Abstract
The husks and fruits of Zanthoxylum species (Rutaceae) are the popular pungent and spicy ingredients of foods and the traditional medicines in many countries. Three Zanthoxylum species, Z. bungeanum, Z. schinifolium, and Z. piperitum, are distributed and intermixed with each other as “Zanthoxyli Pericarpium” in Korean markets. In the present study, we analyzed the ethyl acetate-soluble and nonpolar fractions of Zanthoxylum samples by 1H NMR spectrometry and performed a multivariate analysis for finding the discriminant markers between three species. Xanthoxylin was identified as the metabolic marker for the discrimination of Zanthoxylum species and quantified by the qNMR approach.
1. Introduction
Zanthoxylum species, Rutaceae, are the popular edible ingredients in Asian countries and are used as spices for spicy cuisine and medicinal herbs for treating many syndromes. In Korea, three Zanthoxylum species, Z. bungeanum, Z. schinifolium, and Z. piperitum, were distributed as “Zanthoxyli Pericarpium,” which are used as pungent condiments, spicy seasoning of foods, and medicinal herbs for stimulating intestinal motility and treating dyspepsia [1]. Zanthoxylum species contain bioactive alkaloids, aromatic and aliphatic amides, and phenolic compounds with anti-inflammation, antiplatelet aggregation, antioxidant, and antitumor activities [2, 3]. However, three Zanthoxylum species have different chemical profiles affecting the quality of biological activities and characteristic flavours and hence should be separately used [4].
Nuclear magnetic resonance (NMR) spectroscopy is a versatile technique in metabolomics and can be applied to monitor the metabolic profiles and quantify the target molecules in the biofluid matrix, as well as to elucidate the structures of small and large molecules in natural products chemistry. NMR spectroscopy yields highly accurate, precise, and reproducible spectroscopic data compared with other spectroscopic techniques, mass spectrometry [5]. Recently, quantitative NMR (qNMR) is utilized to determine the concentration and purity of one or more metabolites in the sample and applied to check the quality control of natural products [6]. The qNMR method is a rapid analysis and nondestructive with easy sample preparation which enables the simultaneous quantification of multiple metabolites in the sample without the calibration curves of reference standards [7].
In the present study, we performed the multivariate analysis (MVA) to simultaneously discriminate three Zanthoxylum species which were expected to have different NMR profiles due to their own biosynthetic systems and tried to discover and quantitate the metabolic marker which was contributed to the discrimination of three species using by the qNMR approach.
2. Materials and Methods
2.1. Plant Materials
Thirty-five dried Zanthoxylum samples, 13 of Z. bungeanum, 6 of Z. schinifolium, and 16 of Z. piperitum, were collected from March to September 2016, being purchased from Korea traditional herb markets (Kyungdong market, Seoul, Korea). Z. piperitum and Z. schinifolium samples were originated from Korea and Z. bungeanum from China. They were identified by Professor Yong Soo Kwon, College of Pharmacy of Kangwon National University, and deposited in the Herbarium of the College of Pharmacy of Kangwon National University (Supplementary Materials Table S1). One gram of the samples was extracted with 10 ml of 100% MeOH in an ultrasonic bath for 3 hours, freeze-dried, and kept at −80°C to ensure their sample integrity for further analysis.
2.2. NMR Analysis
10 mg of freeze-dried samples and 1 mg of the reference standard, xanthoxylin (Corescience Inc., Seoul, Korea), were dissolved in 1 ml of CDCl3 (euriso-top, Saint-Aubin, French) mixed with the internal standard, methyl 3,5-dinitrobenzoate (1⟶20) (Alfa Aesar, Massachustts, USA). All the NMR experiments were performed with a Bruker Avance II 600 spectrometer (Bruker Biospin, Rheinstetten, Germany) equipped with a 5 mm BBO probe with Z gradient in the Central Laboratory of Kangwon National University. NMR spectra were acquired at 298 K. Data acquisition and processing were performed with Bruker Topspin 3.0. 1H NMR spectra were acquired using the Bruker zg30 pulse program with the following settings: relaxation delay (d1), 1 s; flip angle, 30°; acquisition time, 1.33 s; free induction decay (FID) data points, 64 kbytes; spectral width, 20.55 ppm; and number of scans, 256. In all cases, the acquired FIDs were Fourier transformed to yield spectra with 128 kbyte data points (zero filling). Manual phase correction and baseline correction were always used. Chemical shift values were referenced to the calibration standard (TMS) signal.
2.3. Multivariate Analysis
1H NMR spectra were processed by binning 0.001 ppm width and normalized by the integration of the peaks separated at δ 9.16∼9.25 ppm of the internal standard, methyl 3,5-dinitrobenzoate, by Mnova (ver. 6.0.2, Mestrelab Research, Compostela, Spain) software. The processed NMR spectra were bucketed with more than 3.00 ppm, excluding the residual MeOH at 3.45∼3.51 ppm, methyl 3,5-dinitrobenzoate at 4.05∼4.10 and 9.16∼9.25 ppm, and the residual non-deuterated chloroform signals at 7.24∼7.28 ppm, respectively. The multivariate analyses, PCA and PLS-DA, were performed with R package ropls (ver. 1.14.0). The variables of the processed data matrix were scaled to the Pareto scaling method. In PLS-DA model, the R2X, R2Y, and Q2 values were calculated to describe the total variation in X and the variation in the response variable Y, and the predictive ability of the models, respectively. The score and loading plots were visualized in the optimal three-dimensional space for identifying differences among groups by R package rgl (ver. 0.99.16).
2.4. Quantitative NMR (qNMR) Analysis of Xanthoxylin
The qNMR analysis for xanthoxylin was performed with a slight modification to a previously published analysis [8]. We used methyl 3,5-dinitrobenzoate (1⟶20) as the internal standard for absolute quantification. For identifying the purest signal and non-overlapped signals of xanthoxylin in the standard and Zanthoxylum samples, the purities of all the signals of xanthoxylin were determined using the following equation:
| (1) |
where Int is the integral, MW is the molecular weight, m is the mass, n is the number of protons, P is the purity expressed as %, IS is the internal standard, and F is xanthoxylin. The intraday and interday variabilities of xanthoxylin were examined by three replicate experiments at a concentration of 10 mg/ml in CDCl3 mixed with the internal standard, methyl 3,5-dinitrobenzoate (1⟶20) in one day for the intraday test and three consecutive days (1, 3, and 5 days) for the interday test. The relative standard deviations (RSD) were calculated as a measure of precision.
3. Results and Discussion
The NMR experimental parameters were optimized to obtain clear resolution and separation of peaks (see Section 2). Methyl 3,5-dinitrobenzoate was added in NMR solvent as the internal standard of which the chemical shifts at δ 9.2 (2H, d, J = 2.13 Hz) and 9.3 (1H, t, J = 2.13 Hz) ppm were totally not overlapped with other peaks (Figure 1). In the 1H NMR of Zanthoxylum samples, we excluded the up-fielded peaks in the range of δ 0.8∼2.5 ppm which were tentatively expected as the aliphatic lipids and overwhelmed other minor peaks. Therefore, we processed the down-fielded peaks in the ranges of above 3.0 ppm which were normalized by the integral of two peaks at δ 9.2 (2H, d, J = 2.13 Hz) and 9.3 (1H, t, J = 2.13 Hz) of the internal standard for the further study.
Figure 1.
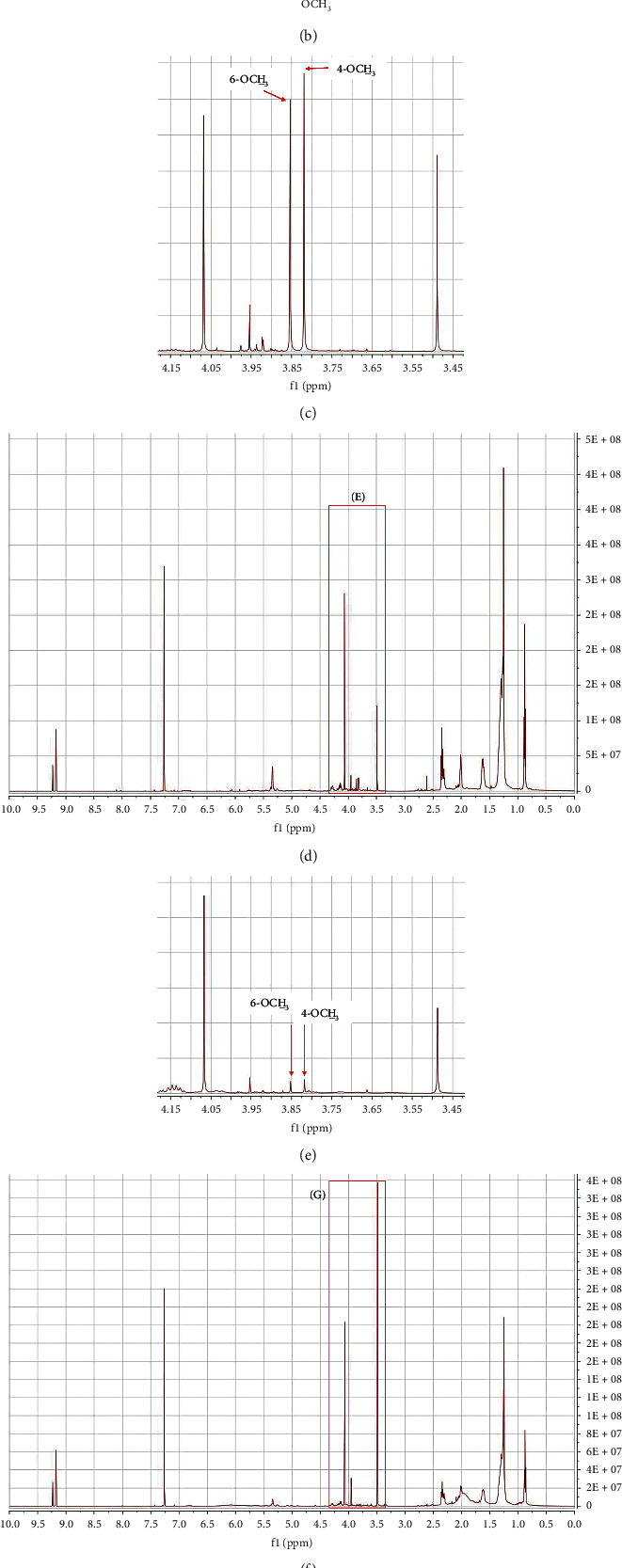
1H NMR spectra of three Zanthoxylum species—Z. bungeanum (a, c), Z. schinifolium (d, e), and Z. piperitum (f, g)—and the structure of xanthoxylin (b). The 1H NMR spectra corresponding to the signals of 4-OCH3 and 6-OCH3 in (a), (d), and (f) were magnified to (c), (e), and (g), respectively.
After the processing steps, such as peaks alignment, normalization, and binning, of the NMR spectral data, we excluded the solvent signals at δ 3.45∼3.51 ppm of the residual MeOH, δ 4.05∼4.10 and δ 9.16∼9.25 ppm of methyl 3,5-dinitrobenzoate, and δ 7.24∼7.28 ppm of the residual non-deuterated chloroform which hindered the interpretation of minor peaks in samples. Firstly, the data matrix was subjected to the unsupervised multivariate analysis (MVA) and the explorative principal components analysis (PCA) to investigate the general trend of clustering between three species, but the samples labelled with species were not clearly clustered by major principal component (PC) 1 (Figure 2). Though Z. bungeanum samples were roughly separated from two species along PC2, the scores for Z. piperitum and Z. schinifolium were nearly identical. Z. piperitum samples were clustered closer together than the other two species, and their qualities were more consistent than others. In the loading plot, δ 3.82 and 3.85 ppm (PC1 > 0.15 and PC2 > 0.11), 4.11 (PC1 > 0.06 and PC2 < -0.03), and 5.34 ppm (PC1 < −0.06 and PC2 > 0.11) were contributed as the significant variables. The supervised partial least squares discriminant analysis (PLS-DA) (R2X = 0.54; R2Y = 0.71; Q2 = 0.61) improved the separation between species (Figure 2). In consistent with PCA results, Z. piperitum samples were more compact than Z. bungeanum and Z. schinifolium, along the three major predictive components. The groups corresponding to Z. schinifolium and Z. piperitum samples were observed at negative values of PC1, while Z. bungeanum samples were found at positive ones of PC1. Next, we investigated the loading plot for identifying the metabolic markers contributing to the separation of three species in the score plot in PLS-DA. The variables for δ 3.82 and 3.85 ppm (PC1 > 0.1, PC2 > 0.1, and PC3 > 0.12) and for δ 5.34 ppm (PC1 < −0.05, PC2 > 0.11 and PC3 > 0.02) were significantly scattered in the direction of Z. bungeanum and Z. schinifolium, respectively, which were similar to the PCA results.
Figure 2.
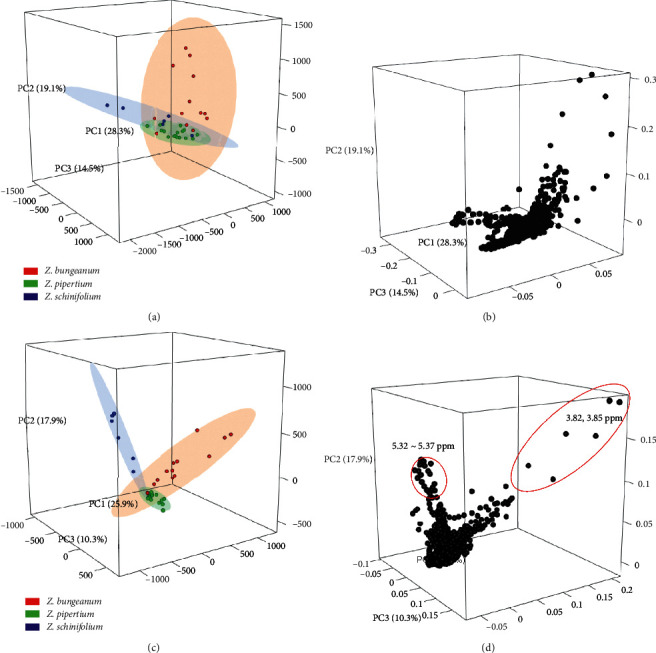
The multivariate plots of three Zanthoxylum species based on the 1H NMR spectra. (a) The PCA score plot with 95% density ellipses. (b) The PCA loading plot. (c) The PLS-DA score plot with 95% density ellipses. (d) The PLS-DA loading plot. The red circles in the PLS-DA loading plot show the variables contributing to the separation of Z. bungeanum and Z. schinifolium samples.
The signals at δ 5.32∼5.37 ppm were tentatively predicted as the protons attached to the olefinic carbons, but the signals at δ 3.82 and 3.85 ppm were identified as a simple phenolic compound, xanthoxylin, which was confirmed with the 1H NMR spectrum of the standard (see Figure S1 in Supplementary Materials) [9–12]. Precision tests of the two signals in xanthoxylin were performed (Table 1). The intraday and interday variations were less than 4.43 and 2.55, respectively. The signals of two methoxy protons were sufficient to use as a quantitative standard in Zanthoxylum species. Xanthoxylin in three Zanthoxylum species was calculated by the qNMR method. Although the variance was large within the same species, xanthoxylin was significantly more abundant in Z. bungeanum than the other two species (Table 2).
Table 1.
Precision for 1H NMR signals of xanthoxylin for proton (ppm); intraday, RSD (%); and interday, RSD (%).
| Proton (ppm) | Intraday, RSD (%) | Interday, RSD (%) |
|---|---|---|
| 4-OCH3 (3.82) | 4.43 | 2.50 |
| 6-OCH3 (3.85) | 4.42 | 2.55 |
Table 2.
The content of xanthoxylin for 16 elutions in three Zanthoxylum species: Z. piperitum, Z. schinifolium, and Z. bungeanum.
| No. | Z. piperitum | Z. schinifolium | Z. bungeanum |
|---|---|---|---|
| (mg/ml) | |||
| 1 | 0.03 | 0.14 | 0.05 |
| 2 | 0.03 | 0.13 | 0.08 |
| 3 | 0.03 | 0.10 | 0.58 |
| 4 | 0.06 | 0.12 | 0.37 |
| 5 | 0.04 | 0.03 | 1.13 |
| 6 | 0.02 | 0.09 | 1.46 |
| 7 | 0.02 | 1.13 | |
| 8 | 0.04 | 0.40 | |
| 9 | 0.06 | 1.43 | |
| 10 | 0.08 | 0.47 | |
| 11 | 0.06 | 0.35 | |
| 12 | 0.07 | 0.11 | |
| 13 | 0.03 | 0.06 | |
| 14 | 0.02 | ||
| 15 | 0.05 | ||
| 16 | 0.05 | ||
| Mean | 0.04 | 0.10 | 0.59 |
| SD | 0.02 | 0.04 | 0.52 |
4. Conclusions
qNMR is the versatile spectroscopic technique to quantify the target molecule in the mixture of metabolites in the extract and to simultaneously inspect the quantitative changes of metabolites with the multivariate analysis. This method can be applied to analyze huge metabolites in natural products samples. In the present study, we measured the 1H NMR spectra of 35 samples of three Zanthoxlyum species and developed the rapid discrimination method of three Zanthoxlyum species by 1H NMR spectrometry. As a result, the metabolic marker, xanthoxylin, contributed to the discrimination of three species. This simple approach could be applied to the quality assessment of dried Zanthoxlyum samples and their extract-containing products.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) and was funded by the Ministry of Science, ICT and Future Planning (NRF-2018R1C1B6002574) and 2017 Research Grant from Kangwon National University (no. 520170395).
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Disclosure
Hyeon Seok Jang and Birang Jeong are considered as co-first authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Authors' Contributions
Hyeon Seok Jang and Birang Jeong contributed equally to this work.
Supplementary Materials
Table S1: the herbarium records of Zanthoxylum species. Figure S1: the 1H NMR spectrum of xanthoxylin mixed with the internal standard, methyl 3,5-dinitrobenzoate.
References
- 1.Yang Y., Ikezoe T., Takeuchi T., et al. Zanthoxyli fructus induces growth arrest and apoptosis of LNCaP human prostate cancer cells in vitro and in vivo in association with blockade of the AKT and AR signal pathways. Oncology Reports. 2006;15(6):1581–1590. [PubMed] [Google Scholar]
- 2.Jeong S. Y., Nguyen P. H., Zhao B. T., Min B. S., Ma E. S., Woo M. H. Bioactive constituents from the leaves of Zanthoxylum schinifolium. Natural Product Sciences. 2015;21(1):1–5. [Google Scholar]
- 3.Jo Y. S., Huong D. T., Bae K., Lee M. K., Kim Y. H. Monoamine oxidase inhibitory coumarin from Zanthoxylum schinifolium. Planta Medica. 2002;68(1):84–85. doi: 10.1055/s-2002-20056. [DOI] [PubMed] [Google Scholar]
- 4.Yang X. Aroma constituents and alkylamides of red and green huajiao (Zanthoxylum bungeanum and Zanthoxylum schinifolium) Journal of Agricultural and Food Chemistry. 2008;56(5):1689–1696. doi: 10.1021/jf0728101. [DOI] [PubMed] [Google Scholar]
- 5.Bharti S. K., Roy R. Quantitative 1H NMR spectroscopy. TrAC Trends in Analytical Chemistry. 2012;35:5–26. doi: 10.1016/j.trac.2012.02.007. [DOI] [Google Scholar]
- 6.Pauli G. F. qNMR ? a versatile concept for the validation of natural product reference compounds. Phytochemical Analysis. 2001;12(1):28–42. doi: 10.1002/1099-1565(200101/02)12:1<28::aid-pca549>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 7.Chen X., Guo Y., Hu Y., Yu B., Qi J. Quantitative analysis of highly similar salvianolic acids with 1 H qNMR for quality control of traditional Chinese medicinal preparation Salvianolate Lyophilized Injection. Journal of Pharmaceutical and Biomedical Analysis. 2016;124:281–287. doi: 10.1016/j.jpba.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 8.Pauli G. F., Chen S.-N., Simmler C., et al. Importance of purity evaluation and the potential of quantitative 1H NMR as a purity assay. Journal of Medicinal Chemistry. 2014;57(22):9220–9231. doi: 10.1021/jm500734a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang M., Wang J., Zhu L., et al. Zanthoxylum bungeanum maxim. (rutaceae): a systematic review of its traditional uses, botany, phytochemistry, pharmacology, pharmacokinetics, and toxicology. International Journal of Molecular Sciences. 2017;18(10):p. 2172. doi: 10.3390/ijms18102172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruangrungsi N., Tantivatana P., Borris R., Cordell G. Traditional medicinal plants of Thailand. III. Constituents of Zanthoxylum budrunga (Rutaceae) Science Asia. 1981;7(3):123–127. doi: 10.2306/scienceasia1513-1874.1981.07.123. [DOI] [Google Scholar]
- 11.Hu K., Wang W., Cheng H., Pan S., Ren J. Synthesis and cytotoxicity of novel chrysin derivatives. Medicinal Chemistry Research. 2011;20(7):838–846. doi: 10.1007/s00044-010-9395-1. [DOI] [Google Scholar]
- 12.Deng Y.-R., Song A.-X., Wang H.-Q. Chemical components ofSeriphidium SantoliumPoljak. Journal of the Chinese Chemical Society. 2004;51(3):629–636. doi: 10.1002/jccs.200400094. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: the herbarium records of Zanthoxylum species. Figure S1: the 1H NMR spectrum of xanthoxylin mixed with the internal standard, methyl 3,5-dinitrobenzoate.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


