Abstract
Background
Abnormal circadian blood pressure (BP) variations during sleep, specifically the non‐dipping (<10% fall in nocturnal BP) and reverse‐dipping patterns (rise in nocturnal BP), have been associated with an increased risk of cardiovascular events and target organ damage. However, the relationship between abnormal sleep BP variations and cerebral small vessel disease markers is poorly established. This study aims to assess the association between non‐dipping and reverse‐dipping BP patterns with markers of silent cerebral small vessel disease.
Methods and Results
MEDLINE, Embase, and Cochrane Databases were searched from inception through November 2019. Studies that reported the odds ratios (ORs) for cerebral small vessel disease markers in patients with non‐dipping or reverse‐dipping BP patterns were included. Effect estimates from the individual studies were extracted and combined using the random‐effect, generic inverse variance method of DerSimonian and Laird. Twelve observational studies composed of 3497 patients were included in this analysis. The reverse‐dipping compared with normal dipping BP pattern was associated with a higher prevalence of white matter hyperintensity with a pooled adjusted OR of 2.00 (95% CI, 1.13–2.37; I2=36%). Non‐dipping BP pattern compared with normal dipping BP pattern was associated with higher prevalence of white matter hyperintensity and asymptomatic lacunar infarction, with pooled ORs of 1.38 (95% CI, 0.95–2.02; I2=52%) and 2.33 (95% CI, 1.30–4.18; I2=73%), respectively. Limiting to only studies with confounder‐adjusted analysis resulted in a pooled OR of 1.38 (95% CI, 0.95–2.02; I2=52%) for white matter hyperintensity and 1.44 (95% CI, 0.97–2.13; I2=0%) for asymptomatic lacunar infarction.
Conclusions
The non‐dipping and reverse‐dipping BP patterns are associated with neuroimaging cerebral small vessel disease markers.
Keywords: blood pressure variability, circadian, meta‐analysis, microbleed, white matter
Subject Categories: Cerebrovascular Disease/Stroke, High Blood Pressure, Magnetic Resonance Imaging (MRI)
Nonstandard Abbreviation and Acronyms
- ALI
asymptomatic lacunar infarction
- CMB
cerebral microbleed
- CSVD
cerebral small vessel disease
- PVS
perivascular space
- WMH
white matter hyperintensity
Clinical Perspective
What Is New?
This systematic review and meta‐analysis of 3497 patients from 12 studies demonstrated an association between abnormal circadian blood pressure pattern, reverse‐dipping and non‐dipping nighttime blood pressure patterns, and prevalent cerebral small vessel disease.
What Are the Clinical Implications?
These results further support the concerns about the abnormal circadian blood pressure pattern and 24‐hour blood pressure control on target organ damages.
This study demonstrates the usefulness of neuroimaging markers of silent cerebral small vessel disease as a presymptomatic marker of cerebrovascular disease.
Circadian rhythm plays an important role in the governance and maintenance of homeostasis in the human body.1 The normal circadian blood pressure pattern is characterized by a mild decrease in blood pressure during sleep that reaches its trough around midnight when the deep sleep stages are most abundant (nocturnal dip), with a subsequent gradual rise towards the end of sleep (morning surge). However, in some individuals during sleep, the nocturnal dip is blunted or reversed. This results in the absence of a nocturnal dip or even a slight increase in blood pressure, termed “non‐dipper” and “reverse‐dipper”, respectively.2, 3
Studies have found that these abnormal blood pressure patterns are linked to an increased risk for cardiovascular events and target organ damage in both normotensive and hypertensive individuals.4, 5, 6, 7, 8, 9, 10, 11 Moreover, better control of sleep blood pressures has been shown to exert greater protection against stroke and cardiovascular events in many studies.12, 13 The insurmountable evidence linking these 2 abnormal blood pressure patterns to target organ damages and cardiovascular events lead us to explore its association to presymptomatic markers of cerebrovascular disease, the neuroimaging markers of cerebral small vessel disease (CSVD).
Silent findings of CSVD on neuroimaging studies, such as white matter hyperintensities (WMH), asymptomatic lacunar infarction (ALI), cerebral microbleeds (CMBs), and enlarged perivascular spaces (PVS), have recently emerged as important surrogate markers for cerebrovascular disease.14 Elevated blood pressures are one of the main risk factors for CSVD, and better control of blood pressure has been shown to decrease WMH progression.15, 16, 17 However, the relationship between abnormal circadian blood pressure variations and CSVD neuroimaging markers has not been well established.
This study investigates the association between abnormal circadian blood pressure variations, specifically the non‐dipping and reverse‐dipping patterns, and silent CSVD neuroimaging markers by performing a systematic review and meta‐analysis of the relevant published literature.
Methods
The protocol for this meta‐analysis is registered with PROSPERO (International Prospective Register of Systematic Reviews; no. CRD42020147729). The data for this systematic review and all potentially eligible studies are publicly available through the Open Science Framework (URL: https://osf.io/gf8pr/).
Search Strategy and Literature Review
A systematic literature search of EMBASE (1988 to November 2019), Ovid MEDLINE (1946 to November 2019), and the Cochrane Database of Systematic Reviews (database inception to November 2019) was performed to assess the association of abnormal circadian sleep blood pressure variations, specifically the non‐dipping and reverse‐dipping blood pressure patterns, on CSVD neuroimaging markers consisting of WMH, ALI, CMBs, and enlarged PVS. The non‐dipping blood pressure pattern refers to a nocturnal blood pressure fall of <10% from the awake blood pressures, and the reverse‐dipping blood pressure pattern refers to a rise in nocturnal blood pressures.18 The systematic literature review was undertaken independently by 2 investigators (A.C. and R.C.) using a search approach that incorporated the terms “dipping” OR “non‐dipping” OR “dipper” OR “non‐dipper” OR “reverse‐dipping” OR “reverse‐dipper” OR “circadian blood pressure” OR “nocturnal blood pressure”, “night‐time blood pressure”, “ambulatory blood pressure”, “ambulatory blood pressure monitoring” AND “silent cerebrovascular disease” OR “silent stroke” OR “silent ischemic stroke” OR “white matter hyperintensity” OR “lacunar infarct” OR “cerebral microbleed” OR “brain microbleed” OR “small vessel disease” OR “cerebrovascular disease” OR “silent cerebral infarct” OR “white matter change” OR “perivascular space”. The search strategy used for each database is provided in Data S1. No language limitation was applied. A manual search for conceivably relevant studies using the references of included articles was also performed. This study was conducted by the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis statement,19 as provided in Data S1.
Selection Criteria
To be included in the meta‐analysis, studies were required to meet the following criteria: (1) observational studies including cohort, case‐control, or cross‐sectional studies (2) reported the association of non‐dipping or reverse‐dipping blood pressure patterns on WMH, ALI, CMBs, or enlarged PVS, (3) an outcome definition was provided, (4) and reported odds ratios (OR) of any CSVD neuroimaging markers. Review articles and case reports were excluded from this meta‐analysis. Retrieved articles were individually reviewed for eligibility by the 2 investigators (A.B. and R.C.). Discrepancies were addressed and solved by mutual agreement. Inclusion was not limited by the size of the study. Newcastle‐Ottawa quality assessment scale was used to appraise the quality of the included observational studies.20
Data Abstraction
Characteristics of the study including first author, study location, publication year, study design, patient demographic data, CSVD outcome definition, exposure and measurement of exposure, follow up time, and confounder adjustments were retrieved. ORs reported in each study were extracted.
Statistical Analysis
All statistical analyses were performed using the Comprehensive Meta‐Analysis version 3 software (Eaglewood, NJ, USA). The pooled ORs of silent CSVD neuroimaging markers in patients with non‐dipping or reverse‐dipping blood pressure patterns were calculated using a generic inverse method of DerSimonian and Laird.21 A random‐effects model was used, given the high likelihood of between study variance. Cochran Q‐test, which is supplemented by I2 statistic, was used to evaluate statistical heterogeneity. The I2 statistic quantifies the proportion of total variation across studies that is because of true heterogeneity rather than chance. A value of I2 of 0% to 25% denotes trivial heterogeneity, 25% to ≤50% denotes low heterogeneity, 50% to ≤75% denotes moderate heterogeneity, and >75% represents high heterogeneity.22
Results
We identified a total of 1334 potentially eligible studies from our search strategy. After title and abstract review, 20 studies were excluded because they were duplicates and 1281 studies were excluded because they did not fulfill the inclusion criteria based on the type of article, study design, study population, or outcome of interest. Thirty‐three articles were left for full‐length review. Nineteen articles were subsequently excluded because they did not report the odds ratios for neuroimaging CSVD findings in relationship to non‐dipping or reverse‐dipping blood pressure pattern. Another 2 studies were excluded because they were not observational studies. Ultimately, 12 cross‐sectional studies comprising 3497 individuals were enrolled.23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34 The literature retrieval, review, and selection process are demonstrated in Figure 1. The characteristics of the included studies are presented in Table.
Figure 1. Outline of our selection process.
Table 1.
Study Characteristics
| Study, Y/Study Design | Country/Population | Number (%Male) | Mean Age±SD | BP Monitoring | BP Pattern | Imaging | CSVD Feature(s) | Confounder Adjusted | Quality Assessment (NOS) |
|---|---|---|---|---|---|---|---|---|---|
| Hamada et al,23 2003/cross‐sectional | Japan/adults aged 50–76 y with depression | 36 (50%) | 64.46±5.9 | Automated 24‐h ambulatory BP | Non‐dipping | 1.5 T MRI | ALI | N/A |
S3 C0 O3 |
| Yamamoto et al,24 2005/cross‐sectional | Japan/acute lacunar infarction | 200 (61%) | 68.8±9.3 | Automated 24‐h ambulatory BP (2–4 wk after stroke) |
Non‐dipping, reverse‐dipping |
1.5 T MRI | ALI, WMH by Fazekas scale | Age, sex |
S4 C2 O3 |
| Henskens et al,25 2008/cross‐sectional | Netherlands/untreated hypertensive patients | 218 (50.5%) | 52.5±12.6 | Automated 24‐h ambulatory BP | Non‐dipping | 1.5 T MRI | CMBs on T2*‐weighted GE image | Age (y), sex, duration of hypertension, prior BP‐lowering agent, smoking, ratio of total/HDL, advanced WMH |
S5 C2 O3 |
| Staals et al,26 2009/cross‐sectional | Netherlands/first lacunar stroke | 97 (61%) | 64.6±11.7 | Automated 24‐h ambulatory BP (1–6 mo after stroke) | Nocturnal SBP dip | 1.5 T MRI | CMBs on T2*‐weighted GE image | Age, sex, number of BP‐lowering agents, asymptomatic lacunar infarction, extensive white matter lesions |
S5 C2 O3 |
| Ma et al,27 2010/cross‐sectional | China/hypertensive patients | 188 (42.5%) | 64±6.6 | Automated 24‐h ambulatory BP | Non‐dipping | MRI | ALI | N/A |
S4 C0 O3 |
| Yamamoto et al,28 2011/cross‐sectional | Japan/acute lacunar infarction | 224 (60%) | 69.8±9.34 | Automated 24‐h ambulatory BP (>2 wk after stroke) | Non‐dipper, reverse‐dipping | 1.5 T MRI |
Multiple ALI gr 3 vs gr 1, WMH Fazekas scale gr 3 vs gr 1 |
eGFR level |
S4 C1 O3 |
| Shimizu et al,29 2011/cross‐sectional | Japan/hypertensive patients | 514 (37%) | 72.3±8.7 | Automated 24‐h ambulatory BP | Non‐dipping | 1.5 T MRI | ALI | N/A |
S4 C2 O3 |
| Lee et al,30 2014/cross‐sectional | Korea/adults aged 40–69 y (exclude hypertension) | 703 (47.5%) | 59.43±6.79 | Automated 24‐h ambulatory BP | Non‐dipping, reverse‐dipping | 1.5 T MRI | WMH by ARWMC scale | Age (y), sex, BMI, total cholesterol, hs‐CRP, DM, smoking, alcohol |
S4 C2 O3 |
| Kwon et al,31 2014/cross‐sectional | Korea/acute ischemic stroke with hypertension | 162 (61.7%) | 65.33±10.32 | Automated 24‐h ambulatory BP | Non‐dipping, reverse‐dipping | 1.5 T MRI | CMBs on T2*‐weighted GE image | Age (y), sex, LDL, 24 h mean SBP/DBP |
S4 C2 O3 |
| Yamashiro et al,32 2018/cross‐sectional | Japan/Parkinson disease | 128 (43%) | 82.1±3.9 | Automated 24‐h ambulatory BP | Non‐dipping | 3 T MRI | CMBs on T2* by Microbleed Anatomical Rating Scale | N/A |
S4 C0 O3 |
| White et al,33 2018/cross‐sectional | USA/elderly aged ≥75 y | 199 (45.7%) | 81.2±4.1 | Automated 24‐h ambulatory BP | Non‐dipping | 1.5 T MRI | WMH volume | N/A |
S4 C0 O3 |
| Nakanishi et al,34 2019/cross‐sectional | /adults aged ≥55 y (exclude history of stroke) | 828 (39.9%) | 70.9±9 | Automated 24‐h ambulatory BP | Non‐dipping | 1.5 T MRI | ALI, High WMH volume (upper quartile) |
ALI : age (y), sex, hypertension, AF, LV mass index, interval between ABPM and MRI WMH : age, race, hypertension, AF, LV mass index, LA diameter, interval between ABPM and MRI |
S4 C2 O3 |
ABPM indicates ambulatory blood pressure monitoring; AF, atrial fibrillation; ALI, asymptomatic lacunar infarct; ARWMC, age‐related white mater changes; BMI, body mass index; CMBs, cerebral microbleeds; CSVD, cerebral small vessel disease; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; GE, gradient echo; HDL, high‐density lipoprotein; hs‐CRP, high‐sensitivity C‐reactive protein; LA, left atrium; LDL, low‐density lipoprotein; LV, left ventricle; MRI, magnetic resonance imaging; N/A, not available; NOS, Newcastle Ottawa Scale; OR, odds ratio; S, C, O, selection, comparability, and outcome; SBP, systolic blood pressure; and WMH, white matter hyperintensity.
Association of a Blood Pressure Reverse‐Dipping Pattern With CSVD Neuroimaging Features
There was a significant association between the nocturnal blood pressure reverse‐dipping pattern and WMH with a pooled adjusted OR of 2.00 (95% CI, 1.13–2.37; I2=36%), when compared with patients with normal nocturnal dip (Figure 2A).24, 28, 30 Sensitivity analysis was performed including only clinic‐based cross‐sectional studies and demonstrated a significant association between the nocturnal blood pressure reverse‐dipping pattern and WMH with a pooled adjusted OR of 3.16 (95% CI, 1.44–6.93; I2=0%).24, 28
Figure 2. Forest plot of the association between reverse‐dipping pattern and silent cerebral small vessel disease neuroimaging features.
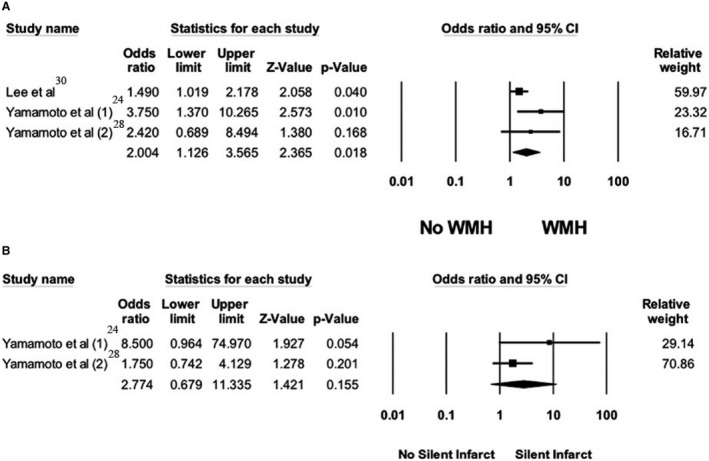
A, Forest plots of included studies assessing the association between reverse‐dipping pattern and white matter hyperintensity. B, Forest plots of the included studies assessing the association between reverse‐dipping pattern and asymptomatic lacunar infarct. A diamond data marker depicts the overall rate from included studies (square data markers) and 95% CI. WMH indicates white matter hypersensitivity.
Two studies assessed the association of the reverse‐dipping pattern and ALI compared with normal dipping pattern. The pooled adjusted OR was 2.77 (95% CI, 0.68–11.34; I2=43%) for reverse‐dipping pattern (Figure 2B).24, 28
Association of Blood Pressure Non‐Dipping Pattern With CSVD Neuroimaging Features
There was a significant association between the non‐dipping blood pressure pattern and WMH with a pooled OR of 1.51 (95% CI, 1.06–2.14, I2=55%), when compared with patients with a normal nocturnal dip (Figure 3A).24, 28, 30, 33, 34 Sensitivity analysis was performed including only clinic‐based cross‐sectional studies and demonstrated a significant association between the non‐dipping blood pressure pattern and WMH with a pooled adjusted OR of 1.99 (95% CI, 1.29–3.05; I2=0%).24, 28, 33 Meta‐analysis limited to studies with confounder‐adjusted analysis was performed. The adjusted pooled OR for WMH was 1.38 (95% CI, 0.95–2.02; I2=52%) among patients with non‐dipping nocturnal blood pressure pattern (Figure S1).24, 28, 30, 34
Figure 3. Forest plot of the association between non‐dipping pattern and silent cerebral small vessel disease neuroimaging features.
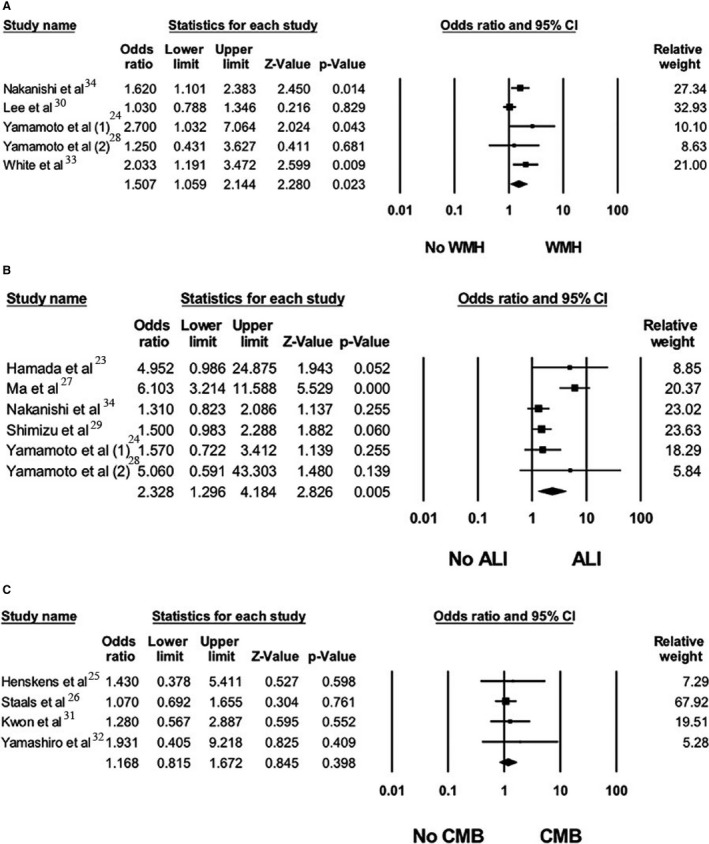
A, Forest plots of the included studies assessing the association between non‐dipping pattern and white matter hyperintensity. B, Forest plots of the included studies assessing association between non‐dipping pattern and asymptomatic lacunar infarction . C, Forest plots of the included studies assessing association between non‐dipping pattern and cerebral microbleeds. A diamond data marker depicts the overall rate from included studies (square data markers) and 95% CI. CMB indicates cerebral microbleeds; and WMH, white matter hypersensitivity.
An analysis of all studies assessing the association between ALI and non‐dipping blood pressure pattern compared with normal dipping blood pressure pattern revealed a significant association with a pooled OR of 2.33 (95% CI, 1.30–4.18; I2=73%) (Figure 3B).23, 24, 27, 28, 29, 34 Sensitivity analysis was performed including only clinic‐based cross‐sectional studies and demonstrated a significant association between the non‐dipping blood pressure pattern and ALI with a pooled adjusted OR of 2.82 (95% CI, 1.34–5.95; I2=74%).23, 24, 27, 28, 29 However, when the analysis was limited to only confounder‐adjusted studies, the association was not statistically significant, with a pooled adjusted OR of 1.44 (95% CI, 0.97–2.13; I2=0%) (Figure S2).24, 28, 34
There was no significant association between nocturnal blood pressure non‐dipping pattern and CMBs when compared with normal nocturnal dipping pattern in all included studies. The pooled OR was 1.17 (95% CI, 0.82–1.67; I2=0%) (Figure 3C)25, 26, 31, 32 and the pooled adjusted OR was 1.14 (95% CI, 0.79–1.64; I2=0%) (Figure S3).25, 26, 31
Data on the association between abnormal circadian blood pressure variations and enlarged PVS were limited and thus a meta‐analysis could not be performed.
Evaluation for Publication Bias
Since tests for funnel plot asymmetry should be used only when there are at least 10 study groups, a funnel plot was not drawn because of the limited number of studies.35 Egger regression asymmetry test was performed and showed no publication bias with P<0.05 for all analyses.
Discussion
Our study demonstrated a significant association between a reverse‐dipping blood pressure pattern and a higher prevalence of WMH, but not ALI. A significant unadjusted association was also found between a non‐dipping blood pressure pattern and an increased prevalence of both WMH and ALI. However, when limiting the analysis to only confounder‐adjusted studies (4 studies for WMH and 3 studies for ALI), the results were no longer statistically significant. We did not find an association between a non‐dipping blood pressure pattern and CMBs.
Hypertension is known to cause damage to both large and small vessels leading to target organ damages, most importantly the heart, brain, and kidneys.36, 37 In hypertensive cerebral small vessel disease, researchers found that hypertension primarily causes endothelial dysfunction leading to subsequent blood‐brain barrier dysfunction, formation of microaneurysms, decreased cerebrovascular reactivity, cerebral hypoperfusion, and neuroinflammation.16, 38, 39, 40, 41 These pathological processes in the brain parenchyma can produce either “silent” findings on neuroimaging studies or symptoms such as strokes.
The circadian rhythm is essential for the regulation and maintenance of normal physiologic functions in the body. The circadian control of the cardiovascular system is most evident with the diurnal blood pressure pattern, characterized by a rise in blood pressure in the morning before awakening and a decrease while sleeping in the night.42, 43 This control is achieved through a synergy of several processes, including neurohormonal factors, vascular tone, the autonomic nervous system, and renal system.1, 5, 42, 44 Abnormal function of these processes or certain environmental changes could lead to disruption of this normal circadian blood pressure pattern through either increased cardiac output or systemic vascular resistance during sleep. Common diseases associated with this disruption include diabetes mellitus, neurodegenerative diseases with autonomic dysfunction, chronic kidney disease, obstructive sleep apnea, and most secondary causes of hypertension.2, 5, 45
An abnormal circadian blood pressure pattern, consisting of non‐dipping or reverse‐dipping, has been shown in several studies to increase the risk of cardiovascular disease. Studies have found an increased risk of myocardial infarction, peripheral artery disease, increased prevalence of vascular disease markers (eg, carotid plaque), and other cardiovascular events in individuals with non‐dipping or reverse‐dipping patterns.4, 5, 6, 7, 9, 10, 46, 47 Karadag et al showed that a non‐dipping blood pressure pattern was associated with a higher risk of microalbuminuria and hypertensive retinopathy in hypertensive patients.8 Another study by Yan et al found a significantly increased risk of lacunar infarction in reverse‐dipping compared with non‐ and normal‐dipping hypertensive patients.11
The pathophysiologic mechanism of non‐dipping and reverse‐dipping blood pressure pattern on vessels remains unclear. Some have proposed that the non‐dipping and the reverse‐dipping blood pressure patterns result in a higher 24‐hour mean blood pressure level, thereby imposing a greater overall pressure load and shear stress to vessels, resulting in accelerated atherosclerotic disease.26, 30, 34 This hypothesis is supported by studies that have demonstrated that reverse‐dippers are more affected than non‐dippers.11, 24, 28, 30 Our findings provide further evidence revealing an association between abnormal circadian blood pressure pattern and silent CSVD markers, and with reverse‐dippers having a more significant risk compared with non‐dippers. However, it remains to be elucidated if other mechanisms are involved in the process.
The neuroimaging features of silent CSVD, such as WMH, ALI, CMBs, and enlarged PVS, have recently been widely adopted as markers of small vessel cerebrovascular disease. Its main advantage is the ability to detect disease in its presymptomatic stage.14, 48 Discrepancies between each neuroimaging markers and their clinical importance have been reported. This may be largely because of the sensitivity of each marker on magnetic resonance imaging, the different techniques used to identify each marker, and the slightly different pathogenic processes for each marker.49 This discrepancy was also evident in our study. In addition, it is also worth noting that the magnetic resonance imaging used in most of the included studies in this meta‐analysis was performed using 1.5 T magnetic resonance imaging, which is less sensitive for pathological changes than the 3 T magnetic resonance imaging used in most current research studies. Moreover, detection of CMBs in the included studies was done on gradient echo (GRE) sequence rather than the currently recommended susceptibility‐weighted imaging, which offers more diagnostic sensitivity.50 Hence, because of the known technical limitations with the neuroimaging techniques used in these prior studies, the actual prevalence of WMH, ALI, and CMBs may be higher and the association more prominent if current and more sensitive neuroimaging techniques were used.
Interestingly, restoration of the normal physiologic blood pressure pattern by decreasing nighttime blood pressure has been explored in several studies.51, 52 The MAPEC (Spanish hypertensive cohort study [PMID: 20854139]) study, which aimed to compare bedtime long‐acting anti‐hypertensive medication administration (chronotherapy) to conventional morning time therapy, reported better control of 24‐hour blood pressure, lower prevalence of non‐dipper, and decreased total and major cardiovascular events in the bedtime treatment group.13 Another cohort study in both normotensive and hypertensive patients found that the decrease in nighttime systolic blood pressure was the most significant predictor for cardiovascular event‐free survival after 5 years of follow‐up, regardless of awake or office systolic blood pressure.4 Finally, a meta‐analysis of 9 cohort studies in hypertensive patients found that an increased nighttime systolic blood pressure was the best predictor of adverse cardiovascular events.53 These studies highlight the importance of nighttime blood pressure in the pathogenesis of vascular diseases.
This study has several limitations. First, a causal relationship between abnormal circadian blood pressure patterns and CSVD cannot be established because of the cross‐sectional design of the studies. Second, the number of studies is relatively limited. There are even fewer studies that explored the prevalence of CMBs in reverse‐dippers,31 and there are no available studies that explored the association of enlarged PVS with abnormal circadian blood pressure patterns. Consequently, the number of available studies exploring the association with certain CSVD neuroimaging findings may be too few to achieve a statistically significant result. Third, most studies used a single 24‐hour ambulatory blood pressure measurement, which could not demonstrate the reproducibility or persistence of the abnormal circadian blood pressure pattern in each individual. Finally, the classification of nocturnal blood pressure decrease into dipping, non‐dipping, and reverse‐dipping groups rather than analyzing the quantitative measurement of the changes in nocturnal blood pressure further limits the extent of the analysis.
Conclusions
The non‐dipping and reverse‐dipping blood pressure patterns are associated with neuroimaging markers of CSVD. However, whether restoration of a normal physiologic nocturnal blood pressure pattern would prevent further progression of CSVD is unknown. Future studies with a longitudinal design assessing the treatment effect of chronotherapy on CSVD neuroimaging features are needed.
Sources of Funding
None.
Disclosures
None.
Supporting information
Data S1 Figures S1–S3References 24–26, 28, 30–31, and 34
Acknowledgments
All authors had access to the data and played significant roles in the writing and review of the article. All authors confirmed they have contributed to the intellectual content of this paper and approved the final article.
(J Am Heart Assoc. 2020;9:e016299 DOI: 10.1161/JAHA.119.016299.)
For Sources of Funding and Disclosures, see page 9.
References
- 1. Smolensky MH, Hermida RC, Portaluppi F. Circadian mechanisms of 24‐hour blood pressure regulation and patterning. Sleep Med Rev. 2017;33:4–16. [DOI] [PubMed] [Google Scholar]
- 2. Hassler C, Burnier M. Circadian variations in blood pressure: implications for chronotherapeutics. Am J Cardiovasc Drugs. 2005;5:7–15. [DOI] [PubMed] [Google Scholar]
- 3. O'Brien E, Parati G, Stergiou G, Asmar R, Beilin L, Bilo G, Clement D, de la Sierra A, de Leeuw P, Dolan E, et al. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31:1731–1768. [DOI] [PubMed] [Google Scholar]
- 4. Hermida RC, Crespo JJ, Otero A, Dominguez‐Sardina M, Moya A, Rios MT, Castineira MC, Callejas PA, Pousa L, Sineiro E, et al. Asleep blood pressure: significant prognostic marker of vascular risk and therapeutic target for prevention. Eur Heart J. 2018;39:4159–4171. [DOI] [PubMed] [Google Scholar]
- 5. Birkenhager AM, van den Meiracker AH. Causes and consequences of a non‐dipping blood pressure profile. Neth J Med. 2007;65:127–131. [PubMed] [Google Scholar]
- 6. Chen Y, Liu JH, Zhen Z, Zuo Y, Lin Q, Liu M, Zhao C, Wu M, Cao G, Wang R, et al. Assessment of left ventricular function and peripheral vascular arterial stiffness in patients with dipper and non‐dipper hypertension. J Investig Med. 2018;66:319–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cuspidi C, Macca G, Sampieri L, Fusi V, Severgnini B, Michev I, Salerno M, Magrini F, Zanchetti A. Target organ damage and non‐dipping pattern defined by two sessions of ambulatory blood pressure monitoring in recently diagnosed essential hypertensive patients. J Hypertens. 2001;19:1539–1545. [DOI] [PubMed] [Google Scholar]
- 8. Karadag B, Ozyigit T, Serindag Z, Ilhan A, Ozben B. Blood pressure profile is associated with microalbuminuria and retinopathy in hypertensive nondiabetic patients. Wien Klin Wochenschr. 2018;130:204–210. [DOI] [PubMed] [Google Scholar]
- 9. Kilic A, Baydar O. The relationship between diurnal blood pressure abnormalities and target organ damage in normotensive subjects. Which is more important? Increased blood pressure levels or circadian blood pressure abnormalities. Clin Exp Hypertens. 2020;42:244–249. [DOI] [PubMed] [Google Scholar]
- 10. Salles GF, Reboldi G, Fagard RH, Cardoso CR, Pierdomenico SD, Verdecchia P, Eguchi K, Kario K, Hoshide S, Polonia J, et al. Prognostic effect of the nocturnal blood pressure fall in hypertensive patients: the ambulatory blood pressure collaboration in patients with hypertension (ABC‐H) meta‐analysis. Hypertension. 2016;67:693–700. [DOI] [PubMed] [Google Scholar]
- 11. Yan B, Peng L, Dong Q, Zheng F, Yang P, Sun L, Gong S, Zeng L, Wang G. Reverse‐dipper pattern of blood pressure may predict lacunar infarction in patients with essential hypertension. Eur J Neurol. 2015;22:1022–1025. [DOI] [PubMed] [Google Scholar]
- 12. Svensson P, de Faire U, Sleight P, Yusuf S, Ostergren J. Comparative effects of ramipril on ambulatory and office blood pressures: a HOPE Substudy. Hypertension. 2001;38:E28–E32. [DOI] [PubMed] [Google Scholar]
- 13. Hermida RC, Ayala DE, Mojon A, Fernandez JR. Influence of circadian time of hypertension treatment on cardiovascular risk: results of the MAPEC study. Chronobiol Int. 2010;27:1629–1651. [DOI] [PubMed] [Google Scholar]
- 14. Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701. [DOI] [PubMed] [Google Scholar]
- 15. Wardlaw JM, Chappell FM, Valdes Hernandez MDC, Makin SDJ, Staals J, Shuler K, Thrippleton MJ, Armitage PA, Munoz‐Maniega S, Heye AK, et al. White matter hyperintensity reduction and outcomes after minor stroke. Neurology. 2017;89:1003–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wardlaw JM, Smith C, Dichgans M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol. 2019;18:684–696. [DOI] [PubMed] [Google Scholar]
- 17. van Middelaar T, Argillander TE, Schreuder F, Deinum J, Richard E, Klijn CJM. Effect of antihypertensive medication on cerebral small vessel disease: a systematic review and meta‐analysis. Stroke. 2018;49:1531–1533. [DOI] [PubMed] [Google Scholar]
- 18. Sander D, Klingelhofer J. Diurnal systolic blood pressure variability is the strongest predictor of early carotid atherosclerosis. Neurology. 1996;47:500–507. [DOI] [PubMed] [Google Scholar]
- 19. Moher D, Liberati A, Tetzlaff J, Altman DG; Group P . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. [DOI] [PubMed] [Google Scholar]
- 20. Stang A. Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol. 2010;25:603–605. [DOI] [PubMed] [Google Scholar]
- 21. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 22. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hamada T, Murata T, Omori M, Takahashi T, Kosaka H, Wada Y, Yoshida H. Abnormal nocturnal blood pressure fall in senile‐onset depression with subcortical silent cerebral infarction. Neuropsychobiology. 2003;47:187–191. [DOI] [PubMed] [Google Scholar]
- 24. Yamamoto Y, Akiguchi I, Oiwa K, Hayashi M, Ohara T, Ozasa K. The relationship between 24‐hour blood pressure readings, subcortical ischemic lesions and vascular dementia. Cerebrovasc Dis. 2005;19:302–308. [DOI] [PubMed] [Google Scholar]
- 25. Henskens LH, van Oostenbrugge RJ, Kroon AA, de Leeuw PW, Lodder J. Brain microbleeds are associated with ambulatory blood pressure levels in a hypertensive population. Hypertension. 2008;51:62–68. [DOI] [PubMed] [Google Scholar]
- 26. Staals J, van Oostenbrugge RJ, Knottnerus IL, Rouhl RP, Henskens LH, Lodder J. Brain microbleeds relate to higher ambulatory blood pressure levels in first‐ever lacunar stroke patients. Stroke. 2009;40:3264–3268. [DOI] [PubMed] [Google Scholar]
- 27. Ma JF, Sun JL, Zhao J, Wei X, Wang BS, Fu Y. Relationship between nocturnal blood pressure variation and silent cerebral infarction in Chinese hypertensive patients. J Neurol Sci. 2010;294:67–69. [DOI] [PubMed] [Google Scholar]
- 28. Yamamoto Y, Ohara T, Nagakane Y, Tanaka E, Morii F, Koizumi T, Akiguchi I. Chronic kidney disease, 24‐h blood pressure and small vessel diseases are independently associated with cognitive impairment in lacunar infarct patients. Hypertens Res. 2011;34:1276–1282. [DOI] [PubMed] [Google Scholar]
- 29. Shimizu M, Ishikawa J, Yano Y, Hoshide S, Shimada K, Kario K. The relationship between the morning blood pressure surge and low‐grade inflammation on silent cerebral infarct and clinical stroke events. Atherosclerosis. 2011;219:316–321. [DOI] [PubMed] [Google Scholar]
- 30. Lee S, Thomas RJ, Kim H, Seo HS, Baik I, Yoon DW, Kim SJ, Lee SK, Shin C. Association between high nocturnal blood pressure and white matter change and its interaction by obstructive sleep apnoea among normotensive adults. J Hypertens. 2014;32:2005–2012; discussion 2012 [DOI] [PubMed] [Google Scholar]
- 31. Kwon HM, Lim JS, Kim YS, Moon J, Park H, Kim HY, Lim YH, Nam H. Cerebral microbleeds are associated with nocturnal reverse dipping in hypertensive patients with ischemic stroke. BMC Neurol. 2014;14:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yamashiro K, Tanaka R, Shimo Y, Oyama G, Ogawa T, Umemura A, Hattori N. Cerebral microbleeds and blood pressure abnormalities in Parkinson's disease. eNeurologicalSci. 2018;10:5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. White WB, Jalil F, Wakefield DB, Kaplan RF, Bohannon RW, Hall CB, Moscufo N, Fellows D, Guttmann CRG, Wolfson L. Relationships among clinic, home, and ambulatory blood pressures with small vessel disease of the brain and functional status in older people with hypertension. Am Heart J. 2018;205:21–30. [DOI] [PubMed] [Google Scholar]
- 34. Nakanishi K, Jin Z, Homma S, Elkind MSV, Rundek T, Schwartz JE, Lee TC, Tugcu A, Yoshita M, DeCarli C, et al. Night‐time systolic blood pressure and subclinical cerebrovascular disease: the Cardiovascular Abnormalities and Brain Lesions (CABL) study. Eur Heart J Cardiovasc Imaging. 2019;20:765–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available at: www.handbook.cochrane.org. [Google Scholar]
- 36. Redon J, Tellez‐Plaza M, Orozco‐Beltran D, Gil‐Guillen V, Pita Fernandez S, Navarro‐Perez J, Pallares V, Valls F, Fernandez A, Perez‐Navarro AM, et al. Impact of hypertension on mortality and cardiovascular disease burden in patients with cardiovascular risk factors from a general practice setting: the ESCARVAL‐risk study. J Hypertens. 2016;34:1075–1083. [DOI] [PubMed] [Google Scholar]
- 37. Leonardi‐Bee J, Bath PM, Phillips SJ, Sandercock PA; Group ISTC . Blood pressure and clinical outcomes in the International Stroke Trial. Stroke. 2002;33:1315–1320. [DOI] [PubMed] [Google Scholar]
- 38. Cannistraro RJ, Badi M, Eidelman BH, Dickson DW, Middlebrooks EH, Meschia JF. CNS small vessel disease: a clinical review. Neurology. 2019;92:1146–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rizzoni D, Rizzoni M, Nardin M, Chiarini G, Agabiti‐Rosei C, Aggiusti C, Paini A, Salvetti M, Muiesan ML. Vascular aging and disease of the small vessels. High Blood Press Cardiovasc Prev. 2019;26:183–189. [DOI] [PubMed] [Google Scholar]
- 40. Meissner A. Hypertension and the brain: a risk factor for more than heart disease. Cerebrovasc Dis. 2016;42:255–262. [DOI] [PubMed] [Google Scholar]
- 41. Ferrer I, Vidal N. Neuropathology of cerebrovascular diseases. Handb Clin Neurol. 2017;145:79–114. [DOI] [PubMed] [Google Scholar]
- 42. Baschieri F, Cortelli P. Circadian rhythms of cardiovascular autonomic function: physiology and clinical implications in neurodegenerative diseases. Auton Neurosci. 2019;217:91–101. [DOI] [PubMed] [Google Scholar]
- 43. Crnko S, Du Pre BC, Sluijter JPG, Van Laake LW. Circadian rhythms and the molecular clock in cardiovascular biology and disease. Nat Rev Cardiol. 2019;16:437–447. [DOI] [PubMed] [Google Scholar]
- 44. Fabbian F, Smolensky MH, Tiseo R, Pala M, Manfredini R, Portaluppi F. Dipper and non‐dipper blood pressure 24‐hour patterns: circadian rhythm‐dependent physiologic and pathophysiologic mechanisms. Chronobiol Int. 2013;30:17–30. [DOI] [PubMed] [Google Scholar]
- 45. Cuspidi C, Tadic M, Sala C, Gherbesi E, Grassi G, Mancia G. Blood pressure non‐dipping and obstructive sleep apnea syndrome: a meta‐analysis. J Clin Med. 2019;8:E1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yan B, Peng L, Han D, Sun L, Dong Q, Yang P, Zheng F, Ong H, Zeng L, Wang G. Blood pressure reverse‐dipping is associated with early formation of carotid plaque in senior hypertensive patients. Medicine (Baltimore). 2015;94:e604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Izzedine H, Launay‐Vacher V, Deray G. Abnormal blood pressure circadian rhythm: a target organ damage? Int J Cardiol. 2006;107:343–349. [DOI] [PubMed] [Google Scholar]
- 48. Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, Lindley RI, O'Brien JT, Barkhof F, Benavente OR, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Das AS, Regenhardt RW, Vernooij MW, Blacker D, Charidimou A, Viswanathan A. Asymptomatic cerebral small vessel disease: insights from population‐based studies. J Stroke. 2019;21:121–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cheng AL, Batool S, McCreary CR, Lauzon ML, Frayne R, Goyal M, Smith EE. Susceptibility‐weighted imaging is more reliable than T2*‐weighted gradient‐recalled echo MRI for detecting microbleeds. Stroke. 2013;44:2782–2786. [DOI] [PubMed] [Google Scholar]
- 51. Hermida RC, Ayala DE, Mojon A, Fernandez JR. Decreasing sleep‐time blood pressure determined by ambulatory monitoring reduces cardiovascular risk. J Am Coll Cardiol. 2011;58:1165–1173. [DOI] [PubMed] [Google Scholar]
- 52. Smolensky MH, Hermida RC, Ayala DE, Mojon A, Fernandez JR. Bedtime chronotherapy with conventional hypertension medications to target increased asleep blood pressure results in markedly better chronoprevention of cardiovascular and other risks than customary on‐awakening therapy. Heart Fail Clin. 2017;13:775–792. [DOI] [PubMed] [Google Scholar]
- 53. Investigators A‐H , Roush GC, Fagard RH, Salles GF, Pierdomenico SD, Reboldi G, Verdecchia P, Eguchi K, Kario K, Hoshide S, Polonia J, et al. Prognostic impact from clinic, daytime, and night‐time systolic blood pressure in nine cohorts of 13,844 patients with hypertension. J Hypertens. 2014;32:2332–2340. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 Figures S1–S3References 24–26, 28, 30–31, and 34


