Abstract
The transient period of regeneration potential in the postnatal heart suggests molecular changes with maturation influence the cardiac response to damage. We have previously demonstrated that injury and exercise can stimulate cardiomyocyte proliferation in the adult heart suggesting a sensitivity to exogenous signals. Here, we consider whether exogenous fetal ECM and mechanically unloading interstitial matrix can drive regeneration after myocardial infarction (MI) surgery in low-regenerative hearts of day5 mice. Compared to controls, exogenous fetal ECM increases cardiac function and lowers fibrosis at 3 weeks post-injury and this effect can be augmented by softening heart tissue. In vitro experiments support a mechano-sensitivity to exogenous ECM signaling. We tested potential mechanisms and observed that fetal ECM increases nuclear YAP localization which could be enhanced by pharmacological stabilization of the cytoskeleton. Blocking YAP expression lowered fetal ECM effects though not completely. Lastly we observed mechanically unloading heart interstitial matrix increased agrin expression, an extracellular node in the YAP signaling pathway. Collectively, these data support a combined effect of exogenous factors and mechanical activity in altering agrin expression, cytoskeletal remodeling, and YAP signaling in driving cardiomyocyte cell cycle activity and regeneration in postnatal non-regenerative mice.
Keywords: Microenvironment stiffness, cardiomyocyte proliferation, heart regeneration, decellularized extracellular matrix, Yes-associated protein
Graphical Abstract

1. Introduction
Heart disease is one of the leading causes of death worldwide. Cardiovascular disease can lead to irreparable damage and lower cardiac function. In the worst scenario, cardiovascular disease progresses to heart failure, which can only be cured by heart transplantation. A critical biological limitation is the low proliferation of beating cardiomyocytes in the adult myocardium that impairs regeneration from tissue damage. In contrast, adult zebrafish and mammalian neonates (pig and rodents) can respond to cardiac injury with proliferation of cardiomyocytes to reverse tissue loss and recover cardiac function [1]–[3]. A consideration for cardiac therapy is targeting proliferative cardiomyocytes that persist in the adult mammalian heart to increase their mitotic capacity.
The cardiac extracellular matrix has been demonstrated to participate in cell fate regulation and proliferative signaling through soluble ECM molecules [4]–[7]. Extracellular matrix can be isolated by decellularization techniques and transplanted as an intramyocardial injection post-MI to stimulate regeneration in adult hearts. Exogenous extracellular matrix also induces cytokinesis of cardiomyocytes in the dish [8]–[11]. The re-introduction of soluble factors associated with fetal cardiac ECM has been reported to increase regenerative effects and partially improve post-MI cardiac function [12]–[14]. The incomplete therapeutic response of exogenous ECM factors suggests other aspects of the injured heart affect induced-regeneration. With injury there are changes in cardiac mechanics that contribute to dysfunction and can intensify with progression of disease [15], [16]. In the clinic, mechanical unloading of diseased hearts can improve adverse remodeling and improve metabolism [17]; similarly stabilization of injured hearts with synthetic patches and compliant injectable gels can improve heart function. Interestingly, cardiomyocyte proliferation was not evaluated in these studies [18], [19]. Experimentally, decreasing tissue stiffness preceding heart injury has also been shown to reduce fibrosis and mitigate the loss of vasculature in neonatal mice [20].
The stiffness of the heart changes with age which overlaps with loss of cardiac regenerative capacity. The neonatal mouse transitions from a highly regenerative response to cardiac injury on day1 to a fibrotic adult-like response if the onset of injury is just a few days after birth. The profile of diffusible cardiac extracellular proteins changes with aging and disease [8]. Similarly, the alteration of extracellular structural proteins is manifested as changes in the physical properties of the heart [21]. The bulk elastic modulus of the rodent heart has been reported to change by 3 fold (12kPa to 39kPa) in the first week post-partum and 20 fold from fetal to adulthood [21]–[23]. Physical analysis of the decellularized cardiac matrix suggests that the extracellular matrix contributes to the intact composite tissue stiffness with an increase from 15kPa to 45kPa in the first 24 hours after birth [20]. Despite differences in reported values likely due to experimental technique and sample preparation, there is consistently an increase in stiffness with postnatal aging, maturation, and disease.
The mechanical properties of cardiac microenvironment regulate cardiac differentiation and fibroblast activity [24]–[26]. The Yes-associated protein (YAP) is a node in the highly conserved Hippo signaling pathway that plays a role in cardiomyocyte proliferation [27]–[29]. There is evidence that YAP pathway is vulnerable to mechano-stimulated signaling in cancer and other systems through agrin, an extracellular matrix protein [29], [30]. Recent reports show alternate responses of cardiomyocyte YAP activity to decreasing microenvironment stiffness [31]–[33]. It is not clear what factors caused the discrepancy between different groups, but the collective observations suggest that YAP activity in cardiomyocyte is highly sensitive to molecular and physical aspects of the microenvironment.
The stiffness of the tissue microenvironment can be decreased by pharmacological inhibitors of ECM remodeling or increased with exogenous crosslinkers. β-aminopropionitrile (BAPN) can decrease tissue stiffness by irreversible inhibition of lysyl oxidase, an enzyme that integrates collagen and elastin into the ECM [33]–[39]. Ribose crosslinks proteins through glycation. Ribose has been used in vitro for increasing the stiffness of collagen hydrogel [41], [42].
Here, we test whether introducing soluble fetal factors in tandem with lowering microenvironment stiffness can stimulate heart regeneration and cardiomyocyte proliferation. Microenvironment stiffness and soluble ECM factors have been investigated separately for a regulatory role in cardiac regeneration [9], [11], [20]. This study investigates a combinatory action of extracellular physical and biological cues to bias the rate of cardiogenesis. We first investigated if manipulating cardiac stiffness influences the regenerative potency of transplanted fetal ECM in non-regenerative day5 mice hearts. With in vivo data supporting a mechano-influence, mouse ventricle explants were used as an in vitro model of cardiomyocytes in their native environment to examine cardiomyocyte proliferation in response to combined treatment of altered tissue stiffness and soluble ECM derived from pig hearts. Decellularized fetal porcine heart matrix was converted to a protein releasing hydrogel for putative promitotic factors and adult porcine heart matrix was used as a control. The stiffness of the microenvironment was modulated using crosslinking agents for explants. We assayed proliferation and YAP dephosphorylation of cardiomyocytes after changing the effective stiffness of the microenvironment with simultaneous exposure to soluble ECM molecules. The results suggest that the bioactivity of age defined extracellular factors can be tuned by tissue stiffness to regulate heart regeneration.
2. Materials and Methods
2.1. Extracellular matrix preparation
Protein based ECM hydrogel preparation was adapted from published work with modifications [8]. Briefly, ventricles from 2–3 porcine hearts were decellularized. Adult tissue was decellularized in 1% wt/vol SDS solution and then in 1% Triton X-100 solution. Fetal tissue was decellularized in 0.5% wt/vol SDS solution and then in 0.5% Triton X-100 solution. Decellularized cardiac ECM was then washed in water and lyophilized. In order to prepare polymerized hydrogels for decellularized matrix, 10mg of cryogenically pulverized decellularized matrix was digested in 1mg pepsin (2,500 U/mg) at pH2. Decellularized adult ECM was digested for 36 hours at room temperature. Decellularized fetal ECM was digested for 6 hours at room temperature. Digested ECM was neutralized to pH8 using 1N NaOH and 10 × phosphate buffered saline (PBS). 100U/ml Penicillin-Streptomycin (P/S) was added after neutralization. The solubilized ECM was stored at −20°C until use.
2.2. Atomic force microscopy
Atomic force microscopy (AFM) was used to measure the elastic modulus of the acellular tissue matrix after decellularization of mouse ventricle explants and hearts from animal experiments after BAPN and ribose treatment. Decellularized samples were adhered to 35mm Petri dishes using a thin layer of fast-drying adhesive. Elastic modulus measurements were conducted using a Keysight 5500 AFM (Keysight Technologies, Santa Rosa, CA) equipped with an infrared laser, using PicoView 1.20 software. Silicon nitride tips (DNP-S, Bruker Corporation, Camarillo, CA) with a nominal spring constant of 0.06 N/m were used for AFM experiments. The spring constant and deflection sensitivity were determined for each tip used. Samples were immersed in PBS solution during the AFM measurements and force-distance (FD) curves were collected at random positions on the immobilized samples by applying a force of 1 nN with a tip speed of 6.25 μm/s. Three different sets of samples were analyzed for control and experimental (BAPN and ribose) explants and (control and BAPN) mice. A Hertzian model modified for pyramidal tips was used for fitting approach force-distance curves to determine the Young’s modulus, using a plug-in package from Keysight Technologies (pico-café.com), as described previously [43].
2.3. Primary cardiomyocyte isolation and explant preparation
All animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee at the Case Western Reserve University according to the guidelines and regulations described in the Guide for the Care and Use of Laboratory Animals (National Academies Press, 2011). All animals were maintained and housed under specific pathogen–free conditions at our animal facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International (AAALAC) at Case Western Reserve University.
Primary rat ventricular cardiomyocytes were isolated from day1 Sprague Dawley rats purchased from Charles River Laboratories US. Primary cardiomyocytes were dissociated from diced ventricular tissues using Neonatal Heart Dissociation Kit, mouse and rat (Miltenyi Biotec). Cardiomyocytes and non-cardiomyocytes were separated by Percoll gradient centrifugation at 1600g for 30 minutes. Cardiomyocytes were then plated at a density of 26,000 cells/cm2.
Cardiac explants were prepared from day1 CD-1 (IGS) mice. After harvesting hearts, the atrium was removed and the ventricle was cut to fragments approximately 1mm3. Solubilized ECM from the fetal and adult porcine sources was injected at 1.5 μl using a Hamilton syringe into freshly cut explants before plating. Treated explants were plated on 1.5mg/ml pre-equilibrated collagen type I hydrogel. Explants were incubated at 37°C for 4 hours to allow for explant adhesion to collagen gel coating and polymerization of injected ECM. Beating explants were cultured for up to 6 days.
2.4. Culture conditions for cardiomyocytes and explants
The cell and explant culture media contain 1U/ml P/S. Primary cardiomyocytes plated with ECM were cultured in DMEM supplemented with 15% fetal bovine serum (FBS) at 37°C overnight. Cells were then cultured in DMEM, followed by culturing in DMEM containing 10μM BrdU. Cells were fixed in 4% paraformaldehyde (PFA).
Cardiac explants were cultured in M199 supplemented with 1% FBS, 1:100 diluted insulin-transferrin-selenium (Gibco, ITS-G 100X), and 2mM L-glutamine [explant culture media] for 3 days [44]. Explants were then cultured in explant culture media with 10μM BrdU for cell cycle labeling for 3 days. Explants were fixed in 4% PFA. In separate experiments, explants were cultured in explant culture media supplemented with 5mM ribose or 0.2mM BAPN for 6 days from plating in combination with ECM treatment conditions. In order to tune cytoskeleton, explants were cultured in explant culture media supplemented with Jasplakinolide (Cayman), Latrunculin A (Cayman), Y27632 (Chemdea), PF573228 (Selleck) for 3 days; no BrdU labeling applied.
2.5. Animal model and echocardiography
BAPN was delivered to neonatal mouse from day1 to day4 after birth through intraperitoneal injection at 0.01mg BAPN per gram body weight. Surgical MI protocol performed on neonatal mouse was adopted from published work[45]. Briefly, anesthesia as induced by hypothermia and maintained on ice during the surgery. A cut was made on the fourth intercostal space to visualize the heart. Permanent MI was induced by ligating coronary artery with a 10–0 nylon suture. 20μg fetal ECM in 2μl PBS or 2μl PBS (negative control) were injected directly into myocardium, 1 injection in infarct area and 1 above the ligation site. Chest cavity was closed by suturing ribs and muscles, and skin was closed using skin glue.
Echocardiography conducted on week 3 post-surgery. M-mode and B-mode were used to examine heart function. A Vevo 3100 (VisualSonics) equipped with a MX550D transducer was used.
2.6. Immunostaining and immunohistochemistry
Fixed cells were immunostained following protocols provided by vendors. DAPI, mouse anti-cardiac Troponin T (TnT) antibody (Developmental Studies Hybridoma Bank), rabbit anti-BrdU antibody (ThermoFisher), and Alexa 488 and Alexa 568 conjugated secondary antibodies (ThermoFisher) were used.
Fixed explants and hearts were embedded in optimal cutting temperature compound or paraffin and sectioned to 4μm sections. Thawed or deparaffinized sections were immunostained. Rabbit anti-TnT (Abcam), chicken anti-BrdU (Abcam), goat anti-TnC (Abcam), rabbit anti-Ki67 (ThermoFisher), rabbit anti-YAP (Cell Signaling Technology), or rabbit anti-phosphorylated histone H3 (Abcam) antibodies were used. DAPI, Alexa 568 conjugated phalloidin (ThermoFisher), and Alexa 488 or 568 conjugated secondary antibodies were used for detection.
Fixed mouse hearts were embedded in optimal cutting temperature compound and sliced to 7μm sections. Thawed sections were stained by Masson’s Trichrome stain. Heart sections were measured to calculate the ventricular wall thickness and the percent of the heart circumference covered by scar tissue. All microscopy imaging was captured at 4 random locations or higher per sample.
Cardiomyocytes were counted automatically using a custom ImageJ Macros. In brief, wavelength channels for troponin T (TnT) and DAPI were set as a threshold to remove the backgrounds. A logic AND operation was used to segment the TnT and DAPI-double positive areas. Small artifacts were removed by size thresholding with opening and dilation operations. The areas above 150 pixels were counted automatically. BrdU, YAP, and PHH3 positive cardiomyocytes were counted manually. The BrdU and PHH3 positive cardiomyocytes have observable signals in nuclei. Equivalent cells with higher YAP nuclear localization than cytoplasm were counted as nuclear YAP positive cardiomyocytes.
2.7. Western blot
Proteins were extracted using Cell Extraction Buffer (Invitrogen) supplemented with phosphatase inhibitors (Roche), protease inhibitors (Roche), and PMSF (Sigma). Protein concentrations were measured by BCA protein assay. 25μg of protein was loaded to each lane for migration by electrophoresis in 4–20% polyacrylamide gel (Bio-Rad). Proteins were transferred to nitrocellulose membrane (Bio-Rad) and blocked before staining. Anti-phospho YAP ser 127 (Cell Signaling), anti-YAP (Cell Signaling), anti-agrin (EMD Millipore), and anti-GAPDH (Cell Signaling) antibodies were used. Membranes were stripped in mild stripping buffer before reprobing with pan antibody or loading control. Horseradish peroxidase conjugated secondary antibody (Cell Signaling) was used for signal detection. The intensity of each band was measured using ImageJ to quantify protein amount.
Explants were washed in PBS and flash froze or lysed immediately. Explants were homogenized in cell extraction buffer using a bead blender, and followed by western blot.
2.8. Statistical analysis
Data of in vivo experiments were collected from 5 animals in each treatment. Data of in vitro experiments were collected from 3 biological repeats at different times with 3 to 4 technical repeats in each biological repeat. Statistical differences between two groups were tested by two-tailed t-test with a confidence level of 95%. Oneway ANOVA test was used to estimate the statistical difference between three or more groups. Tukey test with a confidence interval of 95% was employed for multiple comparisons. Statistical analysis conducted using GraphPad Prism 7. Results were represented as mean ± standard deviation.
3. Results
3.1. ECM hydrogel releases proteins to the environment
Fetal and adult pig hearts were decellularized and processed to generate a thermal-polymerizable solution. The ECM hydrogel, formed by incubating ECM solution at 37°C for 10min, was used as a vehicle for ECM protein delivery (Fig. S1A). In buffer, periodic supernatant sampling over 3 weeks demonstrated a burst release of proteins from both fetal and adult ECM hydrogels in the first 24 hours followed by a sustained release of a small amount of proteins (Fig. S1B). We assayed the age sourced matrix for agrin, an extracellular matrix protein that has been reported to increase cardiomyocyte proliferation[12]. A higher level of agrin protein was measured in solubilized fetal ECM compared to adult ECM (Fig. S1C, D). To validate promitotic potential, neonatal mouse ventricle explants were treated with ECM hydrogels for 6 days and demonstrated significant increases in cardiomyocyte cell cycle activity with fetal ECM treatment in comparison to adult ECM treatment and the control (Fig. S2). The explant results with cardiac cells in their native microenvironment confirmed published work that solubilized ECM from young age mammalian hearts can stimulate proliferation of cultured primary cardiomyocytes in vitro [8].
3.2. Decreasing heart stiffness improves fetal ECM induced heart regeneration in neonatal mouse
A day5 neonatal mouse MI model was used to determine the effect of microenvironment stiffness on fetal ECM induced heart regeneration. BAPN has successfully been used to reduce heart stiffness in animal models below levels for toxicity or aneurysm [20]. AFM measurements of day 5 mouse heart treated with BAPN from day1 to day4 after birth indicated a decrease in the elastic modulus of decellularized heart by 85% to 8kPa (Fig. S6A). Echocardiography of mice at 3-weeks after surgery showed no significant difference in heart function between BAPN treated and the control MI-only animals (Fig. 1A) except for scar circumference (Fig.1G). Fetal ECM treatment significantly increased post-MI ejection fraction (Fig. 1B), fractional shortening (Fig. 1C), and stroke volume (Fig. 1D) in comparison with the MI-sham group. A decreased heart stiffness at the surgery time point resulted in improved heart function in fetal ECM treated animals compared to fetal ECM treatment alone in animals assessed at 3 weeks post-surgery as indicated by ejection fraction, fractional shortening, stroke volume, and cardiac output (Fig. 1E). The results suggest that the effectiveness of fetal ECM induced heart regeneration after acute MI can be augmented by softening the heart in neonatal mouse. Lowering heart stiffness alone had limited benefits to post-MI cardiac function.
Figure 1. Decreasing heart stiffness improves fetal ECM induced heart regeneration in P5 neonatal mice at 3 weeks.

(A) Echocardiography was conducted at week 3 after surgery to measure heart function. (B) Cardiac ejection fraction, (C) fractional shortening, (D) stroke volume, and (E) cardiac output indicated that fetal ECM preserves heart function post-MI, and that decreasing heart stiffness enhances the pro-regenerative effect of fetal ECM. (F) Masson’s trichrome staining was used to examine scarring and heart remodeling. (G) The percentage of heart circumference covered by scar was lowered by fetal ECM treatment, and decrease heart stiffness further inhibited the spreading of scar area. (H) Fetal ECM preserved heart wall thickness compared to the control. (n=5, one-way ANOVA and Tukey’s test, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.)
Heart fibrosis and wall thickness were examined for mediators of the heart function improvement induced by combination therapy of softening heart and fetal ECM treatment. Masson’s trichrome staining (Fig. 1F) showed ECM injected mice had a lower scar region (Fig. 1G), and a larger wall thickness in the infarct zone (Fig. 1H) both significant compared to the control. BAPN treatment significantly reduced scarring in the MI hearts in both fetal ECM and no ECM treated groups. The results suggest that fetal ECM treatment reduces scar expansion in the injured neonatal mouse heart, and the effectiveness can be improved by decreasing the heart stiffness.
3.3. ECM-induced cardiac post-injury response is mechanosensitive in neonatal mouse heart explant
To determine the influence of microenvironment stiffness on cardiac post-injury response in the native physiological environment, we devised a strategy to alter stiffness in neonatal mouse ventricle explants. The extracellular matrix stiffness is primarily mediated by collagen. We directly manipulated the stiffness of extracellular matrix in explants by chemically blocking matrix-integration of new collagen to soften and sugar based induction of matrix crosslinking to stiffen (Fig. 2A). Specifically, BAPN and ribose were used to modulate the stiffness of explants. Culturing day1 ventricle explants in 0.2mM BAPN or 5mM ribose for 6 days modified the elastic modulus of explant extracellular matrix. To directly measure the extracellular matrix stiffness, the explant was decellularized before AFM analysis. BAPN reduced stiffness of decellularized explant by half, conversely, ribose increased it greater than 3-fold (Fig. 2D and Fig. S6B–C). Histological analysis of intact explants showed that cell morphology appeared stable across groups after BAPN and ribose treatments (Fig. 2C).
Figure 2. Changing explant stiffness alters cardiomyocyte cell cycling only in the presence of exogenous extracellular proteins.

(A) BAPN and ribose were used to modulate the stiffness of explants. BAPN reduces collagen crosslinking by inhibiting LOX, ribose crosslinks fibrous proteins through glycation. (B) Adult or fetal ECM hydrogels were injected into ventricle explants before plating on collagen I hydrogel. Explants were then cultured in media supplemented with BAPN or ribose for 6 days and BrdU labeled in the last 3 days. (C) Explant sections were immunostained for TnT, BrdU, and PHH3. No morphological difference was observed in cardiomyocytes after treating explants with 5mM ribose or 0.2mM BAPN for 6 days. (D) AFM measurement indicated that stiffness of the explant after decellularization was altered by ribose and BAPN treatments. (E) Decreasing the stiffness of fetal ECM and (F) adult ECM treated explants promoted cardiomyocyte BrdU incorporation. (G) Tissue stiffness did not modulate cardiomyocyte BrdU incorporation in no ECM treated explant. (H) PHH3 expression was increased by softening fetal ECM treated explant, but not in (I) adult ECM treated and (J) no ECM treated explants. (K) Fetal ECM treated explants had more BrdU incorporated cardiomyocytes compared to adult ECM treated and no ECM treated explants at all stiffness conditions. Adult ECM treatment promoted cardiomyocyte BrdU incorporation compared to no ECM treatment in 27kPa softened explants, but reduced BrdU incorporation in 197kPa stiffened explants. (L) Fetal ECM treatment increased cardiomyocyte PHH3 expression only in 27kPa softened explants compared to adult ECM and no ECM treatment. Adult ECM did not change PHH3 expression compared to no ECM treatment. (n=3, one-way ANOVA and Tukey’s test, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. #p <0.05, ####p < 0.0001 compared to the control. Separate wavelength channels of composite images are shown in supplemental figure 7A and B.)
Next, we examined if the stiffness of the microenvironment influences cardiomyocyte cell cycle activity induced by ECM hydrogels. Day1 explants were cultured for 6 days after an injection of solubilized adult or fetal ECM with BrdU labeling over the last 72 hours (Fig. 2B). BAPN and ribose were added to media to change the explant stiffness. The 27kPa (BAPN) explant treated with fetal ECM yielded significantly more BrdU positive cardiomyocytes compared to the fetal ECM treatment in the 197kPa (ribose) explant (Fig. 2E). The same observation was confirmed by PHH3 expression in cardiomyocytes. Decreasing tissue stiffness by 50% significantly increased the frequency of PHH3 positive cardiomyocytes in fetal ECM treated explants. Conversely, increasing the ECM stiffness by 300% significantly reduced the frequency of PHH3 positive cardiomyocytes compared to control explants (Fig. 2H). Surprisingly, adult ECM treatment with softened (BAPN) explants showed increased cardiomyocyte BrdU incorporation in contrast with suppressed cell cycling from adult treatment in control and stiffened explants (Fig. 2F). In contrast, no significant difference was observed in cardiomyocyte PHH3 expression in adult ECM treated explants of different stiffness (Fig. 2I). Changing the tissue stiffness alone was not sufficient to modulate the cardiomyocyte BrdU incorporation and PHH3 expression without ECM treatment (no ECM treated explants) (Fig. 2G, J). Statistical analysis of all stiffness conditions normalized to each control (no ECM) explants indicated that fetal ECM treatment significantly increased cardiomyocyte BrdU incorporation compared to adult ECM and no-ECM treated explants in all stiffness conditions (Fig. 2K). Fetal ECM significantly promoted cardiomyocyte PHH3 expression compared to adult ECM and non-treatment only in softened (BAPN) explant (Fig. 2L). In parallel experiments, isolated neonatal rat cardiomyocytes cultured on polyacrylamide substrates of different stiffness also demonstrated a mechanosensitive BrdU incorporation that required the presence of fetal ECM treatment (Fig. S3). Collectively, these results suggest that cardiomyocytes in a natural niche can be stimulated to proliferate by age sourced soluble ECM factors and the frequency of mitosis is influenced by the local substrate stiffness.
Collagen deposition and CD31 positive structures in explants were also examined by histological analysis. Masson’s trichrome staining indicated that BAPN treatment inhibits collagen deposition (Fig. S4A). No ECM control (Fig. S4B) and fetal ECM (Fig. S4C) treated explants exhibited reduced collagen deposition at low stiffness compared to high stiffness. The normalized data indicated an inhibitory effect of fetal ECM treatment on collagen deposition at all stiffness levels in comparison with no ECM treated explants (Fig. S4E). In contrast, the same trend was not observed in adult ECM treated explants (Fig. S4D). Adult ECM treatment stimulated collagen deposition at low stiffness. Next, we examined CD31 which is a marker of endothelial cells in vessel wall. The density of CD31 positive structures in explants showed that decreasing tissue stiffness promotes CD31 positive structure density independent of ECM treatments (Fig. S5A). A higher density of CD31 positive structures was observed in softened explants than the stiffened explants in no ECM (Fig. S5B), fetal ECM (Fig. S5C), and adult ECM (Fig. S5D) treated groups. Fetal ECM increased CD31 positive structure density at all stiffness levels compared to no ECM and adult ECM treated explants. In contrast, adult ECM treatment increased vascularization in the stiffened explants but not in the softened explants (Fig. S5E). These results suggest that decreasing tissue stiffness reduces collagen deposition and CD31 positive structure density in heart tissue explant, and the effectiveness of fetal ECM proteins can be modulated by changing tissue stiffness.
3.4. Cytoskeleton polymerization promotes cardiomyocyte cell cycle activity through YAP activation in fetal ECM treated explants
Hippo-YAP signaling has been shown to play a role in cardiomyocyte proliferation. YAP localization to the cardiomyocyte nucleus is an indicator of mitotic activity and was examined for sensitivity to the combination stimulus of solubilized ECM and tissue stiffness (Fig. 3A). In each group, 4,000 to 5,000 cardiomyocytes were analyzed. Altering stiffness did not impact YAP localization in no ECM treated explants (Fig. 3D). With ECM treatments, decreasing explant stiffness increased YAP localization to nuclei. Fetal ECM treatment alone stimulated nuclear localization of YAP. Fetal ECM treated explant showed a significantly higher ratio of YAP positive nuclei with decreased stiffness (Fig. 3B). Adult ECM treatments induced a significant increase in nuclear YAP positive cardiomyocytes in the softened explant compared to the stiffened explant (Fig. 3C). Comparing YAP positive cardiomyocyte nuclei normalized to each ECM treatment control demonstrated fetal ECM softened explants exhibited a significant increase in nuclear YAP in comparison softened explants with adult ECM and no ECM treatments (Fig. 3E). This data supports an influence of the mechanical environment on soluble ECM initiated YAP activity.
Figure 3. Nuclear YAP localization and cytoskeleton organization do not change with stiffness unless exogenous extracellular proteins are present.
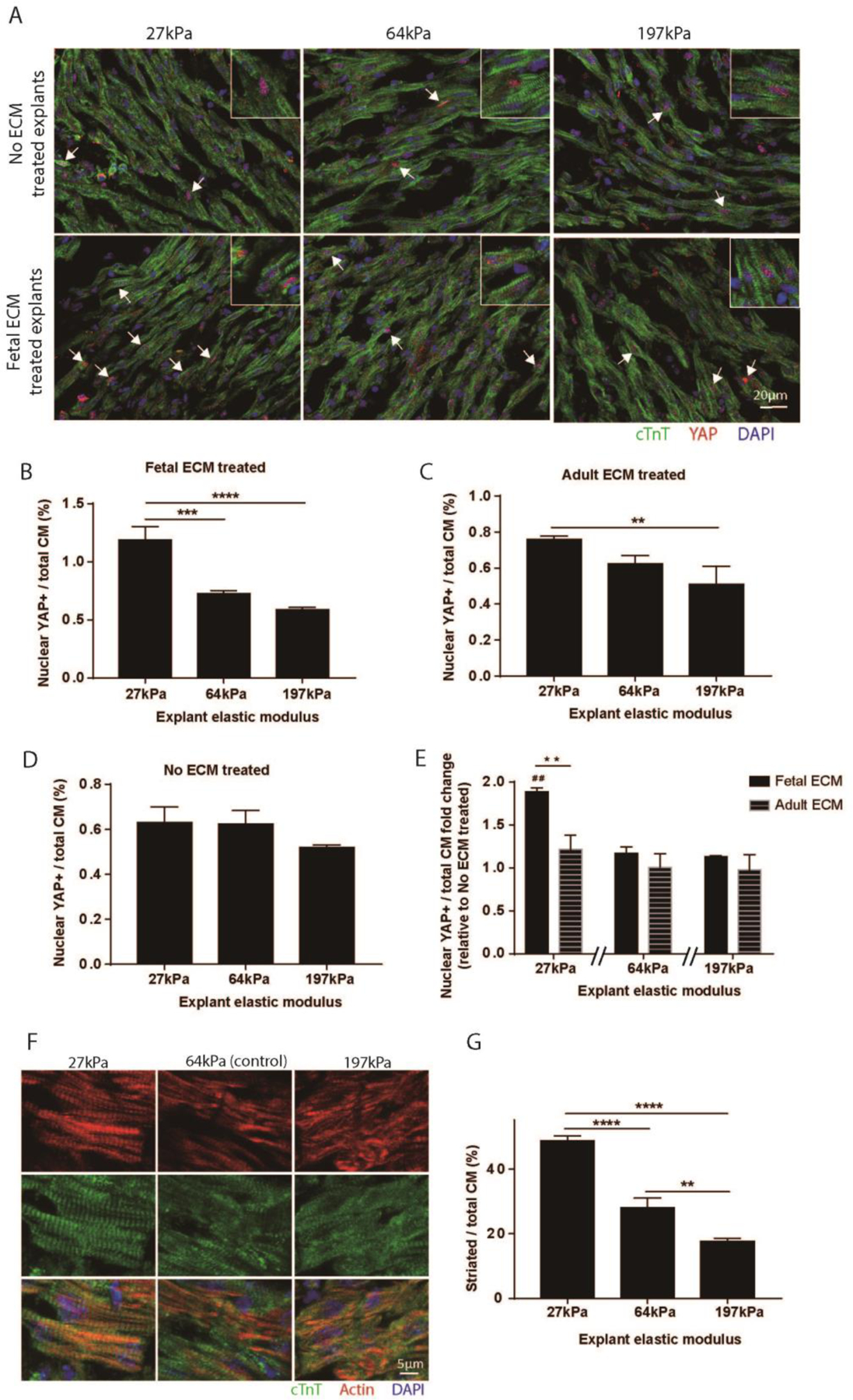
(A) Explant sections were immunostained for YAP and TnT. (B) More YAP positive cardiomyocyte nucleus were observed in 27kPa explants with fetal ECM treatment compared to 64kPa and 197kPa explants. (C) Adult ECM treated explants also showed an increased YAP localization in cardiomyocyte nucleus in 27kPa softened explants compared to 197kPa stiffened explants. (D) YAP localization in no ECM treated explant was not modulated by tissue stiffness. (E) Fetal ECM treatment significantly increased the frequency of nuclear YAP positive cardiomyocytes in 27kPa explants compared to adult ECM and no ECM treated explants, but not in stiffer explants. (F) Explants of different stiffness were immunostained for actin and TnT to examine cardiomyocyte cytoskeleton. (G) Softening explants increased the percentage of fully striated cardiomyocytes in explants. (n=3, one-way ANOVA and Tukey’s test, **p < 0.01, ***p < 0.001, ****p < 0.0001. ##p < 0.01 compared to the control. Separate wavelength channels of composite images are shown in supplemental figure 7C.)
Published works report that cardiomyocytes cultured on polymeric substrates change cytoskeleton organization and proliferate at different stiffness [31]–[33]. We examined the cytoskeleton in (no-ECM treated) explants to determine if local stiffness influences cardiomyocyte cytoskeleton integrity. The cytoskeleton was interrogated by immunolabeling for the percentage of fully striated cardiomyocytes in explants with different stiffness (Fig. 3F). Decreasing explant stiffness promoted cardiomyocyte striations in explants (Fig. 3G). The results suggested that the cytoskeleton may play a role in the cardiomyocyte proliferation response to fetal ECM and the physical microenvironment.
The cytoskeleton and cell adhesion in explants were modulated with pharmaceutical chemicals. Cardiac explants were cultured in media supplemented with focal adhesion kinase (FAK) inhibitor PF573228 (0.1μM), rho-associated protein kinase (ROCK) inhibitor Y27632 (10μM), f-actin polymerization inhibitor Latrunculin A (0.1μM), and f-actin polymerization promotor Jasplakinolide (0.1μM) for 3 days. Promoting f-actin polymerization with jaspakinolide increased YAP activation (nuclear localization) in fetal ECM treated explants compared to all other groups. Conversely, disrupting f-actin polymerization and inhibiting ROCK decreased YAP activation. Blocking FAK activity significantly reduced YAP activation in control (no ECM) explants but not in fetal ECM treated explants (Fig. 4A, C). Similar to changing explant stiffness, modulating the cytoskeleton and ROCK activity did not affect YAP activation in no ECM treated cardiomyocytes. Probing for PHH3, cytoskeleton polymerization increased cardiomyocyte PHH3 expression induced by fetal ECM. Inhibiting FAK, ROCK, or f-actin polymerization eliminates the impact of fetal ECM on cardiomyocytes PHH3 expression (Fig. 4B, D). These results suggest that the stability of cytoskeleton influences YAP activity and fetal ECM induced cardiomyocyte proliferation.
Figure 4. Molecular manipulation of focal adhesion and cytoskeleton stability suggest actin integrity is critical to fetal ECM mediated effects on cardiomyocyte cell cycle activity.

Day1 explants were treated with fetal ECM and pharmaceutical chemicals that modulate f-actin polymerization. (A) PHH3 expression and (B) YAP localization were examined by immunostaining. (C) Promoting cytoskeleton polymerization in fetal ECM treated explants using Jasplakinolide induced a higher ratio of YAP positive nucleus in cardiomyocytes. Inhibiting cytoskeleton polymerization using Latrunculin A and Y27632, on the other hand, hindered YAP localization to cardiomyocyte nucleus. Blocking focal adhesion kinase significantly reduced YAP localization to nucleus in no ECM treated explants. (D) Increasing cytoskeleton polymerization stimulated cardiomyocyte PHH3 expression in response to fetal ECM, and a reversed response was observed by inhibiting cytoskeleton polymerization. (n=3, two-way ANOVA and Tukey’s test, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.)
3.5. Softening explants decreases YAP phosphorylation and increases agrin expression.
The YAP phosphorylation and agrin expression were examined in the explants treated with BAPN/ribose and ECM hydrogels by western blot (Fig. 5A). Phosphorylated YAP is inactive and locates mainly in cytoplasm. A low ratio of phosphorylated YAP to total YAP indicates a high YAP activity. Decreasing explant stiffness significantly reduced YAP phosphorylation in fetal ECM treated explants compared to the control. Increasing the stiffness, on the other hand, promoted YAP phosphorylation in fetal ECM treated explants (Fig. 5B). A similar trend was observed in adult ECM and no ECM treated explants (Fig. 5C, D). Fetal ECM treatment significantly lowered YAP phosphorylation in all stiffness conditions in this study compared to the control (Fig. 5H). Adult ECM treated explants showed significantly reduced YAP phosphorylation in comparison to no ECM at control stiffness levels. Collectively, these observations are consistent with an inverse relationship between fetal ECM mediated YAP phosphorylation and cell mitosis.
Figure 5. Lowering explant stiffness increases YAP dephosphorylation and agrin expression.

(A) Western blot of explant lysates indicated that tissue stiffness regulates agrin level and YAP phosphorylation in ECM treated explants. The observation is consistent with the inverse relationship between YAP phosphorylation and cell division. (B) Decreasing tissue stiffness reduces YAP phosphorylation in fetal ECM treated explants. (C) Adult ECM and (D) no ECM treated explants had decreased YAP phosphorylation in 27kPa softened explants compared to 197kPa stiffened explants. Softening tissue promotes agrin expression in (E) fetal ECM, (F) adult ECM, and (G) no ECM treated explants. (H) Fetal ECM reduced YAP phosphorylation compared to no ECM treated explants at all stiffness conditions. Adult ECM treated explants showed higher YAP phosphorylation in 27kPa and 64kPa explants than fetal ECM treatment. (I) Fetal ECM treated explants had a higher agrin level than adult ECM in 27kPa softened explant. (n=3, one-way ANOVA and Tukey’s test, *p < 0.05, **p < 0.01. #p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001 compared to the control.)
Next, we examined the agrin expression in explants of different stiffness. Agrin promotes cardiomyocyte proliferation in adult mouse heart and has been linked as a mechanosensitive transducer of YAP activity in liver cell lines [12], [30]. The agrin level in explants was negatively correlated with tissue stiffness. Decreasing tissue stiffness by 50% increased agrin levels in fetal ECM treated explants (Fig. 5E). Increasing tissue stiffness by 300% inhibited agrin expression in fetal ECM and adult ECM treated explants(Fig. 5F). A negative correlation between agrin level and explant stiffness was observed in no ECM treated explants, but the changes were not as high as ECM treated explants (Fig. 5G). No significant difference in agrin expression was observed between ECM treated and no ECM treated explants at the control and high stiffness levels (Fig. 5I). Fetal ECM treatment increased agrin level in softened explants compared to adult ECM treatment. The results suggest a correlation between ECM-induced YAP phosphorylation state in cardiac cells and stiffness-stimulation of agrin protein expression, and fetal ECM treatment may also increase agrin expression.
3.6. Fetal ECM induces cardiomyocyte cell cycle activity through YAP dependent and independent signaling pathways
Hippo-YAP pathway mediates the regulation of cell proliferation and has been implicated in cardiac regeneration [46], [47]. Treating isolated neonatal rat cardiomyocytes with media supplemented with fetal cardiac ECM resulted in a decreased YAP phosphorylation consistent with stimulation of mitotic signaling (Fig. 6A, C). The result suggests that soluble extracellular factors trigger YAP activity. Pan YAP expression was negligibly different across all groups (Fig. 6B). Adult ECM treatment did not cause a significant change in YAP phosphorylation compared to no ECM treatment. The western blot results indicate that cardiomyocyte YAP activity is sensitive to soluble ECM proteins.
Figure 6. Fetal ECM induced cell cycle activity is mediated only in part by YAP activity in isolated neonatal cardiomyocytes.

(A) Western blot demonstrated a significantly lower YAP phosphorylation in fetal ECM treated cardiomyocytes than in no ECM and adult ECM treated cardiomyocytes. (B) ECM treatments did not influence total YAP level in cardiomyocytes. (C) The YAP phosphorylation was significantly lower with fetal ECM treatment compared to adult ECM and no ECM treatments. (D) Verteporfin treated cardiomyocytes were immunostained to examine BrdU incorporation. (E) Inhibiting YAP expression reduced cardiomyocyte BrdU incorporation. The fetal ECM induced proliferative response in cardiomyocytes was not fully suppressed by YAP inhibition. (F) YAP level was examined by western blot. (G) Total YAP was significantly reduced by verteporfin treatment in cardiomyocytes. Fetal ECM and verteporfin treated cardiomyocytes showed similar total YAP levels to verteporfin treated cardiomyocytes. (n=3, one-way ANOVA and Tukey’s test, *p < 0.05. **p < 0.01, ***p < 0.001.)
Verteporfin, a clinical pharmaceutical agent with specificity for degradation of YAP, was used in isolated neonatal rat cardiomyocyte cell culture experiments to further investigate the role of YAP with ECM induced mitosis. Verteporfin (0.1μM) significantly lowered pan YAP expression in untreated and fetal ECM treated cardiomyocytes (Fig. 6F, G). Probing for BrdU, verteporfin treatment significantly lowered cardiomyocyte cell cycle activity to negligible levels. Co-treatment with fetal ECM, however, partially reversed the decreased cardiomyocyte cell cycle activity to the level of control groups (Fig. 6D, E). These results suggest that fetal ECM promotes cardiomyocyte proliferation through both YAP-dependent and YAP-independent pathways.
In summary, decreasing tissue stiffness with soluble extracellular matrix factors stimulated cardiomyocyte cell cycle activity mediated through YAP dephosphorylation and actin stability. Lowering stiffness could reverse the repressive effects of adult matrix treatments to become pro-mitotic. Tissue stiffness directly regulated agrin expression and with co-ECM treatment changed YAP phosphorylation state. Using a YAP specific inhibitor, the matrix sensitivity of cardiomyocyte mitosis appears to be mediated by YAP dephosphorylation directly and through YAP-independent signaling pathways. These results have important implications for regenerative strategies in elderly and diseased patients. In particular, mechanosensitive signaling may provide a druggable target for cardiac therapy.
4. Discussion
This work provides evidence that heart tissue stiffness influences neonatal mouse heart post-injury response induced by cardiac ECM hydrogel and ex-vivo cultured cardiomyocyte responding to proliferation-related biomolecules in fetal cardiac ECM. Prior work supports that exogenous ECM factors can induce signaling for heart regeneration[48], that fetal, but not adult, ECM promotes cardiomyocyte proliferation[8], and that several ECM-derived proteins are important for cardiomyocyte proliferation and heart regeneration[49]–[52]. Nevertheless, to our knowledge, there is no report of the microenvironment stiffness as a modulator of cardiomyocyte mitotic response and cardiac regenerative response to extracellular biomolecules. This current study suggests that increased heart stiffness, as anticipated in aging or heart injury, lowers ECM-effectiveness as a mediator of cardiomyocyte proliferation and heart regeneration post-MI; and changing tissue stiffness alone is not enough to modulate cardiomyocyte proliferation in the explant model. Mechanical properties of the microenvironment are an essential but not sufficient factor for regulating heart development and cardiac post-injury response. Our work is the first to indicate that mechanical niche influences cardiomyocytes and injured heart response to diffusible ECM biomolecule in the natural cardiac niche.
Mechanical unloading of the heart has been investigated for reversing maladaptive hypertrophy using invasive mechanical pumps such as the ventricular assist device. Molecular remodeling of the heart may provide a distinct advantage for non-invasive mechanical unloading of the heart and buffering ischemic induction of fibrosis. Irreversible lysyl oxidase inhibitor, BAPN, has been employed to unload collagen and elastin interstitial load both for experimental disease progression and as a therapy. BAPN has not been reported to directly affect cell viability and metabolic function [53]–[57]. However, chronic administration of high doses of BAPN after cardiovascular stress may lead to aneurysms and spontaneous aortic rupture in animal models [58]–[62]. With considerate low and transient dosing, BAPN has demonstrated therapeutic promise for lowering tumor metastatic burden and reducing adverse fibrotic remodeling with cardiovascular and adipose injury [63]–[68]. It remains to be determined whether BAPN administration has potential as an adjunct therapy to be combined with yet to be determined regenerative therapy for reverse remodeling and proliferation. Adult animal studies would allow for cardiac functional analysis to determine whether reduced fibrosis results and putative cardiomyocyte proliferation improves cardiac output after ischemic damage and for high morbidity conditions such as diastolic heart failure. The cardiac post-injury response reported at a single time point does not provide complete tracking of heart recovery process induced by the combination treatment of ECM and tissue softening agents. A longitudinal study is needed in future studies to examine how tissue stiffness modulates ECM hydrogel induced cardiac post-injury response.
Manipulation of actin stability provides a possible mechanism for how microenvironment stiffness induced a biased response to fetal ECM hydrogel in cardiomyocytes. Increasing the microenvironment stiffness has been shown to inhibit myofibril assembly and reduce striations in myocytes cultured in vitro [69]–[71]. Similarly, the diabetic heart with an abnormally high stiffness exhibits diffuse and irregular actin deposition in cardiomyocytes as implied by a clinical study of diabetic patients [72], [73]. Likely cardiomyocyte maturity and disease state influence this mechano-response. Modulating explant extracellular matrix stiffness using BAPN and ribose, and changing f-actin polymerization using jasplakinolide and latrunculin-A achieved similar changes in cardiomyocyte cell cycle activity. The YAP localization data suggests that decreasing matrix stiffness in our explant model stimulates myofibril assembly which then facilitates transduction of fetal ECM initiated YAP activation.
Fetal ECM induces a significant increase in YAP dephosphorylation which correlates with increased proliferation. YAP dephosphorylation triggers interaction with TAZ and translocation to the nucleus to promote cell division [74], [75]. We observed that increase microenvironment stiffness reduces YAP activation in cardiomyocytes. YAP signaling pathway may connect the extracellular matrix stiffness and intracellular mitotic signaling. Agrin regulates YAP activity through focal adhesion, LRP4-MuSK, and dystrophin-glycoprotein complex (DGC), and mediates mechanosignaling of the Hippo/YAP pathway[30], [76], [77]. YAP proteins are sequestered by DGC in cardiomyocytes. Agrin binding destabilizes DGC and dissociates YAP. Agrin treatment leads to a 3 – 4 fold increase in the percentage of nuclear YAP positive cardiomyocytes in adult mice hearts after MI [12]. Fetal ECM contains more agrin proteins compared to adult ECM which may mediate the higher cell cycle activity. We also observed that modulating explant stiffness alters agrin expression from endogenous cells in the explant. The combined effect of ECM factors and tissue softening treatments on YAP dephosphorylation indicates that microenvironment stiffness and ECM soluble factors affect YAP signaling pathway through shared signaling pathways. Gain and loss of function studies will likely determine whether YAP drives the cardiogenic regeneration due to mechanobiological stimuli downstream of agrin. Likely, molecular mapping of additional ECM factors and mechanosensitive signaling pathways are required to fully understand how tissue stiffness affects heart regeneration induced by exogenous ECM.
Our finding resembles data from other groups in that fetal ECM stimulates a more regenerative response in cultured cardiac cells than adult ECM treatment which can suppress proliferation [8], [78]. This similarity in studies is independent of differences in experimental protocol including solubilized ECM preparation (different detergents, freeze-thaw), and treatment conditions (plate adsorption, dissolving in media, and here, hydrogel release). Given that cardiomyocytes can undergo endoreplication (e.g. multinucleation), BrdU labeled cardiomyocytes may not completely comprise newly formed daughter cells. However, PHH3 expression and YAP localization show similar trends to BrdU incorporation which support the conclusion of proliferative cardiomyocyte mechanosensitivity in response to ECM proteins.
Recent studies of cardiomyocyte proliferation in response to matrix rigidity reported seemingly discrepant results. Yaholom-Renon et al. indicated that isolated neonatal mouse cardiomyocytes have a higher proliferation cultured on soft (5kPa and 20kPa) polydimethylsimoxane (PDMS) substrates compared to stiff (5MPa) substrate, and that disrupting cytoskeletal organization promotes cardiomyocyte cell cycle activity [33]. Notari et al. demonstrated that decreasing heart stiffness from ~40kPa to 25kPa in P3 neonatal mouse improves heart regeneration but does not change cardiomyocyte proliferation [20]. On the other hand, Vite et al. reported an increased proliferation and YAP activity in neonatal mouse cardiomyocytes cultured on stiffening polyacrylamide substrates (10kPa, 25kPa, and 50kPa), and that stabilizing cytoskeleton stimulates YAP accumulation in cardiomyocyte nucleus [32]. Corbin et al. also indicated that increasing PDMS substrate stiffness (10 to 55kPa) promotes YAP activity and reduces cytoskeleton organization in induced pluripotent stem cell-derived cardiomyocytes [31]. The differences between published works may have multiple contrasting experimental techniques including stiffness range, cell culture condition, and cell source. However, our study suggests that changing microenvironment stiffness only does not significantly modulate cardiomyocyte proliferation in a wide range (27–197kPa) 3-dimensional native tissue matrix. Nevertheless, microenvironment stiffness influences cardiomyocyte in response to proliferation-related proteins in explants and post-MI response in early-aged mammals. Thus, in addition to microenvironment stiffness, the biomolecules introduced to cardiomyocytes with culture media may need further evaluation. Our results do not suggest a different observation from previous works but draw attention to a new variable that plays an important role in cardiomyocyte-microenvironment interaction.
5. Conclusion
This study demonstrates that microenvironment stiffness influences exogenous ECM induced heart regeneration in neonatal mouse and cardiomyocytes proliferation in heart explant. Physical properties of the microenvironment can modulate the fetal ECM cardio-regenerative effects. Cytoskeleton polymerization and YAP activation are involved in the regulation of cardiomyocyte mechanosensitivity to fetal ECM. Solubilized cardiac extracellular matrix treatments promote cardiomyocyte proliferation potentially through YAP and non-YAP signaling pathways. The results suggest that extracellular biomolecules may have varying effects on cardiomyocytes and heart regeneration at different microenvironment stiffness.
Supplementary Material
Statement of Significance.
With the purpose of developing regenerative strategies, we investigate the influence of the local niche on the cardiac injury response. We conclude tissue stiffness, as anticipated in aging or disease, impairs regenerative therapeutics. Most novel, mechanical unloading facilitates enhanced cardiac regeneration only after cells are pushed into a permissive state by fetal biomolecules. Specifically, mechanical unloading appears to increase extracellular agrin expression that amplifies fetal-stimulation of nuclear YAP signaling which correlates with observed increases of cell cycle activity in cardiomyocytes. The results further suggest the cytoskeleton is critical to this interaction between mechanical unloading and independently actived YAP signaling. Using animal models, tissue explants, and cells, this work indicates that the mechanical niche can augment proliferating-permissive cardiomyocytes in the natural cardiac niche.
Acknowledgment
This work was supported by Faculty Investment Fund RES221997 from Case Western Reserve University (CWRU) (S.E.S), NIH 1 C06 RR12463-01, and R01EY021731 (P.S-.H.P). We would like to acknowledge the use of the Leica SP8 confocal microscope in the Light Microscopy Imaging Facility at CWRU, made available through the Office of Research Infrastructure (NIH-ORIP) Shared Instrumentation Grant S10OD016164. We thank the Hesham Sadek from UT Southwestern for training on the neonatal MI model. We thank Dr. Nicholas Ziats and Sandra Siedlak from the Department of Pathology at CWRU for providing equipment and technical assistance on histology. We thank Nanthawan Avishai from Swagelok Center for Surface Analysis of Materials at CWRU for assistance with SEM imaging. We thank Jessica Berthiaume and Xiaoqin Chen from CWRU for training and access to Vevo 3100 (VisualSonics). We thank Dr. Steven Schomisch from Surgical Training and Research Lab for providing adult pig hearts. We thank Craig Watson, Stephan Nieuwoudt, and Ryan Behmer Hansen for providing technical support.
We thank Valinteshley Pierre for a reviewing all electronic files for data integrity. We thank Horst von Recum and Jeffrey Capadonna from CWRU for helpful edits.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability statement
The datasets generated during the current study are available from the corresponding author by reasonable request.
References
- [1].Lam NT and Sadek HA, “Neonatal Heart Regeneration,” Circulation, vol. 138, no. 4, pp. 412–423, July 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ye L, D’Agostino G, Loo SJ, Wang CX, Su LP, Tan SH, Tee GZ, Pua CJ, Pena EM, Cheng RB, Chen WC, Abdurrachim D, Lalic J, Tan RS, Lee TH, Zhang J, and Cook SA, “Early Regenerative Capacity in the Porcine Heart,” Circulation, vol. 138, no. 24, pp. 2798–2808, December 2018. [DOI] [PubMed] [Google Scholar]
- [3].Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, Wu T-D, Guerquin-Kern J-L, Lechene CP, and Lee RT, “Mammalian heart renewal by pre-existing cardiomyocytes,” Nature, vol. 493, no. 7432, pp. 433–436, December 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bersell K, Arab S, Haring B, and Kühn B, “Neuregulin1/ErbB4 Signaling Induces Cardiomyocyte Proliferation and Repair of Heart Injury,” Cell, vol. 138, no. 2, pp. 257–270, 2009. [DOI] [PubMed] [Google Scholar]
- [5].Chakraborty S and Hong W, “Linking Extracellular Matrix Agrin to the Hippo Pathway in Liver Cancer and Beyond.,” Cancers (Basel)., vol. 10, no. 2, February 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kühn B, del Monte F, Hajjar RJ, Chang Y-S, Lebeche D, Arab S, and Keating MT, “Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair,” Nat. Med, vol. 13, no. 8, pp. 962–969, September 2007. [DOI] [PubMed] [Google Scholar]
- [7].Wey Yong K, Li Y, Huang G, Jian Lu T, Kamarul W Wan Safwani Zaman, Pingguan-Murphy B, and Xu F, “Mechanoregulation of cardiac myofibroblast differentiation: implications for cardiac fibrosis and therapy,” Am J Physiol Hear. Circ Physiol, vol. 309, pp. 532–542, 2015. [DOI] [PubMed] [Google Scholar]
- [8].Williams C, Quinn KP, Georgakoudi I, and Black LD, “Young developmental age cardiac extracellular matrix promotes the expansion of neonatal cardiomyocytes in vitro,” Acta Biomater, vol. 10, no. 1, pp. 194–204, January 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wang Z, Long DW, Huang Y, Chen WCW, Kim K, and Wang Y, “Decellularized neonatal cardiac extracellular matrix prevents widespread ventricular remodeling in adult mammals after myocardial infarction,” Acta Biomater, vol. 87, pp. 140–151, March 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Seif-Naraghi SB, Singelyn JM, Salvatore MA, Osborn KG, Wang JJ, Sampat U, Kwan OL, Strachan GM, Wong J, Schup-Magoffin PJ, Braden RL, Bartels K, DeQuach JA, Preul M, Kinsey AM, DeMaria AN, Dib N, and Christman KL, “Safety and efficacy of an injectable extracellular matrix hydrogel for treating myocardial infarction.,” Sci. Transl. Med, vol. 5, no. 173, p. 173ra25, February 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chen WCW, Wang Z, Missinato MA, Park DW, Long DW, Liu H-J, Zeng X, Yates NA, Kim K, and Wang Y, “Decellularized zebrafish cardiac extracellular matrix induces mammalian heart regeneration,” Sci. Adv, vol. 2, no. 11, pp. e1600844–e1600844, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bassat E, Mutlak YE, Genzelinakh A, Shadrin IY, Baruch Umansky K, Yifa O, Kain D, Rajchman D, Leach J, Riabov Bassat D, Udi Y, Sarig R, Sagi I, Martin JF, Bursac N, Cohen S, and Tzahor E, “The extracellular matrix protein agrin promotes heart regeneration in mice,” Nature, vol. 547, no. 7662, pp. 179–184, July 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wei K, Serpooshan V, Hurtado C, Diez-Cuñado M, Zhao M, Maruyama S, Zhu W, Fajardo G, Noseda M, Nakamura K, Tian X, Liu Q, Wang A, Matsuura Y, Bushway P, Cai W, Savchenko A, Mahmoudi M, et al. , “Epicardial FSTL1 reconstitution regenerates the adult mammalian heart,” Nature, vol. 525, no. 7570, pp. 479–485, September 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bersell K, Arab S, Haring B, and Kühn B, “Neuregulin1/ErbB4 Signaling Induces Cardiomyocyte Proliferation and Repair of Heart Injury,” Cell, vol. 138, no. 2, pp. 257–270, July 2009. [DOI] [PubMed] [Google Scholar]
- [15].Arunachalam SP, Arani A, Baffour F, Rysavy JA, Rossman PJ, Glaser KJ, Lake DS, Trzasko JD, Manduca A, McGee KP, Ehman RL, and Araoz PA, “Regional assessment of in vivo myocardial stiffness using 3D magnetic resonance elastography in a porcine model of myocardial infarction,” Magn. Reson. Med, vol. 79, no. 1, pp. 361–369, January 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gupta KB, Ratcliffe MB, Fallert MA, Edmunds LH, and Bogen DK, “Changes in passive mechanical stiffness of myocardial tissue with aneurysm formation.,” Circulation, vol. 89, no. 5, pp. 2315–26, May 1994. [DOI] [PubMed] [Google Scholar]
- [17].Uriel N, Sayer G, Annamalai S, Kapur NK, and Burkhoff D, “Mechanical Unloading in Heart Failure,” Journal of the American College of Cardiology, vol. 72, no. 5. Elsevier USA, pp. 569–580, 31-July-2018. [DOI] [PubMed] [Google Scholar]
- [18].Plotkin M, Vaibavi SR, Rufaihah AJ, Nithya V, Wang J, Shachaf Y, Kofidis T, and Seliktar D, “The effect of matrix stiffness of injectable hydrogels on the preservation of cardiac function after a heart attack,” Biomaterials, vol. 35, no. 5, pp. 1429–1438, February 2014. [DOI] [PubMed] [Google Scholar]
- [19].Kapnisi M, Mansfield C, Marijon C, Guex AG, Perbellini F, Bardi I, Humphrey EJ, Puetzer JL, Mawad D, Koutsogeorgis DC, Stuckey DJ, Terracciano CM, Harding SE, and Stevens MM, “Auxetic Cardiac Patches with Tunable Mechanical and Conductive Properties toward Treating Myocardial Infarction.,” Adv. Funct. Mater, vol. 28, no. 21, p. 1800618, May 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Notari M, Ventura-Rubio A, Bedford-Guaus SJ, Jorba I, Mulero L, Navajas D, Martí M, and Raya Á, “The local microenvironment limits the regenerative potential of the mouse neonatal heart,” Sci. Adv, vol. 4, no. 5, p. eaao5553, May 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jacot JG, Martin JC, and Hunt DL, “Mechanobiology of cardiomyocyte development.,” J. Biomech, vol. 43, no. 1, pp. 93–8, January 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Young JL, Kretchmer K, Ondeck MG, Zambon AC, and Engler AJ, “Mechanosensitive Kinases Regulate Stiffness-Induced Cardiomyocyte Maturation,” Sci. Rep, vol. 4, no. 1, p. 6425, May 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ward M and Iskratsch T, “Mix and (mis-)match – The mechanosensing machinery in the changing environment of the developing, healthy adult and diseased heart,” Biochimica et Biophysica Acta - Molecular Cell Research, vol. 1867, no. 3. Elsevier B.V., 01-March-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, Funaki M, Zahir N, Ming W, Weaver V, and Janmey PA, “Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion,” Cell Motil. Cytoskeleton, vol. 60, no. 1, pp. 24–34, January 2005. [DOI] [PubMed] [Google Scholar]
- [25].Bhana B, Iyer RK, Chen WLK, Zhao R, Sider KL, Likhitpanichkul M, Simmons CA, and Radisic M, “Influence of substrate stiffness on the phenotype of heart cells,” Biotechnol. Bioeng, vol. 105, no. 6, p. n/a–n/a, April 2010. [DOI] [PubMed] [Google Scholar]
- [26].Wen JH, Vincent LG, Fuhrmann A, Choi YS, Hribar KC, Taylor-Weiner H, Chen S, and Engler AJ, “Interplay of matrix stiffness and protein tethering in stem cell differentiation,” Nat. Mater, vol. 13, no. 10, pp. 979–987, August 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Oliver-De La Cruz J, Nardone G, Vrbsky J, Pompeiano A, Perestrelo AR, Capradossi F, Melajová K, Filipensky P, and Forte G, “Substrate mechanics controls adipogenesis through YAP phosphorylation by dictating cell spreading,” Biomaterials, vol. 205, pp. 64–80, June 2019. [DOI] [PubMed] [Google Scholar]
- [28].Elosegui-Artola A, Andreu I, Beedle AEM, Lezamiz A, Uroz M, Kosmalska AJ, Oria R, Kechagia JZ, Rico-Lastres P, Le Roux A-L, Shanahan CM, Trepat X, Navajas D, Garcia-Manyes S, and Roca-Cusachs P, “Force Triggers YAP Nuclear Entry by Regulating Transport across Nuclear Pores,” Cell, vol. 171, no. 6, pp. 1397–1410.e14, November 2017. [DOI] [PubMed] [Google Scholar]
- [29].Liu F, Lagares D, Choi KM, Stopfer L, Marinković A, Vrbanac V, Probst CK, Hiemer SE, Sisson TH, Horowitz JC, Rosas IO, Fredenburgh LE, Feghali-Bostwick C, Varelas X, Tager AM, and Tschumperlin DJ, “Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis,” Am. J. Physiol. Cell. Mol. Physiol, vol. 308, no. 4, pp. L344–L357, February 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chakraborty S, Njah K, and V Pobbati A, “Agrin as a Mechanotransduction Signal Regulating YAP through the Hippo Pathway,” CellReports, vol. 18, pp. 2464–2479, 2017. [DOI] [PubMed] [Google Scholar]
- [31].Corbin EA, Vite A, Peyster EG, Bhoopalam M, Brandimarto J, Wang X, Bennett AI, Clark AT, Cheng X, Turner KT, Musunuru K, and Margulies KB, “Tunable and Reversible Substrate Stiffness Reveals a Dynamic Mechanosensitivity of Cardiomyocytes,” ACS Appl. Mater. Interfaces, vol. 11, no. 23, pp. 20603–20614, June 2019. [DOI] [PubMed] [Google Scholar]
- [32].Vite A, Zhang C, Yi R, Emms S, and Radice GL, “α-catenin-dependent cytoskeletal tension controls yap activity in the heart,” Dev, vol. 145, no. 5, March 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yahalom-Ronen Y, Rajchman D, Sarig R, Geiger B, and Tzahor E, “Reduced matrix rigidity promotes neonatal cardiomyocyte dedifferentiation, proliferation and clonal expansion,” Elife, vol. 4, August 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mammoto T, Jiang E, Jiang A, and Mammoto A, “Extracellular matrix structure and tissue stiffness control postnatal lung development through the lipoprotein receptor-related protein 5/Tie2 signaling system,” Am. J. Respir. Cell Mol. Biol, vol. 49, no. 6, pp. 1009–1018, December 2013. [DOI] [PubMed] [Google Scholar]
- [35].Brüel A, Ørtoft G, and Oxlund H, “Inhibition of cross-links in collagen is associated with reduced stiffness of the aorta in young rats,” Atherosclerosis, vol. 140, no. 1, pp. 135–145, September 1998. [DOI] [PubMed] [Google Scholar]
- [36].Cheng T, Liu Q, Zhang R, Zhang Y, Chen J, Yu R, and Ge G, “Lysyl oxidase promotes bleomycin-induced lung fibrosis through modulating inflammation,” J. Mol. Cell Biol, vol. 6, no. 6, pp. 506–515, December 2014. [DOI] [PubMed] [Google Scholar]
- [37].Liu SB, Ikenaga N, Peng ZW, Sverdlov DY, Greenstein A, Smith V, Schuppan D, and Popov Y, “Lysyl oxidase activity contributes to collagen stabilization during liver fibrosis progression and limits spontaneous fibrosis reversal in mice,” FASEB J, vol. 30, no. 4, pp. 1599–1609, April 2016. [DOI] [PubMed] [Google Scholar]
- [38].Harlow CR, Wu X, van Deemter M, Gardiner F, Poland C, Green R, Sarvi S, Brown P, Kadler KE, Lu Y, Mason JI, Critchley HOD, and Hillier SG, “Targeting lysyl oxidase reduces peritoneal fibrosis,” PLoS One, vol. 12, no. 8, p. e0183013, August 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Gao L, Wu C, Fu F, You X, Ma X, Qin F, Li T, Wang R, and Yuan J, “Effect of lysyl oxidase (LOX) on corpus cavernous fibrosis caused by ischaemic priapism,” J. Cell. Mol. Med, vol. 22, no. 3, pp. 2018–2022, March 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Mammoto T, Jiang E, Jiang A, and Mammoto A, “Extracellular Matrix Structure and Tissue Stiffness Control Postnatal Lung Development through the Lipoprotein Receptor–Related Protein 5/Tie2 Signaling System,” Am. J. Respir. Cell Mol. Biol, vol. 49, no. 6, pp. 1009–1018, December 2013. [DOI] [PubMed] [Google Scholar]
- [41].Mason BN, Starchenko A, Williams RM, Bonassar LJ, and Reinhart-King CA, “Tuning three-dimensional collagen matrix stiffness independently of collagen concentration modulates endothelial cell behavior,” Acta Biomater, vol. 9, no. 1, pp. 4635–4644, January 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mirahmadi F, Koolstra JH, Lobbezoo F, van Lenthe GH, Ghazanfari S, Snabel J, Stoop R, and Everts V, “Mechanical stiffness of TMJ condylar cartilage increases after artificial aging by ribose,” Arch. Oral Biol, vol. 87, pp. 102–109, March 2018. [DOI] [PubMed] [Google Scholar]
- [43].Senapati S, Poma AB, Cieplak M, Filipek S, and Park PS−H, “Differentiating between Inactive and Active States of Rhodopsin by Atomic Force Microscopy in Native Membranes,” Anal. Chem, vol. 91, no. 11, pp. 7226–7235, June 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].den Haan AD, Veldkamp MW, Bakker D, Boink GJJ, Janssen RB, de Bakker JMT, and Tan HL, “Organ Explant Culture of Neonatal Rat Ventricles: A New Model to Study Gene and Cell Therapy,” PLoS One, vol. 8, no. 3, p. e59290, March 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Blom JN, Lu X, Arnold P, and Feng Q, “Myocardial infarction in neonatal mice, a model of cardiac regeneration,” J. Vis. Exp, vol. 2016, no. 111, May 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Xin M, Kim Y, Sutherland LB, Murakami M, Qi X, McAnally J, Porrello ER, Mahmoud AI, Tan W, Shelton JM, Richardson JA, Sadek HA, Bassel-Duby R, and Olson EN, “Hippo pathway effector Yap promotes cardiac regeneration.,” Proc. Natl. Acad. Sci. U. S. A, vol. 110, no. 34, pp. 13839–44, August 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lin Z, von Gise A, Zhou P, Gu F, Ma Q, Jiang J, Yau AL, Buck JN, Gouin KA, van Gorp PRR, Zhou B, Chen J, Seidman JG, Wang D-Z, and Pu WT, “Cardiac-Specific YAP Activation Improves Cardiac Function and Survival in an Experimental Murine MI Model,” Circ. Res, vol. 115, no. 3, pp. 354–363, July 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].González-Rosa JM, Martín V, Peralta M, Torres M, and Mercader N, “Extensive scar formation and regression during heart regeneration after cryoinjury in zebrafish,” Development, vol. 138, no. 9, pp. 1663–1674, May 2011. [DOI] [PubMed] [Google Scholar]
- [49].Chablais F and Jazwinska A, “The regenerative capacity of the zebrafish heart is dependent on TGFβ signaling.,” Development, vol. 139, no. 11, pp. 1921–30, June 2012. [DOI] [PubMed] [Google Scholar]
- [50].Kühn B, Del Monte F, Hajjar RJ, Chang YS, Lebeche D, Arab S, and Keating MT, “Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair,” Nat. Med, vol. 13, no. 8, pp. 962–969, August 2007. [DOI] [PubMed] [Google Scholar]
- [51].Wang J, Karra R, Dickson AL, and Poss KD, “Fibronectin is deposited by injury-activated epicardial cells and is necessary for zebrafish heart regeneration.,” Dev. Biol, vol. 382, no. 2, pp. 427–35, October 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Ieda M, Tsuchihashi T, Ivey KN, Ross RS, Hong TT, Shaw RM, and Srivastava D, “Cardiac Fibroblasts Regulate Myocardial Proliferation through β1 Integrin Signaling,” Dev. Cell, vol. 16, no. 2, pp. 233–244, February 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Miana M, Galán M, Martínez-Martínez E, Varona S, Jurado-López R, Bausa-Miranda B, Antequera A, Luaces M, Martínez-González J, Rodríguez C, and Cachofeiro V, “The lysyl oxidase inhibitor β-aminopropionitrile reduces body weight gain and improves the metabolic profile in diet-induced obesity in rats,” DMM Dis. Model. Mech, vol. 8, no. 6, pp. 543–551, June 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Nilsson M, Adamo H, Bergh A, and Halin Bergström S, “Inhibition of Lysyl Oxidase and Lysyl Oxidase-Like Enzymes Has Tumour-Promoting and Tumour-Suppressing Roles in Experimental Prostate Cancer,” Sci. Rep, vol. 6, January 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Shi L, Zhang N, Liu H, Zhao L, Liu J, Wan J, Wu W, Lei H, Liu R, and Han M, “Lysyl oxidase inhibition via β-aminoproprionitrile hampers human umbilical vein endothelial cell angiogenesis and migration in vitro,” Mol. Med. Rep, vol. 17, no. 4, pp. 5029–5036, April 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Nelson JM, Diegelmann RF, and Cohen IK, “Effect of -Aminopropionitrile and Ascorbate on Fibroblast Migration,” Exp. Biol. Med, vol. 188, no. 3, pp. 346–352, July 1988. [DOI] [PubMed] [Google Scholar]
- [57].Sasaki T and Yamamoto M, “Potentiating Effect of β-Aminopropionitrile on the DNA Synthesis Induced by Isoproterenol,” Cancer Res, vol. 39, no. 7 Part 1, pp. 2751–2754, July 1979. [PubMed] [Google Scholar]
- [58].SIMPSON CF and BOUCEK RJ, “The B-aminopropionitrile-fed turkey: a model for detecting potential drug action on arterial tissue,” Cardiovasc. Res, vol. 17, no. 1, pp. 26–32, January 1983. [DOI] [PubMed] [Google Scholar]
- [59].Remus EW, O’Donnell RE, Rafferty K, Weiss D, Joseph G, Csiszar K, Fong SFT, and Taylor WR, “The role of lysyl oxidase family members in the stabilization of abdominal aortic aneurysms,” Am. J. Physiol. - Hear. Circ. Physiol, vol. 303, no. 8, October 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Mäki JM, Räsänen J, Tikkanen H, Sormunen R, Mäkikallio K, Kivirikko KI, and Soininen R, “Inactivation of the lysyl oxidase gene Lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice,” Circulation, vol. 106, no. 19, pp. 2503–2509, November 2002. [DOI] [PubMed] [Google Scholar]
- [61].Kurihara T, Shimizu-Hirota R, Shimoda M, Adachi T, Shimizu H, Weiss SJ, Itoh H, Hori S, Aikawa N, and Okada Y, “Neutrophil-derived matrix metalloproteinase 9 triggers acute aortic dissection,” Circulation, vol. 126, no. 25, pp. 3070–3080, December 2012. [DOI] [PubMed] [Google Scholar]
- [62].Ren W, Liu Y, Wang X, Jia L, Piao C, Lan F, and Du J, “β-Aminopropionitrile monofumarate induces thoracic aortic dissection in C57BL/6 mice,” Sci. Rep, vol. 6, June 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Martínez-Martínez E, Galán M, Miana M, Varona S, Jurado-López R, Martínez-González J, Rodríguez C, and Cachofeiro V, “The inhibition of lysyl oxidase improves metabolic alterations and adipose tissue disturbances in obese animals,” Atherosclerosis, vol. 235, no. 2, p. e25, August 2014. [Google Scholar]
- [64].Martínez-Martínez E, Rodríguez C, Galán M, Miana M, Jurado-López R, Bartolomé MV, Luaces M, Islas F, Martínez-González J, López-Andrés N, and Cachofeiro V, “The lysyl oxidase inhibitor (β-aminopropionitrile) reduces leptin profibrotic effects and ameliorates cardiovascular remodeling in diet-induced obesity in rats,” J. Mol. Cell. Cardiol, vol. 92, pp. 96–104, March 2016. [DOI] [PubMed] [Google Scholar]
- [65].Hernandez DR, Wei Y, Andreopoulos F, and Vazquez-Padron R, “Local Delivery of β-Aminopropionitrile Improves Arteriovenous Fistula Maturation and Remodeling | The FASEB Journal,” FASEB J, vol. 31, no. 1, 2017. [Google Scholar]
- [66].Bondareva A, Downey CM, Ayres F, Liu W, Boyd SK, Hallgrimsson B, and Jirik FR, “The Lysyl Oxidase Inhibitor, β-Aminopropionitrile, Diminishes the Metastatic Colonization Potential of Circulating Breast Cancer Cells,” PLoS One, vol. 4, no. 5, p. e5620, May 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Erler JT, Bennewith KL, Nicolau M, Dornhöfer N, Kong C, Le QT, Chi JTA, Jeffrey SS, and Giaccia AJ, “Lysyl oxidase is essential for hypoxia-induced metastasis,” Nature, vol. 440, no. 7088, pp. 1222–1226, April 2006. [DOI] [PubMed] [Google Scholar]
- [68].Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SFT, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, and Weaver VM, “Matrix Crosslinking Forces Tumor Progression by Enhancing Integrin Signaling,” Cell, vol. 139, no. 5, pp. 891–906, November 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Engler AJ, Griffin MA, Sen S, Bonnemann CG, Sweeney HL, and Discher DE, “Myotubes differentiate optimally on substrates with tissue-like stiffness,” J Cell Biol, vol. 166, no. 6, pp. 877–887, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].McCain ML and Parker KK, “Mechanotransduction: The role of mechanical stress, myocyte shape, and cytoskeletal architecture on cardiac function,” Pflugers Archiv European Journal of Physiology, vol. 462, no. 1. Springer Verlag, pp. 89–104, 2011. [DOI] [PubMed] [Google Scholar]
- [71].Engler AJ, Carag-Krieger C, Johnson CP, Raab M, Tang HY, Speicher DW, Sanger JW, Sanger JM, and Discher DE, “Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: Scar-like rigidity inhibits beating,” J. Cell Sci, vol. 121, no. 22, pp. 3794–3802, November 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Benech JC, Benech N, Zambrana AI, Rauschert I, Bervejillo V, Oddone N, and Damián JP, “Diabetes increases stiffness of live cardiomyocytes measured by atomic force microscopy nanoindentation,” Am. J. Physiol. - Cell Physiol, vol. 307, no. 10, pp. C910–C919, November 2014. [DOI] [PubMed] [Google Scholar]
- [73].Van Heerebeek L, Hamdani N, Handoko ML, Falcao-Pires I, Musters RJ, Kupreishvili K, Ijsselmuiden AJJ, Schalkwijk CG, Bronzwaer JGF, Diamant M, Borbély A, Van Der Velden J, Stienen GJM, Laarman GJ, Niessen HWM, and Paulus WJ, “Diastolic stiffness of the failing diabetic heart: Importance of fibrosis, advanced glycation end products, and myocyte resting tension,” Circulation, vol. 117, no. 1, pp. 43–51, January 2008. [DOI] [PubMed] [Google Scholar]
- [74].Varelas X, “The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease.,” Development, vol. 141, no. 8, pp. 1614–26, April 2014. [DOI] [PubMed] [Google Scholar]
- [75].Piccolo S, Dupont S, and Cordenonsi M, “The Biology of YAP/TAZ: Hippo Signaling and Beyond,” Physiol. Rev, vol. 94, no. 4, pp. 1287–1312, October 2014. [DOI] [PubMed] [Google Scholar]
- [76].Morikawa Y, Heallen T, Leach J, Xiao Y, and Martin JF, “Dystrophin–glycoprotein complex sequesters Yap to inhibit cardiomyocyte proliferation,” Nature, vol. 547, no. 7662, pp. 227–231, July 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Xiong WC and Mei L, “Agrin to YAP in Cancer and Neuromuscular Junctions,” Trends in Cancer, vol. 3, no. 4. Cell Press, pp. 247–248, 01-April-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Zhou Y, Horowitz JC, Naba A, Ambalavanan N, Atabai K, Balestrini J, Bitterman PB, Corley RA, Ding B-S, Engler AJ, Hansen KC, Hagood JS, Kheradmand F, Lin QS, Neptune E, Niklason L, Ortiz LA, Parks WC, et al. , “Extracellular matrix in lung development, homeostasis and disease,” Matrix Biol, vol. 73, pp. 77–104, November 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


