Abstract
Background
Atrial fibrillation (AF) is a major, often undetected, cardiac cause of stroke. Markers of atrial cardiopathy, including left atrial enlargement (LAE) or excessive atrial ectopy (EAE) increase the risk of AF and have shown associations with stroke. We sought to determine whether these markers improve stroke risk prediction beyond traditional vascular risk factors (eg CHA 2 DS 2‐VASc score).
Methods and Results
Retrospective longitudinal cohort of 32 454 consecutive community‐dwelling adults aged ≥65 years referred for outpatient echocardiogram or Holter in Ontario, Canada (2010–2017). Moderate‐severe LAE was defined as men >47 mm and women >43 mm, and EAE was defined as >30 APBs per hour. Cause‐specific competing risks Cox proportional hazards used to estimate risk of ischemic stroke (primary), incident AF, and death (secondary). C‐statistics, incremental discrimination improvement and net reclassification were used to compare CHA 2 DS 2‐VASc with LAE and EAE to CHA 2 DS 2‐VASc alone. Each 10 mm increase in left atrial diameter increased 2‐ and 5‐year adjusted cause‐specific stroke hazard almost 2‐fold (LAE: 2‐year hazard ratio (HR), 1.72; P=0.007; 5‐year HR, 1.87; P<0.0001), while EAE showed no significant associations with stroke (2‐year HR, 1.00; P=0.99; 5‐year HR, 1.08, P=0.70), adjusting for incident AF. Stroke risk estimation improved significantly at 2 (C‐statistics=0.68–0.75, P=0.008) and 5 years (C‐statistics=0.70–0.76, P=0.003) with LAE and EAE.
Conclusions
LAE was independently associated with an increased risk of ischemic stroke in the absence of AF and both LAE and EAE improved stroke risk prediction. These findings have implications for stroke risk stratification, AF screening, and stroke prevention before the onset of AF.
Keywords: atrial cardiopathy, atrial fibrillation, CHA2DS2‐VASc score, risk prediction, stroke
Subject Categories: Atrial Fibrillation, Epidemiology, Remodeling, Ischemic Stroke
Nonstandard Abbreviations and Acronyms
- AF
atrial fibrillation
- APB
atrial premature beats
- CHF
congestive heart failure
- EAE
excessive atrial ectopy
- LAE
left atrial enlargement
- NT‐proBNP
N‐terminal pro‐B‐type natriuretic peptide
- OAC
oral anticoagulation
Clinical Perspective
What Is New?
Left atrial enlargement is associated with stroke risk in the absence of atrial fibrillation and independent of incident atrial fibrillation and both left atrial enlargement and excessive atrial ectopy significantly improve CHA2DS2‐VASc risk prediction.
What Are the Clinical Implications?
Markers of left atrial cardiopathy may have implications for atrial fibrillation screening and stroke risk stratification.
Atrial fibrillation (AF) is a major preventable cardiac cause of stroke.1 AF independently increases stroke risk by 5‐fold2 and accounts for >20% of all acute ischemic strokes.3, 4 Oral anticoagulation (OAC) reduces stroke risk by two thirds in patients with AF5 and clinical scoring systems that stratify patients according to traditional vascular risk factors (eg CHA2DS2‐VASc score) are routinely used to estimate stroke risk and guide OAC treatment decisions in non‐anticoagulated patients with non‐valvular AF.6
However, AF is commonly paroxysmal or clinically silent7, 8 and frequently goes undetected before stroke.9 In a recent prospective analysis of 2580 pacemaker patients with vascular risk factors and no known AF, brief subclinical AF was associated with a >2‐fold increased risk of stroke.10 Further, up to one third of patients do not have AF on long‐term continuous rhythm monitoring before stroke,11, 12 suggesting that factors other than manifest AF are involved.
Clinical AF is often preceded by structural and electrical left atrial remodeling,13 referred to as atrial cardiopathy or atrial myopathy.14 Several biomarkers of atrial cardiopathy have been described and include left atrial enlargement (LAE) and excessive atrial ectopic beats.14 These markers may be detected before the onset of clinical AF and are predictive of AF.15, 16 Emerging data also suggest that these markers are associated with incident stroke.17 The purpose of this study was to determine if these markers of atrial cardiopathy increase the risk of ischemic stroke, incident AF, or death in individuals without known AF and whether these markers can improve stroke risk prediction beyond traditional vascular risk factors (eg CHA2DS2‐VASc score).
Methods
Study Cohort and Data
This retrospective longitudinal cohort was comprised of consecutive community‐dwelling adults referred for outpatient echocardiography or Holter monitor at 11 community cardiology laboratories in Ontario, Canada (2010–2017). Exclusion criteria were a history of documented AF, current anticoagulation use, or a history of pacemaker, implantable cardioverter defibrillator, implantable loop recorder, or prosthetic heart valve surgery. Individuals entered the study cohort on the date of their first recorded echocardiogram or Holter study and were followed up to of 5 years. To ensure exclusion of those on anticoagulation at baseline and identify individuals initiating anticoagulation during follow‐up, the cohort for the primary analysis was restricted to those aged ≥65 years, for whom prescribing data were available.
Echocardiography and Holter monitor data were linked with provincial administrative databases housed at ICES using unique encoded patient identifiers for the assessment of study outcomes. Data from the Canadian Institute for Health Information Discharge Abstract Database and the Ontario Health Insurance Plan database, which capture universally available government‐funded coverage for all hospital services, physician visits, and diagnostic tests, were used to document all hospitalizations and physician encounters for primary and secondary outcomes, using the International Classification of Diseases, Tenth Revision (ICD‐10) (Table S1). Data on out‐of‐hospital mortality were obtained from the Ontario Registered Persons Database and data on prescription medications (for those aged ≥65 years) were obtained from the Ontario Drug Benefit Claims database.
The data from this study are held securely in coded form at ICES. Although data sharing agreements prohibit ICES from making the data set publicly available, access may be granted to those who meet pre‐specified criteria for confidential access, available at http://www.ices.on.ca/DAS. The full data set creation plan and underlying analytic code are available from the authors on request, understanding that the programs may rely on coding templates or macros that are unique to ICES. This study was approved by the Research Ethics Board at Sunnybrook Health Sciences Centre. ICES is a prescribed entity under section 45 of Ontario’s Personal Health Information Protection Act. Section 45 authorizes ICES to collect personal health information, without consent, for the purpose of analysis or compiling statistical information with respect to the management of, evaluation or monitoring of, the allocation of resources to or planning for all or part of the health system.
Study Exposures
Measures of atrial cardiopathy included left atrial enlargement (LAE) and excessive atrial ectopy (EAE), obtained from outpatient echocardiography and Holter monitor studies. LAE was measured as antero‐posterior linear left atrial diameter (mm) on the baseline 2D echocardiogram, with moderate‐to‐severe enlargement defined using sex‐specific cut‐offs (≥43 mm for women and ≥47 mm for men).18 EAE was measured as frequency of atrial premature beats per hour on the baseline Holter, with hourly atrial premature beats (APB) count categorized as: (1) normal (0–30 beats/h) and (2) excessive (30+ beats/h), as has previously been reported.19 To examine potential cumulative effects of multiple markers of atrial cardiopathy, a composite measure was also obtained for individuals with both LAE and EAE, as defined above.
Outcomes
The primary outcome was a hospital admission for acute ischemic stroke, ascertained using a previously validated administrative data algorithm for stroke (sensitivity=86%; positive predictive value=90%)20 (Table S1). Secondary outcomes included a diagnosis of incident AF, ascertained using a previously validated administrative data algorithm for AF (sensitivity=79.3%; positive predictive value=80.4%)21 and all‐cause mortality (Table S1).
Statistical Analysis
Descriptive statistics were used to characterize the study cohort with respect to all demographic and clinical variables, with Chi‐square tests, one‐way ANOVA or t‐tests used to compare means between exposure categories. We generated 2‐ and 5‐year absolute person‐time incidence rates (per 1000 person‐years) for ischemic stroke (primary) and incident AF and death (secondary) associated with LAE, EAE, and the composite of both LAE and EAE. Kaplan–Meier curves were generated to estimate 5‐year age direct‐adjusted survival for those with normal versus mild and moderate‐severe LAE and normal APB frequency versus EAE. Cox proportional hazards regression was used to estimate the 2‐ and 5‐year cause‐specific hazard for primary (ischemic stroke) and secondary outcomes (incident AF and death) for each 10‐mm increase in left atrial diameter or for an increased frequency in APBs/hour (EAE: >30). Models for stroke were adjusted for both death and incident AF as a competing risk,22 while models for incident AF were only adjusted for death as a competing risk. All models were adjusted for demographic and clinical comorbidities, including age, sex, prior history of hypertension, diabetes mellitus, congestive heart failure (CHF), ischemic stroke, myocardial infarction, left ventricular hypertrophy, left ventricular mass index, systolic function, and medication status, including prior (<1 year) use of antiplatelet, statin, or anti‐hypertensive therapy. Initiation of oral anticoagulant therapy during follow‐up was entered as a time‐varying covariate into all statistical models. A sensitivity analysis was also performed, entering LAE and EAE as an interaction term to determine if there was an interaction between these markers for the outcomes of stroke, incident AF and death at 2 and 5 years.
We used information on traditional risk factors, including age, sex, and history of CHF, hypertension, stroke, transient ischemic attack, vascular disease, and diabetes mellitus to predict stroke risk, indexed by deriving individual CHA2DS2‐VASc scores for the full cohort (not restricted to individuals aged ≥65 years).23 We then generated C‐statistics to estimate 2‐ and 5‐year stroke risk in this cohort using the CHA2DS2‐VASc alone and compared them to C‐statistics for CHA2DS2‐VASc with the addition of LAE as a continuous variable, EAE as a binary variable and, where available, both LAE and EAE. Chi‐square testing was used to compare C‐statistics for the prediction of stroke risk. Integrated discrimination improvement and net reclassification improvement analyses,24 with bootstrapping to adjust for optimism in the estimates of fit,25 were also performed to independently evaluate the additional predictive utility of LAE and EAE for stroke risk.26
Results
The cohort comprised a total of 32 454 community‐dwelling adults without documented AF: 19 265 with an outpatient echocardiogram, and 13 189 with an outpatient Holter. Demographic and clinical characteristics for those in each exposure category for LAE and EAE are presented in Table 1. Individuals with moderate‐severe LAE and EAE were significantly older than those without, and had significantly more comorbidities, including hypertension, CHF, and diabetes mellitus, had higher values for left ventricular mass index, and had higher median CHA2DS2‐VASc scores (Table 1).
Table 1.
Demographic and Clinical Characteristics for Consecutive Community‐Dwelling Adults Aged ≥65 Years With No Documented Atrial Fibrillation Referred to Outpatient Cardiology Clinics From 2010 to 2017 (N=32 454), Separately for Those With Normal, Mild, or Moderate‐Severe LAE on Echocardiography (n=19 265) and Normal or Excessive Atrial Ectopy on Holter Investigations (n=13 189)
| Echocardiography | P Value | Holter | P Value | ||||
|---|---|---|---|---|---|---|---|
| Normal n=14 658n (%) | Mild LAE n=3601n (%) | Moderate‐Severe LAE (Women >42 mm; Men >47 mm)n=1006n (%) | Normal n=10 377n (%) | EAE (≥30 APBs/h) n=2812n (%) | |||
| Age, y (mean±SD) | 70.8±4.3 | 71.2±4.4 | 71.6±4.6 | <0.0001a | 73.5±6.8 | 76.4±7.2 | <0.0001a |
| Women | 8089 (55.2) | 1736 (48.2) | 564 (56.1) | <0.0001a | 4201 (40.5) | 1327 (47.2) | <0.0001a |
| Rural residence | 408 (2.8) | 125 (3.5) | 41 (4.1) | 0.01 | 191 (1.8) | 57 (2.0) | 0.52 |
| CHA2DS2‐VASc (mean±SD) | 1.2±0.9 | 1.5±1.0 | 1.7±1.0 | <0.0001a | 1.5±2.0 | 1.7±1.0 | <0.0001a |
| PMH | |||||||
| Stroke | 122 (0.8) | 34 (0.9) | 17 (1.7) | 0.02 | 151 (1.5) | 42 (1.5) | 0.88 |
| CHF | 384 (2.6) | 214 (5.9) | 112 (11.1) | <0.0001a | 416 (4.0) | 151 (5.4) | 0.002a |
| Hypertension | 9944 (67.8) | 2783 (77.3) | 827 (82.2) | <0.0001a | 7429 (71.6) | 2117 (75.3) | 0.0001a |
| Diabetes mellitus | 4192 (28.6) | 1293 (35.9) | 379 (37.7) | <0.0001a | 2886 (27.8) | 785 (27.9) | 0.91 |
| Hyperlipidemia | 801 (5.5) | 303 (8.4) | 105 (10.4) | <0.0001a | 731 (7.0) | 165 (5.9) | 0.03 |
| MI | 291 (2.0) | 143 (4.0) | 52 (5.2) | <0.0001a | 288 (2.8) | 61 (2.2) | 0.08 |
| Angina | 300 (2.0) | 144 (4.0) | 39 (3.9) | <0.0001a | 294 (2.8) | 71 (2.5) | 0.38 |
| PCI/CABG | 534 (3.6) | 268 (7.4) | 87 (8.6) | <0.0001a | 432 (4.2) | 104 (3.7) | 0.27 |
| Ischemic heart disease | 979 (6.7) | 462 (12.8) | 163 (16.2) | <0.0001a | 896 (8.6) | 234 (8.3) | 0.86 |
| Vascular disease | 708 (4.8) | 336 (9.3) | 106 (10.5) | <0.0001a | 592 (5.7) | 145 (5.2) | 0.26 |
| Peripheral disease | 119 (0.8) | 55 (1.5) | 12 (1.2) | 0.0003a | 90 (0.9) | 28 (1.0) | 0.52 |
| Charlson ≥2 | 1942 (13.2) | 649 (18.0) | 206 (20.5) | <0.0001a | 1562 (15.1) | 485 (17.2) | 0.004 |
| LV mass index, mean±SD | 123.6±45.3 | 158.5±69.8 | 176.7±52.1 | <0.0001a | 131.2±63.2 | 136.1±42.4 | 0.04 |
| Concentric LVH | 566 (3.9) | 366 (10.2) | 136 (13.5) | <0.0001a | 171 (1.6) | 42 (1.5) | 0.81 |
| Systolic functionb | n=6731 | n=1925 | n=632 | n=1796 | n=365 | ||
| Grade I | 6040 (89.7) | 1517 (78.8) | 431 (68.2) | 1558 (86.7) | 299 (81.9) | ||
| Grade I to II | 342 (5.1) | 171 (8.9) | 62 (9.8) | 112 (6.2) | 32 (8.8) | ||
| Grade II | 226 (3.4) | 130 (6.8) | 64 (10.1) | 61 (3.4) | 13 (3.6) | ||
| Grade II to III | 61 (0.9) | 49 (2.5) | 22 (3.5) | 20 (1.1) | 7 (1.9) | ||
| Grade III | 46 (0.7) | 38 (2.0) | 28 (4.4) | 32 (1.8) | 12 (3.3) | ||
| Grade III to IV | 11 (0.2) | 17 (0.9) | 15 (2.4) | 8 (0.4) | ≤5 | ||
| Grade IV | ≤5 | ≤5 | 10 (1.6) | <0.0001a | ≤5 | ≤5 | 0.18 |
| Prior medication use (<1 y) | |||||||
| Antihypertensive | 8625 (58.8) | 2563 (71.2) | 798 (79.3) | <0.0001a | 6511 (62.7) | 1891 (67.2) | <0.0001a |
| Statin | 7111 (48.5) | 1981 (55.0) | 576 (57.3) | <0.0001a | 5103 (49.2) | 1382 (49.1) | 0.98 |
| Antiplatelet | 926 (6.3) | 309 (8.6) | 109 (10.8) | <0.0001a | 749 (7.2) | 229 (8.1) | 0.10 |
APB indicates atrial premature beats; CHF indicates congestive heart failure; IQR, interquartile range; LAE, left atrial enlargement; LV, left ventricular; LVH, left ventricular hypertrophy; MI, myocardial infarction; PCI/CABG, percutaneous coronary intervention/coronary artery bypass grafting; and PMH, prior medical history;
P<0.05 corrected for multiple comparisons to P<0.002 for significance.
On available data for systolic function.
Kaplan–Meier analyses estimating age direct‐adjusted 5‐year survival showed that survival was reduced for those with LAE, but not for those with EAE (Figure). The absolute rates of ischemic stroke were significantly greater among those with LAE as compared with normal left atrial diameter at 5 years (5.3, 95% CI, 3.4–8.4 versus 2.3, 95% CI, 2.0–2.8, P=0.001), but not at 2 years (2.1, 95% CI, 0.8–5.7 versus 2.3, 95% CI, 1.8–2.9, P=0.15) (Table 2). Those with LAE had significantly increased rates of incident AF and death at both 2 and 5 years (Table 2). Similarly, the absolute rates of ischemic stroke were significantly higher for those with versus without EAE at 5 years (5.9; 95% CI, 4.6–7.6 versus 3.6; 95% CI, 3.1–4.3, P=0.003) but not at 2 years (5.5; 95% CI, 3.8–8.0 versus 3.9; 95% CI, 3.1–4.9, P=0.12) (Table 2). EAE was also associated with significantly increased rates of incident AF and death at 2 and 5 years (Table 2). Among those who had both echocardiography and Holter (n=4688), the composite exposure of moderate‐severe LAE and EAE did not significantly increase either the 2‐ or 5‐year absolute rates of ischemic stroke (P=0.26 and P=0.08, respectively), but did appear to cumulatively increase rates of incident AF (Table S2). As rates of the primary outcome did not differ for the composite exposure of LAE and EAE, this composite exposure was not included in the multivariate analysis.
Figure 1. Age direct‐adjusted 5‐year survival for (A) normal, mild, and moderate‐severe left atrial enlargement and (B) normal and excessive atrial ectopy; P values from age‐adjusted Cox proportional hazards models.
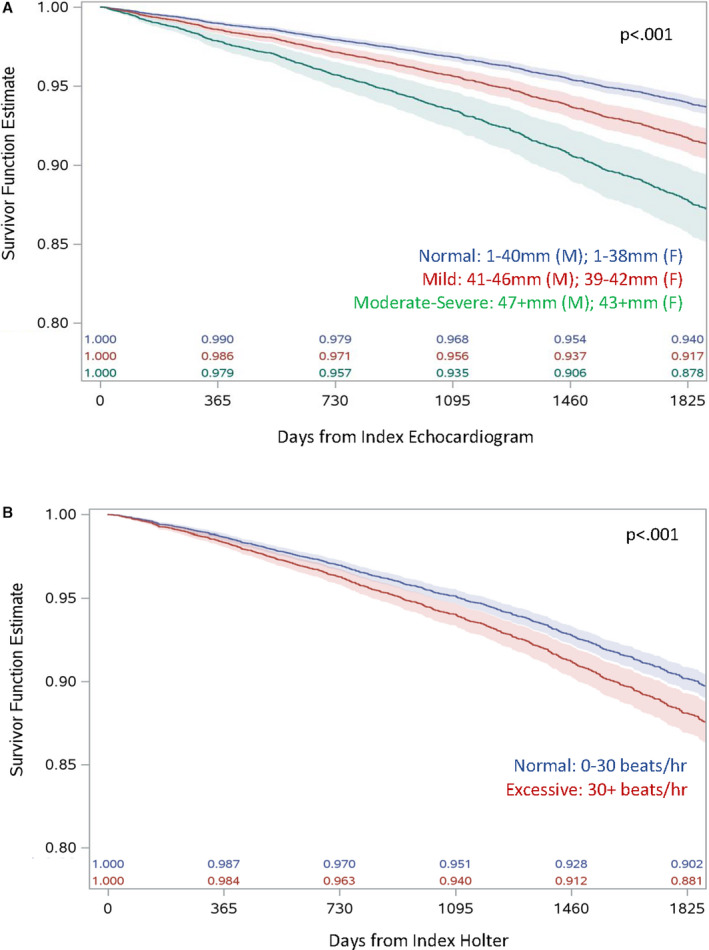
Table 2.
Two‐ and Five‐Year Absolute Person‐Time Incidence Rates (Per 1000 Person‐Years) of Ischemic Stroke (Primary Outcome), Incident AF and Death (Secondary Outcomes) for Those With Normal, Mild, and Moderate‐Severe Left Atrial Enlargement (n=19 265) and Normal and Excessive Atrial Ectopy (n=13 189)
| Outcomes | Left Atrial Enlargement | P Value | Atrial Ectopy | P Value | |||
|---|---|---|---|---|---|---|---|
| Normal | Mild | Moderate‐Severe Women >42 mm; Men >46 mm | Normal | EAE >30 APBs/h | |||
| 2‐y rates | |||||||
| Ischemic stroke | |||||||
| Rate 1000 PY (95% CI) | 2.3 (1.8–2.9) | 3.7 (2.5–5.4) | 2.1 (0.8–5.7) | 3.9 (3.1–4.9) | 5.5 (3.8–8.0) | ||
| Total event counts | 64 | 25 | 4 | 76 | 29 | ||
| Total PY | 27 860.43 | 6834.54 | 1880.46 | 19 559.10 | 5246.58 | ||
| Mean follow‐up PY | 1.9 | 1.9 | 1.87 | 1.88 | 1.87 | ||
| Median follow‐up PY | 2.00 | 2.00 | 2.00 | 0.15 | 2.00 | 2.00 | 0.12 |
| Incident AF | |||||||
| Rate 1000 PY (95% CI) | 20.6 (19.0–22.4) | 43.5 (38.7–48.9) | 87.1 (74.1–102.4) | 40.3 (37.5–43.3) | 118.3 (108.7–128.8) | ||
| Total event counts | 561 | 282 | 147 | 748 | 531 | ||
| Total PY | 27 174.6 | 6874.35 | 1686.83 | 18 567.88 | 4487.26 | ||
| Mean follow‐up PY | 1.85 | 1.80 | 1.68 | 1.79 | 1.60 | ||
| Median follow‐up PY | 2.00 | 2.00 | 2.00 | <0.0001[Link] | 2.00 | 2.00 | <0.0001[Link] |
| Death | |||||||
| Rate 1000 PY (95% CI) | 10.4 (9.3–11.7) | 14.3 (11.7–17.4) | 23.9 (17.8–32.0) | 13.8 (12.3–15.6) | 25.2 (21.3–29.9) | ||
| Total event counts | 290 | 98 | 45 | 271 | 133 | ||
| Total PY | 27 914.82 | 6853.99 | 1884.14 | 19 619.44 | 5273.20 | ||
| Mean follow‐up PY | 1.90 | 1.90 | 1.87 | 1.89 | 1.88 | ||
| Median follow‐up PY | 2.00 | 2.00 | 2.00 | <0.0001[Link] | 2.00 | 2.00 | <0.0001[Link] |
| 5‐y rates | |||||||
| Ischemic stroke | |||||||
| Rate 1000 PY (95% CI) | 2.3 (2.0–2.8) | 3.5 (2.7–4.7) | 5.3 (3.4–8.4) | 3.6 (3.1–4.3) | 5.9 (467–7.6) | ||
| Total event counts | 129 | 48 | 19 | 136 | 58 | ||
| Total PY | 55 074.49 | 13 564.82 | 3551.92 | 37 366.68 | 9838.79 | ||
| Mean follow‐up PY | 3.76 | 3.77 | 3.53 | 3.60 | 3.50 | ||
| Median follow‐up PY | 4.34 | 4.36 | 3.85 | 0.001[Link] | 3.95 | 3.72 | 0.003[Link] |
| Incident AF | |||||||
| Rate 1000 PY (95% CI) | 16.0 (15.0–17.2) | 33.8 (30.7–37.2) | 64.7 (56.3–74.4) | 29.0 (27.3–30.8) | 81.1 (75.1–87.6) | ||
| Total event counts | 851 | 422 | 196 | 1007 | 652 | ||
| Total PY | 53 064.70 | 12 494.43 | 3029.14 | 34 732.98 | 8039.75 | ||
| Mean follow‐up PY | 3.62 | 3.47 | 3.01 | 3.35 | 2.86 | ||
| Median follow‐up PY | 4.13 | 3.93 | 3.04 | <0.0001[Link] | 3.61 | 2.89 | <0.0001[Link] |
| Death | |||||||
| Rate 1000 PY (95% CI) | 11.8 (10.9–12.7) | 16.9 (14.9–19.3) | 26.6 (21.7–32.5) | 17.7 (16.4–19.1) | 29.1 (25.9–32.7) | ||
| Total event counts | 652 | 231 | 95 | 666 | 289 | ||
| Total PY | 55 319.00 | 13 647.78 | 3575.08 | 37 585.93 | 9927.21 | ||
| Mean follow‐up PY | 3.77 | 3.789 | 3.55 | 3.62 | 3.53 | ||
| Median follow‐up PY | 4.38 | 4.42 | 3.88 | <0.0001[Link] | 3.99 | 3.77 | <0.0001[Link] |
P value for comparison of rates between outcome categories. AF indicates atrial fibrillation; APBs, atrial premature beats; and PY, person‐years.
Competing risks Cox proportional hazards analyses indicated that, after adjustment for age, sex, hypertension, diabetes mellitus, CHF, stroke, myocardial infarction, left ventricular hypertrophy, left ventricular mass index, left ventricular systolic function, baseline medication status (antihypertensive, statin, antiplatelet), and adjusting for all‐cause mortality and incident AF as competing risks and time‐varying adjustment for initiation of anticoagulation during follow‐up, each 10‐mm increase in left atrial diameter increased both the 2‐ and 5‐year adjusted cause‐specific hazard of ischemic stroke by almost 2‐fold (2‐year HR, 1.72; 95% CI, 1.16–2.55, P=0.007; 5‐year HR, 1.87; 95% CI, 1.41–2.49, P<0.0001), while EAE (>30 APBs/h) showed no significant associations with stroke risk at either 2 (HR, 1.00; 95% CI, 0.60–1.67, P=0.99) or 5 years (HR, 1.08; 95% CI=0.73–1.59, P=0.71) (Table 3). A >2‐fold increase in the cause‐specific hazard of incident AF was also observed for both LAE and EAE at 2 and 5 years (Table 3). Sensitivity analyses showed no significant interaction between LAE and EAE for any of the models, even after adjustment for age, sex, history of HTN, diabetes mellitus, CHF, myocardial infarction, ischemic heart disease, left ventricular hypertrophy, and systolic function.
Table 3.
Two‐ and Five‐Year Adjusted Cause‐Specific Hazard of Ischemic Stroke (Primary) and Incident AF (Secondary) Associated With Each 10‐mm Increase in LAE and With >30 APBs/h (EAE)
| Outcomes | Model 1: LA Diameter (Per 10 mm Increase) | Model 2: Atrial Ectopy (<30 vs >30 APBs/h) | ||
|---|---|---|---|---|
| Adjusted HRa (95% CI) | Adjusted HR With Selectionb (95% CI) | Adjusted HRa (95% CI) | Adjusted HR With Selectionb (95% CI) | |
| 2 y | ||||
| Primary | ||||
| Ischemic stroke | 1.33 (0.87–2.04) | 1.72 (1.16–2.55) | 1.00 (0.60–1.67) | ··· |
| Secondary | ||||
| Incident AF | 2.34 (2.07–2.65) | 2.36 (2.10–2.65) | 2.55 (2.27–2.86) | 2.54 (2.27–2.85) |
| 5 y | ||||
| Primary | ||||
| Ischemic stroke | 1.57 (1.16–2.13) | 1.87 (1.41–2.49) | 1.08 (0.73–1.59) | ··· |
| Secondary | ||||
| Incident AF | 2.24 (2.03–2.49) | 2.24 (2.04–2.47) | 2.39 (2.16–2.64) | 2.38 (2.15–2.63) |
AF indicates atrial fibrillation; APBs, atrial premature beats; EAE, excessive atrial ectopy; HR, hazard ratio; LA, left atrial ; and LAE, left atrial enlargement.
Competing risks Cox proportional hazards regression: all‐cause mortality, incident AF (for stroke outcome only); time‐varying covariate: follow‐up anticoagulation.
Adjusted for age, sex, prior medical history hypertension, diabetes mellitus, CHF, ischemic stroke, myocardial infarction, left ventricular hypertrophy, systolic function, and baseline medication status (antihypertensive, statin, antiplatelet).
Adjusted for parsimonious predictors only.
For community‐dwelling adults (of any age) with no documented AF referred for echocardiography (n=84 469), we estimated stroke risks at 2 and 5 years based on traditional vascular risk factors using the CHA2DS2‐VASc score. The CHA2DS2‐VASc scores alone had moderate predictive utility, with C‐statistics of 0.71 for both 2‐ and 5‐year stroke risks. However, Chi‐square comparisons showed that the addition of LAE to the CHA2DS2‐VASc scale significantly improved the prediction of ischemic stroke in this cohort, with C‐statistics increasing from 0.71 to 0.75 at both 2 and 5 years, respectively (P<0.0001) (Table 4). For community‐dwelling adults (of any age) with no documented AF referred for Holter (n=48 694), C‐statistics for the CHA2DS2‐VASc score alone were 0.74 at both 2 and 5 years. Similarly, the inclusion of EAE (>30 APBs/h) significantly improved the prediction of ischemic stroke, increasing C‐statistics from 0.74 to 0.75 at 2 years (P=0.010) and 0.74 to 0.76 at 5 years (P=0.002) (Table 4). Notably, the greatest improvement in CHA2DS2‐VASc predictive utility was observed when information for both LAE and EAE was included, with C‐statistics for community‐dwelling adults (of any age) with no documented AF who had both echocardiogram and Holter data (n=20 335) increasing from 0.68 to 0.75 (P=0.008) at 2 years and from 0.70 to 0.76 (P=0.003) at 5 years (Table 4). Consistent with these findings, values for the relative integrated discrimination improvement and category‐free net reclassification improvement all showed that the addition of LAE and EAE significantly improved model prediction for the outcome of ischemic stroke at both 2 and 5 years (Table 5).
Table 4.
C‐Statistics for the Prediction of Ischemic Stroke at 2 and 5 Years Using CHA2DS2‐VASc Score Alone and CHA2DS2‐VASc With Inclusion of Left Atrial Diameter (mm), EAE (Atrial Premature Beats >30) and Both
| Cohort | Outcome | Prediction Rule | C‐Statistic | χ2 | P Value |
|---|---|---|---|---|---|
| Adults with no known AF referred for echocardiography (n=84 469) | Ischemic stroke (2 y) | CHA2DS2‐VASc | 0.68 | ||
| CHA2DS2‐VASc+LA diameter (mm) | 0.74 | 4.69 | 0.03* | ||
| Ischemic stroke (5 y) | CHA2DS2‐VASc | 0.70 | |||
| CHA2DS2‐VASc+LA diameter (mm) | 0.74 | 6.80 | 0.009* | ||
| Adults with no known AF referred for Holter (n=48 838) | Ischemic stroke (2 y) | CHA2DS2‐VASc | 0.68 | ||
| CHA2DS2‐VASc+EAE (>30 APBs/h) | 0.70 | 0.87 | 0.35 | ||
| Ischemic stroke (5 y) | CHA2DS2‐VASc | 0.70 | |||
| CHA2DS2‐VASc+EAE (>30 APBs/h) | 0.73 | 3.17 | 0.07 | ||
| Adults with no known AF referred for both echocardiography and Holter (n=20 370) | Ischemic stroke (2 y) | CHA2DS2‐VASc | 0.68 | ||
| CHA2DS2‐VASc+LA diameter (mm)+EAE (>30 APBs/h) | 0.75 | 7.08 | 0.008* | ||
| Ischemic stroke (5 y) | CHADS‐VASC | 0.70 | |||
| CHA2DS2‐VASc+LA diameter (mm)+EAE (>30 APBs/h) | 0.76 | 8.65 | 0.003* |
P values for the Chi‐square change in log‐likelihood associated with the addition of the variable. AF indicates atrial fibrillation; APB; atrial premature beats; EAE, excessive atrial ectopy; and LA, left atrial.
Table 5.
Integrated Discrimination Improvement and Net Reclassification Improvement for the Prediction of Ischemic Stroke at 2 and 5 Years
| Cohort | Outcome | Prediction Rule | Relative IDI | 95% CI | Category Free NRI | 95% CI |
|---|---|---|---|---|---|---|
| Adults with no known AF referred for echocardiography (n=84 469) | Ischemic stroke (2 y) | CHA2DS2‐VASc+LA diameter (mm) | 0.99 | 0.17 to 1.66 | 0.40 | 0.23 to 0.54 |
| Ischemic stroke (5 y) | CHA2DS2‐VASc+LA diameter (mm) | 0.50 | 0.27 to 0.72 | 0.43 | 0.31 to 0.54 | |
| Adults with no known AF referred for Holter (n=48 838) | Ischemic stroke (2 y) | CHA2DS2‐VASc+EAE (>30 APBs/h) | 0.21 | 0.03 to 0.61 | 0.31 | 0.18 to 0.46 |
| Ischemic stroke (5 y) | CHA2DS2‐VASc+EAE (>30 APBs/h) | 0.24 | 0.08 to 0.52 | 0.36 | 0.24 to 0.48 | |
| Adults with no known AF referred for both echocardiography and Holter (n=20 370) | Ischemic stroke (2 y) | CHA2DS2‐VASc+LA diameter (mm)+EAE (>30 APBs/h) | 0.91 | 0.52 to 5.78 | 0.46 | 0.14 to 0.80 |
| Ischemic stroke (5 y) | CHA2DS2‐VASc+LA diameter (mm)+EAE (>30 APBs/h) | 0.72 | 0.18 to 2.13 | 0.36 | 0.17 to 0.62 |
Estimates for relative integrated discrimination improvement and category‐free net reclassification improvement with 95% CI with bootstrapping. AF indicates atrial fibrillation; APB, atrial premature beats; EAE, excessive atrial ectopy; IDI, integrated discrimination improvement; LA, left atrial; and NRI, net reclassification improvement.
Discussion
This study demonstrated that left atrial enlargement (LAE), a marker of atrial cardiopathy, was associated with an increased risk of ischemic stroke at 2 and 5 years in community dwelling adults without known AF or incident AF or initiation of anticoagulation during follow‐up. Individuals with moderate‐severe LAE showed significantly higher absolute rates of ischemic stroke at 5 years and incident AF and death at both 2 and 5 years. Moderate‐severe LAE without documented AF also reduced survival and increased stroke risk by almost 2‐fold at both 2 and 5 years. While individuals with excessive atrial ectopy (EAE) also showed significantly higher rates of ischemic stroke at 5 years, and incident AF and death at both 2 and 5 years, after accounting for all‐cause mortality and incident AF as competing risks, EAE did not significantly reduce survival or increase the cause‐specific hazard of ischemic stroke in the present cohort. This study also provides novel evidence that the addition of LAE and EAE to the traditional vascular risk factors captured in the CHA2DS2‐VASc score, both independently and cumulatively improved the prediction of stroke risk at 2 and 5 years.
This study adds to prior population‐based findings of associations between multiple markers of atrial cardiopathy, including left atrial size or frequent APBs and incident stroke independent of AF.19 Seminal studies from the Framingham27 and Olmsted County cohorts28 showed that left atrial size was a significant predictor of stroke in both men and women after adjustment for AF27 and that, in those without documented AF at baseline, left atrial volume was independently associated with a composite outcome of major cardiovascular events, including stroke.28 However, others reported that this relationship was attenuated with adjustment for left ventricular function29 or present only for women.30, 31 In more recent analyses, enlarged left atrial diameter was shown to increase ischemic stroke risk by 54% in a large cohort of elderly hypertensive adults, although this study also did not explicitly exclude those with known AF at baseline32 and a recent systematic review of nine cohorts analyzing 67 875 participants and 3093 stroke outcomes confirmed that LAE was significantly associated with increased stroke risk in patients in sinus rhythm across studies.17 The Copenhagen Holter Study cohort reported associations between EAE (≥30 premature atrial contractions) and stroke risk beyond manifest AF,19, 33 although others have only demonstrated this association in those with >97 premature atrial contractions/h,34 or not at all.35
Findings from the present study are consistent with prior work showing significant increases in ischemic stroke risk for those with moderate‐severe LAE, but not for EAE, indicating that, in this cohort, LAE was the strongest marker of stroke risk. Importantly, the present study extends prior findings in several ways. In this large population‐based cohort of >30 000 community‐dwelling adults, we used multiple criteria to ensure the careful exclusion of individuals with AF at baseline, including a history of documented AF, anticoagulation use, pacemaker, implantable cardioverter defibrillator, implantable loop recorder, and prosthetic heart valves and explicitly censored for incident AF as a competing risk and adjusted for initiation of anticoagulation during follow‐up, providing robust evidence to support that LAE significantly increases stroke risk in the absence of any evidence for known or incident AF. The present study was also unique in integrating data from Holter monitor studies to assess the independent and potential cumulative effects of EAE for stroke risk and examining the incremental predictive utility of these markers for stroke risk assessment beyond traditional vascular risk factors captured by the CHA2DS2‐VASc score. Although, similar to prior studies,35 we showed no association between EAE and stroke risk after adjustment for covariates and the onset of incident AF, the present analyses provide new evidence that both LAE and EAE confer additional predictive value for stroke risk compared with the CHA2DS2‐VASc score.
Findings of the present study are consistent with increasing evidence for a new mechanistic model of AF and stroke.36 Although the prevailing view has been that increased thromboembolic risk in AF is related primarily to the dysrhythmia, several recent findings have been incongruent with this model, including evidence that maintaining sinus rhythm with rhythm control therapy does not eliminate the risk of stroke,37 and the lack of a close temporal association between AF episodes and stroke.11, 12 These data have prompted a reconsideration of the mechanisms underlying stroke in AF and the proposal of new model, which considers both the atrial substrate and the dysrhythmia (ie, the overall atrial cardiopathy) in thrombogenesis.36 In this model, while AF may increase thromboembolic risk, it is not a necessary criterion for stroke to occur, and an abnormal atrial substrate may result in thromboembolism independent of AF. This model highlights the complex bidirectional relationship between atrial cardiopathy, AF and outcomes,38 as evidenced by the low observed stroke rates in a recent clinical trial for those on active rhythm therapy,39 and the lack of effectiveness of anticoagulation therapy in recent trials in patients with embolic stroke of undetermined source.40 Results from the present study are consistent with this model, as both LAE and EAE were associated with the development of AF, but only the marker of the abnormal atrial substrate (eg LAE) significantly increased stroke risk in those with no known AF.
The CHADS2 and CHA2DS2‐VASc scores are routinely used to assess stroke risk and determine OAC treatment indications in the setting of AF, with the CHA2DS2‐VASc replacing the CHADS2 score in recent European and American guidelines.6, 41 However, for patients with a diagnosis of clinical AF, a recent meta‐analysis of these scores reported only moderate predictive utility for stroke risk stratification, with pooled median C‐statistics in non‐anticoagulated patients of 0.68 and 0.67 for the CHADS2 and CHA2DS2‐VASc, respectively.42 Although the CHA2DS2‐VASc performs better in identifying low risk patients with AF than the CHADS2,42 it also classifies a higher proportion from the CHADS2 intermediate risk category as high‐risk.42 However, similar to the CHADS2, the CHA2DS2‐VASc incompletely captures stroke risk and those remaining in the intermediate risk category may have a heterogeneous risk and uncertain treatment course. There is thus a recognized need to enhance current risk stratification tools to better identify those who might benefit from treatment with OAC, with recent reviews indicating that the use of novel parameters might improve stroke risk prediction and guide treatment decision‐making.43 Risk stratification among patients with LAE may help identify patients who could benefit from OAC in the absence of AF,17 along with other potential markers, such as NTpro‐BNP (N‐terminal pro‐B‐type natriuretic peptide), which have been shown to significantly improve stroke prediction.44 The present study provides novel evidence that both echocardiographic measures of LAE and Holter measures of EAE independently improved the utility of the CHA2DS2‐VASc score for 2‐ and 5‐year stroke risk prediction. Despite EAE showing no associations with stroke risk in the model adjusted for death and incident AF as competing risks and follow‐up anticoagulation, the inclusion of both LAE and EAE as markers of atrial cardiopathy offered the greatest predictive improvement for the CHA2DS2‐VASc score in individuals with no known AF.
Results of this study have potential implications for the identification of target candidates for AF screening and prevention trials testing new indications for OAC. Although the screening for atrial fibrillation in the elderly (SAFE)45 and other recent screening clinical46 trials showed increased detection of new AF cases with screening (opportunistic pulse screening with follow‐up ECG or systematic ECG screening in SAFE and intermittent ECG screening in other trials 46 ) versus no screening, the optimal strategy for AF screening remains controversial. Recent recommendations from the US Preventive Service Task Force indicate that there is insufficient evidence to assess the benefits and harms of ECG screening for AF in asymptomatic older adults47 and recent cost‐effectiveness analyses in both the UK and Canadian settings have identified opportunistic screening with pulse palpation as the most cost‐effective strategy.48, 49 Results of this study indicate that LAE may be an important selection criterion for screening, with those with moderate‐severe LAE in the absence of AF representing a higher‐risk target group for screening for AF detection. In addition, given increasing evidence for associations between LAE and increased stroke risk independent of AF, LAE also represents a potential therapeutic target for anticoagulant treatment for stroke prevention before the onset of AF. Ongoing trials, such as the ARCADIA (Atrial Cardiopathy and Antithrombotic Drugs In Prevention After Cryptogenic Stroke) and EAST (Early Treatment of Atrial Fibrillation for Stroke Prevention Trial) trials will provide insight into this question for individuals with cryptogenic embolic stroke and atrial cardiopathy.50, 51 In a subgroup of patients from the NAVIGATE ESUS (Secondary Prevention of Stroke and Prevention of Systemic Embolism in Patients With Recent Embolic Stroke of Undetermined Source) trial who had significant left atrial enlargement, anticoagulation with rivaroxaban was associated with a significant reduction in the risk of recurrent strokes as compared with aspirin treatment.52 Additional trials testing the efficacy of screening and anticoagulant therapy in individuals with markers of atrial cardiopathy is required.
This study has several limitations. Although the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group indicate that linear dimensions of the left atrium as the sole measure of left atrial size may be misleading and should be accompanied by left atrial volume determination in both clinical practice and research18 and others have shown volumetric measures of LAE to be more accurate than diameter,53 only 2D measures of left atrial size were available for the present analysis. In addition, given described challenges for the detection of AF in many patients,7, 8 it is possible that the present study was subject to the under‐ascertainment of incident AF cases. As the present findings accounted for incident AF as a competing risk, these data likely represent a more conservative estimate of the association between left atrial cardiopathy and stroke risk. Further, the present cohort of community‐dwelling adults represented a relatively low or moderate‐risk population and reasons for referral for cardiac testing were not available in the current data set. Consequently, the present cohort may have been limited in the accrual of stroke events over the follow‐up period and subject to variation in indication for testing.
Conclusions
Left atrial enlargement and excessive atrial ectopy, both markers of atrial cardiopathy, significantly improve the stroke risk prediction obtained using the CHA2DS2‐VASc risk score. This finding has implications for stroke risk stratification, AF screening, and the development of anticoagulation trials for stroke prevention before the onset of clinical AF. Future work would benefit from the precise estimate of this association with volumetric data, as acknowledged in our limitations section.
Sources of Funding
This work was supported by an operating grant from the Canadian Stroke Prevention Intervention Network (C‐SPIN). Dr Gladstone is supported by a Mid‐Career Investigator Award from the Heart and Stroke Foundation of Canada, the Department of Medicine at Sunnybrook Health Sciences Centre, the Bastable‐Potts Chair in Stroke Research, and the Tory Family. This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long‐Term Care. The opinions, results, and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario Ministry of Health and Long‐Term Care is intended or should be inferred.
Disclosures
Gladstone and Healey are co‐Principal Investigators of the SCREEN‐AF trial, Canadian national co‐PIs of the ARCADIA trial, members of the NAVIGATE ESUS trial Atrial Cardiopathy/Atrial Fibrillation Working Group, and members of the AF‐SCREEN International Collaboration. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S2
Acknowledgments
The authors would also like to acknowledge and thank all KMH Cardiology Laboratory staff involved in the preparation and transfer of data used in this study to ICES.
(J Am Heart Assoc. 2020;9:e013227 DOI: 10.1161/JAHA.119.013227.)
For Sources of Funding and Disclosures, see page 11.
References
- 1. Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. [DOI] [PubMed] [Google Scholar]
- 2. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. [DOI] [PubMed] [Google Scholar]
- 3. Hughes M, Lip GY; Guideline Development Group, National Clinical Guideline for Management of Atrial Fibrillation in Primary and Secondary Care, National Institute for Health and Clinical Excellence . Stroke and thromboembolism in atrial fibrillation: a systematic review of stroke risk factors, risk stratification schema and cost effectiveness data. Thromb Haemost. 2008;99:295–304. [DOI] [PubMed] [Google Scholar]
- 4. Sposato LA, Cipriano LE, Saposnik G, Ruíz Vargas E, Riccio PM, Hachinski V. Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: a systematic review and meta‐analysis. Lancet Neurol. 2015;14:377–387. [DOI] [PubMed] [Google Scholar]
- 5. Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, Flaker G, Avezum A, Hohnloser SH, Diaz R, et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806–817. [DOI] [PubMed] [Google Scholar]
- 6. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, et al. AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1–e76. [DOI] [PubMed] [Google Scholar]
- 7. Gladstone DJ, Spring M, Dorian P, Panzov V, Thorpe KE, Hall J, Vaid H, O'Donnell M, Laupacis A, Côté R, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370:2467–2477. [DOI] [PubMed] [Google Scholar]
- 8. Reiffel JA, Verma A, Kowey PR, Halperin JL, Gersh BJ, Wachter R, Pouliot E, Ziegler PD; REVEAL AF Investigators . Incidence of previously undiagnosed atrial fibrillation using insertable cardiac monitors in a high‐risk population: the REVEAL AF study. JAMA Cardiol. 2017;2:1120–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sanna T, Diener HC, Passman RS, Di Lazzaro V, Bernstein RA, Morillo CA, Rymer MM, Thijs V, Rogers T, Beckers F, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478–2486. [DOI] [PubMed] [Google Scholar]
- 10. Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci A, Lau CP, Fain E, Yang S, Bailleul C, et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366:120–129. [DOI] [PubMed] [Google Scholar]
- 11. Brambatti M, Connolly SJ, Gold MR, Morillo CA, Capucci A, Muto C, Lau CP, Van Gelder IC, Hohnloser SH, Carlson M, et al. Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation. 2014;129:2094–2099. [DOI] [PubMed] [Google Scholar]
- 12. Martin DT, Bersohn MM, Waldo AL, Wathen MS, Choucair WK, Lip GY, Holcomb R, Akar JG, Halperin JL; IMPACT Investigators . Randomized trial of atrial arrhythmia monitoring to guide anticoagulation in patients with implanted defibrillator and cardiac resynchronization devices. Eur Heart J. 2015;36:1660–1668. [DOI] [PubMed] [Google Scholar]
- 13. Goldberger JJ, Arora R, Green D, Greenland P, Lee DC, Lloyd‐Jones DM, Markl M, Ng J, Shah SJ. Evaluating the atrial myopathy underlying atrial fibrillation: identifying the arrhythmogenic and thrombogenic substrate. Circulation. 2015;132:278–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nattel S, Harada M. Atrial remodeling and atrial fibrillation: recent advances and translational perspectives. J Am Coll Cardiol. 2014;63:2335–2345. [DOI] [PubMed] [Google Scholar]
- 15. Tsang TS, Barnes ME, Bailey KR, Leibson CL, Montgomery SC, Takemoto Y, Diamond PM, Marra MA, Gersh BJ, Wiebers DO, et al. Left atrial volume: important risk marker of incident atrial fibrillation in 1655 older men and women. Mayo Clin Proc. 2001;76:467–475. [DOI] [PubMed] [Google Scholar]
- 16. Acharya T, Tringali S, Bhullar M, Nalbandyan M, Ilineni VK, Carbajal E, Deedwania P. Frequent atrial premature complexes and their association with risk of atrial fibrillation. Am J Cardiol. 2015;116:1852–1857. [DOI] [PubMed] [Google Scholar]
- 17. Overvad TF, Nielsen PB, Larsen TB, Sogaard P. Left atrial size and risk of stroke in patients in sinus rhythm. A systematic review. Thromb Haemost. 2016;116:206–219. [DOI] [PubMed] [Google Scholar]
- 18. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 19. Larsen BS, Kumarathurai P, Falkenberg J, Nielsen OW, Sajadieh A. Excessive atrial ectopy and short atrial runs increase the risk of stroke beyond incident atrial fibrillation. J Am Coll Cardiol. 2015;66:232–241. [DOI] [PubMed] [Google Scholar]
- 20. Tirschwell DL, Longstreth WT Jr. Validating administrative data in stroke research. Stroke. 2002;33:2465–2470. [DOI] [PubMed] [Google Scholar]
- 21. Tu K, Nieuwlaat R, Cheng SY, Wing L, Ivers N, Atzema CL, Healey JS, Dorian P. Identifying patients with atrial fibrillation in administrative data. Can J Cardiol. 2016;32:1561–1565. [DOI] [PubMed] [Google Scholar]
- 22. Abdel‐Qadir H, Fang J, Lee DS, Tu JV, Amir E, Austin PC, Anderson GM. Importance of considering competing risks in time‐to‐event analyses: application to stroke risk in a retrospective cohort study of elderly patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2018;11:e004580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Olesen JB, Torp‐Pedersen C, Hansen ML, Lip GY. The value of the CHA2DS2‐VASc score for refining stroke risk stratification in patients with atrial fibrillation with a CHADS2 score 0‐1: a nationwide cohort study. Thromb Haemost. 2012;107:1172–1179. [DOI] [PubMed] [Google Scholar]
- 24. Pencina MJ, D'Agostino RB Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kerr KF, McClelland RL, Brown ER, Lumley T. Evaluating the incremental value of new biomarkers with integrated discrimination improvement. Am J Epidemiol. 2011;174:364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cook NR, Demler OV, Paynter NP. Clinical risk reclassification at 10 years. Stat Med. 2017;36:4498–4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Benjamin EJ, D'Agostino RB, Belanger AJ, Wolf PA, Levy D. Left atrial size and the risk of stroke and death. The Framingham Heart Study. Circulation. 1995;92:835–841. [DOI] [PubMed] [Google Scholar]
- 28. Tsang TS, Abhayaratna WP, Barnes ME, Miyasaka Y, Gersh BJ, Bailey KR, Cha SS, Seward JB. Prediction of cardiovascular outcomes with left atrial size: is volume superior to area or diameter? J Am Coll Cardiol. 2006;47:1018–1023. [DOI] [PubMed] [Google Scholar]
- 29. Nagarajarao HS, Penman AD, Taylor HA, Mosley TH, Butler K, Skelton TN, Samdarshi TE, Aru G, Fox ER. The predictive value of left atrial size for incident ischemic stroke and all‐cause mortality in African Americans: the Atherosclerosis Risk in Communities (ARIC) Study. Stroke. 2008;39:2701–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bouzas‐Mosquera A, Broullon FJ, Alvarez‐Garcia N, Mendez E, Peteiro J, Gandara‐Sambade T, Prada O, Mosquera VX, Castro‐Beiras A. Left atrial size and risk for all‐cause mortality and ischemic stroke. CMAJ. 2011;183:E657–E664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lai CL, Chien KL, Hsu HC, Su TC, Chen MF, Lee YT. Left atrial dimension and risk of stroke in women without atrial fibrillation: the Chin‐Shan Community Cardiovascular Cohort study. Echocardiography. 2011;28:1054–1060. [DOI] [PubMed] [Google Scholar]
- 32. Pierdomenico SD, Pierdomenico AM, Di Carlo S, Di Tommaso R, Cuccurullo F. Left atrial enlargement and risk of ischemic stroke in elderly treated hypertensive patients. Am J Hypertens. 2014;27:1179–1184. [DOI] [PubMed] [Google Scholar]
- 33. Binici Z, Intzilakis T, Nielsen OW, Kober L, Sajadieh A. Excessive supraventricular ectopic activity and increased risk of atrial fibrillation and stroke. Circulation. 2010;121:1904–1911. [DOI] [PubMed] [Google Scholar]
- 34. Marinheiro R, Parreira L, Amador P, Sa C, Duarte T, Caria R. Excessive atrial ectopic activity as an independent risk factor for ischemic stroke. Int J Cardiol. 2017;249:226–230. [DOI] [PubMed] [Google Scholar]
- 35. Ofoma U, He F, Shaffer ML, Naccarelli GV, Liao D. Premature cardiac contractions and risk of incident ischemic stroke. J Am Heart Assoc. 2012;1:e002519 DOI: 10.1161/JAHA.112.002519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kamel H, Okin PM, Elkind MS, Iadecola C. Atrial fibrillation and mechanisms of stroke: time for a new model. Stroke. 2016;47:895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Al‐Khatib SM, Allen LaPointe NM, Chatterjee R, Crowley MJ, Dupre ME, Kong DF, Lopes RD, Povsic TJ, Raju SS, Shah B, et al. Rate‐ and rhythm‐control therapies in patients with atrial fibrillation: a systematic review. Ann Intern Med. 2014;160:760–773. [DOI] [PubMed] [Google Scholar]
- 38. Nattel S, Guasch E, Savelieva I, Cosio FG, Valverde I, Halperin JL, Conroy JM, Al‐Khatib SM, Hess PL, Kirchhof P, et al. Early management of atrial fibrillation to prevent cardiovascular complications. Eur Heart J. 2014;35:1448–1456. [DOI] [PubMed] [Google Scholar]
- 39. Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Poole JE, Noseworthy PA, Rosenberg YD, Jeffries N, Mitchell LB, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1261–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hart RG, Sharma M, Mundl H, Kasner SE, Bangdiwala SI, Berkowitz SD, Swaminathan B, Lavados P, Wang Y, Wang Y, et al. Rivaroxaban for stroke prevention after embolic stroke of undetermined source. N Engl J Med. 2018;378:2191–2201. [DOI] [PubMed] [Google Scholar]
- 41. Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof P; ESC Committee for Practice Guidelines (CPG) . 2012 focused update of the ESC guidelines for the management of atrial fibrillation: an update of the 2010 ESC guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719–2747. [DOI] [PubMed] [Google Scholar]
- 42. Chen JY, Zhang AD, Lu HY, Guo J, Wang FF, Li ZC. CHADS2 versus CHA2DS2‐VASc score in assessing the stroke and thromboembolism risk stratification in patients with atrial fibrillation: a systematic review and meta‐analysis. J Geriatr Cardiol. 2013;10:258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Calenda BW, Fuster V, Halperin JL, Granger CB. Stroke risk assessment in atrial fibrillation: risk factors and markers of atrial myopathy. Nat Rev Cardiol. 2016;13:549–559. [DOI] [PubMed] [Google Scholar]
- 44. Chua W, Purmah Y, Cardoso VR, Gkoutos GV, Tull SP, Neculau G, Thomas MR, Kotecha D, Lip GYH, Kirchhof P, et al. Data‐driven discovery and validation of circulating blood‐based biomarkers associated with prevalent atrial fibrillation. Eur Heart J. 2019;40:1268–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fitzmaurice DA, Hobbs FD, Jowett S, Mant J, Murray ET, Holder R, Raftery JP, Bryan S, Davies M, Lip GY, et al. Screening versus routine practice in detection of atrial fibrillation in patients aged 65 or over: cluster randomised controlled trial. BMJ. 2007;335:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Halcox JPJ, Wareham K, Cardew A, Gilmore M, Barry JP, Phillips C, Gravenor MB. Assessment of remote heart rhythm sampling using the AliveCor heart monitor to screen for atrial fibrillation: the REHEARSE‐AF study. Circulation. 2017;136:1784–1794. [DOI] [PubMed] [Google Scholar]
- 47. Jonas DE, Kahwati LC, Yun JDY, Middleton JC, Coker‐Schwimmer M, Asher GN. Screening for atrial fibrillation with electrocardiography: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;320:485–498. [DOI] [PubMed] [Google Scholar]
- 48. Welton NJ, McAleenan A, Thom HH, Davies P, Hollingworth W, Higgins JP, Okoli G, Sterne JA, Feder G, Eaton D, et al. Screening strategies for atrial fibrillation: a systematic review and cost‐effectiveness analysis. Health Technol Assess. 2017;21:1–236. [DOI] [PubMed] [Google Scholar]
- 49. Tarride JE, Quinn FR, Blackhouse G, Sandhu RK, Burke N, Gladstone DJ, Ivers NM, Dolovich L, Thornton A, Nakamya J, et al. Is screening for atrial fibrillation in Canadian family practices cost‐effective in patients 65 years and older? Can J Cardiol. 2018;34:1522–1525. [DOI] [PubMed] [Google Scholar]
- 50. Kamel H, Longstreth WT Jr, Tirschwell DL, Kronmal RA, Broderick JP, Palesch YY, Meinzer C, Dillon C, Ewing I, Spilker JA, et al. The AtRial cardiopathy and antithrombotic drugs in prevention after cryptogenic stroke randomized trial: rationale and methods. Int J Stroke. 2019;14:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kirchhof P, Breithardt G, Camm AJ, Crijns HJ, Kuck KH, Vardas P, Wegscheider K. Improving outcomes in patients with atrial fibrillation: rationale and design of the Early treatment of Atrial fibrillation for Stroke prevention Trial. Am Heart J. 2013;166:442–448. [DOI] [PubMed] [Google Scholar]
- 52. Healey JS, Gladstone DJ, Swaminathan B, Eckstein J, Mundl H, Epstein AE, Haeusler KG, Mikulik R, Kasner SE, Toni D, et al. Recurrent stroke with rivaroxaban compared with aspirin according to predictors of atrial fibrillation: secondary analysis of the NAVIGATE ESUS randomized clinical trial. JAMA Neurol. 2019;76:764–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Moya‐Mur JL, García‐Martín A, García‐Lledó A, Ruiz‐Leria S, Jiménez‐Nacher JJ, Megias‐Sanz A, Taboada D, Muriel A. Indexed left atrial volume is a more sensitive indicator of filling pressures and left heart function than is anteroposterior left atrial diameter. Echocardiography. 2010;27:1049–1055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2


