Abstract
Chronic inflammation in humans is associated with accelerated development of cardiometabolic diseases such as myocardial infarction, stroke, and diabetes. Strong evidence from animal models and human interventional trials including CANTOS (The Canakinumab Anti-inflammatory Thrombosis Outcome Study) suggests that targeting residual systemic inflammation in humans may impart a benefit in reducing cardiometabolic diseases. Diseases associated with heightened immune-activation and systemic inflammation including psoriasis, rheumatoid arthritis, systemic lupus erythematosus, and human immunodeficiency virus infection are associated with upwards of two to seven-fold risk of future adverse cardiac events even when adjusted for traditional risk factors. Over the past decade, psoriasis has been utilized as a human model to study inflammatory-induced cardiometabolic dysfunction and to better understand residual risk due to inflammation. The high prevalence and early onset of cardiovascular disease in psoriasis enhances the likelihood of discovering novel pathways in vascular disease progression when followed over time. Furthermore, the United States Food and Drug Administration approved treatments for psoriasis include cytokine inhibitors (anti-tumor necrosis factor, anti-interleukin 17, anti-interleukin 12/23) which while treating the skin disease provide a unique opportunity to characterize how treating the inflammatory pathways may impact atherosclerosis. Herein, we provide a review of chronic inflammation, cardiometabolic disease associations, and treatment effects with a focus on psoriasis as a human model of study.
Keywords: Psoriasis, inflammation, cardiometabolic disease, immune activation, lipoprotein dysfunction, adipose dysfunction
Introduction
Despite major advances in our understanding of cardiovascular disease (CVD) and its pathogenesis, 31% of all deaths worldwide are still due to coronary artery disease, stroke, and other vasculopathies (1). Although much of this cardiovascular (CV) risk is due to traditional CVD risk factors including hyperlipidemia, smoking, diabetes, and hypertension, inflammation has been identified as a key driver in the development, progression, and complications of atherosclerosis (2).
Indeed, patients with chronic inflammatory diseases such as rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), and human immunodeficiency virus (HIV) infection have both higher levels of systemic inflammatory markers and a greater incidence of CVD compared to the general population (3). However, RA, SLE and HIV disease pathophysiology and associated treatment modalities including steroid use and retroviral inhibitors hamper using these diseases as clinical models for studying the effects of inflammation, treatment of inflammation, and effects on cardiometabolic disease (CMD). Patients affected by these conditions require ongoing therapy, without which irreversible joint damage or systemic effects would occur (4). Moreover, rarely do these diseases utilize cytokine therapies as in the case of psoriasis, thereby limiting targeted understanding of treatment effects on systemic inflammation in humans.
Inflammation due to innate and adaptive immune cell activation contributes to the development of atherosclerosis in psoriasis (5, 6) and patients with psoriatic arthritis (7). In fact, this past year, psoriasis was noted to be a risk-enhancing condition for developing cardiovascular disease by the American College of Cardiology/American Heart Association prevention guidelines (3). Furthermore, the American Academy of Dermatology/National Psoriasis Foundation guidelines noted these recommendations of psoriasis being a high-risk disease state associated with enhanced cardiovascular disease risk beyond traditional CVD risk factors (8).
While the exact mechanistic links between chronic inflammation and cardiometabolic disease are an active area of investigation, accepted pathways in chronic inflammatory diseases include immune activation and systemic inflammation, lipoprotein dysfunction, and adipose dysfunction including insulin resistance that predispose these patients to cardiometabolic disease. This review aims to describe the links between systemic inflammation and cardiometabolic disease and summarize important evidence of potential mechanisms driving CVD in inflammatory diseases. We highlight the use of psoriasis as a unique human model to study the role of immune activation, lipoprotein dysfunction, and adipose dysfunction in the development of cardiometabolic diseases. Additionally, this review covers effects of treatment of psoriasis on cardiovascular diseases. The long-term goal of these studies in psoriasis is to inform how pathways in inflammation modulate following treatment and utilizing anti-cytokine therapies that may translate into future treatments for atherosclerosis.
Inflammation and Cardiometabolic Disease
Systemic inflammation when defined as elevated high-sensitivity C-reactive protein (hs-CRP) has been shown to be associated with incident cardiovascular events (2). CRP levels were found to be better predictors of CV events than low-density lipoprotein (LDL) cholesterol levels in apparently healthy women followed for ten years (2). Moreover, CANTOS (The Canakinumab Anti-inflammatory Thrombosis Outcome Study) carried out in post-myocardial infarction patients with high inflammatory risk by hs-CRP>2 mg/L demonstrated that treatment with canakinumab, an anti-interleukin (IL)-1β antibody, decreased IL6, hs-CRP levels, and the rate of prospective CV events (9). This landmark trial elucidated the potential role of treating residual inflammation to prevent adverse CV events.
While details of the precise mechanism linking systemic inflammation and atherosclerosis are under active investigation, many preclinical and clinical studies have explored heightened immune activity in the development of cardiometabolic disease. Pre-clinical models utilizing apolipoprotein E-deficient murine demonstrated that neutrophil extracellular traps accelerated atherosclerosis. Furthermore, suppression of pro-inflammatory neutrophil extracellular trap formation by a peptidyl-arginine deiminase inhibitor reduced atherosclerotic lesion size and number (10). Human translational studies in young, healthy humans demonstrated that both high (3 ng/kg) and low-doses (0.6 ng/kg) of IV endotoxin led to a transient systemic inflammatory state with increases in tumor necrosis factor (TNF)-α, IL-6 and hs-CRP. Furthermore, insulin resistance and dysfunctional HDL (high density lipoprotein) ensued concurrently with the development of adipose tissue inflammation (11–14) highlighting that innate immune stimuli drive the development of cardiometabolic disease even in the short term. In addition, the role of intracellular activation of the NLRP3 inflammasome, a macromolecular complex activating caspase-1 and promoting the release of inflammatory components such as cytokines, is increasingly being recognized as an early event initiating atherosclerosis. Of note, the NLRP3 inflammasome is upregulated in states of high systemic inflammation such as psoriasis in turn leading to early atherogenesis (15). Furthermore, immune cell subsets of myeloid origin (neutrophils, monocytes and platelets) are elevated and activated in inflammatory disease states and associate with high aortic vascular inflammation (16). Specifically, two subsets of myeloid cells, low-density neutrophils and classical monocytes, were found to be elevated in psoriasis and associated with skin disease severity, destruction of endothelial cells in culture, and coronary artery disease by CCTA (coronary computed tomography angiography) in vivo (17, 18). Additionally, a composite inflammatory biomarker GlycA (a subset of glycan N-acetylglucosamine residues on glycosylated acute-phase reactant proteins), was associated with myeloid cells and systemic inflammation by hs-CRP (19), and a prospective observational study demonstrated a positive association between GlycA, aortic vascular inflammation by positron emission tomography computed tomography (PET/CT), and coronary artery disease by CCTA (20). Finally, vascular inflammation itself drives atherosclerotic plaque development, and a recent study demonstrated that 18F-FDG (18F-labeled fluoro-2-deoxyglucose) uptake was high where an atherosclerotic plaque was not yet formed. However, 18F-FDG uptake was not high where an atherosclerotic plaque had already developed, suggesting that high vascular inflammation preceded the development of atherosclerotic plaque (21).
Traditional cardiovascular risk factors are more prevalent in patients with chronic inflammatory diseases including increasing hypertension, metabolic syndrome, and dyslipidemia. For example, patients with psoriasis have lipid profiles with increased apolipoprotein B-containing lipoproteins (LDL and VLDL) (22) and a reduction in apolipoprotein A1-containing lipoproteins (HDL). Furthermore, a triglyceride-rich environment is driven in part by systemic inflammation and an insulin-resistant state termed metabolic dysfunction. Most importantly, HDL function is impaired (~10% less removal of cholesterol by HDL efflux assay). This leads to augmented retention and accelerated oxidation of cholesterol (23), a major driver of early atherosclerosis. Additionally, the receptors of these oxidized lipids were recently demonstrated to be associated with systemic inflammation and in vivo coronary burden by CCTA (Dey et al., JAMA Dermatol., in press). Thus, heightened systemic inflammation leads to underlying cardiometabolic disease (24, 25) and adverse CV outcomes (26) driven by immune-activation and its sequelae.
Psoriasis: A Chronic Inflammatory Skin Disease Associated with Cardiometabolic Disease
Psoriasis is a chronic inflammatory skin disease that affects 125 million people worldwide, and 7 million people in the United States, with more than 3 million people undiagnosed (27). Psoriasis affects the skin most commonly with erythematous, thickened plaques on extensor surfaces. Psoriasis is associated with development of psoriatic arthritis in upwards of 35% of patients (28), which imparts more risk for co-morbid disease (4). Psoriasis is multifactorial in etiology, partly genetically driven and associated with heightened innate and adaptive immune activation (28). T helper (Th)1 and Th17 cells drive pro-inflammatory cytokines including TNFα, interferon-γ, IL17A, and IL23 (28, 29). As such, psoriasis has underlying systemic effects (28, 30) more pronounced as skin disease worsens driving the development of cardiometabolic dysfunction (26, 31), especially when the psoriatic diseases are untreated.
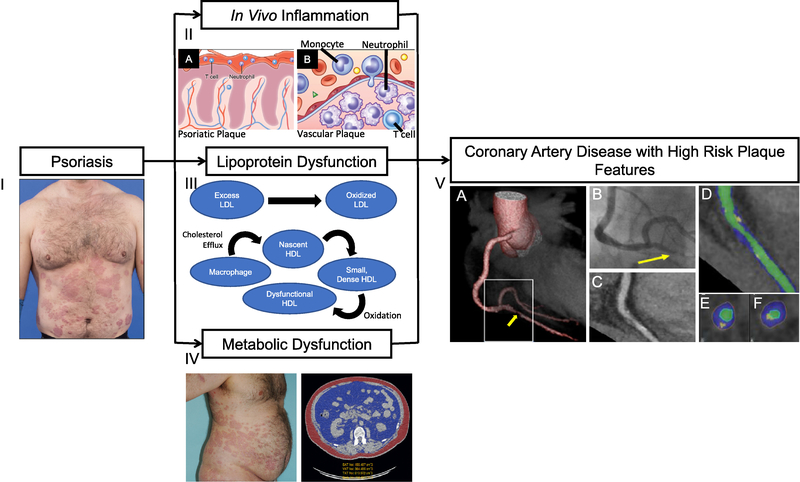
Psoriasis is associated with a greater risk of co-morbid CV risk factors (32, 33) as well as elevated CV events including myocardial infarction (26, 34), stroke (35), and cardiovascular death (36, 37). Patients with psoriasis have increased vascular inflammation by 18F-FDG PET/CT (16, 38), lipid-rich non-calcified coronary burden (39), coronary artery calcium by coronary computed tomography (40), and carotid/femoral atherosclerotic plaques by ultrasound (41). Immune activation (32, 33), lipoprotein dysfunction (22, 42), and adipose dysfunction (26, 31) are three of the main factors driving accelerated cardiovascular diseases in psoriasis (Figure 1).
Figure 1:
Psoriasis is a chronic inflammatory skin disease associated with cardiometabolic disease. (I) Psoriasis patients may develop thick, extensive cutaneous plaques. (II) Psoriasis exhibits infiltration of T cells and neutrophils in (A) the cutaneous plaque as well as high systemic inflammation where the (B) vessel wall is infiltrated through a complex interplay of pro-inflammatory cellular components including T cells, monocytes, and neutrophils, leading to atherosclerotic cardiovascular disease. (III) Psoriasis associates with abnormal lipid profile and impaired HDL function, which in combination with residual inflammatory stress accelerates the formation of oxidation-modified lipoproteins, such as oxidized LDL and oxidized HDL. (IV) Transverse computed tomography (CT) images of an obese patient with psoriasis at the level of the abdominal aorta depicting high visceral adiposity (blue) in comparison with subcutaneous adiposity (red). Psoriasis and concomitant cardiometabolic disease are associated with high body mass index and waist-to-hip ratio. (V) CT 3D reconstruction model of a heart (A) depicting critical stenosis (B) due to a high-risk plaque, which was subsequently found to be a culprit for an ST-elevation myocardial infarction during follow-up. (C) Planar reconstruction of vessel demonstrating high-risk plaque. (D) Color-coded, curved multiplanar reconstruction (green, vessel lumen; blue, non-calcified plaque component; yellow, calcified plaque component). (E) and (F) Cross-sectional images. The plaque is only partially calcified, showing spotty calcification and some positive remodeling.
Immune Activation and Pro-Inflammatory Cytokines in Psoriasis
There are multiple immune cells that are active in psoriatic skin plaque (Figure 1, Panel IIA). Early on, acute inflammatory neutrophils begin the cascade resulting in T cells, macrophages, and response-to-injury mediators secreting cytokines and chemokines (28, 43). Heightened myeloid and lymphoid immune cell activity drives pro-inflammatory cytokines, such as IL1β, IL6, and TNFα. Systemic inflammatory effects in psoriasis and early atherosclerosis are amplified by a broader class of cytokines, including IL17A, IL1β, IL6 and TNFα (Figure 1, Panel IIB) (15, 44). In turn, these cells secrete additional cytokines such as IL12/23, leading to the differentiation of helper T cells (Th1 and Th17) (44). Subsequently, T cells secrete intermediates (e.g., IL17F, IL17A, and IL22) which in turn activate keratinocytes and stimulate more pro-inflammatory cytokines, antimicrobial peptides, chemokines, and S100 proteins (29, 44). The vessel wall is then infiltrated by pro-inflammatory cellular components, cholesterol crystals (45), and various lipoproteins contributing to atherosclerotic cardiovascular disease.
Lipoprotein Dysfunction
Systemic inflammation impacts lipoprotein composition and function (23). Patients with psoriasis have a high prevalence of dyslipidemia by traditional lipid panels independent of obesity (46). While it is evident that patients with psoriasis are at a greater risk of developing dyslipidemia, the relationship is most likely bi-directional (47). Dyslipidemia affects the distribution of lipid subsets and also impairs their functional components, augmenting the risk of atherosclerotic cardiovascular diseases (22). Increased apolipoprotein B:A1 ratios in psoriasis suggest a paucity of anti-atherogenic (apolipoprotein A1) and an increase in atherogenic species (apolipoprotein B) (22). Furthermore, oxidation modified lipoproteins (OMLs) are elevated in psoriasis. Because of reduced clearance (22) and increased reactive oxygen species, both oxidized LDL and oxidized HDL have been shown to be elevated in psoriasis and associated with non-calcified coronary burden (Figure 1, Panel III) (23). The implications of HDL impairment are tightly linked to a higher burden of inflammation (12), which ultimately drives cholesterol in tissues, thus accelerating atherosclerosis. Reduced HDL function in psoriasis relates directly to the burden of non-calcified coronary burden (48) and to psoriasis severity (45).
Adipose Dysfunction
Metabolic syndrome is increased in moderate-to-severe psoriasis independent of cardiometabolic disease risk factors (49). Obesity is highly prevalent in psoriasis and may be an indication of underlying inflammation and metabolic dysfunction (50) since psoriasis exhibits a dose response relationship between psoriasis severity and obesity. However traditional measures of obesity, such as body mass index (BMI) and waist-to-hip ratio, do not fully capture the increased cardiovascular risk associated with obesity. Patients with psoriasis have demonstrated increased adipose tissue by computed compartmental measures of subcutaneous adipose tissue and visceral adipose distribution (Figure 1, Panel IV) (51). Additionally, psoriasis is associated with a higher prevalence of diabetes as well as diabetes-related complications when compared to controls (52, 53). Furthermore, elevated visceral adipose volume in psoriasis positively associates with higher sub-clinical aortic vascular inflammation by FDG PET/CT (54) as well as non-calcified coronary burden (55). Finally, modulation of weight in those with BMI >40 kg/m2 undergoing weight loss by standard methods or gastric bypass procedure was associated with improvement in their psoriasis (56); however relationships between psoriasis and associated adiposity are still under study (57).
Treatment of Psoriasis
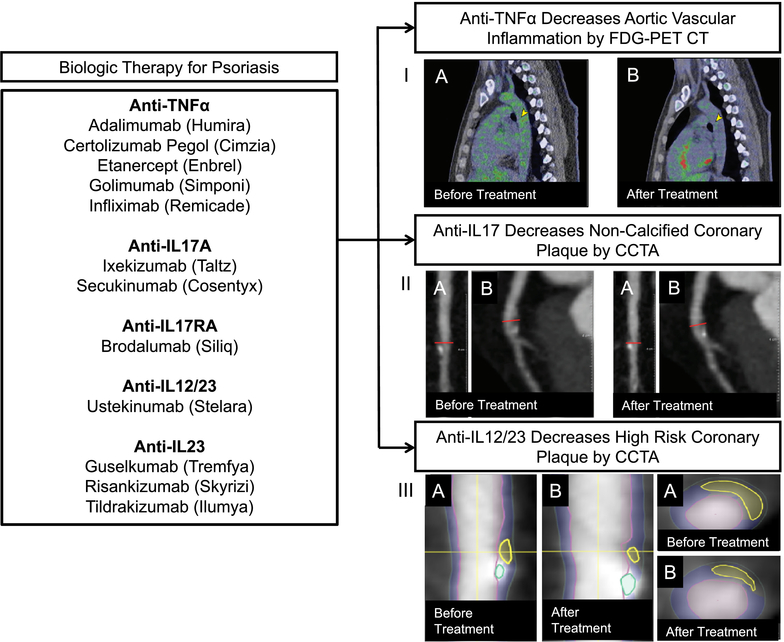
Pro-inflammatory cytokines and other immune pathways provide potential common links between the pathogenesis of psoriasis and atherosclerosis. Therefore, pro-inflammatory cytokines and immune pathways serve as potential targets in the treatment of psoriasis, as well as psoriasis-associated atherosclerosis. Although psoriasis lacks a definitive therapy, newer therapies targeting cytokines of interest are emerging (58). Mild psoriasis [body surface area (BSA) < 3%)] is usually treated with local therapies including ultraviolet phototherapy and topical treatments, which act on the surface of the skin. Systemic therapies targeting more specific immune pathways are reserved for moderate (3–10% BSA)-to-severe (>10% BSA) psoriasis (58). These include systemic agents (such as cyclosporine, methotrexate, and apremilast), and biologics (including anti-TNFα, anti-IL12/23s, anti-IL17s, and anti-IL23s) (58) (Figure 2).
Figure 2:
Anti-inflammatory biologic therapy for psoriasis reduces subclinical vascular disease. (I) The images show a sagittal section at the level of the mid-aorta at baseline (A) and 1 year of biologic psoriasis treatment (B). Green represents 18-fluorodeoxyglucose tracer uptake in the aorta, which is higher at baseline compared with 1 year later (yellow arrowheads). (II) Left anterior descending artery plaque identified before (left) and after (right) biologic therapy for psoriasis (Medis QAngio, Netherlands). (A) Longitudinal planar and (B) curved planar reformat showing reduction of coronary plaque after biologic treatment for psoriasis. (III, left) Longitudinal planar view with color overlap displaying reduction in high-risk coronary plaque characteristics (vascuCAP, Elucid Bioimaging, USA). Yellow horizontal line indicates the cross-sectional view in image; (A) is before and (B) is after biologic treatment. (III, right) Representative cross-sectional view with color-overlap displaying plaque characteristics (A) before and (B) after biologic therapy for psoriasis.
Treatment with cyclosporine has not been associated with reduction in CV events (59). In fact, intermediate to long-term use of cyclosporine has been associated with a number of adverse side effects, including renal dysfunction, hypertension, and hyperlipidemia (60). When compared to cyclosporine, methotrexate was associated with reduction in CRP and CV events in observational studies of psoriasis and rheumatoid arthritis (61, 62). These results were not observed in a recent randomized controlled trial CIRT (Cardiovascular Inflammation Reduction Trial) comparing the effects of methotrexate with placebo on prospective CV events in patients with established coronary artery disease. This trial was neutral compared to CANTOS. However, the baseline populations included in these trials were different in that CANTOS recruited based on hs-CRP and CIRT on the basis of metabolic syndrome. Median hs-CRP at baseline in CANTOS was 4.20 mg/L and in CIRT was 1.60 mg/L suggesting that the CIRT population was not as inflamed on the IL-6/hs-CRP axis as in CANTOS (63).
Anti-TNFα therapy has shown both positive and negative effects on cardiometabolic risk in psoriasis. A large observational study associated anti-TNFα therapy with a 50% reduction in myocardial infarction risk compared to risk with treatment with topical agents (64). Furthermore, patients receiving anti-TNFα therapy have shown a reduction in hs-CRP at 24-week follow-up (65). However, incidence of new-onset diabetes has been observed along with documented worsening of BMI and lipid profiles (66). Treating psoriasis with anti-TNFα therapy in an observational study reduced vascular inflammation by positron emission tomography by ~6–8%, similar to the effect of a low-dose statin on vascular inflammation (Figure 2, Panel I). Anti-TNFα therapy reduced intima media thickness by carotid Doppler ultrasound and the development of coronary artery disease by coronary artery calcium score in observational studies (38, 46, 67). Similarly, echocardiographic data demonstrated improvement of myocardial function in a small sample of psoriatic subjects treated with anti-TNFα therapy (68). Treatment with anti-TNFα therapy has been associated with stabilization of coronary burden over time and reduction in fibrofatty coronary burden by CCTA (69). Additionally, there was a decrease in peri-coronary inflammation, as assessed by perivascular fat attenuation index, associated with anti-TNFα therapy (70).
Randomized controlled trials (RCTs) of anti-TNFα therapy in patients with severe psoriasis have differed from observational evidence. In a large RCT, adalimumab, a TNFα inhibitor, did not reduce aortic vascular inflammation compared to placebo (71), and there was an increase in carotid vascular inflammation by 18F-FDG PET/CT after 52 weeks of treatment with adalimumab. Another study demonstrated anti-TNFα therapy might not have beneficial effects on aortic vascular inflammation when compared to placebo (72). The placebo group had improved vascular inflammation at 12 weeks, which may be because aortic vascular inflammation is subject to modulation by subtle lifestyle changes. Second, when compared to an observational study that demonstrated beneficial effects with anti-TNFα therapy (38), the study populations in the RCTs were younger with a paucity of CV risk factors (71, 72). Third, anti-TNFα therapy did not have a beneficial effect on vascular inflammation when compared to placebo in psoriasis, but there was a reduction in inflammatory CV disease markers such as GlycA and TNFα, suggesting perhaps longer duration and other vascular beds may be impacted (72). Moreover, vascular inflammation usually precedes the development of atherosclerotic plaque but is usually not high in areas of co-existing atherosclerotic plaque. Therefore, it is possible that vascular inflammation by PET does not capture similar effects observed in the coronary arteries, and ongoing studies over time are needed to better understand vascular inflammation by PET in the context of presence or absence of atherosclerotic plaque (21). Future studies evaluating major adverse cardiovascular events are needed to discern the effects of anti-TNFα therapy on vascular disease.
More recent biologics such as an IL12/23 inhibitor, ustekinumab, have been shown to have effects on coronary and myocardial function in psoriasis patients (73). Following treatment with IL-12/23, vascular inflammation was reduced at 12 weeks. These findings however did not persist at 52 weeks compared to placebo (70) but provide early evidence that selectively blocking the IL-23 pathway may be beneficial.
Additionally, anti-IL17 therapy carries great interest in psoriasis given the immense success it has on reducing psoriasis disease severity (74). Recent pre-clinical, murine studies in psoriasis have shown anti-IL17 therapy has positive effects on peripheral oxidative stress levels, pro-inflammatory cytokines, and vascular inflammation (75). However, in humans, anti-IL17 therapy was not associated with improvement in endothelial function at 1-year (76). Additionally, a randomized controlled trial of secukinumab, an anti-IL17 therapeutic agent, demonstrated no reduction in aortic vascular inflammation (data not published) when compared to placebo. By contrast, when coronary artery disease was evaluated, observational studies found that therapy with anti-IL12/23 and anti-IL17 was associated with a reduction of coronary artery disease indices (Figure 2, Panels II and III) when compared to non-biologic therapy over one year (69, 70). Whether inflammation is an independent risk factor in cardiovascular disease was answered in a recent, large, non-psoriasis RCT, the CANTOS trial. In this study, 10,061 patients with a history of MI and with a high inflammatory burden as measured by hs-CRP>2mg/L were treated with the IL1β inhibitor, canakinumab, for 48 months (9). There was a dose-dependent reduction in hs-CRP and a reduction in second myocardial event rate with canakinumab compared to placebo, suggesting modulation of residual inflammation leading to a reduction in cardiovascular diseases (9). Randomized control trials of biologic therapies are needed in psoriasis especially since observational studies have shown that treating inflammation is associated with improvement in coronary burden in psoriasis independent of traditional risk factors (69).
Conclusion
Chronic inflammatory diseases such as psoriasis are associated with increased and accelerated cardiometabolic events, potentially through elevated systemic inflammation, lipoprotein dysfunction, and metabolic dysregulation. Moreover, treatment of psoriasis, especially when severe, has been associated with favorable effects on cardiometabolic dysfunction. Continued follow-up and evaluation of treatment effects on this disease state will inform pathways important both in psoriasis and in the understanding of inflammatory induced cardiometabolic disease.
Footnotes
Declarations
- NNM is a full-time US government employee and has served as a consultant for Amgen, Eli Lilly, and Leo Pharma receiving grants/other payments; as a principal investigator and/or investigator for AbbVie, Celgene, Janssen Pharmaceuticals, Inc, and Novartis receiving grants and/or research funding and as a principal investigator for the National Institute of Health receiving grants and/or research funding.
- SSL is funded by the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and contributions to the Foundation for the NIH from the Doris Duke Charitable Foundation, the American Association for Dental Research, the Colgate-Palmolive Company, and other private donors.
- All other authors have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135(10):e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347(20):1557–65. [DOI] [PubMed] [Google Scholar]
- 3.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease. Circulation. 2019;0(0):CIR0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogdie A, Yu Y, Haynes K, Love TJ, Maliha S, Jiang Y, et al. Risk of major cardiovascular events in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a population-based cohort study. Ann Rheum Dis. 2015;74(2):326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sajja AP, Joshi AA, Teague HL, Dey AK, Mehta NN. Potential Immunological Links Between Psoriasis and Cardiovascular Disease. Front Immunol. 2018;9:1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boehncke WH. Systemic Inflammation and Cardiovascular Comorbidity in Psoriasis Patients: Causes and Consequences. Front Immunol. 2018;9:579.29675020 [Google Scholar]
- 7.Eder L, Dey A, Joshi AA, Boehncke WH, Mehta NN, Szentpetery A. Cardiovascular Diseases in Psoriasis and Psoriatic Arthritis. J Rheumatol Suppl. 2019;95:20–7. [DOI] [PubMed] [Google Scholar]
- 8.Menter A, Strober BE, Kaplan DH, Kivelevitch D, Prater EF, Stoff B, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80(4):1029–72. [DOI] [PubMed] [Google Scholar]
- 9.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377(12):1119–31. [DOI] [PubMed] [Google Scholar]
- 10.Knight JS, Luo W, O’Dell AA, Yalavarthi S, Zhao W, Subramanian V, et al. Peptidylarginine deiminase inhibition reduces vascular damage and modulates innate immune responses in murine models of atherosclerosis. Circ Res. 2014;114(6):947–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson PD, Mehta NN, Wolfe ML, Hinkle CC, Pruscino L, Comiskey LL, et al. Innate immunity modulates adipokines in humans. J Clin Endocrinol Metab. 2007;92(6):2272–9. [DOI] [PubMed] [Google Scholar]
- 12.de la Llera Moya M, McGillicuddy FC, Hinkle CC, Byrne M, Joshi MR, Nguyen V, et al. Inflammation modulates human HDL composition and function in vivo. Atherosclerosis. 2012;222(2):390–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehta NN, Heffron SP, Patel PN, Ferguson J, Shah RD, Hinkle CC, et al. A human model of inflammatory cardio-metabolic dysfunction; a double blind placebo-controlled crossover trial. J Transl Med. 2012;10:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehta NN, McGillicuddy FC, Anderson PD, Hinkle CC, Shah R, Pruscino L, et al. Experimental endotoxemia induces adipose inflammation and insulin resistance in humans. Diabetes. 2010;59(1):172–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garshick MS, Barrett TJ, Wechter T, Azarchi S, Scher JU, Neimann A, et al. Inflammasome Signaling and Impaired Vascular Health in Psoriasis. Arterioscler Thromb Vasc Biol. 2019;39(4):787–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naik HB, Natarajan B, Stansky E, Ahlman MA, Teague H, Salahuddin T, et al. Severity of Psoriasis Associates With Aortic Vascular Inflammation Detected by FDG PET/CT and Neutrophil Activation in a Prospective Observational Study. Arterioscler Thromb Vasc Biol. 2015;35(12):2667–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teague HL, Varghese NJ, Tsoi LC, Dey AK, Garshick MS, Silverman JI, et al. Neutrophil Subsets, Platelets, and Vascular Disease in Psoriasis. JACC Basic Transl Sci. 2019;4(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teague HL, Aksentijevich M, Stansky E, Silverman JI, Varghese NJ, Dey AK, et al. Cells of myeloid origin partly mediate the association between psoriasis severity and coronary plaque. J Invest Dermatol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ritchie SC, Wurtz P, Nath AP, Abraham G, Havulinna AS, Fearnley LG, et al. The Biomarker GlycA Is Associated with Chronic Inflammation and Predicts Long-Term Risk of Severe Infection. Cell Syst. 2015;1(4):293–301. [DOI] [PubMed] [Google Scholar]
- 20.Joshi AA, Lerman JB, Aberra TM, Afshar M, Teague HL, Rodante JA, et al. GlycA Is a Novel Biomarker of Inflammation and Subclinical Cardiovascular Disease in Psoriasis. Circ Res. 2016;119(11):1242–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernandez-Friera L, Fuster V, Lopez-Melgar B, Oliva B, Sanchez-Gonzalez J, Macias A, et al. Vascular Inflammation in Subclinical Atherosclerosis Detected by Hybrid PET/MRI. J Am Coll Cardiol. 2019;73(12):1371–82. [DOI] [PubMed] [Google Scholar]
- 22.Mehta NN, Li R, Krishnamoorthy P, Yu Y, Farver W, Rodrigues A, et al. Abnormal lipoprotein particles and cholesterol efflux capacity in patients with psoriasis. Atherosclerosis. 2012;224(1):218–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sorokin AV, Kotani K, Elnabawi YA, Dey AK, Sajja AP, Yamada S, et al. Association Between Oxidation-Modified Lipoproteins and Coronary Plaque in Psoriasis. Circ Res. 2018;123(11):1244–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Rooij SR, Nijpels G, Nilsson PM, Nolan JJ, Gabriel R, Bobbioni-Harsch E, et al. Low-grade chronic inflammation in the relationship between insulin sensitivity and cardiovascular disease (RISC) population: associations with insulin resistance and cardiometabolic risk profile. Diabetes Care. 2009;32(7):1295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khovidhunkit W, Kim MS, Memon RA, Shigenaga JK, Moser AH, Feingold KR, et al. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J Lipid Res. 2004;45(7):1169–96. [DOI] [PubMed] [Google Scholar]
- 26.Mehta NN, Li K, Szapary P, Krueger J, Brodmerkel C. Modulation of cardiometabolic pathways in skin and serum from patients with psoriasis. J Transl Med. 2013;11:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Armstrong AW, Robertson AD, Wu J, Schupp C, Lebwohl MG. Undertreatment, treatment trends, and treatment dissatisfaction among patients with psoriasis and psoriatic arthritis in the United States: findings from the National Psoriasis Foundation surveys, 2003–2011. JAMA dermatology. 2013;149(10):1180–5. [DOI] [PubMed] [Google Scholar]
- 28.Greb JE, Goldminz AM, Elder JT, Lebwohl MG, Gladman DD, Wu JJ, et al. Psoriasis. Nat Rev Dis Primers. 2016;2:16082. [DOI] [PubMed] [Google Scholar]
- 29.Brembilla NC, Senra L, Boehncke WH. The IL-17 Family of Cytokines in Psoriasis: IL-17A and Beyond. Front Immunol. 2018;9:1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim N, Thrash B, Menter A. Comorbidities in psoriasis patients. Semin Cutan Med Surg. 2010;29(1):10–5. [DOI] [PubMed] [Google Scholar]
- 31.Alexandroff AB, Pauriah M, Camp RD, Lang CC, Struthers AD, Armstrong DJ. More than skin deep: atherosclerosis as a systemic manifestation of psoriasis. The British journal of dermatology. 2009;161(1):1–7. [DOI] [PubMed] [Google Scholar]
- 32.Boehncke S, Salgo R, Garbaraviciene J, Beschmann H, Hardt K, Diehl S, et al. Effective continuous systemic therapy of severe plaque-type psoriasis is accompanied by amelioration of biomarkers of cardiovascular risk: results of a prospective longitudinal observational study. Journal of the European Academy of Dermatology and Venereology : JEADV. 2011;25(10):1187–93. [DOI] [PubMed] [Google Scholar]
- 33.Boehncke WH, Boehncke S, Tobin AM, Kirby B. The ‘psoriatic march’: a concept of how severe psoriasis may drive cardiovascular comorbidity. Experimental dermatology. 2011;20(4):303–7. [DOI] [PubMed] [Google Scholar]
- 34.Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. Jama. 2006;296(14):1735–41. [DOI] [PubMed] [Google Scholar]
- 35.Kimball AB, Guerin A, Latremouille-Viau D, Yu AP, Gupta S, Bao Y, et al. Coronary heart disease and stroke risk in patients with psoriasis: retrospective analysis. The American journal of medicine. 2010;123(4):350–7. [DOI] [PubMed] [Google Scholar]
- 36.Gelfand JM, Troxel AB, Lewis JD, Kurd SK, Shin DB, Wang X, et al. The risk of mortality in patients with psoriasis: results from a population-based study. Archives of dermatology. 2007;143(12):1493–9. [DOI] [PubMed] [Google Scholar]
- 37.Mehta NN, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2010;31(8):1000–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dey AK, Joshi AA, Chaturvedi A, Lerman JB, Aberra TM, Rodante JA, et al. Association Between Skin and Aortic Vascular Inflammation in Patients With Psoriasis: A Case-Cohort Study Using Positron Emission Tomography/Computed Tomography. JAMA Cardiol. 2017;2(9):1013–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lerman JB, Joshi AA, Chaturvedi A, Aberra TM, Dey AK, Rodante JA, et al. Coronary Plaque Characterization in Psoriasis Reveals High-Risk Features That Improve After Treatment in a Prospective Observational Study. Circulation. 2017;136(3):263–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mansouri B, Kivelevitch D, Natarajan B, Joshi AA, Ryan C, Benjegerdes K, et al. Comparison of Coronary Artery Calcium Scores Between Patients With Psoriasis and Type 2 Diabetes. JAMA dermatology. 2016;152(11):1244–53. [DOI] [PubMed] [Google Scholar]
- 41.Eder L, Jayakar J, Shanmugarajah S, Thavaneswaran A, Pereira D, Chandran V, et al. The burden of carotid artery plaques is higher in patients with psoriatic arthritis compared with those with psoriasis alone. Ann Rheum Dis. 2013;72(5):715–20. [DOI] [PubMed] [Google Scholar]
- 42.Cohen AD, Sherf M, Vidavsky L, Vardy DA, Shapiro J, Meyerovitch J. Association between psoriasis and the metabolic syndrome. A cross-sectional study. Dermatology. 2008;216(2):152–5. [DOI] [PubMed] [Google Scholar]
- 43.Krueger JG. The immunologic basis for the treatment of psoriasis with new biologic agents. J Am Acad Dermatol. 2002;46(1):1–23; quiz −6. [DOI] [PubMed] [Google Scholar]
- 44.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135–43. [DOI] [PubMed] [Google Scholar]
- 45.Baumer Y, Ng Q, Sanda GE, Dey AK, Teague HL, Sorokin AV, et al. Chronic skin inflammation accelerates macrophage cholesterol crystal formation and atherosclerosis. JCI Insight. 2018;3(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jokai H, Szakonyi J, Kontar O, Marschalko M, Szalai K, Karpati S, et al. Impact of effective tumor necrosis factor-alfa inhibitor treatment on arterial intima-media thickness in psoriasis: results of a pilot study. J Am Acad Dermatol. 2013;69(4):523–9. [DOI] [PubMed] [Google Scholar]
- 47.Wu S, Li WQ, Han J, Sun Q, Qureshi AA. Hypercholesterolemia and risk of incident psoriasis and psoriatic arthritis in US women. Arthritis & rheumatology (Hoboken, NJ). 2014;66(2):304–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salahuddin T, Natarajan B, Playford MP, Joshi AA, Teague H, Masmoudi Y, et al. Cholesterol efflux capacity in humans with psoriasis is inversely related to non-calcified burden of coronary atherosclerosis. European Heart Journal. 2015;36(39):2662–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Langan SM, Seminara NM, Shin DB, Troxel AB, Kimmel SE, Mehta NN, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132(3 Pt 1):556–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and obesity: a systematic review and meta-analysis of observational studies. Nutrition & diabetes. 2012;2:e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rose S, Stansky E, Dagur PK, Samsel L, Weiner E, Jahanshad A, et al. Characterization of immune cells in psoriatic adipose tissue. J Transl Med. 2014;12:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Armstrong AW, Guerin A, Sundaram M, Wu EQ, Faust ES, Ionescu-Ittu R, et al. Psoriasis and risk of diabetes-associated microvascular and macrovascular complications. J Am Acad Dermatol. 2015;72(6):968–77 e2. [DOI] [PubMed] [Google Scholar]
- 53.Gyldenlove M, Storgaard H, Holst JJ, Vilsboll T, Knop FK, Skov L. Patients with psoriasis are insulin resistant. J Am Acad Dermatol. 2015;72(4):599–605. [DOI] [PubMed] [Google Scholar]
- 54.Rivers JP, Powell-Wiley TM, Dey AK, Rodante JA, Chung JH, Joshi AA, et al. Visceral Adiposity in Psoriasis is Associated With Vascular Inflammation by (18)F-Fluorodeoxyglucose Positron-Emission Tomography/Computed Tomography Beyond Cardiometabolic Disease Risk Factors in an Observational Cohort Study. JACC Cardiovasc Imaging. 2018;11(2 Pt 2):349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rivers Joshua P, Dey Amit K, Chung Jonathan H, Rana A, Chaturvedi A, Rodante Justin A, et al. Abstract 549: Visceral Adipose Tissue Associates With Coronary Plaque Burden Beyond Cardiovascular Risk Factors in Psoriasis. Arteriosclerosis, thrombosis, and vascular biology. 2017;37(suppl_1):A549–A. [Google Scholar]
- 56.Egeberg A, Sorensen JA, Gislason GH, Knop FK, Skov L. Incidence and Prognosis of Psoriasis and Psoriatic Arthritis in Patients Undergoing Bariatric Surgery. JAMA surgery. 2017;152(4):344–9. [DOI] [PubMed] [Google Scholar]
- 57.Kunz M, Simon JC, Saalbach A. Psoriasis: Obesity and Fatty Acids. Front Immunol. 2019;10:1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schon MP, Boehncke WH. Psoriasis. N Engl J Med. 2005;352(18):1899–912. [DOI] [PubMed] [Google Scholar]
- 59.Ahlehoff O, Skov L, Gislason G, Gniadecki R, Iversen L, Bryld LE, et al. Cardiovascular outcomes and systemic anti-inflammatory drugs in patients with severe psoriasis: 5-year follow-up of a Danish nationwide cohort. Journal of the European Academy of Dermatology and Venereology : JEADV. 2015;29(6):1128–34. [DOI] [PubMed] [Google Scholar]
- 60.Christophers E, Mrowietz U, Henneicke H, Farber L, Welzel D, Participantsinthegermanmultic. Cyclosporine in psoriasis: A multicenter dose-finding study in severe plaque psoriasis. Journal of the American Academy of Dermatology. 1992;26(1):86–90. [DOI] [PubMed] [Google Scholar]
- 61.Prodanovich S, Ma F, Taylor JR, Pezon C, Fasihi T, Kirsner RS. Methotrexate reduces incidence of vascular diseases in veterans with psoriasis or rheumatoid arthritis. J Am Acad Dermatol. 2005;52(2):262–7. [DOI] [PubMed] [Google Scholar]
- 62.Flytstrom I, Stenberg B, Svensson A, Bergbrant IM. Methotrexate vs. ciclosporin in psoriasis: effectiveness, quality of life and safety. A randomized controlled trial. The British journal of dermatology. 2008;158(1):116–21. [DOI] [PubMed] [Google Scholar]
- 63.Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, et al. Low-Dose Methotrexate for the Prevention of Atherosclerotic Events. New England Journal of Medicine. 2018;380(8):752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu JJ, Poon KY, Channual JC, Shen AY. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Archives of dermatology. 2012;148(11):1244–50. [DOI] [PubMed] [Google Scholar]
- 65.Strober B, Teller C, Yamauchi P, Miller JL, Hooper M, Yang YC, et al. Effects of etanercept on C-reactive protein levels in psoriasis and psoriatic arthritis. The British journal of dermatology. 2008;159(2):322–30. [DOI] [PubMed] [Google Scholar]
- 66.Solomon DH, Massarotti E, Garg R, Liu J, Canning C, Schneeweiss S. Association between disease-modifying antirheumatic drugs and diabetes risk in patients with rheumatoid arthritis and psoriasis. Jama. 2011;305(24):2525–31. [DOI] [PubMed] [Google Scholar]
- 67.Hjuler KF, Bottcher M, Vestergaard C, Botker HE, Iversen L, Kragballe K. Association Between Changes in Coronary Artery Disease Progression and Treatment With Biologic Agents for Severe Psoriasis. JAMA dermatology. 2016;152(10):1114–21. [DOI] [PubMed] [Google Scholar]
- 68.Ahlehoff O, Hansen PR, Gislason GH, Frydland M, Bryld LE, Elming H, et al. Myocardial function and effects of biologic therapy in patients with severe psoriasis: a prospective echocardiographic study. Journal of the European Academy of Dermatology and Venereology : JEADV. 2016;30(5):819–23. [DOI] [PubMed] [Google Scholar]
- 69.Elnabawi YA, Dey AK, Goyal A, Groenendyk JW, Chung JH, Belur AD, et al. Coronary artery plaque characteristics and treatment with biologic therapy in severe psoriasis: results from a prospective observational study. Cardiovascular Research. 2019;115(4):721–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Elnabawi YA, Oikonomou EK, Dey AK, Mancio J, Rodante JA, Aksentijevich M, et al. Association of Biologic Therapy With Coronary Inflammation in Patients With Psoriasis as Assessed by Perivascular Fat Attenuation IndexAssociation of Biologic Therapy With Coronary Inflammation in PsoriasisAssociation of Biologic Therapy With Coronary Inflammation in Psoriasis. JAMA Cardiology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bissonnette R, Harel F, Krueger JG, Guertin MC, Chabot-Blanchet M, Gonzalez J, et al. TNF-alpha Antagonist and Vascular Inflammation in Patients with Psoriasis Vulgaris: A Randomized Placebo-Controlled Study. J Invest Dermatol. 2017;137(8):1638–45. [DOI] [PubMed] [Google Scholar]
- 72.Mehta NN, Shin DB, Joshi AA, Dey AK, Armstrong AW, Duffin KC, et al. Effect of 2 Psoriasis Treatments on Vascular Inflammation and Novel Inflammatory Cardiovascular Biomarkers: A Randomized Placebo-Controlled Trial. Circ Cardiovasc Imaging. 2018;11(6):e007394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ikonomidis I, Papadavid E, Makavos G, Andreadou I, Varoudi M, Gravanis K, et al. Lowering Interleukin-12 Activity Improves Myocardial and Vascular Function Compared With Tumor Necrosis Factor-a Antagonism or Cyclosporine in Psoriasis. Circ Cardiovasc Imaging. 2017;10(9):e006283. [DOI] [PubMed] [Google Scholar]
- 74.Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CEM, Papp K, et al. Secukinumab in Plaque Psoriasis — Results of Two Phase 3 Trials. New England Journal of Medicine. 2014;371(4):326–38. [DOI] [PubMed] [Google Scholar]
- 75.Schuler R, Brand A, Klebow S, Wild J, Veras FP, Ullmann E, et al. Antagonization of IL-17A Attenuates Skin Inflammation and Vascular Dysfunction in Mouse Models of Psoriasis. J Invest Dermatol. 2019;139(3):638–47. [DOI] [PubMed] [Google Scholar]
- 76.von Stebut E, Reich K, Thaci D, Koenig W, Pinter A, Korber A, et al. Impact of Secukinumab on Endothelial Dysfunction and Other Cardiovascular Disease Parameters in Psoriasis Patients over 52 Weeks. J Invest Dermatol. 2019;139(5):1054–62. [DOI] [PubMed] [Google Scholar]