Abstract
Background
Polypharmacy is highly prevalent in elderly people with chronic conditions, including atrial fibrillation (AF). The impact of polypharmacy on adverse outcomes and on treatment effectiveness in elderly patients with AF remains unaddressed.
Methods and Results
We studied 338 810 AF patients ≥75 years of age enrolled in the MarketScan Medicare Supplemental database in 2007–2015. Polypharmacy was defined as ≥5 active prescriptions at AF diagnosis (defined by the presence of International Classification of Diseases, Ninth Revision, Clinical Modification [ICD‐9‐CM] codes) based on outpatient pharmacy claims. AF treatments (oral anticoagulation, rhythm and rate control) and cardiovascular end points (ischemic stroke, bleeding, heart failure) were defined based on inpatient, outpatient, and pharmacy claims. Multivariable Cox models were used to estimate associations of polypharmacy with cardiovascular end points and the interaction between polypharmacy and AF treatments in relation to cardiovascular end points. Prevalence of polypharmacy was 52%. Patients with polypharmacy had increased risk of major bleeding (hazard ratio [HR], 1.16; 95% CI, 1.12–1.20) and heart failure (HR, 1.33; 95% CI, 1.29–1.36) but not ischemic stroke (HR, 0.96; 95% CI, 0.92–1.00), compared with those not receiving polypharmacy. Polypharmacy status did not consistently modify the effectiveness of oral anticoagulants. Rhythm control (versus rate control) was more effective in preventing heart failure hospitalization in patients not receiving polypharmacy (HR, 0.87; 95% CI, 0.76–0.99) than among those with polypharmacy (HR, 0.98; 95% CI, 0.91–1.07; P=0.02 for interaction).
Conclusion
Polypharmacy is common among patients ≥75 with AF, is associated with adverse outcomes, and may modify the effectiveness of AF treatments. Optimizing management of polypharmacy in AF patients ≥75 may lead to improved outcomes.
Keywords: adverse outcomes, atrial fibrillation, polypharmacy
Subject Categories: Atrial Fibrillation, Quality and Outcomes
Nonstandard Abbreviations and Acronyms
- AF
atrial fibrillation
- CPT
Current Procedural Terminology
- ICD‐9‐CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- OAC
oral anticoagulant
Clinical Perspective
What Is New?
Using a large healthcare claims database, we demonstrated that polypharmacy is common among patients ≥75 with atrial fibrillation and is associated with adverse outcomes.
Polypharmacy may also modify the effectiveness of certain atrial fibrillation treatments in this vulnerable population.
What Are the Clinical Implications?
Optimizing management of polypharmacy in patients ≥75 with atrial fibrillation may lead to improved outcomes.
Further research is necessary to assess the impact of prescription dosage and subsequent dose reductions on the association of polypharmacy with cardiovascular and bleeding outcomes.
Polypharmacy is commonly defined as the concurrent use of ≥5 drugs by an individual patient, regardless of the indications for which they have been prescribed.1 Using this definition, the prevalence of polypharmacy is >15% in the general US population and ≈40% in those aged ≥65 years.2 The prevalence is even higher among patients with chronic conditions.
Atrial fibrillation (AF) is a common cardiac arrhythmia that disproportionately affects older adults. It is often associated with multiple chronic conditions, resulting in high prevalence of polypharmacy. Among AF patients included in recent clinical trials, the prevalence of polypharmacy ranged between 40% and 75% and was linked to increased rates of cardiovascular mortality, bleeding, and stroke.3, 4, 5
Polypharmacy likely leads to worse outcomes in patients with AF given higher likelihood of drug–drug interaction and reduced treatment adherence.6, 7 Polypharmacy may also have a negative impact on the effectiveness of AF treatments, including oral anticoagulation and rate or rhythm control therapies. These concerns are of particular importance in the oldest individuals because of their frailty and high prevalence of comorbidities.8 Because of an increased risk of gastrointestinal bleeding among adults aged ≥75 years, the 2019 American Geriatrics Society Beers Criteria list 2 oral anticoagulants (OACs; dabigatran and rivaroxaban) as drugs to be used with caution in older adults.9 To date, however, minimal evidence exists regarding the impact of polypharmacy on outcomes and treatment effectiveness in patients ≥75 with AF.
To address existing gaps in the literature and to inform future guidelines for AF treatment in older adults, we evaluated the association of polypharmacy with adverse outcomes and interactions between polypharmacy and AF treatments in a large sample of AF patients aged ≥75 years identified in a healthcare claims database. We hypothesized that AF patients receiving polypharmacy are more likely to have an adverse outcome than those not receiving polypharmacy.
Methods
Study Population
We used data from the MarketScan Commercial and the MarketScan Medicare Supplemental Databases (Truven Health Analytics). These MarketScan databases contain paid claims and encounter data with >20 billion service records for the medical experience of insured employees and their dependents and for retirees with Medicare supplemental insurance paid by employers. The claims and encounter data were linked to detailed patient information of the enrollees. Claims and enrollment data are linked via a common synthetic patient identifier created by Truven Health Analytics as part of the data preparation to facilitate analysis while ensuring patient confidentiality. For the current analysis, we used data for the period January 1, 2007, through September 30, 2015. Because of licensing restrictions, data and study materials cannot be made available to other investigators to reproduce results, but researchers may contact Truven Health Analytics Inc to obtain and license the data.
We included patients with nonvalvular AF who were aged ≥75 years at the time of diagnosis. Valvular AF is a contraindication for direct OACs, and in general, these patients have different management. Consequently, they were excluded from the present study.10 AF was defined by an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) code 427.31 or 427.32 in any position based on at least 1 inpatient claim or 2 outpatient claims separated by at least 7 days but <1 year for enrollees without history of mitral stenosis (ICD‐9‐CM 394.0) or mitral valve disorder (ICD‐9‐CM 424.0). Using this definition, we identified 1 194 111 patients with AF in the databases. From these, we restricted the sample to the 480 313 (40%) who were aged ≥75 years. In addition, because 3 months has been shown to have positive predictive value of 80% in terms of predicting episodes of polypharmacy,11 we excluded participants with <90 days of enrollment before AF diagnosis and those who were followed <30 days after AF diagnosis (n=141 503). The final sample size for analysis was 338 810. The institutional review board at Emory University reviewed and approved this study and waived the need for patient consent.
Polypharmacy Use
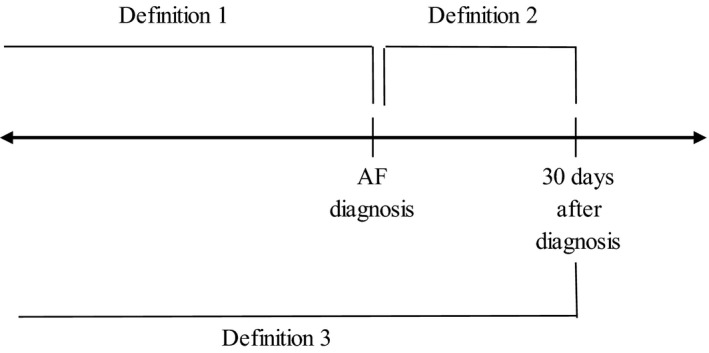
Information on drug prescriptions was obtained from outpatient pharmacy claims. Participants who had ≥5 concurrently active medication prescriptions based on the date of the prescription and days supply were defined as polypharmacy users, a commonly used definition for polypharmacy.12 To characterize risk among those considered to be the most vulnerable to complications from polypharmacy,13, 14 we examined the category of substantial polypharmacy, which we defined as patients having ≥10 active prescriptions simultaneously. The primary polypharmacy definition utilized in the main analysis was based on the number of active prescriptions at the time of AF diagnosis, including those prescribed on the day of diagnosis (Figure). To account for potential inaccuracies in defining the date of AF diagnosis within claims data, we used 2 alternative definitions of polypharmacy to account for changes in prescriptions following AF diagnosis, one considering prescriptions that started up in the 30 days after AF diagnosis and the other considering prescriptions at time of AF diagnosis plus new prescriptions up to 30 days after AF diagnosis, which is the combination of the 2 other definitions.
Figure 1. Polypharmacy definitions based on active prescriptions up through atrial fibrillation (AF) diagnosis and new prescriptions after diagnosis.
AF Treatment
We ascertained use of OACs, rhythm control therapy, and rate control therapy in a 30‐day period after AF diagnosis based on inpatient claims, outpatient claims, and outpatient pharmacy claims. Participants who had at least 1 prescription for warfarin, dabigatran, rivaroxaban, apixaban, or edoxaban were categorized as OAC users. Rhythm control therapy was defined as having an antiarrhythmic drug prescription, a catheter ablation procedure, or cardioversion during the 30‐day window after AF diagnosis. We defined catheter ablation as the presence of Current Procedural Terminology (CPT) codes 93651 (before 2013) and 93656 or 93657 (after January 2013) or the presence of ICD‐9‐CM procedure code 37.34 in the absence of codes for pacemaker or implantable cardioverter‐defibrillator implementation or for atrioventricular node ablation.15, 16, 17 Cardioversion was defined by the presence of ICD‐9‐CM codes 99.61 or 99.62 or CPT codes 92960 or 92961 in any position in an inpatient admission or CPT codes 92960 or 92961 in the primary position in an outpatient claim.16 Finally, we defined rate control as the presence of CPT code 93650 (atrioventricular node ablation) in any position in an inpatient or outpatient claim or at least 1 prescription for β‐blockers, nondihydropyridine calcium channel antagonists, or digoxin in the 30 days after AF diagnosis.
Other Covariates
We defined comorbidities at the time of AF considering the 20 conditions identified by the US Department of Health and Human Services (excluding autism and HIV infection), and some additional conditions. The final list included the following comorbidities: congestive heart failure, coronary artery disease, hyperlipidemia, stroke, arthritis, myocardial infarction, peripheral artery disease, gastrointestinal bleeding, cerebral bleeding, other bleeding, anemia, coagulopathy, mood disorder, cognitive impairment, liver disease, alcohol abuse, asthma, cancer, chronic kidney disease, chronic pulmonary disease, dementia, depression, diabetes mellitus, hepatitis, osteoporosis, schizophrenia, and substance abuse.18 We defined a frailty index using a published algorithm that utilizes inpatient and outpatient ICD‐9‐CM codes.19 Table S1 provides the ICD‐9‐CM codes used to define comorbidities and the frailty index.
Cardiovascular and Bleeding End Points
Three end points were evaluated in our analysis as independent adverse outcomes. Incident ischemic stroke was defined as the presence of ICD‐9‐CM codes 434 (occlusion of cerebral arteries) and 436 (acute but ill‐defined cerebrovascular disease) as the primary discharge diagnosis in inpatient claims, with positive predictive values >80% in different validation studies.20 A composite measure of bleeding was defined using the algorithms developed by Cunningham et al.21 This algorithm considers only those with a primary diagnosis associated with bleeding and excludes bleeding related to trauma. The positive predictive values were between 89% and 99%.21 Heart failure was also considered as one of the outcomes given its high incidence in AF patients22 and was defined as the occurrence of ICD‐9‐CM codes 402.x1, 404.x1, 404.x3, or 428 recorded as the principal discharge diagnosis in any inpatient claim, with positive predictive values ranging from 84% to 100%.23 For these 3 outcomes, we accounted only for events occurring at least 30 days after AF diagnosis.
Statistical Analysis
All statistical analyses were performed in SAS version 9.4 (SAS Institute). We examined patient characteristics and prevalent use of major therapeutic classes by polypharmacy status.
The associations of polypharmacy with incident ischemic stroke, major bleeding, and hospitalization of heart failure were assessed separately. Multivariable Cox proportional hazards regression was used to calculate hazard ratios (HRs) and their 95% CIs after adjusting for age, sex, comorbidities, and AF treatments. In the primary analysis, we defined polypharmacy status at the time of AF diagnosis. Secondary analyses used the alternative definitions of polypharmacy and created a separate category for patients receiving polypharmacy (≥10 prescriptions). For all analyses, time to event was calculated starting at 30 days after AF diagnosis to allow time to evaluate AF‐related treatments following diagnosis.
We conducted stratified analyses to identify the interaction between polypharmacy and AF treatment. We considered the following treatment comparisons: OAC use versus no OAC use, warfarin versus dabigatran versus rivaroxaban versus apixaban, and rhythm control versus rate control. Participants were classified into exclusive groups for each comparison. For the comparison of different OACs, we included 97 335 participants who had prescriptions for only 1 type of OAC in the 30‐day window after AF diagnosis. For the rhythm versus rate control comparison, we restricted the analysis to 163 506 participants receiving rhythm or rate control therapy in the 30‐day window after AF diagnosis, and participants receiving both rhythm and rate control therapy were considered to be in the rhythm control group. In the analysis, we considered AF treatment as the exposure and tested the effect of AF treatment on the 3 adverse outcomes, stratified by polypharmacy status. P values for the significance of the multiplicative interaction between polypharmacy use and AF treatment were also calculated.
Results
At the time of AF diagnosis, 338 810 patients ≥75 with AF had 1 761 660 active prescriptions (mean±SD, 5.1±3.8 per patient). Among these active prescriptions, anticoagulants, β‐blockers, and antihyperlipidemic drugs were the 3 most common classes. Calcium channel blockers, angiotensin‐converting enzyme inhibitors, loop diuretics, thyroid hormones, and gastrointestinal drugs (eg, antacids, proton pump inhibitors) were the next most prevalent drugs in the cohort. In total, they composed about half the prescribed medications (Table S2). In the 30‐day period after AF diagnosis (definition 2), 1 596 888 new medications were prescribed (mean±SD, 4.5±4.2 per patient). Under this definition of polypharmacy, anticoagulants were the most commonly prescribed medication class, followed by β‐blockers, and lipid‐lowering drugs. Besides the classes of medication mentioned, there was an increase in the prescription of opiate agonists, potassium supplements, and antiarrhythmic agents during the 30 days after AF diagnosis (Table S2). The prevalence of prescribed drugs of each therapeutic class was similar for participants receiving polypharmacy and those not receiving polypharmacy (Table S3).
Based on active prescriptions at the time of AF diagnosis, 176 007 patients (52%) were categorized as polypharmacy users (≥5 prescriptions). Table 1 presents patient characteristics by polypharmacy status. Those receiving polypharmacy had a higher prevalence of several comorbidities and were more likely to receive AF treatment within the 30 days after AF diagnosis compared with those taking <5 medications. However, polypharmacy users were less likely to have experienced cerebral bleeding, cognitive impairment, and dementia. Age and sex did not differ by polypharmacy status. Comorbidities such as hepatitis, schizophrenia, and alcohol and substance abuse also did not seem to be related to polypharmacy status. Patient characteristics stratified by polypharmacy use defined as ≥5 medications in the 30‐day period after AF diagnosis (143 362 polypharmacy users and 195 448 non–polypharmacy users were identified) or combining prescriptions at time of AF plus the 30‐day period after AF diagnosis (264 023 polypharmacy users and 74 787 non–polypharmacy users were identified) followed a pattern similar to the primary definition (Tables S4 and S5). Prevalence of substantial polypharmacy was 12%. Table S6 presents patient characteristics by category of polypharmacy (0–4, 5–9, and ≥10 prescriptions) at the time of AF diagnosis.
Table 1.
Characteristics by Polypharmacy Use Among AF Patients Aged ≥75 Years, MarketScan, 2007–2015
| No Polypharmacy | Polypharmacy | P Valuea | |
|---|---|---|---|
| n (%) | 162 803 (48.0) | 176 007 (52.0) | |
| Age, mean±SD | 83.3±5.5 | 82.8±5.2 | |
| Female, % | 50.5 | 51.3 | |
| Comorbidities, % | |||
| Hypertension | 64.1 | 72.1 | <0.0001 |
| Congestive heart failure | 26.5 | 33.9 | <0.0001 |
| Coronary artery disease | 38.7 | 48.8 | <0.0001 |
| Hyperlipidemia | 40.8 | 47.1 | <0.0001 |
| Stroke | 25.3 | 27.2 | <0.0001 |
| Arthritis | 30.2 | 33.5 | <0.0001 |
| Myocardial infarction | 9.3 | 10.3 | <0.0001 |
| Peripheral artery disease | 15.8 | 19.2 | <0.0001 |
| Gastrointestinal bleeding | 8.9 | 9.5 | <0.0001 |
| Cerebral bleeding | 1.9 | 1.5 | <0.0001 |
| Other bleeding | 10.3 | 11.2 | <0.0001 |
| Anemia | 23.8 | 25.7 | <0.0001 |
| Coagulopathy | 5.9 | 6.5 | <0.0001 |
| Mood disorder | 6.6 | 8.2 | <0.0001 |
| Cognitive impairment | 6.6 | 5.3 | <0.0001 |
| Liver disease | 3.4 | 3.4 | 0.97 |
| Alcohol abuse | 0.9 | 0.7 | <0.0001 |
| Asthma | 5.7 | 8.3 | <0.0001 |
| Cancer | 30.2 | 31 | <0.0001 |
| Chronic kidney disease | 19.9 | 25 | <0.0001 |
| Chronic pulmonary disease | 21.5 | 26.2 | <0.0001 |
| Dementia | 13.1 | 12 | <0.0001 |
| Depression | 6.8 | 8.4 | <0.0001 |
| Diabetes mellitus | 23.2 | 36.7 | <0.0001 |
| Hepatitis | 0.5 | 0.5 | 0.32 |
| Osteoporosis | 9.5 | 9.8 | 0.002 |
| Schizophrenia | 3.7 | 3.4 | <0.0001 |
| Substance abuse | 1.5 | 1.3 | 0.0003 |
| AF treatment during 30 d after AF, % | |||
| OACs | 24.5 | 32.7 | <0.0001 |
| Antiarrhythmic drugs | 1.7 | 2.1 | <0.0001 |
| Catheter ablation | 0.2 | 0.3 | 0.003 |
| Cardioversion | 1.3 | 1.8 | <0.0001 |
| Rate control therapy | 41.2 | 51.9 | <0.0001 |
Polypharmacy defined as ≥5 prescriptions at the time of AF diagnosis (polypharmacy definition 1). AF indicates atrial fibrillation; and OAC, oral anticoagulant.
χ2 P values.
Main Effect of Polypharmacy Use on Outcomes
After a mean±SD follow‐up of 2.1±1.8 years, enrollees receiving polypharmacy experienced 4860 ischemic strokes, 9967 major bleeding episodes, and 14 851 heart failure hospitalizations. The corresponding figures among non–polypharmacy users were 4582 ischemic strokes, 7212 major bleeding events, and 8718 heart failure hospitalizations, after a mean follow‐up of 2.0±1.8 years. After multivariable adjustment, enrollees receiving polypharmacy had increased risk of major bleeding (HR, 1.16; 95% CI, 1.12–1.20; Figure S1) and heart failure (HR, 1.33; 95% CI, 1.29–1.36; Figure S2) but not of ischemic stroke (HR, 0.96; 95% CI, 0.92–1.00; Figure S3) compared with those not receiving polypharmacy (Table 2). The results were comparable when using alternative definitions of polypharmacy (Tables S7 and S8) or when considering substantial polypharmacy (Table S9).
Table 2.
HRs and 95% CIsa of Selected Outcomes After 30 Days Following AF Diagnosis, Comparing Polypharmacy Users With Non–Polypharmacy Users Among AF Patients Aged ≥75 Years, MarketScan, 2007–2015
| No Polypharmacy | Polypharmacy | |
|---|---|---|
| Patients, n | 162 803 | 176 007 |
| Stroke | ||
| Event, n (%) | 4582 (2.8) | 4860 (2.8) |
| Follow‐up, y, mean±SD | 2.0±1.8 | 2.1±1.8 |
| Incident rateb | 14.0 | 13.2 |
| HR (95% CI) | 1 | 0.96 (0.92, 1.00) |
| Major bleeding | ||
| Patients, n (%) | 7212 (4.4) | 9967 (5.7) |
| Follow‐up, y, mean±SD | 2.0±1.8 | 2.0±1.8 |
| Incident rateb | 22.3 | 27.8 |
| HR (95% CI) | 1 | 1.16 (1.12, 1.20) |
| Heart failure | ||
| Patients, n (%) | 8718 (5.4) | 14 851 (8.4) |
| Follow‐up, y, mean±SD | 2.0±1.8 | 2.0±1.8 |
| Incident rateb | 27.0 | 41.8 |
| HR (95% CI) | 1 | 1.33 (1.29, 1.36) |
Polypharmacy defined as ≥5 prescriptions at the time of AF diagnosis (polypharmacy definition 1). AF indicates atrial fibrillation; and HR, hazard ratio.
Models adjusted for age, sex, frailty index, comorbidities (congestive heart failure, coronary artery disease, hyperlipidemia, stroke, arthritis, myocardial infarction, peripheral artery disease, gastrointestinal bleeding, cerebral bleeding, other bleeding, anemia, coagulopathy, mood disorder, cognitive impairment, liver disease, alcohol abuse, asthma, cancer, chronic kidney disease, chronic pulmonary disease, dementia, depression, diabetes mellitus, hepatitis, osteoporosis, schizophrenia, and substance abuse), and AF treatment (oral anticoagulation, antiarrhythmic drugs, catheter ablation, cardioversion, rate control therapy).
Per 1000 person‐years.
Polypharmacy and OAC Use Effectiveness
OAC use was associated with reduced ischemic stroke risk, increased risk of major bleeding, and small increased risk of heart failure in both AF patients who were receiving polypharmacy and those not receiving polypharmacy (Table 3). Associations were of similar magnitude except for major bleeding risk (P=0.03 for interaction), with higher bleeding risk among patients not receiving polypharmacy (HR, 1.32; 95% CI, 1.25–1.39), compared with those receiving polypharmacy (HR, 1.19; 95% CI, 1.14–1.24). Similar trends were seen for all outcomes when polypharmacy was redefined to include substantial polypharmacy (Table S10). However, the direction of this interaction was sensitive to the definition of polypharmacy, with risk of bleeding associated with OAC use modestly greater among those receiving polypharmacy using alternative definitions (HR, 1.25 [95% CI, 1.20–1.31] and 1.23 [95% CI, 1.18–1.27]) compared with those not receiving polypharmacy (HR, 1.15 [95% CI, 1.09–1.22] and 1.13 [95% CI, 0.99–1.30]). In addition, OAC use was associated with only a marginal increase in the risk of heart failure hospitalization among polypharmacy users according to alternative definitions (HR, 1.08 and 1.07) but not among non–polypharmacy users (HR, 0.98 and 0.85; Tables S11 and S12).
Table 3.
HRs and 95% CIsa of Selected Outcomes After 30 Days Following AF Diagnosis. Comparing AF Treatments by Polypharmacy Use Among AF Patients Aged ≥75 Years, MarketScan, 2007–2015
| HR (95% CI) | Stroke | Major Bleeding | Heart Failure | |||
|---|---|---|---|---|---|---|
| No Polypharmacy | Polypharmacy | No Polypharmacy | Polypharmacy | No Polypharmacy | Polypharmacy | |
| OAC vs No OAC (N=338 810) | ||||||
| No OAC | 1 | 1 | 1 | 1 | 1 | 1 |
| OAC | 0.92 (0.86–0.98) | 0.89 (0.84–0.95) | 1.32 (1.25–1.39) | 1.19 (1.14–1.24) | 1.09 (1.04–1.15) | 1.07 (1.04–1.11) |
| P for interaction | 0.51 | 0.03 | 0.82 | |||
| Warfarin vs dabigatran vs rivaroxaban vs apixaban (n=97 335) | ||||||
| Warfarin | 1 | 1 | 1 | 1 | 1 | 1 |
| Dabigatran | 1.16 (0.92–1.48) | 1.06 (0.84–1.32) | 0.87 (0.72–1.05) | 0.96 (0.83–1.10) | 0.68 (0.56–0.84) | 0.87 (0.77–0.99) |
| Rivaroxaban | 0.79 (0.57–1.11) | 0.90 (0.68–1.21) | 0.95 (0.77–1.17) | 1.08 (0.92–1.26) | 0.87 (0.71–1.07) | 0.81 (0.70–0.95) |
| Apixaban | 0.65 (0.33–1.25) | 0.56 (0.30–1.04) | 0.65 (0.42–1.00) | 0.76 (0.56–1.03) | 0.79 (0.56–1.12) | 0.81 (0.63–1.04) |
| P for interaction | 0.87 | 0.93 | 0.57 | |||
| Rhythm control vs rate control (n=163 506) | ||||||
| Rate control | 1 | 1 | 1 | 1 | 1 | 1 |
| Rhythm control | 0.78 (0.64–0.93) | 0.79 (0.68–0.93) | 0.85 (0.74–0.98) | 0.93 (0.84–1.03) | 0.87 (0.76–0.99) | 0.98 (0.91–1.07) |
| P for interaction | 0.85 | 0.20 | 0.02 | |||
Polypharmacy defined as ≥5 prescriptions at the time of AF diagnosis (polypharmacy definition 1). AF indicates atrial fibrillation; HR, hazard ratio; and OAC, oral anticoagulant.
Models adjusted for age, sex, frailty index, comorbidities (congestive heart failure, coronary artery disease, hyperlipidemia, stroke, arthritis, myocardial infarction, peripheral artery disease, gastrointestinal bleeding, cerebral bleeding, other bleeding, anemia, coagulopathy, mood disorder, cognitive impairment, liver disease, alcohol abuse, asthma, cancer, chronic kidney disease, chronic pulmonary disease, dementia, depression, diabetes mellitus, hepatitis, osteoporosis, schizophrenia, and substance abuse), and AF treatment (oral anticoagulation, antiarrhythmic drugs, catheter ablation, cardioversion, rate control therapy).
Among the 97 335 patients ≥75 with AF who were OAC users in our study, apixaban users had a consistently lower—albeit statistically insignificant—ischemic stroke risk in both polypharmacy and non–polypharmacy groups compared with warfarin users (Table 3). Similarly, risk of bleeding associated with the different types of OAC was not modified by polypharmacy status, with risk lowest among apixaban users, intermediate among dabigatran users, and highest among rivaroxaban and warfarin users (Table 3). All three OACs (dabigatran, rivaroxaban, and apixaban) were associated with lower risk of heart failure hospitalization compared with warfarin independent of polypharmacy status (Table 3). Results were similar using alternative definitions of polypharmacy (Tables S11 and S12) and when considering substantial polypharmacy (Table S10).
Polypharmacy and Rate Versus Rhythm Control Effectiveness
Among the 163 506 patients ≥75 who received either rate or rhythm control therapy in the 30‐day period after AF diagnosis, rhythm control therapy compared with rate control was associated with a similar reduction in risk of ischemic stroke and major bleeding in patients receiving polypharmacy and those not receiving polypharmacy (Table 3). Similarly, rhythm control was associated with lower risk of heart failure hospitalization compared with rate control, but this association was observed only in non–polypharmacy users (P=0.02 for interaction; Table 3). Using alternative definitions of polypharmacy (Tables S11 and S12) or redefining polypharmacy to include substantial polypharmacy (Table S10) results in overall similar patterns of association.
Discussion
In a large cohort of individuals ≥75 with AF, we observed that the average number of active prescriptions at the time of AF diagnosis was ≈5 and that 1 in 2 patients met our definition of polypharmacy, with 12% having substantial polypharmacy (≥10 prescriptions). We also found that polypharmacy users had a 33% increased risk of heart failure and 16% increased risk of major bleeding compared with patients not receiving polypharmacy. Risk of ischemic stroke, however, did not differ by polypharmacy status. Overall, polypharmacy status did not clearly modify the effectiveness and risks of OACs in AF patients ≥75. Similarly, rhythm versus rate control comparisons were not modified by polypharmacy status, except for a potential stronger benefit of rhythm control in preventing heart failure hospitalizations among those not receiving polypharmacy.
Ischemic Stroke
In contrast to the association of polypharmacy with heart failure and bleeding, stroke risk was not elevated in older AF patients receiving polypharmacy, after adjusting for comorbidities and frailty. Similarly, effectiveness of OACs for stroke prevention was not modified by polypharmacy status. Secondary analyses of randomized trials of OACs in AF have reported similar efficacy of diverse OACs in those receiving or not receiving polypharmacy, which is consistent with our findings.4, 5
Bleeding Risk
We observed that patients ≥75 with AF receiving polypharmacy had a higher risk of bleeding than those not receiving polypharmacy. This association was independent of frailty, comorbidities, and AF‐related treatments. Prior studies have reported similar findings. In the ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) trial, compared with AF patients using 0 to 5 drugs, bleeding risk was 24% higher in those using 6 to 8 drugs and 72% higher in those using ≥9 drugs.5 Comparable results were observed in the ROCKET‐AF (Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation) trial.4 Consistent with our observations, polypharmacy did not modify bleeding risk associated with OAC type in the ARISTOTLE and ROCKET‐AF trials.4, 5
Polypharmacy may lead to increased bleeding risk by increasing the possibility of drug–drug interactions, by direct effect of some medications (including OACs, platelet inhibitors, and NSAIDs), or by increasing the risk of fall‐related bleeding (due to sedatives and antihypertensive medications).24 Polypharmacy is also a marker of higher comorbidity burden and frailty, and despite extensive covariate adjustment, the observed increased risk of bleeding may be due to uncontrolled confounding by multimorbidity. Because of the potential for drug–drug interactions, we expected that bleeding risk associated with OAC use would be higher in patients receiving polypharmacy. This was not consistently the case, which may be due to changes in medication after OAC initiation to reduce bleeding risk.
Heart Failure
Heart failure is common in patients with AF because of their shared risk factors and pathology.25, 26 The observed increased risk of heart failure in patients with AF receiving polypharmacy could be the result of polypharmacy being a marker of higher prevalence of heart failure risk factors, such as hypertension or coronary artery disease. It could also stem from adverse effects of medications commonly used in AF patients with multimorbidities, such as fluid retention with some oral antidiabetic medications or a cardiodepressant effect with selected antiarrhythmic drugs.27
Polypharmacy status significantly modified the association of rhythm versus rate control therapy with heart failure risk in our study. Specifically, rhythm control therapy, compared with rate control, was associated with lower risk of heart failure only in AF patients ≥75 patients not receiving polypharmacy. Polypharmacy could be a correlate of more severe atrial disease or presence of structural heart disease, which would reduce the effectiveness of rhythm control approaches.28 Polypharmacy may also limit the rhythm control options available to a particular patient because of potential drug interactions in the case of antiarrhythmic drugs or perceived increased risk of complications in catheter ablation, reducing the potential effectiveness of this approach.
We did not examine the influence of medication dosage on these outcomes. Prescription dose reduction is common for patients receiving polypharmacy in order to avoid potential drug–drug interactions and adverse outcomes.11 For instance, AF patients with chronic heart failure may be more susceptible to bleeds when on both warfarin and β‐blockers.29 Furthermore, a perceived benefit of OAC over warfarin use is the viability of fixed doses: warfarin's narrow therapeutic window requires involved monitoring and constant dose adjustments that are not associated with OAC use.30 Therefore, the associations observed with polypharmacy status may not represent the true underlying association by not taking medication dosage into account.
Our analysis has a number of strengths including the large sample size, the availability of extensive healthcare utilization information, and the ability to control for potential confounders. However, limitations of this study should be noted. First, these results may not be generalizable to noninsured populations. Second, although our model adjusts for a sizeable number of comorbidities, other socioeconomic, anthropomorphic, and lifestyle factors may be associated with the outcomes that were not accounted for in this analysis. Third, definitions of AF, polypharmacy, end points, and comorbidities are based on claims data, which may have suboptimal validity. Fourth, this study does not take OAC dosage into account, which may bias our estimates. Fifth, we did not account for any potential changes in polypharmacy status (eg, initiation of new medications or discontinuance of previous medications) after the date of AF diagnosis, which may also bias our estimates.
Conclusions
Our study confirmed the sizable prevalence of polypharmacy in patients ≥75 with AF and found evidence of increased rates of bleeding and heart failure hospitalizations associated with polypharmacy, although there is potential for more severe disease in the polypharmacy group when taking prescription dose reduction into account. These results, together with the high prevalence of multimorbidity in AF patients, highlight the need to develop and test best practices for integrating management of polypharmacy and multimorbidity in treatment guidelines for AF.
Sources of Funding
Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health under award number R21AG058445 and the National Heart, Lung, and Blood Institute of the National Institutes of Health under award numbers R01HL122200 and K24HL148521. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was also supported by American Heart Association grant 16EIA26410001 (Alonso).
Disclosures
None.
Supporting information
Tables S1–S12 Figures S1–S3
(J Am Heart Assoc. 2020;9:e015089 DOI: 10.1161/JAHA.119.015089.)
For Sources of Funding and Disclosures, see page 8.
References
- 1. Gnjidic D, Hilmer SN, Blyth FM, Naganathan V, Waite L, Seibel MJ, McLachlan AJ, Cumming RG, Handelsman DJ, Le Couteur DG. Polypharmacy cutoff and outcomes: five or more medicines were used to identify community‐dwelling older men at risk of different adverse outcomes. J Clin Epidemiol. 2012;65:989–995. [DOI] [PubMed] [Google Scholar]
- 2. Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States From 1999‐2012. JAMA. 2015;314:1818–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Proietti M, Raparelli V, Olshansky B, Lip GY. Polypharmacy and major adverse events in atrial fibrillation: observations from the AFFIRM trial. Clin Res Cardiol. 2016;105:412–420. [DOI] [PubMed] [Google Scholar]
- 4. Piccini JP, Hellkamp AS, Washam JB, Becker RC, Breithardt G, Berkowitz SD, Halperin JL, Hankey GJ, Hacke W, Mahaffey KW, et al. Polypharmacy and the efficacy and safety of rivaroxaban versus warfarin in the prevention of stroke in patients with nonvalvular atrial fibrillation. Circulation. 2016;133:352–360. [DOI] [PubMed] [Google Scholar]
- 5. Jaspers Focks J, Brouwer MA, Wojdyla DM, Thomas L, Lopes RD, Washam JB, Lanas F, Xavier D, Husted S, Wallentin L, et al. Polypharmacy and effects of apixaban versus warfarin in patients with atrial fibrillation: post hoc analysis of the ARISTOTLE trial. BMJ. 2016;353:i2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dumbreck S, Flynn A, Nairn M, Wilson M, Treweek S, Mercer SW, Alderson P, Thompson A, Payne K, Guthrie B. Drug‐disease and drug‐drug interactions: systematic examination of recommendations in 12 UK national clinical guidelines. BMJ. 2015;350:h949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fincke BG, Miller DR, Spiro A III. The interaction of patient perception of overmedication with drug compliance and side effects. J Gen Intern Med. 1998;13:182–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rolland Y, Morley JE. Editorial: frailty and polypharmacy. J Nutr Health Aging. 2016;20:645–646. [DOI] [PubMed] [Google Scholar]
- 9. By the American Geriatrics Society Beers Criteria Update Expert Panel . American Geriatrics Society 2019 updated AGS Beers Criteria(R) for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67:674–694. [DOI] [PubMed] [Google Scholar]
- 10. Fauchier L, Philippart R, Clementy N, Bourguignon T, Angoulvant D, Ivanes F, Babuty D, Bernard A. How to define valvular atrial fibrillation? Arch Cardiovasc Dis. 2015;108:530–539. [DOI] [PubMed] [Google Scholar]
- 11. Bjerrum L, Rosholm JU, Hallas J, Kragstrup J. Methods for estimating the occurrence of polypharmacy by means of a prescription database. Eur J Clin Pharmacol. 1997;53:7–11. [DOI] [PubMed] [Google Scholar]
- 12. Masnoon N, Shakib S, Kalisch‐Ellett L, Caughey GE. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Herr M, Robine JM, Pinot J, Arvieu JJ, Ankri J. Polypharmacy and frailty: prevalence, relationship, and impact on mortality in a French sample of 2350 old people. Pharmacoepidemiol Drug Saf. 2015;24:637–646. [DOI] [PubMed] [Google Scholar]
- 14. Kennel PJ, Kneifati‐Hayek J, Bryan J, Banerjee S, Sobol I, Lachs MS, Safford MM, Goyal P. Prevalence and determinants of hyperpolypharmacy in adults with heart failure: an observational study from the National Health and Nutrition Examination Survey (NHANES). BMC Cardiovasc Disord. 2019;19:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reynolds MR, Gunnarsson CL, Hunter TD, Ladapo JA, March JL, Zhang M, Hao SC. Health outcomes with catheter ablation or antiarrhythmic drug therapy in atrial fibrillation: results of a propensity‐matched analysis. Circ Cardiovasc Qual Outcomes. 2012;5:171–181. [DOI] [PubMed] [Google Scholar]
- 16. Noseworthy PA, Kapa S, Deshmukh AJ, Madhavan M, Van Houten H, Haas LR, Mulpuru SK, McLeod CJ, Asirvatham SJ, Friedman PA, et al. Risk of stroke after catheter ablation versus cardioversion for atrial fibrillation: a propensity‐matched study of 24,244 patients. Heart Rhythm. 2015;12:1154–1161. [DOI] [PubMed] [Google Scholar]
- 17. Noseworthy PA, Yao X, Deshmukh AJ, Van Houten H, Sangaralingham LR, Siontis KC, Piccini JP Sr, Asirvatham SJ, Friedman PA, Packer DL, et al. Patterns of anticoagulation use and cardioembolic risk after catheter ablation for atrial fibrillation. J Am Heart Assoc. 2015;4:e002597 DOI: 10.1161/JAHA.115.002597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goodman RA, Posner SF, Huang ES, Parekh AK, Koh HK. Defining and measuring chronic conditions: imperatives for research, policy, program, and practice. Prev Chronic Dis. 2013;10:E66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim DH, Schneeweiss S. Measuring frailty using claims data for pharmacoepidemiologic studies of mortality in older adults: evidence and recommendations. Pharmacoepidemiol Drug Saf. 2014;23:891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Andrade SE, Harrold LR, Tjia J, Cutrona SL, Saczynski JS, Dodd KS, Goldberg RJ, Gurwitz JH. A systematic review of validated methods for identifying cerebrovascular accident or transient ischemic attack using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(suppl 1):100–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cunningham A, Stein CM, Chung CP, Daugherty JR, Smalley WE, Ray WA. An automated database case definition for serious bleeding related to oral anticoagulant use. Pharmacoepidemiol Drug Saf. 2011;20:560–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Piccini JP, Hammill BG, Sinner MF, Hernandez AF, Walkey AJ, Benjamin EJ, Curtis LH, Heckbert SR. Clinical course of atrial fibrillation in older adults: the importance of cardiovascular events beyond stroke. Eur Heart J. 2014;35:250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Saczynski JS, Andrade SE, Harrold LR, Tjia J, Cutrona SL, Dodd KS, Goldberg RJ, Gurwitz JH. A systematic review of validated methods for identifying heart failure using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(suppl 1):129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leiss W, Mean M, Limacher A, Righini M, Jaeger K, Beer HJ, Osterwalder J, Frauchiger B, Matter CM, Kucher N, et al. Polypharmacy is associated with an increased risk of bleeding in elderly patients with venous thromboembolism. J Gen Intern Med. 2015;30:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Crijns HJ, Van den Berg MP, Van Gelder IC, Van Veldhuisen DJ. Management of atrial fibrillation in the setting of heart failure. Eur Heart J. 1997;18(suppl C):C45–C49. [DOI] [PubMed] [Google Scholar]
- 26. Chamberlain AM, Gersh BJ, Alonso A, Kopecky SL, Killian JM, Weston SA, Roger VL. No decline in the risk of heart failure after incident atrial fibrillation: a community study assessing trends overall and by ejection fraction. Heart Rhythm. 2017;14:791–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Masoudi FA, Krumholz HM. Polypharmacy and comorbidity in heart failure. BMJ. 2003;327:513–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schreiber D, Rostock T, Frohlich M, Sultan A, Servatius H, Hoffmann BA, Luker J, Berner I, Schaffer B, Wegscheider K, et al. Five‐year follow‐up after catheter ablation of persistent atrial fibrillation using the stepwise approach and prognostic factors for success. Circ Arrhythm Electrophysiol. 2015;8:308–317. [DOI] [PubMed] [Google Scholar]
- 29. Berlowitz DR, Miller DR, Oliveria SA, Cunningham F, Gomez‐Caminero A, Rothendler JA. Differential associations of beta‐blockers with hemorrhagic events for chronic heart failure patients on warfarin. Pharmacoepidemiol Drug Saf. 2006;15:799–807. [DOI] [PubMed] [Google Scholar]
- 30. Mekaj YH, Mekaj AY, Duci SB, Miftari EI. New oral anticoagulants: their advantages and disadvantages compared with vitamin K antagonists in the prevention and treatment of patients with thromboembolic events. Ther Clin Risk Manag. 2015;11:967–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S12 Figures S1–S3


