Abstract
Background
Patient selection and outcomes for percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG) have changed over the past decade. However, there is limited information on outcomes for both revascularization strategies in the same population. The study evaluated temporal changes in risk profile, procedural characteristics, and clinical outcomes for PCI‐ and CABG‐treated patients.
Methods and Results
We analyzed all PCI and isolated CABG between 2005 and 2017 in nonfederal hospitals in Washington State. Descriptive analysis was performed to evaluate temporal changes in risk profile and, risk‐adjusted in‐hospital mortality. Over the study period, 178 474 PCI and 36 592 CABG procedures were performed. PCI and CABG volume decreased by 2.9% and 22.6%, respectively. Compared with 2005–2009, patients receiving either form of revascularization between 2014 and 2017 had a higher prevalence of comorbidities including diabetes mellitus and hypertension and dialysis. Presentation with ST‐segment–elevation myocardial infarction (17% versus 20%) and cardiogenic shock (2.4% versus 3.4%) increased for patients with PCI compared with CABG. Conversely, clinical acuity decreased for patients receiving CABG over the study period. From 2005 to 2017, mean National Cardiovascular Data Registry CathPCI mortality score increased for patients treated with PCI (20.1 versus 22.4, P<0.0001) and decreased for patients treated with CABG (18.8 versus 17.8, P<0.0001). Adjusted observed/expected in‐hospital mortality ratio increased for PCI (0.98 versus 1.19, P<0.0001) but decreased for CABG (1.21 versus 0.74, P<0.0001) over the study period.
Conclusions
Clinical acuity increased for patients treated with PCI rather than CABG. This resulted in an increase in adjusted observed/expected mortality ratio for patients undergoing PCI and a decrease for CABG. These shifts may reflect an increased use of PCI instead of CABG for patients considered to be at high surgical risk.
Keywords: bypass surgery, outcomes, percutaneous coronary intervention
Subject Categories: Catheter-Based Coronary and Valvular Interventions, Cardiovascular Surgery, Risk Factors
Nonstandard Abbreviations and Acronyms
- CABG
coronary artery bypass grafting
- COAP
Cardiac Care Outcomes Assessment Program
- CTO
chronic total occlusion
- LM
left main
- MI
myocardial infarction
- NCDR
National Cardiovascular Data Registry
- NSTEMI
non–ST‐segment–elevation myocardial infarction
- NYHA
New York Heart Association
- O/E
observed to expected
- PCI
percutaneous coronary intervention
- PAD
peripheral artery disease
- STEMI
ST‐segment–elevation myocardial infarction
- STROBE
Strengthening the Reporting of Observational Studies in Epidemiology
- STS
Society of Thoracic Surgery
Clinical Perspective
What Is New?
In an analysis from the Cardiac Care Outcomes Assessment Program from Washington State, 178 474 patients undergoing percutaneous coronary intervention and 36 592 patients undergoing coronary artery bypass grafting between 2005 to 2017 were evaluated.
While medical comorbidities increased for both groups, the percutaneous coronary intervention cohort demonstrated an increase in both clinical acuity (ST‐segment–elevation myocardial infarction, cardiogenic shock) and procedural complexity (left main, atherectomy, chronic total occlusion).
Risk‐adjusted in‐hospital mortality increased for percutaneous coronary intervention and decreased for coronary artery bypass grafting.
What Are the Clinical Implications?
Patient selection for coronary revascularization has shifted over the past decade with higher risk patients undergoing percutaneous coronary intervention with an accompanying increase in risk‐adjusted mortality.
As a result of improved risk factor control, declining rates of acute myocardial infarction, and similar clinical outcomes between initial medical and invasive management for stable coronary disease, rates of coronary revascularization have decreased.1, 2, 3 In addition, the proportion of elderly patients and prevalence of medical comorbidities among patients treated with coronary revascularization have increased in the contemporary era.4, 5
Concurrent innovation in percutaneous coronary intervention (PCI) has enabled treatment of complex coronary lesions in high‐risk patients.6 High‐risk PCI has increased as a result of trials demonstrating the safety of unprotected left main (LM) stenting, atherectomy devices for treatment of calcified lesions, percutaneous techniques for chronic total occlusion (CTO) PCI, and use of temporary mechanical circulatory support.7, 8, 9, 10, 11 The influence of these shifts on the risk profiles and clinical outcomes of patients undergoing PCI or coronary artery bypass grafting (CABG) are not well understood. Previous studies have either evaluated revascularization trends for PCI or CABG in isolation5, 12, 13 or studied an older cohort of patients.3, 14, 15, 16, 17
The objective of our study was to evaluate temporal trends in the risk profiles and clinical outcomes for a contemporary cohort of patients undergoing PCI or CABG in nonfederal hospitals in Washington State. The COAP (Cardiac Care Outcomes Assessment Program) registry captures all PCI and cardiac surgery procedures performed in nonfederal hospitals in Washington State, providing a unique opportunity to examine shifts in revascularization strategies in the same population. We hypothesized that the risk profile and in‐hospital mortality will increase for both PCI and CABG. Furthermore, procedural complexity was expected to increase for PCI.
Methods
Data Source
Deidentified data can be made available for other investigators by reasonable request. We used the COAP database, which captures all PCI and cardiac surgery procedures performed in nonfederal hospitals in Washington State. The database has been previously described.18 Briefly, COAP is a physician‐lead initiative with universal participation from all nonfederal hospitals in Washington State. Participating sites provide clinical information on consecutive PCI and cardiac surgeries for the purposes of quality improvement. Deidentified hospital‐level quality metrics are discussed at monthly meetings to minimize care variation, initiate quality improvement initiatives, and share best practices. All nonfederal PCI‐capable hospitals (33) and CABG hospitals (17) are currently compliant with data submission. The quality of the data is maintained through routine audits.
From 2005 to 2007, COAP collected data for CABG using its own case report form. Beginning in 2008, the Society of Thoracic Surgery (STS) data collection form was used for CABG. Between 2005 and 2008, COAP used its own data collection form for PCI and subsequently transitioned to the American College of Cardiology NCDR (National Cardiovascular Data Registry) CathPCI data collection form in 2009. In addition to demographic information, clinical variables, procedural characteristics, and outcomes were captured.
Patient Population
We included all patients older than 18 years who were treated with PCI or isolated CABG between May 9, 2005, and December 31, 2017. Patients who received concurrent CABG at the time of valve surgery or had missing clinical variables (5.6% for PCI and 4.1% for CABG) were excluded from the analysis.
Statistical Analysis
We evaluated baseline demographics and clinical presentation over 3 time periods: (1) 2005–2009, (2) 2010–2013, and (3) 2014–2017. Three time periods of approximately equal length were selected to compare baseline variables to evaluate secular trends; the remainder of the analysis evaluated annual trends. Because of the large sample size, we calculated the standardized mean difference between the first and last time period for baseline variables. Standardized mean difference estimates the effect size and is calculated as the difference between groups divided by the standard deviation.
Annual volumes for PCI and CABG were analyzed from 2005 to 2017. Linear trend statistics were utilized to analyze significance. The analysis further stratified the indication for the invasive procedure as ST‐segment–elevation myocardial infarction (STEMI), non‐STEMI (NSTEMI), and elective. Temporal trends were evaluated for a subpopulation of patients with diabetes mellitus who had multivessel coronary artery disease undergoing elective revascularization.
We calculated the NCDR CathPCI mortality score for PCI‐ and CABG‐treated patients. The model was derived from 181 775 PCI procedures and subsequently validated with prospective cohorts with excellent discriminatory function (c‐statistic 0.926) for 30‐day mortality.19 Variables included in the model were age, presence of cardiogenic shock, prior congestive heart failure, peripheral artery disease, chronic lung disease, glomerular filtration rate, New York Heart Association class IV symptoms, presence of STEMI, and PCI status. We used all variables in the risk score calculation except New York Heart Association class IV, which was not collected for the years 2005 through 2007 for CABG and 2005 through 2008 for PCI. We stratified the NCDR CathPCI mortality score into tertiles of low risk (<20), intermediate risk (20–40), and high risk (>40). These tertiles reflect the distribution of the risk score in our population. In addition, the difference in the mean NCDR CathPCI mortality score from 2005 to 2017 was calculated for the CABG and PCI groups. The absolute difference was then compared with a 95% CI.
Linear trends in PCI procedural characteristics were evaluated with a focus on atherectomy use, LM interventions, and treatment of CTOs. The data collection only captured CTO PCIs following 2010.
Finally, linear trends for in‐hospital mortality were analyzed for the CABG and PCI groups and expressed as an observed to expected (O/E) ratio. Statistical analyses were performed with the chi‐square test for linear trend. The expected number of deaths was calculated from the logistic function adjusting for NCDR CathPCI mortality risk score for the PCI and CABG groups. For the PCI group, we performed additional adjustment for LM intervention and atherectomy use. A sensitivity analysis was performed by calculating the O/E mortality for patients with CABG using the STS risk score between 2008 and 2016. The STS risk score was not reliably available for other time periods and, therefore, not utilized for the primary analysis. We examined the association between year and the ratio of O/E deaths with linear regression and used the Durbin‐Watson statistic to check for autocorrelation. SPSS version 19.0 (IBM) was used for data analysis.
All data are reported in accordance with STROBE Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. The study was exempt from institutional review board approval because the analysis was conducted for quality improvement and did not involve human participants.
Results
Our study evaluated patients treated with PCI and CABG between 2005 and 2017. In total, 178 474 patients treated with PCI and 36 592 treated with CABG were included in the analysis. PCI volume decreased by 2.9% over the entire study period, with a 19.1% decrease from 2005 to 2014 followed by a subsequent increase (Figure 1A). Annual CABG volume decreased consistently by 22.6% from 2005 to 2017 (Figure 1B), with a relative 39.0% reduction in elective and 60.7% reduction in STEMI indications. For a subpopulation of patients with diabetes mellitus and multivessel coronary artery disease undergoing elective revascularization, utilization of CABG (n=9429) and PCI (n=14 031) was evaluated over the study period. Total revascularization volume decreased from 2005 to 2017 for both strategies (2433 to 1581, P<0.0001), driven by reduction in PCI volume (decrease from 1624 to 856, P=0.0003). CABG volume did not change substantially over the study period. Proportional utilization of PCI decreased from 66.7% to 54.1% (P<0.0001) and CABG increased from 33.3% to 45.9% (P<0.0001) over the study period.
Figure 1. Annual percutaneous coronary intervention (PCI) volume (A) and annual coronary artery bypass grafting (CABG) volume (B).
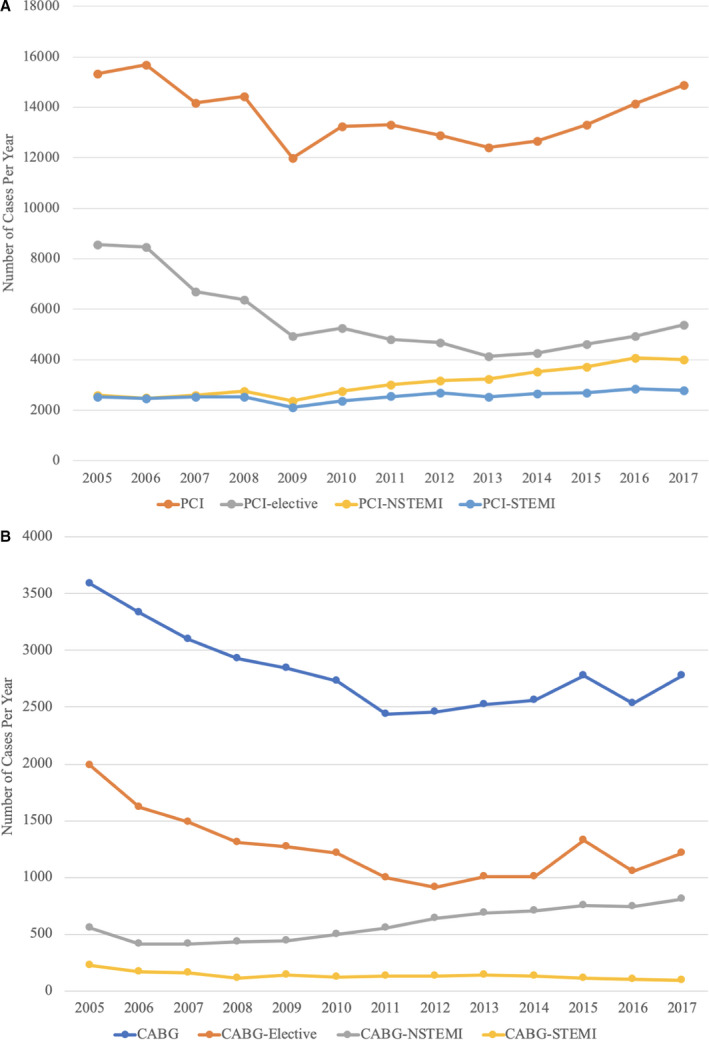
NSTEMI indicates non–ST‐segment–elevation myocardial infarction; STEMI, ST‐segment–elevation myocardial infarction.
Table 1 summarizes the clinical characteristics for PCI over 3 time periods 2005–2009, 2010–2013, and 2014–2017. Compared with 2005–2009, a higher proportion of patients undergoing PCI between 2014 and 2017 were elderly patients older than 80 years (12% versus 14%) and had increased medical comorbidities such as diabetes mellitus (30% versus 35%,), hypertension (74% versus 78%), requirement of dialysis (1.4% versus 2.6%), cerebrovascular disease (11% versus 13%), congestive heart failure (10% versus 15%), and chronic kidney disease, defined as preprocedure creatinine >2.0 mg/dL (3.7% versus 5.0%). History of previous CABG decreased over the study period (18% versus 16%). Clinical acuity for patients undergoing PCI also increased over the study period, with higher prevalance of STEMI (17% versus 20%), NSTEMI (18% versus 28%), cardiogenic shock (2.4% versus 3.4%), emergent or urgent procedures (50% versus 64%), and use of intra‐aortic balloon pump (0.9% versus 2.4%).
Table 1.
Demographic and Clinical Characteristics for PCI
| Variable | Time Periods | Mean Standardized Difference (95% CI) | |||
|---|---|---|---|---|---|
| All Years | June 9, 2005, to October 12, 2009 | October 13, 2009, to December 31, 2013 | January 1, 2014, to December 31, 2017 | ||
| PCI, No. | 178 474 | 71 590 | 51 880 | 55 004 | |
| Demographic variables and risk factors | |||||
| Age, mean±SD, y | 66±12 | 65±12 | 66±12 | 66±12 | −0.109 (−0.120 to −0.098) |
| Age ≥80 y | 23 381 (13) | 8911 (12) | 6960 (13) | 7510 (14) | −0.036 (−0.947 to −0.025) |
| Women | 50 965 (29) | 20 924 (29) | 14 711 (28) | 15 330 (28) | 0.030 (0.019–0.041) |
| Race | 50 965 (29) | ||||
| White | 157 609 (88) | 62 119 (87) | 46 613 (90) | 48 877 (89) | −0.064 (−0.075 to −0.053) |
| Nonwhite | 16 637 (10) | 7316 (10) | 4162 (8) | 5159 (9) | 0.028 (0.017–0.039) |
| Unknown | 4228 (2.4) | 2155 (3.0) | 1105 (2.1) | 968 (1.8) | 0.081 (0.070–0.092) |
| Hypertension | 135 119 (76) | 52 644 (74) | 39 822 (77) | 42 653 (78) | −0.092 (−0.104 to −0.081) |
| Diabetes mellitus | 57 747 (32) | 21 365 (30) | 16 960 (33) | 19 422 (35) | −0.117 (−0.128 to −0.106) |
| Dialysis | 3371 (1.9) | 977 (1.4) | 955 (1.8) | 1439 (2.6) | −0.091 (−0.102 to −0.080) |
| Cerebrovascular disease | 21 234 (12) | 8051 (11) | 6095 (12) | 7088 (13) | −0.050 (−0.061 to −0.039) |
| COPD | 23 573 (13) | 10 058 (14) | 6389 (12) | 7306 (13) | 0.022 (0.011–0.034) |
| PAD | 18 859 (11) | 7411 (10) | 5278 (10) | 6170 (11) | −0.028 (−0.039 to −0.017) |
| CABG | 29 723 (17) | 12 580 (18) | 8656 (17) | 8487 (15) | 0.058 (0.046–0.069) |
| Valve surgery | 2904 (1.6) | 795 (1.1) | 907 (1.7) | 1202 (2.2) | −0.086 (−0.097 to −0.075) |
| PCI | 65 691 (37) | 25 674 (36) | 19 696 (38) | 20 321 (37) | −0.022 (−0.033 to −0.011) |
| CHF | 20 522 (12) | 7061 (10) | 5307 (10) | 8154 (15) | −0.153 (−0.164 to −0.142) |
| NYHA class IV | 3085 (2.8) | 80 (2.2) | 1142 (2.2) | 1864 (3.4) | −0.064 (−0.098 to −0.030) |
| Creatinine >2.0 mg/dL | 7138 (4.2) | 2548 (3.7) | 1962 (4.2) | 2628 (5.0) | −0.064 (−0.075 to −0.052) |
| Clinical presentation | |||||
| Cardiogenic shock | 4883 (2.7) | 1700 (2.4) | 1339 (2.6) | 1844 (3.4) | −0.059 (−0.070 to −0.048) |
| Cardiac arrest | 5170 (3.2) | 1812 (3.2) | 1635 (3.2) | 1723 (3.1) | 0.005 (−0.007 to 0.017) |
| Balloon pump | 3177 (1.8) | 678 (0.9) | 1186 (2.3) | 1313 (2.4) | −0.023 (−0.057 to 0.011) |
| Cardiac presentation | |||||
| No angina | 11 524 (6) | 6589 (9) | 3048 (6) | 1887 (3) | 0.233 (0.222–0.244) |
| Atypical CP | 5196 (2.9) | 3578 (5) | 857 (1.7) | 761 (1.4) | 0.200 (0.189–0.211) |
| Stable angina | 30 443 (17) | 13 710 (19) | 7902 (15) | 8831 (16) | 0.081 (0.070–0.092) |
| Unstable angina | 57 333 (32) | 22 444 (31) | 17 728 (34) | 17 161 (31) | 0.004 (−0.007, 0.015) |
| NSTEMI | 40 307 (23) | 12 802 (18) | 12 185 (24) | 15 320 (28) | −0.241 (−0.252 to −0.230) |
| STEMI | 12 189 (17) | 12 189 (17) | 10 148 (20) | 10 993 (20) | −0.076 (−0.087 to −0.065) |
| Diseased vessels, No. | |||||
| 0 | 4384 (2.5) | 2568 (3.6) | 850 (1.6) | 966 (1.8) | 0.111 (0.100–0.122) |
| 1 | 88 983 (50) | 36 348 (51) | 25 715 (50) | 26 920 (49) | 0.037 (0.026–0.048) |
| 2 | 49 195 (28) | 18 955 (26) | 14 567 (28) | 15 673 (28) | −0.045 (−0.056 to −0.034) |
| 3 | 35 912 (20) | 13 719 (19) | 10 748 (21) | 11 445 (21) | −0.041 (−0.052 to −0.030) |
| Ejection fraction | 54+13 | 55±13 | 54±13 | 52±13 | 0.165 (0.151–0.179) |
| Procedure priority | |||||
| Elective | 68 410 (42) | 30 106 (50) | 15 852 (39) | 22 452 (35) | 0.286 (0.275–0.297) |
| Urgent | 64 805 (36) | 20 865 (29) | 20 989 (40) | 22 951 (42) | −0.267 (−0.279 to −0.256) |
| Emergent | 39 275 (22) | 15 319 (21) | 11 649 (22) | 12 307 (22) | −0.024 (−0.035 to −0.013) |
| Salvage | 1093 (0.6) | 314 (0.4) | 318 (0.6) | 461 (0.8) | −0.051 (−0.062 to −0.040) |
Values are expressed as number (percentage) unless otherwise indicated.
CABG indicates coronary artery bypass grafting; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CP, chest pain; NSTEMI, non–ST‐segment–elevation myocardial infarction; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; PAD, peripheral artery disease; and STEMI, ST‐segment–elevation myocardial infarction.
Compared with 2005–2009, CABG‐treated patients between 2014 and 2017 also demonstrated higher prevalance of diabetes mellitus (37% versus 45%), hypertension (80% versus 85%), requirement of dialysis (1.6% versus 2.5%), cerebrovascular disease (14% versus 21%), and previous PCI (23% versus 29%) (Table 2). However, there was a decrease in the proportion of elderly patients older than 80 years (9% versus 7%), prevalence of chronic kidney disease (4.7–4.1%), presentation with cardiogenic shock (2.4% versus 1.3%), and STEMI (5.0% versus 4.3%). Re‐do CABG also decreased (4.0% versus 1.5%).
Table 2.
Demographic and Clinical Characteristics for CABG
| Variable | Time Periods | Mean Standardized Difference (95% CI) | |||
|---|---|---|---|---|---|
| All Years | June 9, 2005, to October 12, 2009 | October 13, 2009, to December 31, 2013 | January 1, 2014, to December 31, 17 | ||
| CABG volume | 36 592 | 15 793 | 10 151 | 10 648 | |
| Demographic variables and risk factors | |||||
| Age, mean±SD, y | 66±11 | 66+11 | 65+10 | 66±10 | 0.004 (0.021–0.029) |
| Age ≥80 y | 2981 (8) | 1350 (9) | 856 (8) | 775 (7) | 0.062 (0.037–0.086) |
| Women | 8198 (22) | 3649 (23) | 2357 (23) | 2192 (21) | 0.061 (0.036–0.085) |
| Race | |||||
| White | 32 294 (88) | 14 210 (90) | 9045 (89) | 9078 (85) | 0.157 (0.132–0.181) |
| Nonwhite | 3775 (11) | 1428 (9) | 1055 (10) | 1292 (12) | −0.102 (−0.126 to −0.077) |
| Unknown | 523 (1.4) | 155 (1.0) | 51 (0.5) | 375 (3.0) | −0.151 (−0.176 to −0.127) |
| Hypertension | 30 299 (83) | 12 570 (80) | 8651 (85) | 9078 (85) | −0.147 (−0.172 to −0.077) |
| Diabetes mellitus | 14 739 (40) | 5812 (37) | 4156 (41) | 4771 (45) | −0.164 (−0.188 to −0.139) |
| Dialysis | 742 (2.0) | 256 (1.6) | 220 (2.2) | 266 (2.5) | −0.060 (−0.088 to −0.038) |
| Cerebrovascular disease | 5822 (16) | 2153 (14) | 1451 (14) | 2218 (21) | −0.195 (−0.219 to −0.170) |
| COPD | 6740 (18) | 2801 (18) | 2132 (21) | 1537 (14) | 0.089 (0.065–0.114) |
| PAD | 4886 (13) | 22 358 (15) | 1283 (13) | 1245 (12) | 0.094 (0.070–0.119) |
| CABG | 1039 (2.8) | 632 (4.0) | 244 (2.4) | 163 (1.5) | 0.145 (0.120–0.170) |
| Valve surgery | 115 (0.3) | 54 (0.3) | 26 (0.3) | 35 (0.3) | 0.002 (−0.022 to 0.027) |
| PCI | 9399 (26) | 3602 (23) | 2754 (27) | 3043 (29) | −0.133 (−0.158 to −0.109) |
| MI | 17 855 (49) | 6402 (41) | 5473 (54) | 5980 (56) | −0.317 (−0.341 to −0.292) |
| CHF | 5845 (16) | 2051 (13) | 1709 (17) | 2085 (20) | −0.182 (−0.207 to 0.157) |
| NYHA class IV | 896 (3.4) | 189 (3.3) | 357 (3.5) | 350 (3.3) | −0.001 (−0.003 to 0.031) |
| Creatinine >2.0 mg/dL | 1614 (4.4) | 741 (4.7) | 437 (4.3) | 436 (4.1) | 0.030 (0.006–0.055) |
| Clinical presentation | |||||
| Cardiogenic shock | 764 (2.1) | 379 (2.4) | 244 (2.5) | 141 (1.3) | 0.078 (0.053–0.102) |
| Balloon pump | 2392 (6.5) | 1014 (6.4) | 745 (7.3) | 633 (6.2) | 0.053 (0.021–0.085) |
| Cardiac presentation | |||||
| No angina | 2418 (7) | 1791 (11) | 253 (2.5) | 374 (4) | 0.281 (0.256–0.306) |
| Atypical CP | 1109 (3) | 438 (2.8) | 229 (2.3) | 442 (4) | −0.086 (−0.111 to −0.061) |
| Stable angina | 9162 (25) | 4602 (29) | 2376 (24) | 2184 (21) | 0.178 (0.153 to −0.203) |
| Unstable angina | 13 850 (38) | 5733 (36) | 4376 (43) | 3741 (37) | −0.006 (−0.031 to 0.019) |
| NSTEMI | 7638 (21) | 2253 (14) | 2378 (24) | 3007 (30) | −0.385 (−0.410 to −0.359) |
| STEMI | 1759 (5) | 806 (5) | 515 (5) | 438 (4.3) | 0.039 (0.014–0.063) |
| Missing MI type | 110 (0.3) | 110 (0.7) | 0 (0.0) | 0 (0.0) | −0.287 (−0.312 to −0.263) |
| Diseased vessels, No. | |||||
| 0 | 482 (1.3) | 435 (2.8) | 39 (0.4) | 8 (0.1) | 0.201 (0.186–0.235) |
| 1 | 2967 (8.1) | 2157 (13.7) | 404 (4.0) | 402 (3.8) | 0.340 (0.316–0.365) |
| 2 | 8148 (22.3) | 3780 (24.1) | 2173 (21.4) | 2195 (20.5) | 0.081 (0.056–0.105) |
| 3 | 24 884 (68.2) | 9329 (59.4) | 7533 (74.2) | 8002 (75.4) | −0.343 (−0.368 to −0.318) |
| Ejection fraction | 53±13 | 53±14 | 53±13 | 53±12 | −0.113 (−0.036 to −0.014) |
| Procedure priority | |||||
| Elective | 16 401 (45) | 7691 (49) | 4131 (41) | 4599 (43) | 0.111 (0.087–0.136) |
| Urgent | 18 539 (51) | 7311 (46) | 5533 (54) | 5696 (54) | −0.141 (−0.166 to −0.116) |
| Emergent | 1518 (4) | 704 (4) | 472 (5) | 342 (3) | 0.065 (0.040–0.089) |
| Salvage | 77 (0.2) | 51 (0.3) | 15 (0.1) | 11 (0.1) | 0.046 (0.021–0.070) |
Values are expressed as number (percentage) unless otherwise indicated.
CABG indicates coronary artery bypass grafting; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CP, chest pain; MI, myocardial infarction; NSTEMI, non–ST‐segment–elevation myocardial infarction; NYHA, New York Heart Association; PCI, percutaneous; PAD, peripheral artery disease; and STEMI, ST‐segment–elevation myocardial infarction.
Annual risk profiles of patients treated with PCI and CABG stratified by tertiles of NCDR CathPCI mortality score are displayed in Figure 2A and 2B. Low risk was defined as an NCDR CathPCI score <20, medium risk was defined as a score of 20 to 40, and high risk was defined as a score >40. Over the study period, the proportion of patients undergoing PCI who were considered at low risk decreased from 54.4% to 46.0%, while those considered at high risk increased from 4.8% to 6.2% (Figure 2). Conversely, the proportion of patients undergoing CABG who were considered at low risk increased from 62.3% to 63.3%, while those considered at high risk decreased from 3.0% to 1.6% (Table S1). The mean NCDR CathPCI mortality score increased for PCI by 2.3 points (95% CI, 2.0–2.6) from 2005 to 2017 and decreased for CABG by −1.0 points (95% CI, −1.5 to −0.5), with an absolute difference between the groups of 3.3 points (95% CI, 2.7–3.9) (Table S2 and S3).
Figure 2. Annual risk profile of percutaneous coronary intervention coronary intervention (PCI) (A) and coronary artery bypass grafting (CABG) (B) by NCDR (National Cardiovascular Data Registry) CathPCI mortality score.
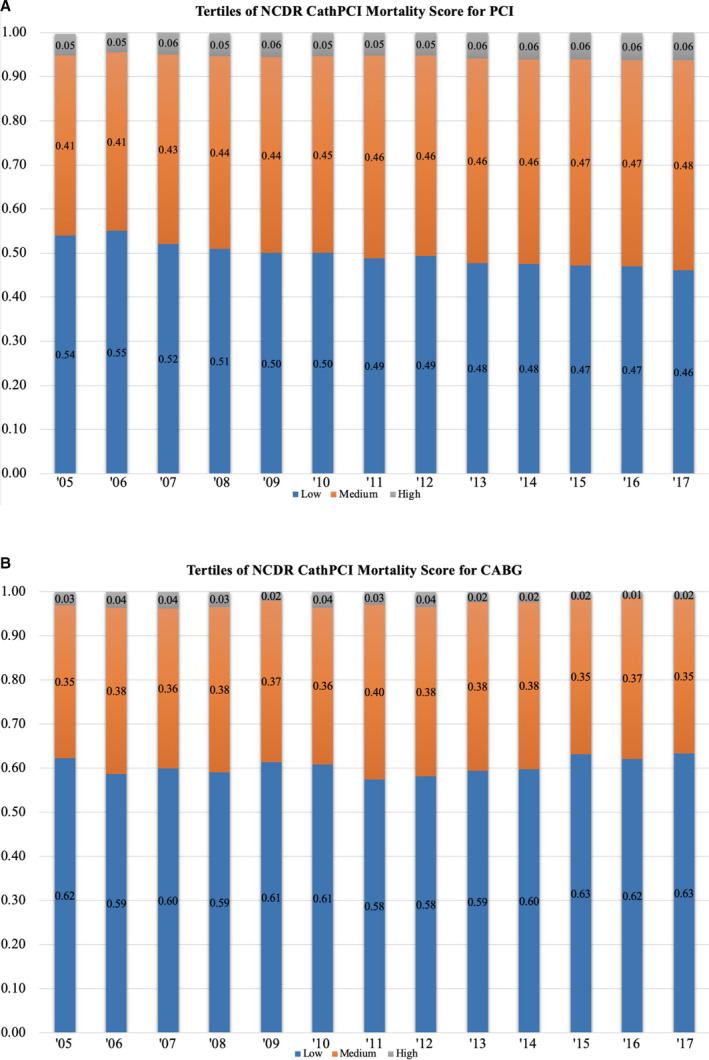
High indicates NCDR CathPCI morality score >40; low, NCDR CathPCI morality score <20; and medium, NCDR CathPCI mortality score 20 to 40.
For patients treated with PCI, high‐risk interventions such as atherectomy use (1.3–3.0%, P<0.0001) and LM intervention (1.6–4.3%, P<0.0001) (Figure 3) increased from 2005 to 2017. PCI for CTOs also increased from 2010 to 2017 (4.4% versus 7.6%, P<0.0001).
Figure 3. Temporal trend left main (LM) coronary artery intervention, atherectomy use, and chronic total occlusion (CTO) revascularization.
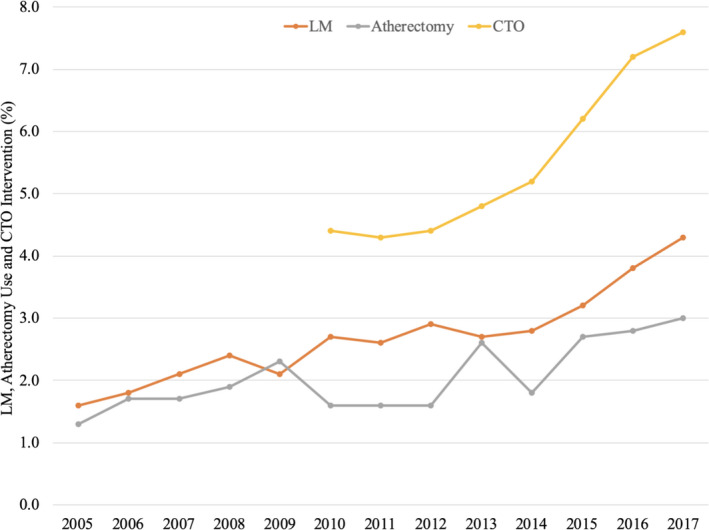
PCI indicates percutaneous coronary intervention.
Over the study period, unadjusted in‐hospital mortality increased for PCI (1.5–2.3%, P<0.0001) and decreased for CABG (2.3–1.2%, P<0.0001). After adjustment for baseline NCDR CathPCI mortality score and procedural characteristics unique to the PCI group, the ratio of O/E deaths decreased for CABG (1.21–0.74, P<0.0001) but increased for PCI (0.98–1.19, P<0.0001) (Figure 4). Durbin‐Watson statistics for both CABG and PCI indicated that there was no autocorrelation. Additional sensitivity analysis for O/E ratio for CABG adjusting for NCDR CathPCI mortality score and STS risk score between 2008 and 2016 showed no difference in mortality (Table S4).
Figure 4. Risk‐adjusted observed to expected ratio for in‐hospital mortality for percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG).

*Outcomes adjusted for NCDR (National Cardiovascular Data Registry) CathPCI score and procedural characteristic for PCI group (left main and atherectomy use).
Discussion
The present study evaluated temporal trends in the procedural volume, risk profile, PCI procedural characteristics, and clinical outcomes for a contemporary cohort of patients treated with PCI and CABG in nonfederal hospitals in Washington State between 2005 and 2017. Several important findings emerged from our analysis. First, there was an overall 6.6% decrease in all revascularization procedures, a finding primarily driven by a relative 22.6% reduction in CABG volume and a modest 2.9% reduction in PCI volume. A unique finding from our analysis was an increase in PCI in the recent era from 2013 to 2017, with a 20.0% increase in overall PCI and 30.3% increase in elective PCI. Second, the percentage of patients with moderate to high composite risk scores increased for PCI and decreased for CABG despite higher medical comorbidities in both groups, a finding driven by higher clinical acuity including cardiogenic shock and STEMI among PCI‐treated patients. Third, high‐risk PCI such as LM intervention, atherectomy use, and CTO PCI increased. Finally, risk adjusted O/E in‐hospital mortality ratio increased for PCI and decreased for CABG over the study period.
Rates of coronary revascularization declined between 2005 and 2017, particularly for CABG. The decreasing rates of revascularization for stable coronary disease reflect cumulative forces of improved risk factor control, lack of improvement in clinical outcomes between initial medical and invasive management, adoption of appropriate use criteria for coronary revascularization, and use of physiologic hemodynamic assessment.18, 20, 21, 22, 23, 24, 25, 26 A unique finding from our analysis was a relative 20.0% increase in PCI procedures from 2013 to 2017, with a 30.3% increase in elective PCIs. While previous studies demonstrated steady reduction in annual PCI volume, a contemporary cohort had not been evaluated.3, 18, 26 Reporting of PCI volume in California also demonstrated a 14.3% increase from 2013 to 2016,27 similar to our findings. Multiple forces may influence the growth in elective PCI including increasing LM and CTO interventions, which may shift patients who were previously treated with CABG or untreated entirely. Furthermore, Medicaid expansion with the Affordable Care Act may have influenced a rise in elective PCI. Other states participating in Medicaid expansion have also observed increased cardiovascular procedural volumes.28
Among patients who underwent any form of coronary revascularization, the prevalence of older age and medical comorbidities increased in our study and national registries.5, 13, 29 The risk profile as assessed by the NCDR CathPCI mortality score for patients with CABG and PCI, however, diverged despite the increased medical comorbidities in both groups. The mean NCDR CathPCI mortality score increased for PCI (from 20.1 to 22.2) and decreased for CABG (from 18.8 to 17.8) from 2005 to 2017. In particular, the proportion of high and moderate NCDR CathPCI mortality score increased for PCI and decreased for CABG. An analysis from the Department of Veterans Affairs demonstrated a similar increase in the median NCDR CathPCI mortality score from 2009 to 2015 (14–15, P=0.005).13 Conversely, an analysis of 65 097 patients treated with CABG between 1997 and 2011 demonstrated a lower predicted operative morality (3.1–1.7%) despite an increase in medical comorbidities.5 In our study, the discordance between medical comorbidities and risk profile resulted from both higher‐risk patients, such as octogenarians and those with chronic kidney disease, and acute clinical presentations, such as STEMI and cardiogenic, for patients treated with PCI. The higher prevalence of cardiogenic shock in the PCI group is particularly important as 30‐day mortality from cardiogenic shock complicating acute MI has remained unchanged at ≈45% over the past decade despite the use of various percutaneous left ventricular assist devices.30, 31, 32 Together, our findings highlight an important clinical and therapeutic shift in coronary revascularization with increasing utilization of PCI rather than CABG among moderate‐ and high‐risk patients, especially those with cardiogenic shock or STEMI.
In addition to higher‐risk patient profiles, procedural complexity of PCI increased over the study period. We discovered a consistent uptrend in LM intervention, CTO PCI, and atherectomy use over the study period. Current guidelines favor CABG over PCI in the presence of LM or multivessel disease in the context of complex coronary anatomy, diabetes mellitus, and cardiomyopathy.33 Despite the guidelines, studies from national registries demonstrate increased utilization of PCI in patients with complex coronary anatomy, including those with a class I indication for CABG.34, 35 Our study demonstrated a reduction in combined revascularization volume for patients with diabetes mellitus and multivessel coronary artery disease, consistent with overall findings. However, PCI remained the most common revascularization strategy in this patient population. Similarly, from an NCDR analysis, PCI was the most common form of revascularization for patients with diabetes mellitus and multivessel coronary artery disease hospitalized with NSTEMI.35 Increase in LM intervention may be related to numerous factors. With the increased prevalence of octogenarians and medical comorbidities, patients may be deemed surgically ineligible for CABG. Alternatively, increased operator comfort and patient preference for PCI may drive shared decision‐making towards a percutaneous procedure.
The adjusted O/E ratio for in‐hospital mortality decreased for CABG and increased for PCI in our analysis. Similar results were seen in a large study of Medicare beneficiaries, which showed increased in‐hospital mortality for PCI and decreased in‐hospital mortality for CABG between 2008 and 2012.16 In our study, adjusted O/E ratio for PCI initially remained stable between 2005 and 2013 (O/E ratio, 0.94–0.95) followed by an increase from 2014 to 2017 (O/E ratio, 1.0–1.17). This temporally correlates with an uptrend in high‐risk PCI. However, higher mortality persisted despite adjusting for LM intervention and atherectomy device utilization, factors known to be associated with increased procedural risk.36, 37 While previous studies have demonstrated improved short‐ and long‐term mortality for PCI, a contemporary cohort had not been evaluated.4, 29 In an analysis of US veterans treated with PCI, Waldo et al13 demonstrated a trend towards decreased mortality from 2009 to 2015 (hazard ratio, 0.98; 95% CI, 0.96–1.00). However, the population had lower clinical acuity with a minor increase in STEMI (2–4%) and unchanged rates of LM intervention (2%) and intra‐aortic balloon pump use (1%). Therefore, the increased clinical acuity with STEMI and cardiogenic shock along with the rise in PCI for high‐risk lesions may explain the increase in in‐hospital mortality in our cohort.
Finally, the adjusted O/E ratio for in‐hospital mortality decreased for patients with CABG over the study period from 1.21 to 0.74 (P<0.0001). Analysis of the VA and Medicare population undergoing CABG also demonstrated similar improvement in clinical outcomes.5, 38 The national and regional shifts in CABG outcomes may reflect changes in patient selection owing to increased scrutiny on outcomes of cardiac surgery, proposed changes for financial reimbursement leading to risk avoidance in patients at high or extreme risk, and increasing percutaneous options for complex lesions.39 These factors may prompt the transition to PCI of patients who were previously thought to only be amenable to surgical revascularization. As the selection of the optimal revascularization strategy continues to evolve, a heart‐team approach will be needed to determine the optimal revascularization strategy.
Study Limitations
Our study has several limitations that warrant discussion. First, long‐term outcomes of PCI and CABG were not available. Therefore, conclusions regarding the appropriate choice for revascularization cannot be drawn from this analysis. Second, we could not evaluate for staged PCI procedures (which may underestimate the number of lesions treated) or crossover between revascularization strategies. Third, the STS risk score could not be calculated for the CABG group for all years because of lack of available data. However, the NCDR CathPCI mortality score and STS risk score for isolated CABG overlap on many variables and our sensitivity analysis demonstrated similar risk‐adjusted mortality between 2008 and 2016. Fourth, we cannot exclude potential upcoding of procedural urgency by providers. The rising rates of STEMI, cardiogenic shock, and intra‐aortic balloon pump use in the PCI group, however, suggest an objective increase in clinical acuity and emergent procedures. Finally, changes in the data collection form for PCI and CABG may have artificially altered variable definition. We attempted to minimize the effect of these changes by focusing on common variables between the data collection forms.
Conclusions
Rates of coronary revascularization decreased for both elective PCI and CABG from 2005 to 2017 in nonfederal hospitals in Washington State. Risk profile and procedural complexity increased for the PCI group, whereas risk profile decreased for CABG. Our findings suggest a shift in revascularization patterns, with increasing rates of PCI in higher‐risk patients and acute clinical presentations. Adjusted in‐hospital mortality increased for PCI and decreased for CABG, reflecting changes in patient selection for surgical procedures.
Sources of Funding
Kataruka is supported by training grant T32HL007828 from the National Heart, Lung, and Blood Institute.
Disclosures
McCabe receives honoraria from Cardiovascular Systems Inc and Boston‐Scientific, and grant/honoraria from Abiomed. Gurm receives research funding from National Institutes of Health Centers for Accelerated Innovations and Blue Cross Blue Shield of Michigan, and is a consultant for Osprey Medical. Hira is a consultant for Abbott Vascular Inc. and ASAHI Intech. Virani receives research funding from the Department of Veterans Affairs Health Services Research & Development (IIR 16‐072), World Heart Federation, and Tahir and Jooma Family. Virani receives honorarium from the American College of Cardiology (Associate Editor for Innovations, acc.org) and serves on the steering committee for the PALM (Patient and Provider Assessment of Lipid Management) registry at the Duke Clinical Research Institute (no financial remuneration). Ring is a proctor for Medtronic, consultant for Boston‐Scientific, and on the speakers’ bureau for Amgen. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S4
(J Am Heart Assoc. 2020;9:e015317 DOI: 10.1161/JAHA.119.015317.)
See Editorial by Lahoud and Dauerman
For Sources of Funding and Disclosures, see page 12.
This manuscript was handled independently by Ik‐Kyung Jang, MD, PhD as a guest editor. The editors had no role in the evaluation of the manuscript or in the decision about its acceptance.
References
- 1. Boden WE, O'Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, et al Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–1516. [DOI] [PubMed] [Google Scholar]
- 2. Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362:2155–2165. [DOI] [PubMed] [Google Scholar]
- 3. Yeh RW, Mauri L, Wolf RE, Romm IK, Lovett A, Shahian D, Normand SL. Population trends in rates of coronary revascularization. JAMA Intern Med. 2015;175:454–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rao SV, Hess CN, Dai D, Green CL, Peterson ED, Douglas PS. Temporal trends in percutaneous coronary intervention outcomes among older patients in the united states. Am Heart J. 2013;166:273–281.e274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cornwell LD, Omer S, Rosengart T, Holman WL, Bakaeen FG. Changes over time in risk profiles of patients who undergo coronary artery bypass graft surgery: the veterans affairs surgical quality improvement program (VASQIP). JAMA Surg. 2015;150:308–315. [DOI] [PubMed] [Google Scholar]
- 6. Bass TA. High‐risk percutaneous coronary interventions in modern day clinical practice: current concepts and challenges. Circ Cardiovasc Interv. 2015;8:e003405. [DOI] [PubMed] [Google Scholar]
- 7. Madhavan MV, Tarigopula M, Mintz GS, Maehara A, Stone GW, Genereux P. Coronary artery calcification: pathogenesis and prognostic implications. J Am Coll Cardiol. 2014;63:1703–1714. [DOI] [PubMed] [Google Scholar]
- 8. Patel VG, Brayton KM, Tamayo A, Mogabgab O, Michael TT, Lo N, Alomar M, Shorrock D, Cipher D, Abdullah S, et al Angiographic success and procedural complications in patients undergoing percutaneous coronary chronic total occlusion interventions: a weighted meta‐analysis of 18,061 patients from 65 studies. JACC Cardiovasc Interv. 2013;6:128–136. [DOI] [PubMed] [Google Scholar]
- 9. Stone GW, Sabik JF, Serruys PW, Simonton CA, Genereux P, Puskas J, Kandzari DE, Morice MC, Lembo N, Brown WM III, et al Everolimus‐eluting stents or bypass surgery for left main coronary artery disease. N Engl J Med. 2016;375:2223–2235. [DOI] [PubMed] [Google Scholar]
- 10. Makikallio T, Holm NR, Lindsay M, Spence MS, Erglis A, Menown IB, Trovik T, Eskola M, Romppanen H, Kellerth T, et al Percutaneous coronary angioplasty versus coronary artery bypass grafting in treatment of unprotected left main stenosis (NOBLE): a prospective, randomised, open‐label, non‐inferiority trial. Lancet. 2016;388:2743–2752. [DOI] [PubMed] [Google Scholar]
- 11. Khera R, Cram P, Lu X, Vyas A, Gerke A, Rosenthal GE, Horwitz PA, Girotra S. Trends in the use of percutaneous ventricular assist devices: analysis of national inpatient sample data, 2007 through 2012. JAMA Intern Med. 2015;175:941–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vora AN, Dai D, Gurm H, Amin AP, Messenger JC, Mahmud E, Mauri L, Wang TY, Roe MT, Curtis J, et al Temporal trends in the risk profile of patients undergoing outpatient percutaneous coronary intervention: a report from the National Cardiovascular Data Registry's CathPCI registry. Circ Cardiovasc Interv. 2016;9:e003070. [DOI] [PubMed] [Google Scholar]
- 13. Waldo SW, Gokhale M, O'Donnell CI, Plomondon ME, Valle JA, Armstrong EJ, Schofield R, Fihn SD, Maddox TM. Temporal trends in coronary angiography and percutaneous coronary intervention: insights from the VA clinical assessment, reporting, and tracking program. JACC Cardiovasc Interv. 2018;11:879–888. [DOI] [PubMed] [Google Scholar]
- 14. Blumenfeld O, Na'amnih W, Shapira‐Daniels A, Lotan C, Shohat T, Shapira OM. Trends in coronary revascularization and ischemic heart disease‐related mortality in Israel. J Am Heart Assoc. 2017;6:e004734 DOI: 10.1161/JAHA.116.004734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ko W, Tranbaugh R, Marmur JD, Supino PG, Borer JS. Myocardial revascularization in New York state: variations in the PCI‐to‐CABG ratio and their implications. J Am Heart Assoc. 2012;1:e001446 DOI: 10.1161/JAHA.112.001446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Culler SD, Kugelmass AD, Brown PP, Reynolds MR, Simon AW. Trends in coronary revascularization procedures among medicare beneficiaries between 2008 and 2012. Circulation. 2015;131:362–370; discussion 370. [DOI] [PubMed] [Google Scholar]
- 17. Epstein AJ, Polsky D, Yang F, Yang L, Groeneveld PW. Coronary revascularization trends in the united states, 2001–2008. JAMA. 2011;305:1769–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bradley SM, Bohn CM, Malenka DJ, Graham MM, Bryson CL, McCabe JM, Curtis JP, Lambert‐Kerzner A, Maynard C. Temporal trends in percutaneous coronary intervention appropriateness: insights from the clinical outcomes assessment program. Circulation. 2015;132:20–26. [DOI] [PubMed] [Google Scholar]
- 19. Peterson ED, Dai D, DeLong ER, Brennan JM, Singh M, Rao SV, Shaw RE, Roe MT, Ho KK, Klein LW, et al Contemporary mortality risk prediction for percutaneous coronary intervention: results from 588,398 procedures in the national cardiovascular data registry. J Am Coll Cardiol. 2010;55:1923–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang D, Lv S, Song X, Yuan F, Xu F, Zhang M, Yan S, Cao X. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention: a meta‐analysis. Heart. 2015;101:455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van't Veer M, Klauss V, Manoharan G, Engstrom T, Oldroyd KG, et al Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213–224. [DOI] [PubMed] [Google Scholar]
- 22. De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, Piroth Z, Jagic N, Mobius‐Winkler S, Rioufol G, Witt N, et al Fractional flow reserve‐guided pci versus medical therapy in stable coronary disease. N Engl J Med. 2012;367:991–1001. [DOI] [PubMed] [Google Scholar]
- 23. Gotberg M, Frobert O. Instantaneous wave‐free ratio versus fractional flow reserve. N Engl J Med. 2017;377:1596–1597. [DOI] [PubMed] [Google Scholar]
- 24. Davies JE, Sen S, Escaned J. Instantaneous wave‐free ratio versus fractional flow reserve. N Engl J Med. 2017;377:1597–1598. [DOI] [PubMed] [Google Scholar]
- 25. Mohan AV, Fazel R, Huang PH, Shen YC, Howard D. Changes in geographic variation in the use of percutaneous coronary intervention for stable ischemic heart disease after publication of the clinical outcomes utilizing revascularization and aggressive drug evaluation (courage) trial. Circ Cardiovasc Qual Outcomes. 2014;7:125–130. [DOI] [PubMed] [Google Scholar]
- 26. Desai NR, Bradley SM, Parzynski CS, Nallamothu BK, Chan PS, Spertus JA, Patel MR, Ader J, Soufer A, Krumholz HM, et al Appropriate use criteria for coronary revascularization and trends in utilization, patient selection, and appropriateness of percutaneous coronary intervention. JAMA. 2015;314:2045–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. California Cardiac Surgery Intervention Project (CCSIP). https://www.californiacardiacsurgery.com/CCSIP2018/. Accessed January 10, 2020
- 28. Sukul D, Bhatt DL, Seth M, Zakroysky P, Wojdyla D, Rumsfeld JS, Wang T, Rao SV, Gurm HS. Appropriateness and outcomes of percutaneous coronary intervention at top‐ranked and nonranked hospitals in the united states. JACC Cardiovasc Interv. 2018;11:342–350. [DOI] [PubMed] [Google Scholar]
- 29. Fokkema ML, James SK, Albertsson P, Akerblom A, Calais F, Eriksson P, Jensen J, Nilsson T, de Smet BJ, Sjogren I, et al Population trends in percutaneous coronary intervention: 20‐year results from the SCAAR (Swedish Coronary Angiography and Angioplasty Registry). J Am Coll Cardiol. 2013;61:1222–1230. [DOI] [PubMed] [Google Scholar]
- 30. Ouweneel DM, Eriksen E, Sjauw KD, van Dongen IM, Hirsch A, Packer EJ, Vis MM, Wykrzykowska JJ, Koch KT, Baan J, et al Percutaneous mechanical circulatory support versus intra‐aortic balloon pump in cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol. 2017;69:278–287. [DOI] [PubMed] [Google Scholar]
- 31. Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, Richardt G, Hennersdorf M, Empen K, Fuernau G, et al Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367:1287–1296. [DOI] [PubMed] [Google Scholar]
- 32. Schrage B, Ibrahim K, Loehn T, Werner N, Sinning JM, Pappalardo F, Pieri M, Skurk C, Lauten A, Landmesser U, et al Impella support for acute myocardial infarction complicated by cardiogenic shock. Circulation. 2019;139:1249–1258. [DOI] [PubMed] [Google Scholar]
- 33. Patel MR, Calhoon JH, Dehmer GJ, Grantham JA, Maddox TM, Maron DJ, Smith PK. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 appropriate use criteria for coronary revascularization in patients with stable ischemic heart disease: a report of the American College of Cardiology appropriate use criteria task force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2017;69:2212–2241. [DOI] [PubMed] [Google Scholar]
- 34. Frutkin AD, Lindsey JB, Mehta SK, House JA, Spertus JA, Cohen DJ, Rumsfeld JS, Marso SP; NCDR . Drug‐eluting stents and the use of percutaneous coronary intervention among patients with class I indications for coronary artery bypass surgery undergoing index revascularization: analysis from the NCDR (National Cardiovascular Data Registry). JACC Cardiovasc Interv. 2009;2:614–621. [DOI] [PubMed] [Google Scholar]
- 35. Pandey A, McGuire DK, de Lemos JA, Das SR, Berry JD, Brilakis ES, Banerjee S, Marso SP, Barsness GW, Simon DN, et al Revascularization trends in patients with diabetes mellitus and multivessel coronary artery disease presenting with non‐ST elevation myocardial infarction: insights from the National Cardiovascular Data Registry Acute Coronary Treatment and Intervention Outcomes Network Registry‐Get With the Guidelines (NCDR Action Registry‐GWTG). Circ Cardiovasc Qual Outcomes. 2016;9:197–205. [DOI] [PubMed] [Google Scholar]
- 36. Al‐Lamee R, Ielasi A, Latib A, Godino C, Ferraro M, Mussardo M, Arioli F, Carlino M, Montorfano M, Chieffo A, et al Incidence, predictors, management, immediate and long‐term outcomes following grade III coronary perforation. JACC Cardiovasc Interv. 2011;4:87–95. [DOI] [PubMed] [Google Scholar]
- 37. Brilakis ES, Banerjee S, Karmpaliotis D, Lombardi WL, Tsai TT, Shunk KA, Kennedy KF, Spertus JA, Holmes DR Jr, Grantham JA. Procedural outcomes of chronic total occlusion percutaneous coronary intervention: a report from the NCDR (National Cardiovascular Data Registry). JACC Cardiovasc Interv. 2015;8:245–253. [DOI] [PubMed] [Google Scholar]
- 38. McNeely C, Markwell S, Vassileva C. Trends in patient characteristics and outcomes of coronary artery bypass grafting in the 2000 to 2012 medicare population. Ann Thorac Surg. 2016;102:132–138. [DOI] [PubMed] [Google Scholar]
- 39. Mehaffey JH, Hawkins RB, Byler M, Charles EJ, Fonner C, Kron I, Quader M, Speir A, Rich J, Ailawadi G; Virginia Cardiac Services Quality I . Cost of individual complications following coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2018;155:875–882.e871. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4


