Abstract
Background
FGF21 (fibroblast growth factor 21), a novel hepatokine regulating lipid metabolism, has been linked to atherosclerotic disease. However, whether this relationship exists in patients without nonalcoholic fatty liver disease is unclear. We assessed the association between serum FGF21 levels and atherosclerosis in patients without nonalcoholic fatty liver disease, and investigated whether baseline FGF21 could predict incident atherosclerotic cardiovascular disease in a 7‐year prospective cohort.
Methods and Results
Baseline serum FGF21 was measured in a cross‐sectional cohort of 371 patients with type 2 diabetes mellitus without nonalcoholic fatty liver disease (determined by hepatic magnetic resonance spectroscopy), and in a population‐based prospective cohort of 705 patients from the Shanghai Diabetes Study. In the cross‐sectional study, FGF21 was significantly higher in patients with than in those without subclinical carotid atherosclerosis (P<0.01). The association remained significant after adjusting for demographic and traditional cardiovascular risk factors. In the prospective cohort, 80 patients developed atherosclerotic cardiovascular disease during follow‐up. Baseline FGF21 was significantly higher in those who developed ischemic heart disease or cerebral infarction than in those who did not. Using a cutoff serum concentration of 232.0 pg/mL, elevated baseline FGF21 independently predicted incident total atherosclerotic cardiovascular disease events, ischemic heart disease, and cerebral infarction in a nondiabetic population (all P<0.05), and significantly improved the discriminatory and reclassifying abilities of our prediction model after adjustment for established cardiovascular risk factors.
Conclusions
This study provides the first evidence that FGF21 levels are elevated in patients without nonalcoholic fatty liver disease with subclinical atherosclerosis. Baseline FGF21 is an independent predictor of atherosclerotic cardiovascular disease and represents a novel biomarker for primary prevention in the general population.
Keywords: atherosclerosis, biomarker, cardiovascular events, fibroblast growth factor 21, nonalcoholic fatty liver disease
Subject Categories: Biomarkers, Atherosclerosis, Coronary Artery Disease, Ischemic Stroke
Nonstandard Abbreviations and Acronyms
- ASCVD
atherosclerotic cardiovascular disease
- BMI
body mass index
- C‐IMT
carotid intima‐media thickness
- CVD
cardiovascular disease
- DHS
Dallas Heart Study
- DM
diabetes mellitus
- eGFR
estimated glomerular filtration rate
- FGF21
fibroblast growth factor 21
- FIELD
Fenofibrate Intervention and Event Lowering in Diabetes
- HDL‐C
high‐density lipoprotein cholesterol
- HOMA‐IR
homeostatic model assessment for insulin resistance index
- HKDR
Hong Kong West Diabetes Registry
- hsCRP
high‐sensitivity C‐reactive protein
- IHD
ischemic heart disease
- IQR
interquartile range
- LDL‐C
low‐density lipoprotein cholesterol
- MESA
Multi‐Ethnic Study of Atherosclerosis
- NAFLD
nonalcoholic fatty liver disease
- SHDS
Shanghai Diabetes Study
- TNT
Treating to New Targets study
- T2DM
type 2 diabetes mellitus
- WHO
World Health Organization
Clinical Perspective
What Is New?
We used liver proton magnetic resonance spectroscopy to exclude the confounder factor of nonalcoholic fatty liver disease, and determined that serum FGF21 (fibroblast growth factor 21) levels were still elevated in patients with subclinical atherosclerosis.
Baseline serum FGF21 was an independent predictor for atherosclerotic cardiovascular disease, including ischemic heart disease and cerebral infarction, among patients without diabetes mellitus.
What Are the Clinical Implications?
Circulating FGF21 could be utilized as a novel serum biomarker for risk evaluation and primary prevention of atherosclerotic cardiovascular disease in the general population.
The major cause of cardiovascular disease (CVD) is atherosclerosis, which develops insidiously throughout life and is usually advanced by the time symptoms occur.1 In 2015, ≈17.92 million people died from CVD worldwide, mostly as a result of ischemic heart disease (IHD) and stroke.2 The World Health Organization (WHO) has set a goal to reduce the risk of premature death from noncommunicable diseases, including CVD, by 25% by 2025.3 Early detection and management of cardiovascular risk factors is key in the prevention of incident CVD events. However, it is difficult to identify individuals with higher CVD risk for early intervention, highlighting the need to identify additional biomarkers for CVD risk screening and stratification.
FGF21 (fibroblast growth factor 21) is a novel metabolic regulator predominantly produced by the liver.4, 5 As it lacks a conventional heparin‐binding domain, FGF21 can be secreted into the circulation, where it acts as an endocrine hormone.6 In animal studies, FGF21 has several beneficial metabolic effects, including anti‐inflammatory, antidiabetes mellitus, antiobesity, and lipid‐lowering profiles.7, 8, 9 However, in human studies, elevated serum FGF21 levels are found in cardiometabolic diseases such as type 2 diabetes mellitus (T2DM), obesity, dyslipidemia, and, in particular, nonalcoholic fatty liver disease (NAFLD).10, 11, 12 NAFLD is one of the biggest influences on FGF21 levels. Both hepatic and circulating FGF21 levels are significantly higher in patients with NAFLD and are strongly correlated with intrahepatic triglycerides.12 Elevated circulating FGF21 levels in patients with impaired glucose tolerance are mediated by steatosis rather than insulin sensitivity.13
Despite all of this work, the association between circulating FGF21 levels and CVD remains unclear. First, contradictory results were reported in several cross‐sectional studies. In some studies, serum FGF21 levels were elevated in patients with atherosclerosis or coronary heart disease,14, 15, 16, 17 while other studies reported negative results.18, 19 In these studies, liver steatosis, the determining factor of serum FGF21 levels and also a risk factor for atherosclerosis, was not evaluated or considered. Second, in long‐term prospective studies, increased serum FGF21 levels can predict CVD events in patients with T2DM or established CVD at baseline.20, 21, 22 However, because circulating FGF21 levels are already elevated in patients with diabetes mellitus (DM), whether it could predict incident CVD in the general population and in patients without DM remains to be explored.
In this study, we investigated the association between serum FGF21 levels and subclinical carotid atherosclerosis in a cross‐sectional study, in which participants were evaluated for the presence of NAFLD by liver proton magnetic resonance spectroscopy, and patients with NAFLD were excluded. Furthermore, we investigated whether serum FGF21 could be a useful biomarker to predict the development of atherosclerotic CVD (ASCVD) events in a population‐based 7‐year prospective cohort.
Materials and Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Participants
Two cohorts of participants were recruited for this study. The first was a cross‐sectional cohort consisting of 371 patients with T2DM without NAFLD. The patients were enrolled from the Department of Endocrinology and Metabolism, Shanghai Jiao Tong University Affiliated Sixth People's Hospital between August 2015 and July 2016. All patients underwent liver proton magnetic resonance spectroscopy and carotid ultrasonography to measure their hepatic fat fractions and determine the occurrence of carotid atherosclerosis. Exclusion criteria included: (1) hepatic fat fraction ≥5.56%23; (2) clinical symptoms of atherosclerosis or the presence of CVD, including angina, myocardial infarction, heart failure, stroke, transient ischemic attack, or a prior invasive cardiovascular procedure; (3) age younger than 45 or older than 75 years; (4) liver or kidney dysfunction (alanine transaminase >40 U/L or estimated glomerular filtration rate [eGFR] <30 mL/min per 1.73 m2); (5) alcoholism (≥140 g per week for men or ≥70 g per week for women); (6) known history of viral hepatitis, autoimmune hepatitis, severe infection, or cancer; and (7) current use of lipid‐lowering drugs such as fenofibrate (which significantly increases hepatic FGF21 expression).24
The second cohort was a prospective cohort from the second study of the SHDS (Shanghai Diabetes Study), which has previously been described.25 A flow diagram of the cohort is provided in Figure S1. Briefly, in 2004, a total of 1651 patients from Caoyang community aged 33 to 96 years were enrolled in a baseline survey. From 2010 to 2011, these patients were invited for follow‐up assessments. Of the 1651, 642 patients were not included because of emigration and 112 patients died during the follow‐up period as a result of non‐CVD causes. Of the 897 patients enrolled in the follow‐up, 63 were excluded because of known CVD at baseline and 129 were excluded because of insufficient sample volume for serum FGF21 measurement. All patients except for those taking antidiabetic drugs underwent a 75‐g oral glucose tolerance test. The study was performed in accordance with the Declaration of Helsinki and the protocol was approved by the human research ethics committee of the Shanghai Sixth People's Hospital. Written informed consent was obtained from all participants.
Clinical and Biochemical Assessment
All participants were assessed after overnight fasting and underwent comprehensive physical examinations and routine biochemical analyses of blood samples. Anthropometric measurements included body weight, height, waist circumference, and blood pressure. Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). Triglycerides, total cholesterol, high‐density lipoprotein cholesterol (HDL‐C), and low‐density lipoprotein cholesterol (LDL‐C) were measured using standard enzymatic methods on a biochemical analyzer (7600‐120; Hitachi). Plasma glucose was determined using the glucose oxidase method. Glycated hemoglobin levels, using high‐performance liquid chromatography on a VARIANT II Hemoglobin Testing System (Bio‐Rad Laboratories, Inc). Basal insulin resistance was evaluated using the homeostatic model assessment for insulin resistance index (HOMA‐IR).26 The eGFR was calculated using the formula developed by the Chronic Kidney Disease Epidemiology Collaboration.27 Detailed medical, drug, and family histories, including any history of CVD, were obtained using a standardized questionnaire.
Circulating FGF21 levels were quantified using an ELISA kit. The intra‐assay and interassay coefficients of variation were 4.5% and 6.9%, respectively. The serum lipocalin‐2 concentration was also measured using an ELISA kit, with intra‐assay and interassay variations of 3.1% and 9.0%, respectively. Serum C‐reactive protein levels were measured using a hsCRP (high‐sensitivity C‐reactive protein) assay. The intra‐assay and interassay variations were 2.0% and 9.8%, respectively. The abovementioned assays were obtained from Antibody and Immunoassay Services, The University of Hong Kong.
Carotid Ultrasonography
A high‐resolution B‐mode scanner (VOLUSON 730 pro, GE) and a 10.0‐MHz probe were used for carotid artery scanning. An experienced ultrasound doctor blinded to the study scanned both common carotid arteries. Carotid intima‐media thickness (C‐IMT) was measured on the far wall of the common carotid arteries, 1 cm proximal to the carotid bulb. The C‐IMT value was measured as the highest value of intima‐media thickness of each carotid artery in both sides. Plaque was defined by C‐IMT ≥1.3 mm or the presence of a focal protrusion into the lumen with a thickness at least 50% greater than the adjacent intima‐media complex. Subclinical atherosclerosis was defined as C‐IMT >1.0 mm and/or the presence of plaque without clinical manifestations.28
Diagnosis of NAFLD
NAFLD was diagnosed by hepatic steatosis at proton magnetic resonance spectroscopy in the absence of history of excessive alcohol consumption and any other specific causes of hepatic steatosis. Proton magnetic resonance spectroscopy was performed on an Ingenia 3T magnetic resonance system (Philips). The fat fraction was measured in a single voxel (2×2×2 cm3) placed at the right lobe of the liver, avoiding liver edges, large vessels, and bile ducts. Hepatic steatosis was defined by fat fraction ≥5.56%, the 95th percentile of hepatic triglyceride content among 2287 healthy participants in the DHS (Dallas Heart Study).23
Diagnosis of Metabolic Disorders and ASCVD
DM was diagnosed according to 1999 WHO classifications.29 Hypertension was defined as a sitting blood pressure ≥140/90 mm Hg or current use of antihypertensive medication. Dyslipidemia was defined as having ≥1 of the following criteria: (1) triglycerides ≥1.7 mmol/L; (2) total cholesterol ≥5.2 mmol/L; (3) HDL‐C <1.04 mmol/L; (4) LDL‐C ≥3.4 mmol/L; and (5) current use of lipid‐lowering medications.30
Diagnoses of ASCVD events (IHD and cerebral infarction) were obtained from individual health records, using categories I21 to 25 and I63 of the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, respectively. Mortality information was ascertained by the Shanghai Municipal Center for Disease Control and Prevention.
Statistical Analysis
Normally distributed data were expressed as mean±SD. Data with a skewed distribution were expressed as the median with interquartile range (IQR) and were natural log‐transformed before analysis. One‐way ANOVA was used to compare continuous variables between groups. Chi‐square tests were used to compare differences in the proportions of categorical variables between groups. Multivariable linear regression was used to examine the associations between serum FGF21 levels and various parameters. Logistic regression was used to identify independent factors of subclinical atherosclerosis. The Youden index was calculated as sensitivity+specificity−1.31 To identify independent predictors of incident ASCVD events, baseline variables were analyzed using univariable and multivariable Cox proportion hazards regression. C‐statistics were calculated using R package survC1. The incremental value of FGF21 with reference to the baseline model in predicting incident ASCVD was evaluated by net reclassification index and integrated discrimination improvement.32 Cumulative hazard curves of incident ASCVD was tested by log‐rank test. Interaction by sex was evaluated in multivariable logistic regression and Cox proportional hazards regression by including the interaction term in the fully adjusted models. No significant interaction with sex was detected. Thus, we did not stratify the results by sex. All statistical analyses were performed in SPSS 22.0 (IBM) and R software 3.3.3 (Package survC1 and PredictABEL). Two‐sided P<0.05 values were considered statistically significant.
Results
Serum FGF21 Levels are Independently Associated With Subclinical Carotid Atherosclerosis in Patients Without NAFLD: Results From the Cross‐Sectional Study
A total of 371 patients with T2DM but without NAFLD were included in the cross‐sectional study. Their demographic and biochemical characteristics are summarized in Table 1. The average age and BMI in this cohort were 56.5±8.9 years and 23.3±2.7 kg/m2, respectively. Patients with subclinical carotid atherosclerosis tended to be older, men, and smokers; they also had higher BMI, C‐IMT, systolic blood pressure, diastolic blood pressure, triglycerides, total cholesterol, LDL‐C, and HDL‐C values. After adjustment for age and sex, BMI, diastolic blood pressure, triglycerides, HDL‐C, and LDL‐C remained statistically different between patients with and without atherosclerosis. Notably, serum FGF21 levels were significantly higher in patients with atherosclerosis (median, 266.7 pg/mL; IQR, 135.5–415.2) versus those without (198.4 pg/mL; IQR, 99.9–373.6 [P=0.005]), and this remained statistically significant after age and sex adjustment (P=0.002).
Table 1.
Characteristics of Patients in the Cross‐Sectional Cohort
| Variables | Total | Nonatherosclerosis | Atherosclerosis | P Valuea |
|---|---|---|---|---|
| N=371 | n=185 | n=186 | ||
| Age, y | 56.5±8.9 | 54.4±9.0 | 58.6±8.4 | <0.001 |
| Men | 210 (56.6) | 95 (51.4) | 115 (61.8) | 0.042 |
| BMI, kg/m2 | 23.3±2.7 | 23.0±2.6 | 23.7±2.8 | 0.023 |
| Waist circumference, cm | 87.1±8.6 | 85.9±8.3 | 88.3±8.8 | 0.070 |
| C‐IMT, mm | 0.79±0.16 | 0.73±0.13 | 0.87±0.16 | <0.001 |
| SBP, mm Hgb | 125 (120–135) | 120 (118–132) | 129 (120–135) | 0.012 |
| DBP, mm Hgb | 78 (70–80) | 78 (70–80) | 79 (70–80) | 0.030 |
| FBG, mmol/Lb | 6.7 (5.5–8.6) | 6.5 (5.3–8.6) | 6.8 (5.7–8.5) | 0.403 |
| 2hBG, mmol/Lb | 11.5 (8.0–14.8) | 11.5 (7.5–14.7) | 11.6 (8.4–15.0) | 0.395 |
| Glycated hemoglobin, mmol/molb | 7.80 (6.525–9.70) | 7.70 (6.30–9.90) | 7.85 (6.70–9.60) | 0.757 |
| Fasting insulin, mmol/Lb | 9.7 (5.1–17.1) | 9.9 (5.2–16.5) | 9.2 (4.9–17.9) | 0.994 |
| HOMA‐IRb | 2.9 (1.4–5.7) | 2.9 (1.4–5.7) | 3.0 (1.5–6.3) | 0.777 |
| Triglycerides, mmol/Lb | 1.1 (0.8–1.5) | 1.0 (0.7–1.4) | 1.1 (0.8–1.6) | 0.032 |
| Total cholesterol, mmol/Lb | 4.4 (3.8–5.1) | 4.2 (3.7–4.9) | 4.5 (3.9–5.3) | 0.013 |
| HDL‐C, mmol/Lb | 1.1 (0.9–1.4) | 1.2 (1.0–1.4) | 1.1 (0.9–1.3) | 0.042 |
| LDL‐C, mmol/Lb | 2.6 (2.1–3.3) | 2.4 (2.1–3.1) | 2.8 (2.3–3.3) | 0.005 |
| hsCRP, mg/Lb | 0.60 (0.29–1.34) | 0.59 (0.30–1.32) | 0.61 (0.28–1.55) | 0.147 |
| Ever smoker | 137 (36.9) | 55 (29.7) | 82 (44.1) | 0.004 |
| Dyslipidemia | 215 (58.0) | 99 (53.5) | 116 (62.4) | 0.090 |
| Hypertension | 153 (41.2) | 69 (37.3) | 84 (45.2) | 0.140 |
| FGF21, pg/mLb | 233.5 (117.6–390.2) | 198.4 (99.9–373.6) | 266.7 (135.5–415.2) | 0.005 |
Data are presented as mean±SD, median (interquartile range), or number (percentage). 2hBG indicates 2‐hour blood glucose; BMI, body mass index; C‐IMT, carotid intima‐media thickness; DBP, diastolic blood pressure; FBG, fasting blood glucose; FGF21, fibroblast growth factor 21; HDL‐C, high‐density lipoprotein cholesterol; HOMA‐IR, homeostatic model assessment for insulin resistance index; hsCRP, high‐sensitivity C‐reactive protein; LDL‐C, low‐density lipoprotein cholesterol; and SBP, systolic blood pressure.
Atherosclerosis group vs nonatherosclerosis group.
Log‐transformed before analysis.
We next analyzed the association of serum FGF21 levels with related factors. Serum FGF21 levels were higher in patients with dyslipidemia (303.1 pg/mL; IQR, 154.1–459.7) versus without (168.2 pg/mL; IQR, 93.1–295.5 [P<0.001]) and with hypertension (268.0 pg/mL; IQR, 136.0–440.3) versus without (208.9 pg/mL; IQR, 107.4–374.2 [P=0.017]), but no significant difference was observed between smokers and nonsmokers (Table S1). In linear regression analysis of log‐transformed serum FGF21 levels with other parameters, FGF21 levels had a positive correlation with triglycerides and an inverse correlation with HDL‐C after adjusted for age and BMI (both P<0.001, Table S2).
To evaluate independent risk factors for carotid atherosclerosis, we conducted a multivariable logistic regression model with log‐transformed serum FGF21 levels and other conventional atherosclerosis risk factors, including sex, age, BMI, waist circumference, HOMA‐IR, hsCRP, smoking, hypertension, and dyslipidemia. Only FGF21 (odds ratio [OR], 1.315; 95% CI, 1.033–1.674 [P=0.026]), age (OR, 1.062; 95% CI, 1.033–1.091 [P<0.001]), and smoking (OR, 2.007; 95% CI, 1.099–3.665 [P=0.023]) were significantly correlated with carotid atherosclerosis (Table 2).
Table 2.
Multivariable Logistic Regression Showing the Independent Factors for Subclinical Atherosclerosis
| Variables | OR (95% CI) | P Value |
|---|---|---|
| FGF21a | 1.315 (1.033–1.674)a | 0.026a |
| Age | 1.062 (1.033–1.091)a | <0.001a |
| Ever smoker | 2.007 (1.099–3.665)a | 0.023a |
| Men | 1.060 (0.585–1.921) | 0.847 |
| Hypertension | 0.951 (0.588–1.538) | 0.837 |
| Dyslipidemia | 1.005 (0.625–1.617) | 0.984 |
| BMI | 1.077 (0.958–1.211) | 0.214 |
| Waist circumference | 1.005 (0.968–1.043) | 0.812 |
| HOMA‐IR | 0.991 (0.953–1.030) | 0.634 |
| hsCRP | 1.012 (0.990–1.034) | 0.291 |
BMI indicates body mass index; HOMA‐IR, homeostatic model assessment for insulin resistance index; and hsCRP, high‐sensitivity C‐reactive protein.
Odds ratios (ORs) were expressed as per SD increase in log‐transformed fibroblast growth factor 21 (FGF21).
Serum FGF21 Levels are a Predictor of Incident CVD Events in a Community‐Based Population: Results From the 7‐Year Prospective Study
In the prospective cohort, there were no significant differences in age, sex, BMI, or rate of DM at baseline between the patients included in the analysis and the 946 excluded patients. Of the 705 patients with complete follow‐up information and sufficient baseline serum samples, 97 patients (13.8%) developed CVD events during the follow‐up period, with a median follow‐up duration of 74 months. Among these patients, 80 developed ASCVD; 44 with cerebral infarction and 36 with IHD. Baseline characteristics of this cohort are shown in Table 3. Compared with patients without incident CVD, patients who developed ASCVD were older; had higher blood glucose, glycated hemoglobin, systolic blood pressure, and hsCRP levels; and were more likely to have DM and hypertension. In addition, patients who developed IHD had higher waist circumference and lipocalin‐2 and lower eGFR. Serum FGF21 levels were significantly elevated both in patients with incident IHD (479.5 pg/mL, 302.4–627.0; P=0.004) and those with incident cerebral infarction (401.6 pg/mL, 238.3–616.4; P=0.021) compared with patients without incident CVD (325.2 pg/mL, 189.0–498.9). In linear regression analysis of log‐transformed serum FGF21 levels with other parameters, FGF21 levels had a positive correlation with triglycerides and an inverse correlation with HDL‐C after adjusted for age and BMI (both P<0.001, Table S3).
Table 3.
Baseline Characteristics of Patients in the Prospective Cohort
| Variables | No Incident CVD (n=608) | Developed IHD (n=36) | Developed Cerebral Infarction (n=44) |
|---|---|---|---|
| Age, y | 57.6±13.5 | 71.6±14.1† | 71.4±10.6† |
| Men | 236 (38.8) | 20 (55.6) | 18 (40.9) |
| BMI, kg/m2 a | 24.1 (21.7–26.2) | 24.9 (22.5–26.6) | 23.4 (21.6–25.9) |
| Waist circumference, cm | 82.2±9.7 | 86.1±8.4* | 83.6±10.8 |
| SBP, mm Hga | 120 (110–134) | 132 (119–150)† | 130 (120–140)† |
| DBP, mm Hga | 75 (70–80) | 79 (71–86) | 80 (71–80) |
| Hypertension | 189 (31.1) | 22 (61.1)† | 27 (61.4) |
| Ever smoker | 155 (25.5%) | 10 (27.8%) | 8 (18.2%) |
| DM | 73 (12.0%) | 9 (25.0%)* | 16 (36.4%)† |
| FBG, mmol/La | 5.19 (4.85–5.60) | 5.54 (5.15–6.35)* | 5.46 (5.17–6.81)† |
| 2hBG, mmol/La | 6.20 (5.10–7.21) | 6.75 (6.27–8.20)* | 6.75 (6.17–8.75)† |
| HOMA‐IRa | 0.84 (0.50–1.42) | 0.96 (0.68–1.43) | 0.97 (0.56–1.60) |
| Glycated hemoglobin, %a | 5.7 (5.3–6.1) | 6.0 (5.7–6.6)† | 5.8 (5.5–6.6)† |
| Triglycerides, mmol/La | 1.45 (1.02–2.25) | 1.87 (1.24–2.83) | 1.66 (1.14–2.79)* |
| Total cholesterol, mmol/La | 4.80 (4.20–5.50) | 4.95 (4.33–5.70) | 5.20 (4.25–5.95) |
| HDL‐C, mmol/La | 1.32 (1.10–1.55) | 1.24 (1.05–1.42) | 1.25 (1.10–1.48) |
| LDL‐C, mmol/L | 2.75±0.77 | 3.01±0.99 | 2.81±0.80 |
| Dyslipidemia | 349 (57.4) | 24 (66.7) | 32 (72.7) |
| eGFR, mL/min per 1.73 m2 a | 100.3 (85.6–114.7) | 80.1 (60.5–102.6)† | 89.6 (79.0–114.2) |
| hsCRP, mg/La | 0.9 (0.4–2.2) | 1.5 (0.7–7.1)† | 2.4 (0.9–5.4)† |
| Lipocalin‐2, pg/mLa | 15.4 (10.6–22.7) | 18.6 (13.6–27.8)† | 18.5 (9.9–25.0) |
| FGF21, pg/mLa | 325.2 (189.0–498.9) | 479.5 (302.4–627.0)† | 401.6 (238.3–616.4)* |
Data are presented as mean±SD, median (interquartile range), or number (percentage). 2hBG indicates 2‐hour blood glucose; BMI, body mass index; DBP, diastolic blood pressure; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; FBG, fasting blood glucose; FGF21, fibroblast growth factor 21; HDL‐C, high‐density lipoprotein cholesterol; HOMA‐IR, homeostatic model assessment for insulin resistance index; hsCRP, high‐sensitivity C‐reactive protein; IHD, ischemic heart disease; LDL‐C, low‐density lipoprotein cholesterol; and SBP, systolic blood pressure.
*P<0.05 and † P<0.01 compared with no incident cardiovascular disease (CVD) group.
Log‐transformed before analysis.
The predictive ability of baseline serum FGF21 concentrations for incident ASCVD, IHD, and cerebral infarction during the follow‐up period was evaluated with Cox proportional hazard models (Table 4). For the 705 patients in this cohort, using an optimal FGF21 level cutoff of 232.0 pg/mL (obtained from the Youden index), elevated serum FGF21 was associated with incident ASCVD, IHD, and cerebral infarction in the unadjusted model. In the fully adjusted model, after adjusting for other conventional cardiovascular risk factors (age, sex, BMI, fasting blood glucose, glycated hemoglobin, HOMA‐IR, eGFR, hsCRP, smoking status, dyslipidemia, and hypertension) and lipocalin‐2 levels, higher serum FGF21 concentration remained an independent predictor for incident total ASCVD and IHD. In our study, 602 of 705 patients did not have DM at baseline. In this subsample of patients, serum FGF21 concentration remained an independent predictor for all atherosclerotic outcomes in the fully adjusted model. However, as there were only 103 patients with DM, we did not observe a statistically significant association among patients with DM, presumably because of inadequate sample size.
Table 4.
HRs of Incident ASCVD, IHD, and Cerebral Infarction Related to Elevated Baseline Circulating FGF21 Levels of >232.0 pg/mL
| Cases, No. (%) | Unadjusted | Fully Adjusted Model | |||
|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | ||
| Whole cohort (N=705) | |||||
| Total ASCVD | 80 (11.3) | 2.984 (1.607–5.540) | 0.001 | 2.176 (1.156–4.096) | 0.016 |
| IHD | 36 (5.10) | 5.691 (1.736–18.654) | 0.004 | 3.904 (1.153–13.213) | 0.029 |
| Cerebral infarction | 44 (6.2) | 2.276 (1.057–4.900) | 0.036 | 1.918 (0.862–4.266) | 0.11 |
| Patients without DM at baseline (n=602) | |||||
| Total ASCVD | 55 (9.1) | 4.602 (1.958–10.813) | <0.001 | 3.185 (1.319–7.693) | 0.01 |
| IHD | 27 (4.5) | 6.497 (1.534–27.515) | 0.011 | 4.536 (1.013–20.307) | 0.048 |
| Cerebral infarction | 28 (4.7) | 4.523 (1.364–15.000) | 0.014 | 4.188 (1.193–14.704) | 0.025 |
| Patients with DM at baseline (n=103) | |||||
| Total ASCVD | 25 (24.3) | 1.098 (0.430–2.803) | 0.845 | 1.398 0.478–4.086) | 0.541 |
| IHD | 9 (8.7) | 3.622 (0.423–31.018) | 0.24 | 14.317 (0.281–730.75) | 0.185 |
| Cerebral infarction | 16 (15.5) | 0.691 (0.240–1.990) | 0.494 | 0.696 (0.186–2.603) | 0.59 |
Fully adjusted model: adjusted for age, sex, body mass index, fasting blood glucose, glycated hemoglobin, homeostatic model assessment for insulin resistance index, estimated glomerular filtration rate, high‐sensitivity C‐reactive protein, lipocalin‐2, smoking status (never, ever‐smoker), dyslipidemia (yes/no), and hypertension (yes/no). ASCVD indicates atherosclerotic cardiovascular disease; DM, diabetes mellitus; FGF21, fibroblast growth factor 21; HR, hazard ratio; and IHD, ischemic heart disease.
Figure shows the Kaplan–Meier curve of the cumulative hazard for total incident ASCVD in the entire cohort, stratified by serum FGF21 concentration. For patients with baseline serum FGF21 levels >232.0 pg/mL, the overall incident rate for ASCVD events was 14.4%, significantly higher than in patients with lower baseline FGF21 levels (5.6%; log‐rank test, P=0.001).
Figure 1. Cumulative hazard of incident atherosclerotic cardiovascular disease (ASCVD) in patients below and above the cutoff values of fibroblast growth factor 21 (FGF21).
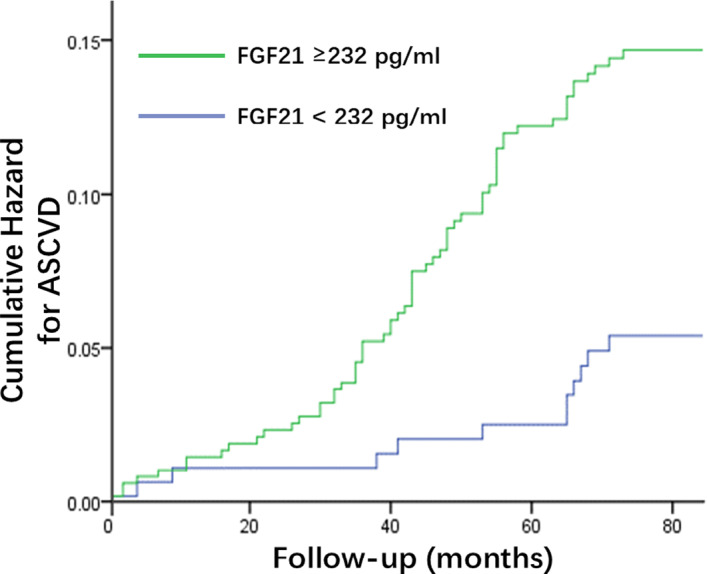
Green line: above the FGF21 cutoff; blue line: below the FGF21 cutoff. Log‐rank test: P<0.001.
The ability of serum FGF21 measurements to improve discrimination and reclassification for total ASCVD events is shown in Table 5. The addition of FGF21 level to a baseline model containing traditional CVD risk factors (age, sex, BMI, eGFR, and hsCRP) and the novel biomarker lipocalin‐2, as either a continuous variable or optimal cutoff, did not result in significant improvement in the C‐statistics for the prediction of incident ASCVD events. However, it did significantly improve the net reclassification index of the baseline model (net reclassification index +0.240 and 0.389, P=0.041 and P<0.001, as a continuous variable and as an optimal cutoff, respectively). In addition, using serum FGF21 levels as an optimal cutoff resulted in a modest but significant improvement in the integrated discrimination improvement for incident ASCVD prediction (integrated discrimination improvement +0.008, P=0.037).
Table 5.
Discrimination and Reclassification Performance of the Addition of Circulating FGF21 Levels in Predicting Incident ASCVD
| Model | C‐Statistics (95% CI) | P Value | NRI (95% CI) | P Value | IDI (95% CI) | P Value |
|---|---|---|---|---|---|---|
| Baseline modela | 0.827 (0.782–0.871) | ··· | ··· | ··· | ··· | ··· |
| + FGF21 levelsb | 0.828 (0.785–0.871) | 0.795 | 0.240 (0.009–0.471) | 0.042 | 0.005 (−0.002 to 0.012) | 0.162 |
| + FGF21 >232 pg/mL | 0.828 (0.784–0.872) | 0.822 | 0.389 (0.210–0.568) | <0.001 | 0.008 (0.001–0.0163) | 0.037 |
ASCVD indicates atherosclerotic cardiovascular disease; FGF21, fibroblast growth factor 21; IDI, integrated discrimination improvement; and NRI, net reclassification index.
Baseline model included age, sex, body mass index, smoking status, high‐sensitivity C‐reactive protein, estimated glomerular filtration rate, and lipocalin‐2.
Log‐transformed before analysis.
Discussion
In the cross‐sectional study of patients with T2DM, we demonstrated that elevated serum FGF21 is independently associated with subclinical carotid atherosclerosis in patients without NAFLD. In the prospective study, we provided the first evidence that baseline circulating FGF21 levels can independently predict incident ASCVD events in a community‐based cohort of patients without known CVD at baseline.
Our study explores the association between FGF21 levels and atherosclerosis, the subject of conflicting results in recent years. Several cross‐sectional studies showed that serum FGF21 levels were significantly elevated in patients with compared with those without coronary heart disease.14, 15 In a study of 212 newly diagnosed patients with T2DM, serum FGF21 was independently associated with the presence of carotid or iliac lesions in patients with atherosclerosis.16 Another study showed that circulating FGF21 was associated with carotid atherosclerosis and C‐IMT, independent of established risk factors.17 However, it was reported that serum FGF21 levels were not significantly correlated with coronary heart disease in BMI‐ or glucose status–matched cohorts.18, 19 A major limitation of these studies is that they did not measure the degree of hepatic steatosis, and, hence, lacked adjustment for NAFLD.15, 16, 17, 18, 19 NAFLD is a major determinant of serum FGF21 levels.12, 33 Circulating FGF21 levels are 2‐fold higher in patients with NAFLD than in controls, and hepatic FGF21 mRNA expression positively correlates with intrahepatic triglyceride levels.12 A 3‐year prospective study of 712 Chinese patients suggested that high serum FGF21 level is a predictor of NAFLD.33 As NAFLD is a significant risk factor for CVD,34 further investigation is required to determine whether elevated FGF21 levels are the result of higher NAFLD prevalence in patients with subclinical atherosclerosis. In this study, we applied proton magnetic resonance spectroscopy to precisely measure patients’ liver fat fractions and used this information to entirely exclude the confounding effects of NAFLD. Our results demonstrate that elevated serum FGF21 levels are indeed associated with subclinical carotid atherosclerosis, independent of liver steatosis and other cardiovascular risk factors.
In the past decade, the beneficial metabolic effects of FGF21 have been extensively studied. In mice, administration of recombinant FGF21 resulted in weight loss, improved insulin sensitivity, reduction of triglyceride and LDL‐C levels, and amelioration of liver steatosis.7, 8 In human studies, injection of FGF21 analogs led to significant improvement in the lipid profiles of patients with T2DM.9, 35 As FGF21 exerts favorable metabolic effects, it may seem contradictory that higher serum FGF21 levels were observed in patients with atherosclerosis. FGF21 elevation could be explained by a compensatory response to underlying metabolic stress, or by FGF21 resistance as a result of impaired FGF21 signaling. As with insulin resistance, this may suggest a requirement for a supraphysiological dose of FGF21 to meet concentration demands.36 FGF21 resistance has been reported in several cardiometabolic diseases and may occur well in advance of clinical manifestation.17, 33, 37, 38 Therefore, underlying FGF21 resistance might lead to multiple CVD risk factors, such as dyslipidemia and DM, which then promote the development of atherosclerosis.
Animal studies have demonstrated direct or indirect protective effects of FGF21 on atherosclerotic disease. Exogenous FGF21 inhibited apoptosis in rat cardiac microvascular endothelial cells in atherosclerosis‐like conditions, suggesting a protective effect during early‐stage atherosclerosis.39 Apolipoprotein E and FGF21 double‐knockout mice demonstrated accelerated atherosclerotic plaque formation, worsened hypercholesterolemia, and increased expression of proinflammatory factors compared with mice with apolipoprotein E deficiency alone.40 Treatment with recombinant FGF21 showed a significant reduction in plaque formation. This antiatherosclerotic effect could be caused by increased expression of adiponectin, a downstream factor of FGF21 that suppresses the proliferation and migration of smooth muscle cells and reduces oxidized LDL‐C uptake by macrophages,41 and/or direct inhibition of sterol regulatory element‐binding protein 2.40 The increased serum FGF21 observed in our study may be a protective response to metabolic stress underlying the atherosclerotic process.
The close correlations between FGF21, the pathophysiological process of atherosclerosis, and cardiometabolic disorders make FGF21 a promising biomarker for ASCVD risk. At present, only limited prospective studies have examined the relationship between baseline serum FGF21 levels and incident CVD, and most of these studies focused on patients with T2DM or established CVD. As circulating FGF21 levels are increased in patients with T2DM and established CVD, the result of these studies may not translate well to use with the general population. In the FIELD (Fenofibrate Intervention and Event Lowering in Diabetes) study, baseline circulating FGF21 levels were measured in 9697 patients with T2DM. Over a follow‐up period of 5 years, plasma FGF21 levels were associated with total CVD events, stroke, and coronary/carotid revascularization, but not incident coronary heart disease events, after adjustment for conventional CVD risk factors.20 Another study of 3538 Chinese patients with T2DM from the HKDR (Hong Kong West Diabetes Registry) reported that baseline serum FGF21 level was an independent predictor for coronary heart disease events over a median follow‐up period of 3.8 years.21 The TNT (Treating to New Targets) study reported that higher plasma FGF21 levels were associated with higher CVD risk in high‐risk patients treated with statins.22 A recent study of the MESA (Multi‐Ethnic Study of Atherosclerosis) cohort did not observe a significant association between baseline FGF21 levels with incident total CVD events.42 In this study, some end point events not related to atherosclerosis were not excluded, such as hemorrhagic stroke. Our study provides the evidence of an independent, prospective relationship between increased circulating FGF21 levels and incident ASCVD events in the general population. The optimal cutoff for serum FGF21, 232.0 pg/mL, could be used as an independent predictor for both IHD and cerebral infarction among patients without DM.
Our previous study demonstrated that lipocalin‐2 is an independent predictor for incident CVD events in men.25 In this study, the addition of serum FGF21 levels to the previously established model consisting of conventional cardiovascular risk factors and lipocalin‐2 resulted in significant improvements in the model's discriminatory and reclassifying abilities to predict incident ASCVD events. Our results suggest that application of those novel biomarkers could improve risk stratification ability in the primary prevention of CVD.
A major limitation of this study is that hepatic steatosis measurements were not routinely performed in our prospective cohort, preventing further evaluation of the impact of NAFLD on the relationship between serum FGF21 levels and incident ASCVD events. Additionally, this was a single‐centered study of Chinese patients. Our cohort is relatively small and has lower mean BMI compared with Western patients. For this reason, the optimal cutoff for serum FGF21 should be interpreted carefully, and the results may not be generalizable to other groups of patients. To obtain more robust results, future studies should be performed using larger cohorts of multiethnic patients with well‐documented baseline information on liver steatosis.
Conclusions
Our study demonstrates that elevated serum FGF21 occurs in subclinical carotid atherosclerosis in patients without NAFLD, independent of established risk factors. Baseline serum FGF21 level is an independent predictor of future ASCVD events in the general population and is a promising novel biomarker for use in the primary prevention of CVD.
Sources of Funding
This work was supported by the National Science Foundation of China (NSFC)–National Health and Medical Research Council joint research grant (81561128016) and NSFC major international (regional) joint research project (81220108006), Shanghai Belt and Road Joint Laboratory of Intelligent Diagnosis and Treatment of Metabolic Diseases (18410750700), the National Key Research and Development Program of China (2018YFA0800402), and Shanghai Municipal Key Clinical Specialty to Jia; and Hong Kong Scholars Program (XJ2013035), General Program of NSFC (81870598), Shanghai Pujiang Program (17PJ1407500), Two Hundred Program from Shanghai Jiao Tong University School of Medicine (20191830), and Municipal Human Resources Development Program for Outstanding Young Talents in Medical and Health Sciences in Shanghai (2017YQ009) to H. Li.
Disclosures
None.
Supporting information
Tables S1–S3Figure S1
Acknowledgments
We acknowledge Anthony Keech, Alicia Jenkins, and Andrzej Januszewski for their assistance with statistical analysis and helpful advice. This research was conducted using the Metabolic Diseases Biobank Resource at Shanghai Jiao Tong University Affiliated Sixth People's Hospital.
Author contributions: H. Li and W. Jia conceived and designed the study. L. Wu, L. Qian, L. Zhang, and J. Zhang contributed to FGF21 level measurement. J. Zhou and Y. Li contributed to the measurement of hepatic fat fraction. L. Wu and L. Qian contributed to data analysis. L. Wu, L. Qian, X. Hou, Q. Fang, and H. Li contributed to data interpretation. L. Wu and H. Li drafted the article. All authors approved the final version of the article.
(J Am Heart Assoc. 2020;9:e015226 DOI: 10.1161/JAHA.119.015226.)
For Sources of Funding and Disclosures, see page 9.
References
- 1. Perk J, De Backer G, Gohlke H, Graham I, Reiner Ž, Verschuren WM, Albus C, Benlian P, Boysen G, Cifkova R. European guidelines on cardiovascular disease prevention in clinical practice (version 2012): the Fifth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts). Atherosclerosis. 2012;223:1–68. [DOI] [PubMed] [Google Scholar]
- 2. Roth GA, Johnson C, Abajobir A, Abd‐Allah F, Abera SF, Abyu G, Ahmed M, Aksut B, Alam T, Alam K. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization . Global action plan for the prevention and control of noncommunicable diseases 2013–2020. 2013. Available at: https://www.who.int/nmh/events/ncd_action_plan/en/. Accessed May 11, 2020.
- 4. Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA. FGF‐21 as a novel metabolic regulator. J Clin Invest. 2005;115:1627–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Woo Y, Xu A, Wang Y, Lam KS. Fibroblast growth factor 21 as an emerging metabolic regulator: clinical perspectives. Clin Endocrinol. 2013;78:489–496. [DOI] [PubMed] [Google Scholar]
- 6. Kharitonenkov A. FGFs and metabolism. Curr Opin Pharmacol. 2009;9:805–810. [DOI] [PubMed] [Google Scholar]
- 7. Kharitonenkov A, Wroblewski VJ, Koester A, Chen YF, Clutinger CK, Tigno XT, Hansen BC, Shanafelt AB, Etgen GJ. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor‐21. Endocrinology. 2007;148:774–781. [DOI] [PubMed] [Google Scholar]
- 8. Singhal G, Chee MJ, Tan TG, El Ouaamari A, Adams AC, Najarian R, Kulkarni RN, Benoist C, Flier JS, Maratos‐Flier E. Fibroblast growth factor 21 (FGF21) protects against high fat diet induced inflammation and islet hyperplasia in pancreas. PLoS One. 2016;11:e0148252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Talukdar S, Zhou Y, Li D, Rossulek M, Dong J, Somayaji V, Weng Y, Clark R, Lanba A, Owen BM. A long‐acting FGF21 molecule, PF‐05231023, decreases body weight and improves lipid profile in non‐human primates and type 2 diabetic subjects. Cell Metab. 2016;23:427–440. [DOI] [PubMed] [Google Scholar]
- 10. Xiao Y, Xu A, Law LS, Chen C, Li H, Li X, Yang L, Liu S, Zhou Z, Lam KS. Distinct changes in serum fibroblast growth factor 21 levels in different subtypes of diabetes. J Clin Endocrinol Metab. 2012;97:E54–E58. [DOI] [PubMed] [Google Scholar]
- 11. Yu H, Xia F, Lam KS, Wang Y, Bao Y, Zhang J, Gu Y, Zhou P, Lu J, Jia W. Circadian rhythm of circulating fibroblast growth factor 21 is related to diurnal changes in fatty acids in humans. Clin Chem. 2011;57:691–700. [DOI] [PubMed] [Google Scholar]
- 12. Li H, Fang Q, Gao F, Fan J, Zhou J, Wang X, Zhang H, Pan X, Bao Y, Xiang K. Fibroblast growth factor 21 levels are increased in nonalcoholic fatty liver disease patients and are correlated with hepatic triglyceride. J Hepatol. 2010;53:934–940. [DOI] [PubMed] [Google Scholar]
- 13. Li H, Bao Y, Xu A, Pan X, Lu J, Wu H, Lu H, Xiang K, Jia W. Serum fibroblast growth factor 21 is associated with adverse lipid profiles and gamma‐glutamyltransferase but not insulin sensitivity in Chinese subjects. J Clin Endocrinol Metab. 2009;94:2151–2156. [DOI] [PubMed] [Google Scholar]
- 14. Shen Y, Ma X, Zhou J, Pan X, Hao Y, Zhou M, Lu Z, Gao M, Bao Y, Jia W. Additive relationship between serum fibroblast growth factor 21 level and coronary artery disease. Cardiovasc Diabetol. 2013;12:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lin Z, Wu Z, Yin X, Liu Y, Yan X, Lin S, Xiao J, Wang X, Feng W, Li X. Serum levels of FGF‐21 are increased in coronary heart disease patients and are independently associated with adverse lipid profile. PLoS One. 2010;5:e15534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xiao Y, Liu L, Xu A, Zhou P, Long Z, Tu Y, Chen X, Tang W, Huang G, Zhou Z. Serum fibroblast growth factor 21 levels are related to subclinical atherosclerosis in patients with type 2 diabetes. Cardiovasc Diabetol. 2015;14:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chow WS, Xu A, Woo YC, Tso AW, Cheung SC, Fong CH, Tse HF, Chau MT, Cheung BM, Lam KS. Serum fibroblast growth factor‐21 levels are associated with carotid atherosclerosis independent of established cardiovascular risk factors. Arterioscler Thromb Vasc Biol. 2013;33:2454–2459. [DOI] [PubMed] [Google Scholar]
- 18. Lee Y, Lim S, Hong ES, Kim JH, Moon MK, Chun EJ, Choi SI, Kim YB, Park YJ, Park KS. Serum FGF21 concentration is associated with hypertriglyceridaemia, hyperinsulinaemia and pericardial fat accumulation, independently of obesity, but not with current coronary artery status. Clin Endocrinol. 2014;80:57–64. [DOI] [PubMed] [Google Scholar]
- 19. Kim WJ, Kim SS, Lee HC, Song SH, Bae MJ, Yi YS, Jeon YK, Kim BH, Kim YK, Kim IJ. Association between serum fibroblast growth factor 21 and coronary artery disease in patients with type 2 diabetes. J Korean Med Sci. 2015;30:586–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ong KL, Januszewski AS, O'Connell R, Jenkins AJ, Xu A, Sullivan DR, Barter PJ, Hung WT, Scott RS, Taskinen MR. The relationship of fibroblast growth factor 21 with cardiovascular outcome events in the Fenofibrate Intervention and Event Lowering in Diabetes study. Diabetologia. 2015;58:464–473. [DOI] [PubMed] [Google Scholar]
- 21. Lee CH, Woo YC, Chow WS, Cheung CY, Fong CH, Yuen MM, Xu A, Tse HF, Lam KS. Role of circulating fibroblast growth factor 21 measurement in primary prevention of coronary heart disease among Chinese patients with type 2 diabetes mellitus. J Am Heart Assoc. 2017;6:e005344 DOI: 10.1161/JAHA.116.005344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ong KL, Hui N, Januszewski AS, Kaakoush NO, Xu A, Fayyad R, DeMicco DA, Jenkins AJ, Keech AC, Waters DD. High plasma FGF21 levels predicts major cardiovascular events in patients treated with atorvastatin (from the Treating to New Targets [TNT] Study). Metabolism. 2019;93:93–99. [DOI] [PubMed] [Google Scholar]
- 23. Reeder SB, Cruite I, Hamilton G, Sirlin CB. Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy. J Magn Reson Imaging. 2011;34:729–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ong KL, Rye KA, O'connell R, Jenkins AJ, Brown C, Xu A, Sullivan DR, Barter PJ, Keech AC; Investigators FS . Long‐term fenofibrate therapy increases fibroblast growth factor 21 and retinol‐binding protein 4 in subjects with type 2 diabetes. J Clin Endocrinol Metab. 2012;97:4701–4708. [DOI] [PubMed] [Google Scholar]
- 25. Wu G, Li H, Fang Q, Jiang S, Zhang L, Zhang J, Hou X, Lu J, Bao Y, Xu A. Elevated circulating lipocalin‐2 levels independently predict incident cardiovascular events in men in a population‐based cohort. Arterioscler Thromb Vasc Biol. 2014;34:2457–2464. [DOI] [PubMed] [Google Scholar]
- 26. Matthews D, Hosker J, Rudenski A, Naylor B, Treacher D, Turner R. Homeostasis model assessment: insulin resistance and β‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- 27. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Touboul PJ, Hennerici M, Meairs S, Adams H, Amarenco P, Desvarieux M, Ebrahim S, Fatar M, Hernandez RH, Kownator S. Mannheim intima‐media thickness consensus. Cerebrovasc Dis. 2004;18:346–349. [DOI] [PubMed] [Google Scholar]
- 29. Alberti KG, Zimmet PF. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. [DOI] [PubMed] [Google Scholar]
- 30. Prevention JCfDCGo, Adults ToDi . Chinese guidelines on prevention and treatment of dyslipidemia in adults (no abstract). Chin J Cardiol. 2007;35:390. [PubMed] [Google Scholar]
- 31. Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biom J. 2005;47:458–472. [DOI] [PubMed] [Google Scholar]
- 32. Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. [DOI] [PubMed] [Google Scholar]
- 33. Li H, Dong K, Fang Q, Hou X, Zhou M, Bao Y, Xiang K, Xu A, Jia W. High serum level of fibroblast growth factor 21 is an independent predictor of non‐alcoholic fatty liver disease: a 3‐year prospective study in China. J Hepatol. 2013;58:557–563. [DOI] [PubMed] [Google Scholar]
- 34. Oni ET, Agatston AS, Blaha MJ, Fialkow J, Cury R, Sposito A, Erbel R, Blankstein R, Feldman T, Al‐Mallah MH. A systematic review: burden and severity of subclinical cardiovascular disease among those with nonalcoholic fatty liver; should we care? Atherosclerosis. 2013;230:258–267. [DOI] [PubMed] [Google Scholar]
- 35. Gaich G, Chien JY, Fu H, Glass LC, Deeg MA, Holland WL, Kharitonenkov A, Bumol T, Schilske HK, Moller DE. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab. 2013;18:333–340. [DOI] [PubMed] [Google Scholar]
- 36. Díaz‐Delfín J, Hondares E, Iglesias R, Giralt M, Caelles C, Villarroya F. TNF‐α represses β‐Klotho expression and impairs FGF21 action in adipose cells: involvement of JNK1 in the FGF21 pathway. Endocrinology. 2012;153:4238–4245. [DOI] [PubMed] [Google Scholar]
- 37. Chen C, Cheung BM, Tso AW, Wang Y, Law LS, Ong KL, Wat NM, Xu A, Lam KS. High plasma level of fibroblast growth factor 21 is an independent predictor of type 2 diabetes. Diabetes Care. 2011;34:2113–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee C, Hui E, Woo Y, Yeung C, Chow W, Yuen M, Fong C, Xu A, Lam K. Circulating fibroblast growth factor 21 levels predict progressive kidney disease in subjects with type 2 diabetes and normoalbuminuria. J Clin Endocrinol Metab. 2015;100:1368–1375. [DOI] [PubMed] [Google Scholar]
- 39. Lü Y, Liu JH, Zhang LK, Du J, Zeng XJ, Hao G, Huang J, Zhao DH, Wang GZ, Zhang YC. Fibroblast growth factor 21 as a possible endogenous factor inhibits apoptosis in cardiac endothelial cells. Chin Med J. 2010;123:3417–3421. [PubMed] [Google Scholar]
- 40. Lin Z, Pan X, Wu F, Ye D, Zhang Y, Wang Y, Jin L, Lian Q, Huang Y, Ding H. Fibroblast growth factor 21 prevents atherosclerosis by suppression of hepatic sterol regulatory element‐binding protein‐2 and induction of adiponectin in mice. Circulation. 2015;131:1861–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lin Z, Tian H, Lam KS, Lin S, Hoo RC, Konishi M, Itoh N, Wang Y, Bornstein SR, Xu A. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metab. 2013;17:779–789. [DOI] [PubMed] [Google Scholar]
- 42. Yuan D, Wu BJ, Henry A, Rye KA, Ong KL. Role of fibroblast growth factor 21 in gestational diabetes mellitus: a mini‐review. Clin Endocrinol. 2019;90:47–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3Figure S1


