs into spherical structures with an encapsulation efficiency close to 100% [145, 146]. Goncalves et al. [136] developed alginate microparticles which were shown to have perfluorocarbon breakthrough capacity when subjected to vibration by ultrasound waves. Results showed a disruption of these microparticles after 15 min of exposure, suggesting that such structures are promising DDSs controlled externally by acoustic stimuli.
Another strategy to synthesize particles relies on complexation, based on the electrostatic interactions between alginate at neutral and alkaline pH values, bioactive agents, and other kinds of naturally occurring polymers, such as the polycation chitosan [147–149].
3.1. Preparation Techniques for Production of Alginate Nanoparticles
3.1.1. Oligopeptide-Side Chained Alginate via the Amidation Method
A melittin-targeting drug carrier was successfully synthesized by the grafting of sodium alginate to an oligopeptide via an amidation method at different oligopeptide: alginate unit molar ratios [150]. The average sizes of the oligopeptide-alginate nanoparticles formed decreased with increasing oligopeptide contents, indicating intramolecular interactions between oligopeptide-side chains. The results confirm that the derivation of an oligopeptide-side chain in alginate offers a specific binding site for melittin and effectively works in cancer chemotherapy.
3.1.2. Chitosan/Alginate Nanoparticles by Emulsification and Ionotropic Gelification
Curcumin-diglutaric acid (CG) is a prodrug of curcumin encapsulated into chitosan/alginate polysaccharide-based nanoparticles [151]. CG-loaded chitosan/alginate nanoparticles were prepared by o/w emulsification and ionotropic gelification, with the conditions optimized using response surface methodology. The CG-loaded chitosan/alginate nanoparticles showed better stability compared to a CG dispersion in water. The nanoparticles showed slow cumulative release and the release pattern was mainly controlled by Fickian diffusion and erosion of polymer materials. CG-loaded chitosan/alginate nanoparticles showed higher in vitro cellular uptake in human epithelial colorectal adenocarcinoma (Caco-2 cells) and better anticancer activity against Caco-2, human hepatocellular carcinoma (HepG2), and human breast cancer (MDA-MB-231) cells.
3.1.3. Alginate/Chitosan Nanoparticles for Controlled Release of Vitamin B2
Work by Azevedo et al. [152] encapsulating vitamin B2 with alginate/chitosan nanoparticles using ionotropic polyelectrolyte pregelation was conducted. Alginate/chitosan nanoparticles were 104.0 ± 67.2 nm, PDI of 0.319 ± 0.068, encapsulation efficiency, and loading capacity values of 55.9 ± 5.6% and 2.2 ± 0.6%, respectively. Sizes and PDI during 5 months showed that vitamin B2-loaded nanoparticles were stable.
3.1.4. Nutraceutical Nanodelivery System
Alginate nano/microspheres were produced by emulsification/internal gelation of sodium alginate within vegetable oils containing surfactant, followed by CaCl2 addition resulting in hardened particles [153]. Size of nanoparticles decreased at higher oil and surfactant contents, higher molarity of CaCl2, and lower alginate concentrations. Moreover, encapsulation efficiency was inversely proportional to the size of nanoparticles.
3.1.5. Alginate/Chitosan Formulations for Ciprofloxacin-Controlled Delivery
Kyziol et al. loaded ciprofloxacin in alginate beads with an emulsification technique in combination with an internal gelation method [154]. Hydrodynamic diameter and zeta potential showed of 160 nm and −32 mV in the case of AL_CP and ca. 240 nm and ca. +14 mV in the case of AL_CP_CS, respectively. They found that alginate beads with encapsulated ciprofloxacin covered with chitosan were effective oral delivery system since limited ciprofloxacin was release in gastric.
Various techniques which have been used to produce alginate nanoparticles are presented in Table 5.
Table 5.
Various techniques used to produce alginate nanoparticles.
| Drugs | Polymer | Method | Size | Main findings | References |
|---|---|---|---|---|---|
| Recombinant hepatitis B surface antigen (rHBsAg) | Sodium alginate | Ionic crosslinking | 80–400 nm | Size and surface charge could be modulated by adjusting the ratio of polymer | [155] |
| Curcumin | Alginate, chitosan, and pluronic | Ionic gelation | 100 ± 20 nm | Composite nanoparticles (NPs) were successfully prepared | [156] |
| Doxorubicin | Alginate and chitosan | Novel ionic gelation method | 100 nm | Chitosan-alginate nanoparticle produced higher zeta potential and encapsulation efficiency than chitosan nanoparticles | [157] |
| Hyaluronic acid | Chitosan and alginate | Ionic gelation | 100 nm | Cryoprotectants provided stability for the NPs | [158] |
| Tobramycin | Alginate and chitosan | Isothermal titration calorimetry | ±500 nm | High survival rates and low toxicity were observed | [159] |
| ZnO | Alginate | Pumped dropwise using a peristaltic pump and tubing | 120 to 236 nm | Inactivation of antibiotic-resistant bacteria by ZnO NP-alginate beads was improved by increasing the nanocomposite amount and contact time | [160] |
| Curcumin-loaded zein | Sodium caseinate (SC) and sodium alginate (SA) | Liquid-liquid dispersion and encapsulation | nm | A significantly improved encapsulation efficiency and controlled release was successfully produced | [161] |
| trans-Cinnamaldehyde | Chitosan-alginate | Ionic gelation and polyelectrolyte complexation technique | 166.26 nm | (i) Small size and high encapsulation efficiency was found | [162] |
| Imazapic and imazapyr herbicides | Alginate/chitosan and chitosan/tripolyphosphate nanoparticles | Ionic encapsulation | 400 nm | (ii) High efficiency and stable nanoparticles resulted during 30 days of storage at ambient temperature | [163] |
| Genipin | Silver nanoparticles (AgNPs)-loaded alginate in gelatin scaffolds | Electrospraying and freeze-drying | 154 and 171 μm | Swelling and weight loss behaviors of the AgNPs-loaded alginate beads embedded in gelatin scaffolds increased and nontoxic as wound dressings | [164, 165] |
| Vancomycin (VCM) and glyceryl tripalmitate | Oleic acid (OA), chitosan (CHT), and sodium alginate (ALG) | Hot high-pressure homogenization followed by ultrasonication | 202.5 ± 3.81 to 250.9 ± 9.04 | (i) Rod-shaped LPNs with suitable size, PDI, zeta potential, higher encapsulation efficiency, and potency as antibacterial activity | [87] |
| CM-chitin | Polypyrrole (PPY)/sodium alginate | Oxidative polymerization and templating | 117–217 ± 17 nm | (ii) Negative viscosity change of the dispersions resulting in a decrease in bulk alginate concentration | [166] |
3.2. Preparation Techniques for Production of Alginate Microparticles
Some techniques were used to produce alginate microparticles. Production is by conventional emulsification using sodium alginate single or combination polymer with chitosan to encapsulate a variety of drugs including glucose oxidase [167], paclitaxel [168], cocoa extract [169], and diclofenac sodium [170] or double emulsification techniques [171].
Another method is internal gelation technique, which by using sodium alginate polymer to entrap drug of doxorubicin was done by Giovagnoli et al. [35], diclofenac by Ahmed et al. [172], L-α-phosphatidylcholine by Semmling et al. [173], and sulfasalazine by Tavakol et al. [174]. Extrusion dripping method was also used to optimize sphericity of particles and shape deformation [175].
The more recent technique to produce microparticles was an impinging aerosol technique to successfully encapsulate propranolol HCl by Hariyadi et al. [89] and high-voltage electrostatic bead generator for BSA-alginate microparticles by Ørning et al. [176]. Mishra et al. [177] used gas blowing technique to contain verapamil HCl resulting in faster/burst drug release; however, importantly a strong mechanical strength and drug integrity were maintained in hydrogel polymeric network.
4. Mechanism of Drug Release from Alginate Nano/Microparticles
Some researchers focused on investigating release behavior of polymer in nanoparticles and microparticles by modified polymers which are used to form hydrogels or other ways such as producing smart polymers consisting of copolymerized agents as additional polymer, change the pH of the encapsulation process, temperature changes, and others [178–184]. James et al. designed smart polymers in order to achieve mechanism of release of swelling, contraction, and disintegration mechanism, although these additional agents must be programmable to show depot mechanism for sustained release, for example, the formation of complex from chitosan and glycerophosphate [179]. There are different mechanisms of release of a bioactive agent from the carrier, such as through variations of temperature and pH and the use of biodegradable materials or enzymatic degradation, among other chemical and physical stimuli-responsive methods [42, 185–189]. Hadijev et al. [180] studied hydrogels which mostly applied drug diffusion as a release mechanism; however, this can be changed with the properties to broadly change the solute diffusion coefficient as the gel system swells. According to Gao et al. [183], mechanism of release of hydrogels can be modified to have more steady release behavior by adding some copolymer which is able to interact and may change the chemical structure, morphology, and rheology characteristics, thus affecting release behavior and mechanism.
5. Toxicity and In Vivo Study
5.1. Toxicity
Alginate nanoparticles and microparticles were considered safe, although some studies about safety and toxicity were widely conducted. For example, Spadari et al. [120] investigated alginate nanoparticles as a nontoxic delivery system for miltefosine (MFS) in the treatment of candidiasis and cryptococcosis. Alginate nanoparticles were produced using the external emulsification/gelation method and toxicity on red blood cells and Galleria mellonella larvae were assessed. MFS in alginate nanoparticles presented no hemolytic effect and no toxicity in G. mellonella larvae. These results showed the potential and nontoxic use of alginate-based drug-delivery systems as carriers to control the fungal infection in the in vivo model of G. mellonella.
5.2. In Vivo Study for Alginate Nano/Microparticles
In vivo study is usually not directly related to the in vitro achievement. Here are some potential in vivo studies for alginate nanoparticles and microparticles. Wang et al. demonstrated that BaSO4/alginate microspheres possessed excellent visibility under X-ray and histopathology analysis for transcatheter arterial embolization (TAE) therapy. In vivo study verified that the embolic efficacy of microspheres was similar to that of commercially available alginate microsphere embolic agents [14]. For colon study, Patole and Pandit entrapped mesalamine in variety of polymers including alginate, HPMC, and Eudragit FS-30D and found histopathologically no signs of ulceration or bleeding of the released microspheres [190]. Other in vivo studies including anti-inflammatory, mucoadhesion test, and histopathological were conducted by researchers [191–195].
For vaccine delivery, research using chitosan, trimethyl chitosan (TMC), and alginate was conducted by Mosafer et al. using inactivated PR8 influenza virus for mucosal vaccine delivery. PR8-chitosan formulation elicited higher IgG2a and IgG1 antibody titers compared with PR8-TMC. Alginate coating significantly decreased the antibody titers and less immune response was induced [121].
In vivo study for the transdermal application was done by Hariyadi et al. [196]. They showed the effectiveness of glutathione-alginate microspheres in decreasing matrix metalloproteinase-1 (MMP-1) expression in the dermis tissue of mice.
Natural products have been investigated by researchers in vivo. Alginate polymer-encapsulated black seed oil for intestine-targeted drug delivery has been studied by Azad et al. (2020) in the forms of gastrointestinal distribution study [197]. They found uniform distribution of beads after oral administration in rats.
Beside in vivo investigation, Thai et al. indicated low toxicity of lovastatin-alginate and chitosan nanoparticles in mice in the acute toxicity test [198].
6. Conclusions
This paper provides a comprehensive review of the current status of alginate and its progress in drug and protein delivery. Alginate as a potential carrier has been investigated for the delivery of a variety of low and high molecular weight drugs. Applications of alginate polymer in pharmaceutical and biomedical research have a promising future. The most important properties of alginate include safety, biocompatibility, and simple methods of preparations. This review highlights the recent advances in the alginate polymers in pharmaceutical and biomedical fields. Because of its biocompatibility, biodegradability, and nontoxicity, it is applied to various drug-delivery technologies. Thus, researchers need to update the advances in the alginate-based drug-delivery systems and this review is a source of guidance for future research.
Acknowledgments
The authors acknowledge the financial support received from Universitas Airlangga and their support in carrying out this research review.
Disclosure
The authors alone are responsible for the content and writing of this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Lee K. Y., Mooney D. J. Alginate: properties and biomedical applications. Progress in Polymer Science. 2012;37(1):106–126. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jain D., Bar-Shalom D. Alginate drug delivery systems: application in context of pharmaceutical and biomedical research. Drug Development and Industrial Pharmacy. 2014;40(12):1576–1584. doi: 10.3109/03639045.2014.917657. [DOI] [PubMed] [Google Scholar]
- 3.Ching S. H., Bansal N., Bhandari B. Alginate gel particles-a review of production techniques and physical properties. Critical Reviews in Food Science and Nutrition. 2017;57(6):1133–1152. doi: 10.1080/10408398.2014.965773. [DOI] [PubMed] [Google Scholar]
- 4.Sosnik A. Alginate particles as platform for drug delivery by the oral route: state-of-the-art. ISRN Pharmaceutics. 2014;2014:17. doi: 10.1155/2014/926157.926157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang A., Jung K., Li A., Liu J., Boyer C. Recent advances in stimuli-responsive polymer systems for remotely controlled drug release. Progress in Polymer Science. 2019;99 doi: 10.1016/j.progpolymsci.2019.101164.101164 [DOI] [Google Scholar]
- 6.Jana S., Kumar Sen K., Gandhi A. Alginate based nanocarriers for drug delivery applications. Current Pharmaceutical Design. 2016;22(22):3399–3410. doi: 10.2174/1381612822666160510125718. [DOI] [PubMed] [Google Scholar]
- 7.Szekalska M., Puciłowska A., Szymańska E., Ciosek P., Winnicka K. Alginate: current use and future perspectives in pharmaceutical and biomedical applications. International Journal of Polymer Science. 2016;2016:17. doi: 10.1155/2016/7697031.7697031 [DOI] [Google Scholar]
- 8.Cardoso M., Costa R., Mano J. Marine origin polysaccharides in drug delivery systems. Marine Drugs. 2016;14(2):p. 34. doi: 10.3390/md14020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hariyadi D. M., Lin S. C.-Y., Wang Y., et al. Diffusion loading and drug delivery characteristics of alginate gel microparticles produced by a novel impinging aerosols method. Journal of Drug Targeting. 2010;18(10):831–841. doi: 10.3109/1061186x.2010.525651. [DOI] [PubMed] [Google Scholar]
- 10.Mirtič J., Ilaš J., Kristl J. Influence of different classes of crosslinkers on alginate polyelectrolyte nanoparticle formation, thermodynamics and characteristics. Carbohydrate Polymers. 2018;181:93–102. doi: 10.1016/j.carbpol.2017.10.040. [DOI] [PubMed] [Google Scholar]
- 11.Agulhon P., Robitzer M., David L., Quignard F. Structural regime identification in ionotropic alginate gels: influence of the cation nature and alginate structure. Biomacromolecules. 2012;13(1):215–220. doi: 10.1021/bm201477g. [DOI] [PubMed] [Google Scholar]
- 12.Guo X., Huang S., Sun J., Wang F. Comparison of the cytotoxicities and wound healing effects of hyaluronan, carbomer, and alginate on skin cells in vitro. Advances in Skin & Wound Care. 2015;28(9):410–414. doi: 10.1097/01.asw.0000467303.39079.59. [DOI] [PubMed] [Google Scholar]
- 13.Aramwit P., Yamdech R., Ampawong S. Controlled release of chitosan and sericin from the microspheres-embedded wound dressing for the prolonged anti-microbial and wound healing efficacy. The AAPS Journal. 2016;18(3):647–658. doi: 10.1208/s12248-016-9897-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang T., Zheng Y., Shi Y., Zhao L. pH-Responsive calcium alginate hydrogel laden with protamine nanoparticles and hyaluronan oligosaccharide promotes diabetic wound healing by enhancing angiogenesis and antibacterial activity. Drug Delivery and Translational Research. 2019;9(1):227–239. doi: 10.1007/s13346-018-00609-8. [DOI] [PubMed] [Google Scholar]
- 15.Liakos I., Rizzello L., Bayer I. S., Pompa P. P., Cingolani R., Athanassiou A. Controlled antiseptic release by alginate polymer films and beads. Carbohydrate Polymers. 2013;92(1):176–183. doi: 10.1016/j.carbpol.2012.09.034. [DOI] [PubMed] [Google Scholar]
- 16.Bhujbal S. V., de Vos P., Niclou S. P. Drug and cell encapsulation: alternative delivery options for the treatment of malignant brain tumors. Advanced Drug Delivery Reviews. 2014;67-68:142–153. doi: 10.1016/j.addr.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 17.Rokstad A. M. A., Lacík I., de Vos P., Strand B. L. Advances in biocompatibility and physico-chemical characterization of microspheres for cell encapsulation. Advanced Drug Delivery Reviews. 2014;67-68:111–130. doi: 10.1016/j.addr.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 18.Gurruchaga H., Saenz del Burgo L., Ciriza J., Orive G., Hernández R. M., Pedraz J. L. Advances in cell encapsulation technology and its application in drug delivery. Expert Opinion on Drug Delivery. 2015;12(8):1251–1267. doi: 10.1517/17425247.2015.1001362. [DOI] [PubMed] [Google Scholar]
- 19.Haghi M., Ong H. X., Traini D., Young P. Across the pulmonary epithelial barrier: integration of physicochemical properties and human cell models to study pulmonary drug formulations. Pharmacology & Therapeutics. 2014;144(3):235–252. doi: 10.1016/j.pharmthera.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Li P., Luo Z., Liu P., et al. Bioreducible alginate-poly(ethylenimine) nanogels as an antigen-delivery system robustly enhance vaccine-elicited humoral and cellular immune responses. Journal of Controlled Release. 2013;168(3):271–279. doi: 10.1016/j.jconrel.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida K., Onoe H. Functionalized core-shell hydrogel microsprings by anisotropic gelation with bevel-tip capillary. Scientific Reports. 2017;7(1):1–9. doi: 10.1038/srep45987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Q., Chiu A., Wang L.-H., et al. Zwitterionically modified alginates mitigate cellular overgrowth for cell encapsulation. Nature Communications. 2019a;10(1):1–14. doi: 10.1038/s41467-019-13238-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Vos P., Lazarjani H. A., Poncelet D., Faas M. M. Polymers in cell encapsulation from an enveloped cell perspective. Advanced Drug Delivery Reviews. 2014;67-68:15–34. doi: 10.1016/j.addr.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Malpique R., Osório L. M., Ferreira D. S., et al. Alginate encapsulation as a novel strategy for the cryopreservation of neurospheres. Tissue Engineering Part C: Methods. 2010;16(5):965–977. doi: 10.1089/ten.tec.2009.0660. [DOI] [PubMed] [Google Scholar]
- 25.Christ G. J., Saul J. M., Furth M. E., Andersson K.-E. The pharmacology of regenerative medicine. Pharmacological Reviews. 2013;65(3):1091–1133. doi: 10.1124/pr.112.007393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poels J., Abou-Ghannam G., Decamps A., Leyman M., Rieux A. d., Wyns C. Transplantation of testicular tissue in alginate hydrogel loaded with VEGF nanoparticles improves spermatogonial recovery. Journal of Controlled Release. 2016;234:79–89. doi: 10.1016/j.jconrel.2016.05.037. [DOI] [PubMed] [Google Scholar]
- 27.Richardson T., Kumta P. N., Banerjee I. Alginate encapsulation of human embryonic stem cells to enhance directed differentiation to pancreatic islet-like cells. Tissue Engineering Part A. 2014;20(23-24):3198–3211. doi: 10.1089/ten.tea.2013.0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen X., Fan M., Tan H., et al. Magnetic and self-healing chitosan-alginate hydrogel encapsulated gelatin microspheres via covalent cross-linking for drug delivery. Materials Science and Engineering: C. 2019;101:619–629. doi: 10.1016/j.msec.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 29.Jalayeri M., Pirnia A., Najafabad E. P., Varzi A. M., Gholami M. Evaluation of alginate hydrogel cytotoxicity on three-dimensional culture of type a spermatogonial stem cells. International Journal of Biological Macromolecules. 2017;95:888–894. doi: 10.1016/j.ijbiomac.2016.10.074. [DOI] [PubMed] [Google Scholar]
- 30.Mao A. S., Shin J.-W., Utech S., et al. Encapsulation of single cells in thin tunable microgels for niche modelling and therapeutic delivery. Nature Materials. 2017;16(2):236–243. doi: 10.1038/nmat4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poojari R., Srivastava R. Composite alginate microspheres as the next-generation egg-box carriers for biomacromolecules delivery. Expert Opinion on Drug Delivery. 2013;10(8):1061–1076. doi: 10.1517/17425247.2013.796361. [DOI] [PubMed] [Google Scholar]
- 32.Choi Y. H., Han H.-K. Nanomedicines: current status and future perspectives in aspect of drug delivery and pharmacokinetics. Journal of Pharmaceutical Investigation. 2018;48(1):43–60. doi: 10.1007/s40005-017-0370-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang K.-S., Yang C.-H., Lin Y.-S., et al. Electrostatic droplets assisted synthesis of alginate microcapsules. Drug Delivery and Translational Research. 2011;1(4):289–298. doi: 10.1007/s13346-011-0020-8. [DOI] [PubMed] [Google Scholar]
- 34.Giovagnoli S., Blasi P., Luca G., et al. Bioactive long-term release from biodegradable microspheres preserves implanted ALG-PLO-ALG microcapsules from in vivo response to purified alginate. Pharmaceutical Research. 2009;27(2):285–295. doi: 10.1007/s11095-009-0017-x. [DOI] [PubMed] [Google Scholar]
- 35.Giovagnoli S., Tsai T., DeLuca P. P. Formulation and release behavior of doxycycline-alginate hydrogel microparticles embedded into pluronic F127 thermogels as a potential new vehicle for doxycycline intradermal sustained delivery. AAPS PharmSciTech. 2010;11(1):212–220. doi: 10.1208/s12249-009-9361-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holkem A. T., Raddatz G. C., Nunes G. L., et al. Development and characterization of alginate microcapsules containing bifidobacterium BB-12 produced by emulsification/internal gelation followed by freeze drying. LWT-Food Science and Technology. 2016;71:302–308. doi: 10.1016/j.lwt.2016.04.012. [DOI] [Google Scholar]
- 37.Cañibano-Hernández A., Saenz del Burgo L., Espona-Noguera A., et al. Alginate microcapsules incorporating hyaluronic acid recreate closer in vivo environment for mesenchymal stem cells. Molecular Pharmaceutics. 2017;14(7):2390–2399. doi: 10.1021/acs.molpharmaceut.7b00295. [DOI] [PubMed] [Google Scholar]
- 38.Finotelli P. V., Da Silva D., Sola-Penna M., et al. Microcapsules of alginate/chitosan containing magnetic nanoparticles for controlled release of insulin. Colloids and Surfaces B: Biointerfaces. 2010;81(1):206–211. doi: 10.1016/j.colsurfb.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 39.Choi D. H., Park C. H., Kim I. H., Chun H. J., Park K., Han D. K. Fabrication of core-shell microcapsules using PLGA and alginate for dual growth factor delivery system. Journal of Controlled Release. 2010;147(2):193–201. doi: 10.1016/j.jconrel.2010.07.103. [DOI] [PubMed] [Google Scholar]
- 40.Ma G. Microencapsulation of protein drugs for drug delivery: strategy, preparation, and applications. Journal of Controlled Release. 2014;193:324–340. doi: 10.1016/j.jconrel.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 41.Zou L., Zhang Z., Zhang R., et al. Encapsulation of protein nanoparticles within alginate microparticles: impact of pH and ionic strength on functional performance. Journal of Food Engineering. 2016;178:81–89. doi: 10.1016/j.jfoodeng.2016.01.010. [DOI] [Google Scholar]
- 42.Jin C., Jin C., Siyu X., Xueyong Q., Song S., Yanru G. Alginate/chitosan microcapsules for in-situ delivery of the protein, interleukin-1 receptor antagonist (IL-1Ra), for the treatment of dextran sulfate sodium (DSS)-induced colitis in a mouse model. European Journal of Pharmaceutics and Biopharmaceutics. 2019;137:112–121. doi: 10.1016/j.ejpb.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 43.Yu L., Sun Q., Hui Y., Seth A., Petrovsky N., Zhao C.-X. Microfluidic formation of core-shell alginate microparticles for protein encapsulation and controlled release. Journal of Colloid and Interface Science. 2019;539:497–503. doi: 10.1016/j.jcis.2018.12.075. [DOI] [PubMed] [Google Scholar]
- 44.Deepika R., Girigoswami K., Murugesan R., Girigoswami A. Influence of divalent cation on morphology and drug delivery efficiency of mixed polymer nanoparticles. Current Drug Delivery. 2018;15(5):652–657. doi: 10.2174/1567201814666170825160617. [DOI] [PubMed] [Google Scholar]
- 45.Kotagale N., Raut N., Umekar M., Deshmukh P. Zinc cross-linked hydroxamated alginates for pulsed drug release. International Journal of Pharmaceutical Investigation. 2013;3(4):p. 194. doi: 10.4103/2230-973x.121292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Şanli O., Olukman M. Preparation of ferric ion crosslinked acrylamide grafted poly (vinyl alcohol)/sodium alginate microspheres and application in controlled release of anticancer drug 5-fluorouracil. Drug Delivery. 2014;21(3):213–220. doi: 10.3109/10717544.2013.844743. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y., Wei W., Lv P., Wang L., Ma G. Preparation and evaluation of alginate-chitosan microspheres for oral delivery of insulin. European Journal of Pharmaceutics and Biopharmaceutics. 2011;77(1):11–19. doi: 10.1016/j.ejpb.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 48.Bhattacharyya A., Mukherjee D., Mishra R., Kundu P. P. Preparation of polyurethane-alginate/chitosan core shell nanoparticles for the purpose of oral insulin delivery. European Polymer Journal. 2017;92:294–313. doi: 10.1016/j.eurpolymj.2017.05.015. [DOI] [Google Scholar]
- 49.Chen K., Zhang H. Alginate/pectin aerogel microspheres for controlled release of proanthocyanidins. International Journal of Biological Macromolecules. 2019;136:936–943. doi: 10.1016/j.ijbiomac.2019.06.138. [DOI] [PubMed] [Google Scholar]
- 50.Del Gaudio P., Russo P., Rosaria Lauro M., Colombo P., Aquino R. P. Encapsulation of ketoprofen and ketoprofen lysinate by prilling for controlled drug release. AAPS PharmSciTech. 2009;10(4):1178–1185. doi: 10.1208/s12249-009-9309-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Auriemma G., Cerciello A., Aquino R. P., Del Gaudio P., Fusco B. M., Russo P. Pectin and zinc alginate: the right inner/outer polymer combination for core-shell drug delivery systems. Pharmaceutics. 2020;12(2):p. 87. doi: 10.3390/pharmaceutics12020087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palombo M., Deshmukh M., Myers D., Gao J., Szekely Z., Sinko P. J. Pharmaceutical and toxicological properties of engineered nanomaterials for drug delivery. Annual Review of Pharmacology and Toxicology. 2014;54(1):581–598. doi: 10.1146/annurev-pharmtox-010611-134615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu L., Ge F., Yang L., et al. Alginate particles with ovalbumin (OVA) peptide can serve as a carrier and adjuvant for immune therapy in B16-OVA cancer model. Medical Science Monitor Basic Research. 2017;23:166–172. doi: 10.12659/msmbr.901576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu L., Sun C., Song A., et al. Alginate encapsulated mesoporous silica nanospheres as a sustained drug delivery system for the poorly water-soluble drug indomethacin. Asian Journal of Pharmaceutical Sciences. 2014;9(4):183–190. doi: 10.1016/j.ajps.2014.05.004. [DOI] [Google Scholar]
- 55.Barros J. A. R., Melo L. D. R. d., Silva R. A. R. d., et al. Encapsulated bacteriophages in alginate-nanohydroxyapatite hydrogel as a novel delivery system to prevent orthopedic implant-associated infections. Nanomedicine: Nanotechnology, Biology and Medicine. 2020;24:p. 102145. doi: 10.1016/j.nano.2019.102145. [DOI] [PubMed] [Google Scholar]
- 56.Colom J., Cano-Sarabia M., Otero J., et al. Microencapsulation with alginate/CaCO3: a strategy for improved phage therapy. Scientific Reports. 2017;7(1) doi: 10.1038/srep41441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scott R., Antoniadou E., Kong H. Enzymatically cross-linked injectable alginate-g-pyrrole hydrogels for neovascularization. Journal of Controlled Release. 2013;172(1):30–37. doi: 10.1016/j.jconrel.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 58.Gamboa A., Araujo V., Caro N., Gotteland M., Abugoch L., Tapia C. Spray freeze-drying as an alternative to the ionic gelation method to produce chitosan and alginate nano-particles targeted to the colon. Journal of Pharmaceutical Sciences. 2015;104(12):4373–4385. doi: 10.1002/jps.24617. [DOI] [PubMed] [Google Scholar]
- 59.Gupta P. K., Jaiswal A. K., Asthana S., et al. Self assembled ionically sodium alginate cross-linked amphotericin B encapsulated glycol chitosan stearate nanoparticles: applicability in better chemotherapy and non-toxic delivery in visceral leishmaniasis. Pharmaceutical Research. 2015;32(5):1727–1740. doi: 10.1007/s11095-014-1571-4. [DOI] [PubMed] [Google Scholar]
- 60.Liang Y.-H., Liu C.-H., Liao S.-H., et al. Cosynthesis of cargo-loaded hydroxyapatite/alginate core-shell nanoparticles (HAP@alg) as pH-responsive nanovehicles by a pre-gel method. ACS Applied Materials & Interfaces. 2012;4(12):6720–6727. doi: 10.1021/am301895u. [DOI] [PubMed] [Google Scholar]
- 61.Ragab D., Sabra S., Xia Y., Goodale D., Allan A. L., Rohani S. On-chip preparation of amphiphilic nanomicelles-in-sodium alginate spheroids as a novel platform against triple-negative human breast cancer cells: fabrication, study of microfluidics flow hydrodynamics and proof of concept for anticancer and drug delivery applications. Journal of Pharmaceutical Sciences. 2019;108(11):3528–3539. doi: 10.1016/j.xphs.2019.07.015. [DOI] [PubMed] [Google Scholar]
- 62.Saralkar P., Dash A. K. Alginate nanoparticles containing curcumin and resveratrol: preparation, characterization, and in vitro evaluation against DU145 prostate cancer cell line. AAPS PharmSciTech. 2017;18(7):2814–2823. doi: 10.1208/s12249-017-0772-7. [DOI] [PubMed] [Google Scholar]
- 63.Sohail A., Turner M. S., Coombes A., Bhandari B. The Viability of Lactobacillus rhamnosus GG and Lactobacillus acidophilus NCFM following double encapsulation in alginate and maltodextrin. Food and Bioprocess Technology. 2012;6(10):2763–2769. doi: 10.1007/s11947-012-0938-y. [DOI] [Google Scholar]
- 64.Şanlı O., Olukman M. Preparation of ferric ion crosslinked acrylamide grafted poly (vinyl alcohol)/sodium alginate microspheres and application in controlled release of anticancer drug 5-fluorouracil. Drug Delivery. 2013;21(3):213–220. doi: 10.3109/10717544.2013.844743. [DOI] [PubMed] [Google Scholar]
- 65.Zhang B., Yan Y., Shen Q., et al. A colon targeted drug delivery system based on alginate modificated graphene oxide for colorectal liver metastasis. Materials Science and Engineering: C. 2017;79:185–190. doi: 10.1016/j.msec.2017.05.054. [DOI] [PubMed] [Google Scholar]
- 66.Zhao D., Liu C.-J., Zhuo R.-X., Cheng S.-X. Alginate/CaCO3 hybrid nanoparticles for efficient codelivery of antitumor gene and drug. Molecular Pharmaceutics. 2012;9(10):2887–2893. doi: 10.1021/mp3002123. [DOI] [PubMed] [Google Scholar]
- 67.Zhou K., Wang X., Chen D., et al. Enhanced treatment effects of tilmicosin against Staphylococcus aureus cow mastitis by self-assembly sodium alginate-chitosan nanogel. Pharmaceutics. 2019;11(10):p. 524. doi: 10.3390/pharmaceutics11100524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kang S.-M., Lee G.-W., Huh Y. S. Centrifugal force-driven modular micronozzle system: generation of engineered alginate microspheres. Scientific Reports. 2019;9(1):1–10. doi: 10.1038/s41598-019-49244-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kearney C. J., Skaat H., Kennedy S. M., et al. Switchable release of entrapped nanoparticles from alginate hydrogels. Advanced Healthcare Materials. 2015;4(11):1634–1639. doi: 10.1002/adhm.201500254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kroneková Z., Pelach M., Mazancová P., et al. Changes in alginate-based microspheres exposed to in vivo environment as revealed by confocal Raman microscopy. Scientific Reports. 2018;8(1) doi: 10.1038/s41598-018-20022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sagiri S. S., Pal K., Basak P., Rana U. A., Shakir I., Anis A. Encapsulation of sorbitan ester-based organogels in alginate microparticles. AAPS PharmSciTech. 2014;15(5):1197–1208. doi: 10.1208/s12249-014-0147-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Samak Y. O., El Massik M., Coombes A. G. A. A comparison of aerosolization and homogenization techniques for production of alginate microparticles for delivery of corticosteroids to the colon. Journal of Pharmaceutical Sciences. 2017;106(1):208–216. doi: 10.1016/j.xphs.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 73.Unagolla J. M., Jayasuriya A. C. Drug transport mechanisms and in vitro release kinetics of vancomycin encapsulated chitosan-alginate polyelectrolyte microparticles as a controlled drug delivery system. European Journal of Pharmaceutical Sciences. 2018;114:199–209. doi: 10.1016/j.ejps.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bochenek M. A., Veiseh O., Vegas A. J., et al. Alginate encapsulation as long-term immune protection of allogeneic pancreatic islet cells transplanted into the omental bursa of macaques. Nature Biomedical Engineering. 2018;2(11):810–821. doi: 10.1038/s41551-018-0275-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Braim S., Śpiewak K., Brindell M., Heeg D., Alexander C., Monaghan T. Lactoferrin-loaded alginate microparticles to target Clostridioides difficile infection. Journal of Pharmaceutical Sciences. 2019;108(7):2438–2446. doi: 10.1016/j.xphs.2019.02. [DOI] [PubMed] [Google Scholar]
- 76.Haffner F. B., Pasc A. Freeze-dried alginate-silica microparticles as carriers of probiotic bacteria in apple juice and beer. LWT. 2018;91:175–179. doi: 10.1016/j.lwt.2018.01.050. [DOI] [Google Scholar]
- 77.Han J., Guenier A.-S., Salmieri S., Lacroix M. Alginate and chitosan functionalization for micronutrient encapsulation. Journal of Agricultural and Food Chemistry. 2008;56(7):2528–2535. doi: 10.1021/jf703739k. [DOI] [PubMed] [Google Scholar]
- 78.Mawad A., Helmy Y. A., Shalkami A.-G., Kathayat D., Rajashekara G. E. coli nissle microencapsulation in alginate-chitosan nanoparticles and its effect on Campylobacter jejuni in vitro. Applied Microbiology and Biotechnology. 2018;102(24):10675–10690. doi: 10.1007/s00253-018-9417-3. [DOI] [PubMed] [Google Scholar]
- 79.Praveen R., Verma P. R. P., Singh S. K., George J. K. Cross linked alginate gel beads as floating drug delivery system for cefdinir: optimization using Box-Behnken design. Journal of Pharmaceutical Investigation. 2014;45(2):187–199. doi: 10.1007/s40005-014-0164-x. [DOI] [Google Scholar]
- 80.Pungrasmi W., Intarasoontron J., Jongvivatsakul P., Likitlersuang S. Evaluation of microencapsulation techniques for micp bacterial spores applied in self-healing concrete. Scientific Reports. 2019;9(1) doi: 10.1038/s41598-019-49002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu S.-H., Sun N.-N., Chau C.-F. Microspheres as carriers for lipase inhibitory substances to reduce dietary triglyceride absorption in mice. Journal of Food and Drug Analysis. 2016;24(1):129–135. doi: 10.1016/j.jfda.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wu S. Y., Chang H.-I., Burgess M., McMillan N. A. J. Vaginal delivery of siRNA using a novel PEGylated lipoplex-entrapped alginate scaffold system. Journal of Controlled Release. 2011;155(3):418–426. doi: 10.1016/j.jconrel.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 83.Tong K. Preparation and biosorption evaluation of Bacillus subtilis/alginate-chitosan microcapsule. Nanotechnology, Science and Applications. 2017;10:35–43. doi: 10.2147/nsa.s104808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Park H., Lee K. Y. Alginate/hyaluronate hydrogels for cartilage regeneration. Journal of Controlled Release. 2011;152:e233–e234. doi: 10.1016/j.jconrel.2011.09.031. [DOI] [PubMed] [Google Scholar]
- 85.Wawrzyńska E., Kubies D. Alginate matrices for protein delivery-a short review. Physiological Research. 2018;67(Suppl 2):319–334. doi: 10.33549/physiolres.933980. [DOI] [PubMed] [Google Scholar]
- 86.Loira-Pastoriza C., Todoroff J., Vanbever R. Delivery strategies for sustained drug release in the lungs. Advanced Drug Delivery Reviews. 2014;75:81–91. doi: 10.1016/j.addr.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 87.Seedat N., Kalhapure R. S., Mocktar C., et al. Co-encapsulation of multi-lipids and polymers enhances the performance of vancomycin in lipid-polymer hybrid nanoparticles: in vitro and in silico studies. Materials Science and Engineering: C. 2016;61:616–630. doi: 10.1016/j.msec.2015.12.053. [DOI] [PubMed] [Google Scholar]
- 88.Möbus K., Siepmann J., Bodmeier R. Zinc-alginate microparticles for controlled pulmonary delivery of proteins prepared by spray-drying. European Journal of Pharmaceutics and Biopharmaceutics. 2012;81(1):121–130. doi: 10.1016/j.ejpb.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 89.Hariyadi D. M., Bostrom T., Bhandari B., Coombes A. G. A. A novel impinging aerosols method for production of propranolol hydrochloride-loaded alginate gel microspheres for oral delivery. Journal of Microencapsulation. 2012;29(1):63–71. doi: 10.3109/02652048.2011.629746. [DOI] [PubMed] [Google Scholar]
- 90.Gombotz W. R., Wee S. F. Protein release from alginate matrices. Advanced Drug Delivery Reviews. 2012;64:194–205. doi: 10.1016/j.addr.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 91.Hariyadi D. M., Wang Y., Lin S. C.-Y., Bostrom T., Bhandari B., Coombes A. G. A. Novel alginate gel microspheres produced by impinging aerosols for oral delivery of proteins. Journal of Microencapsulation. 2012;29(3):250–261. doi: 10.3109/02652048.2011.646329. [DOI] [PubMed] [Google Scholar]
- 92.Morachis J. M., Mahmoud E. A., Almutairi A. Physical and chemical strategies for therapeutic delivery by using polymeric nanoparticles. Pharmacological Reviews. 2012;64(3):505–519. doi: 10.1124/pr.111.005363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Severino P., Chaud M. V., Shimojo A., et al. Sodium alginate-cross-linked polymyxin B sulphate-loaded solid lipid nanoparticles: antibiotic resistance tests and HaCat and NIH/3T3 cell viability studies. Colloids and Surfaces B: Biointerfaces. 2015;129:191–197. doi: 10.1016/j.colsurfb.2015.03.049. [DOI] [PubMed] [Google Scholar]
- 94.Joddar B., Garcia E., Casas A., Stewart C. M. Development of functionalized multi-walled carbon-nanotube-based alginate hydrogels for enabling biomimetic technologies. Scientific Reports. 2016;6(1):1–12. doi: 10.1038/srep32456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ciriza J., Saenz del Burgo L., Gurruchaga H., et al. Graphene oxide enhances alginate encapsulated cells viability and functionality while not affecting the foreign body response. Drug Delivery. 2018;25(1):1147–1160. doi: 10.1080/10717544.2018.1474966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yoncheva K., Merino M., Shenol A., et al. Optimization and in-vitro/in-vivo evaluation of doxorubicin-loaded chitosan-alginate nanoparticles using a melanoma mouse model. International Journal of Pharmaceutics. 2019;556:1–8. doi: 10.1016/j.ijpharm.2018.11.070. [DOI] [PubMed] [Google Scholar]
- 97.Ling K., Wu H., Neish A. S., Champion J. A. Alginate/chitosan microparticles for gastric passage and intestinal release of therapeutic protein nanoparticles. Journal of Controlled Release. 2019;295:174–186. doi: 10.1016/j.jconrel.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 98.Wang X., Wenk E., Hu X., et al. Silk coatings on PLGA and alginate microspheres for protein delivery. Biomaterials. 2007;28(28):4161–4169. doi: 10.1016/j.biomaterials.2007.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Moebus K., Siepmann J., Bodmeier R. Novel preparation techniques for alginate-poloxamer microparticles controlling protein release on mucosal surfaces. European Journal of Pharmaceutical Sciences. 2012;45(3):358–366. doi: 10.1016/j.ejps.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 100.Nesamony J., Singh P. R., Nada S. E., Shah Z. A., Kolling W. M. Calcium alginate nanoparticles synthesized through a novel interfacial cross-linking method as a potential protein drug delivery system. Journal of Pharmaceutical Sciences. 2012;101(6):2177–2184. doi: 10.1002/jps.23104. [DOI] [PubMed] [Google Scholar]
- 101.Pescosolido L., Piro T., Vermonden T., et al. Biodegradable IPNs based on oxidized alginate and dextran-HEMA for controlled release of proteins. Carbohydrate Polymers. 2011;86(1):208–213. doi: 10.1016/j.carbpol.2011.04.033. [DOI] [Google Scholar]
- 102.Heo Y., Akimoto J., Kobatake E., Ito Y. Gelation and release behavior of visible light-curable alginate. Polymer Journal. 2020;52(3):323–332. doi: 10.1038/s41428-019-0280-6. [DOI] [Google Scholar]
- 103.Davis M. S., Marrero-Berrios I., Perez X. I., et al. Alginate-liposomal construct for bupivacaine delivery and MSC function regulation. Drug Delivery and Translational Research. 2018;8(1):226–238. doi: 10.1007/s13346-017-0454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Petchsomrit A., Sermkaew N., Wiwattanapatapee R. Alginate-based composite sponges as gastroretentive carriers for curcumin-loaded self-microemulsifying drug delivery systems. Scientia Pharmaceutica. 2017;85(1):p. 11. doi: 10.3390/scipharm85010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hazra M., Dasgupta Mandal D., Mandal T., Bhuniya S., Ghosh M. Designing polymeric microparticulate drug delivery system for hydrophobic drug quercetin. Saudi Pharmaceutical Journal. 2015;23(4):429–436. doi: 10.1016/j.jsps.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kajjari P. B., Manjeshwar L. S., Aminabhavi T. M. Novel pH- and temperature-responsive blend hydrogel microspheres of sodium alginate and PNIPAAm-g-GG for controlled release of isoniazid. AAPS PharmSciTech. 2012;13(4):1147–1157. doi: 10.1208/s12249-012-9838-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Scolari I. R., Páez P. L., Sánchez-Borzone M. E., Granero G. E. Promising chitosan-coated alginate-tween 80 nanoparticles as rifampicin coadministered ascorbic acid delivery carrier against Mycobacterium tuberculosis. AAPS PharmSciTech. 2019;20(2) doi: 10.1208/s12249-018-1278-7. [DOI] [PubMed] [Google Scholar]
- 108.Banks S. R., Enck K., Wright M., Opara E. C., Welker M. E. Chemical modification of alginate for controlled oral drug delivery. Journal of Agricultural and Food Chemistry. 2019;67(37):10481–10488. doi: 10.1021/acs.jafc.9b01911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang G. A., Mukherjee A., Castro G. R. Development of biopolymer nanocomposite for silver nanoparticles and ciprofloxacin controlled release. International Journal of Biological Macromolecules. 2015;72:740–750. doi: 10.1016/j.ijbiomac.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 110.Islan G. A., Castro G. R. Tailoring of alginate-gelatin microspheres properties for oral ciprofloxacin-controlled release against pseudomonas aeruginosa. Drug Delivery. 2014;21(8):615–626. doi: 10.3109/10717544.2013.870257. [DOI] [PubMed] [Google Scholar]
- 111.Kadir A., Mokhtar M. T. M., Wong T. W. Nanoparticulate assembly of mannuronic acid-and guluronic acid-rich alginate: oral insulin carrier and glucose binder. Journal of Pharmaceutical Sciences. 2013;102(12):4353–4363. doi: 10.1002/jps.23742. [DOI] [PubMed] [Google Scholar]
- 112.Stilhano R. S., Madrigal J. L., Wong K., et al. Injectable alginate hydrogel for enhanced spatiotemporal control of lentivector delivery in murine skeletal muscle. Journal of Controlled Release. 2016;237:42–49. doi: 10.1016/j.jconrel.2016.06.047. [DOI] [PubMed] [Google Scholar]
- 113.Cetin M., Sahin S. Microparticulate and nanoparticulate drug delivery systems for metformin hydrochloride. Drug Delivery. 2016;23(8):2796–2805. doi: 10.3109/10717544.2015.1089957. [DOI] [PubMed] [Google Scholar]
- 114.Sriamornsak P., Thirawong N., Korkerd K. Swelling, erosion and release behavior of alginate-based matrix tablets. European Journal of Pharmaceutics and Biopharmaceutics. 2007;66(3):435–450. doi: 10.1016/j.ejpb.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 115.Song S. E.-S., Sokar M. S., Abdelmonsif D. A., El-Kamel A. H. Mucopenetrating nanoparticles for enhancement of oral bioavailability of furosemide: in vitro and in vivo evaluation/sub-acute toxicity study. International Journal of Pharmaceutics. 2017;526(1-2):366–379. doi: 10.1016/j.ijpharm.2017.04.072. [DOI] [PubMed] [Google Scholar]
- 116.Jayapal J. J., Dhanaraj S. Exemestane loaded alginate nanoparticles for cancer treatment: formulation and in vitro evaluation. International Journal of Biological Macromolecules. 2017;105:416–421. doi: 10.1016/j.ijbiomac.2017.07.064. [DOI] [PubMed] [Google Scholar]
- 117.Alipour S., Montaseri H., Tafaghodi M. Preparation and characterization of biodegradable paclitaxel loaded alginate microparticles for pulmonary delivery. Colloids and Surfaces B: Biointerfaces. 2010;81(2):521–529. doi: 10.1016/j.colsurfb.2010.07.050. [DOI] [PubMed] [Google Scholar]
- 118.Kaur G., Narang R. K., Rath G., Goyal A. K. Advances in pulmonary delivery of nanoparticles. Artificial Cells, Blood Substitutes, and Biotechnology. 2011;40(1-2):75–96. doi: 10.3109/10731199.2011.592494. [DOI] [PubMed] [Google Scholar]
- 119.Zhou Q., Leung S. S. Y., Tang P., Parumasivam T., Loh Z. H., Chan H.-K. Inhaled formulations and pulmonary drug delivery systems for respiratory infections. Advanced Drug Delivery Reviews. 2015;85:83–99. doi: 10.1016/j.addr.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 120.Abdelaziz H. M., Gaber M., Abd-Elwakil M. M., et al. Inhalable particulate drug delivery systems for lung cancer therapy: nanoparticles, microparticles, nanocomposites and nanoaggregates. Journal of Controlled Release. 2018;269:374–392. doi: 10.1016/j.jconrel.2017.11.036. [DOI] [PubMed] [Google Scholar]
- 121.Mosafer J., Sabbaghi A.-H., Badiee A., Dehghan S., Tafaghodi M. Preparation, characterization and in vivo evaluation of alginate-coated chitosan and trimethylchitosan nanoparticles loaded with PR8 influenza virus for nasal immunization. Asian Journal of Pharmaceutical Sciences. 2019;14(2):216–221. doi: 10.1016/j.ajps.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hosseini M., Dobakhti F., Pakzad S. R., Ajdary S. Immunization with single oral dose of alginate-encapsulated BCG elicits effective and long-lasting mucosal immune responses. Scandinavian Journal of Immunology. 2015;82(6):489–497. doi: 10.1111/sji.12351. [DOI] [PubMed] [Google Scholar]
- 123.Hill M., Twigg M., Sheridan E. A., et al. Alginate/chitosan particle-based drug delivery systems for pulmonary applications. Pharmaceutics. 2019;11(8):p. 379. doi: 10.3390/pharmaceutics11080379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Migaud P. S., Kesarwani A., Sahu P., Upadhyay P. Aerosol immunization by alginate coated Mycobacterium (BCG/MIP) particles provide enhanced immune response and protective efficacy than aerosol of plain Mycobacterium against M.tb. H37Rv infection in mice. BMC Infectious Diseases. 2019;19(1) doi: 10.1186/s12879-019-4157-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Muralidharan P., Malapit M., Mallory E., Hayes D., Mansour H. M. Inhalable nanoparticulate powders for respiratory delivery. Nanomedicine: Nanotechnology, Biology and Medicine. 2015;11(5):1189–1199. doi: 10.1016/j.nano.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 126.Ahmed A., Getti G., Boateng J. Ciprofloxacin-loaded calcium alginate wafers prepared by freeze-drying technique for potential healing of chronic diabetic foot ulcers. Drug Delivery and Translational Research. 2018;8(6):1751–1768. doi: 10.1007/s13346-017-0445-9. [DOI] [PubMed] [Google Scholar]
- 127.Sanna V., Roggio A. M., Siliani S., et al. Development of novel cationic chitosan-and anionic alginate–coated poly (D,L-lactide-co-glycolide) nanoparticles for controlled release and light protection of resveratrol. International Journal of Nanomedicine. 2012;7:5501–5516. doi: 10.2147/IJN.S36684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sarheed O., Abdul Rasool B. K., Abu-Gharbieh E., Aziz U. S. An investigation and characterization on alginate hydogel dressing loaded with metronidazole prepared by combined inotropic gelation and freeze-thawing cycles for controlled release. AAPS PharmSciTech. 2014;16(3):601–609. doi: 10.1208/s12249-014-0237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhao Y., Shen W., Chen Z., Wu T. Freeze-thaw induced gelation of alginates. Carbohydrate Polymers. 2016;148:45–51. doi: 10.1016/j.carbpol.2016.04.047. [DOI] [PubMed] [Google Scholar]
- 130.Du J., El-Sherbiny I. M., Smyth H. D. Swellable ciprofloxacin-loaded nano-in-micro hydrogel particles for local lung drug delivery. AAPS PharmSciTech. 2014;15(6):1535–1544. doi: 10.1208/s12249-014-0176-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rokstad T., Tagami T. Drug/polymer nanoparticles prepared using unique spray nozzles and recent progress of inhaled formulation. Asian Journal of Pharmaceutical Sciences. 2014;9(5):236–243. doi: 10.1016/j.ajps.2014.06.005. [DOI] [Google Scholar]
- 132.Zhang C., Shi G., Zhang J., et al. Targeted antigen delivery to dendritic cell via functionalized alginate nanoparticles for cancer immunotherapy. Journal of Controlled Release. 2017;256:170–181. doi: 10.1016/j.jconrel.2017.04.020. [DOI] [PubMed] [Google Scholar]
- 133.Fenn S. L., Miao T., Scherrer R. M., Oldinski R. A. Dual-cross-linked methacrylated alginate sub-microspheres for intracellular chemotherapeutic delivery. ACS Applied Materials & Interfaces. 2016;8(28):17775–17783. doi: 10.1021/acsami.6b03245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Floyd J. A., Galperin A., Ratner B. D. Drug encapsulated polymeric microspheres for intracranial tumor therapy: a review of the literature. Advanced Drug Delivery Reviews. 2015;91:23–37. doi: 10.1016/j.addr.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 135.Ausili E., Paolucci V., Triarico S., et al. Treatment of pressure sores in Spina bifida patients with calcium alginate and foam dressing. European Review for Medical and Pharmacological Sciences. 2013;17(12):1642–1647. [PubMed] [Google Scholar]
- 136.Gonçalves V. S. S., Gurikov P., Poejo J., et al. Alginate-based hybrid aerogel microparticles for mucosal drug delivery. European Journal of Pharmaceutics and Biopharmaceutics. 2016;107:160–170. doi: 10.1016/j.ejpb.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 137.Nagarwal R. C., Kumar R., Pandit J. K. Chitosan coated sodium alginate-chitosan nanoparticles loaded with 5-FU for ocular delivery: in vitro characterization and in vivo study in rabbit eye. European Journal of Pharmaceutical Sciences. 2012;47(4):678–685. doi: 10.1016/j.ejps.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 138.Santos E., Orive G., Calvo A., et al. Optimization of 100 μm alginate-poly-l-lysine-alginate capsules for intravitreous administration. Journal of Controlled Release. 2012;158(3):443–450. doi: 10.1016/j.jconrel.2011.09.079. [DOI] [PubMed] [Google Scholar]
- 139.Costa J. R., Silva N. C., Sarmento B., Pintado M. Potential chitosan-coated alginate nanoparticles for ocular delivery of daptomycin. European Journal of Clinical Microbiology & Infectious Diseases. 2015;34(6):1255–1262. doi: 10.1007/s10096-015-2344-7. [DOI] [PubMed] [Google Scholar]
- 140.Leslie S. K., Cohen D. J., Sedlaczek J., Pinsker E. J., Boyan B. D., Schwartz Z. Controlled release of rat adipose-derived stem cells from alginate microbeads. Biomaterials. 2013;34(33):8172–8184. doi: 10.1016/j.biomaterials.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 141.Maia F. R., Barbosa M., Gomes D. B., et al. Hydrogel depots for local co-delivery of osteoinductive peptides and mesenchymal stem cells. Journal of Controlled Release. 2014;189:158–168. doi: 10.1016/j.jconrel.2014.06.030. [DOI] [PubMed] [Google Scholar]
- 142.Ferreira N. N., Caetano B. L., Boni F. I., et al. Alginate-based delivery systems for bevacizumab local therapy: in vitro structural features and release properties. Journal of Pharmaceutical Sciences. 2019;108(4):1559–1568. doi: 10.1016/j.xphs.2018.11.038. [DOI] [PubMed] [Google Scholar]
- 143.Park H., Woo E. K., Lee K. Y. Ionically cross-linkable hyaluronate-based hydrogels for injectable cell delivery. Journal of Controlled Release. 2014;196:146–153. doi: 10.1016/j.jconrel.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 144.Ulker Z., Erkey C. An emerging platform for drug delivery: aerogel based systems. Journal of Controlled Release. 2014;177:51–63. doi: 10.1016/j.jconrel.2013.12.033. [DOI] [PubMed] [Google Scholar]
- 145.Zelikin A. N. Drug releasing polymer thin films: new era of surface-mediated drug delivery. ACS Nano. 2010;4(5):2494–2509. doi: 10.1021/nn100634r. [DOI] [PubMed] [Google Scholar]
- 146.Kodiyan A., Silva E. A., Kim J., Aizenberg M., Mooney D. J. Surface modification with alginate-derived polymers for stable, protein-repellent, long-circulating gold nanoparticles. ACS Nano. 2012;6(6):4796–4805. doi: 10.1021/nn205073n. [DOI] [PubMed] [Google Scholar]
- 147.Lin N., Gèze A., Wouessidjewe D., Huang J., Dufresne A. Biocompatible double-membrane hydrogels from cationic cellulose nanocrystals and anionic alginate as complexing drugs codelivery. ACS Applied Materials & Interfaces. 2016;8(11):6880–6889. doi: 10.1021/acsami.6b00555. [DOI] [PubMed] [Google Scholar]
- 148.Lopedota A., Denora N., Laquintana V., et al. Alginate-based hydrogel containing minoxidil/hydroxypropyl-β-cyclodextrin inclusion complex for topical alopecia treatment. Journal of Pharmaceutical Sciences. 2018;107(4):1046–1054. doi: 10.1016/j.xphs.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 149.Jeong S., Kim B., Lau H.-C., Kim A. Gelatin-alginate complexes for EGF encapsulation: effects of h-bonding and electrostatic interactions. Pharmaceutics. 2019;11(10):p. 530. doi: 10.3390/pharmaceutics11100530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wattanakul K., Imae T., Chang W.-W., Chu C.-C., Nakahata R., Yusa S.-i. Oligopeptide-side chained alginate nanocarrier for melittin-targeted chemotherapy. Polymer Journal. 2019;51(8):771–780. doi: 10.1038/s41428-019-0191-6. [DOI] [Google Scholar]
- 151.Sorasitthiyanukarn F. N., Muangnoi C., Ratnatilaka Na Bhuket P., Rojsitthisak P., Rojsitthisak P. Chitosan/alginate nanoparticles as a promising approach for oral delivery of curcumin diglutaric acid for cancer treatment. Materials Science and Engineering: C. 2018;93:178–190. doi: 10.1016/j.msec.2018.07.069. [DOI] [PubMed] [Google Scholar]
- 152.Azevedo M. A., Bourbon A. I., Vicente A. A., Cerqueira M. A. Alginate/chitosan nanoparticles for encapsulation and controlled release of vitamin B2. International Journal of Biological Macromolecules. 2014;71:141–146. doi: 10.1016/j.ijbiomac.2014.05.036. [DOI] [PubMed] [Google Scholar]
- 153.Mokhtari S., Jafari S. M., Assadpour E. Development of a nutraceutical nano-delivery system through emulsification/internal gelation of alginate. Food Chemistry. 2017;229:286–295. doi: 10.1016/j.foodchem.2017.02.071. [DOI] [PubMed] [Google Scholar]
- 154.Kyziol A., Mazgala A., Michna J., Regiel-Futyra A., Sebastian V. Preparation and characterization of alginate/chitosan formulations for ciprofloxacin-controlled delivery. Journal of Biomaterials Applications. 2017;32(2):162–174. doi: 10.1177/0885328217714352. [DOI] [PubMed] [Google Scholar]
- 155.Zhang A., Olmo R., Iglesias I., Teijón J. M., Blanco M. D. Folate-targeted nanoparticles based on albumin and albumin/alginate mixtures as controlled release systems of tamoxifen: synthesis and in vitro characterization. Pharmaceutical Research. 2013;31(1):182–193. doi: 10.1007/s11095-013-1151-z. [DOI] [PubMed] [Google Scholar]
- 156.Das R. K., Kasoju N., Bora U. Encapsulation of curcumin in alginate-chitosan-pluronic composite nanoparticles for delivery to cancer cells. Nanomedicine: Nanotechnology, Biology and Medicine. 2010;6(1):153–160. doi: 10.1016/j.nano.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 157.Katuwavila N. P., Perera A. D. L. C., Samarakoon S. R., et al. Chitosan-alginate nanoparticle system efficiently delivers doxorubicin to MCF-7 cells. Journal of Nanomaterials. 2016;2016:12. doi: 10.1155/2016/3178904.3178904 [DOI] [Google Scholar]
- 158.Almalik A., Alradwan I., Kalam M. A., Alshamsan A. Effect of cryoprotection on particle size stability and preservation of chitosan nanoparticles with and without hyaluronate or alginate coating. Saudi Pharmaceutical Journal. 2017;25(6):861–867. doi: 10.1016/j.jsps.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Deacon J., Abdelghany S. M., Quinn D. J., et al. Antimicrobial efficacy of tobramycin polymeric nanoparticles for Pseudomonas aeruginosa infections in cystic fibrosis: formulation, characterisation and functionalisation with dornase alfa (DNase) Journal of Controlled Release. 2015;198:55–61. doi: 10.1016/j.jconrel.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 160.Baek S., Joo S. H., Toborek M. Treatment of antibiotic-resistant bacteria by encapsulation of ZnO nanoparticles in an alginate biopolymer: insights into treatment mechanisms. Journal of Hazardous Materials. 2019;373:122–130. doi: 10.1016/j.jhazmat.2019.03.072. [DOI] [PubMed] [Google Scholar]
- 161.Liu Q., Jing Y., Han C., Zhang H., Tian Y. Encapsulation of curcumin in zein/caseinate/sodium alginate nanoparticles with improved physicochemical and controlled release properties. Food Hydrocolloids. 2019;93:432–442. doi: 10.1016/j.foodhyd.2019.02.003. [DOI] [Google Scholar]
- 162.Loquercio A., Castell-Perez E., Gomes C., Moreira R. G. Preparation of chitosan-alginate nanoparticles forTrans-cinnamaldehyde entrapment. Journal of Food Science. 2015;80(10):N2305–N2315. doi: 10.1111/1750-3841.12997. [DOI] [PubMed] [Google Scholar]
- 163.Maruyama C. R., Guilger M., Pascoli M., et al. Nanoparticles based on chitosan as carriers for the combined herbicides imazapic and imazapyr. Scientific Reports. 2016;6(1) doi: 10.1038/srep19768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Pankongadisak P., Ruktanonchai U. R., Supaphol P., Suwantong O. Preparation and characterization of silver nanoparticles-loaded calcium alginate beads embedded in gelatin scaffolds. AAPS PharmSciTech. 2014;15(5):1105–1115. doi: 10.1208/s12249-014-0140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wang C., Jiang W., Zuo W., Han G., Zhang Y. Effect of heat-transfer capability on micropore structure of freeze-drying alginate scaffold. Materials Science and Engineering: C. 2018;93:944–949. doi: 10.1016/j.msec.2018.08.055. [DOI] [PubMed] [Google Scholar]
- 166.Dai Tran T., Li Z., Rujiravanit R., Sirivat A., Jamieson A. M. Anomalous rheology of polypyrrole nanoparticle/alginate suspensions: effect of solids volume fraction, particle size, and electronic state. Rheologica Acta. 2011;50(9-10):809–823. doi: 10.1007/s00397-011-0566-x. [DOI] [Google Scholar]
- 167.Zhu H., Srivastava R., Brown J. Q., McShane M. J. Combined physical and chemical immobilization of glucose oxidase in alginate microspheres improves stability of encapsulation and activity. Bioconjugate Chemistry. 2005;16(6):1451–1458. doi: 10.1021/bc050171z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wu A. C., Sher P., Mano J. F. Production methodologies of polymeric and hydrogel particles for drug delivery applications. Expert Opinion on Drug Delivery. 2012;9(2):231–248. doi: 10.1517/17425247.2012.652614. [DOI] [PubMed] [Google Scholar]
- 169.Lupo B., Maestro A., Porras M., Gutiérrez J. M., González C. Preparation of alginate microspheres by emulsification/internal gelation to encapsulate cocoa polyphenols. Food Hydrocolloids. 2014;38:56–65. doi: 10.1016/j.foodhyd.2013.11.003. [DOI] [Google Scholar]
- 170.Qi X., Qin X., Yang R., et al. Intra-articular administration of chitosan thermosensitive in situ hydrogels combined with diclofenac sodium-loaded alginate microspheres. Journal of Pharmaceutical Sciences. 2016;105(1):122–130. doi: 10.1016/j.xphs.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 171.Giri T. K., Choudhary C., Alexander A. A., Alexander H., Tripathi D. K. Prospects of pharmaceuticals and biopharmaceuticals loaded microparticles prepared by double emulsion technique for controlled delivery. Saudi Pharmaceutical Journal. 2013;21(2):125–141. doi: 10.1016/j.jsps.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ahmed M. M., El-Rasoul S. A., Auda S. H., Ibrahim M. A. Emulsification/internal gelation as a method for preparation of diclofenac sodium-sodium alginate microparticlesfication/internal gelation as a method for preparation of diclofenac sodium–sodium alginate microparticles. Saudi Pharmaceutical Journal. 2013;21(1):61–69. doi: 10.1016/j.jsps.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Semmling B., Nagel S., Sternberg K., Weitschies W., Seidlitz A. Development of hydrophobized alginate hydrogels for the vessel-simulating flow-through cell and their usage for biorelevant drug-eluting stent testing. AAPS PharmSciTech. 2013;14(3):1209–1218. doi: 10.1208/s12249-013-0011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Silva M., Vasheghani-Farahani E., Hashemi-Najafabadi S. The effect of polymer and CaCl2 concentrations on the sulfasalazine release from alginate-N,O-carboxymethyl chitosan beads. Progress in Biomaterials. 2013;2(1):p. 10. doi: 10.1186/2194-0517-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Davarcı F., Turan D., Ozcelik B., Poncelet D. The influence of solution viscosities and surface tension on calcium alginate microbead formation using dripping technique. Food Hydrocolloids. 2017;62:119–127. doi: 10.1016/j.foodhyd.2016.06.029. [DOI] [Google Scholar]
- 176.Ørning P., Hoem K. S., Coron A. E., et al. Alginate microsphere compositions dictate different mechanisms of complement activation with consequences for cytokine release and leukocyte activation. Journal of Controlled Release. 2016;229:58–69. doi: 10.1016/j.jconrel.2016.03.021. [DOI] [PubMed] [Google Scholar]
- 177.Mishra D., Nagpal M., Singh S. Synthesis characterization and in vitro drug release from acrylamide and sodium alginate based superporous hydrogel devices. International Journal of Pharmaceutical Investigation. 2013;3(3):p. 131. doi: 10.4103/2230-973x.119215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wells L. A., Sheardown H. Photosensitive controlled release with polyethylene glycol-anthracene modified alginate. European Journal of Pharmaceutics and Biopharmaceutics. 2011;79(2):304–313. doi: 10.1016/j.ejpb.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 179.Priya James H., John R., Alex A., Anoop K. R. Smart polymers for the controlled delivery of drugs - a concise overview. Acta Pharmaceutica Sinica B. 2014;4(2):120–127. doi: 10.1016/j.apsb.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hadjiev N. A., Amsden B. G. An assessment of the ability of the obstruction-scaling model to estimate solute diffusion coefficients in hydrogels. Journal of Controlled Release. 2015;199:10–16. doi: 10.1016/j.jconrel.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 181.Kamaly N., Yameen B., Wu J., Farokhzad O. C. Degradable controlled-release polymers and polymeric nanoparticles: mechanisms of controlling drug release. Chemical Reviews. 2016;116(4):2602–2663. doi: 10.1021/acs.chemrev.5b00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Setti C., Suarato G., Perotto G., Athanassiou A., Bayer I. S. Investigation of in vitro hydrophilic and hydrophobic dual drug release from polymeric films produced by sodium alginate-MaterBi drying emulsions. European Journal of Pharmaceutics and Biopharmaceutics. 2018;130:71–82. doi: 10.1016/j.ejpb.2018.06.019. [DOI] [PubMed] [Google Scholar]
- 183.Gao B., Chen L., Zhao Y., et al. Methods to prepare dopamine/polydopamine modified alginate hydrogels and their special improved properties for drug delivery. European Polymer Journal. 2019;110:192–201. doi: 10.1016/j.eurpolymj.2018.11.025. [DOI] [Google Scholar]
- 184.Zhang L., Zhang F., Fang Y., Wang S. Alginate-shelled SPI nanoparticle for encapsulation of resveratrol with enhanced colloidal and chemical stability. Food Hydrocolloids. 2019;90:313–320. doi: 10.1016/j.foodhyd.2018.12.042. [DOI] [Google Scholar]
- 185.Déat-Lainé E., Hoffart V., Garrait G., et al. Efficacy of mucoadhesive hydrogel microparticles of whey protein and alginate for oral insulin delivery. Pharmaceutical Research. 2012;30(3):721–734. doi: 10.1007/s11095-012-0913-3. [DOI] [PubMed] [Google Scholar]
- 186.Truong-Le V., Lovalenti P. M., Abdul-Fattah A. M. Stabilization challenges and formulation strategies associated with oral biologic drug delivery systems. Advanced Drug Delivery Reviews. 2015;93:95–108. doi: 10.1016/j.addr.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 187.Welch R. P., Lee H., Luzuriaga M. A., Brohlin O. R., Gassensmith J. J. Protein-polymer delivery: chemistry from the cold chain to the clinic. Bioconjugate Chemistry. 2018;29(9):2867–2883. doi: 10.1021/acs.bioconjchem.8b00483. [DOI] [PubMed] [Google Scholar]
- 188.Spadari C. d. C., de Bastiani F. W. M. D. S., Lopes L. B., Ishida K. Alginate nanoparticles as non-toxic delivery system for miltefosine in the treatment of Candidiasis and Cryptococcosis. International Journal of Nanomedicine. 2019;14:5187–5199. doi: 10.2147/ijn.s205350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Chorvát P. L., Gambari R. Advanced progress of microencapsulation technologies: in vivo and in vitro models for studying oral and transdermal drug deliveries. Journal of Controlled Release. 2014;178:25–45. doi: 10.1016/j.jconrel.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 190.Patole V. C., Pandit A. P. Mesalamine-loaded alginate microspheres filled in enteric coated HPMC capsules for local treatment of ulcerative colitis: in vitro and in vivo characterization. Journal of Pharmaceutical Investigation. 2017;48(3):257–267. doi: 10.1007/s40005-017-0304-1. [DOI] [Google Scholar]
- 191.Agüero L., Zaldivar-Silva D., Peña L., Dias M. L. Alginate microparticles as oral colon drug delivery device: a review. Carbohydrate Polymers. 2017;168:32–43. doi: 10.1016/j.carbpol.2017.03.033. [DOI] [PubMed] [Google Scholar]
- 192.Paredes-Juarez G. A., de Haan B. J., Faas M. M., de Vos P. The role of pathogen-associated molecular patterns in inflammatory responses against alginate based microcapsules. Journal of Controlled Release. 2013;172(3):983–992. doi: 10.1016/j.jconrel.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 193.Sohail R., Abbas S. R. Evaluation of amygdalin-loaded alginate-chitosan nanoparticles as biocompatible drug delivery carriers for anticancerous efficacy. International Journal of Biological Macromolecules. 2020;153:36–45. doi: 10.1016/j.ijbiomac.2020.02.191. [DOI] [PubMed] [Google Scholar]
- 194.Wang Q., Qian K., Liu S., et al. X-ray visible and uniform alginate microspheres loaded with in situ synthesized BaSO4 nanoparticles for in vivo transcatheter arterial embolization. Biomacromolecules. 2015;16(4):1240–1246. doi: 10.1021/acs.biomac.5b00027. [DOI] [PubMed] [Google Scholar]
- 195.Wang Q., Jamal S., Detamore M. S., Berkland C. PLGA-chitosan/PLGA-alginate nanoparticle blends as biodegradable colloidal gels for seeding human umbilical cord mesenchymal stem cells. Journal of Biomedical Materials Research Part A. 2011;96(3):520–527. doi: 10.1002/jbm.a.33000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hariyadi D. M., Rosita N., Nugrahaeni F. Formulation, characteristic evaluation, stress test and effectiveness study of matrix metalloproteinase-1 (MMP-1) expression of glutathione loaded alginate microspheres and gel. Pharmaceutical Sciences. 2018;24(4):304–312. doi: 10.15171/ps.2018.44. [DOI] [Google Scholar]
- 197.Azad A. K., Al-Mahmood S. M. A., Chatterjee B., Wan Sulaiman W. M. A., Elsayed T. M., Doolaanea A. A. Encapsulation of black seed oil in alginate beads as a pH-sensitive carrier for intestine-targeted drug delivery: in vitro, in vivo and ex vivo study. Pharmaceutics. 2020;12(3):219–226. doi: 10.3390/pharmaceutics12030219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Thai H., Thuy Nguyen C., Thi Thach L., et al. Characterization of chitosan/alginate/lovastatin nanoparticles and investigation of their toxic effects in vitro and in vivo. Scientific Reports. 2020;10(1) doi: 10.1038/s41598-020-57666-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


