Abstract
The Fontan procedure has provided patients with single ventricle physiology extended survival into adulthood and in many cases has improved their quality of life. Atrioventricular valve regurgitation (AVVR) is common in single ventricle patients and is associated with increased risk of mortality. AVVR is more common in patients with a systemic tricuspid or common atrioventricular valve but is generally progressive irrespective of underlying valve morphology. AVVR can be attributable to diverse structural and functional abnormalities at multiple levels of the valvar apparatus, as well as ventricular dysfunction and dilation. Multiple imaging modalities including recent advances in 3‐dimensional echocardiography and cross‐sectional imaging have been used to further understand AVVR. Surgery to address AVVR must be tailored to the underlying mechanism and the timing of surgical repair should be chosen carefully. In this review, we discuss the etiologies, treatment options, surgical timing, and outcomes of valve repair or replacement for AVVR in patients with single ventricle congenital heart disease, with a focus on those with a Fontan circulation as AVVR is associated with increased risk for Fontan failure and mortality. In‐depth understanding of the current literature will help guide clinicians in their approach and management of AVVR in this population.
Keywords: atrioventricular valve regurgitation, Fontan, single ventricle congenital heart disease
Subject Categories: Congenital Heart Disease
Nonstandard Abbreviations and Acronyms
- AVV
atrioventricular valve
- AVVR
atrioventricular valve regurgitation
- CMR
cardiac magnetic resonance
- FF
Fontan failure
- HLHS
hypoplastic left heart syndrome
- PM
papillary muscle
- SV
single ventricle
- TEE
transesophageal echocardiography
- TV
tricuspid valve
- 2D
2‐dimensional
- 3D
3‐dimensional
The Fontan procedure has transformed the lives of patients with single ventricle (SV) congenital heart disease, providing improved survival and quality of life into adulthood.1 Since it was first performed in 1968, surgical techniques have evolved along with improvements in cardiac intensive care and cardiac catheterization approaches. As a consequence, outcomes have progressively improved but the incidence of cardiovascular and associated adverse events remains high and life expectancy is decades less than the population average.2, 3 The 5‐year risk of death for a 40‐year‐old Fontan patient is similar to that of a 75‐year‐old without congenital heart disease.4
Atrioventricular valve regurgitation (AVVR) is associated with increased risk of Fontan circulation failure, morbidity and premature mortality.5, 6, 7, 8 In this paper, we review the problem of AVVR in SV patients, with a focus on the Fontan circulation. Imaging and surgical repair options are discussed, along with an overview of outcomes after atrioventricular valve (AVV) repair. Lastly, we hope to provide a concise review of the literature to support a more uniform approach in the evaluation and management of patients with AVVR.
AVVR Incidence and Associated Risk for Fontan Failure
Up to 75% of Fontan patients have some degree of AVVR, with moderate or severe AVVR in a sizable minority (≈20%).8 King et al recently analyzed the outcomes of almost 1200 Fontan patients from an Australia and New Zealand Fontan registry.5 With a median follow‐up of 11.7 years, the cumulative incidence of AVV failure, defined as moderate or greater regurgitation or referral for AVV operation, was 56% among those with a common AVV and 46% with hypoplastic left heart syndrome (HLHS) (single tricuspid valve) at 25 years of age. Patients with tricuspid atresia and those with 2 AVVs had a substantially lower cumulative incidence of AVV failure at 25 years, 8% and 26%, respectively. The authors further reported AVV failure to be associated with a 2‐fold higher incidence of Fontan failure (FF), in this case defined as either: death, heart transplantation, Fontan takedown or conversion, plastic bronchitis, protein losing enteropathy or New York Heart Association functional class III or IV symptoms.5 By 20 years post‐Fontan, freedom from FF was 54% and 77% in those with and without AVV failure, respectively.5 Importantly, this study makes apparent that AVVR is a progressive disease with long‐term, life‐limiting implications. Several smaller studies have similarly reported that AVVR is a risk factor for FF and long‐term mortality in Fontan patients.9, 10 Furthermore, patients who require AVV surgery have lower survival compared with those who do not require AVV surgery.11
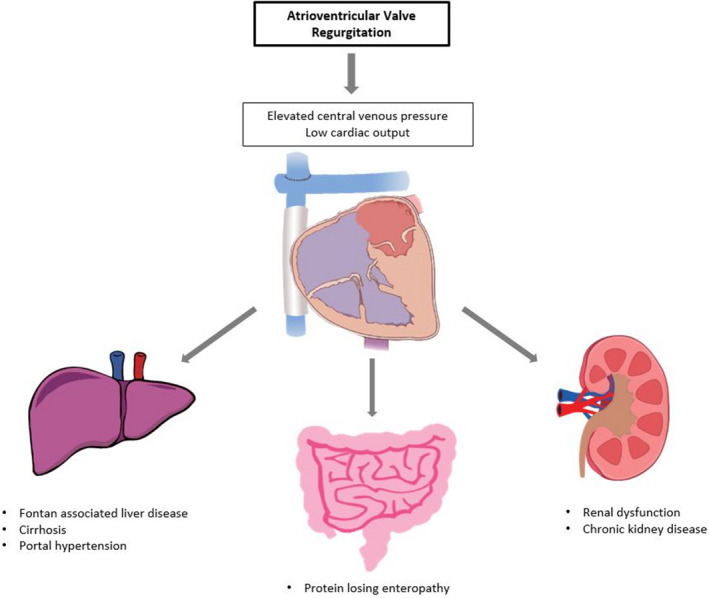
AVVR can lead to FF, involving multiple organ systems such as the liver, intestines, and kidneys. Figure 1 demonstrates the relationship between AVVR and end organ dysfunction in FF. AVVR causes elevated atrial and Fontan pressures; the increase in systemic venous pressure leads to a number of consequences including worsening Fontan associated liver disease and lymphatic dysfunction.12 Increased Fontan pressure likely also contributes to formation of venovenous collaterals and exacerbates systemic arterial hypoxemia. Furthermore, a high regurgitant volume increases SV preload and decreases effective cardiac output, leading to SV dilation, adverse remodeling, and systolic dysfunction.5, 13 The resulting decreased cardiac output activates the sympathetic nervous system and increases systemic vascular resistance. Progressive atrial enlargement secondary to AVVR contributes to an increased risk of atrial arrhythmias, which is strongly associated with worse outcomes.6, 14, 15
Figure 1. Atrioventricular valve regurgitation as a mechanism for multi‐organ system involvement in Fontan failure.
AVVR Mechanisms
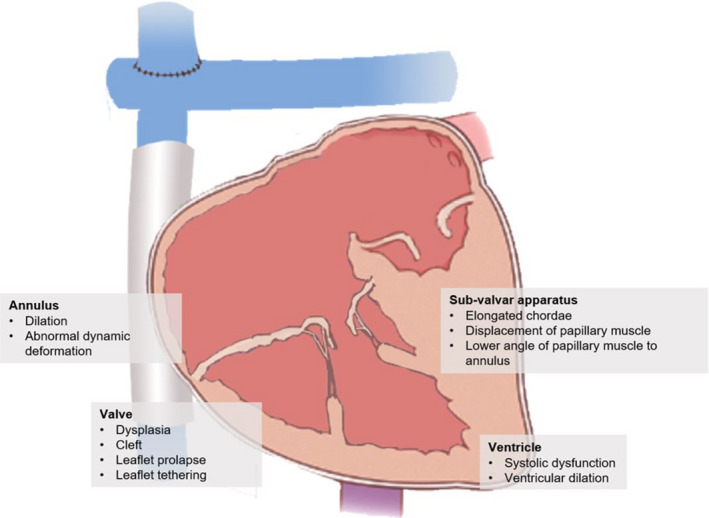
AVVR can be caused by many distinct structural valvar abnormalities and functional ventricular etiologies (Figure 2). Structural abnormalities are the most common cause of AVVR and can occur at all levels of the valve apparatus, including the leaflets, chordae, papillary muscles (PM) and annulus.11, 16 .
Figure 2. Atrioventricular valve regurgitation mechanisms.
Etiologies for Tricuspid Regurgitation in HLHS
In HLHS, a small septal leaflet and anterior leaflet prolapse are associated with the development of tricuspid valve regurgitation (Videos S1 and S2).17 Frequently encountered abnormalities are valve leaflet dysplasia and leaflet prolapse that alter AVV geometry resulting in malcoaptation.16, 18, 19
Leaflet tethering can result in restricted leaflet motion, deficient coaptation, and regurgitation. A higher tethering volume has been correlated with increased AVVR.20 Tethering can also occur as a consequence of lateral displacement of the anterior PM because of the abnormal geometry and dilation of a single right ventricle in HLHS.17, 20, 21, 22 Abnormal chordae tendinae, including elongation, deficiency, or malattachment, are pathologic findings that result in AVVR.
HLHS is also associated with systolic distortion and flattening of the typically saddle‐shaped tricuspid valve (TV) during systole, resulting in decreased annular bending and limited septal‐lateral coaptation at the anteroseptal commissure.23 This results in abnormal 3‐dimensional deformation throughout the cardiac cycle and increased dynamic changes in annular septo‐lateral dimensions, which is associated with worse tricuspid regurgitation.21
Etiologies for Common AVVR in Unbalanced Atrioventricular Septal Defects
The common AVV is particularly prone to AVVR, with a 10‐year cumulative incidence of valve failure of 34%.9 These valves have variable anatomy and are poorly adapted to sustain systemic afterload.24 Valve dysplasia, leaflet prolapse, and tethering are common etiologies of poor valve coaptation in this population (Videos S3 through S5). In a study of SV patients with unbalanced atrioventricular septal defect, increased tenting height, which is a measure of leaflet tethering, was found to be a predictor of severe AVVR. Leaflet tenting is measured from leaflet coaptation to leaflet annulus; the more distant the coaptation is from the annulus, the worse the leaflet tethering and the AVVR.25 Moreover, those with progressive reduction in tenting height maintained valve competency. This suggests that leaflet tethering is an important factor to consider in this subset of patients.
Functional AVVR
Functional AVVR occurs in the absence of structural abnormalities of the valve apparatus secondary to ventricular and annular dilation. This results in a stretched annulus and deficient coaptation of valve leaflets. Causes of functional AVVR can occur at any time in staged palliation. The interstage period between Stage I and Stage II palliation exposes the SV to an increased volume load and AVVR can evolve over time as a consequence of the resulting remodeling.26 In addition, etiologies of functional AVVR that are more common post‐Fontan include chronic volume overload secondary to aortopulmonary collaterals, aortic (or neo‐aortic) valve regurgitation, patent fenestration, or ventricular systolic dysfunction.16, 26
Imaging of AVVR
Various imaging modalities can assess AVV anatomy and function (Table 1). Transthoracic echocardiography is universally available, non‐invasive, and relatively inexpensive, and thus is most often used as the initial assessment. It usually provides excellent visualization of valve morphology, clefts, and leaflet motion in multiple planes.27 The degree of regurgitation can be determined by color flow pattern or quantification of vena contracta and regurgitant area.28 Grading of severity remains largely qualitative because of heterogeneous valve morphology, eccentric flow jets, and the frequency of multiple regurgitant jets. Additional pathologies that could contribute to the volume of AVVR independent of AVV regurgitant orifice size, including neo‐aortic outflow or aortic arch obstruction, can also be readily evaluated by echocardiography. The valve and subvalvar apparatus are important to examine closely by transthoracic echocardiography. Potential etiologies of AVVR including the presence of leaflet prolapse, clefts, or leaflet tethering should be described as this can help dictate surgical repair options. PM and chordae attachments should be looked at closely, as abnormalities in these structures can be addressed during repair. Annular dilation, ventricular dilation, and ventricular function are important to assess as well to provide guidance for surgeons on type and timing of repair.
Table 1.
Imaging Modalities
| Imaging Modality | Pros | Cons | Study |
|---|---|---|---|
| Transthoracic echocardiography |
|
|
Shah et al, 201627 Buber et al, 201928 |
| Transesophageal echocardiography |
|
|
Bharucha et al, 201330 Hahn 201629 Buber et al, 201928 |
| 3D echocardiography |
|
|
Nii et al, 200622 Mart et al, 201431 Bautista‐Hernandez et al, 201423 Kutty et al, 201420 Nguyen et al, 201921 |
| Computed tomography |
|
|
Hauser et al, 201732 |
| Cardiac magnetic resonance |
|
|
Hauser et al, 201732 |
Summary of imaging modalities that can be used to assess atrioventricular valve regurgitation.
In patients with poor acoustic windows, transesophageal echocardiography (TEE) can provide a more detailed visualization of valve structure and motion.28, 29, 30 Benefits of TEE include the ability to visualize the AVV in multiple planes to improve understanding of the location of leaflet abnormalities and origin of the regurgitant jet. Since sedation or even general anesthesia may be required, TEE is less commonly used in the outpatient setting.
Nevertheless, identifying the mechanism of AVVR pre‐operatively can be challenging. One study reported the experience of a single‐center, comparing the expected pathophysiology based on echocardiography and direct surgical inspection during repair.30 By 2‐dimensional (2D) echocardiography, valve prolapse, and tethering were the most common mechanisms of AVVR while direct surgical visualization diagnosed annular dilation and leaflet dysplasia as most common.30 There was poor agreement between echocardiography and surgical assessment; echocardiography described leaflet motion abnormalities well but was insensitive to structural abnormalities and leaflet dysplasia.
The use of 3‐dimensional (3D) echocardiography has emerged recently and addressed some of the limitations of 2D echocardiography; 3D echocardiography can describe surface and volumetric details of AVVs as well as clarify spatial relationships of the leaflets and sub‐valvar apparatus, measure tethering volumes, and describe dynamic geometric changes of the annulus.20, 21, 22, 23, 31 Although 3D echocardiography is an excellent new technology, its use is limited in the pediatric population. Most pediatric echocardiography probes do not have 3D technology. Additionally, 3D TEE generally cannot be performed adequately in small children who weigh <20 kg.
Cross‐sectional imaging can also provide information about AVVR. Cardiac magnetic resonance (CMR) is especially useful in adults or children with poor echocardiographic windows (Video S6). CMR is better suited to evaluate flow and quantify AVVR.32 Serial CMR can assess the progression of ventricular dilation or systolic dysfunction, each of which predisposes to mortality after AVV intervention post‐Fontan.16 The presence of stainless steel coils, Fontan pathway stents, or fenestration occlusion devices may cause imaging artifacts and limit evaluation by CMR. Computed tomography is an alternative as it requires a short imaging time and has high spatial resolution to provide some detail of the AVV anatomy and annular size (Video S7). One of the side effects of computed tomography is exposure to ionizing radiation.32 Although this is not an ideal method of imaging compared with CMR, it can be useful in patients with metallic stents or pacemakers that preclude CMR evaluation.
Because of the limitations and constraints of each available imaging modality, multimodality imaging is often useful to inform decision making. Initial evaluation should include precise transthoracic echocardiography. If the mechanism of AVVR is unclear or if there are poor acoustic windows, then we would recommend additional evaluation with TEE or CMR to quantify the regurgitation, ventricular size, and function, and assess valve anatomy. We also recommend that TEE be used pre‐ and intraoperatively to guide surgical repair.
Surgical Techniques to Address AVVR in SV Patients
There are many surgical techniques that address AVVR (Table 2). Surgical planning should include careful imaging of the valve as detailed above to determine mechanisms of AVVR and thus guide repair. In addition, intraoperative assessment with TEE is imperative to verify the mechanism of AVVR and can also provide information that may affect the surgeon's approach to valve repair.
Table 2.
Surgical Repairs of the Atrioventricular Valve
| Type of repair | Indication |
|---|---|
| Annuloplasty |
Annular dilation Leaflet restriction |
| Commissuroplasty | Atrioventricular valve clefts |
| Valvuloplasty | Coaptation defects |
| Chordal procedure (shortening, elongation, repositioning, artificial chords) |
Chordal absence Chordal rupture Elongated chordae Leaflet prolapse |
Types of surgical techniques to address specific structural abnormalities causing atrioventricular valve regurgitation.
The most common surgical techniques are partial annuloplasty and commissuroplasty (Figure 3A and 3B).18, 19, 23 Annuloplasties can be performed using extended polytetrafluoroethylene strips, pericardial strips, or rings.24 Ring annuloplasties decrease annular size and improves coaptation. A partial annuloplasty addresses leaflet restriction and/or annular dilation that results in deficient coaptation and central regurgitation. Two examples of partial annuloplasties to address tricuspid regurgitation include the DeVega and Kay annuloplasty. A DeVega suture annuloplasty involves placing parallel semicircular sutures along the annulus to decrease annular size typically from the septal‐posterior commissure to the fibrous trigone (Figure 3C). With the Kay annuloplasty, sutures are placed at the annulus from the antero‐posterior commissure to the posteroseptal commissure and tied down to exclude the posterior leaflet, creating a functional bicuspid valve and improving regurgitation (Figure 3D).33, 34 Clefts or fenestrations can be successfully repaired by primary closure or lateral leaflet plication.19, 23
Figure 3. Surgical repair techniques for atrioventricular valve regurgitation.
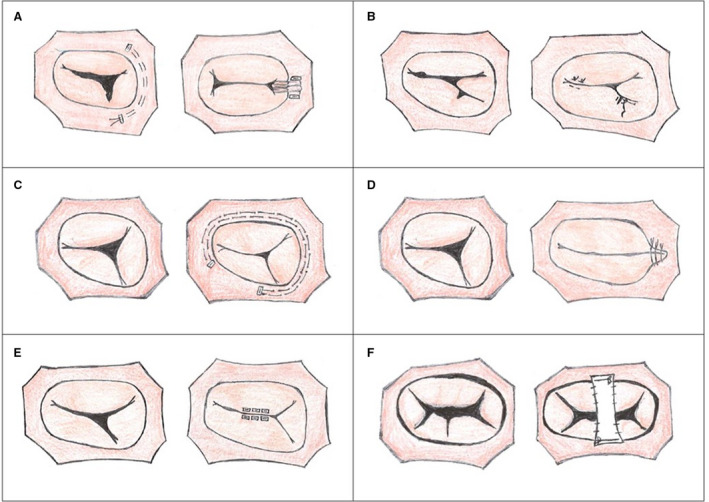
A, Tricuspid valve (TV), partial annuloplasty; (B) TV, commisuroplasty; (C) TV, DeVega annuloplasty; (D) TV, Kay annuloplasty; (E) TV, edge‐to‐edge repair; (F) Common atrioventricular valve, polytetrafluoroethylene bridge repair.
Valvuloplasty is another common method of repair. Edge‐to‐edge repair has been used successfully in both TV and common AVV repairs (Figure 3E).24, 35 For a TV, the free edge of the septal and anterior leaflets are sutured together to close the anteroseptal commissure or a suture is placed where the regurgitant jet arises, creating a double orifice. In a common AVV, the superior and inferior bridging leaflets are sutured together to create a double orifice, commonly referred to as an “Alfieri stitch”. If coaptation is particularly poor, the free edges can be completely sutured together to form a single orifice.36
In common AVVs, another repair strategy involves approximation of the leaflets with a polytetrafluoroethylene bridge or glutaraldehyde‐treated autologous pericardial strip to provide reinforcement for the valve (Figure 3F).24, 37 This strip is sutured to the annulus and on the anterior and inferior leaflets of the common AVV; the anterior and inferior leaflets are approximated and provides improved apposition of the bridging leaflets.24 Circular annuloplasties has also been found to be effective in reducing regurgitation in the common AVV.38 Leaflet apposition for clefts and defects results in improvement in AVVR.39, 40
Additional methods of repair include chordal shortening, elongation, and use of artificial chords. PM advancement and repositioning can address valve prolapse and papillotomy can address leaflet tethering. Repositioning of the anterior PM can alter ventricular geometry by increasing its spherical shape and improve valve tethering in HLHS.18, 19, 23 Leaflet size can be increased by incising and patching the restricted or deficient leaflet, thereby increasing the area of coaptation. Multiple techniques can be used simultaneously to address AVVR. Ultimately, the repair must be individualized to the patient's anatomy and the mechanism of regurgitation.
Surgical valve repair is preferred to valve replacement, as it preserves native valve structure and has demonstrated good early results.16, 19 However, AVV replacement may be necessary in severely dysplastic AVV where repair is unlikely to be adequate. Replacement is also more appealing in older patients or adults to improve the likelihood of long‐term freedom from regurgitation. AVV replacement is associated with postoperative in‐hospital mortality of almost 30% and 5‐year mortality rate of nearly 40%.41, 42 Heart block after valve replacement is also high at 20% to 40%.41 Lastly, AVV replacements have been shown to be associated with higher mortality in those aged <2 years compared with those >2 years.41 Poor outcomes may be partly because of patient selection since AVV replacement is often considered after multiple attempts to repair the valve.
Finally, the recent revolution in transcatheter AVV interventions for mitral and TV in adults with structurally normal hearts raises the possibility that these techniques or modifications thereof may be useful in some SV patients. Case reports of transcatheter valve interventions in Fontan patients, such as the use of the Mitraclip device, valve‐in‐valve or heterotopic valve implantation, have emerged.43, 44 Further studies are needed to define appropriate candidates and percutaneous techniques in this population.
Timing of Surgical Repair or Replacement
Significant AVVR and valve repair itself are known risk factors for ventricular dysfunction, FF, transplantation, or death after Fontan palliation.10, 11, 16, 45 Questions remain about which patients benefit from valve repair or replacement and also about the optimal timing of surgery. Without intervention, chronic AVVR can cause pulmonary vascular disease, arrhythmias, and heart failure. Few data exist to aid in this decision. Much of the literature on surgical timing and outcomes has included AVV interventions across all stages of palliation, with most interventions performed before or at the time of Fontan. We will discuss the considerations on timing of AVV repair from infancy to the Fontan procedure, and end with the adult Fontan population.
Young SV patients with AVVR pose a particularly difficult conundrum. Early AVV repair between initial palliation and Stage II is independently associated with increased mortality.10 Some argue that with moderate AVVR, valve intervention is not required, based on the expectation that AVVR usually improves with the volume unloading intrinsic to completing Stage II.46 To further support delaying intervention, repair at Stage II or earlier predicts recurrent AVVR and reintervention, and some have seen a reduction in regurgitation after the Fontan operation even without an accompanying AVV repair.10, 36, 47, 48 Younger age and smaller size are associated with re‐intervention and mortality, suggesting potential deleterious consequences if repair is needed early in the surgical palliative course.10, 16, 48, 49
Of particular interest are patients with severe AVVR before the Fontan procedure. AVV repair can be performed in isolation, before staged palliation, or at the time of the Fontan procedure.45, 47, 50 Isolated AVV repair before Fontan is beneficial in that it simplifies Fontan surgery and reduces cardiopulmonary bypass time.51 Early AVV intervention may ameliorate the postoperative risks at the time of Fontan and turn a patient who is a high‐risk Fontan candidate into a good Fontan candidate.10, 16 Earlier repair at a separate stage before Fontan may be associated with fewer postoperative complications, such as decreased intensive care unit stay, chylothorax, and ascites, though data are inconclusive.11, 50 In the short‐term, patients who underwent a prior initial AVV repair had improved AVVR at discharge from Fontan completion and were less likely to undergo AVV reoperations.11 This approach also allows the surgeon to evaluate and re‐repair the valve at the time of the Fontan as needed. However, it has also been shown that valve operation between Stage II and Fontan completion predicts late repair failure which is likely because of recurrence of AVVR after the Fontan.10
AVV repair at the time of Fontan may be a reasonable option in selected patients. One single center retrospective study demonstrated that patients undergoing concomitant AVV repair at time of Fontan did not have differences in postoperative complications and mortality compared with those who did not require AVV intervention.50 Comparing the approach of isolated AVV repair versus repair at time of Fontan has not been well studied because of possible selection bias, as symptomatic patients with more severe AVVR are likely to get an isolated repair rather than a repair during the Fontan operation.
Repair can also be performed after Fontan completion or during Fontan revision with postoperative improvement in the degree of regurgitation.51, 52, 53 However, those requiring late AVV repair have worse survival of 57% at 10 years and a higher risk of transplantation compared with Fontan patients who do not require AVV repair.45, 51 AVV repair in the adult Fontan population carries a high risk, with mortality up to 13% in the postoperative period and 33% at 5 years.51 The development of protein‐losing enteropathy has also been described to be a risk factor for mortality in valve repair in Fontan patients.51 On long‐term follow‐up, patients who underwent AVV repair after Fontan completion commonly developed arrhythmias (72%), protein‐losing enteropathy (20%), and required pacemaker placement (28%).51 Adult congenital heart disease patients have higher complication rates and mortality after cardiac surgery in general and AVV surgery in the adult Fontan is a particularly high‐risk surgery.54
Lastly, patients with common AVVs, especially those with heterotaxy, are more likely to require AVV reintervention and are challenging to manage.5 There are limited data on the optimal timing of repair in this subset of single ventricle patients. Sano et al describes their 18‐year experience with patients with heterotaxy syndrome who underwent AVV repair.24 The majority (87.5%) had a common AVV, which has been shown to be a risk factor for mortality.24, 55 The 4‐year freedom of AVV failure was ≈50% with an early mortality of 18% and estimated survival after repair of 54% at 10 years.9, 24, 56 This emphasizes the difficulty in repairing common AVV in this population and the persistent risk of recurrent AVVR and mortality.
The timing of AVV replacements is equally controversial and there is even less literature on this topic. Most are commonly performed between Stage II and Fontan or after the Fontan procedure. Patients who require AVV replacement before the Fontan often require a second valve replacement.42 The risks of early AVV replacement include thrombosis and patient prosthesis mismatch because of rapid somatic growth that leads to valve failure, especially in smaller children. AVV replacement at the time of Fontan completion has been also associated with increased mortality.2
Identifying patients with risk factors for recurrent AVVR and mortality after initial repair is essential to improve patient selection and outcomes, and the timing of repair can affect the recurrence rate of AVVR. Although the literature has provided some evidence, there have been variable results which are limited by the retrospective nature of most studies. AVVR intervention at a younger age and lower body weight, and in the older Fontan population have been shown on multiple occasions to be a risk factor for morbidity and mortality. Additionally, ventricular dysfunction, even in the setting of a successful valve repair, is associated with worse outcomes.19, 51 One study found that 75% of patients with moderate to severely diminished ventricular function on post repair imaging required a heart transplantation or had the outcome of death.16 Earlier AVV intervention, especially in setting of progressive ventricular dilation, could prevent further irreversible changes in ventricular geometry, systolic dysfunction, or need for repeat interventions.16 Valve and ventricular morphology are also important to consider; those with 2 separate AVVs and a dominant left ventricle have the highest freedom from repair failure.5, 56, 57 Optimal timing, precise identification of AVVR mechanism and appropriate repair are imperative, as early failed repair or recurrent moderate to severe postoperative regurgitation are predictors of late reoperations.10
It is important to mention that it remains unclear if there is a subcategory of patients who may benefit from transplant rather than AVV intervention. Given the challenge of successful TV or common AVV repair, some recommend cardiac transplantation for patients with systolic ventricular dysfunction and moderate or worse tricuspid regurgitation or common AVVR post Fontan.52 Transplantation may also be preferable to high‐risk surgical intervention among adults with significant Fontan‐related comorbidities.
Thus, management of AVVR in this population remains challenging. Our institutional practice is to repair the AVV during a planned palliative staged surgery, ie, during Stage I, Stage II, or Fontan surgeries, if there is moderate or greater AVVR in asymptomatic patients. In symptomatic patients, repair is typically performed in between staged palliations or after Fontan completion. For those patients with unfavorable hemodynamics before the Fontan, we will repair the AVV first and re‐evaluate candidacy for Fontan at a later stage. Table 3 summarizes the available data on timing of surgery across all stages of palliation.
Table 3.
Atrioventricular Valve Interventions and Outcomes
| First Author | Y | Number of AVV Interventions | Age, Y (IQR or Range) or Mean±SD | Failed AVV Interventions | Timing of Repair Failure From Original Intervention, Y (IQR) or Mean±SD | Risk Factors for Repair Failure or Re‐intervention |
|---|---|---|---|---|---|---|
| King et al5 | 2019 |
120 110 repairs 10 replacements |
3.4 (1.6–6.9) | 41% re‐interventions or recurrent moderate or greater AVVR | 3.4 (1.6–4.8) |
Common AVV and tricuspid valve Systemic right ventricle |
| Sughimoto et al42 | 2018 | 56 replacements | 2.1 (0.8–10.5) | 20% repeat AVV replacement | 0.25 (0.04–0.78) |
Tricuspid valve Valve replacement between Stage II and Fontan |
| King et al9 | 2017 |
28 24 repairs 4 replacements |
3.5 (2.0–7.0) | 67% re‐interventions or recurrent moderate or greater AVVR | 2.9±0.75 | NR |
| Laux et al48 | 2015 | 31 | 3.6 (range 0.1–36.5) | 19% reoperations | 2 (range 0.2–7.6) |
Failed first AVV repair Higher total number of surgeries Lower body mass index Male sex Need for early repair before Stage II |
| Kotani et al36 | 2012 | 58 | 0.62* (range 0.02–13.33) |
81% recurrent AVVR 21% repeat interventions |
1.75 (range 0.17–5.33) |
Recurrent moderate‐severe AVVR: repair at Stage II, CPB time Re‐intervention: valvuloplasty repair technique, CPB time, aortic cross‐clamp time, significant residual AVVR on intraoperative transesophageal echo, poor postoperative ventricular function |
| Sano et al24 | 2012 | 32 | 1.25 (range 0.07–36.92) | 38% repeat interventions for recurrent AVVR | NR |
Moderate or more pre‐operative AVVR Early failure of initial repair (moderate or more AVVR within 1 mo after operation) Younger age at initial AVV repair |
| Wong et al10 | 2012 |
76 66 repairs 5 replacements 5 valve closures |
1 (range 0.27–3.3) | 26% reoperations | 2.8 (1–5) |
Moderate or severe postoperative regurgitation Timing between Stage II and Fontan completion |
| Honjo et al16 | 2011 | 57 | 0.57 (range 0.025–17.4) | 17% recurrent AVVR requiring repeat repair or replacement | 1.75 (range 0.17–5.17) |
Younger age at repair Small body surface area Increased indexed AVV annular and ventricular dimensions Leaflet dysplasia Residual AVVR |
| Ando and Takahashi57 | 2011 |
103 93 repairs 10 replacements |
9.9±9.6 | 24% repeat interventions | NR | NR |
| Menon et al51 | 2010 |
61 38 repairs 23 replacements |
14 (range 3–41) | 13% of repairs | NR | NR |
| Nakata et al49 | 2010 | 65 | 0.80 (range 0–17) | 30% reoperations | 0.49 (0.02–3.93) |
Univariate analysis: concomitant systemic to pulmonary shunt, age <3 mo, body weight <4 kg, palliative stage Multivariate analysis: no risk factors |
| Mavroudis et al53 | 2005 | 15 | 20.3±8.4 | N/A | 12.0±4.7 | 15/80 patients required AVV repair at time of Fontan revision |
Description of studies reporting atrioventricular valve interventions across all stages of palliation, failed repairs, and risk factors for valve failure in single ventricle patients. AVV, atrioventricular valve; AAVR, atrioventricular valve regurgitation; CPB, cardiopulmonary bypass; IQR, interquartile range; and NR, not reported. *, mean.
Conclusions
AVVR is common in SV patients with Fontan circulation and is associated with worse outcomes including FF, arrhythmia, heart failure, and death. Mechanisms of AVVR are diverse, relating to both structural and functional abnormalities. These mechanisms are best assessed by a combination of imaging modalities. Optimal timing for AVVR intervention remains controversial and should be individualized; both early and late surgical repair have associated risk. In adult Fontan patients, AVV interventions are often performed in conjunction with other procedures and AVV replacement may be reasonable. Patients with significant AVVR who have already developed ventricular systolic dysfunction are at particularly high risk for adverse outcomes after AVV intervention and cardiac transplantation should be considered.
Disclosures
None.
Supporting information
Video S1
Video S2
Video S3
Video S4
Video S5
Video S6
Video S7
Acknowledgments
We would like to acknowledge Asmaa Minkara, MSIS for her help with creating animations for the figures.
(J Am Heart Assoc. 2020;9:e015737 DOI: 10.1161/JAHA.119.015737.)
For Disclosures, see page 10.
References
- 1. Gersony WM. Fontan operation after 3 decades: what we have learned. Circulation. 2008;117:13–15. [DOI] [PubMed] [Google Scholar]
- 2. Pundi KN, Johnson JN, Dearani JA, Pundi KN, Li Z, Hinck CA, Dahl SH, Cannon BC, O'Leary PW, Driscoll DJ, et al. 40‐year follow‐up after the Fontan operation: long‐term outcomes of 1,052 patients. J Am Coll Cardiol. 2015;66:1700–1710. [DOI] [PubMed] [Google Scholar]
- 3. Iyengar AJ, Winlaw DS, Galati JC, Wheaton GR, Gentles TL, Grigg LE, Justo RN, Radford DJ, Weintraub RG, Bullock A, et al. The extracardiac conduit Fontan procedure in Australia and New Zealand: hypoplastic left heart syndrome predicts worse early and late outcomes. Eur J Cardiothorac Surg. 2014;46:465–473. [DOI] [PubMed] [Google Scholar]
- 4. Diller GP, Kempny A, Alonso‐Gonzalez R, Swan L, Uebing A, Li W, Babu‐Narayan S, Wort SJ, Dimopoulos K, Gatzoulis MA. Survival prospects and circumstances of death in contemporary adult congenital heart disease patients under follow‐up at a large tertiary centre. Circulation. 2015;132:2118–2125. [DOI] [PubMed] [Google Scholar]
- 5. King G, Ayer J, Celermajer D, Zentner D, Justo R, Disney P, Zannino D, d'Udekem Y. Atrioventricular valve failure in Fontan palliation. J Am Coll Cardiol. 2019;73:810–822. [DOI] [PubMed] [Google Scholar]
- 6. Alsaied T, Bokma JP, Engel ME, Kuijpers JM, Hanke SP, Zuhlke L, Zhang B, Veldtman GR. Factors associated with long‐term mortality after Fontan procedures: a systematic review. Heart. 2017;103:104–110. [DOI] [PubMed] [Google Scholar]
- 7. d'Udekem Y, Xu MY, Galati JC, Lu S, Iyengar AJ, Konstantinov IE, Wheaton GR, Ramsay JM, Grigg LE, Millar J, et al. Predictors of survival after single‐ventricle palliation: the impact of right ventricular dominance. J Am Coll Cardiol. 2012;59:1178–1185. [DOI] [PubMed] [Google Scholar]
- 8. Anderson PA, Sleeper LA, Mahony L, Colan SD, Atz AM, Breitbart RE, Gersony WM, Gallagher D, Geva T, Margossian R, et al. Contemporary outcomes after the Fontan procedure: a Pediatric Heart Network multicenter study. J Am Coll Cardiol. 2008;52:85–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. King G, Gentles TL, Winlaw DS, Cordina R, Bullock A, Grigg LE, Alphonso N, Radford DJ, Zannino D, Buratto E, et al. Common atrioventricular valve failure during single ventricle palliation. Eur J Cardiothorac Surg. 2017;51:1037–1043. [DOI] [PubMed] [Google Scholar]
- 10. Wong DJ, Iyengar AJ, Wheaton GR, Ramsay JM, Grigg LE, Horton S, Konstantinov IE, Brizard CP, d'Udekem Y. Long‐term outcomes after atrioventricular valve operations in patients undergoing single‐ventricle palliation. Ann Thorac Surg. 2012;94:606–613. [DOI] [PubMed] [Google Scholar]
- 11. Ono M, Cleuziou J, Pabst von Ohain J, Beran E, Burri M, Strbad M, Hager A, Horer J, Schreiber C, Lange R. Atrioventricular valve regurgitation in patients undergoing total cavopulmonary connection: impact of valve morphology and underlying mechanisms on survival and reintervention. J Thorac Cardiovasc Surg. 2018;155:701–709.e706. [DOI] [PubMed] [Google Scholar]
- 12. Rychik J. The relentless effects of the Fontan paradox. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2016;19:37–43. [DOI] [PubMed] [Google Scholar]
- 13. Rathod RH, Prakash A, Kim YY, Germanakis IE, Powell AJ, Gauvreau K, Geva T. Cardiac magnetic resonance parameters predict transplantation‐free survival in patients with Fontan circulation. Circ Cardiovasc Imaging. 2014;7:502–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Giannakoulas G, Dimopoulos K, Yuksel S, Inuzuka R, Pijuan‐Domenech A, Hussain W, Tay EL, Gatzoulis MA, Wong T. Atrial tachyarrhythmias late after Fontan operation are related to increase in mortality and hospitalization. Int J Cardiol. 2012;157:221–226. [DOI] [PubMed] [Google Scholar]
- 15. Ghai A, Harris L, Harrison DA, Webb GD, Siu SC. Outcomes of late atrial tachyarrhythmias in adults after the Fontan operation. J Am Coll Cardiol. 2001;37:585–592. [DOI] [PubMed] [Google Scholar]
- 16. Honjo O, Atlin CR, Mertens L, Al‐Radi OO, Redington AN, Caldarone CA, Van Arsdell GS. Atrioventricular valve repair in patients with functional single‐ventricle physiology: impact of ventricular and valve function and morphology on survival and reintervention. J Thorac Cardiovasc Surg. 2011;142:326–335.e322. [DOI] [PubMed] [Google Scholar]
- 17. Takahashi K, Inage A, Rebeyka IM, Ross DB, Thompson RB, Mackie AS, Smallhorn JF. Real‐time 3‐dimensional echocardiography provides new insight into mechanisms of tricuspid valve regurgitation in patients with hypoplastic left heart syndrome. Circulation. 2009;120:1091–1098. [DOI] [PubMed] [Google Scholar]
- 18. Tsang VT, Raja SG. Tricuspid valve repair in single ventricle: timing and techniques. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2012;15:61–68. [DOI] [PubMed] [Google Scholar]
- 19. Ohye RG, Gomez CA, Goldberg CS, Graves HL, Devaney EJ, Bove EL. Tricuspid valve repair in hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 2004;127:465–472. [DOI] [PubMed] [Google Scholar]
- 20. Kutty S, Colen T, Thompson RB, Tham E, Li L, Vijarnsorn C, Polak A, Truong DT, Danford DA, Smallhorn JF, et al. Tricuspid regurgitation in hypoplastic left heart syndrome: mechanistic insights from 3‐dimensional echocardiography and relationship with outcomes. Circ Cardiovasc Imaging. 2014;7:765–772. [DOI] [PubMed] [Google Scholar]
- 21. Nguyen AV, Lasso A, Nam HH, Faerber J, Aly AH, Pouch AM, Scanlan AB, McGowan FX, Mercer‐Rosa L, Cohen MS, et al. Dynamic three‐dimensional geometry of the tricuspid valve annulus in hypoplastic left heart syndrome with a Fontan circulation. J Am Soc Echocardiogr. 2019;32:655–666.e613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nii M, Guerra V, Roman KS, Macgowan CK, Smallhorn JF. Three‐dimensional tricuspid annular function provides insight into the mechanisms of tricuspid valve regurgitation in classic hypoplastic left heart syndrome. J Am Soc Echocardiogr. 2006;19:391–402. [DOI] [PubMed] [Google Scholar]
- 23. Bautista‐Hernandez V, Brown DW, Loyola H, Myers PO, Borisuk M, del Nido PJ, Baird CW. Mechanisms of tricuspid regurgitation in patients with hypoplastic left heart syndrome undergoing tricuspid valvuloplasty. J Thorac Cardiovasc Surg. 2014;148:832–838; discussion 838–840. [DOI] [PubMed] [Google Scholar]
- 24. Sano S, Fujii Y, Arai S, Kasahara S, Tateishi A. Atrioventricular valve repair for patient with heterotaxy syndrome and a functional single ventricle. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2012;15:88–95. [DOI] [PubMed] [Google Scholar]
- 25. Vijarnsorn C, Khoo NS, Tham EB, Colen T, Rebeyka IM, Smallhorn JF. Increased common atrioventricular valve tenting is a risk factor for progression to severe regurgitation in patients with a single ventricle with unbalanced atrioventricular septal defect. J Thorac Cardiovasc Surg. 2014;148:2580–2588. [DOI] [PubMed] [Google Scholar]
- 26. Gewillig M, Brown SC. The Fontan circulation after 45 years: update in physiology. Heart. 2016;102:1081–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shah S, Jenkins T, Markowitz A, Gilkeson R, Rajiah P. Multimodal imaging of the tricuspid valve: normal appearance and pathological entities. Insights Imaging. 2016;7:649–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Buber J, Schwaegler RG, Mazor Dray E. Echocardiographic evaluation of univentricular physiology and cavopulmonary shunts. Echocardiography. 2019;36:1381–1390. [DOI] [PubMed] [Google Scholar]
- 29. Hahn RT. State‐of‐the‐art review of echocardiographic imaging in the evaluation and treatment of functional tricuspid regurgitation. Circ Cardiovasc Imaging. 2016;9:e005332. [DOI] [PubMed] [Google Scholar]
- 30. Bharucha T, Honjo O, Seller N, Atlin C, Redington A, Caldarone CA, van Arsdell G, Mertens L. Mechanisms of tricuspid valve regurgitation in hypoplastic left heart syndrome: a case‐matched echocardiographic‐surgical comparison study. Eur Heart J Cardiovasc Imaging. 2013;14:135–141. [DOI] [PubMed] [Google Scholar]
- 31. Mart CR, Eckhauser AW, Murri M, Su JT. A systematic method for using 3D echocardiography to evaluate tricuspid valve insufficiency in hypoplastic left heart syndrome. Ann Pediatr Cardiol. 2014;7:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hauser JA, Taylor AM, Pandya B. How to image the adult patient with Fontan circulation. Circ Cardiovasc Imaging. 2017;10:e004273. [DOI] [PubMed] [Google Scholar]
- 33. Boyd JH, Edelman JJB, Scoville DH, Woo YJ. Tricuspid leaflet repair: innovative solutions. Ann Cardiothorac Surg. 2017;6:248–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Belluschi I, Del Forno B, Lapenna E, Nisi T, Iaci G, Ferrara D, Castiglioni A, Alfieri O, De Bonis M. Surgical techniques for tricuspid valve disease. Front Cardiovasc Med. 2018;5:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ando M, Takahashi Y. Edge‐to‐edge repair of common atrioventricular or tricuspid valve in patients with functionally single ventricle. Ann Thorac Surg. 2007;84:1571–1576; discussion 1576–1577 [DOI] [PubMed] [Google Scholar]
- 36. Kotani Y, Chetan D, Atlin CR, Mertens LL, Jegatheeswaran A, Caldarone CA, Van Arsdell GS, Honjo O. Longevity and durability of atrioventricular valve repair in single‐ventricle patients. Ann Thorac Surg. 2012;94:2061–2069. [DOI] [PubMed] [Google Scholar]
- 37. Sughimoto K, Konstantinov IE, Brizard CP, d'Udekem Y. Polytetrafluoroethylene bridge for atrioventricular valve repair in single‐ventricle palliation. J Thorac Cardiovasc Surg. 2015;149:641–643. [DOI] [PubMed] [Google Scholar]
- 38. Imai Y, Takanashi Y, Hoshino S, Terada M, Aoki M, Ohta J. Modified Fontan procedure in ninety‐nine cases of atrioventricular valve regurgitation. J Thorac Cardiovasc Surg. 1997;113:262–268; discussion 269 [DOI] [PubMed] [Google Scholar]
- 39. Ota N, Fujimoto Y, Hirose K, Tosaka Y, Nakata T, Ide Y, Sakamoto K. Improving results of atrioventricular valve repair in challenging patients with heterotaxy syndrome. Cardiol Young. 2010;20:60–65. [DOI] [PubMed] [Google Scholar]
- 40. Acharya G, Wilsgaard T, Berntsen GK, Maltau JM, Kiserud T. Reference ranges for serial measurements of umbilical artery Doppler indices in the second half of pregnancy. Am J Obstet Gynecol. 2005;192:937–944. [DOI] [PubMed] [Google Scholar]
- 41. Mahle WT, Gaynor JW, Spray TL. Atrioventricular valve replacement in patients with a single ventricle. Ann Thorac Surg. 2001;72:182–186. [DOI] [PubMed] [Google Scholar]
- 42. Sughimoto K, Hirata Y, Hirahara N, Miyata H, Suzuki T, Murakami A, Miyaji K, Takamoto S. Mid‐term result of atrioventricular valve replacement in patients with a single ventricle. Interact Cardiovasc Thorac Surg. 2018;27:895–900. [DOI] [PubMed] [Google Scholar]
- 43. Kuo CC, Shih JY, Wu NC, Cheng BC. Transcatheter atrioventricular valve‐in‐valve implantation for Fontan circulation and dextrocardia. Interact Cardiovasc Thorac Surg. 2019;28:496–498. [DOI] [PubMed] [Google Scholar]
- 44. Ghobrial J, Aboulhosn J. Transcatheter valve replacement in congenital heart disease: the present and the future. Heart. 2018;104:1629–1636. [DOI] [PubMed] [Google Scholar]
- 45. Liu VJ, Yong MS, d'Udekem Y, Weintraub RG, Praporski S, Brizard CP, Konstantinov IE. Outcomes of atrioventricular valve operation in patients with Fontan circulation. Ann Thorac Surg. 2015;99:1632–1638. [DOI] [PubMed] [Google Scholar]
- 46. Mahle WT, Cohen MS, Spray TL, Rychik J. Atrioventricular valve regurgitation in patients with single ventricle: impact of the bidirectional cavopulmonary anastomosis. Ann Thorac Surg. 2001;72:831–835. [DOI] [PubMed] [Google Scholar]
- 47. Sallehuddin A, Bulbul Z, Otero F, Al Dhafiri K, Al‐Halees Z. Repair of atrioventricular valve regurgitation in the modified Fontan operation. Eur J Cardiothorac Surg. 2004;26:54–59. [DOI] [PubMed] [Google Scholar]
- 48. Laux D, Vergnat M, Lambert V, Gouton M, Ly M, Peyre M, Roussin R, Belli E. Atrio‐ventricular valve regurgitation in univentricular hearts: outcomes after repair. Interact Cardiovasc Thorac Surg. 2015;20:622–629. [DOI] [PubMed] [Google Scholar]
- 49. Nakata T, Fujimoto Y, Hirose K, Tosaka Y, Ide Y, Tachi M, Sakamoto K. Atrioventricular valve repair in patients with functional single ventricle. J Thorac Cardiovasc Surg. 2010;140:514–521. [DOI] [PubMed] [Google Scholar]
- 50. Kerendi F, Kramer ZB, Mahle WT, Kogon BE, Kanter KR, Kirshbom PM. Perioperative risks and outcomes of atrioventricular valve surgery in conjunction with Fontan procedure. Ann Thorac Surg. 2009;87:1484–1488; discussion 1488–1489 [DOI] [PubMed] [Google Scholar]
- 51. Menon SC, Dearani JA, Cetta F. Long‐term outcome after atrioventricular valve surgery following modified Fontan operation. Cardiol Young. 2011;21:83–88. [DOI] [PubMed] [Google Scholar]
- 52. Mavroudis C, Deal BJ, Backer CL, Stewart RD, Franklin WH, Tsao S, Ward KM, DeFreitas RA. J. Maxwell Chamberlain Memorial Paper for congenital heart surgery. 111 fontan conversions with arrhythmia surgery: surgical lessons and outcomes. Ann Thorac Surg. 2007;84:1457–1465; discussion 1465‐1456 [DOI] [PubMed] [Google Scholar]
- 53. Mavroudis C, Stewart RD, Backer CL, Deal BJ, Young L, Franklin WH. Atrioventricular valve procedures with repeat Fontan operations: influence of valve pathology, ventricular function, and arrhythmias on outcome. Ann Thorac Surg. 2005;80:29–36. [DOI] [PubMed] [Google Scholar]
- 54. Setton M, He W, Benavidez OJ. Morbidity during adult congenital heart surgery admissions. Pediatr Cardiol. 2019;40:987–993. [DOI] [PubMed] [Google Scholar]
- 55. d'Udekem Y, Iyengar AJ, Cochrane AD, Grigg LE, Ramsay JM, Wheaton GR, Penny DJ, Brizard CP. The Fontan procedure: contemporary techniques have improved long‐term outcomes. Circulation. 2007;116:I157–I164. [DOI] [PubMed] [Google Scholar]
- 56. Buratto E, Ye XT, King G, Shi WY, Weintraub RG, d'Udekem Y, Brizard CP, Konstantinov IE. Long‐term outcomes of single‐ventricle palliation for unbalanced atrioventricular septal defects: Fontan survivors do better than previously thought. J Thorac Cardiovasc Surg. 2017;153:430–438. [DOI] [PubMed] [Google Scholar]
- 57. Ando M, Takahashi Y. Long‐term functional analysis of the atrioventricular valve in patients undergoing single ventricle palliation. Ann Thorac Surg. 2011;92:1767–1773; discussion 1773 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1
Video S2
Video S3
Video S4
Video S5
Video S6
Video S7


