Abstract
Argininosuccinic aciduria (ASA) is an inherited urea cycle disorder and has a highly variable phenotypic spectrum ranging from individuals with lethal hyperammonemic encephalopathy, liver dysfunction and cognitive deterioration, to individuals with a mild disease course. Since it is difficult to predict the phenotypic severity, we aimed at identifying a reliable disease prediction model. We applied a biallelic expression system to assess the functional impact of pathogenic argininosuccinate lyase (ASL) variants and to determine enzymatic activity of ASL in 58 individuals with ASA. This cohort represented 42 ASL gene variants, and 42 combinations in total. Enzymatic ASL activity was compared to biochemical and clinical endpoints from the UCDC and E-IMD databases. Enzymatic ASL activity correlated with peak plasma ammonium concentration at initial presentation and with number of hyperammonemic events (HAEs) per year of observation. Individuals with ≤ 9% of enzymatic activity had more severe initial decompensations and a higher annual frequency of HAEs than individuals above this threshold. Enzymatic ASL activity also correlated with the cognitive outcome and the severity of liver disease, enabling a reliable severity prediction for individuals with ASA. Thus, enzymatic activity measured by this novel expression system can serve as an important marker of phenotypic severity.
Keywords: argininosuccinic aciduria, enzymatic ASL activity, predictive biomarker, disease course, clinical outcome
Introduction
Argininosuccinic aciduria (ASA) is an autosomal recessive urea cycle disorder (UCD) caused by deficiency of the cytosolic enzyme argininosuccinate lyase (ASA; MIM# 207900) due to pathogenic variants in the argininosuccinate lyase (ASL) gene located on chromosome 7q11.21. ASA is the second or third most common UCD with an estimated overall incidence of 1 in 220,000 newborns (Batshaw, Tuchman, Summar, Seminara, & Members of the Urea Cycle Disorders, 2014; Posset, Garbade, et al., 2019). Individuals with ASA can present with a variable clinical phenotype ranging from severe life-threatening hyperammonemic events (HAEs) within the neonatal period (early onset, EO) to mild-to-moderate symptoms, such as headache, recurrent vomiting, and cognitive impairment any time after the newborn period (late onset, LO) even in the absence of recurrent HAEs (Baruteau et al., 2019; Kolker, Garcia-Cazorla, et al., 2015; Kolker, Valayannopoulos, et al., 2015). A recent meta-analysis demonstrated that neonatal mortality in EO ASA is up to 20% of newborns with only one third of survivors having a normal cognitive outcome by the end of the first year of life (Burgard, Kolker, Haege, Lindner, & Hoffmann, 2016). Recently, it was demonstrated that early identification of individuals with ASA by newborn screening programs (NBS) might improve the cognitive outcome; however, ASA is still not consistently included in NBS programs worldwide (Lindner et al., 2011; Mercimek-Mahmutoglu et al., 2010; Posset, Gropman, et al., 2019). Noteworthy, individuals with ASA often suffer from progressive hepatic disease, neurological and cognitive deficits in spite of having lower frequencies of HAEs. This is substantiated by recent findings that the severity of the initial HAE is an important but not the only factor associated with a favorable or poor cognitive outcome (Baruteau et al., 2019; Posset, Gropman, et al., 2019; Waisbren, Gropman, Members of the Urea Cycle Disorders, & Batshaw, 2016). The paradox reflecting that individuals with ASA perform worse in cognitive testing as compared to other UCDs despite lower frequencies of HAEs, suggests that other ammonia-independent pathomechanisms might underlie the disease, such as disturbed NO metabolism causing cerebral oxidative/nitrosative stress (Baruteau et al., 2018). Moreover, interallelic complementation between specific variants has been shown to contribute to phenotypic variability in ASA (McInnes, Shih, & Chilton, 1984; Walker et al., 1997).
Recently, we have been able to show that enzymatic activity of argininosuccinate synthetase 1, as determined by a newly established biallelic expression system, predicts the clinical disease course and neurocognitive outcome in individuals with citrullinemia type 1 (CTLN1) (Zielonka, Kolker, et al., 2019). To analyse whether this approach might also be of predictive value for the heterogeneous clinical phenotype in individuals with ASA and to quantitatively determine the effect of the various pathogenic variant combinations, we applied the mammalian biallelic expression system to ASA for the functional investigation of exonic variants and correlated enzymatic ASL activity with individuals’ biochemical, clinical and neurological follow-up data, retrieved from the two largest observational natural history studies worldwide, i.e. the North American Urea Cycle Disorders Consortium (UCDC; https://www.rarediseasesnetwork.org/cms/ucdc) and the European registry and network for Intoxication type Metabolic Disease (E-IMD; https://www.eimd-registry.org/).
Materials and Methods
Eligibility criteria
Only individuals from the UCDC and E-IMD patient registries with confirmed ASA and unequivocal molecular genetic test results of ASL were included into this analysis. A detailed description of the data model of both registries, information on written informed consent as well as the follow-up protocols used have been recently described in detail (Posset, Garbade, et al., 2019; Posset, Gropman, et al., 2019). Requirements set forth by the ICMJE (International Committee of Medical Journal Editors) were met. All procedures followed were in accordance with the ethical standards of the Helsinki Declaration of 1975, as revised in 2013. Data were retrieved from the UCDC and E-IMD electronic databases with cut-off for data pull on October 10, 2018.
Plasmids
Strategy of FLAG-and MYC-tagged wildtype expression vector generation has been described in detail previously (Zielonka, Kolker, et al., 2019). Briefly, the ASL coding sequence was amplified by PCR from cDNA (human fibroblasts) with FLAG or MYC-tags introduced at the C-or N-terminus using specific primer pairs and inserted into the BamHI and NotI restriction sites in the open-reading frame of the eukaryotic expression vector pcDNA5 (Thermo Fisher Scientific). The amplified and subcloned ASL coding sequence corresponded to NCBI reference sequence NM_001024943.2 (https://www.ncbi.nlm.nih.gov/nuccore/NM_001024943). Pathogenic ASL gene variants from individuals with ASA reported in the E-IMD and UCDC registries were introduced into the tagged ASL expression vectors using the QuickChange II site-directed mutagenesis kit (Agilent) applying the manufacturer’s protocol. Missense variants were introduced into the C-terminally tagged ASL expression vectors, whereas nonsense variants (variants associated with a premature stop codon or frameshift variants) were introduced into expression vectors harboring a N-terminal tag. All ASL variants reported in this study have been checked including the provided nomenclature applying the Mutalyzer 2.0.31 software (https://mutalyzer.nl/). The wildtype ASL expression vectors and the correct insertion of the variants were confirmed by Sanger-sequencing. N. Himmelreich (Heidelberg University, Germany) kindly provided the pSV-β-galactosidase control vector (Promega).
Cell culture and transfections
COS-7 cells were maintained as adherent cell culture in 10 cm petri-dishes in DMEM medium (Thermo Fisher Scientific) supplemented with 10% heat-inactivated fetal bovine serum in a humified incubator at 37°C and 5% CO2. Transfections were performed with 2.5 μg of each FLAG-and MYC-tagged ASL-plasmid and 1 μg of β-galactosidase reporter plasmid using Lipofectamin 2000 reagent (Thermo Fisher Scientific). Forty-eight hours after transfection, cells were washed twice with ice-cold phosphate-buffered saline (PBS), and lysed. Subsequently, the lysates were subjected to qRT-PCR, Western blot analysis or spectrophotometric determination of enzymatic ASL activity.
qRT-PCR
RNA was extracted with TRIzol reagent (Invitrogen) by standard procedure as previously described (Zielonka, Kolker, et al., 2019). Briefly, equal amounts from each sample (500 to 800 ng) were used for cDNA syntheses using the Maxima first strand cDNA synthesis kit (Thermo Fisher Scientific). Real-time qPCR was performed on a CFX Connect™ 180 Real-Time cycler (Biorad) (denaturation step: 95°C for 25s, annealing and elongation step: 60°C for 30s) using SensiFast SYBR™ Hi-ROX mix (Bioline) with the following primers:
Tagged-ASL-plasmids (N-terminus):
N-FLAG_forward: 5’-CTACAAAGACGATGACGACAAG-3’
N-MYC_forward: 5’-GAAGAGGATCTGGGAGGTTCAGG-3’
N-tag_reverse: 3’-TAGGCTTTGCTGCCTTGAA-5’
Tagged-ASL-plasmids (C-terminus):
C-tag_forward: 5’-TTCTCGGGCGACGTGATCT-3’
C-FLAG_reverse: 3’-GTCGTCATCGTCTTTGTAGTCG-5’
C_MYC_reverse: 3’-GTTTTTGTTCGCTGCCTCCTG-5’
β-galactosidase:
Galactosidase_forward: 5’-CAGGGTTTTCCCAGTCACGA-3’
Galactosidase_reverse: 3’-CAGGGTTTTCCCAGTCACGA-3’
The expression level of β-galactosidase was used for normalization.
Western Blot
COS-7 cells were washed twice in ice-cold PBS and lysed in 1x ice-cold radioimmunoprecipitation buffer (600 mM NaCl, 100 mM TRIS-HCl pH 7.4, 10 mM EDTA, 2% Triton X-100, 0.2% SDS, 1% sodium deoxycholate) prior to sonification. Lysates were then centrifuged at 13,000 x g and 4°C for 10 minutes. Western blotting was carried out using supernatants according to standard laboratory protocols. For protein visualization, membranes were probed with the following primary antibodies, each at a dilution of 1:2,000: anti-FLAG (BioLegend), anti-MYC (Cell Signaling) and anti-β-Actin (Sigma-Aldrich).
Spectrophotometric analysis of enzymatic ASL activity
Purified citrate synthase, malate dehydrogenase, and fumarase from porcine heart were obtained from Sigma-Aldrich. ASL enzyme activity was determined in transfected COS-7 cell lysates (triplicates) in a buffer containing 10 mmol/l potassium phosphate, 10 mmol/l TRIS-HCl, 650 mU/ml fumarase, 660 mU/ml malate dehydrogenase, 400 mU/ml citrate synthase, 2 mmol/l argininosuccinic acid, 330 μmol/l NAD and 100 μmol/l acetyl-CoA, which was adjusted to pH 7.4 (25°C). ASL enzyme activity was determined as NAD reduction by measuring the absorbance change at λ=340 using the absorbance at λ=400 nm as baseline (identical for NAD and NADH). Lysates of COS-7 cells transfected with the empty pcDNA5 vector served as negative control. To adjust for background ASL activity, negative control values were subtracted from ASL activities.
ASL activities were normalized to the β-galactosidase activity in the respective samples as determined by the β-galactosidase enzyme assay system (Promega) in order to control for transfection efficacy. The adjusted ASL activities were normalized to the protein content in each sample. Activities are depicted as percentage of total (%) by dividing the normalized ASL activity of a specific variant combination (homozygous/compound heterozygous) by the normalized wildtype ASL activities.
Clinical variables used for data analyses
Clinical variables used for data analyses were retrieved from the UCDC and E-IMD databases as recently reported (Zielonka, Kolker, et al., 2019). The following numerical clinical variables were used: Peak plasma ammonium concentration (NH4+max), number of HAEs (with NH4+max > 100 μmol/l) per year of observation (defined as time between date of birth and last regular visit), NH4+max during the most severe hyperammonemic decompensation, and cognitive standard deviation scores (SDS) at the most recent visit being calculated using the normative data from the standardization sample of each cognitive test. The most recent visit was chosen since this most likely represents the true outcome. For each Hamburg-Wechsler-Intelligenztest für Kinder/Erwachsene (n=3/30), Wechsler Abbreviated Scale of Intelligence (n=6/30), Wechsler Intelligence Scale for Children (n=3/30), and Wechsler Preschool and Primary Scale of Intelligence (n=3/30) full scale IQ was selected. For Bayley Scales of Infant Development (n=12/30) mental developmental index and cognitive scale were applied; for Adaptive Behavior Assessment System (n=3/30) the general adaptive composite was used (Buerger et al., 2019).
The following categorical clinical variables were also included in this study: disease onset (EO, LO, asymptomatic), presence or absence of movement disorder (dystonia and/or chorea and/or ataxia), tone change (muscular hypotonia and/or muscular hypertonia and/or spasticity), hepatocellular injury (alanine aminotransferase ≥ 250 U/l or aspartate aminotransferase ≥ 250 U/l), and kidney dysfunction [full age spectrum glomerular filtration rate (FAS-GFR) ≥ 90 ml/min/1.73m2 versus FAS-GFR < 90 ml/min/1.73m2] (Gallagher, Lam, Wong, Cederbaum, & Sokol, 2014; Pottel et al., 2016).
For symptomatic individuals with or without reported HAE during the initial presentation/decompensation, NH4+max represents the highest value prior to initiation of treatment. For asymptomatic individuals, NH4+max represents the highest reported NH4+ concentration during the observation period; only untreated asymptomatic individuals were considered eligible for this analysis. We investigated the impact of the cumulative enzymatic ASL activity as determined by the established biallelic expression system on clinical outcome parameters outlined above. Mortality could not be examined (Posset, Garbade, et al., 2019).
Statistical analysis
Statistical analyses were performed as previously described (Zielonka, Kolker, et al., 2019). All analyses were performed using R (http://www.r-project.org). To evaluate the relationship between a continuous dependent variable and the cumulative enzymatic ASL activity as predictor variable, a generalized additive regression model (GAM) with automated smoothing selections was used (Wood, 2011). In GAM, the linear relationship between the response variable and predictors are replaced by nonlinear smooth functions. The R package “mgcv” was used to fit GAM regressions. Unbiased recursive partitioning was used to determine cut-off values for the impact of enzymatic ASL activity on different outcome variables (Hothorn, Hornik, & Zeileis, 2006). Two groups where compared with a t-test with Welch correction. P values reported were two-sided. P ≤ 0.05 was considered statistically significant.
Results
Description of study population and ASL protein functions
We systematically assessed the impact of pathogenic ASL variants on the mRNA and protein expression levels and determined enzymatic ASL activities in a group of 58 individuals with ASA, representing 42 ASL gene variants, and 42 different, mostly compound heterozygous combinations in total. Detailed descriptive characteristics of the study population for each subsequent analysis are illustrated in Table 1. Information on pathogenic ASL variant combinations (on the cDNA and protein level) per patient including corresponding biochemical and clinical data are depicted in Supp. Table S1.
Table 1.
Descriptive characteristics for correlation analyses in ASA
| Correlation between enzymatic ASL activity and peak plasma NH4+ concentration | |||
|---|---|---|---|
| ASL activity (%) | Peak plasma NH4+ concentration (μmol/l) | Peak plasma NH4+ concentration μmol/l), if ASL activity ≤ 7.9% | Peak plasma NH4+ concentration (μmol/l), if ASL activity > 7.9% |
| Mean, SD | Mean, SD | Mean, SD | Mean, SD |
| Median [Q1, Q3] | Median [Q1, Q3] | Median [Q1, Q3] | Median [Q1, Q3] |
| Min, Max; n | Min, Max; n | Min, Max; n | Min, Max; n |
| 31.1, 25.8 | 410.3, 704.7 | 998.0, 894.1 | 65.7, 92.6 |
| 34.2 [2.7, 57.3] | 61.5 [33.4, 466.0] | 737.6 [312.0, 1240.0] | 35.8 [28.0, 55.0] |
| 0, 70.5; 46 | 9.0, 3600.0; 46 | 186.0, 3600.0; 17 | 9.0, 478.0; 29 |
| Correlation between enzymatic ASL activity and number of hyperammonemic events (HAE) per year | |||
|
ASL activity
(%) |
Number of HAE
per year |
Number of HAE per year,
if ASL acitivity ≤ 8.7% |
Number of HAE per year,
if ASL acitivity > 8.7% |
| Mean, SD | Mean, SD | Mean, SD | Mean, SD |
| Median [Q1, Q3] | Median [Q1, Q3] | Median [Q1, Q3] | Median [Q1, Q3] |
| Min, Max; n | Min, Max; n | Min, Max; n | Min, Max; n |
| 35.0, 24.7 | 0.30, 0.56 | 0.85, 0.75 | 0.07, 0.21 |
| 41.5 [7.2, 58.0] | 0 [0, 0.43] | 0.51 [0.42, 1.04] | 0 [0, 0] |
| 0, 66.0; 35 | 0, 2.56; 35 | 0, 2.56; 10 | 0, 0.95; 25 |
| Correlation between enzymatic ASL activity and peak plasma NH4+ concentration during most severe HAE | |||
| ASL activity (%) | Peak plasma NH4+ concentration (μmol/l) | n/a | |
| Mean, SD | Mean, SD | ||
| Median [Q1, Q3] | Median [Q1, Q3] | ||
| Min, Max; n | Min, Max; n | ||
| 28.7, 27.6 | 581.3, 866.4 | ||
| 18.5 [1.5, 53.6] | 200.0 [34.0, 909.0] | ||
| 0, 66.0; 19 | 13.0, 3600.0; 19 | ||
| Correlation between enzymatic ASL activity and cognitive SDS at last regular visit | |||
| ASL activity (%) | Cognitive SDS (z-score) |
Cognitive SDS,
if ASL activity ≤ 24.3% |
Cognitive SDS,
if ASL activity > 24.3% |
| Mean, SD | Mean, SD | Mean, SD | Mean, SD |
| Median [Q1, Q3] | Median [Q1, Q3] | Median [Q1, Q3] | Median [Q1, Q3] |
| Min, Max; n | Min, Max; n | Min, Max; n | Min, Max; n |
| 35.5, 24.7 | −1.4, 1.3 | −2.3, 1.0 | −0.9, 1.1 |
| 43.3 [8.8, 56.9] | −1.3 [−2.3, −0.6] | −2.3 [−3.0, −1.6] | −1.0 [−1.4, −0.2] |
| 0, 70.5; 30 | −3.5, 1.3; 30 | −3.5, −0.7; 11 | −3.1, 1.3; 19 |
| Correlation between enzymatic ASL activity and presence of hepatocellular injury (HCI) | |||
| ASL activity of individuals with HCI (%) | ASL acitivity of individuals w/o HCI (%) | HCI, if ASL activity ≤ 8.7% (% of total) | HCI, if ASL activity > 8.7% (% of total) |
| Mean, SD | Mean, SD | ||
| Median [Q1, Q3] | Median [Q1, Q3] | ||
| Min, Max; n | Min, Max; n | ||
| 9.1, 19.5 | 40.5, 23.5 | 8/17 = 47.0% | 1/39 = 2.6% |
| 1.2 [1.2, 5.6] | 49,4 [20.5, 59.1] | ||
| 0, 60.6; 9 | 0, 70.5; 47 | ||
Descriptive characteristics for the correlation between enzymatic ASL activity in 58 individuals with ASA and peak plasma NH4+ concentration, number of HAE per year, peak plasma NH4+ concentration during most severe HAE, cognitive SDS at last regular visit, and presence of HCI, respectively. Since the numbers of individuals (n) with information on specific biochemical or clinical endpoints vary, the n for each specific analysis is presented in the leftmost cell of each row. For the correlation between ASL activity and presence of HCI, altogether 56 individuals were considered appropriate. Of note, correlation between enzymatic ASL activity and peak plasma NH4+ concentration, number of HAE per year, and HCI showed nearly identical threshold values for ASL activity. ASL(-D), argininosuccinic aciduria, HAEs, hyperammonemic events defined as NH4+ > 100 μmol/l, HCI, hepatocellular injury defined as presence of alanine aminotransferase and or aspartate aminotransferase ≥ 250 U/l; n, number of patients with information for the respective biochemical or clinical endpoint.
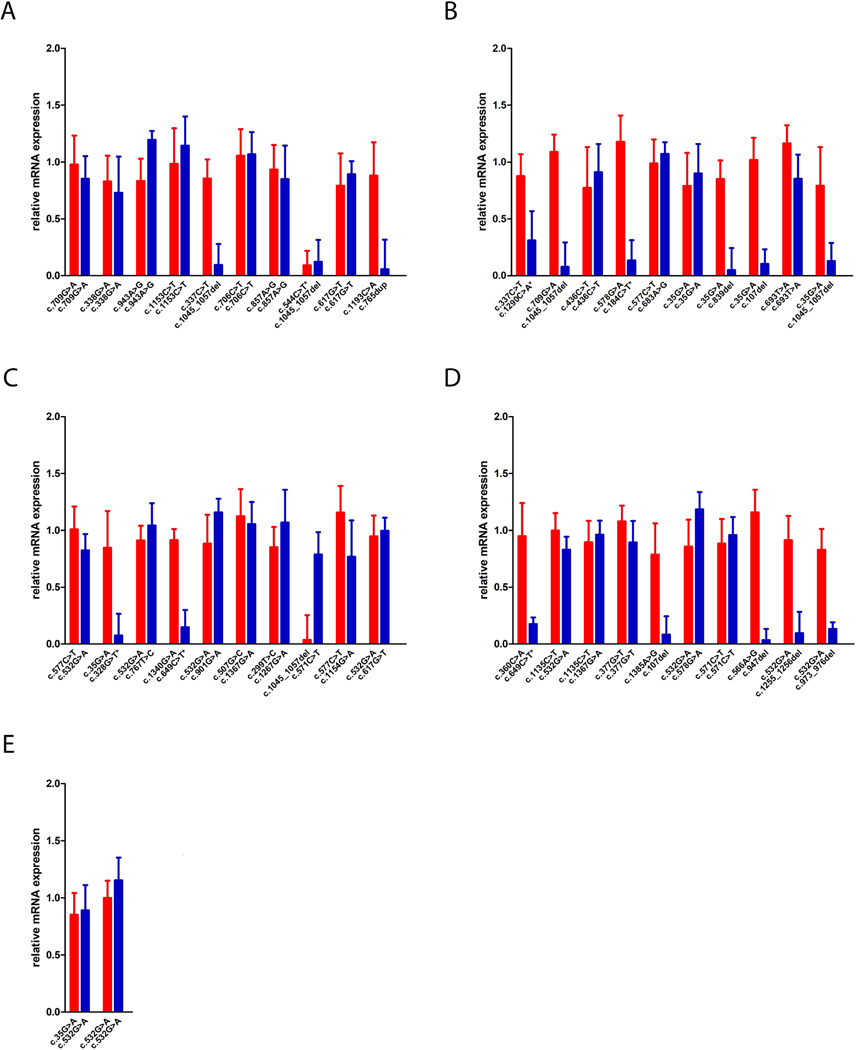
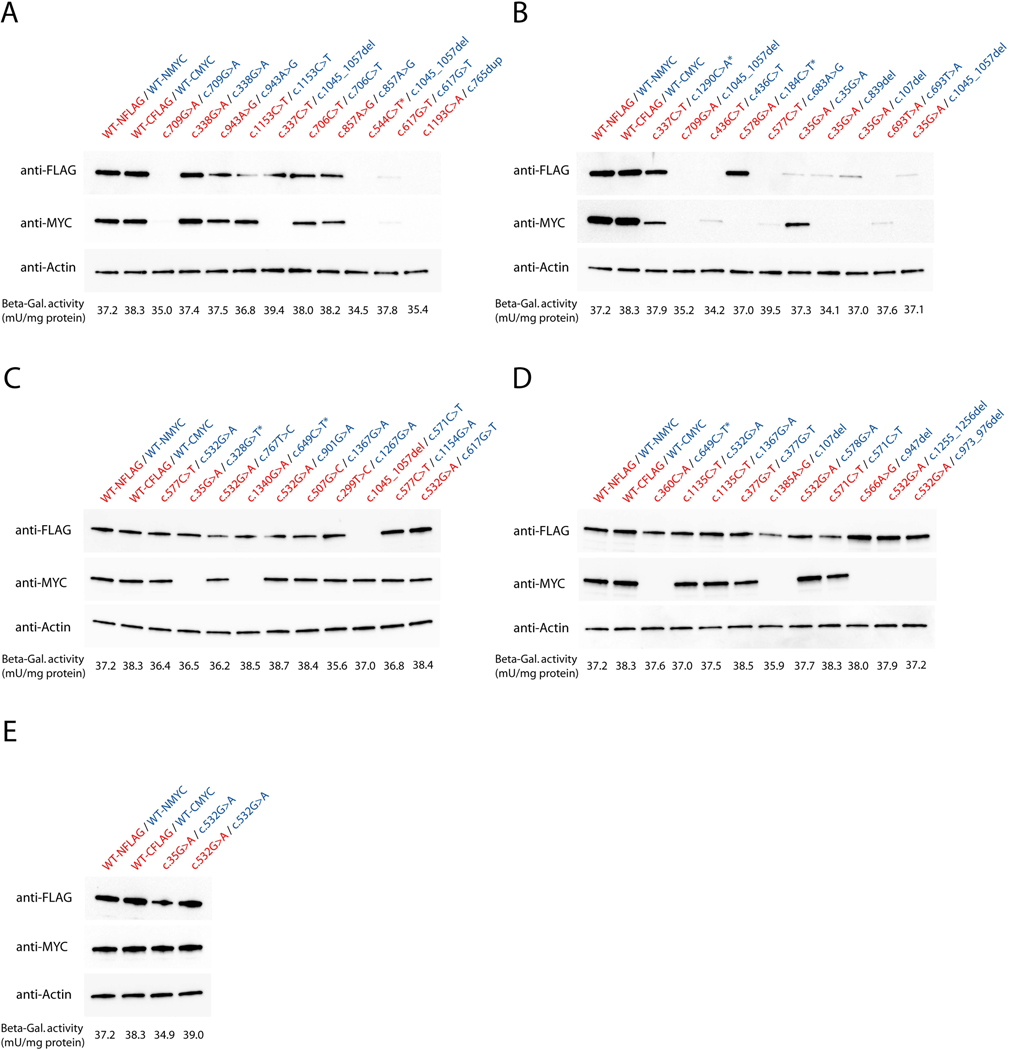
Whereas the majority of ASL missense variants showed unaltered mRNA expression levels as determined by qRT-PCR, the mRNA expression of nonsense variants (c.107del, c.765dup, c.947del, c.973_976del, c.1045_1057del, c.1255_1256del) as well as variants with the presence of a premature stop codon (c.184C>T, c.328G>T, c.544C>T, c.649C>T, c.1290C>A) was significantly reduced (Figure 1), which was associated with complete lack of protein expression (Figure 2). In contrast, ASL missense variants showed variable expression levels depending on the underlying variant. Intriguingly, protein expression for a specific variant was in part dependent on the second variant, which was most pronounced for the variant c.35C>G (Figure 2). Full-size images of Western blot analysis are depicted in Supp. Figure S1.
Figure 1. Overview of relative mRNA expression levels per variant combination.
COS-7 cells were transfected with 2.5 μg of each FLAG-and MYC-tagged ASL expression vectors and 1 μg of β-galactosidase reporter plasmid, cultured for 48 hours and subjected to quantitative analysis of mRNA expression applying qRT-PCR. Data are expressed as fold-change (mean +/− SD) normalized to the relative expression of the respective ASL wildtype plasmids (A-E; n=3 for each experiment). Red columns illustrate FLAG-tagged expression vectors, blue columns represent MYC-tagged plasmids. Variants associated with a premature stop codon are indicated by an asterisk (*).
Figure 2. Overview of protein expression levels per variant combination.
COS-7 cells were transfected with 2.5 μg of each FLAG-and MYC-tagged ASL expression vectors and 1 μg of β-galactosidase reporter plasmid, cultured for 48 hours and subjected to protein expression analysis using standard Western blot technique. Briefly, proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and blotted on nitrocellulose membranes using the Trans-Blot® Turbo Transfer System (BioRad). Expression of FLAG-or MYC-tagged ASL variants was visualized using anti-FLAG-or anti-MYC antibodies on two identical gels, which were carried in parallel (A-E). Equal protein loading in cell lysates was confirmed by immunoblotting using an anti-β-actin antibody. Activity of β-galactosidase per variant combination was measured applying the β-galactosidase enzyme assay system (Promega). Data are expressed as mean in mU/mg protein (A-E, n=3). Of note, full-size images of anti-FLAG and anti-MYC stainings per variant combination are depicted in Supp. Figure S1. Variants associated with a premature stop codon are indicated by an asterisk (*). Red letters illustrate FLAG-tagged expression vectors, blue letters represent MYC-tagged plasmids.
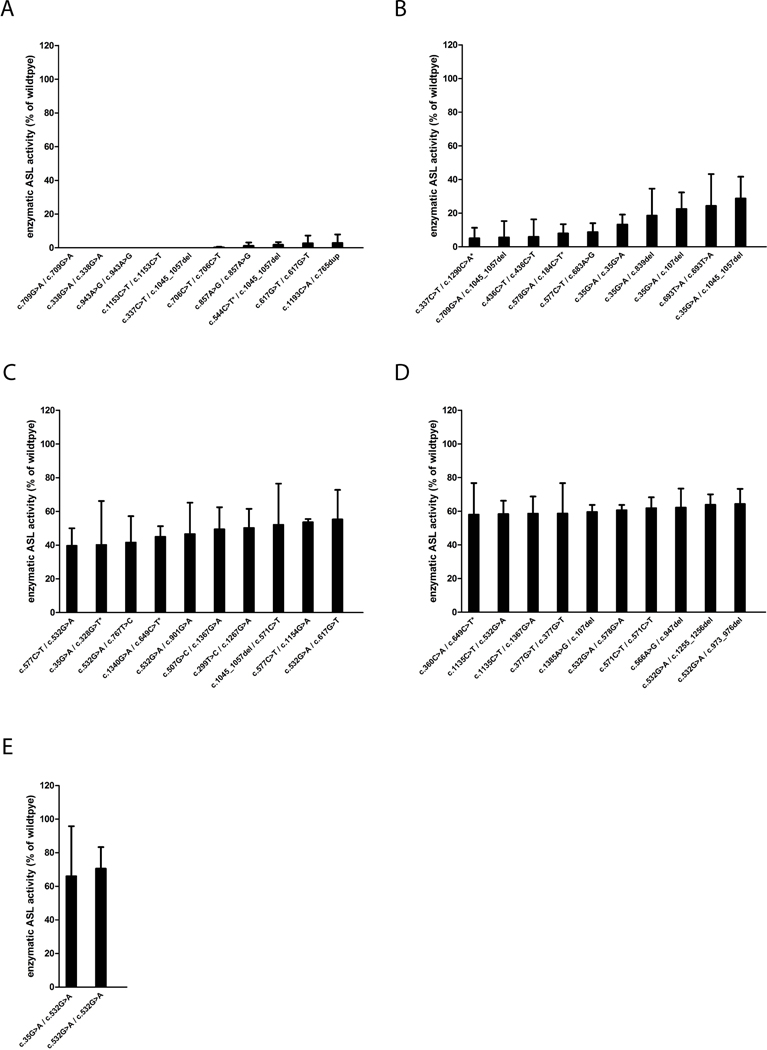
Results of enzymatic ASL activity per variant are depicted in Figure 3. Notably, individuals with EO ASA showed lower enzymatic ASL activities than LO (p=0.004, t-test) or asymptomatic individuals (p<0.001, t-test); further details are depicted in Supp. Figure S2.
Figure 3. Overview of enzymatic ASL activities per variant combination.
COS-7 cells were transfected with 2.5 μg of each FLAG-and MYC-tagged ASL expression vectors and 1 μg of β-galactosidase reporter plasmid, cultured for 48 hours and enzymatic ASL activities determined applying a spectrophotometric assay as described under Material and methods. Data are expressed as mean +/− SD in % of ASL wildtype activity (A-E, n=3 for each experiment). Variants associated with a premature stop codon are indicated by an asterisk (*).
Enzymatic ASL activity correlates with the disease course and cognitive outcome in ASA
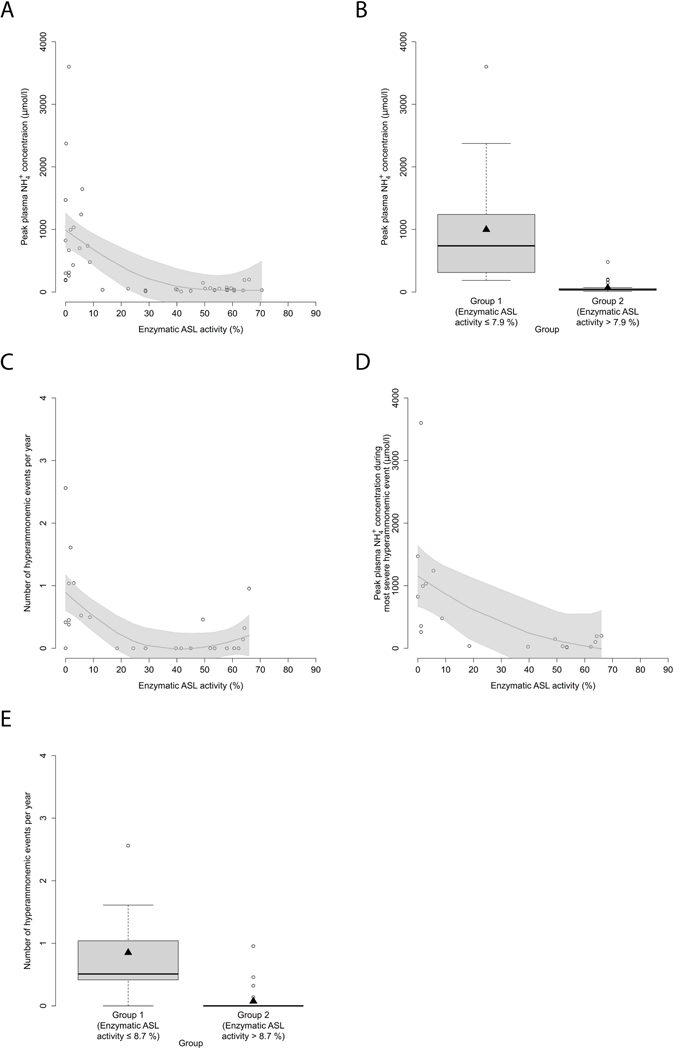
Since we have recently been able to show that NH4+max and L-citrulline concentrations at initial decompensation are determined by enzymatic ASS1 activity in CTLN1 (Zielonka, Kolker, et al., 2019), we first investigated whether biochemical parameters at initial decompensation are also associated with the degree of enzymatic dysfunction in ASA and hence reflect disease severity. Notably, initial NH4+max inversely correlated with the underlying enzymatic ASL activity (n=46, p<0.001, R2=0.36, GAM analysis; Figure 4A) as determined in the mammalian biallelic expression system. Moreover, we identified a threshold distribution for ASA with a enzymatic ASL activity below or equal to 7.9% leading to higher initial NH4+max as opposed to individuals with a enzymatic ASL activity above this threshold (n=46, p<0.001, recursive partitioning, Figure 4B, Table 1). Intriguingly, individuals with an enzymatic ASL activity below or equal to 7.9% identified by newborn screening, high risk family screening or prenatal testing exhibited low NH4+max at initial decompensation comparable to individuals with an enzymatic ASL activity above the threshold (Supp. Table S1), suggesting that early diagnosis and treatment might have a beneficial effect on the metabolic course in ASA. In the next step, we assessed whether enzymatic ASL activity correlates and therefore might predict disease severity as reflected by the reported number of hyperammonemic events (HAEs) per year of observation, NH4+max during the most severe HAE and the cognitive SDS of individuals with ASA at last follow-up visit. Enzymatic ASL activity was not only associated with the number of HAEs per year (n=35, p<0.001, R2=0.39, GAM analysis, Figure 4C), but also with NH4+max during the most severe hyperammonemic decompensation (n=19, p=0.019, R2=0.32, GAM-analysis, Figure 4D). Intriguingly, individuals with a enzymatic ASL activity below or equal to 8.7% showed a significantly higher number of HAEs per year as opposed to individuals with a ASL activity above this threshold (n=35, p=0.004, recursive partitioning, Figure 4E, Table 1).
Figure 4. Enzymatic ASL activity correlates with initial NH4+max as well as annual frequency and severity of HAEs.
(A) NH4+max (μmol/l) subject to enzymatic ASL activity as determined in the mammalian biallelic expression system. Each point represents a single patient (n=46). Gray line displays estimated regression curve. GAM analysis, p<0.001, R2=0.36. (B) Boxplot illustrating NH4+max (μmol/l) with a enzymatic activity below or equal to 7.9% (n=17) and above 7.9% (n=29). Data are shown as median (black thick line) and mean (triangle), length of the box corresponds to interquartile range (IQR), upper and lower whiskers correspond to max. 1.5 x IQR, each point represents an outlier. Recursive partitioning, p<0.001. (C) Number of HAEs (NH4+max ≥ 100 μmol/l) per year subject to enzymatic ASL activity (%). Each point represents a single patient (n=35). Gray line displays estimated regression curve. GAM analysis, p<0.001, R2=0.39. (D) NH4+max during most severe HAE subject to enzymatic ASL activity (%). Each point represents a single patient (n=19). Gray line displays estimated regression curve. GAM analysis, p=0.019, R2=0.32. (E) Boxplot illustrating number of HAEs (NH4+max ≥ 100 μmol/l) per year with a enzymatic ASS1 activity below or equal to 8.7% (n=10) or above 8.7% (n=25). Data are shown as median (black thick line) and mean (triangle), length of the box corresponds to IQR, upper and lower whiskers correspond to max. 1.5 x IQR, each point represents an outlier. Recursive partitioning, p=0.004. ASL, argininosuccinate lyase.
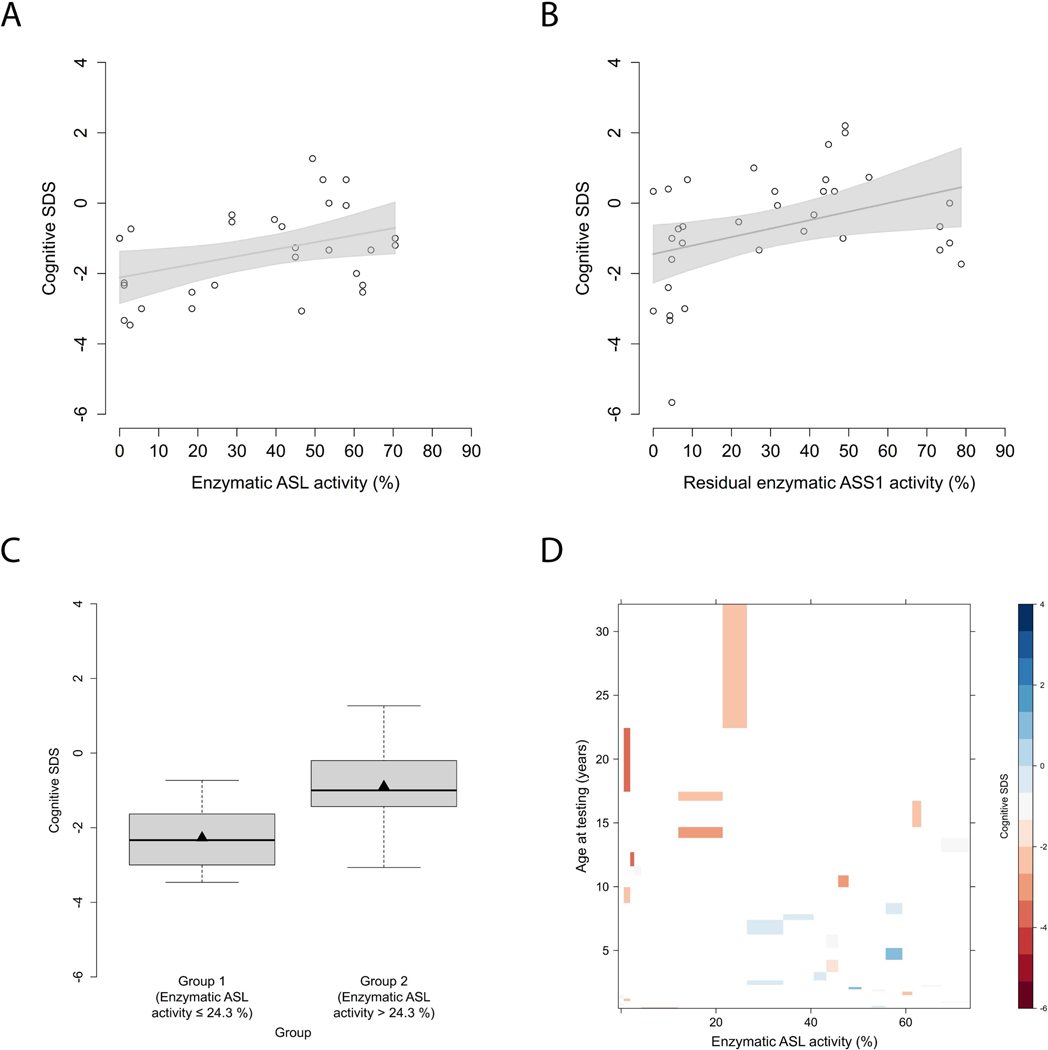
Next, we investigated whether enzymatic ASL activity is associated with the neurocognitive outcome as reflected by the cognitive SDS at last follow-up visit, and found an association (n=30, p=0.032, R2=0.15, linear analysis; Figure 5A), analogously to CTLN1 (Figure 5B) (Zielonka, Kolker, et al., 2019). Age-adjusted individuals with a enzymatic ASL activity below or equal to 24.3% performed worse at last cognitive testing than individuals above 24.3% (n=30, p=0.034, recursive partitioning, Figure 5C, Table 1). Of note, older individuals with ASA perform worse in cognitive testing than younger individuals (Figure 5D).
Figure 5. Neurocognitive outcome is associated with enzymatic ASL activity.
(A) Cognitive SDS subject to enzymatic ASL activity (%). Each point represents a single patient (n=30). Gray line indicates linear regression curve. Linear regression, p=0.032, R2=0.15. (B) Cognitive SDS subject to enzymatic ASS1 activity (%). Each point represents a single patient (n=34). Gray line indicates linear regression curve. Linear regression, p=0.029, R2=0.14. Figure adapted from (Zielonka, Kolker, et al., 2019). (C) Boxplot illustrating cognitive SDS with an enzymatic ASL activity below or equal to 24.3% (n=11) and above 24.3% (n=19). Data are shown as median (black thick line) and mean (triangle), length of the box corresponds to IQR, upper and lower whiskers correspond to max. 1.5 x IQR. Recursive partitioning, p=0.034. (D) Levelplot for cognitive SDS, enzymatic ASL activity (%) and age at testing (years). Cognitive SDS values are indicated by color coding in grading from blue to red with descending cognitive SDS. ASL, argininosuccinate lyase; ASS1, argininosuccinate synthetase 1.
Enzymatic ASL activity is associated with hepatic, but not with renal or neurological outcome parameters
Given the correlations of enzymatic ASL activity with the metabolic disease course and neurocognitive outcome, we next investigated whether this observation also holds true for other clinical outcome parameters, such as presence of hepatocellular injury, movement disorder, tone change and impaired kidney function. Individuals with a enzymatic ASL activity below or equal to 8.7% experienced more often episodes of hepatocellular injury as opposed to individuals with a enzymatic ASL activity above this threshold (n=56, p<0.001, recursive partitioning, Supp. Figure S3, Table 1). In contrast to this observation, enzymatic ASL activity did neither discriminate between individuals with or without movement disorders (n=55, p=0.881, Welch two sample t-test) nor between individuals with or without abnormalities of muscular tone (n=56, p=0.23, Welch two sample t-test). In addition, individuals with (FAS-GFR < 90 ml/min/1.73m2) or without (FAS-GFR ≥ 90 ml/min/1.73m2) reported episodes of impaired kidney function could not be differentiated by enzymatic ASL activity (n=48, p=0.21, Wilcoxon rank sum test).
Discussion
By applying a recently established biallelic mammalian expression system for the functional investigation of all exonic ASL variants reported in the UCDC and E-IMD databases, we demonstrate that enzymatic ASL activity reliably determines the metabolic disease course and phenotypic severity in ASA. Individuals with a enzymatic ASL activity below or equal to 8.7% not only had higher initial NH4+max and more frequent HAEs per year of observation, but also experienced more often episodes of hepatocellular injury. In addition, enzymatic ASL activity at a threshold of 24.3% enabled to discriminate between individuals with a poor (cognitive SDS ≤ −2) and favorable (cognitive SDS > −2) cognitive outcome.
Impact of pathogenic ASL variants on mRNA and protein expression as basis for future pathomechanistic investigations
The systematic characterization of the functional impact of specific pathogenic ASL variants on mRNA and protein expression levels is of importance for further detailed pathomechanistic investigations and might have direct implications for the development of individualized therapeutic approaches. ASL variants with the presence of a premature stop codon as well as nonsense variants did show a significantly decreased mRNA expression associated with complete lack of protein expression. Notably, premature termination codon read-through has been shown to increase protein expression and enzymatic activity in a subset of variants in aspartylglucosaminuria and might therefore be a novel therapeutic option for ASA in the future (Banning, Schiff, & Tikkanen, 2018). Moreover, further detailed analysis of variants associated with decreased ASL protein expression might reveal enhanced protein degradation or decreased protein stability as underlying pathomechanisms (e.g., missense variants), which might be therapeutically addressed by specific chaperones as already proven clinically beneficial for Fabry disease (Germain et al., 2019).
Modelling evidence-based thresholds for the clinical disease course of individuals with ASA
Since it has been recently shown that enzymatic ASS1 activity is a predictive biomarker in CTLN1, we wondered whether this might also hold true for the clinically more complex disease ASA, caused by inherited deficiency of ASL, the enzymatic step succeeding ASS1 in the urea cycle. Indeed enzymatic ASL activity reliably predicts the (initial) metabolic derangement as reflected by correlation with the severity of initial NH4+max, and the annual frequency of HAEs. Whereas threshold distributions in ASA for the initial NH4+max and annual frequency of HAEs correspond to 7.9% and 8.7%, respectively, the threshold distribution for both clinical endpoints corresponds to 8.1% in CTLN1 (Zielonka, Kolker, et al., 2019). Moreover, individuals with ASA below and above the respective threshold developed a similar annual frequency of HAEs as individuals with CTLN1 (Table 1) (Zielonka, Kolker, et al., 2019). These evidence-based data suggest essentially identical threshold values in the context of measuring accuracy of our biallelic expression system, demonstrating a similar biochemical (initial NH4+max) and metabolic disease course (number of HAEs per year of observation) for both disorders. Moreover, individuals with an activity below or equal to 8.7% do also exhibit more often episodes of hepatocellular injury in ASA. Similar thresholds for biochemical (initial NH4+max), metabolic (number of HAEs per year of observation), and hepatological (hepatocellular injury) endpoints are not unexpected, since it has recently been demonstrated that elevated ammonium concentrations induce a transamination-dependent withdrawal of 2-oxoglutarate from the tricarboxylic acid cycle, ultimately causing bioenergetic failure and cellular dysfunction in a zebrafish model of acute hyperammonemic encephalopathy and primary hepatocytes (Wang et al., 2014; Zielonka et al., 2018; Zielonka, Probst, et al., 2019). However, the association of enzymatic ASL activity and hepatocellular injury should be considered somewhat exploratory, given that individuals with ASA might have asymptomatic elevations of liver enzymes that might not have been captured appropriately in this study. In addition, severity of hepatocellular injury is hard to reliably judge by solely investigating for transaminase elevations. Given these limitations, future imaging studies characterizing morphological liver alterations are indispensable to unequivocally assess severity of liver damage in ASA.
Enzymatic ASL activity showed a correlation with cognitive function of individuals with ASA at last follow-up visit. We identified a robust threshold value of 24.3%, thereby confirming that enzymatic ASL activity is a determinant of cognitive function in ASA. However, in contrast to the number of HAEs per year of observation and hepatocellular injury, the cut-off of enzymatic ASL activity discriminating between individuals with poor and favorable cognitive outcome was markedly higher. In addition, older individuals with ASA performed worse than younger individuals in cognitive testing at last follow-up visit pointing at a potential chronic neurotoxic or neurodegenerative disease course of ASA. Intriguingly, this effect was even more pronounced in individuals with higher enzymatic ASL activity above the cut-off of 24.3%. These findings suggest additional modifying disease mechanisms underlying cognitive dysfunction in ASA. Recently, several alternative mechanisms in the pathophysiology of ASA have been postulated such as cumulative exposure to disease-specific neurotoxic biomarkers, high cerebral concentrations of argininosuccinic and guanidinosuccinic acid and cerebral deficiency of nitric oxide due to NOS deficiency (Baruteau et al., 2017; Erez, Nagamani, & Lee, 2011; Erez, Nagamani, Shchelochkov, et al., 2011; Posset, Gropman, et al., 2019). Moreover, neuronal ammonia-independent cerebral disease was shown to be caused by oxidative/nitrosative stress through disturbed imbalance of NO metabolism in an ASL-deficient mouse model, a mechanism which is not targeted by current therapeutic principles (Ashley, Nordin, Buza, Greig, & Wilson, 2018; Baruteau et al., 2018). The importance of brain-specific pathomechanisms in ASA is also highlighted by the fact, that the cognitive outcome at last regular visit of these patients is not relevantly affected by the initial peak plasma ammonium concentration unlike in other UCDs (Posset, Gropman, et al., 2019). Despite a follow-up period of approximately two decades, there is still a substantial lack of intra-individual long-term data regarding cognitive functioning, which hampers to unequivocally elucidate the natural cognitive disease course of individuals with ASA as well as CTLN1. Furthermore, we lack systematic cerebral imaging that might be an important tool to unravel morphological changes pointing at potential underlying neurodegenerative pathomechanisms in ASA. In light of our observation, that older individuals with ASA perform worse in cognitive testing than younger individuals, length of disease might also have an impact on other disease manifestations such as neurological or renal disease features, which might have blurred association with enzymatic ASL activity in this study.
Enzymatic ASL activity qualifies as predictive biomarker in ASA enabling risk-stratification of affected individuals
Enzymatic ASL activity, as determined in our recently established biallelic mammalian expression system, qualifies as prognostic and predictive biomarker, since it reflects disease severity and reliably predicts the clinical and cognitive outcome in ASA, which are defined prerequisites for biomarkers (Katz, 2004; “US Food and Drug Administration. Center for Drug Evaluation and Research. Guidance for Industry and Staff; Qualification Process for Drug Development Tools, 2014.,”). Besides capturing the severity of a disease, biomarkers that serve as truly validated surrogate markers are also required to reflect the net effect of treatment on the true outcome (Prentice, 1989). Since there are currently no specific therapies available that aim at increasing activity of the ASL enzyme in ASA, the latter requirement of demonstrating the reflection of net treatment effects on defined outcome parameters cannot be addressed at the moment. However, the identification that the metabolic disease course and cognitive outcome clearly correlate with the enzymatic ASL activity is encouraging and might stimulate the development of novel therapies in the future. It is tempting to speculate, that increasing the enzymatic ASL activity above the identified threshold of 8.7% [e.g. by application of chaperones as proven beneficial for Fabry disease (Germain et al., 2019) or antisense nucleotides targeting altered splicing of the precursor mRNA to increase the concentration of normally translated protein as described for spinal muscular atrophy (Chiriboga et al., 2016)] might have major beneficial effects on the disease course by reducing the annual frequency and severity of HAEs and by improving hepatic function of afflicted individuals with ASA. However, given the evidence for alternative disease mechanisms underlying the cognitive dysfunction in ASA, we hypothesize that solely increasing enzymatic ASL activity might only have limited effects on the cognitive outcome. Notably, the established mammalian expression system can be reliably used to systematically investigate the efficacy of novel therapeutic approaches mechanistically and assess the expected impact on the disease course based on the improvement of enzymatic ASL activity in an evidence-based manner.
The identification of robust cut-off values of enzymatic ASL activity for the prediction of clinical outcomes in ASA enables the risk-stratification of affected individuals discriminating between individuals with a severe (enzymatic ASL activity ≤ 9%) and mild-to-moderate (> 9%) phenotype, which is of importance for 1) the counselling of patients and their families regarding the expected disease course and clinical outcome at a very early stage of the disease, 2) the development of severity-adapted individualized concepts of therapy and care, and 3) planning and interpretation of future clinical studies evaluating the impact of therapeutic interventions such as newborn screening as well as potential future (gene) therapies on the health outcomes of affected individuals.
Limitations and directions for future research
This study has some inherent limitations. Firstly, effects of intronic variants associated with defective splicing have not been investigated, due to the applied technique of cloning the coding sequence of ASL into the expression vector, which only allowed the modelling of exonic gene variants. Our study does not claim to systematically analyze the underlying molecular events of individual gene variants (homozygous or compound heterozygous state) leading to decreased enzymatic ASL activity such as but not limited to mRNA decay, impaired protein folding or decreased protein stability. Results of mRNA and protein expression as illustrated in Figures 1 and 2 should be considered exploratory. These data might serve as a systematic basis for the investigation regarding the underlying molecular mechanisms of reduced enzymatic ASL activity for each of the listed variants in the future. Moreover, as the ASL enzyme exerts its catalytic function as a tetramer, future studies comparing individual activity values for a specific pathogenic variant to cumulative values of a respective (compound heterozygous) variant combination are indispensable to investigate for synergisms or antagonisms induced by hybrid tetramer assembly on ASL protein functions. The presence of epilepsy and anticonvulsant treatment was not assessed in this study due to low data density. This might have introduced some noise into the cognitive outcome analysis, since early exposure to antiepileptic drugs was shown to be associated with cognitive impairment in ASA (Wiwattanadittakul et al., 2018).
Importantly, given the evident disease progression in ASA over time, intra-individual long-term data are indispensable to substantiate our findings, in particular with regard to a potential neurodegenerative disease course associated with neurocognitive deterioration in older individuals with ASA. Moreover, longer observation periods and inclusion of more individuals will help to further substantiate the impact of gene variants and their genetic localization on the cognitive outcome.
Conclusion
We suggest a new severity-adjusted classification system, based on the biallelic expression method, for individuals with ASA having a severe (enzymatic ASL activity ≤ 9%) and mild-to-moderate (enzymatic ASL activity > 9%) phenotype, which can be regarded complementary to the currently existing clinical classification system (EO, LO, Asymptomatic) for individuals with ASA. The threshold is in line with data from individuals with CTLN1 and thus, appears to be a robust cut-off value for distal (i.e. cytosolic) UCDs. Given the impact of enzymatic ASS1 and ASL activities as predictive biomarkers for the clinical disease course and cognitive outcome of individuals with CTLN1 and ASA, we are confident that this method can be applied to proximal urea cycle disorders and further inherited metabolic diseases in the future, enabling the early identification of affected individuals with severe phenotypes ideally before irreversible organ manifestations might be present.
Supplementary Material
Acknowledgements
All UCDC and E-IMD sites contributed to the datasets of the longitudinal studies used in this publication. Principal investigators and personnel with key contributions are listed as UCDC and E-IMD consortia study group members. Furthermore, we gratefully acknowledge subsequent study coordinators – Jennifer Seminara, Saima Ali, Sondra Bloxam, Kia Bryan, Sara Elsbecker, Joan Hart, Melanie Horn, Elijah Kravets, Audrey Lynn, Mary Mullins, Maya Muldowney, Kendall Parks, Ulrike Mütze, Thu Quan, Kara Simpson, Julia Smith, Suzanne Hollander, and Hayden Vreugdenhil – and study neuropsychologists – Fabienne Dietrich Alber, Talin Babikian, Heidi Bender, Christopher Boys, David Breiger, Mina Nguyen-Driver, Benjamin Goodlett, Elizabeth Kerr, Casey Krueger, Eva Mamak, Jacqueline H. Sanz, David Schwartz, Arianna K. Stefanatos, Rachel Tangen, and Greta N. Wilkening. We would also like to acknowledge the contributions of (former) longitudinal study PIs: Mark L. Batshaw, Stephen Cederbaum, Annette Feigenbaum, Douglas S. Kerr, Brendan Lee, Uta Lichter-Konecki, Margretta R. Seashore, Marshall L. Summar, Peter Burgard, Curtis R. Coughlin II, Gregory Enns, Renata C. Gallagher, Cynthia Le Mons, Shawn E. McCandless, Tamar Stricker, Mendel Tuchman, Susan Waisbren, and James D. Weisfeld-Adams. In particular, we are indebted to all our UCD individuals and their families for their trust, patience and participation in both longitudinal registry studies for many years.
Funding Information (with grant numbers)
The Urea Cycle Disorders Consortium (UCDC; U54HD061221) is part of the National Institutes of Health (NIH) Rare Disease Clinical Research Network (RDCRN), supported through collaboration between the Office of Rare Diseases Research (ORDR), the National Center for Advancing Translational Science (NCATS) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). The Urea Cycle Disorders Consortium is also supported by the O’Malley Foundation, the Rotenberg Family Fund, the Dietmar Hopp Foundation, the Kettering Fund, and the National Urea Cycle Disorders Foundation. In addition, support for neuropsychological testing is provided by a NIH grant for Intellectual and Developmental Disability Research Centers (U54HD090257). This work was also supported in part by the Clinical Translational Core at Baylor College of Medicine which is supported by the IDDRC grant number U54HD083092 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The E-IMD patient registry has received funding by the European Union (E-IMD; EAHC no 2010 12 01; coordinator: Stefan Kölker), in the framework of the Health Programme. After the end of the EU funding period the E-IMD patient registry has been sustained by funding from the Kindness-for-Kids Foundation (Munich, Germany), the Kettering Fund, and Dietmar Hopp Foundation. This work was supported by a Trainee Research Fellowship Award 2018–2019 provided by the UCDC. No external funding was secured for the study. MZ (Heidelberg, Germany) was supported by the Physician-Scientist Program at University of Heidelberg and by a Career Development Fellowship provided by the Heidelberg Research Center for Molecular Medicine (HRCMM) in the framework of Excellence Initiative II of the German Research Foundation.
SK receives funding from Horizon Pharma Ireland Limited for the European Post-Authorization Registry for Ravicti® (glycerol phenylbutyrate) oral liquid in partnership with the E-IMD (RRPE) (EU PAS Register no. EUPAS17267; http://www.encepp.eu/). GFH received lecture fees from Nutricia. ABB has received speaker honoraria and travel support from Sanofi Genzyme, Biomarin, Takeda, PIAM, and Nutricia Danone. The sponsors have in no way influenced the design, conductance, analysis and report of the present study.
Footnotes
Data availability
The datasets generated and analyzed during the current study are not publicly available due to existing data protection laws. Furthermore, data ownership is retained by the members of the UCDC and E-IMD consortia making data available for specific research purposes. Data availability is subject to the consent of both consortia upon request.
Conflict of Interest Statement
All other authors declare that they have no conflict of interest.
References
- Ashley SN, Nordin JML, Buza EL, Greig JA, & Wilson JM (2018). Adeno-associated viral gene therapy corrects a mouse model of argininosuccinic aciduria. Mol Genet Metab, 125(3), 241–250. doi: 10.1016/j.ymgme.2018.08.013 [DOI] [PubMed] [Google Scholar]
- Banning A, Schiff M, & Tikkanen R. (2018). Amlexanox provides a potential therapy for nonsense mutations in the lysosomal storage disorder Aspartylglucosaminuria. Biochim Biophys Acta Mol Basis Dis, 1864(3), 668–675. doi: 10.1016/j.bbadis.2017.12.014 [DOI] [PubMed] [Google Scholar]
- Baruteau J, Diez-Fernandez C, Lerner S, Ranucci G, Gissen P, Dionisi-Vici C, … Haberle J. (2019). Argininosuccinic aciduria: Recent pathophysiological insights and therapeutic prospects. J Inherit Metab Dis. doi: 10.1002/jimd.12047 [DOI] [PubMed] [Google Scholar]
- Baruteau J, Jameson E, Morris AA, Chakrapani A, Santra S, Vijay S, … Davison JE (2017). Expanding the phenotype in argininosuccinic aciduria: need for new therapies. J Inherit Metab Dis, 40(3), 357–368. doi: 10.1007/s10545-017-0022-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruteau J, Perocheau DP, Hanley J, Lorvellec M, Rocha-Ferreira E, Karda R, … Waddington SN (2018). Argininosuccinic aciduria fosters neuronal nitrosative stress reversed by Asl gene transfer. Nat Commun, 9(1), 3505. doi: 10.1038/s41467-018-05972-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batshaw ML, Tuchman M, Summar M, Seminara J, & Members of the Urea Cycle Disorders, C. (2014). A longitudinal study of urea cycle disorders. Mol Genet Metab, 113(1–2), 127–130. doi: 10.1016/j.ymgme.2014.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buerger C, Garbade SF, Dietrich Alber F, Waisbren SE, McCarter R, Kolker S, … Urea Cycle Disorders, C. (2019). Impairment of cognitive function in ornithine transcarbamylase deficiency is global rather than domain-specific and is associated with disease onset, sex, maximum ammonium, and number of hyperammonemic events. J Inherit Metab Dis, 42(2), 243–253. doi: 10.1002/jimd.12013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgard P, Kolker S, Haege G, Lindner M, & Hoffmann GF (2016). Neonatal mortality and outcome at the end of the first year of life in early onset urea cycle disorders--review and meta-analysis of observational studies published over more than 35 years. J Inherit Metab Dis, 39(2), 219–229. doi: 10.1007/s10545-015-9901-1 [DOI] [PubMed] [Google Scholar]
- Chiriboga CA, Swoboda KJ, Darras BT, Iannaccone ST, Montes J, De Vivo DC, … Bishop KM (2016). Results from a phase 1 study of nusinersen (ISIS-SMN(Rx)) in children with spinal muscular atrophy. Neurology, 86(10), 890–897. doi: 10.1212/WNL.0000000000002445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erez A, Nagamani SC, & Lee B. (2011). Argininosuccinate lyase deficiency-argininosuccinic aciduria and beyond. Am J Med Genet C Semin Med Genet, 157C(1), 45–53. doi: 10.1002/ajmg.c.30289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erez A, Nagamani SC, Shchelochkov OA, Premkumar MH, Campeau PM, Chen Y, … Lee B. (2011). Requirement of argininosuccinate lyase for systemic nitric oxide production. Nat Med, 17(12), 1619–1626. doi: 10.1038/nm.2544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher RC, Lam C, Wong D, Cederbaum S, & Sokol RJ (2014). Significant hepatic involvement in patients with ornithine transcarbamylase deficiency. J Pediatr, 164(4), 720–725 e726. doi: 10.1016/j.jpeds.2013.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain DP, Nicholls K, Giugliani R, Bichet DG, Hughes DA, Barisoni LM, … Viereck C. (2019). Efficacy of the pharmacologic chaperone migalastat in a subset of male patients with the classic phenotype of Fabry disease and migalastat-amenable variants: data from the phase 3 randomized, multicenter, double-blind clinical trial and extension study. Genet Med. doi: 10.1038/s41436-019-0451-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hothorn T, Hornik K, & Zeileis A. (2006). Unbiased recursive partitioning: a conditional inference framework. J Comput Graph Stat 15, 651–674. [Google Scholar]
- Katz R. (2004). Biomarkers and surrogate markers: an FDA perspective. NeuroRx, 1(2), 189–195. doi: 10.1602/neurorx.1.2.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolker S, Garcia-Cazorla A, Valayannopoulos V, Lund AM, Burlina AB, Sykut-Cegielska J, … Burgard P. (2015). The phenotypic spectrum of organic acidurias and urea cycle disorders. Part 1: the initial presentation. J Inherit Metab Dis, 38(6), 1041–1057. doi: 10.1007/s10545-015-9839-3 [DOI] [PubMed] [Google Scholar]
- Kolker S, Valayannopoulos V, Burlina AB, Sykut-Cegielska J, Wijburg FA, Teles EL, … Garcia-Cazorla A. (2015). The phenotypic spectrum of organic acidurias and urea cycle disorders. Part 2: the evolving clinical phenotype. J Inherit Metab Dis, 38(6), 1059–1074. doi: 10.1007/s10545-015-9840-x [DOI] [PubMed] [Google Scholar]
- Lindner M, Gramer G, Haege G, Fang-Hoffmann J, Schwab KO, Tacke U, … Hoffmann GF (2011). Efficacy and outcome of expanded newborn screening for metabolic diseases--report of 10 years from South-West Germany. Orphanet J Rare Dis, 6, 44. doi: 10.1186/1750-1172-6-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnes RR, Shih V, & Chilton S. (1984). Interallelic complementation in an inborn error of metabolism: genetic heterogeneity in argininosuccinate lyase deficiency. Proc Natl Acad Sci U S A, 81(14), 4480–4484. doi: 10.1073/pnas.81.14.4480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercimek-Mahmutoglu S, Moeslinger D, Haberle J, Engel K, Herle M, Strobl MW, … Stockler-Ipsiroglu S. (2010). Long-term outcome of patients with argininosuccinate lyase deficiency diagnosed by newborn screening in Austria. Mol Genet Metab, 100(1), 24–28. doi: 10.1016/j.ymgme.2010.01.013 [DOI] [PubMed] [Google Scholar]
- Posset R, Garbade SF, Boy N, Burlina AB, Dionisi-Vici C, Dobbelaere D, … the, c EIMD (2019). Transatlantic combined and comparative data analysis of 1095 patients with urea cycle disorders-A successful strategy for clinical research of rare diseases. J Inherit Metab Dis, 42(1), 93–106. doi: 10.1002/jimd.12031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posset R, Gropman AL, Nagamani SCS, Burrage LC, Bedoyan JK, Wong D, … the, c. s. g EIMD (2019). Impact of diagnosis and therapy on cognitive function in urea cycle disorders. Ann Neurol. doi: 10.1002/ana.25492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottel H, Hoste L, Dubourg L, Ebert N, Schaeffner E, Eriksen BO, … Delanaye P. (2016). An estimated glomerular filtration rate equation for the full age spectrum. Nephrol Dial Transplant, 31(5), 798–806. doi: 10.1093/ndt/gfv454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice RL (1989). Surrogate endpoints in clinical trials: definition and operational criteria. Stat Med, 8(4), 431–440. [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administration. Center for Drug Evaluation and Research. Guidance for Industry and Staff; Qualification Process for Drug Development Tools, 2014. Retrieved 23 February 2019 http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM230597.pdf.
- Waisbren SE, Gropman AL, Members of the Urea Cycle Disorders, C., & Batshaw ML (2016). Improving long term outcomes in urea cycle disorders-report from the Urea Cycle Disorders Consortium. J Inherit Metab Dis, 39(4), 573–584. doi: 10.1007/s10545-016-9942-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DC, Christodoulou J, Craig HJ, Simard LR, Ploder L, Howell PL, & McInnes RR (1997). Intragenic complementation at the human argininosuccinate lyase locus. Identification of the major complementing alleles. J Biol Chem, 272(10), 6777–6783. doi: 10.1074/jbc.272.10.6777 [DOI] [PubMed] [Google Scholar]
- Wang Q, Wang Y, Yu Z, Li D, Jia B, Li J, … Kan Q. (2014). Ammonia-induced energy disorders interfere with bilirubin metabolism in hepatocytes. Arch Biochem Biophys, 555–556, 16–22. doi: 10.1016/j.abb.2014.05.019 [DOI] [PubMed] [Google Scholar]
- Wiwattanadittakul N, Prust M, Gaillard WD, Massaro A, Vezina G, Tsuchida TN, & Gropman AL (2018). The utility of EEG monitoring in neonates with hyperammonemia due to inborn errors of metabolism. Mol Genet Metab, 125(3), 235–240. doi: 10.1016/j.ymgme.2018.08.011 [DOI] [PubMed] [Google Scholar]
- Wood SN (2011). Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J R Stat Soc B, 73, 3–36. [Google Scholar]
- Zielonka M, Breuer M, Okun JG, Carl M, Hoffmann GF, & Kolker S. (2018). Pharmacologic rescue of hyperammonemia-induced toxicity in zebrafish by inhibition of ornithine aminotransferase. PLoS One, 13(9), e0203707. doi: 10.1371/journal.pone.0203707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielonka M, Kolker S, Gleich F, Stutzenberger N, Nagamani SCS, Gropman AL, … Network for Intoxication type Metabolic Diseases Consortia Study, G. (2019). Early prediction of phenotypic severity in Citrullinemia Type 1. Ann Clin Transl Neurol. doi: 10.1002/acn3.50886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielonka M, Probst J, Carl M, Hoffmann GF, Kolker S, & Okun JG (2019). Bioenergetic dysfunction in a zebrafish model of acute hyperammonemic decompensation. Exp Neurol, 314, 91–99. doi: 10.1016/j.expneurol.2019.01.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.