Abstract
Background
Atherosclerotic vertebrobasilar disease is a significant etiology of posterior circulation stroke. The prospective observational VERiTAS (Vertebrobasilar Flow Evaluation and Risk of Transient Ischemic Attack and Stroke) study demonstrated that distal hemodynamic status is a robust predictor of subsequent vertebrobasilar stroke risk. We sought to compare predictive models using thresholds for posterior circulation vessel flows standardized to age and vascular anatomy to optimize risk prediction.
Methods and Results
VERiTAS enrolled patients with recent vertebrobasilar transient ischemic attack or stroke and ≥50% atherosclerotic stenosis/occlusion in vertebral and/or basilar arteries. Quantitative magnetic resonance angiography measured large‐vessel vertebrobasilar territory flow, and patients were designated as low or normal flow based on a prespecified empiric algorithm considering distal territory regional flow and collateral capacity. For the present study, post hoc analysis was performed to generate additional predictive models using age‐specific normalized flow measurements. Sensitivity, specificity, and time‐to‐event analyses were compared between the algorithms. The original prespecified algorithm had 50% sensitivity and 79% specificity for future stroke risk prediction; using a predictive model based on age‐normalized flows in the basilar and posterior cerebral arteries, standardized to vascular anatomy, optimized flow status thresholds were identified. The optimized algorithm maintained sensitivity and increased specificity to 84%, while demonstrating a larger and more significant hazard ratio for stroke on time‐to‐event analysis.
Conclusions
These results indicate that flow remains a strong predictor of stroke across different predictive models, and suggest that prediction of future stroke risk can be optimized by use of vascular anatomy and age‐specific normalized flows.
Keywords: blood flow, magnetic resonance angiography, magnetic resonance imaging, quantitative magnetic resonance angiography, stroke vertebrobasilar disease
Subject Categories: Magnetic Resonance Imaging (MRI), Prognosis, Cerebrovascular Disease/Stroke, Ischemic Stroke, Transient Ischemic Attack (TIA)
Nonstandard Abbreviations and Acronyms
- BA
basilar artery
- PCA
posterior cerebral arteries
- QMRA
quantitative magnetic resonance angiography
- VERiTAS
Vertebrobasilar Flow Evaluation and Risk of Transient Ischemic Attack and Stroke
Clinical Perspective
What Is New?
This report uses data from the prospective observational multicenter VERiTAS (Vertebrobasilar Flow Evaluation and Risk of Transient Ischemic Attack and Stroke) study and details the methodical consideration of normal age‐related changes in vertebrobasilar distribution flow to optimize the algorithm allowing stratification of patients into low or normal flow categories with improved prediction of recurrent vertebrobasilar stroke.
What Are the Clinical Implications?
The optimized algorithm improves the selection criteria for secondary stroke prevention strategies and research: low versus normal distal flow is predictive of recurrent vertebrobasilar stroke.
Posterior circulation stroke accounts for up to 30% of all ischemic stroke, a significant source of which is atherosclerotic vertebrobasilar disease.1, 2, 3, 4, 5, 6 Hemodynamic compromise, as assessed by large‐vessel flow measurements, has proven to be a robust predictor of stroke in symptomatic vertebrobasilar disease based on prospective observational data from the VERiTAS (Vertebrobasilar Flow Evaluation and Risk of Transient Ischemic Attack and Stroke) study. In VERiTAS, patients classified as low flow on the basis of evaluation of relevant distal territory flow had a 4‐fold higher risk of stroke.7 The flow algorithm used for determination of flow status was originally established from retrospective data and proved highly predictive in VERiTAS. However, this algorithm relies on absolute thresholds for flow in the relevant arteries, without adjustment for age, and can generate equivocal (ie, borderline) cases dependent on vascular anatomy. We sought to determine if the algorithm could be optimized by using age‐normalized thresholds and accounting for posterior circulation anatomic variations.
Methods
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
The VERiTAS study design has been published previously.8 Briefly, the study was a multicenter prospective cohort study of adult patients suffering recent (within 60 days) vertebrobasilar territory transient ischemic attack or nondisabling stroke, and ≥50% vertebrobasilar stenosis or occlusion demonstrated on conventional or computed tomographic angiography.9, 10 Cases of dissection, fibromuscular dysplasia, vasculitis, radiation‐induced vasculopathy, and other nonatherosclerotic disease were excluded; unilateral isolated vertebral artery occlusion was also excluded due to uncertainty of the underlying etiology of disease. Patients were excluded for inability to return for follow‐up or comorbidity with <12‐month life expectancy, as well as for any known cardiac disease associated with cardioembolic risk (eg, atrial fibrillation, prosthetic valves, cardiomyopathy with low ejection fraction) and blood dyscrasias (eg, polycythemia vera, sickle cell disease). Seventy‐two patients were recruited prospectively at 5 centers in North America from 2008 to 2014. This cohort underwent quantitative magnetic resonance angiography (QMRA) for flow measurements at prespecified locations at the vertebral artery straight segment proximal to the posterior inferior cerebellar artery, basilar artery (BA) proximal to superior cerebellar arteries, and posterior cerebral arteries (PCAs) distal to the posterior communicating artery. The results of the QMRA were kept blinded from the patients and investigators, and patients were followed prospectively on standard medical therapy for 12 to 24 months for a primary end point of ischemic stroke in the vertebrobasilar territory. The study was approved by the local institutional review boards, and all subjects provided informed consent.
Original Flow Algorithm
The algorithm is depicted in Figure 1A. Low flow was designated a priori as more than 20% reduction below normative lower limits of flow (as available at that time) in the BA (<120 cc/min) or PCA (<40 cc/min).11 The PCA flow was not considered in flow status determination if the PCA anatomy was fetal (defined as absent P1 segment on QMRA). In patients with reduced distal demand from bilateral fetal PCAs, the BA flow threshold was adjusted to <40 cc/min. In patients with 2 normal‐configuration PCAs but flow in 1 PCA below and the other above the normative limit, flow status was considered borderline and additional clinical/radiographic criteria were applied to determine the flow status.
Figure 1. Flow stratification algorithms.
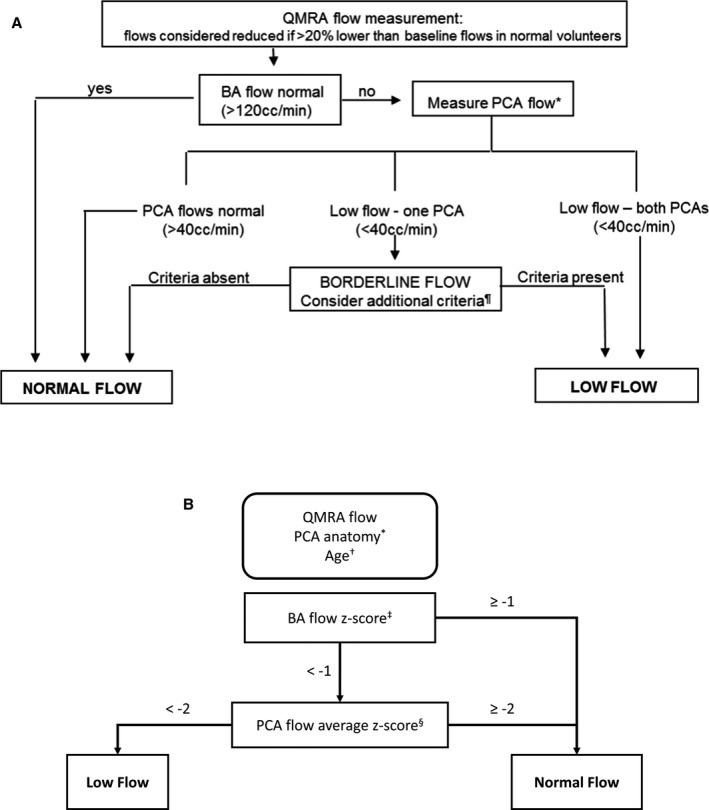
A, The original algorithm for determining a low‐flow vs normal flow state. Flow algorithm for symptomatic VB disease. *In the case of fetal PCA, determination of flow status is as follows. If 1 PCA is fetal, only the flow in nonfetal PCA is considered; if both PCAs are fetal, only flow in the BA is considered (low flow if <40 mL/min). ¶Additional criteria in borderline cases: ominous BA flow waveform oscillating ≈0, ominous symptom complex (symptoms exacerbated with head position, cannot be on anti‐coagulation/antiplatelets, requires very elevated blood pressure to avert symptoms); flow in nonoccluded proximal BA <40 mL/min. B, The optimized anatomy‐specific and age‐stratified normalizing algorithm for determining a low or normal flow state. BA indicates basilar artery; PCA, posterior cerebral artery; and QMRA, quantitative magnetic resonance angiography. *The PCA anatomy is classified as bilateral fetal PCA, unilateral fetal PCA, or no fetal PCA. †The age of patients with no fetal PCA is stratified as 18 to 60 or >60 years old. Each stratification has distinct averages and standard deviations of normal BA and PCA flows (Table 1). ‡The BA flow Z score is calculated . §The PCA flow Z score is calculated as the average of normal configuration PCA Z scores, where PCA Z scores are .
Development of Optimized Algorithm
To develop an optimized algorithm, we examined a strategy to define vascular anatomy–specific and age‐normalized thresholds for flow in the BA and PCA. Data for flow measurements from a large cohort of healthy adults (n=323, 18‐ to 84‐year‐old nonsmokers with no history of cerebrovascular, cardiac, respiratory, liver, kidney, or neoplastic disease, diabetes mellitus, or untreated hypertension), published subsequent to the original designation of flow thresholds, was used as our reference population for normative flows.12 This cohort conforms to the distribution of healthy adults and therefore is enriched in younger cases compared with cases >60 years old (mean 48±15). Age stratification was not able to be performed on all anatomic configurations because of infrequent occurrence of bilateral and unilateral fetal PCA (8 and 32 normal subjects, respectively). In the 283 patients with normal PCA configuration, age stratification was dichotomized into 2 age groupings based on our previously observed threshold for reduction in cerebral blood flow from the published normative cohort: ≤60 years old cohort (n=219) and the >60 years old cohort (n=64).
VERiTAS patients were assigned a calculated Z score from their flows in the BA and each PCA normalized to the normative means±standard deviation derived from the healthy adult cohort (Table 1). In patients with bilateral fetal PCAs, the BA Z score determined the flow state. In patients with unilateral fetal PCA, the BA Z score, and nonfetal PCA Z score only were used to determine flow status. In patients with bilateral normal (nonfetal) PCAs, the Z scores of the 2 PCAs were averaged, and the BA Z score and averaged PCA Z score were used to determine flow status. The Z scores for the BA and PCA were then applied at various thresholds ranging from 0 to −3 (Table 2). These thresholds were applied to the algorithm shown in Figure 1B.
Table 1.
Normal BA and PCA Flows Dependent on PCA Anatomy and Age
| BA Flow (Relative to PCA Anatomy an Age) | BA Flow Mean±SD |
|---|---|
| Normal PCA anatomy | |
| ≤60 y (n=219) | 150±37 |
| >60 y (n=64) | 131±33 |
| Unilateral fetal PCA (n=32) | 92±22 |
| Bilateral fetal PCA (n=8) | 50±17 |
| PCA Flow (Relative to Age) |
Left PCA Flow Mean±SD |
Right PCA Flow Mean±SD |
|---|---|---|
| ≤60 y (n=252) | 72±16 | 68±16 |
| >60 y (n=73) | 63±14 | 59±14 |
BA indicates basilar artery; and PCA, posterior cerebral artery.
Table 2.
Subset of Thresholds in the Age‐Stratified Algorithm and Resulting Test Characteristics in Comparison With the Original Algorithm
| BA Z Score | PCA Z Score | Sensitivity | Specificity | PPV | NPV | χ2 P Value | |
|---|---|---|---|---|---|---|---|
| −0.5 | −2 | 0.5 | 0.82 | 0.31 | 0.91 | 0.04 | |
| −1 | −0.5 | 0.6 | 0.63 | 0.21 | 0.91 | 0.19 | |
| −1 | −1 | 0.5 | 0.74 | 0.24 | 0.90 | 0.14 | |
| −1 | −1.5 | 0.5 | 0.79 | 0.28 | 0.91 | 0.11 | |
| Optimized algorithm | −1 | −2 | 0.5 | 0.84 | 0.33 | 0.91 | 0.03 |
| −1 | −2.5 | 0.4 | 0.85 | 0.31 | 0.90 | 0.07 | |
| −1 | −3 | 0.3 | 0.90 | 0.33 | 0.89 | 0.10 | |
| −1.5 | −2 | 0.4 | 0.84 | 0.29 | 0.90 | 0.10 | |
| −2 | −2 | 0.3 | 0.87 | 0.27 | 0.89 | 0.17 | |
| Old algorithm | NA | NA | 0.5 | 0.79 | 0.28 | 0.91 | 0.05 |
The shaded row highlights the optimum threshold. BA indicates basilar artery; NPV, negative predictive value; PCA, posterior cerebral artery; and PPV, positive predictive value.
Applying the anatomy and age‐normalized algorithm, 2‐way tables were constructed on the basis of the flow classification and event occurrence to calculate discrete sensitivity, specificity, positive predictive value, and a negative predictive value of various Z score thresholds. The receiver operating characteristic curve was calculated for the age‐stratified analysis to aid in selecting the optimum BA and PCA threshold. The optimum threshold was selected by simultaneously varying the BA and PCA thresholds independently for a maximum Youden's J statistic.13
Algorithm Comparison
To compare the relative performance of the optimized algorithm to the original algorithm, Kaplan‐Meier analysis with log‐rank testing, and Cox proportional hazards model to calculate a hazard ratio (HR) for stroke events was performed for each algorithm.
Results
The VERiTAS cohort consisted of 72 patients, 44% women, aged 40 to 90 years (mean 65.6±10.3). Forty percent had disease of the BA only, 30% had disease of the vertebral artery only; 78% had intracranial disease only, and 10% had extracranial disease only. The cohort was stratified into 18 low‐flow and 54 normal‐flow cases based on the original algorithm. On the basis of the optimized algorithm, 15 were classified as low flow and 57 were classified as normal flow.
The receiver operating characteristic curve of the optimized algorithm demonstrates a maximum Youden's J statistic of 0.35 (Figure 2). The optimum threshold for the Z statistic in the anatomy‐specific age‐adjusted algorithm was found at −1 for the BA and −2 for the PCA. In comparison, the original algorithm had a Youden's statistic of 0.29 (Figure 2). This is the result of 5 reclassifications (7%), all occurring in patients >60 years of age: 4 patients designated as low flow with the original algorithm were reclassified as normal flow, 1 of which had suffered a stroke end point; 1 patient designated originally as normal flow, who had suffered a stroke end point, was reclassified as low flow. With these reclassifications, 5 of 15 low‐flow patients reached a primary stroke end point as compared with 5 of 18 patients with the original algorithm. The specificity of the optimized algorithm reached 84%, compared with 79% in the original algorithm (Table 2).
Figure 2. The receiver operating characteristic shows the behavior of the optimized algorithm along the range of possible basilar artery and posterior cerebral artery cutoffs.
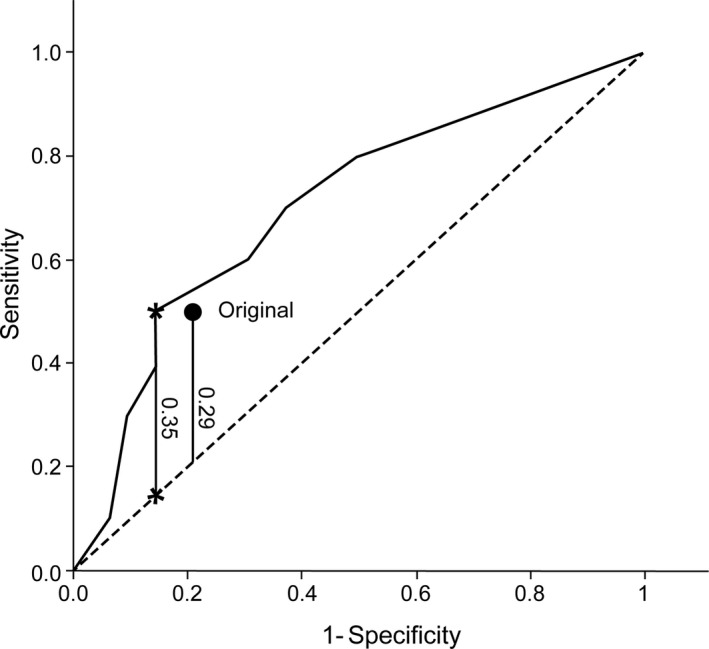
The optimum threshold is found at a Youden's J statistic shown as a vertical line, delineated by the 2 asterisks. The prior algorithm is plotted as a singular point, below the receiver operating characteristic of the optimized algorithm, with a smaller Youden's J statistic shown as the vertical line below this point.
Although the original algorithm demonstrated a statistically significant difference by log‐rank analysis in the low‐flow versus normal‐flow patient cohorts (P=0.04), the age‐adjusted algorithm improved upon this statistical significance (P=0.01) (Figure 3), and better distinguished the stroke risk in low‐flow versus normal‐flow patients (Table 2). Furthermore, the HR in a Cox model was more robust and significant: 4.5 (95% CI, 1.3–15.5; P=0.02) in the optimized algorithm compared with 3.4 (95% CI, 0.99–11.8; P=0.05).
Figure 3. Cumulative probabilities of stroke.
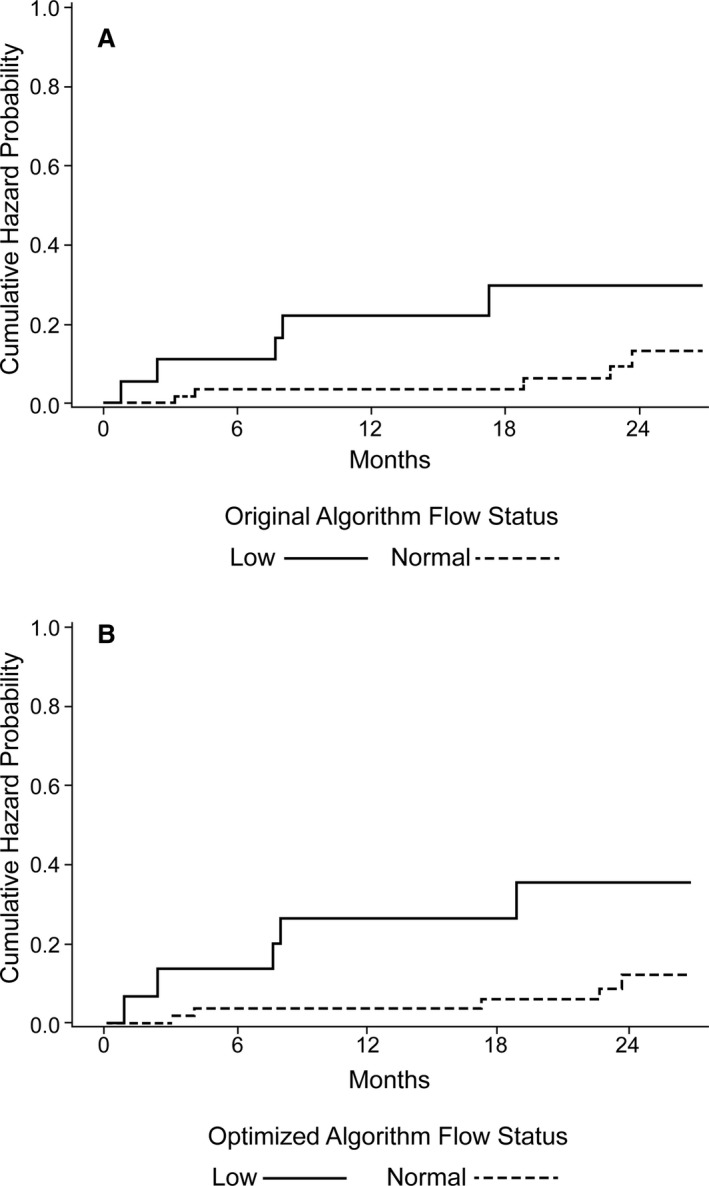
A, The log‐rank analysis of event occurrence with the original algorithm. B, The optimized algorithm distinguishes the low‐ and high‐flow groups in a more statistically significant fashion.
Discussion
Patients with vertebrobasilar circulation transient ischemic attack or stroke secondary to athero‐occlusive disease are at an increased risk of a recurrent event.14 QMRA has been shown to be an effective means of stratifying these patients into high‐ and low‐risk categories for subsequent stroke based on vessel‐specific flows.7 Given the well‐recognized flow variations attributable to vascular anatomic variants and the reduction in cerebral blood flow that occurs with age, we sought to optimize the existing flow algorithm by accounting for both PCA anatomy and age. Our optimized algorithm enhances specificity without sacrificing sensitivity and is less complex because of the elimination of a borderline flow category and the associated subjective clinical criteria involved in the original algorithm. Decreased algorithm complexity may facilitate clinical and research use and simplifies automated stratification.
Despite the significant effect of age on blood flow, Table 1 demonstrates that anatomic configuration remains a primary driver of BA flow rate. Fetal supply to a PCA, which is accounted for in both algorithms, is more impactful on the BA flow than the age of the patient. As a result, the 2 algorithms differ in only 7% of assignments. However, the age proves critical in the determination of every discrepancy. All the restratifications occurred in patients >60 years old. The optimized algorithm demonstrates that accounting for both anatomic variation and age is important in determining normal vertebrobasilar blood flow and regional risk of secondary stroke.
Randomized controlled trials of interventions such as angioplasty/stenting have not demonstrated benefit for patients with vertebrobasilar, or other intracranial stenosis, in part due to high peri‐procedural complication rates.15, 16, 17, 18, 19 However, such trials did not restrict inclusion to high‐risk flow‐compromised patients and may have thus precluded determination of benefit. Consequently, optimal performance of an algorithm for flow stratification is critical to enriching the at‐risk population in this context. More so than sensitivity, higher specificity is particularly important in patient stratification by honing the candidate population for higher‐risk interventions to those who are likely to glean benefit by virtue of their elevated risk for recurrent vertebrobasilar stroke without intervention. Highly specific stratification is therefore paramount and more useful in identifying appropriate target patients. Although achieving a high sensitivity is also favorable, the clinical imperative is to avoid unnecessary interventions and their associated risk of complications, in order to achieve a favorable balance between recurrent stroke prevention and procedural risk.
Although several diagnostic metrics are available to measure the quality of the optimized algorithm, predictive values (both negative and positive) are distorted by prevalence of the event, in this case recurrent vertebrobasilar stroke, which remains relatively low at 14% in the overall patient population. The log rank and HR are valuable in comparing the prior and optimized algorithms, as, unlike sensitivity, specificity, positive predictive value, and negative predictive value, these statistics account for the exposure to time‐at‐risk. The optimized algorithm has better predictive performance on these time‐to‐event analyses.
Limitations of this study include the post hoc nature of the algorithm validation, although testing of the algorithm in a prospective blinded centrally adjudicated cohort closely simulates a priori testing. External validation with larger cohorts will be useful in the future. The normalizing data set used in algorithm development was insufficiently large to allow age‐specific stratification of patients with unilateral or bilateral fetal PCA, which could theoretically further improve the algorithm. These variants, however, are relatively uncommon. Although the age distribution of the normalizing data is skewed younger, this is a natural reflection of an increasing frequency of medical disease in older people, which excludes them from a healthy cohort; although this reduces the relative sample size for determination of normative flow ranges in the older age group, the sample of healthy elderly was large enough to generate reference data with similar standard deviations as for the younger healthy cohort.
Notably, the findings here are applicable to atherosclerotic disease but not dissection as an etiology of decreased flow, since the original study was designed to exclude cases of potential dissecting mechanism. Patients with unilateral vertebral occlusion were excluded from the original study because of difficulty in confirming underlying pathology as atherosclerotic versus dissection; in principle, this would preclude generalizing this model to such cases.
Conclusions
Although QMRA flows have already been demonstrated to stratify the vertebrobasilar stroke population into high‐ and low‐risk subgroups, using anatomic and age‐normalization further improves the effectiveness of the stratification. This is useful in optimizing the identification of particularly high‐risk patients in whom procedural interventions could reduce the risk of recurrent vertebrobasilar stroke. This methodology should be preferentially used for patient selection for future trials.
Appendix
VERiTAS Study Group
Clinical Coordinating Center
University of Illinois at Chicago; PI: Sepideh Amin‐Hanjani, MD; Project Manager: Linda Rose‐Finnell, MPA CCRA.
Data Management Center
Center for Stroke Research, University of Illinois at Chicago; Director: DeJuran Richardson, PhD, Dilip Pandey, MD, PhD; Biostatisticians: Xinjian Du, MD MPH, Hui Xie, PhD; Database Administrator: Xinjian Du, MD, MPH.
Participating Sites (in Descending Order of Number of Enrollees)
University of Illinois at Chicago: Site PI: Sepideh Amin‐Hanjani, MD; Project Manager: Linda Rose‐Finnell, MPA CCRA; Site MR Team: Keith Thulborn, MD, PhD, Michael P. Flannery, Hagai Ganin; Study Physician(s): Sean Ruland, DO, Rebecca Grysiewicz, DO, Aslam Khaja, MD, Laura Pedelty, MD, Fernando Testai, MD, Archie Ong, MD, Noam Epstein, MD, Hurmina Muqtadar, MD; Coordinator(s): Karriem Watson, MD, Nada Mlinarevich, RN, Maureen Hillmann, RN.
Columbia University, New York: Site PI: Mitchell S. V. Elkind, MD; Site MR Team: Joy Hirsch, PhD, Stephen Dashnaw; Study Physician(s): Philip M. Meyers, MD, Josh Z. Willey, MD; Coordinator(s): Edwina McNeill‐Simaan, BS, Veronica Perez, MA, Alberto Canaan, MD, Wayna Paulino‐Hernandez, MD.
Washington University, St. Louis: Site PI: Gregory J. Zipfel, MD; Site MR Team: Katie Vo, MD, Glenn Foster; Study Physicians: Andria Ford, MD, Abdullah Nassief, MD; Coordinator(s): Abbie Bradley, RN, BSN, MSW, Jannie Serna‐Northway, RN, BSN, Kristi Kraus, RN, Lina Shiwani, BS, Nancy Hantler, BS, CCRC.
University of California, Los Angeles: Site PI: David S. Liebeskind, MD; Site MR Team: Jeffrey Alger, PhD, Sergio Godinez; Study Physician(s): Jeffrey L. Saver, MD, Latisha Ali, MD, Doojin Kim, MD, Matthew Tenser, MD, Michael Froehler, MD, Radoslav Raychev, MD, Sarah Song, MD, Bruce Ovbiagele, MD, Hermelinda Abcede, MD, Peter Adamczyk, MD, Neal Rao, MD, Anil Yallapragada, MD, Royya Modir, MD, Jason Hinman, MD, Aaron Tansy, MD, Mateo Calderon‐Arnulphi, MD, Sunil Sheth, MD, Alireza Noorian, MD, Kwan Ng, MD, Conrad Liang, MD; Coordinator: Jignesh Gadhia, BS, Hannah Smith, BS, Gilda Avila, BS, Johanna Avelar, BA.
University of Toronto ‐Toronto Western Hospital, Toronto: Site PI: Frank L. Silver, MD; Site MR Team: David Mikulis, MD, Jorn Fierstra, Eugen Hlasny; Study Physician(s): Leanne K. Casaubon, MD, Mervyn Vergouwen, MD, J. C. Martin del Campo, MD, Cheryl S. Jaigobin, MD; Coordinator(s): Cherissa Astorga, RN, Libby Kalman, RN.
Satellite Site
Mercy Hospital and Medical Center, Chicago: Site PI: Jeffrey Kramer, MD; Study Physician(s): Susan Vaughan, MD; Coordinator(s): Laura Owens, RN.
Committees and Panels
Operations Committee: Sepideh Amin‐Hanjani, MD, FACS (Chair); Fady T. Charbel, MD, FACS; Dilip K. Pandey, MD, PhD; DeJuran Richardson, PhD; Keith R. Thulborn, MD, PhD.
Advisory Committee: Colin P. Derdeyn, MD (Chair); Louis R. Caplan, MD; Philip B. Gorelick, MD, MPH, FACP.
Adjudication Committee: Scott E. Kasner, MD (Chair); Brett Kissela, MD; Tanya N. Turan, MD.
Central Angiography Review: Victor Aletich, MD.
National Institutes of Health/National Institute of Neurological Disorders and Stroke Program Officer(s): Tom P. Jacobs, MD/Scott Janis PhD.
Sources of Funding
The study was funded by grant R01 NS 059745 from the National Institutes of Health/National Institute of Neurological Disorders and Stroke. Additional funding and support was provided by the Dr Ralph and Marian Falk Research Trust Foundation. Material research support was provided by VasSol Inc (supplying NOVA technology and technical support).
Disclosures
Dr Amin‐Hanjani reported receiving material research support (no direct funds) from GE Healthcare and VasSol Inc for the VERiTAS study. The remaining authors have no disclosures to report.
(J Am Heart Assoc. 2020;9:e016406 DOI: 10.1161/JAHA.120.016406.)
This article was sent to Ik‐Kyung Jang, MD, PhD, Guest Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 7.
Contributor Information
Sepideh Amin‐Hanjani, Email: hanjani@uic.edu.
for the VERiTAS Study Group*:
DeJuran Richardson, Hui Xie, Keith Thulborn, Michael P. Flanner, Hagai Ganin, Sean Ruland, Rebecca Grysiewicz, Aslam Khaja, Laura Pedelty, Fernando Testai, Archie Ong, Noam Epstein, Hurmina Muqtadar, Karriem Watson, Nada Mlinarevich, Maureen Hillmann, Mitchell S. V. Elkind, Joy Hirsch, Stephen Dashnaw, Philip M. Meyers, Josh Z. Willey, Edwina McNeill‐Simaan, Veronica Perez, Alberto Canaan, Wayna Paulino‐Hernandez, Gregory J. Zipfel, Katie Vo, Glenn Foster, Andria Ford, Abdullah Nassief, Abbie Bradley, Jannie Serna‐Northway, Kristi Kraus, Lina Shiwani, Nancy Hantler, David S. Liebeskind, Jeffrey Alger, Sergio Godinez, Jeffrey L. Saver, Latisha Ali, Doojin Kim, Matthew Tenser, Michael Froehler, Radoslav Raychev, Sarah Song, Bruce Ovbiagele, Hermelinda Abcede, Peter Adamczyk, Neal Rao, Anil Yallapragada, Royya Modir, Jason Hinman, Aaron Tansy, Mateo Calderon‐Arnulphi, Sunil Sheth, Alireza Noorian, Kwan Ng, Conrad Liang, Jignesh Gadhia, Hannah Smith, Gilda Avila, Johanna Avelar, Frank L. Silver, David Mikulis, Jorn Fierstra, Eugen Hlasny, Leanne K. Casaubon, Mervyn Vergouwen, J. C. Martin del Campo, Cheryl S. Jaigobin, Cherissa Astorga, Libby Kalman, Jeffrey Kramer, Susan Vaughan, Laura Owens, DeJuran Richardson, Keith R. Thulborn, Louis R. Caplan, Philip B. Gorelick, Scott E. Kasner, Brett Kissela, Tanya N. Turan, Victor Aletich, Tom P. Jacobs, and Scott Janis
References
- 1. Caplan LR, Wityk RJ, Glass TA, Tapia J, Pazdera L, Chang HM, Teal P, Dashe JF, Chaves CJ, Breen JC, et al. New England Medical Center Posterior Circulation registry. Ann Neurol. 2004;56:389–398. [DOI] [PubMed] [Google Scholar]
- 2. Qureshi AI, Qureshi MH, Lien LM, Lee JT, Jeng JS, Hu CJ, Lai TC, Liu CH, Chen CH, Chen PL, et al. One‐year risk of recurrent stroke and death associated with vertebrobasilar artery stenosis and occlusion in a cohort of 10,515 patients. Cerebrovasc Dis. 2019;47:40–47. [DOI] [PubMed] [Google Scholar]
- 3. Michel P, Odier C, Rutgers M, Reichhart M, Maeder P, Meuli R, Wintermark M, Maghraoui A, Faouzi M, Croquelois A, et al. The acute stroke registry and analysis of Lausanne (ASTRAL): design and baseline analysis of an ischemic stroke registry including acute multimodal imaging. Stroke. 2010;41:2491–2498. [DOI] [PubMed] [Google Scholar]
- 4. Vemmos KN, Takis CE, Georgilis K, Zakopoulos NA, Lekakis JP, Papamichael CM, Zis VP, Stamatelopoulos S. The Athens stroke registry: results of a five‐year hospital‐based study. Cerebrovasc Dis. 2000;10:133–141. [DOI] [PubMed] [Google Scholar]
- 5. Labropoulos N, Nandivada P, Bekelis K. Stroke of the posterior cerebral circulation. Int Angiol. 2011;30:105–114. [PubMed] [Google Scholar]
- 6. Marquardt L, Kuker W, Chandratheva A, Geraghty O, Rothwell PM. Incidence and prognosis of > or = 50% symptomatic vertebral or basilar artery stenosis: prospective population‐based study. Brain. 2009;132:982–988. [DOI] [PubMed] [Google Scholar]
- 7. Amin‐Hanjani S, Pandey DK, Rose‐Finnell L, Du X, Richardson D, Thulborn KR, Elkind MSV, Zipfel GJ, Liebeskind DS, Silver FL, et al. Effect of hemodynamics on stroke risk in symptomatic atherosclerotic vertebrobasilar occlusive disease. JAMA Neurol. 2016;73:178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Amin‐Hanjani S, Rose‐Finnell L, Richardson D, Ruland S, Pandey D, Thulborn KR, Liebeskind DS, Zipfel GJ, Elkind MSV, Kramer J, et al. Vertebrobasilar flow evaluation and risk of transient ischaemic attack and stroke study (VERiTAS): rationale and design. Int J Stroke. 2010;5:499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Special report from the National Institute of Neurological Disorders and Stroke. Classification of cerebrovascular diseases III. Stroke. 1990;21:637–676. [DOI] [PubMed] [Google Scholar]
- 10. Amin‐Hanjani S, Du X, Rose‐Finnell L, Pandey DK, Richardson D, Thulborn KR, Elkind MSV, Zipfel GJ, Liebeskind DS, Silver FL, et al. Hemodynamic features of symptomatic vertebrobasilar disease. Stroke. 2015;46:1850–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Amin‐Hanjani S, Du X, Zhao M, Walsh K, Malisch TW, Charbel FT. Use of quantitative magnetic resonance angiography to stratify stroke risk in symptomatic vertebrobasilar disease. Stroke. 2005;36:1140–1145. [DOI] [PubMed] [Google Scholar]
- 12. Amin‐Hanjani S, Du X, Pandey DK, Thulborn KR, Charbel FT. Effect of age and vascular anatomy on blood flow in major cerebral vessels. J Cereb Blood Flow Metab. 2015;35:312–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. [DOI] [PubMed] [Google Scholar]
- 14. Gulli G, Marquardt L, Rothwell PM, Markus HS. Stroke risk after posterior circulation stroke/transient ischemic attack and its relationship to site of vertebrobasilar stenosis: pooled data analysis from prospective studies. Stroke. 2013;44:598–604. [DOI] [PubMed] [Google Scholar]
- 15. Zaidat OO, Fitzsimmons B‐F, Woodward BK, Wang Z, Killer‐Oberpfalzer M, Wakhloo A, Gupta R, Kirshner H, Megerian JT, Lesko J, et al. Effect of a balloon‐expandable intracranial stent vs medical therapy on risk of stroke in patients with symptomatic intracranial stenosis: the VISSIT randomized clinical trial. JAMA. 2015;313:1240–1248. [DOI] [PubMed] [Google Scholar]
- 16. Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, Janis LS, Lutsep HL, Barnwell SL, Waters MF, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365:993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Derdeyn CP, Chimowitz MI, Lynn MJ, Fiorella D, Turan TN, Janis LS, Montgomery J, Nizam A, Lane BF, Lutsep HL, et al. Aggressive medical treatment with or without stenting in high‐risk patients with intracranial artery stenosis (SAMMPRIS): the final results of a randomised trial. Lancet. 2014;383:333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Markus HS, Larsson SC, Kuker W, Schulz UG, Ford I, Rothwell PM, Clifton A; VIST Investigators . Stenting for symptomatic vertebral artery stenosis: the Vertebral Artery Ischaemia Stenting Trial. Neurology. 2017;89:1229–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Compter A, van der Worp HB, Algra A, Kappelle LJ; VAST Investigators . Risks of stenting in patients with extracranial and intracranial vertebral artery stenosis. Lancet Neurol. 2015;14:875. [DOI] [PubMed] [Google Scholar]


