Abstract
Background
Neurodevelopmental impairments are common in survivors of complex congenital heart defects (CHD). We report neuropsychological and brain imaging assessments in adults operated for isolated septal defects.
Methods and Results
Patients (mean age 25.6 yrs) who underwent childhood surgery for isolated atrial septal defect (n=34) or ventricular septal defect (n=32), and healthy matched peers (n=40), underwent a standard battery of neuropsychological tests and a 3.0T brain magnetic resonance imaging scan. Patient intelligence was affected with lower scores on Full‐Scale intelligence quotient (P<0.001), Verbal Comprehension (P<0.001), Perceptual Reasoning (P=0.007), and Working Memory (P<0.001) compared with controls. Also, the CHD group had poorer visuospatial abilities (Immediate Recall, P=0.033; Delayed Recall, P=0.018), verbal memory (Trial 1, P=0.015; Total Learning, P<0.001; Delayed Recall, P=0.007), executive function (Executive Composite Score, P<0.001), and social recognition (Reading the Mind in the Eyes Test, P=0.002) compared with controls. Self‐reported levels of executive dysfunction, attention deficits and hyperactivity behavior, and social cognition dysfunction were higher in the CHD group compared with population means and controls. We found similar global and regional morphometric brain volumes and a similar frequency of brain magnetic resonance imaging abnormalities in the 2 groups. The CHD group had a high occurrence of psychiatric disease and a larger need for special teaching during school age.
Conclusions
Children operated for simple CHD demonstrate poorer neurodevelopmental outcomes in adulthood when compared with healthy controls and expected population means.
REGISTRATION
URL: https://www.clinicaltrials.gov. Unique identifier: NCT03871881.
Keywords: atrial septal defect, magnetic resonance imaging, neurodevelopmental outcome, neuropsychology, ventricular septal defect
Subject Categories: Clinical Studies, Congenital Heart Disease, Magnetic Resonance Imaging (MRI), Cardiovascular Surgery, Quality and Outcomes
Nonstandard Abbreviations and Acronyms
- ADHD
Attention deficit hyperactivity disorder
- ASD
Atrial septal defect
- BRIEF‐A
Behavior Rating Inventory of Executive Function–Adult Version
- CAARS
Conners’ Adult Attention Deficit and Hyperactivity Disorder Rating Scales
- CHD
Congenital heart defect
- D‐KEFS
Delis‐Kaplan Executive Function System
- FDR
False discovery rate
- MRI
Magnetic resonance imaging
- RAVLT
Rey Auditory Verbal Learning Test
- RCFT
Rey‐Osterreith Complex Figure Test
- RMET
Reading the Mind in the Eyes Test
- SRS‐2
Social Responsiveness Scale, Second Edition
- VSD
Ventricular septal defect
- WAIS‐IV
Wechsler Adult Intelligence Scale–Fourth Edition
- WMHs
White matter hyperintensities of presumed vascular origin
1.
Clinical Perspective
What Is New?
Neurodevelopment is challenged in a significant proportion of adults operated in childhood for atrial septal or ventricular septal defects.
Simple congenital heart defect patients may have impairments across a majority of important neuropsychological domains with verbal comprehension, working memory, perceptual reasoning, visuospatial and verbal memory, and social recognition being particularly affected.
Major structural brain abnormalities were not detected in the simple congenital heart defects.
What Are the Clinical Implications?
Attention should be paid to patients with surgical closed atrial septal and ventricular septal heart defects as they are at risk of neurodevelopmental impairments.
Patients with atrial septal and ventricular septal defects should be made aware of the potential risk of neurodevelopmental challenges in adulthood.
2.
The management of congenital heart defect (CHD) has improved dramatically over recent decades, but little is known about the morbidity and medical needs of the growing number of CHD survivors1, 2, 3. Cyanotic CHD has been linked to neurodevelopmental impairments in both children and adolescents4, 5, 6, 7, 8, 9 and includes neurocognitive domains such as lower IQ (intelligence quotient) scores, visuospatial integration deficits, problems with social cognition, and increased risk of attention deficit hyperactivity disorder (ADHD)9, 10, 11. These neuropsychological deficits are associated with changes in brain morphology11, 12, 13 and persist through childhood and into early adulthood7, 9, 12.
Surprisingly, adult patients with isolated septal heart defects, in which peripheral blood oxygen saturation is normal, also experience increased morbidity14, 15, mortality3, 16, and increased risk of psychiatric disorders17, 18 compared to the background population. There is hence a need to examine brain function and brain structure in adult CHD patients with isolated septal heart defects in order to disclose potential altered neurodevelopment and need for targeted support.
The aim of this study was to compare neuropsychological scores and structural brain magnetic resonance imaging (MRI) in adult patients who underwent surgical closure of atrial septal defect (ASD) or ventricular septal defect (VSD) during childhood to normative population data and healthy adult controls.
Materials and Methods
The study complies with the World Medical Association's Declaration of Helsinki, amended in 2013, and was approved by the Regional Committee on Biomedical Research Ethics of the Central Denmark Region (chart: 1‐10‐72‐233‐17) and the Danish Data Protection Agency (chart: 2012‐58‐006). The study is registered on clinicaltrials.gov (identifier: NCT03871881). In compliance with Danish law, all participants provided written informed consent prior to enrolment. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Design
In a prospective, cross‐sectional study, participants underwent a battery of neuropsychological tests and a brain MRI separated by a 60‐minute break. Participants filled out 3 self‐reporting questionnaires and were given 3 informant questionnaires to be passed on to a close relative as described later.
Study Population
Inclusion criteria were patients with (1) isolated ASD, (2) isolated VSD closed surgically between 1990 and 2000, and (3) healthy volunteers, matched for age, sex, and education to the patient groups. These controls were recruited through flyers and announcements on official webpages. Exclusion criteria were other congenital cardiac abnormalities, associated syndromes (eg, Down's syndrome), previous stroke, recent head trauma, pregnancy, non‐MRI compatible implants such as pacemakers, lack of Danish language skills, and age under 18. Treatment was performed at Aarhus University Hospital, a tertiary referral hospital, by a specialized, homogeneous group of anesthetists, cardiologists, and cardiac surgeons. The surgical procedures were performed through a median sternotomy on cardiopulmonary bypass with a cross‐clamp on the aorta. Defects were closed through right atrial approach. Moderate hypothermia and crystalloid cardioplegia were used during the procedures.
Neuropsychological Assessment
The neuropsychological test battery consisted of validated neurocognitive tests and questionnaires assessing intelligence, executive functions, learning and memory, and social cognition. All tests were performed by trained and experienced research assistants under the supervision of an experienced research neuropsychologist (L.E).
Intelligence
The intellectual functioning was tested using Wechsler Adult Intelligence Scale–Fourth Edition (WAIS‐IV)19. The end points were the 5 composite scores: Full‐scale IQ, Verbal Comprehension Index, Perceptual Reasoning Index, Working Memory Index, and Processing Speed Index. For all 5 indexes the scaled scores correspond to the population mean of 100 (SD±15) with higher scores indicating better performance.
Executive Functions
An executive function summary was derived using Delis‐Kaplan Executive Function System (D‐KEFS) standard scores20. An average score was calculated in each of the (D‐KEFS) subtests using the following conditions; Trail Making Test (conditions 1–5), Verbal Fluency Test (letter fluency, category fluency, category switching total correct responses and category switching total switching accuracy), Design Fluency Test (filled dots, empty dots only, and switching total correct designs) and Color‐Word Interference Test (color naming, word reading, inhibition, inhibition/switching). End points were the subtest calculated means and a summary score calculated by averaging the 4 subtests. All scaled scores have an expected mean of 10 (SD±3) with higher scores indicating better performance. The Behavior Rating Inventory of Executive Function–Adult Version (BRIEF‐A) was completed by 2 informants: participant self‐report and an informant report21. The informant was defined as the person with the closest relation to the participant and could be a family member, spouse, partner, or friend. The expected mean scaled score is 50 (SD±10), with a higher score indicating less optimal function. End point was the Global Executive Composite.
The questionnaire Conners’ Adult Attention Deficit and Hyperactivity Disorder Rating Scales (CAARS) was completed by participants and informants by a close relative as described previously22. The expected scaled score mean is 50 (SD±10), with higher scores indicating greater attention problems. End point was ADHD index T score.
Learning and Memory
Visuospatial learning and memory skills were assessed using the Rey‐Osterreith Complex Figure Test (RCFT)23, 24, 25. End points were scores from the copy, immediate recall, delayed recall, and recognition total correct trials.
Verbal learning and memory were tested using the Rey Auditory Verbal Learning Test (RAVLT) (Rey A. Mémorisation d'une série de 15 mots en 5 répetitions. Paris, France: Presses Universitaires des France; 1958). End points were the Trial 1, Total Learning, Delayed Recall, and Recognition Trials.
Social Cognition
A measure of social cognition was derived from the Reading the Mind in the Eyes Test (RMET)26. The RMET involves viewing 36 photographs of eyes and, using a multiple‐choice format, to select the term that best describes the emotion expressed on the picture. A total score was summarized from the correct answers.
The questionnaire Social Responsiveness Scale, Second Edition (SRS‐2) was completed by participants and informants by a close relative as described previously27. End points were a total score calculated by addition of the 5 subscales: social awareness, social cognition, social communication, social motivation and restricted interests, and repetitive behavior.
Magnetic Resonance Imaging
Data Acquisition
Morphometric brain‐MRI was performed using a Siemens Magnetom Prisma 3T MRI system with a 32‐channel head coil and a Magnetization‐Prepared 2 Rapid Acquisition Gradient Echo sequence. Magnetization‐Prepared 2 Rapid Acquisition Gradient Echo parameters were acquired with pulse repetition time=6.5s, inversion time 1=0.5s, inversion time 2=2.9s, α1=4°, α2=7°, a 3D sequence imaged at isotropic 0.9 mm resolution (acquisition matrix: 240×256, 192 sagittal slices) and turbo factor of 144 as defined by others28. Fluid‐attenuated inversion recovery images were acquired for assessing hyperintense T2 lesions with pulse repetition time=5 seconds, echo time=387 ms, at isotropic 0.9 mm resolution (acquisition matrix: 256×256, 192 sagittal slices). Multishell diffusion weighted imaging was acquired with both anterior‐posterior and posterior‐anterior phase encoding directions at b‐values 0, 700, 1200, and 2800 s/mm2. For each phase encoding direction, a total of 191 image volumes were acquired (distribution over b‐values: 11/30/60/90) with pulse repetition time=2972 ms, echo time=65 ms, at istotropic 1.8 mm resolution (acquisition matrix: 112×112, 60 axial slices). Finally, blood oxygenation level dependent imaging was acquired during 12 minutes resting state (600 image volumes with pulse repetition time=1170 ms, echo time=29.6 ms, and isotropic 2.5 mm resolution [acquisition matrix: 76×76, 63 axial slices]).
1. Analyses
Background noise in Magnetization‐Prepared 2 Rapid Acquisition Gradient Echo T1w images were initially suppressed by applying a head mask found by thresholding the image of the second gradient echo acquisition. The corrected images were then processed using the proposed framework from Aubert‐Broche et al29 consisting of tissue denoising30, bias fields correction31, and linear32 and nonlinear33 spatial normalization to Montreal Neurological Institute space. Images were then skull stripped34 and classified into grey matter, white matter, and cerebrospinal fluid35. Tissue segmentations were parcellated into main cerebral lobes using an atlas36 in Montreal Neurological Institute space. Subcortical nuclei were segmented either by multi‐atlas segmentation37 (hippocampus and thalamus) or by the atlas in Montreal Neurological Institute space (caudate and putamen).
For the purpose of clinical assessment, fractional anisotropy and apparent diffusion coefficient were calculated using FMRIB Software Library v5.0.9 (FMRIB, Oxford, UK). blood oxygenation level dependent images were corrected for motion and averaged as supplement for the clinical assessment.
Brain MRIs were clinically assessed by a single blinded neuroradiologist (R.B.D) by visual inspection to identify abnormalities. Abnormalities were classified by origin (acquired or developmental), extent (focal, diffuse, generalized), type (infarction, cortical dysplasia, ventricular enlargement, or white matter hyperintensities of presumed vascular origin38 [WMHs]), and anatomic location. Ventricular enlargement was assessed using the Evans Index with a score <0.3 indicating normal ventricular size.
Statistical Analysis
Continuous results are, if appropriate, reported as mean±SD, otherwise as median with 95% CI or total range. Continuous data were compared using unpaired Student t tests and noncontinuous data were compared with the Mann‐Whitney‐Wilcoxon rank‐sum test. The chi‐square test was used for binominal data. Statistical significance was considered as P<0.05.
Normative data for the WAIS‐IV and D‐KEFS scores were obtained from their scoring manuals19, 20. Normative data for RAVLT and RCFT was obtained from Mitrushina using regression equations derived from meta‐analysis of multiple normative data sets39. For the RMET, normative data from a British cohort23 was used. Age and sex matched normative data for BRIEF‐A, CAARS, and SRS‐2 questionnaires, were obtained from interpretation and scoring manuals21, 22, 27. Multiple testing was accounted for by calculating a false discovery rate (FDR) q‐value40. Statistical analysis was performed on a blinded data set. Extreme scores for a poorer outcome were calculated as the percentage of participants whose neuropsychological outcomes deviated more than 1 SD from the expected population mean.
The MRI brain volumes, except the total intracranial volume, were normalized by calculating the ratio between the participants regional volume and the total intracranial volume, then multiplied by a constant to maintain absolute values. The constant was defined as mean total intracranial volume of the control group.
All data were analyzed using Stata/SE 15.1 for Mac (StataCorp, College Station, Texas).
Sample Size Justification
The sample size estimate was based on previously published full‐scale IQ data8. In order to determine a difference between groups on our primary outcome with a power of 80% and a significance level of 0.05 using the Student t test, the minimal sample size was determined to be 35 CHD participants. To adjust for participant dropout, we enrolled more than 35 CHD participants.
Results
From March 2018 to November 2018, 66 participants with a surgically closed septal defect (34 ASD and 32 VSD) and 40 healthy controls were enrolled. Three patients got claustrophobic/anxious and did not complete the MRI study. MRI scans of 2 control subjects were excluded due to inadequate image quality caused by metal retainers.
Demographics
Demographics and clinical characteristics are shown in Table 1. The participants were all in their mid‐twenties. No differences in race or ethnicity were observed. The CHD group received special teaching 3 times as often and pedagogical psychological counseling 5 times as often as the control group. Also, they had a higher level of dyslexia.
Table 1.
Demographics and Clinical Characteristics for CHD and Control Participants
| Variable | CHD (n=66) | Control (n=40) | P Value |
|---|---|---|---|
| At inclusion | |||
| Age, y | 25.6±5.2 | 25.6±4.7 | 0.961 |
| Height, cm | 170±11 | 175±8 | 0.029 |
| BMI, kg/m2 | 24.4±4.1 | 23.0±3.2 | 0.062 |
| Male [n, (%)] | 20 (30) | 14 (35) | 0.616 |
| Education | |||
| ISCED primary education [n, (%)] | 3 (5) | 0 (0) | 0.171 |
| ISCED secondary education [n, (%)] | 49 (74) | 26 (65) | 0.311 |
| ISCED tertiary education [n, (%)] | 14 (21) | 14 (35) | 0.119 |
| Pedagogical psychological counselinga [n, (%)] | 10 (15) | 1 (3) | 0.038 |
| Special teachingb [n, (%)] | 30 (45) | 6 (15) | 0.001 |
| Dyslexia [n, (%)] | 11 (17) | 0 (0) | 0.006 |
| Dyscalculia [n, (%)] | 4 (6) | 1 (3) | 0.353 |
Data are presented as mean±SD or as absolute numbers with relative percentages. BMI indicates body mass index; CHD, congenital heart defect; and ISCED, International Standard Classification of Education 2011.
Received pedagogical psychological counseling during primary or secondary school.
Received special teaching during primary or secondary school.
Cardiac Status and History
Perioperative information is displayed in Table 2. All the patients had undergone simple surgery with no complications. None had significant left or right ventricular outflow tract obstructions, aortic valve regurgitation, or right ventricular hypertrophy prior to surgery.
Table 2.
Perioperative Status for ASD and VSD Participants
| Variable | ASD (n=34) | VSD (n=32) |
|---|---|---|
| Age at diagnosis | 6.5±8.0 | 0.5±1.0 |
| Age at surgery | 7.8±7.7 | 1.7±1.4 |
| Defect size, mm | 16.7±8.8 | 8.1±3.9 |
| Total bypass time, min | 44±20 | 59±20 |
| Cross clamp time, min | 16±9 | 30±13 |
| Hospital stay [days, (min–max)] | 7 (2–11) | 10 (6–27) |
| ICU stay [days, (min–max)] | 1 (1–2) | 1 (1–5) |
| Preoperative catherization [n, (%)] | 8 (24) | 12 (38) |
| Mean pulmonary‐to‐systemic blood flow (Qp/Qs) | 2.7±0.9 | 2.9±1.3 |
| ASD type [n, (%)] | ||
| Primum | 2 (6) | … |
| Secundum | 32 (94) | … |
| ASD closure [n, (%)] | ||
| Direct suture | 17 (50) | … |
| Dacron patch | 13 (38) | … |
| Pericardial patch | 4 (12) | … |
| VSD type [n, (%)] | ||
| Perimembranous | … | 17 (53) |
| Muscular | … | 15 (47) |
| VSD closure [n, (%)] | ||
| Patch | … | 21 (66) |
| Direct suture | … | 11 (34) |
| Pulmonary artery banding [n, (%)] | … | 3 (9) |
| Persisting ductus arteriosus closure | … | 3 (9) |
Data are presented as mean±SD, median with range (min–max) or as absolute numbers with relative percentages. ASD indicates atrial septal defect; ICU, intensive care unit; and VSD, ventricular septal defect.
Medical history at the time of this examination is displayed in Table 3. The patients were asymptomatic with no significant residual shunt. Postsurgery checkups had been concluded.
Table 3.
Medical History for CHD and Control Participants
| Variable | CHD (n=66) | Control (n=40) |
|---|---|---|
| Psychiatric diagnosis | ||
| ≥1 Psychiatric diagnoses [n, (%)]a | 22 (33) | 4 (10) |
| ≥2 Psychiatric diagnoses [n, (%)]a | 12 (18) | 2 (5) |
| Mental and behavioral disorders due to psychoactive substance use | 1 (2) | 0 (0) |
| Schizophrenia, schizotypal and delusional disorders | 1 (2) | 0 (0) |
| Mood (affective) disorders | 18 (27) | 4 (10) |
| Depression [n, (%)] | 15 (23) | 4 (10) |
| Bipolar affective disorder [n, (%)] | 3 (5) | 0 |
| Neurotic, stress‐related and somatoform disorders | 10 (15) | 0 (0) |
| Obsessive‐compulsive disorder [n, (%)] | 6 (9) | … |
| Anxiety [n, (%)] | 4 (6) | … |
| Behavioral syndromes associated with physiological disturbances and physical factors | 7 (11) | 2 (5) |
| Eating disorders | 3 (5) | 2 (5) |
| Nonorganic sleep disorders | 4 (6) | … |
| Disorders of adult personality and behavior | 3 (5) | 0 (0) |
| Personality disorder | 3 (5) | … |
| Disorders of psychological development | 2 (3) | 0 |
| Infantile autism | 1 (2) | … |
| Asperger's syndrome | 1 (2) | … |
| Behavioral and emotional disorders with onset usually occurring in childhood and adolescence | 8 (12) | 0 (0) |
| ADD or ADHD | 8 (12) | … |
| Somatic history | ||
| Brain infections (meningitis, encephalitis etc.) | 0 (0) | 0 (0) |
| Other brain diseases (neoplasms etc.) | 0 (0) | 0 (0) |
| Previous head trauma | 0 (0) | 0 (0) |
| Epilepsy | 1 (2) | 0 (0) |
| Stroke | 0 (0) | 0 (0) |
Data are presented as absolute numbers with relative percentages. ADD indicates attention deficit disorder; ADHD, attention deficit hyperactivity disorder; and CHD, congenital heart defect.
Diagnosed with a psychiatric disease and received treatment.
There was a high amount of psychiatric disease in the CHD group with no differences between the ASD and the VSD group.
Neuropsychological Assessment
Table 4 presents the neuropsychological scores of the ASD and VSD adults, collectively and split by defect type, along with the results of comparisons with the control group scores and with the scores of the expected population mean. The Figure presents the extreme scores of the CHD and control group. The CHD group as a whole performed poorer than the control group in all tested domains even after FDR adjustment. When compared to the expected population mean scores, most outcomes were lower. Particular weaknesses were detected in the areas of verbal comprehension, working memory, auditory and visual learning and memory, and social cognition. Neuropsychological outcomes were comparable in adults with ASD and VSD. The following sections focus on assessment of outcomes in the different neuropsychological categories.
Table 4.
Neuropsychological Outcomes for CHD and Control Participants
| Variable | ASD (n=34) | VSD (n=32) | CHD (n=66) | Control (n=40) | P Value, ASD Vs VSD | P Value, All CHD Vs Controls | P Value, CHD Vs Expected Population Mean |
|---|---|---|---|---|---|---|---|
| Mean±SD | |||||||
| Wechsler Adult Intelligence Scale Version IV | |||||||
| Full‐scale IQ | 92.6±13.3 | 95.8±13.5 | 94.2±13.4 | 105.6±12.8 | 0.346 | <0.001a | <0.001a |
| Verbal comprehension (index scores) | 89.9±13.5 | 91.2±13.8 | 90.5±13.6 | 105.0±16.4 | 0.719 | <0.001a | <0.001a |
| Perceptual reasoning (index scores) | 92.8±14.9 | 97.1±16.7 | 94.8±15.8 | 103.0±12.9 | 0.273 | 0.007a | 0.010a |
| Working memory (index scores) | 90.8±11.9 | 94.4±13.7 | 92.5±12.9 | 102.2±14.4 | 0.265 | <0.001a | <0.001a |
| Processing speed (index scores) | 103.7±16.2 | 106.7±16.5 | 105.2±16.3 | 111.7±16.9 | 0.461 | 0.054 | 0.012a |
| Delis‐Kaplan executive function system | |||||||
| Executive Composite score (scaled scores) | 10.3±1.6 | 10.2±2.0 | 10.3±1.8 | 11.8±1.8 | 0.677 | <0.001a | 0.275 |
| Trail making (scaled scores) | 10.4±2.1 | 10.3±2.7 | 10.3±2.4 | 11.6±1.5 | 0.902 | 0.003a | 0.249 |
| Verbal fluency (scaled scores) | 11.3±2.4 | 10.9±3.0 | 11.1±2.7 | 13.0±2.2 | 0.503 | <0.001a | 0.001a |
| Design fluency (scaled scores) | 10.0±2.3 | 10.2±2.1 | 10.1±2.2 | 12.0±2.9 | 0.729 | <0.001a | 0.766 |
| Color‐word interference (scaled scores) | 9.2±2.2 | 9.2±2.3 | 9.2±2.3 | 10.9±2.3 | 0.928 | <0.001a | 0.006a |
| Rey‐Osterreith Complex Figure Test | |||||||
| Copy (impaired, n [%])b | 4 (12) | 9 (29) | 13 (20) | 3 (8) | 0.082 | 0.082 | ‐ |
| Time to copy (sec) | 205.5±60.6 | 225.9±63.4 | 215.1±62.3 | 186.5±71.3 | 0.193 | 0.035a | ‐ |
| Immediate recall (T‐scores) | 38.8±12.1 | 44.4±13.5 | 41.5±13.0 | 46.9±11.9 | 0.084 | 0.033a | <0.001a |
| Delayed recall (T‐scores) | 41.4±9.7 | 44.8±10.0 | 43.0±9.9 | 47.5±8.4 | 0.169 | 0.018a | <0.001a |
| Recognition total correct (impaired, no [%])c | 12 (35) | 12 (39) | 24 (37) | 11 (28) | 0.776 | 0.320 | ‐ |
| Rey Auditory Verbal Learning Test | |||||||
| Trial 1 (T‐scores) | 43.4±9.5 | 43.7±10.4 | 43.5±9.9 | 48.5±10.1 | 0.884 | 0.015a | <0.001a |
| Total learning (T‐scores) | 41.8±10.4 | 41.8±11.2 | 41.8±10.7 | 49.4±9.1 | 0.992 | <0.001a | <0.001a |
| Delayed recall (T‐scores) | 42.0±9.3 | 44.1±10.1 | 43.0±9.7 | 48.2±9.0 | 0.375 | 0.007a | <0.001a |
| Recognition (impaired, n [%])d | 0 (0) | 2 (6) | 2 (3) | 0 (0) | 0.132 | 0.263 | ‐ |
| Reading the Mind in the Eyes Test (T‐scores) | 45.2±9.3 | 45.1±9.8 | 45.2±9.5 | 51.0±6.9 | 0.982 | 0.002a | <0.001a |
| BRIEF‐A self‐report (T‐scores)e | 56.0±12.1 | 55.3±13.3 | 55.7±12.6 | 46.8±8.3 | 0.836 | <0.001a | 0.005a |
| BRIEF‐A Informant (T‐scores)e | 48.6±10.4 | 49.6±11.9 | 49.1±11.1 | 42.5±6.6 | 0.726 | 0.005a | 0.531 |
| CAARS self‐report (T‐scores)f | 55.7±12.8 | 53.5±12.7 | 54.6±12.7 | 44.0±11.1 | 0.473 | <0.001a | 0.004a |
| CAARS Informant (T‐scores)f | 48.8±11.2 | 51.1±13.3 | 49.9±12.2 | 45.6±8.2 | 0.474 | 0.178 | 0.942 |
| SRS‐2 self‐report (T‐scores)g | 55.9±15.2 | 57.3±14.6 | 56.6±14.8 | 46.5±8.5 | 0.704 | <0.001a | <0.001a |
| SRS‐2 Informant (T‐scores)g | 50.2±11.5 | 53.4±14.2 | 51.7±12.8 | 44.5±7.9 | 0.329 | 0.007a | 0.314 |
Data are presented as mean±SD or as absolute numbers with relative percentages. Missing <2% of outcomes except for BRIEF‐A self‐report (CHD: n=63; controls: n=38) and informant report (CHD: n=60; controls: n=33), CAARS self‐report (CHD: n=66; controls: n=39), CAARS informant‐report (CHD: n=61; controls: n=34), SRS‐2 self‐report (CHD: n=66; controls: n=38), SRS‐2 self‐report (CHD: n=60; controls: n=33). Calculated expected population means: RFCT (Immediate Recall raw‐score=24.9±5.2, Delayed Recall raw‐score=25.0±6.7, T‐score=50±10); RAVLT (Trial 1 raw score=7.1±1.7, Total learning raw score=54.9±8.85, Delayed recall raw score=11.9±2.9, T‐score=50±10); Reading the Mind in the Eyes Test (raw score=26.2±3.6, T‐score=50±10); BRIEF‐A (self‐report (GEC) raw score=107.28±22.2, T‐score=51±10, Informant (GEC) raw score=110.41±27.5, T‐score=50±10); CAARS (ADHD‐index=50±10); SRS‐2 (50±10). ASD indicates atrial septal defect; BRIEF‐A, Behavior Rating Inventory of Executive Function–Adult; CAARS, Connors’ Adult ADHD Rating Scales; CHD, congenital heart defect; FDR, false discovery rate; GEC, Global Executive Composite; RAVLT, Rey Auditory Verbal Learning Test; RCFT, Rey‐Osterreith Complex Figure Test; SRS‐2, Social Responsiveness Scale Second Edition; and VSD, ventricular septal defect.
FDR q<0.05.
RCFT copy ≤32=impaired.
RCFT recognition total correct (true positives+true negatives) ≤19=Impaired.
RAVLT recognition (true positives) ≤11=impaired.
BRIEF‐A report the Global Executive Composite T‐score.
CAARS report the ADHD‐index T‐score.
SRS‐2 report the Total T‐score.
Figure 1.
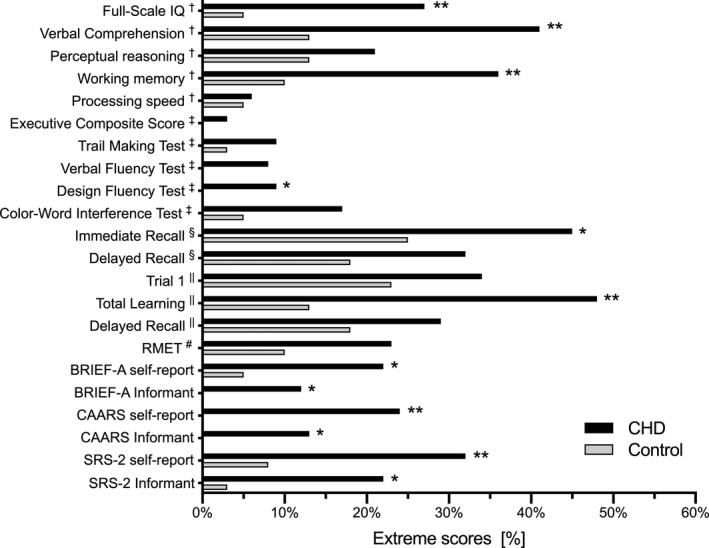
Extreme scores for CHD and Control Participants. Data are presented as percentages. *P<0.05, **FDR q<0.05. †Wechsler Adult Intelligence Scale Version IV; ‡Delis‐Kaplan Executive Function System; §Rey‐Osterreith Complex Figure Test; ||Rey Auditory Verbal Learning Test; #Reading the Mind in the Eyes Test. CHD indicates congenital heart defect and FDR, false discovery rate.
Intelligence
The CHD participants performed worse on the full‐scale IQ and in 3 of 4 subdomains (verbal comprehension, perceptual reasoning, working memory), though the fourth subdomain (processing speed) was also bordering significance compared with the control group and when compared to the expected population mean. Significant differences remained after FDR adjustment. Although IQ scores were within the normal range (IQ 85–115), the proportion of CHD participants in whom IQ was more than 1SD below the expected population mean was higher than in the control group (full‐scale IQ 27% versus 5%, P=0.004; verbal comprehension 41% versus 13%, P=0.002; working memory 36% versus 10%, P=0.003).
Executive Functions
The D‐KEFS mean executive composite score and the 4 subdomains were lower in CHD participants compared with the mean scores of the referent group (P<0.01). All D‐KEFS scores were within the normal range (7–13); however, the proportions of CHD participants in whom the Design Fluency Test score was more than 1SD below the expected population mean was higher than in the control group (9% versus 0%, P=0.04). The proportions of participants who performed more than 1SD lower in the Trail Making Test, Verbal Fluency Test, or Color‐Word Interference Test compared to the expected population mean (9% versus 3%, P=0.18; 8% versus 0%, P=0.07; 17% versus 5%, P=0.07, respectively), did not differ significantly between groups.
On the BRIEF‐A self‐report, the mean Global Executive Composite scores of the CHD group were worse compared with the control group and compared to the expected population mean scores. Approximately every fifth (22%) participant of the CHD group scored ≥65 (ie, “potential clinical significance”), compared with 5% of the control group (P=0.02). On the BRIEF‐A Informant, these figures were 12% and 0%, respectively (P=0.04). The self‐reported Global Executive Composite was significantly higher than the informant Global Executive Composite in the CHD group, but the same difference also occurred in the control group (P=0.003 and 0.02, respectively).
The CAARS self‐report revealed a significantly higher ADHD‐index in the CHD group. Approximately every fourth (24%) of the participants in the CHD group and 0% in the referent group scored >65, which is considered to be “moderately atypical.” The self‐reported ADHD‐index was significantly higher compared with the informant ADHD‐index in the CHD group (P=0.03).
Visuospatial Learning and Memory
The CHD group had an overall worse performance in visuospatial learning and memory (RCFT). They used longer time in the copy trial, and moreover, they had a lower score in the immediate recall and delayed recall trails compared with the referent group (P=0.02–0.04). Adjusted for FDR, the CHD group demonstrated significantly lower scores in immediate recall and delayed recall trials compared to the expected population mean (P <0.001). The RCFT performances were, however, within the normal range.
Verbal Learning and Memory
Overall verbal learning and memory performance (RAVLT) were worse in the CHD group. They performed significantly lower than the control group on all subtests except the recognition trial. The RAVLT scores were all within the normal range, yet nearly half (48%) of the CHD participants had a Total Learning score of more than 1SD below the expected population mean, compared with 13% in the referent group (P<0.001). A high significance level in all RAVLT measures withstood after FDR adjustment, when comparing the CHD participants with the expected population means.
Social Cognition
The CHD group performed lower on the RMET compared with the control group (P=0.002), and when compared to the expected population mean (P<0.001). The proportions of CHD participants in whom RMET score was more than 1SD below the expected population mean was 23% whereas it was 10% in the control group (P =0.09).
The CHD group had significantly worse scores in the SRS‐2 self‐report and the informant report when compared with the control group. On the self‐report, every third (32%) of the CHD group scored ≥60 (ie, “indicating mild to moderate deficits, or higher”), compared with 8% of the control group (P=0.005). On the informant reports, these figures were 22% and 3%, respectively (P=0.02).
The self‐reported perception of social dysfunction was borderline higher in the CHD participants when compared with their informant reports, (P=0.05). There was no difference between the control group's self‐report and their informant reports on the perception of social cognition (P=0.33).
1. Data
Brain MRI data were available for 63 adults who underwent surgical closure of an ASD (n=33) or VSD (n=30) and 38 controls. Brain morphometric volumes and comparisons are displayed in Table 5. Overall, there were no significant differences in global or regional brain volumes after FDR adjustment.
Table 5.
Global and Regional Brain Volumes for CHD and Control Participants
| Variable | ASD (n=33) | VSD (n=30) | CHD (n=63) | Control (n=38) | P Value, ASD Vs VSD | P Value, All CHD Vs Control |
|---|---|---|---|---|---|---|
| Global | ||||||
| Total intracranial volume, cm3 | 1342.9±126.3 | 1372.2±133.9 | 1357.4±129.7 | 1400.4±141.2 | 0.392 | 0.122 |
| Grey matter, cm3 | 788.3±32.9 | 784.6±49.3 | 786.6±41.2 | 771.7±51.0 | 0.726 | 0.112 |
| White matter, cm3 | 434.6±27.7 | 428.1±26.6 | 431.5±27.2 | 437.8±26.8 | 0.260 | 0.349 |
| Corpus callosum, cm2 | 6.0±1.0 | 6.0±0.7 | 5.9±0.8 | 6.2±0.8 | 0.613 | 0.113 |
| Cerebellum, cm3 | 134.2±8.3 | 135.6±11.1 | 134.9±9.7 | 132.4±8.9 | 0.595 | 0.206 |
| Lobes | ||||||
| Frontal right, cm3 | 212.8±10.3 | 207.1±8.5 | 210.1±9.8 | 208.4±10.2 | 0.020 | 0.404 |
| Frontal left, cm3 | 210.2±9.5 | 204.4±8.8 | 207.4±9.5 | 206.7±9.9 | 0.016 | 0.711 |
| Parietal right, cm3 | 102.7±6.4 | 101.5±5.7 | 102.2±6.0 | 103.8±7.1 | 0.434 | 0.237 |
| Parietal left, cm3 | 101.9±6.0 | 101.3±6.6 | 101.6±6.3 | 101.4±7.3 | 0.682 | 0.850 |
| Temporal right, cm3 | 126.2±4.7 | 126.6±7.9 | 126.4±6.4 | 126.2±5.7 | 0.821 | 0.866 |
| Temporal left, cm3 | 121.0±4.9 | 121.9±6.6 | 121.4±5.8 | 121.1±6.2 | 0.538 | 0.841 |
| Occipital right, cm3 | 56.6±4.3 | 57.8±5.5 | 57.2±4.9 | 54.5±5.5 | 0.330 | 0.012 |
| Occipital left, cm3 | 53.7±4.2 | 53.7±5.7 | 53.7±4.9 | 52.9±4.9 | 0.973 | 0.456 |
| Basal nuclei | ||||||
| Thalami, cm3 | 12.6±1.0 | 12.5±0.8 | 12.6±0.9 | 12.7±0.8 | 0.912 | 0.523 |
| Putamen, cm3 | 10.8±8.5 | 11.1±9.4 | 10.9±9.0 | 10.7±9.7 | 0.218 | 0.177 |
| Nucleus Caudate, cm3 | 11.8±9.6 | 11.7±8.3 | 11.8±0.9 | 11.7±0.9 | 0.535 | 0.551 |
| Limbic lobes | ||||||
| Hippocampi, cm3 | 6.0±0.7 | 5.9±0.5 | 5.9±0.6 | 5.9±0.6 | 0.492 | 0.622 |
| Ventricles | ||||||
| Lateral ventricles, cm3 | 15.3±5.9 | 15.2±6.6 | 15.2±6.2 | 13.6±5.6 | 0.938 | 0.184 |
Data are presented as mean±SD. ASD, atrial septal defect; CHD, congenital heart defect; and VSD, ventricular septal defect.
Visually identified brain abnormalities and comparisons are displayed in Table 6. The frequency of any brain abnormality was 24% in CHD participants compared with 29% in controls (P=0.567). The majority of abnormalities were minor developmental abnormalities. The occurrence of WMHs in the CHD group was not different from that in controls. However, there was a higher frequency of WMHs in the ASD group compared with the control group (P=0.047, not reported in Table 6.). Notably, more than twice as many of the ASD participants had “several” WMHs (>1 and ≤20 WMHs) compared with the controls (42% versus 18%). In the patients and controls with WMHs, the most common anatomical location was the frontal lobe followed by the parietal and temporal lobe.
Table 6.
Structural Magnetic Resonance Imaging Findings for CHD and Control Participants
| Variable | ASD (n=33) | VSD (n=30) | CHD (n=63) | Control (n=38) | P Value, ASD Vs VSD | P Value, All CHD Vs Control |
|---|---|---|---|---|---|---|
| Any abnormalitya | 7 (21) | 8 (27) | 15 (24) | 11 (29) | 0.612 | 0.567 |
| Acquired focal or multifocal abnormality | 1 (3) | 0 (0) | 1 (2) | 2 (5) | 0.336 | 0.292 |
| Focal infarction | 0 (0) | 0 (0) | 0 (0) | 0 (0) | … | … |
| Focal cortical dysplasia | 0 (0) | 0 (0) | 0 (0) | 1 (3) | … | 0.196 |
| Otherb | 1 (3) | 0 (0) | 1 (2) | 1 (3) | 0.336 | 0.715 |
| Diffuse abnormalityc | 3 (9) | 3 (10) | 6 (10) | 4 (11) | 0.902 | 0.870 |
| Generalized abnormality | 0 (0) | 0 (0) | 0 (0) | 0 (0) | … | … |
| Developmental abnormality | 3 (9) | 5 (17) | 8 (13) | 6 (16) | 0.367 | 0.663 |
| Major malformationd | 1 (3) | 0 (0) | 1 (2) | 0 (0) | 0.336 | 0.435 |
| Minor malformatione | 2 (6) | 5 (17) | 7 (11) | 6 (16) | 0.181 | 0.496 |
| White matter hyperintensities | 0.108 | 0.227 | ||||
| None or a single punctate WMH (≤1) | 19 (58) | 23 (77) | 42 (67) | 29 (76) | ||
| Several punctate WMHs (>1 & ≤20) | 14 (42) | 6 (20) | 20 (32) | 7 (18) | ||
| Many punctate WMHs (>20) | 0 (0) | 1 (3) | 1 (2) | 2 (5) |
Data are presented as absolute numbers with relative percentages (n [%]). ASD indicates atrial septal defect; CHD, congenital heart defect; VSD, ventricular septal defect; and WMHs, white matter hyperintensities.
Any abnormality does not include WMHs.
Other focal or multifocal abnormalities comprise unspecific subcortical hyperintensity and gliotic scar around venule.
Diffuse abnormality is Evans’ Index ≥0.3.
The major malformation is a malformation of corpus callosum.
Minor malformations include developmental venous anomaly, Chiari 1 malformation, arachnoid cysts, empty sella and cavum septi pellucidi with and without cavum vergae.
Potential Risk Factors
We could not identify any clinical or surgery‐related risk factors (extracorporeal circulation time, cross‐clamp time, defect size, ASD and VSD subtypes, pulmonary banding in VSD, length of intensive care unit stay, and length of hospital stay) for poor neuropsychological performance.
Discussion
The main result of our study is that neuropsychological functioning is impaired in adults operated for ASD or VSD compared to population means and a healthy, local control group. Impairments were manifest across the majority of the neuropsychological domains with verbal comprehension, working memory, perceptual reasoning, visuospatial and verbal memory, and social recognition being particularly affected. We did not identify any major risk factors for poor neurodevelopmental outcome. ASD patients were not different from VSD, on neither neuropsychological outcomes nor brain morphometric measures.
Neuropsychological Profile
Although the CHD participants generally performed worse than healthy controls across all cognitive domains some aspects of cognitive function appeared to be more impaired than others. In terms of intelligence, the verbal comprehension, perceptual reasoning, and working memory appeared to be more impaired than other WAIS‐IV subdomains, whereas processing speed was a relative strength. More than one‐fourth of CHD participants had a full‐scale IQ of more than 1SD below the expected population mean. Our findings on lower full‐scale IQ intelligence compares to what have been reported in CHD adolescents with either cyanotic CHD such as tetralogy of Fallot9 and single ventricle defects11 or in a mixed cyanotic‐acyanotic cohort8. In cyanotic CHD adolescents, the processing speed is particularly impaired, and the verbal comprehension is the least impaired7, 11. We find the opposite in our cohort, with the largest difference in verbal comprehension and no difference in processing speed. Worse outcomes in perceptual reasoning and working memory are unanimously reported in both ours and previous studies8, 9, 11. In a mixed cohort of acyanotic and cyanotic CHD adolescents 9% had a full‐scale IQ below 85 (−1SD)13, where we report a substantial higher occurrence of 27%.
With regard to executive function, the composite score was lower compared with the controls but not when compared to the population norm. The Color‐Word Interference Task was particularly troublesome for the CHD participants and they performed significantly lower than the population mean. Disabilities in executive function have previously been reported in children and adolescents with complex CHD7, 9, 11, 41, compared with the population mean.
In terms of self‐perceived executive function, the CHD participants reported greater difficulties than both the healthy controls and their informant reports. This is in contrast to previous studies on dextro‐transposition of the great arteries adolescents, where parents and teachers reported a greater executive dysfunction than was noted by the adolescents themselves7. Also, it has previously been suggested that children with CHD lack insight into their own weaknesses42. As the CHD adults in our study did recognize an executive dysfunction, their self‐awareness may have evolved when entering adulthood.
The CHD participants self‐reported a high level of attention deficits and hyperactivity behavior, as one‐fourth (24%) had an ADHD‐index considered “moderately atypical.” However, their informants did not recognize a higher ADHD‐index. These findings are in accordance with those reported in adolescents with tetralogy of Fallot (21% with cutoff value ≥66) and dextro‐transposition of the great arteries (19% with cutoff value ≥65)7, 9. Importantly, in these studies, the ADHD‐index was reported by parents and not by the adolescents themselves, and this may partly explain the slightly higher rate of behavioral problems in our study.
Visuospatial learning and memory were also found particularly weak in the CHD participants. In the RCFT, the immediate and delayed recall trials were significantly lower in the CHD participants, demonstrating a clear‐cut deficit in visual memory capacities. This discrepancy in visual information processing is also manifested on the intelligence profile through lower perceptual reasoning. Our findings are in accordance with previously reported findings, which documented visuospatial deficits in acyanotic CHD children43 and mixed acyanotic and cyanotic CHD adolescents8, 44. The auditory verbal learning and memory (RAVLT) performance were poorer in the CHD participants, who had significantly lower scores in 3 of 4 tasks, and half (48%) scored more than 1SD below population means in the Total Learning Trial, indicating a clear deficit in verbal memory capacities. Our findings support the conclusion by others that deficits in the verbal memory domain are present in patients with acyanotic CHD43, and in addition we find that these impairments continue into adulthood.
Like children and adolescents with other forms of CHD, our ASD and VSD participants manifested difficulties in social cognition7, 9, 11, 41. They had poorer performance than controls and population means in their ability to identify the emotions behind facial expressions (RMET). In terms of self‐perceived social cognition (SRS‐2), the CHD participants had a lower self‐reported performance with 32% reporting mild to moderate or higher deficits. CHD participants and their informants shared the perception of a social cognition dysfunction. These findings emphasize the presence of social cognition dysfunction in CHD adults and support findings that previously reported social cognition deficits in childhood41 continue into adulthood independent of CHD type and severity.
The practical importance of these neuropsychological impairments is emphasized by the high rates of academic and behavioral services that our CHD participants received during school age. Furthermore, their medical history (33% diagnosed with a psychiatric disease) reveals a coexistence of neurocognitive impairments and mental health vulnerability. These results are in accordance with our previous findings in a nationwide cohort study showing increased risk of developmental and other psychiatric disorders in CHD45 and our study on adults with unrepaired ASDs where 17% had 1 or more psychiatric diagnoses17.
Pathophysiology
The neurodevelopmental impairments are notable in these seemingly simple types of CHD. The ASD and VSD participants had no diagnosed genetic syndromes or extracardiac anomalies and were operated on only once during a short‐term hospitalization and intensive care unit visit. Furthermore, they have not been exposed to in utero reduced cerebral oxygen delivery and consumption, as is the case with cyanotic and complex CHD46. Yet our findings mirror the neurodevelopmental discrepancies demonstrated in complex CHD, though less severe.
With increasing evidence of a genetic link between heart and brain development and a genetic burden in severe CHD47, the possibility of clinically undiagnosed genetic syndromes, subchromosomal gene abnormalities, and/or epigenetic factors in our CHD participants cannot be ruled out.
Neurodevelopmental Outcome and Brain Morphology
The coexistence of CHD and abnormal brain volumes has been described in adolescents with acyanotic CHD who demonstrate reduced volume in the cortical grey matter, cerebellum, basal ganglia, and hippocampus12. Furthermore, global and regional brain volumes were shown to correlate with neurodevelopmental functioning. We did not find differences in brain morphometric volumes in our cohort and consequently did not find the basis for examining such associations in this study. We have previously, in a nationwide cohort study, reported that ASD and VSD are associated with large head circumference relative to birth weight whereas transposition of the great arteries is associated with smaller head circumference relative to birth weight48. Those findings indicate that small brain size is associated with more severe CHD but it is not typically a feature in septal heart defects.
Complex CHDs have a high frequency of abnormalities on MRI11. This do not apply to septal CHD in general, as we did not find differences in the amount of brain abnormalities in CHD participants and controls. We did however find a noteworthy higher level of WMHs and a higher number of “several” WMHs (between >1 and ≤20 WMHs chosen as an arbitrary clinical cutoff entity) in the ASD group compared with the control group.
Implications for Clinical Practice and Future Research
Children and adolescents with septal CHD are at risk of long‐term neurodevelopmental deficits and in need of educational support. A wide variety of cognitive areas for academic learning (executive function, visuospatial skills, and verbal learning among others) are impaired and supportive initiatives need to embrace this variety. The disparity is in accordance with previous findings of a poor affiliation to the work force, as well as a lower education rate in ASD adults as shown in a nationwide cohort study49. Also, the current recommendations relating to medical and developmental surveillance, screening, and periodic reevaluation for children treated for CHD outlined by American Heart Association and American Academy of Pediatrics50 should be taken into count when dealing with patients with isolated septal heart defects.
Even though we did not demonstrate any macroscopic brain abnormalities, a brain‐behavior relationship may still exist between neurocognitive deficits and neuroanatomy. Future studies should address brain surface morphology, grey and white matter microstructural differences, or functional MRI measures, as well as associations between MRI and neurocognitive impairments. Questions also arise regarding the fundamental basis of these neurodevelopmental impairments and the possible genetic and epigenetic involvement.
Limitations
The cross‐sectional design entails the possibility of selection bias, a known disadvantage in this study design. Further, it should be noted that mean neurodevelopmental scores for the CHD participants fell in the average range and mostly fell within 1 SD from the population‐based mean. This reveals mild impairments rather than significant impairments on a group level.
The CHD participants in this study were operated on in the 1990s and with today's younger age at operation, they may not reflect today's patients. However, a large number of patients operated in the last century live with the consequences of their congenital heart defect and they deserve attention.
We did not adjust for socioeconomic status, which in CHD is a well‐known predictor of neurodevelopmental outcome in CHD51. As substantial negative socioeconomic consequences have been reported in ASD patients49, an adjustment for socioeconomic status may underestimate true deficits in the patient cohort. Of relevance it should be noted that the public and freely accessible nature of the Danish education system reduced any potential confounding from differences in socioeconomic status among the CHD participants and the controls.
To account for the impact of CHD‐related treatment and potential long‐term sequelae on neurodevelopmental outcomes, the matching of the control group may have benefited from an implementation of several other social and educational factors.
Other studies on mixed CHD cohorts have found differences in brain size. The lack of major structural brain morphology in our CHD participants does not exclude the presence of anatomically confined structural abnormalities. Furthermore, the small sample size should also be taken into consideration.
Finally, birth‐related information such as weight, gestational age, and Apgar score was not obtained and is a potential source of confounding related to the neurodevelopmental outcomes, as these factors are known to influence early life development.
Conclusions
We found that impaired neuropsychological performance is common in adults who in childhood have undergone surgical closure of ASD or VSD compared with healthy peers and population means. However, these 2 groups had similar global and regional morphometric brain volumes and a similar frequency of MRI abnormalities. Our findings imply the presence of long‐term neurocognitive impairments in surgically approached ASD and VSD patients. Further studies are needed to clarify the extent of these patients’ neurodevelopmental challenges and to reveal the brain‐behavior relationship. It seems advisable to clinicians to consider patients with septal heart defects at risk of neurodevelopmental impairments, and we emphasize the importance of early detection and intervention in such developmental problems.
Sources of Funding
This study was supported by Aarhus University, Aase & Ejnar Danielsens Foundation, The Danish Medical Association, A.P. Møller Foundation for the Advancement of Medical Science, Helga and Peter Korning Foundation and the Health Research Fund of Central Denmark Region.
Disclosures
None.
Acknowledgements
The authors warmly acknowledge research nurse Vibeke Laursen for her appreciated contribution and our neuropsychologists’ team (K.A, A.M.B, E.E.P, K.W.J, R.H.P) for the assistance with data collection.
(J Am Heart Assoc. 2020;9:e015843 DOI: 10.1161/JAHA.120.015843.)
For Sources of Funding and disclosures, see page 12.
See Editorial by Jonas
References
- 1. Oster ME, Lee KA, Honein MA, Riehle‐Colarusso T, Shin M, Correa A. Temporal trends in survival among infants with critical congenital heart defects. Pediatrics. 2013;131:e1502–e1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marelli AJ, Ionescu‐Ittu R, Mackie AS, Guo L, Dendukuri N, Kaouache M. Lifetime prevalence of congenital heart disease in the general population from 2000 to 2010. Circulation. 2014;130:749–756. [DOI] [PubMed] [Google Scholar]
- 3. Larsen SH, Olsen M, Emmertsen K, Hjortdal VE. Interventional treatment of patients with congenital heart disease: nationwide Danish experience over 39 years. J Am Coll Cardiol. 2017;69:2725–2732. [DOI] [PubMed] [Google Scholar]
- 4. Wernovsky G, Stiles KM, Gauvreau K, Gentles TL, DuPlessis AJ, Bellinger DC, Walsh AZ, Burnett J, Jonas RA, Mayer JE, et al. Cognitive development after the Fontan operation. Circulation. 2000;102:883–889. [DOI] [PubMed] [Google Scholar]
- 5. Newburger JW, Sleeper LA, Bellinger DC, Goldberg CS, Tabbutt S, Lu M, Mussatto KA, Williams IA, Gustafson KE, Mital S, et al. Early developmental outcome in children with hypoplastic left heart syndrome and related anomalies: the single ventricle reconstruction trial. Circulation. 2012;125:2081–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Licht DJ, Shera DM, Clancy RR, Wernovsky G, Montenegro LM, Nicolson SC, Zimmerman RA, Spray TL, Gaynor JW, Vossough A. Brain maturation is delayed in infants with complex congenital heart defects. J Thorac Cardiovasc Surg. 2009;137:529–536; discussion 536–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bellinger DC, Wypij D, Rivkin MJ, Demaso DR, Robertson RL, Dunbar‐Masterson C, Rappaport LA, Wernovsky G, Jonas RA, Newburger JW. Adolescents with d‐transposition of the great arteries corrected with the arterial switch procedure: neuropsychological assessment and structural brain imaging. Circulation. 2011;124:1361–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schaefer C, von Rhein M, Knirsch W, Huber R, Natalucci G, Caflisch J, Landolt MAMA, Latal B. Neurodevelopmental outcome, psychological adjustment, and quality of life in adolescents with congenital heart disease. Dev Med Child Neurol. 2013;55:1143–1149. [DOI] [PubMed] [Google Scholar]
- 9. Bellinger DC, Rivkin MJ, Demaso D, Robertson RL, Stopp C, Dunbar‐Masterson C, Wypij D, Newburger JW. Adolescents with tetralogy of Fallot: neuropsychological assessment and structural brain imaging. Cardiol Young. 2015;25:338–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shillingford AJ, Glanzman MM, Ittenbach RF, Clancy RR, Gaynor JW, Wernovsky G. Inattention, hyperactivity, and school performance in a population of school‐age children with complex congenital heart disease. Pediatrics. 2008;121:e759–e767. [DOI] [PubMed] [Google Scholar]
- 11. Bellinger DC, Watson CG, Rivkin MJ, Robertson RL, Roberts AE, Stopp C, Dunbar‐Masterson C, Bernson D, DeMaso DR, Wypij D, et al. Neuropsychological status and structural brain imaging in adolescents with single ventricle who underwent the Fontan procedure. J Am Heart Assoc. 2015;4:e002302 DOI: 10.1161/JAHA.115.002302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Von Rhein M, Buchmann A, Hagmann C, Huber R, Klaver P, Knirsch W, Latal B. Brain volumes predict neurodevelopment in adolescents after surgery for congenital heart disease. Brain. 2014;137:268–276. [DOI] [PubMed] [Google Scholar]
- 13. Von Rhein M, Scheer I, Loenneker T, Huber R, Knirsch W, Latal B. Structural brain lesions in adolescents with congenital heart disease. J Pediatr. 2011;158:984–989. [DOI] [PubMed] [Google Scholar]
- 14. Nyboe C, Olsen MS, Nielsen‐Kudsk JE, Hjortdal VE. Atrial fibrillation and stroke in adult patients with atrial septal defect and the long‐term effect of closure. Heart. 2015;101:706–711. [DOI] [PubMed] [Google Scholar]
- 15. Karunanithi Z, Nyboe C, Hjortdal VE. Long‐term risk of atrial fibrillation and stroke in patients with atrial septal defect diagnosed in childhood. Am J Cardiol. 2017;119:461–465. [DOI] [PubMed] [Google Scholar]
- 16. Nyboe C, Karunanithi Z, Nielsen‐Kudsk JE, Hjortdal VE. Long‐term mortality in patients with atrial septal defect: a nationwide cohort‐study. Eur Heart J. 2018;39:993–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Udholm S, Nyboe C, Dantoft TM, Jørgensen T, Rask CU, Hjortdal VE. Small atrial septal defects are associated with psychiatric diagnoses, emotional distress, and lower educational levels. Congenit Heart Dis. 2019;14:803–810. [DOI] [PubMed] [Google Scholar]
- 18. Benderly M, Kalter‐Leibovici O, Weitzman D, Blieden L, Buber J, Dadashev A, Mazor‐Dray E, Lorber A, Nir A, Yalonetsky S, et al. Depression and anxiety are associated with high health care utilization and mortality among adults with congenital heart disease. Int J Cardiol. 2019;276:81–86. [DOI] [PubMed] [Google Scholar]
- 19. Wechsler D. Wechsler Adult Intelligence Scale IV. San Antonio, TX: USA Pearson, Psychol Corp; 2008. [Google Scholar]
- 20. Delis DC, Kaplan E, Kramer J. Delis‐Kaplan Executive Function System (D‐KEFS). San Antonio, TX: USA Pearson, Psychol Corp; 2001:21. [Google Scholar]
- 21. Roth RM, Isquith PK, Gioia GA. Behavior Rating Inventory of Executive Function—Adult Version (BRIEF‐A). 2005.
- 22. Conners CK, Erhardt D, Sparrow EP. CAARS: Conners’ Adult ADHD Rating Scale. North Tonawanda, NY: Multi‐Health Systems; 1999. [Google Scholar]
- 23. Rey A. L’examen psychologique dans les cas d’encéphalopathie traumatique: (Les problems.) Arch Psychol; 1941;28:286–340. [Google Scholar]
- 24. Osterreith PA, Le test de copie d'une figure complex: contribution a l’étude de la perception et de la mémoire: Arch Psychol; 1944;30:286–356. [Google Scholar]
- 25. Meyers JE, Meyers KR, Rey complex figure test and recognition trial: Professional manual. Odessa, FL: Psychological Assessment Resources; 1995. [Google Scholar]
- 26. Baron‐Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. Reading the Mind in the Eyes Test revised version: a study with normal adults, and adults with Asperger syndrome or high‐functioning autism. J Child Psychol Psychiatry. 2001;42:241–251. [PubMed] [Google Scholar]
- 27. Constantino JN, Gruber CP. The Social Responsiveness Scale‐Second Edition. Los Angeles, CA: Western Psychological Services; 2012. [Google Scholar]
- 28. Marques JP, Kober T, Krueger G, van der Zwaag W, Van de Moortele PF, Gruetter R. MP2RAGE, a self bias‐field corrected sequence for improved segmentation and T1‐mapping at high field. NeuroImage. 2010;49:1271–1281. [DOI] [PubMed] [Google Scholar]
- 29. Aubert‐Broche B, Fonov VS, García‐Lorenzo D, Mouiha A, Guizard N, Coupé P, Eskildsen SF, Collins DL. A new method for structural volume analysis of longitudinal brain MRI data and its application in studying the growth trajectories of anatomical brain structures in childhood. NeuroImage. 2013;82:393–402. [DOI] [PubMed] [Google Scholar]
- 30. Coupe P, Yger P, Prima S, Hellier P, Kervrann C, Barillot C. An optimized blockwise nonlocal means denoising filter for 3‐D magnetic resonance images. IEEE Trans Med Imaging. 2008;27:425–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sied JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. [DOI] [PubMed] [Google Scholar]
- 32. Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- 33. Collins DL, Evans AC. Animal: validation and application of nonlinear registration‐based segmentation. Int J Pattern Recognit Artif Intell. 1997;11:1271–1294. [Google Scholar]
- 34. Eskildsen SF, Coupé P, Fonov V, Manjón JV, Leung KK, Guizard N, Wassef SN, Østergaard LR, Collins DL. BEaST: brain extraction based on nonlocal segmentation technique. NeuroImage. 2012;59:2362–2373. [DOI] [PubMed] [Google Scholar]
- 35. Zijdenbos A, Forghani R, Evans A. Automatic quantification of MS lesions in 3D MRI brain data sets: validation of INSECT In: Wells, WM, Colchester A, Delp S, eds, the First International Conference on Medical Image Computing and Computer‐Assisted Intervention MICCAI’98. volume 1496 of Lecture Notes in Computer Science. Boston, 1998:439–448. [Google Scholar]
- 36. Fonov V, Evans AC, Botteron K, Almli CR, McKinstry RC, Collins DL. Unbiased average age‐appropriate atlases for pediatric studies. NeuroImage. 2011;54:313–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Coupé P, Manjón JV, Fonov V, Pruessner J, Robles M, Collins DL. Patch‐based segmentation using expert priors: application to hippocampus and ventricle segmentation. NeuroImage. 2011;54:940–954. [DOI] [PubMed] [Google Scholar]
- 38. Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, Lindley RI, O'Brien JT, Barkhof F, Benavente OR, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mitrushina MN, Boone KB, D'Elia LF. Handbook of Normative Data for Neuropsychological Assessment. 2 ed. New York, NY: Oxford University Press, Inc.; 2005. [Google Scholar]
- 40. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- 41. Calderon J, Bonnet D, Courtin C, Concordet S, Plumet MH, Angeard N. Executive function and theory of mind in school‐aged children after neonatal corrective cardiac surgery for transposition of the great arteries. Dev Med Child Neurol. 2010;52:1139–1144. [DOI] [PubMed] [Google Scholar]
- 42. Bellinger DC, Newburger JW. Neuropsychological, psychosocial, and quality‐of‐life outcomes in children and adolescents with congenital heart disease. Prog Pediatr Cardiol. 2010;29:87–92. [Google Scholar]
- 43. Sarrechia I, Miatton M, François K, Gewillig M, Meyns B, Vingerhoets G, De Wolf D. Neurodevelopmental outcome after surgery for acyanotic congenital heart disease. Res Dev Disabil. 2015;45:58–68. [DOI] [PubMed] [Google Scholar]
- 44. von Rhein M, Kugler J, Liamlahi R, Knirsch W, Latal B, Kaufmann L. Persistence of visuo‐constructional and executive deficits in adolescents after open‐heart surgery. Res Dev Disabil. 2015;36:303–310. [DOI] [PubMed] [Google Scholar]
- 45. Olsen M, Sørensen HT, Hjortdal VE, Christensen TD, Pedersen L. Congenital heart defects and developmental and other psychiatric disorders: a Danish nationwide cohort study. Circulation. 2011;124:1706–1712. [DOI] [PubMed] [Google Scholar]
- 46. Sun L, Macgowan CK, Sled JG, Yoo S‐J, Manlhiot C, Porayette P, Grosse‐Wortmann L, Jaeggi E, McCrindle BW, Kingdom J, et al. Reduced fetal cerebral oxygen consumption is associated with smaller brain size in fetuses with congenital heart disease. Circulation. 2015;131:1313–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Homsy J, Zaidi S, Shen Y, Ware JS, Samocha KE, Karczewski KJ, DePalma SR, McKean D, Wakimoto H, Gorham J, et al. De novo mutations in congenital heart disease with neurodevelopmental and other congenital anomalies. Science. 2015;350:1262–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Matthiesen NB, Henriksen TB, Gaynor JW, Agergaard P, Bach CC, Hjortdal VE, Østergaard JR. Congenital heart defects and indices of fetal cerebral growth in a nationwide cohort of 924 422 liveborn infants. Circulation. 2016;133:566–575. [DOI] [PubMed] [Google Scholar]
- 49. Nyboe C, Fonager K, Larsen ML, Andreasen JJ, Lundbye‐Christensen S, Hjortdal V. Effect of atrial septal defect in adults on work participation (from a Nation Wide Register‐based follow‐up study regarding work participation and use of permanent social security benefits). Am J Cardiol. 2019;124:1775–1779. [DOI] [PubMed] [Google Scholar]
- 50. Marino BS, Lipkin PH, Newburger JW, Peacock G, Gerdes M, Gaynor JW, Mussatto KA, Uzark K, Goldberg CS, Johnson WH, et al. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management a scientific statement from the American Heart Association. Circulation. 2012;126:1143–1172. [DOI] [PubMed] [Google Scholar]
- 51. Wernovsky G. Current insights regarding neurological and developmental abnormalities in children and young adults with complex congenital cardiac disease. Cardiol Young. 2006;16:92–104. [DOI] [PubMed] [Google Scholar]


