Abstract
Stunting has been negatively associated with children’s development. We examined the range of height by testing hypotheses: 1) height is positively associated with children’s development, with associations moderated by inflammation and 2) home environments characterized by nurturance and early learning opportunities are positively associated with children’s development over time and attenuate associations with height. Data included 513 infants (mean age 8.6 months) and 316 preschoolers (mean age 36.6 months) in rural India from a randomized controlled trial of multiple micronutrient powders. Measures included height (height-for-age z-scores based on WHO standards), inflammation (C-reactive protein concentration >5 mg/L), nurturance (HOME Inventory), child development (Mullens Scales of Early Learning), and inhibitory control (preschoolers). Linear mixed effects models accounting for repeated measures, clustering, and confounders were used to assess associations between height and child development over time (infants: enrollment, 6- and 12-months; preschoolers: enrollment and 8-months). Moderating effects of inflammation and nurturance were tested with interaction terms. Among infants and preschoolers, height and nurturance were positively associated with all domains of child development over time, with the exception of inhibitory control. Among preschoolers, in the presence of inflammation, height was not associated with child development. Among infants, but not preschoolers, a nurturant home environment attenuated significant associations between height with fine motor and receptive language development. The mechanisms associated with children’s development over time are multi-factorial and include direct and indirect associations among nutrition, health, and the home environment, as supported by the Nurturing Care Framework.
Keywords: Stunting, Height, Inflammation, Infant development, Preschool development, HOME, Child development, Global, 1000 days
The first 1000 days, from conception to age 24 months, are a foundational period for early growth and development with life-span extensions to adult health and well-being (Hanson & Gluckman, 2014; Victora et al., 2008). Although less attention has been focused on the second 1000 days (age 2–5 years), early brain development continues at a rapid rate and experiences during this period influence children’s life-course developmental trajectories through adulthood (Black, Perez-Escamilla, & Fernandez Rao, 2015). Early physiological processes related to maternal and child health and nutrition form the origins of children’s development and of non-communicable diseases (Hanson & Gluckman, 2014), giving rise to the recommendation that investigations of children’s early development consider children’s health and nutrition (Shonkoff, 2010). The integration of health into children’s development is consistent with the bioecological theory of human development, which establishes the conceptual framework for the time-dependent interactions influenced by genetics and environmental conditions that underlie children’s development (Bronfenbrenner & Morris, 2006). Nutritional deficiencies and inflammatory processes have been associated with deficits to children’s growth and health, and potentially to their development (Harper, Mutasa, Prendergast, Humphrey, & Manges, 2018). At the same time, there is strong evidence that maternal nurturance, characterized as a home environment with responsive parenting and opportunities for early learning, can promote children’s early development through direct processes (Black et al., 2017; Britto et al., 2017). Based on bioecological theory, nurturance may also operate through indirect processes by attenuating the effects of threats, such as nutritional deficiencies.
Nutritional Deficiencies
Nutritional deficiencies along with stress and other adversities during early life can create disparities that disrupt children’s growth and developmental trajectories, compromising their future health, educational attainment, psychosocial well-being, and economic productivity (Black, et al., 2017). Stunting (length-for-age measured at 2 standard deviations below the World Health Organization (WHO)-established median) prior to age 24 months has been associated with poor school performance (Sudfeld et al., 2015), reduced economic income during adulthood (Hoddinott, Alderman, Behrman, Haddad, & Horton, 2013), and both low birth weight and poor cognitive performance in the subsequent generation (Victora, de Onis, Hallal, Blossner, & Shrimpton, 2010; Walker, Chang, Wright, Osmond, & Grantham-McGregor, 2015). Although the association between stunting and child development has been demonstrated across multiple sites and study designs (Machin, Day, & Green, 2007; Miller, Murray, Thomson, & Arbour, 2016; Sudfeld, et al., 2015), there is no consensus on the mechanisms linking stunting and children’s development (Perkins et al., 2017). Possible mechanisms include neurological, hormonal, infection, stress, and functional isolation, as well as combinations or interactions among mechanisms. The lack of consensus regarding mechanisms is further complicated by the diversity of child development measures and metrics, variability in the samples and in the timing of stunting, and inconsistent inclusion of confounders. However, based on the consistency of the associations across multiple countries, stunting prior to age 24 months is accepted globally as an indicator of risk to children’s well-being (de Onis & Branca, 2016). Using stunting and poverty as proxies, estimates from 141 low and middle-income countries (LMIC) indicate that 43% of the world’s children under age 5 years (249 million children) are at risk for not reaching their developmental potential (Black, et al., 2017; Lu, Black, & Richter, 2016). The economic consequences from lost developmental potential are estimated at approximately a quarter of average adult income per year (Richter et al., 2017). These negative consequences can undermine the well-being not only of individuals, but of entire societies, and have made the prevention of stunting and the promotion of children’s development urgent targets among multiple international agencies (Chan, Lake, & Hansen, 2017).
Inflammation
Recent evidence, including a special section in Pediatrics (Kutlesic, Brewinski Isaacs, Freund, Hazra, & Raiten, 2017), has drawn attention to gaps in the intersection of nutrition, inflammation, and neurodevelopment, particularly in low- and middle-income countries (LMIC). Chronic inflammation resulting from infectious and noninfectious causes during infancy may impact early neurological development (Krebs, Lozoff, & Georgieff, 2017). Inflammation is part of the healing process in response to pathogens that may result from multiple sources, including stress and lack of hygienic conditions. An observational study in an urban slum in Bangladesh found that biomarkers of inflammation (C-reactive protein (CRP) and soluble CD14) were negatively related to children’s performance on the Bayley Scales of Infant and Toddler Development (BSID) prior to 24 months of age (Jiang et al., 2017). Unhygienic conditions, such as poor sanitation with fecal-oral bacteria exposure, may lead to environmental enteropathy (EE), a subclinical, asymptomatic abnormality of the small intestine, characterized by villous atrophy, malabsorption, and inflammation (Humphrey, 2009). EE is not well understood or easily measured, but may compromise nutritional status and interfere with nutritional absorption (Korpe & Petri Jr, 2012). The gut becomes hyperpermeable, interfering with nutrient absorption, facilitating the translocation of microbes, and triggering metabolic changes of the immune response (Prendergast & Kelly, 2012). Thus, EE may increase the risk for both stunting and poor child development (Humphrey, 2009; Ngure et al., 2014). Chronic inflammation related to EE has been associated with stunting among infants in Zimbabwe (Prendergast et al., 2014) and a recent study in Tanzania found that biomarkers of gastrointestinal function (citrulline, antibodies to lipopolysaccharide, and flagellin) were negatively associated with children’s cognitive development at 15 months, but a biomarker of chronic inflammation (CRP) was not (Etheredge et al., 2018). Subclinical enteropathogens measured from non-diarrhea stools have been associated with poor cognitive development in a multi-site study among children in LMIC at 24 months, (Murray-Kolb & MAL-ED Network Investigators, 2018). In the MAL-ED study, the association between enteropathogens and poor cognitive development was mediated through anemia, illustrating the potential interplay between inflammation and nutrition. Several studies have found associations between diarrhea and child development (Guerrant et al., 1999; Lorntz et al., 2006; Niehaus et al., 2002; Tarleton et al., 2006). However, a re-analysis of these studies with length-for-age in the model found no direct associations between diarrhea and cognition, (Fischer-Walker et al., 2012), illustrating the complex associations among inflammation, nutritional status, and child development, and the need to examine the mechanisms among infants and preschoolers.
Maternal nurturance
Maternal nurturance, operationalized as parent-child interactions that are responsive, emotionally supportive, and developmentally stimulating and appropriate (Black & Aboud, 2011), promotes children’s early development (Black et al., 2016). Neuroscientific evidence has shown that maternal nurturance during early childhood provides protection from the detrimental effects of low socioeconomic status on early brain development (Luby, 2015). Maternal nurturance is facilitated by maternal education, which has been positively related to children’s health and development in LMIC (Walker, Chang, Vera-Hernandez, & Grantham-McGregor, 2011). Higher education enables mothers to manage household resources and provide the protection, nurturance, and early learning opportunities that children need for healthy growth and development (Bornstein & Putnick, 2012).
Multiple studies in LMIC have shown positive associations between the quality of the home environment, often measured by local adaptations to the HOME inventory, a measure of maternal responsiveness (Bradley et al., 1989), and children’s development (Britto, et al., 2017). Parenting interventions can positively impact the quality of responsive parenting, measured by the HOME, raising the possibility that children’s development is mediated through the home environment (Aboud & Yousafzai, 2015; Britto, et al., 2017), a suggestion consistent with bioecological theory. The home environment has been shown to mediate the effects of poverty on children’s development in LMIC (Hamadani, Nahar, Huda, & Tofail, 2014; Rubio-Codina, Attanasio, & Grantham-McGregor, 2016), and to mediate the impact of a parenting intervention on children’s development in Pakistan (Obradović, Yousafzai, Finch, & Rasheed, 2016). We identified only one study that examined whether the home environment can offset the negative associations between stunting and child development. That study, from Vietnam, found that a high-quality home environment protected 24-month-old children from a negative association between stunting and child development (Nguyen et al., 2017). We did not locate any studies that examined whether the home environment can offset children’s development related to stunting over time, thereby measuring development at multiple time points and adjusting for associated factors, and focusing exclusively on health, nutrition, and home environment.
Study Location
The current study was conducted in India, selected because India has the second largest population of children under age 6 in the world, including over 117,586 children in the rural areas and 41,204 children in the urban areas, and a prevalence of stunting estimated at 38% among children under age 5 years (National Family Health Survey-4, 2015–16). Water, sanitation and hygiene are major public health concerns in India, in spite of recent economic growth. Based on a 2017 UNICEF report, up to 44% of the population in India practice open defecation (UNICEF, 2018), a practice that has been associated with stunting (Spears, Ghosh, & Cumming, 2013). The prevalence of households with improved sanitation facilities1 is estimated at 48% overall and 37% in rural areas (National Family Health Survey-4, 2015–16). Thus, rural India is an ideal site to examine how stunting, inflammation, and the home environment relate to children’s development over time.
This longitudinal investigation examines how height, inflammation, and the home environment relate to child development scores over time among infants and preschoolers in rural India. The study tests two hypotheses: 1) height is positively associated with children’s development over time, moderated (worsened) by inflammation and 2) a home environment characterized by nurturance and early learning opportunities is positively associated with children’s development over time and attenuates the associations with height. These hypotheses enhance our understanding of the complex roles that height, inflammation, and nurturance play related to child development over time among infants and preschoolers in LMIC.
Methods
This is a secondary data analysis of a randomized controlled trial designed to assess the impact of micronutrient powders (MNP) and early childhood development interventions on child development, growth, anemia, and micronutrient status in rural India (Project Grow Smart) (Fernandez-Rao et al., 2014).
Setting
The study, Project Grow Smart, was conducted in 26 villages in the Nalgonda district in the state of Telangana in India. Telengana, the newest state in India, was separated from Andhra Pradesh in 2014. In the rural districts where the study was conducted, approximately 85% of the residents are Hindu and 13% are Muslim, 52% of the women age 15–49 are literate, most households have electricity (97%), but only 39% have improved sanitary facilities (National Family Health Survey-4, 2015–16). Among children under age 5 years, rates of stunting are 33%, underweight 47%, and wasting 53% (Challa & Challa, 2015). The study site was selected because the prevalence of anemia among preschoolers was above 70% (International Institute for Population Sciences and Macro International, 2007; National Nutrition Monitoring Bureau, 2003) with inadequate intake of several micronutrients among infants and toddlers (Vazir et al., 2013). In addition, the Integrated Child Development Services (ICDS), the government program in India that provides food, preschool education, and primary healthcare to children under 6 years of age and their mothers, was not distributing either micronutrient supplementation or fortified foods in this district. Ethical approval was obtained from the Institutional Ethical Committee of the National Institute of Nutrition (NIN), Hyderabad, India and the Institutional Review Board of the University of Maryland School of Medicine, Baltimore, Maryland. Permission from village leaders and the Department of Women’s Development and Child Welfare was obtained before study initiation. Household demographic surveys were conducted in 26 villages located within one hour of the National Institute of Nutrition to determine the eligibility of children and government-sponsored preschools (Anganwadi Centers). Each participating village had a field site that was used for evaluations.
Participants and procedure
Eligibility included age 6–14 months for infants and 29–49 months for preschoolers, and parents who intended to remain in the area for the following year. Exclusionary criteria for both infants and preschoolers included chronic morbidities, developmental delays, or physical disabilities. Upon maternal consent, children were enrolled in the study. Children with severe anemia (hemoglobin <7 g/dL) were referred to a local hospital and excluded from the study. The study design of the infant and preschool components differed slightly; a detailed description has been provided elsewhere (Fernandez-Rao, et al., 2014).
Infants were identified from the village demographic surveys conducted in all 26 villages. Of the 551 infants aged 6–14 months assessed for eligibility, 29 were ineligible and nine refused to participate leaving a sample of 513 infants.
Preschoolers were recruited from participating preschools. Preschools had to have an enrollment of at least 15 children per preschool and agree to add point-of-use fortification (MNP or placebo) to the midday meal. Of the 46 government-sponsored preschools located within the study area, 10 did not meet enrollment criteria and 22 were selected using a computer-generated randomization procedure. All invited preschools agreed to participate. We recruited preschoolers from the 22 government-sponsored preschools enrolled in the project. Of the 336 preschoolers who were assessed for eligibility, 14 were ineligible and one refused to participate leaving 321 preschoolers.
Interventions
Infants were randomized, using a computer-generated randomization procedure, into 2 by 2 design (MNP vs. placebo powders and early learning intervention vs. control), with a mid-line follow-up at six months and end-line at twelve months. All groups received nutrition education. Preschoolers were randomized, using a computer-generated randomization procedure, into MNP or placebo powders groups nested within high- and low- preschool quality groups (as described in the ‘Preschool quality’ section below); they were followed with end-line at eight months, to align with the academic year. Both trials yielded significant reductions in rates of anemia; small, but significant improvements in certain domains of child development; and no significant effects on weight or height.
Micronutrient/placebo powders
MNP were formulated based on recommendations from the WHO (World Health Organization, 2012) and the Indian Council of Medical Research (Indian Council of Medical Research, 2010). The formulation for MNP included iron, folic acid, zinc, and vitamins A, C, B12, and B2 and formulation for the placebo included only vitamin B2 (Fernandez-Rao, et al., 2014).
For the infant component, individual sachets of 0.5 g of MNP or placebo powders were delivered to mothers every other week. Mothers were trained to mix MNP into the first bites of the infant’s food on a daily basis (1 sachet per day). For the preschool component, bulk packages of 200g were provided to government-sponsored preschools. The preschool staff were trained to mix the MNP or placebo powders into the midday meal.
Early learning
The infant early learning intervention was delivered every other week, along with the sachets. The intervention promoted responsive parenting by including age-appropriate play and communication activities for parents and infants, using a flip chart, demonstrations, modeling, and coaching. Materials used were adapted from the WHO’s Care for Child Development Module (World Health Organization, 2012) and the Pakistan Early Development Study (Yousafzai, Rasheed, Rizvi, Armstrong, & Bhutta, 2014). The intervention was delivered by study staff (trained local mothers) in participants’ homes. Participants in the control condition did not receive an intervention. There was no early learning intervention in the preschool component.
Preschool quality
Government-sponsored preschools were classified based on the quality of early learning opportunities and interactions between teachers and students. The Early Childhood Environment Rating Scale-Revised (ECERS-R) (Harmes, Clifford, & Cryer, 1998) was used as a template and modified for the government-sponsored preschools. Items from the HOME inventory (Bradley, et al., 2003) were incorporated into the new tool. The final version of the modified tool yielded a 109-item observation scale (Fernandez-Rao, et al., 2014). A four-hour assessment was conducted in each preschool by two evaluators, who completed independent observations. The inter-rater reliability, based on intra-class correlation (ICC) exceeded 0.92. A median-split across schools was used to classify preschools into high vs. low quality. The nested design ensured that equal numbers of high- and low- quality preschools were in the MNP and placebo powders groups.
Measures
Child development
The Mullen Scales of Early Learning (MSEL) were used to evaluate child development (Mullen, 1995). MSEL are appropriate for children from birth to age 68 months. The tool has excellent psychometric properties and has been used in LMIC (Koura et al., 2013; Miller, Chan, Comfort, & Tirella, 2005). Among infants, MSEL yields scores in five domains including gross motor, visual reception, fine motor, expressive language, and receptive language. Among preschoolers, MSEL measures four domains: visual reception, fine motor, receptive and expressive language. Gross motor refers to large muscle skills such as sitting, standing and walking; fine motor refers to touching and using hands; visual reception refers to recognizing similarities and differences; receptive language refers to understanding; and expressive language refers to making sounds and speaking. In the absence of domain-specific evidence associated with stunting, we examined all five domains among infants and four domains among preschoolers. Raw scores were converted into age-adjusted t scores (mean = 50, SD = 10) based on the MSEL standardization sample since there were no standardized data for India.
Among preschoolers, inhibitory control was added to the child development battery evaluated to test children’s ability to follow instructions and inhibit responses related to size and color, based on a protocol developed by Dowsett and Livesey (2000). Test materials included shapes (square and circle) that varied in size (small and large) and color (white and black). Trials of sorting and selecting were presented in a game-like format. Success and failure scores were recorded and summed. Raw scores, unadjusted by age, were used in the analysis.
The evaluation team included 5 local psychologists, supervised by a doctoral level psychologist. All child development measures were modified by the psychology team by adding local pictures and translating instructions to Telugu (Fernandez-Rao, et al., 2014). The validity of the tools was tested with local children. A pilot test was conducted to ensure that tools were appropriate for the study population and evaluators were reliable in administration and scoring. Training was reviewed prior to each round of testing. Inter-rater reliability based on ICC exceeded 0.90. All measures were assessed at enrollment, 6-month and 12-month follow-up in the infant component and at enrollment and 8-month follow-up in the preschool component.
Child hemoglobin
At enrollment, two mL of non-fasting blood was collected from each child’s ante-cubital vein into a heparinized vacutainer. Blood was transported to the National Institute of Nutrition in thermally insulated boxes containing ice. Plasma was separated and stored in aliquots at −20°C. The same day, a commercial kit (HemoCor-D, Coral Systems; Tulip Group, Alto Santacruz, India) was used to perform hemoglobin analysis in whole blood. The laboratory and its personnel were under external quality assurance for micronutrient estimations (VITAL-EQA program and Centers for Disease Control and Prevention, Atlanta, Georgia, USA). Anemia was defined as hemoglobin <11g/dL based on WHO guidelines1.
Child anthropometry
Anthropometry was measured at enrollment by two trained anthropometrists, following a standardized protocol. Child weight was measured at enrollment using a calibrated digital scale to 0.1kg (Seca, Birmingham, UK). Weight represents a measure of acute undernutrition, whereas height represents a measure of chronic undernutrition. Therefore, weight was used to describe the sample, but not included in the analyses.
Among infants, length was measured using an infantometer to measure crown-to-heel length to the nearest 0.1 cm. Among preschoolers, height was measured with a portable stadiometer to the nearest 0.1 cm (both from Galaxy Scientifics, India). Length and height were measured two times and repeated until both measurements agreed within 0.2 cm.
Using the average of the two closest measurements, length-for-age z-scores (LAZ) and height-for-age z-scores (HAZ) were calculated using WHO standards (World Health Organization, 2007). Weight-for-age, weight-for-height, and height-for-age, were converted to z-scores (WAZ, WHZ, HAZ, respectively) using WHO standards (Furlong et al., 2016). Underweight, wasting, and stunting were defined as <−2 WAZ, WHZ, and HAZ, respectively. Throughout this report, the terms height and HAZ refer to length and LAZ among infants and height and HAZ among preschoolers. It is likely that chronic undernutrition impacts the entire height-for-age distribution (Perumal, Bassani, & Roth, 2018). Because limiting analyses to stunted vs. not stunted may mask the effects of changes in HAZ that do not reach the defined threshold, we used continuous measures of HAZ in the analyses.
Child Inflammation
C-reactive protein (CRP) from the blood draw at enrollment was used as an indicator of systemic inflammation. CRP is an acute-phase protein produced by the liver in response to inflammation. CRP levels rise rapidly in response to infectious or inflammatory disease states. CRP from whole blood was analyzed using a human CRP assay kit (Alpha Diagnostic International, San Antonio, TX, USA) with a minimum detectable limit of 10 ng/mL and assay range of 100–10 000 ng/mL. CRP higher than 5 mg/L is indicative of current inflammation (World Health Organization, 2014). Prior investigations have been inconsistent in using either continuous CRP or categorical CRP > 5 mg/L (Jiang, et al., 2017 Etheredge, et al., 2018). We used continuous CRP and graphed significant interactions with CRP > 5 mg/L vs CRP<=5 mg/L for interpretation.
Household asset index
A 27-item questionnaire developed for India and recommended in LMIC (Filmer & Pritchett, 2001) was used to assess economic resources based on family ownership of household assets. Assets included type of house, water source, electrification, furniture, telephone/mobile, etc. Scores were weighted by relative significance of asset owned and ranged from −0.2 to 8.3. High scores indicate more economic resources.
Maternal education
Demographic data, including maternal education, were collected at enrollment. Mothers reported the number of years of schooling. Maternal education was classified as low literacy (primary education or below) and high literacy (beyond primary education).
Maternal nurturance in the home environment
The Home Observation for the Measurement of the Environment (HOME) inventory was used to assess maternal nurturance at enrollment. The HOME inventory measures the quality of early learning opportunities in the home environment (Bradley, Caldwell, & Corwyn, 2003). For the infant, the Infant-Toddler version was used; it includes 45 Yes/No questions and six scales (Emotional and Verbal Responsivity, Avoidance of Restriction and Punishment, Organization of Environment, Provision of Appropriate Play Material, Maternal Involvement with Child, and Opportunities for Variety in Daily Stimulation). For preschoolers, a modified Early Childhood version adapted for India (Mohite, 1992; Black et al., 2007; Jones et al., 2017) was used; it includes 55 items and eight scales (Learning Materials, Language Stimulation, Physical Environment, Responsivity, Academic Stimulation, Modeling, Variety, and Acceptance). These tools were revised by altering and deleting items that were not culturally appropriate (Fernandez-Rao, et al., 2014). Instructions were translated to Telugu and pilot tested among local children. Scores were summed with high scores indicating higher quality of nurturance in the home environment. Inter-rater reliability based on ICC exceeded 0.90.
Statistical analysis
Descriptive analyses on selected sample characteristics were conducted (Table 1). We examined changes over time in the standard MSEL domain scores and inhibitory control by conducting repeated measures ANOVA, followed by paired comparisons from enrollment to 6-month and 6-month to 12-month follow-up for infants and enrollment to 8-month follow-up for preschoolers, adjusted for covariates and using Bonferroni correction (Tables 2a and 2b, Figure 1).
Table 1.
Sample characteristics at enrollment
| Infants (n=513) | Preschoolers (n=321) | |
|---|---|---|
| Child | ||
| Female sex, n (%) | 241 (47.0) | 166 (51.7) |
| Age (months), mean (SD) | 8.6 (2.2) | 36.6 (5.7) |
| Stunted, n (%) | 100 (19.5) | 130 (40.6) |
| Height-for-age z score, mean (SD) | −1.1 (1.1) | −1.8 (1.0) |
| Anemic, n (%) | 316 (66.4) | 151 (47.8) |
| Underweight, n (%) | 97 (18.9) | 147 (45.9) |
| Wasted, n (%) | 50 (9.8) | 64 (20.0) |
| HOME score, mean (SD) | 23.8 (3.7) | 25.1 (8.5) |
| Inflammation (CRP>5 mg/L), n (%) | 78 (15.2) | 33 (10.3) |
| CRP concentration, median (IQR) | 0.6 (2.7) | 0.0 (1.7) |
| Randomized to multiple micronutrient powders intervention, n (%) | 262 (51.1) | 170 (53.0) |
| Randomized to early learning intervention, n (%) | 256 (49.9) | |
| Attended high quality preschool, n (%) | 158 (49.2) | |
| Mother | ||
| Low literacy, n (%) | 128 (25.0) | 143 (44.5) |
| Household | ||
| Household assets, mean (SD) | 3.7 (1.5) | 3.0 (1.5) |
Table 2a.
Infants: Standardized MSEL scores by domain and adjusted mean differences from enrollment to 6-months and from 6-months to 12-months
| Unadjusted scores | Adjusted mean difference† | Unadjusted scores | Adjusted mean difference† | ||||
|---|---|---|---|---|---|---|---|
| Enrollment | 6-months | Enrollment to 6-month difference | 12-months | 6-month to 12-month difference | |||
| Mean (SD) | Mean (SD) | MD (95% CI) | P | Mean (SD) | MD (95% CI) | P | |
| Visual Reception | 52.27 (8.35) | 41.96 (7.88) | −10.31 (−11.50, −9.32) | 0.001 | 41.85 (7.74) | −0.11 (−1.21, 1.00) | ns |
| Fine Motor | 48.29 (9.50) | 43.55 (8.35) | −4.71 (−6.02, −3.40) | 0.001 | 42.43 (6.60) | −1.11 (−2.41, −0.18) | ns |
| Gross Motor | 45.83 (10.36) | 50.88 (11.56) | 5.05 (3.46, 6.63) | 0.001 | 49.40 (10.86) | −1.47 (−3.28, −0.33) | ns |
| Receptive Language | 41.72 (8.05) | 38.15 (7.23) | −3.57 (−4.74, −2.41) | 0.001 | 44.91 (9.05) | 6.75 (5.60, 7.90) | 0.001 |
| Expressive Language | 49.29 (9.40) | 39.25 (6.71) | −10.08 (−10.99, −9.17) | 0.001 | 38.60 (6.47) | −0.65 (−1.57, −0.28) | ns |
MSEL=Mullens Scales of Early Development; MD= mean difference; 95%CI= 95% confidence interval; SD= standard deviation
Model adjusted for child age, maternal literacy, household assets, and randomization groups (MNP vs. placebo powder and early learning vs. control). Bonferroni corrected.
Table 2b.
Preschoolers: Standardized MSEL scores by domain and adjusted mean differences from enrollment to 8-months
| Unadjusted scores | Adjusted mean difference† | |||
|---|---|---|---|---|
| Enrollment | 8-month follow-up | MD (95% CI) | P | |
| Mean (SD) | Mean (SD) | |||
| Visual Reception | 42.08 (11.49) | 41.42 (10.34) | −0.67 (−1.91, 0.58) | ns |
| Fine Motor | 45.02 (11.94) | 45.75 (13.69) | 0.73 (−0.61, 2.08) | ns |
| Receptive Language | 42.16 (7.97) | 36.01 (8.07) | −6.15 (−7.12, −5.18) | 0.001 |
| Expressive Language | 38.85 (10.49) | 38.62 (10.53) | −0.23 (−1.59, 1.13) | ns |
| Inhibitory Control | 2.40 (3.09) | 4.09 (3.54) | 1.68 (1.28, 2.09) | 0.001 |
MSEL=Mullens Scales of Early Development; MD= mean difference; 95%CI= 95% confidence interval; SD= standard deviation
Model adjusted for child age, maternal literacy, household assets, randomization group (MNP vs. placebo powder), and preschool quality (high vs. low). Bonferroni corrected.
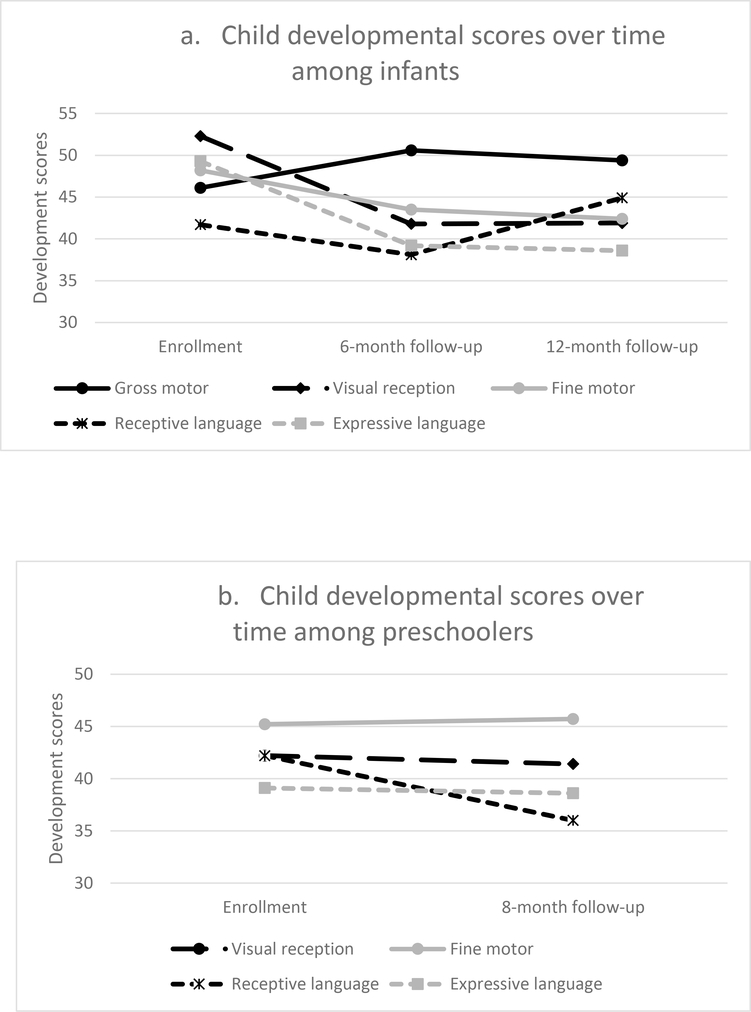
Figure 1. Child developmental scores over time.
1a. Infants: enrollment through 6- and 12-months.
1b. Preschoolers: enrollment through 8-months.
For hypothesis testing, continuous measures were used for HAZ, inflammation (CRP), and the home environment (HOME) at enrollment. Linear mixed effects models accounting for repeated measures and clustering within preschools/villages were used to assess the association between enrollment HAZ and child development scores over time (enrollment, 6- and 12-month follow-up for infants and enrollment and 8-month follow-up for preschoolers). Multivariate models were built separately for infants and preschoolers adjusting for child, maternal, household, and intervention variables: child age, maternal literacy, household assets, randomization group (MNP vs. placebo powder). For infants, models also included early learning intervention vs. control and for preschoolers, models included preschool quality (high vs. low). Since baseline anemia was significantly related to only one outcome measure (inhibitory control in preschoolers), baseline anemia was adjusted for in the models with inhibitory control only.
We tested whether the associations between HAZ and outcome differed over time by including interaction terms between HAZ and time in models. Interaction terms were not significantly different suggesting that the association between HAZ and outcome was constant over time. Interaction terms were removed and time was included in the models to account for the differences in child development scores over time. To estimate the association between HAZ at enrollment and child development over time, multivariate models were fitted and estimates of main effects were reported (Table 3).
Table 3.
Association between enrollment height-for-age z-score and child development scores
| Among infants (over 12 months)† | Among preschoolers (over 8-months)‡ | ||||
|---|---|---|---|---|---|
| b (SE) | p | b (SE) | p | ||
| Gross motor | 1.30 (0.30) | <0.01 | |||
| Visual reception | 1.07 (0.22) | <0.01 | 2.54 (0.50) | <0.01 | |
| Fine motor | 0.85 (0.26) | <0.01 | 4.08 (0.59) | <0.01 | |
| Receptive language | 0.55 (0.23) | 0.02 | 1.74 (0.33) | <0.01 | |
| Expressive language | 0.68 (0.21) | <0.01 | 1.62 (0.46) | <0.01 | |
| Inhibitory control▯ | 0.55 (0.13) | <0.01 | |||
Models adjusted for child age, maternal literacy, household assets, randomization group (MNP vs. placebo powder and early learning vs. control), and time
Models adjusted for child age, maternal literacy, household assets, randomization group (MNP vs. placebo powder and high vs. low preschool quality), and time
Anemia included in the model
To assess whether inflammation (CRP) moderates the relation between height and child development, interactions between enrollment CRP and HAZ were included in separate models for infants and preschoolers. Results were stratified by inflammation (CRP > 5 vs. CRP ≤ 5mg/L) following significant or marginally significant interactions (Figures 2a, 2b, and 2c) (Cohen, Cohen, West, & Aiken, 2003). Analyses were repeated after removing non-significant interaction terms from remaining models to assess main effects of CRP (Table 4).
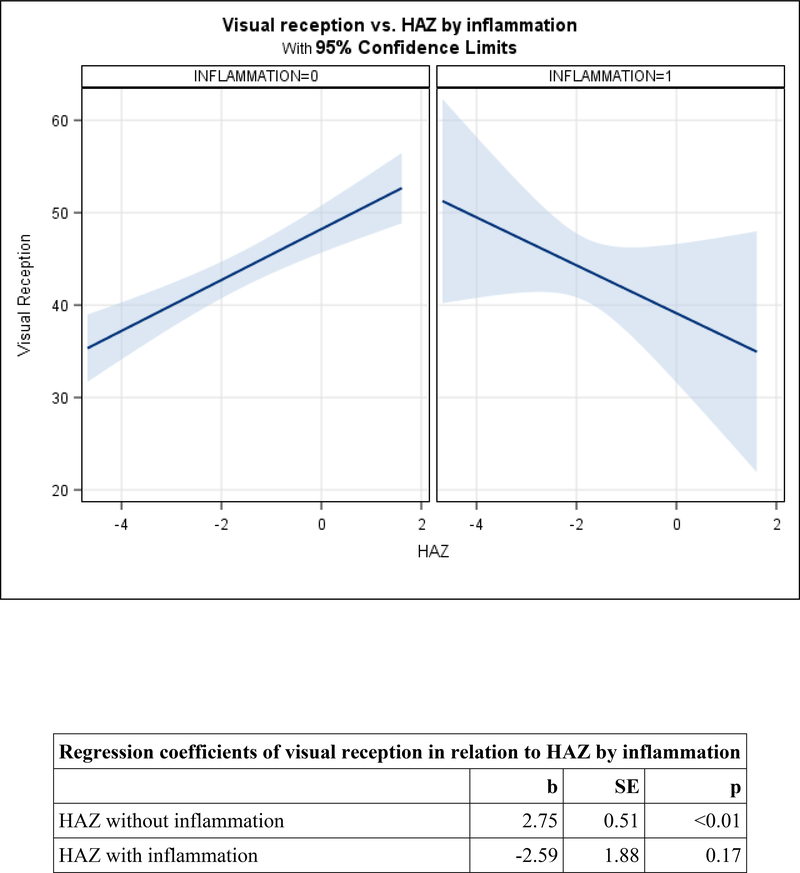
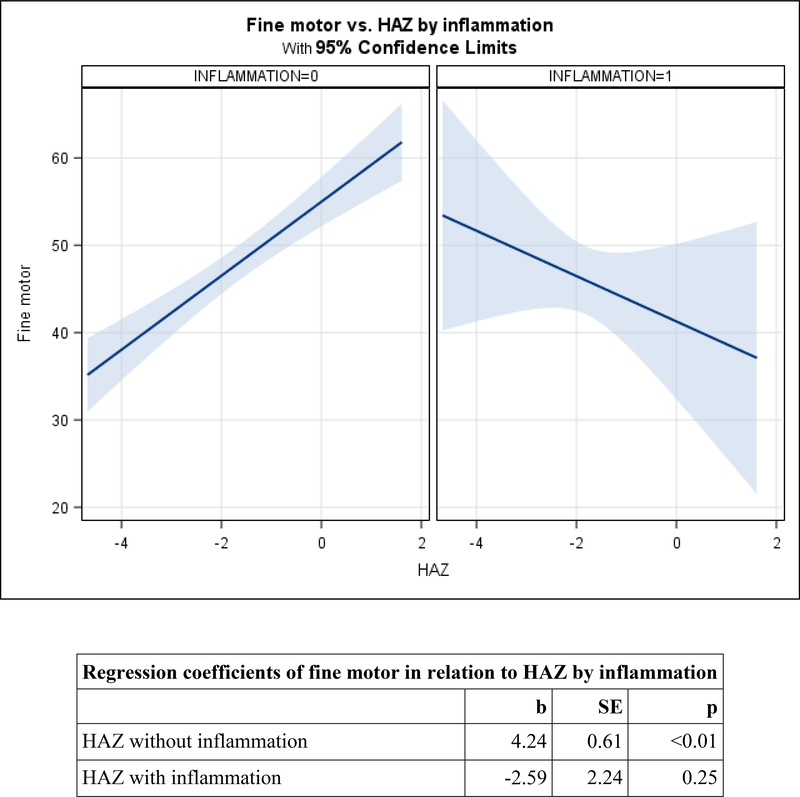
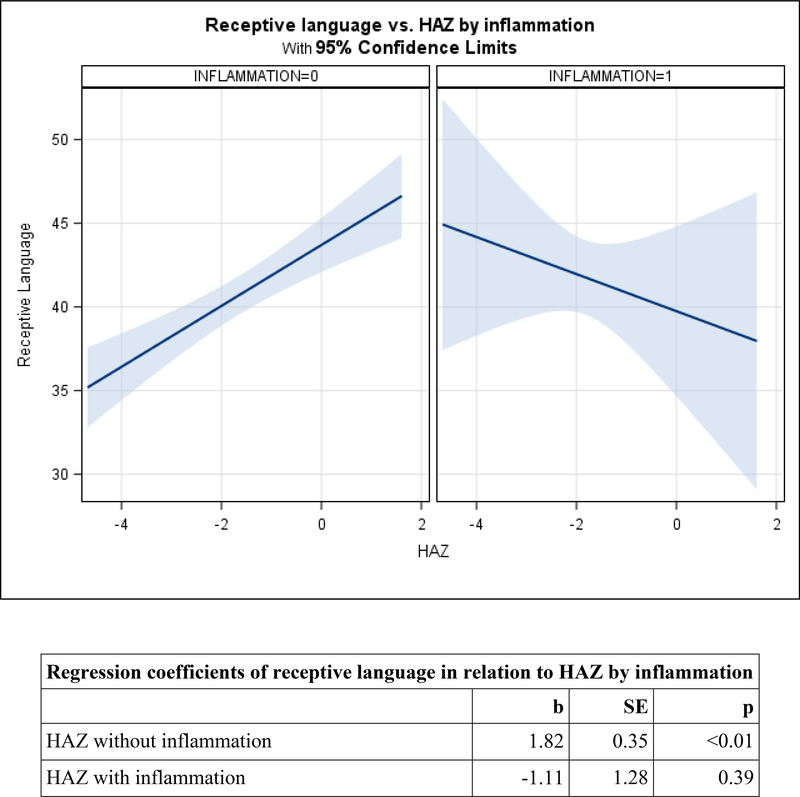
Figure 2. Adjusted mean change in child development by HAZ, moderated by inflammation (CRP>5) among preschoolers.
2a. Visual reception.
2b. Fine Motor.
2c. Receptive language.
Table 4.
Association between enrollment height-for-age z-score and inflammation with child development scores
| Among infants (over 12 months)† | Among preschoolers (over 8-months)‡ | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Height-for-age z-score | CRP (continuous) | Height-for-age z-score | CRP (continuous) | ||||||
| b (SE) | p | b (SE) | p | b (SE) | p | b (SE) | p | ||
| Gross motor | 1.24 (0.32) | <0.01 | −0.02 (0.06) | 0.68 | |||||
| Visual reception | 1.17 (0.23) | <0.01 | −0.02 (0.04) | 0.61 | |||||
| Fine motor | 0.80 (0.28) | <0.01 | −0.05 (0.05) | 0.30 | |||||
| Receptive language | 0.49 (0.24) | 0.04 | −0.08 (0.04) | 0.08 | |||||
| Expressive language | 0.66 (0.22) | <0.01 | −0.00 (0.04) | 0.97 | 1.51 (0.48) | <0.01 | 0.04 (0.17) | 0.81 | |
| Inhibitory control▯ | 0.53 (0.13) | <0.01 | −0.08 (0.05) | 0.09 | |||||
Models adjusted for child age, maternal literacy, household assets, randomization group (MNP vs. placebo powder and early learning vs. control), and time
Models adjusted for child age, maternal literacy, household assets, randomization group (MNP vs. placebo powder and high vs. low preschool quality), and time
Anemia included in the model
To assess whether the home environment moderates the relation between HAZ and child development, interactions between enrollment HOME scores and height were included in separate models for infants and preschoolers. Results were stratified following significant or marginally significant interactions. Stratified results were plotted and shown in graphs at differing levels of HOME scores (mean −1 standard deviation (SD), mean, and mean + 1 SD) (Figures 3a and 3b) (Cohen et al., 2003). Analyses were repeated after removing non-significant interaction terms from remaining models to assess main effects of the home environment (Table 5).
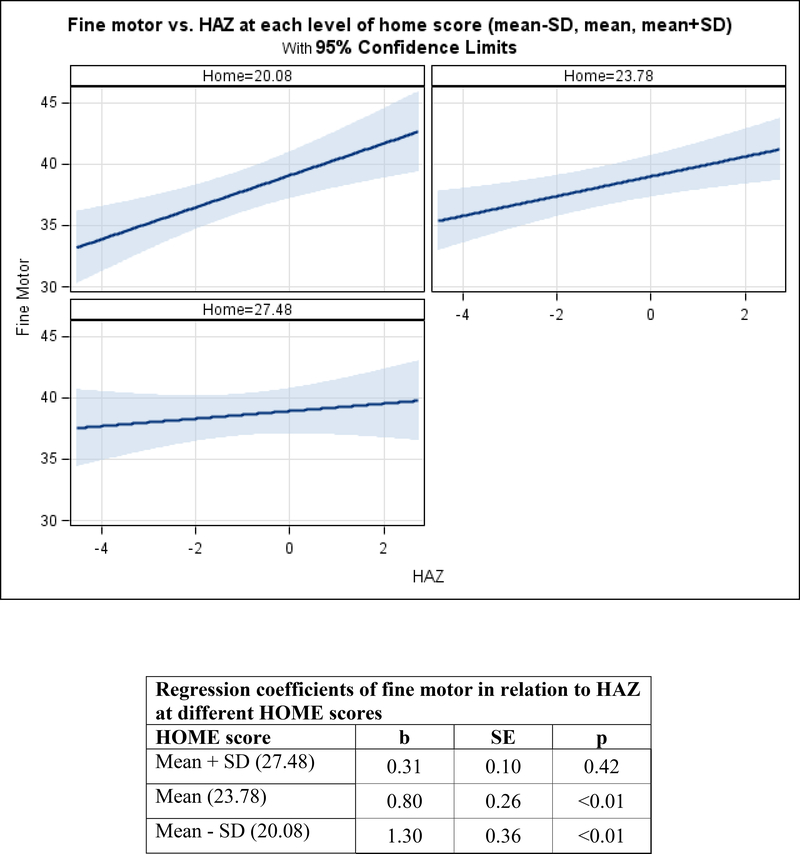
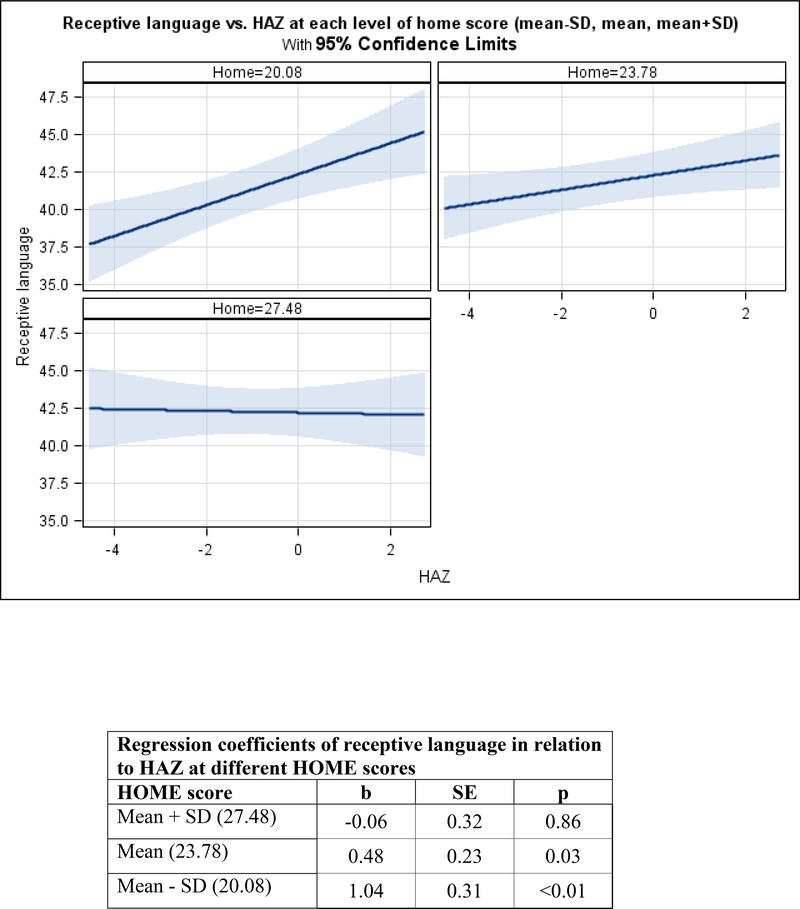
Figure 3. Adjusted mean change in child development by HAZ, moderated by nurturance among infants.
3a. Fine Motor.
3b. Receptive language.
Table 5.
Association between enrollment height-for-age z-score and nurturance with child development scores
| Among infants (over 12 months)† | Among preschoolers (over 8-months)‡ | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Height-for-age z-score | Nurturance (continuous) | Height-for-age z-score | Nurturance (continuous) | ||||||
| b (SE) | p | b (SE) | p | b (SE) | p | b (SE) | p | ||
| Gross motor | 1.29 (0.31) | <0.01 | 0.05 (0.09) | 0.59 | |||||
| Visual reception | 1.00 (0.22) | <0.01 | 0.27 (0.06) | <0.01 | 2.37 (0.49) | <0.01 | 0.24 (0.06) | <0.01 | |
| Fine motor | 3.92 (0.58) | <0.01 | 0.24 (0.08) | <0.01 | |||||
| Receptive language | 1.63 (0.33) | <0.01 | 0.16 (0.04) | <0.01 | |||||
| Expressive language | 0.62 (0.21) | <0.01 | 0.25 (0.06) | <0.01 | 1.50 (0.46) | <0.01 | 0.20 (0.06) | <0.01 | |
| Inhibitory control▯ | 0.54 (0.13) | <0.01 | 0.02 (0.02) | 0.36 | |||||
Models adjusted for child age, maternal literacy, household assets, randomization group (MNP vs. placebo powder and early learning vs. control), and time
Models adjusted for child age, maternal literacy, household assets, randomization group (MNP vs. placebo powder and high vs. low preschool quality), and time
Anemia included in the model
Analyses were conducted using SAS 9.4 (Cary, NC). Significance level was at p<0.05 and marginal significance at p<0.10 for interactions.
Results
A total sample of 513 infants (aged 6–14 months) and 321 preschoolers (aged 29–49 months) were enrolled in this study. Among infants, 47% were girls, 20% stunted, 66% anemic, 19% underweight, 10% wasted, 15% had CRP>5 mg/L, mean age at enrollment was 8.6 months (SD= 2.2), and 25% of mothers were at the low literacy level (Table 1). Among preschoolers, 52% were girls, 41% stunted, 48% anemic, 46% underweight, 20% wasted, 10.3% had CRP>5 mg/L, mean age at enrollment was 36.6 months (SD=5.7), and 44% of mothers were at the low literacy level.
Among infants, from enrollment to the 6-month follow-up, standard scores on the MSEL declined significantly in visual reception, fine motor, receptive language, and expressive language and increased in gross motor, adjusting for covariates. From the 6-month to the 12-month follow-up, standard scores did not change significantly in visual reception, fine motor, gross motor, and expressive language domains. There were significant gains in receptive language (Table 2a, Figure 1a).
Among preschoolers, from enrollment to the 8-month follow-up, standard scores on the MSEL significantly increased in inhibitory control, and significantly decreased in receptive language, with no significant change in visual reception, fine motor, and expressive language (Table 2b, Figure 1b).
Height at enrollment and child development over time
Among infants, HAZ at enrollment was significantly positively associated with all domains of child development over time, adjusting for covariates (Table 3). Among preschoolers, HAZ at enrollment was significantly positively associated with all domains of child development and with inhibitory control over time (Table 3).
Height and inflammation in relation to child development over time
Among infants, there were no significant interactions between HAZ and inflammation. After removing interactions, CRP was not related to any child development domains (Table 4).
Among preschoolers, the HAZ-by-inflammation interaction was marginally significant for visual reception (p=0.078), and significant for fine motor (p=0.046) and receptive language (p=0.009). Patterns were similar across the three domains. In the absence of inflammation, HAZ was positively related to visual reception (b=2.75, SE=0.51, p<0.01), fine motor (b=4.24, SE=0.61, p<0.01), and receptive language (b=1.82, SE=0.35, p<0.01). In the presence of inflammation, relations between HAZ and each domain were non-significant and the slopes were negative (Figures 2a, 2b, and 2c). After removing the interactions, there were no direct relations between CRP and either expressive language or inhibitory control among preschoolers (Table 4).
Height and home environment in relation to child development over time
Among infants, the HAZ by HOME interaction was marginally related to fine motor (p=0.058) and significantly related to receptive language development (p=0.015). In the context of HOME scores at the mean or below (1 SD below the mean), HAZ was significantly related to fine motor (mean: b=1.30, SE=0.36, p<0.01 and below the mean: b=0.80, SE=0.26, p<0.01). In contrast, in the context of high HOME scores (1 SD above the mean), HAZ was not significantly related to fine motor scores. There was a similar pattern with among receptive language. In the context of HOME scores at the mean or below (1 SD below the mean), HAZ was significantly related to receptive language (mean: b=0.48, SE=0.23, p=0.03 and below the mean: b=1.04, SE=0.31, p<0.01). In contrast, in the context of high HOME scores (1 SD above the mean), HAZ was not significantly related to receptive language scores (Figures 3a and 3b). After removing the interactions, HOME scores were significantly related to visual reception (b=0.27, SE=0.06, p<0.01) and expressive language (b=0.25, SE=0.1, p<0.01), but not to gross motor (Table 5).
Among preschoolers, there were no significant interactions between HAZ and the HOME scores for any child development domains (all ps>0.10). After removing the interactions, HOME scores were significantly related to visual reception (b=0.24, SE=0.06, p<0.01), fine motor (b=0.24, SE=0.08, p<0.01), receptive language (b=0.16, SE=0.04, p<0.01), and expressive language (b=0.20, SE=0.06, p<0.01), but not to inhibitory control (Table 5).
Discussion
Consistent with bioecological theory (Bronfenbrenner & Morris, 2006), this study highlights the importance of considering children’s health, nutrition, and home environment in evaluating child development over time among both infants and preschoolers. The study has four major findings. First, the associations documented previously in both cross-sectional and longitudinal studies in LMIC between stunting (or HAZ) and child development (Sudfeld, et al., 2015) (Hoddinott, et al., 2013) (Miller, et al., 2016), apply to associations between HAZ and child development scores among both infants and preschoolers over time. Second, non-specific inflammation, measured from the biomarker, CRP, is not clearly associated with child development among infants or preschoolers. In the presence of inflammation, there is no evidence of an association between height and child development, although the slope is negative. Third, a home environment characterized by high maternal nurturance and early learning opportunities attenuates the relation between HAZ and visual reception and fine motor scores among infants. The home environment is positively associated with multiple domains of child development scores over time, among both infants and preschoolers. Finally, the patterns of children’s development and relations with height, inflammation, and the home environment vary between infants and preschoolers, illustrating the importance of timing in studies of children’s early development.
Height and Child Development
HAZ had a positive relation to multiple domains of infant and preschooler development over time, even after adjusting for a broad range of child, maternal, household, and intervention variables. These findings confirm previous findings that stunting is negatively related to children’s development in LMIC (Black, et al., 2015; Sudfeld, et al., 2015; Walker, et al., 2011), including a recent meta-analysis among almost 60,000 preschool-age children from fifteen LMIC that reported associations between stunting and multiple domains of child development, based on caregiver report (Miller et al., 2016). Conducting the analyses with a continuous measure of HAZ has shown that the associations between height and children’s development are not limited to the extremes of stunting, as previously suggested (Perumal et al., 2018). Thus, this study reinforces recommendations for strategies to promote healthy growth and prevent slowdowns in height acceleration throughout the distribution, not only at the extreme of stunting.
Globally, the prevalence of stunting has decreased significantly from an estimated 39.7% in 1990 to 26.7% in 2010 and is expected to reach 21.8% in 2020 (de Onis, Blossner, & Borghi, 2012), with an estimated 156 million children under the age of 5 stunted in 2015 (UNICEF, 2017). At least part of the decrease in the prevalence of stunting may be attributed to the economic growth that has occurred over the same time period (Alderman, Haddad, Headey, & Smith, 2014). The prevalence of stunting is higher in LMIC (often exceeding 30%), compared to high-income countries, suggesting that poverty plays an important role in stunting, as it does in compromising child development (Lu, et al., 2016). In high-income countries, including the United States, the prevalence of stunting has been stable since 1990 at 6.0%, is expected to remain stable through 2020 (de Onis, et al., 2012), and is not associated with children’s development.
The WHO Multicentre Growth Reference Study (de Onis, 2006) found that children from multiple countries (Norway, Oman, Brazil, India, Ghana, and the United States) born to middle-income, non-smoking women, and breastfed according to the WHO guidelines grew along similar weight and length trajectories for the first two years of life. These findings illustrate that in the context of a healthy background and recommended feeding practices, children’s weight and length grow along similar trajectories without significant differences in linear growth.
Recognizing that stunting often begins prenatally (Victora, et al., 2010) and continues throughout the first 2 years of a child’s life, nutritional efforts to prevent or reduce stunting have been directed toward pregnant women and infants and have included dietary diversification, provision of nutrient rich food, food fortification, and micronutrient supplementation. However, efforts have met with limited success, prompting consideration of additional causes of stunting beyond undernutrition (Dewey, 2016). In addition to poor maternal health/nutrition and suboptimal infant and young child feeding practices, important causes of stunting include infectious diseases and subclinical infections (both of which cause inflammation), as well as underlying causes such as poverty, food insecurity, and inadequate care practices. Additional efforts to prevent stunting may consider strategies to prevent or reduce inflammation (Prendergast, et al., 2014).
Inflammation
The negative (although non-significant) association between HAZ and visual reception, fine motor, and receptive language in the context of inflammation among preschoolers suggests that inflammation may have an unidentified indirect association with child development. This possibility is consistent with findings that inflammation (EE) was associated with child development through anemia (Murray-Kolb & MAL-ED Network Investigators, 2018) and that diarrhea was not associated with child development once length-for-age was included in the model (Fischer-Walker, et al., 2012). Thus, additional research related to inflammation and child development is warranted, ensuring that indicators of nutrition, such as stunting or HAZ, along with other potential confounders are included in the models.
Nurturant home environment
Among infants, a nurturant home environment attenuated the association between HAZ and both fine motor and receptive language development. These findings are consistent with a recent study from Vietnam showing that a high quality home environment protected stunted children from low child development scores (Nguyen, et al., 2017). Among infants in both studies, there were non-significant associations between stunting (or HAZ) and child development in the context of a high quality home environment. These findings illustrate the multifactorial aspects of early child development, suggesting that in the context of a home characterized by nurturance and learning opportunities, children can acquire fine motor and receptive language skills, perhaps through play with objects and responsive social interactions, in spite of having low HAZ or being stunted. Opportunities for play and responsive social interactions may also be an explanation for the positive associations demonstrated in this study between a nurturant home environment and infants’ visual reception and expressive language over time.
These findings are also consistent with studies showing that a high quality, nurturant home environment mediates the association between poverty and children’s development through age 3½ years in Colombia (Rubio-Codina, et al., 2016) and age 5 years in Bangladesh (Hamadani et al., 2014), the association between a responsive caregiving intervention and children’s cognitive development at age 4 in Pakistan (Obradović, et al., 2016), and the association between maternal depressive symptoms and children’s development at 12 months in Bangladesh (Black et al., 2007). Taken together, the evidence supports recommendations for integrated programming that incorporates both stunting prevention and promotion of responsive caregiving and early learning in the home environment.
Among preschoolers, a nurturant home environment was positively associated with all domains on the MSEL (visual reception, fine motor, receptive language, and expressive language), but a nurturant home environment did not mitigate the associations between HAZ and low child development scores. Possible explanations are the high rate of stunting among the preschoolers (41%), suggesting ongoing nutritional deprivation, and the need for more intensive opportunities for early learning and responsive interactions.
The finding of positive associations between the home environment and multiple domains of child development among both infants and preschoolers is consistent with the bioecological model (Bronfenbrenner & Morris, 2006) and illustrates the importance of a nurturing home and early learning opportunities regardless of children’s nutritional status (Britto, et al., 2017; Shonkoff, 2010).
One explanation for the absence of an association between the home environment and infants’ gross motor development may be the canalization that occurs during infancy as children are acquiring time-dependent developmental skills, such as gross motor skills (Fox, Levitt, & Nelson, 2010). The absence of a relationship between a nurturant home environment and inhibitory control over time among preschoolers was unexpected, based on findings that nurturant caregiving during infancy has been shown to be positively associated with executive functioning during children’s preschool years (Bernier, Carlson, Deschênes, & Matte-Gagné, 2012). Skills in inhibitory control increased over time and were associated with children’s height and anemia (which was adjusted in the analyses), suggesting a role linking nutritional status with inhibitory control.
Developmental patterns
The examination of developmental patterns found more variability in developmental scores across domains among infants than among preschoolers, consistent with variability in timing and the emergence of developmental skills (Fox et al., 2010). The finding that nurturance and early learning in the home could attenuate associations between children’s height and some domains of development during infancy, but not during the preschool period suggests that preschoolers may need more intensive early learning opportunities. Many caregivers in LMIC are providing developmental opportunities involving preschoolers, as shown in a representative sample from over 163,000 three- and four-year-old children from 63 countries (McCoy et al., 2018). Almost three-quarters of the preschool-age children experienced at-home learning opportunities (e.g., reading, counting, drawing), one-third attended center-based early childhood care and education programming, and slightly under one-quarter experienced neither.
Implications and future directions
The findings have both scientific and programmatic implications. From a scientific perspective, development over time during both infancy and preschool years is independently associated with children’s early health and nutrition as well as with maternal nurturance and opportunities for learning in the home environment. These behavioral findings support the neuroscientific changes to the hippocampus associated with maternal nurturance (Luby, 2015). Young children who reach their developmental potential are better prepared to take advantage of schooling and to follow a life-course trajectory with health, economic, and social capital benefits in adulthood, as demonstrated by effective early interventions with long-term follow-up (Grantham-McGregor & Smith, 2016).
From a programmatic perspective, the prevention of stunting is likely to enhance children’s development. However, if the goal is to promote early child development, the prevention of stunting is not enough. As data from this study and others have shown, children need home environments that support maternal nurturance and access to early learning. What is needed to achieve these goals is a comprehensive framework of Nurturing Care that incorporates health, nutrition, responsive caregiving, early learning, and child protection (Black, et al., 2017). Recommendations include integrated interventions and cross-sectoral collaborations that incorporate the domains of Nurturing Care and are scaled up to include programs and national policies (Richter, et al., 2017). In spite of the enthusiasm for early opportunities and for integrated interventions, there have been few evaluated trials or programs, illustrating the difficulties in scaling and sustainability (Grantham-McGregor, Fernald, Kagawa, & Walker, 2014). For example, a recent follow-up report from Colombia on a psychosocial intervention that had been successfully incorporated into a cash transfer program with positive effects on children’s development and home environment at ages 2.5—3.5 years found no effects on children’s cognition and behavior, or on the home environment at age 4.5–5.5 years (Andrew et al., 2018). These findings illustrate that implementation research and partnerships with implementing partners may be necessary to achieve lasting findings and to scale integrated interventions.
Future directions include investigations of timing, individual differences, and mechanisms that enable children to acquire developmental skills under adverse conditions, such as ongoing nutritional deficiencies. This study found differences in developmental patterns between infants and preschoolers. Although the home environment was positively associated with children’s development over time across both age ranges, the mitigating effects of the home environment were more apparent during infancy than during preschool years. While the high rates of stunting among preschoolers may be a factor, additional attention to threats, such as inflammation, and to the quality of interactions and opportunities within the home environment warrant investigation. Our measure of inflammation, CRP, is an acute phase indicator of systemic inflammation, which rises and falls rapidly, and may be a result of infection. An indicator of enteric inflammation may be more informative in understanding how inflammation relates to development in childhood. Additional within-person aspects of children, such as gender or temperament, and of the caregivers may also contribute to our understanding of mechanisms driving early development and underlying variability across developmental domains. Although center-based early childhood care and education programming is increasing in LMIC (McCoy et al., 2018), little is known about the immediate or long-term impact of these programs on children and families. Finally, translating analyses conducted with continuous variables into clinically relevant cut points, such as stunting, may enhance interpretability and inform programmatic recommendations.
Methodological Considerations
There are several methodological considerations to be addressed in interpreting the findings. First, CRP was measured once, at enrollment, meaning that we could not examine the possibility of chronic or repeated bouts of acute infection or inflammation. We used the continuous range of CRP in the analyses, followed by the recommended cut-point of CRP >5 mg/L to define inflammation. However, others have found associations between inflammation and child development using other cut-points (median split, Jiang, et al., 2017), raising the possibility that other cut-points could be considered. Second, the children in this sample also experienced high rates of underweight and wasting, measures of acute nutritional status. We focused on stunting because it is a measure of chronic undernutrition, is strongly related to child development over time, and is the focus of global policies and reporting. Future investigations are needed to examine how patterns of weight loss and recovery relate to children’s development. Third, the limited sample size reduced our power to detect small effects. In addition, we accepted a marginal significance level for interactions, with findings that should be replicated. Finally, the child development measures were not validated for children in India. However, we used the age-appropriate version of the same domains of child development for infants and preschoolers (MSEL), administered by an Indian team of masters-level psychologists who were trained and supervised by a doctoral level psychologist. In addition, we conducted several pilot studies to ensure that children could understand the items and all analyses were conducted within the Indian samples.
This study fills several critical gaps in our understanding of how height, inflammation, and the home environment relate to child development scores over time. First, the study was conducted among two samples, infants and preschoolers, thus extending beyond the first 1000 days, to the second 1000 days (ages 2–5), an age group that has often been ignored (Black, et al., 2015). Second, child development scores were examined over 12 months among infants and over 8 months among preschoolers, thereby incorporating either two or three measures for each child. Third, the analysis adjusted for potential confounders at the child, maternal, and household level, thereby increasing the plausibility of the findings. Finally, the design enabled us to study how mechanisms among height, inflammation, and the home environment relate to specific domains of child development over time, while adjusting for prior influences.
Conclusion
As the current study has shown, the mechanisms contributing to longitudinal advances in children’s development are multi-factorial, including children’s health and nutrition, as well as maternal nurturance and early learning opportunities in the home environment. This perspective is consistent with the Nurturing Care Framework (Black, et al., 2017), suggesting that support for child development consider the policies and programs that are necessary to enable families to provide the nurturance and opportunities that children require to reach their developmental potential (Richter, et al., 2017). The recognition that development during the infancy and preschool years is part of a life-course trajectory from the first 1000 days through adulthood and into the next generation illustrates the importance of attention to the modifiable factors of early child development, including prevention of stunting, better understanding of inflammation, and promotion of a home environment that includes maternal nurturance and early learning opportunities (Black, et al., 2017). The Sustainable Development Goals support these recommendations by calling for the elimination of malnutrition and the assurance that children have early access to pre-primary educational opportunities (United Nations, 2015). Current findings support the Sustainable Development Goals and illustrate that the pathways to success and well-being for entire societies are through investments in the health, nutrition, and development of young children and their families.
Research Highlights.
Height and nurturance are positively associated with child development among infants and preschoolers in rural India.
Among infants and preschoolers, a nurturant home environment is positively associated with child development over time. Among infants only, a nurturant home environment attenuates associations between height and child development.
Inflammation is not associated with child development among either infants or preschoolers. Among preschoolers, in the presence of inflammation height is not associated with child development.
Mechanisms contributing to early child development are multi-factorial supported by the Nurturing Care Framework, which encompasses nutrition, health, and a nurturant home environment.
Acknowledgement
We acknowledge the leadership and contributions of Dr. Shahnaz Vazir, former deputy director and emeritus scientist, National Institute of Nutrition; Dr. B. Sesikeran, former director, National Institute of Nutrition; Dr. Annie Wesley, former employee of Micronutrient Initiative; and Dr. Yan Wang, Department of Pediatrics, University of Maryland School of Medicine.
Footnotes
Conflict of Interest: None of the authors has a conflict of interest. Kimberly Harding is an employee of Nutrition International (formerly Micronutrient Initiative) and Gregory Reinhart was formerly an employee of The Mathile Institute for the Advancement of Human Nutrition.
Flush to piped sewer system, flush to septic tank, flush to pit latrine, ventilated improved pit (VIP)/biogas latrine, pit latrine with slab, twin pit/composting toilet, which is not shared with any other household.
References
- Aboud FE, & Yousafzai AK (2015). Global health and development in early childhood. Annual Review of Psychology, 66, 433–457. doi: 10.1146/annurev-psych-010814-015128 [DOI] [PubMed] [Google Scholar]
- Algarin C, Nelson CA, Peirano P, Westerlund A, Reyes S, Lozoff B (2013). Iron-deficiency anemia in infancy and poorer cognitive inhibitory control at age 10 years. Developmental medicine and child neurology, 55 (5), 453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderman H, Haddad L, Headey DD, & Smith L (2014). Association between economic growth and early childhood nutrition. The Lancet Global Health, 2(9), e500. [DOI] [PubMed] [Google Scholar]
- Andrew A, Attanasio O, Fitzsimons E, Grantham-McGregor S, Meghir C, & Rubio-Codina M (2018). Impacts 2 years after a scalable early childhood development intervention to increase psychosocial stimulation in the home: A follow-up of a cluster randomised controlled trial in Colombia. PLoS Medicine, 15(4), e1002556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlinski S, & Schady N (2015). The early years: Child well-being and the role of public policy. Development in the americas series. New York: MacMillan, and Washington, DC: Inter-American Development Bank. [Google Scholar]
- Bernier A, Carlson SM, Deschênes M, & Matte-Gagné C (2012). Social factors in the development of early executive functioning: A closer look at the caregiving environment. Developmental Science, 15(1), 12–24. [DOI] [PubMed] [Google Scholar]
- Black MM, & Aboud FE (2011). Theoretical basis of responsive feeding among infants and young children in high and low income countries. The Journal of Nutrition, 141(3), 490–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black MM, Baqui AH, Zaman K, McNary SW, Le K, Arifeen SE,… Black RE (2007). Depressive symptoms among rural Bangladeshi mothers: implications for infant development. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 48(8), 764–772. doi: 10.1111/j.1469-7610.2007.01752.x [DOI] [PubMed] [Google Scholar]
- Black MM, Perez-Escamilla R, & Fernandez Rao S (2015). Integrating nutrition and child development interventions: Scientific Basis, evidence of impact, and implementation considerations. Advances in Nutrition(6), 852–859. doi: 10.3945/an.115.010348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black MM, Tilton N, Harding KB, Hurley KM, Fernandez-Rao S, Balakrishna N,… Nair KM (2016). Economic inequities and growth and development among infants and preschoolers in rural India: Caregiver protective/promotive factors. International Journal of Behavior and Development, 40(6), 526–535. [Google Scholar]
- Black MM, Walker SP, Fernald LC, Andersen CT, DiGirolamo AM, Lu C,… Grantham-McGregor S (2017). Early childhood development coming of age: science through the life course. The Lancet, 389(10064), 77–90. doi: 10.1016/s0140-6736(16)31389-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein MH, & Putnick DL (2012). Cognitive and socioemotional caregiving in developing countries. Child Development, 83(1), 46–61. doi: 10.1111/j.1467-8624.2011.01673.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley RH, Caldwell BM, & Corwyn RF (2003). The child care HOME Inventories: assessing the quality of family child care homes. Early Childhood Research Quarterly, 18(3), 294–309. doi: 10.1016/S0885-2006(03)00041-3 [DOI] [Google Scholar]
- Bradley RH, Caldwell BM, Rock SL, Ramey CT, Barnard KE, Gray C,… Siegel L (1989). Home environment and cognitive development in the first 3 years of life: A collaborative study involving six sites and three ethnic groups in North America. Developmental Psychology, 25(2), 217. [Google Scholar]
- Britto PR, Lye SJ, Proulx K, Yousafzai AK, Matthews SG, Vaivada T,… Bhutta ZA (2017). Nurturing care: promoting early childhood development. The Lancet, 389(10064), 91–102. doi: 10.1016/s0140-6736(16)31390-3 [DOI] [PubMed] [Google Scholar]
- Bronfenbrenner U, & Morris PA (2006). The bioecological model of human development In Damon W & Lerner RM (Eds.), Handbook of Child Psychology (6th ed., Vol. 1, pp. 793–828). New York: John Wiley & Sons, Inc. [Google Scholar]
- Challa S, & Challa P (2015). Nutritional status of under-5 children in the newlycarved states of India. International Journal of Medical Science and Public Health, 4(7), 1019–1022. [Google Scholar]
- Chan M, Lake A, & Hansen K (2017). The early years: silent emergency or unique opportunity? The Lancet, 389(10064), 11–13. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West S, & Aiken L (Eds.). (2003). Applied multiple regression/correlation analysis for the behavioral sciences. New York: Wiley. [Google Scholar]
- de Onis M (2006). Assessment of differences in linear growth among populations in the WHO Multicentre Growth Reference Study. Acta Paediatrica, 95(S450), 56–65. [DOI] [PubMed] [Google Scholar]
- de Onis M, Blossner M, & Borghi E (2012). Prevalence and trends of stunting among pre-school children, 1990–2020. Public Health Nutrition, 15(1), 142–148. doi: 10.1017/s1368980011001315 [DOI] [PubMed] [Google Scholar]
- de Onis M, & Branca F (2016). Childhood stunting: a global perspective. Maternal & Child Nutrition, 12(S1), 12–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey KG (2016). Reducing stunting by improving maternal, infant and young child nutrition in regions such as South Asia: evidence, challenges and opportunities. Maternal & Child Nutrition, 12(S1), 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowsett SM, & Livesey DJ (2000). The development of inhibitory control in preschool children: effects of “executive skills” training. [Comparative Study]. Developmental Psychobiology, 36(2), 161–174. [DOI] [PubMed] [Google Scholar]
- Etheredge AJ, Manji K, Kellogg M, Tran H, Liu E, McDonald CM,… Duggan CP (2018). Markers of Environmental Enteric Dysfunction Are Associated With Neurodevelopmental Outcomes in Tanzanian Children. Journal Of Pediatric Gastroenterology And Nutrition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Rao S, Hurley KM, Nair KM, Balakrishna N, Radhakrishna KV, Ravinder P,… Black MM (2014). Integrating nutrition and early child-development interventions among infants and preschoolers in rural India. Annals of the New York Academy of Sciences, 1308, 218–231. doi: 10.1111/nyas.12278 [DOI] [PubMed] [Google Scholar]
- Filmer D, & Pritchett LH (2001). Estimating wealth effects without expenditure data--or tears: an application to educational enrollments in states of India. Demography, 38(1), 115–132. [DOI] [PubMed] [Google Scholar]
- Fischer-Walker CL, Lamberti L, Adair L, Guerrant RL, Lescano AG, Martorell R,… Black RE (2012). Does childhood diarrhea influence cognition beyond the diarrhea-stunting pathway? PloS One, 7(10), e47908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong KR, Anderson LN, Kang H, Lebovic G, Parkin PC, Maguire JL…. TARGet Kids! Collaboration. (2016). BMI-for-Age and Weight-for-Length in Children 0 to 2 Years. Pediatrics, 138(1), e20153809. [DOI] [PubMed] [Google Scholar]
- Fox SE, Levitt P, & Nelson CAI (2010). How the timing and quality of early experiences influence the development of brain architecture. Child Development, 81(1), 28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham-McGregor S, & Smith JA (2016). Extending the jamaican early childhood development intervention. Journal of Applied Research on Children: Informing Policy for Children at Risk, 7(2), 4. [Google Scholar]
- Grantham-McGregor SM, Fernald LC, Kagawa RM, & Walker S (2014). Effects of integrated child development and nutrition interventions on child development and nutritional status. Annals of the New York Academy of Sciences, 1308, 11–32. doi: 10.1111/nyas.12284 [DOI] [PubMed] [Google Scholar]
- Guerrant DI, Moore SR, Lima AA, Patrick PD, Schorling JB, & Guerrant RL (1999). Association of early childhood diarrhea and cryptosporidiosis with impaired physical fitness and cognitive function four-seven years later in a poor urban community in northeast Brazil. The American Journal of Tropical Medicine and Hygiene, 61(5), 707–713. [DOI] [PubMed] [Google Scholar]
- Hamadani JD, Nahar B, Huda SN, & Tofail F (2014). Integrating early child development programs into health and nutrition services in Bangladesh: benefits and challenges. Annals of the New York Academy of Sciences, 1308, 192–203. doi: 10.1111/nyas.12366 [DOI] [PubMed] [Google Scholar]
- Hamadani JD, Tofail F, Huda SN, Alam DS, Ridout DA, Attanasio O, & Grantham-McGregor SM (2014). Cognitive deficit and poverty in the first 5 years of childhood in Bangladesh. Pediatrics, 134(4), e1001–1008. doi: 10.1542/peds.2014-0694 [DOI] [PubMed] [Google Scholar]
- Hanson M. a., & Gluckman P (2014). Early developmental conditioning of later health and disease: physiology or pathophysiology? Physiological Reviews, 94(4), 1027–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmes T, Clifford R, & Cryer D (1998). Early childhood environment rating scale-revised: New York: Teachers College Press. [Google Scholar]
- Harper KM, Mutasa M, Prendergast AJ, Humphrey J, & Manges AR (2018). Environmental enteric dysfunction pathways and child stunting: A systematic review. PLoS Neglected Tropical Diseases, 12(1), e0006205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoddinott J, Alderman H, Behrman JR, Haddad L, & Horton S (2013). The economic rationale for investing in stunting reduction. Maternal & Child Nutrition, 9 Suppl 2, 69–82. doi: 10.1111/mcn.12080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey JH (2009). Child undernutrition, tropical enteropathy, toilets, and handwashing. The Lancet, 374(9694), 1032–1035. doi: 10.1016/s0140-6736(09)60950-8 [DOI] [PubMed] [Google Scholar]
- Indian Council of Medical Research. (2010). Nutrient requirements and recommended dietary allowances for Indians. a report of the expert working group of the Indian council of medical research.. Hyderabad, India: National Institute of Nutrition. [Google Scholar]
- International Institute for Population Sciences and Macro International. (2007). National Family Health Survey, 2005–2006. Mumbai, India: International Institute for population Sciences. [Google Scholar]
- Jiang NM, Tofail F, Ma JZ, Haque R, Kirkpatrick B, Nelson CA III, & Petri WA Jr. (2017). Early Life Inflammation and Neurodevelopmental Outcome in Bangladeshi Infants Growing Up in Adversity. The American Journal of Tropical Medicine and Hygiene, 97(3), 974–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PC, Pendergast LL, Schaefer BA, Rasheed M, Svensen E, Scharf R, … MAL-ED Network Investigators. (2017). Measuring home environments across cultures: Invariance of the HOME scale across eight international sites from the MAL-ED study. Journal of School Psychology, 64, 109–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpe PS, & Petri WA Jr (2012). Environmental enteropathy: critical implications of a poorly understood condition. Trends in Molecular Medicine, 18(6), 328–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koura KG, Boivin MJ, Davidson LL, Ouedraogo S, Zoumenou R, Alao MJ,… Bodeau-Livinec F (2013). Usefulness of child development assessments for low-resource settings in francophone Africa. Journal of Developmental & Behavioral Pediatrics, 34(7), 486–493. doi: 10.1097/DBP.0b013e31829d211c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs NF, Lozoff B, & Georgieff MK (2017). Neurodevelopment: The Impact of Nutrition and Inflammation During Infancy in Low-Resource Settings. Pediatrics, 139(Supplement 1), S50–S58. [DOI] [PubMed] [Google Scholar]
- Kutlesic V, Brewinski Isaacs M, Freund LS, Hazra R, & Raiten DJ (2017). Executive Summary: Research Gaps at the Intersection of Pediatric Neurodevelopment, Nutrition, and Inflammation in Low-Resource Settings. Pediatrics, 139(Supplement 1), S1–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorntz B, Soares AM, Moore SR, Pinkerton R, Gansneder B, Bovbjerg VE,… Guerrant RL (2006). Early childhood diarrhea predicts impaired school performance. The Pediatric Infectious Disease Journal, 25(6), 513–520. [DOI] [PubMed] [Google Scholar]
- Lu C, Black MM, & Richter LM (2016). Risk of poor development in young children in low-income and middle-income countries: an estimation and analysis at the global, regional, and country level. Lancet Glob Health, 4(12), e916–e922. doi: 10.1016/s2214-109x(16)30266-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby JL (2015). Poverty’s most insidious damage: The developing brain. JAMA. doi: 10.1001/jamapediatrics.2015.1682 [DOI] [PubMed] [Google Scholar]
- Machin D, Day S, & Green S (Eds.). (2007). Textbook on clinical trials (2nd ed.). New York: Wiley. [Google Scholar]
- McCoy DC, Salhi C, Yoshikawa H, Black M, Britto P, & Fink G (2018). Home-and center-based learning opportunities for preschoolers in low-and middle-income countries. Children and Youth Services Review, 88, 44–56. [Google Scholar]
- Miller AC, Murray MB, Thomson DR, & Arbour MC (2016). How consistent are associations between stunting and child development? Evidence from a meta-analysis of associations between stunting and multidimensional child development in fifteen low-and middle-income countries. Public Health Nutrition, 19(8), 1339–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L, Chan W, Comfort K, & Tirella L (2005). Health of children adopted from Guatemala: comparison of orphanage and foster care. Pediatrics, 115(6), e710–e717. [DOI] [PubMed] [Google Scholar]
- Mohite K (1992). Indian Adaptation of the HOME Scale. New Delhi, India: University of Delhi Library. [Google Scholar]
- Mullen EM (1995). Mullens Scales of Early Learning. AGS Edition. Circle Pines, MN; American Guidance Service. [Google Scholar]
- Murray-Kolb L, & MAL-ED Network Investigators. (2018). Early childhood cognitive development is affected by interactions among illness, diet, enteropathogens and the home environment: findings from the MAL-ED birth cohort study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Family Health Survey-4. (2015–16). http://rchiips.org/nfhs/pdf/NFHS4/TG_FactSheet.pdf. (accessed April 25, 2018).
- National Nutrition Monitoring Bureau. (2003). Prevalence of micronutrient deficiencies. National Institute of Nutrition, Indian Council of Medical Research. [Google Scholar]
- Ngure FM, Reid BM, Humphrey JH, Mbuya MN, Pelto G, & Stoltzfus RJ (2014). Water, sanitation, and hygiene (WASH), environmental enteropathy, nutrition, and early child development: making the links. Annals of the New York Academy of Sciences, 1308(1), 118–128. [DOI] [PubMed] [Google Scholar]
- Nguyen PH, Headey D, Frongillo EA, Tran LM, Rawat R, Ruel MT, & Menon P (2017). Changes in Underlying Determinants Explain Rapid Increases in Child Linear Growth in Alive & Thrive Study Areas between 2010 and 2014 in Bangladesh and Vietnam. The Journal of Nutrition, 147(3), 462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehaus MD, Moore SR, Patrick PD, Derr LL, Lorntz B, Lima AA, & Guerrant RL (2002). Early childhood diarrhea is associated with diminished cognitive function 4 to 7 years later in children in a northeast Brazilian shantytown. The American Journal of Tropical Medicine and Hygiene, 66(5), 590–593. [DOI] [PubMed] [Google Scholar]
- Obradović J, Yousafzai AK, Finch JE, & Rasheed MA (2016). Maternal scaffolding and home stimulation: Key mediators of early intervention effects on children’s cognitive development. Developmental Psychology, 52(9), 1409. [DOI] [PubMed] [Google Scholar]
- Perkins JM, Kim R, Krishna A, McGovern M, Aguayo VM, & Subramanian S (2017). Understanding the association between stunting and child development in low-and middle-income countries: Next steps for research and intervention. Social Science & Medicine. [DOI] [PubMed] [Google Scholar]
- Perumal N, Bassani DG, & Roth DE (2018). Use and misuse of stunting as a measure of child health.Journal of Nutrition,.148(3), 311–315 [DOI] [PubMed] [Google Scholar]
- Prendergast A, & Kelly P (2012). Enteropathies in the developing world: neglected effects on global health. The American Journal of Tropical Medicine and Hygiene, 86(5), 756–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast AJ, Rukobo S, Chasekwa B, Mutasa K, Ntozini R, Mbuya MN,… Humphrey JH (2014). Stunting is characterized by chronic inflammation in Zimbabwean infants. PloS one, 9(2), e86928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter LM, Daelmans B, Lombardi J, Heymann J, Boo FL, Behrman JR,… Dua T (2017). Investing in the foundation of sustainable development: pathways to scale up for early childhood development. The Lancet, 389(10064), 103–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Codina M, Attanasio O, & Grantham-McGregor S (2016). Mediating pathways in the socio-economic gradient of child development: Evidence from children 6–42 months in Bogota. International Journal of Behavioral Development, 40(6), 483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff JP (2010). Building a new biodevelopmental framework to guide the future of early childhood policy. Child Development, 81(1), 357–367. [DOI] [PubMed] [Google Scholar]
- Spears D, Ghosh A, & Cumming O (2013). Open defecation and childhood stunting in India: an ecological analysis of new data from 112 districts. PLoS One, 8(9), e73784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudfeld CR, Charles McCoy D, Danaei G, Fink G, Ezzati M, Andrews KG, & Fawzi WW (2015). Linear growth and child development in low- and middle-income countries: a meta-analysis. Pediatrics, 135(5), e1266–e1275. doi: 10.1542/peds.2014-3111 [DOI] [PubMed] [Google Scholar]
- Tarleton JL, Haque R, Mondal D, Shu J, Farr BM, & Petri WA Jr (2006). Cognitive effects of diarrhea, malnutrition, and Entamoeba histolytica infection on school age children in Dhaka, Bangladesh. The American Journal of Tropical Medicine and Hygiene, 74(3), 475–481. [PubMed] [Google Scholar]
- UNICEF. (2017). Reducing Stunting in Children Under 5 Years of Age: A Comprehensive Evaluation of UNICEF’s Strategies and Programme Performance, 2017. https://www.unicef.org/evaluation/files/Stunting_Evaluation_Synthesis_Report_Final_13_June_2017.pdf.
- UNICEF. (2018). Monitoring the situation for children and women. https://data.unicef.org/country/ind/.
- United Nations. (2015). Transforming our world: The 2030 agenda for sustainable development. New York: United Nations General Assembly; https://sustainabledevelopment.un.org/post2015/transformingourworld/publication. (accessed April 18, 2018). [Google Scholar]
- Vazir S, Engle P, Balakrishna N, Griffiths PL, Johnson SL, Creed-Kanashiro H,… Bentley ME (2013). Cluster-randomized trial on complementary and responsive feeding education to caregivers found improved dietary intake, growth and development among rural Indian toddlers. Maternal & Child Nutrition, 9, 99–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victora CG, Adair L, Fall C, Hallal PC, Martorell R, Richter L, & Sachdev HS (2008). Maternal and child undernutrition: consequences for adult health and human capital. The Lancet, 371, 340–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victora CG, de Onis M, Hallal PC, Blossner M, & Shrimpton R (2010). Worldwide timing of growth faltering: revisiting implications for interventions. Pediatrics, 125(3), e473–480. doi: 10.1542/peds.2009-1519 [DOI] [PubMed] [Google Scholar]
- Walker SP, Chang SM, Vera-Hernandez M, & Grantham-McGregor S (2011). Early childhood stimulation benefits adult competence and reduces violent behavior. Pediatrics, 127(5), 849–857. doi: 10.1542/peds.2010-2231 [DOI] [PubMed] [Google Scholar]
- Walker SP, Chang SM, Wright A, Osmond C, & Grantham-McGregor SM (2015). Early childhood stunting is associated with lower developmental levels in the subsequent generation of children. The Journal of Nutrition, 145(4), 823–828. doi: 10.3945/jn.114.200261 [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2007). The WHO Child Growth Standards, http://www.who.int/childgrowth/en/index.html.
- World Health Organization (2011) Hemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Vitamin and Mineral Nutrition Information System, WHO/NMH/NHD/MNM/11.1. Geneva: WHO; http://www.who.int/vmnis/indicators/haemoglobin/en/. (Accessed April 23, 2018). [Google Scholar]
- World Health Organization. (2012). Care for child development: http://www.who.int/maternal_child_adolescent/documents/care_child_development/en/. (Accessed April 23, 2018).
- World Health Organization. (2014). C-reactive protein concentrations as a marker of inflammation or infection for interpreting biomarkers of micronutrient status, http://www.who.int/vmnis/indicators/c-reactive_protein/en/ (accessed April 25, 2018).
- Yousafzai AK, Rasheed MA, Rizvi A, Armstrong R, & Bhutta ZA (2014). Effect of integrated responsive stimulation and nutrition interventions in the Lady Health Worker programme in Pakistan on child development, growth, and health outcomes: a cluster-randomised factorial effectiveness trial. The Lancet, 384(9950), 1282–1293. [DOI] [PubMed] [Google Scholar]