Abstract
Background
There are limited data to inform policy mandating primary percutaneous coronary intervention (PPCI) volume benchmarks for catheterization laboratories in low‐ and middle‐income countries.
Methods and Results
This prospective state‐wide registry included ST‐segment–elevation myocardial infarction patients with symptoms of <12 hours, or with ongoing ischemia at 12 to 24 hours, reperfused with PPCI. From June 2013 to March 2016, we recruited 5560 consecutive patients. We categorized hospitals on the basis of annual PPCI volumes into low, medium, and high volume (<100, 100–199, and ≥200 PPCIs per year, respectively). Kaplan‐Meier curves and Cox regression models were used to examine the association between PPCI volume and 1‐year mortality. Among 42 recruiting hospitals, there were 24 (57.2%) low‐volume, 8 (19%) medium‐volume, and 10 (23.8%) high‐volume hospitals. The median (25th–75th percentile) TIMI (Thrombolysis in Myocardial Infarction) ST‐segment–elevation myocardial infarction risk score was 3 (2–5). Cardiac arrest before admission occurred in 4.2%, 2.1%, and 2.9% of cases at low‐, medium‐, and high‐volume hospitals, respectively (P=0.02). Total ischemic time differed significantly among low‐volume (median [25th–75th percentile], 3.5 [2.4–5.5] hours), medium‐volume (median, 3.8 [25th–75th percentile, 2.58–6.05] hours), and high‐volume hospitals (median, 4.16 [25th–75th percentile 2.8–6.3] hours) (P=0.01). Vascular access was radial in 61.5%, 71.3%, and 63.2% of cases at low‐, medium‐, and high‐volume hospitals, respectively (P=0.01). The observed 1‐year mortality rate was 6.5%, 3.4%, and 8.6% at low‐, medium‐ and high‐volume hospitals, respectively (P<0.01), and the difference did not attenuate after multivariate adjustment (low versus medium: hazard ratio [95% CI], 1.80 [1.12–2.90]; high versus medium: hazard ratio [95% CI], 2.53 [1.78–3.58]) (P<0.01).
Conclusions
Low‐ and middle‐income countries, like India, may have a nonlinear relationship between institutional PPCI volume and outcomes, partly driven by procedural variations and inequalities in access to care.
Keywords: percutaneous coronary intervention, ST‐segment–elevation myocardial infarction, stents
Subject Categories: Percutaneous Coronary Intervention, Quality and Outcomes
Nonstandard Abbreviations and Acronyms
- ACS
acute coronary syndrome
- LMIC
low‐ and middle‐income country
- PCI
percutaneous coronary intervention
- PPCI
primary percutaneous coronary intervention
- STEMI
ST‐segment–elevation myocardial infarction
- TIMI
Thrombolysis in Myocardial Infarction
Clinical Perspective
What Is New?
This large multicenter primary percutaneous coronary intervention (PPCI) registry from Kerala, India, reports long‐term outcomes of patients presenting with ST‐segment–elevation myocardial infarction to percutaneous coronary intervention–capable hospitals categorized according to institutional PPCI volume.
There were significant variations in equity of access to ST‐segment–elevation myocardial infarction care across low‐, moderate‐, and high‐volume hospitals. Also, there were significant PPCI procedural variations as well as differences in the rates of guideline‐directed medical therapy between low‐, moderate‐, and large‐volume hospitals.
Low‐ and middle‐income countries, like India, may have a unique nonlinear relationship between institutional‐level PPCI volumes and outcomes, with high‐volume hospitals having the highest 1‐year mortality rates.
What Are the Clinical Implications?
ST‐segment–elevation myocardial infarction PPCI quality improvement initiatives in Kerala should at first focus on the relatively few high‐volume hospitals.
Process‐of‐care metrics, like timeliness of reperfusion and PPCI procedural characteristics, may be the key to improving ST‐segment–elevation myocardial infarction outcomes in India rather than institutional‐level PPCI volumes.
Current global institutional‐level PPCI volume benchmarks may not be appropriate for low‐ and middle‐income countries, like India.
Global cardiovascular disease burden has been steadily increasing, with the disparate contribution of low‐ and middle‐income countries (LMICs), like India, coming into focus.1, 2, 3 Disease epidemiology in India, a country with 1.34 billion population, has made a dramatic shift, with noncommunicable diseases accounting for a sizeable burden of disability‐adjusted life years.1, 2 The southern Indian state of Indian has been spearheading this national epidemiological transition to noncommunicable diseases.1 Consequently, Kerala has had to pivot its healthcare delivery system traditionally designed to care for infectious diseases to increasingly face a significant burden of noncommunicable diseases, especially acute coronary syndromes (ACSs).4, 5 This shift is also apparent in the expansion of primary percutaneous coronary intervention (PPCI)–capable facilities in Kerala.4 The rapid increase in the number of PPCI‐capable hospitals in Kerala, with the attendant burgeoning of hospitals with low PPCI volumes, calls into question the safety and outcomes of PPCI at such hospitals.
An inverse relationship between both institutional‐ and operator‐level percutaneous coronary intervention (PCI) volume and mortality has been observed.6, 7 Several international societies and regulatory bodies have issued institutional‐ and operator‐level PPCI volume benchmarks, with wide variance in the recommended volume cutoffs.8, 9, 10 However, there are limited data to inform policy mandating volume benchmarks for cardiac catheterization laboratories in LMICs that lack an organized prehospital system of ST‐segment–elevation myocardial infarction (STEMI) care.11, 12 Prior ACS studies from Kerala showed the presence of extensive heterogeneity across hospitals in process and outcome measures.5, 13 Lower PPCI procedural volume may be driving some of this variability across hospitals. Analyzing the relationship between PPCI volumes and outcomes may enable LMICs to regionally adapt global PPCI institutional volume benchmarks and focus the limited healthcare resources on quality improvement initiatives across specific hospital groups.
The Cardiology Society of India–Kerala chapter developed and implemented a prospective state‐wide PPCI registry to analyze the regional quality, procedural variations, and outcomes of PPCI services for STEMI. We report these results with a specific focus on the institutional PPCI volume–patient outcome relationship.
Methods
Data Source and Study Population
The data that support the findings of this study are available from the corresponding author on reasonable request. The PPCI registry of Kerala is an investigator‐initiated prospective state‐wide multicenter registry of consecutive undergoing PPCIs at hospitals across Kerala, a state with a population of 33.4 million as per the 2011 Census. We performed a baseline survey to identify PPCI‐capable healthcare facilities in the state and updated it annually during the study period. During the study period, Kerala had a total of 255 hospitals providing emergency reperfusion services for STEMI. Of these hospitals, 92 had cardiac catheterization laboratories. Nine of the catheterization laboratory‐equipped hospitals were public. After excluding 5 hospitals that were not offering PPCI services, we invited the remaining 87 hospitals to take part in the registry. Eighty‐one percutaneous coronary intervention (PCI)–capable centers responded, of which 48 hospitals finally participated in the registry.
All adult patients presenting with acute STEMI, with ischemic symptoms of <12‐hours’ duration, designated to reperfusion therapy with PPCI were included. We also included patients with acute STEMI presenting to PCI‐capable centers 12 to 24 hours after the onset of symptoms if the patient had clinical and electrocardiographic evidence of ongoing ischemia. At least one measurement of troponin I, troponin T, or creatinine kinase‐MB above the upper reference limit was required for confirmation of diagnosis. New‐onset left bundle branch block was also considered as a STEMI equivalent. We excluded patients presenting with STEMI >24 hours after symptom onset and those presenting between 12 and 24 hours with no clinical and electrocardiographic evidence of ongoing ischemia.
We enrolled patients in 2 phases. The first phase, extending from June 2013 to March 2015, enrolled 783 STEMI patients undergoing PPCI at hospitals in central Kerala. The second phase extended across the entire state and enrolled 5274 patients from April 2015 to March 2016. Follow‐up was completed by March 2017. We obtained ethics committee clearance from participating hospitals and the central Cardiology Society of India–Kerala chapter ethics committee. Consent was obtained from all participating patients, except for very sick patients. An ethics committee waiver was obtained for patients who were deemed too ill to administer informed consent.
Assessment of Hospital Volume and Outcomes
We stratified hospitals according to the annual PPCI volumes into low‐, medium‐, and high‐volume centers (<100, 100–199, and >200 PPCIs per year, respectively) as well as based on whether they belonged to the public or private sector. The annual individual hospital PPCI volumes were corroborated with the numbers separately reported to the Interventional Cardiology Council of Kerala, an autonomous organization dedicated to the advancement of interventional cardiology regionally. We excluded hospitals performing <10 PPCIs per year, as well as hospitals deemed to have recruited nonconsecutive patients, from the final analysis.
We recruited 6057 patients presenting with STEMI and undergoing PPCI across 48 hospitals in Kerala. We exclude the following groups of patients: 23 patients treated at 4 hospitals that submitted <10 PPCIs during the study period, 161 patients treated at 2 hospitals deemed to have recruited nonconsecutive patients, and 313 patients lost to follow‐up after the index hospitalization. We also excluded 64 patients who did not consent to take part in the study. There was no difference between hospital groups in the proportion of nonconsenting patients. Thus, the overall study cohort included 5560 patients (Figure 1). The primary outcome was all‐cause mortality within 1 year. Secondary outcomes included in‐hospital mortality rates and stent thrombosis rates. We have provided additional details on methods in Data S1.
Figure 1. CONSORT diagram of exclusions from the primary angioplasty registry of Kerala.
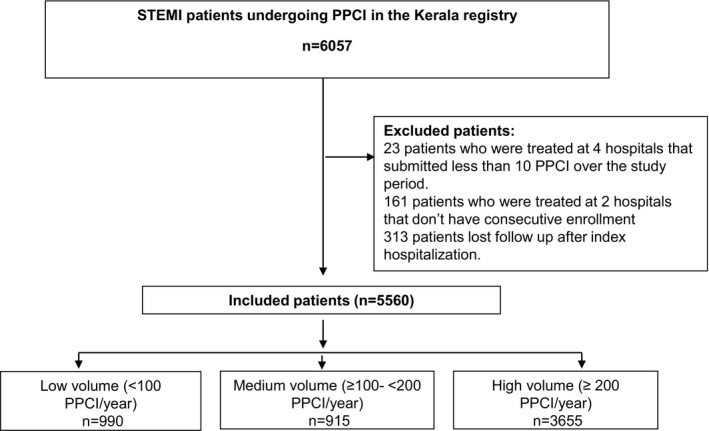
PPCI indicates primary percutaneous coronary intervention; and STEMI, ST‐segment–elevation myocardial infarction.
Statistical Analysis
We summarized categorical variables as numbers and percentages, whereas continuous variables were presented as mean with SD. Continuous variables with skewed distribution were expressed as medians with 25th and 75th percentiles. Differences among groups (low‐, medium‐, and high‐volume hospitals) were tested using Cochran‐Mantel‐Haenszel row mean scores for categorical variables and Cochran‐Mantel‐Haenszel correlation tests for continuous variables, thereby identifying trends. A descriptive analysis between public and private institutions in the high‐volume group was performed to examine the difference in the quality of care and mortality within 1 year.
We plotted Kaplan‐Meier curves to display the unadjusted relationship between hospital type and clinical outcomes (ie, the time to death within 30 days or 1 year). The groups were compared using the log‐rank test. Follow‐up time was censored at 365 days after symptom onset or discontinuation, whichever occurs first. The relative associations were estimated using Cox proportional hazard regression. We applied robust sandwich covariance estimates, by using maximum partial likelihood estimates in the model, to account for within‐hospital clustering. We reported unadjusted and adjusted hazard ratios (HRs) and 95% CIs, with medium‐volume hospitals as the reference group. To account for selection bias and confounders, we adjusted the PPCI volume‐outcome association for sex, Thrombolysis in Myocardial Infarction (TIMI) risk score, insurance coverage, living below the poverty threshold, family history of coronary artery disease, smoking status, culprit lesion involving the proximal left anterior descending artery, number of years catheterization laboratory was performing PCI, and year of enrollment. We used a restricted cubic spline function to test the linearity assumption for continuous variables in the model. Spline transformations were applied when the linearity assumption was violated. As an exploratory analysis, we also examined the annual hospital volume as a continuous variable for mortality within 1 year using Cox proportional hazard regression model; hospital annual PPCI volume >300 was truncated to 300 because no hospital had yearly PPCI volumes between 300 and 500.
We performed a sensitivity analysis using individual components of the TIMI risk score for adjustment in the model instead of the score itself. We also repeated the analysis after excluding patients without insurance coverage treated at high‐volume hospitals to see if this modified the mortality differential observed at high‐volume centers.
All statistical tests were 2 sided, with P<0.05 considered nominally significant. We performed statistical analyses using SAS (version 9.4; SAS Institute, Cary, NC).
Results
Hospital Characteristics
Forty‐eight hospitals across the state of Kerala participated in the registry. We compared the institutional characteristics of participating and nonparticipating hospitals. Hospital‐level characteristics, including the proportion of urban hospitals (participating, 87.5%, versus nonparticipating, 92.7%; P=0.49), the number of hospital beds (participating, 600±513 beds, versus nonparticipating, 469±253 beds; P=0.07), and the number of years the catheterization laboratory has been performing PCI (participating, 7.4±5.4 years, versus nonparticipating, 6.5±5.9 years; P=0.24), were similar between participating and nonparticipating hospitals. However, participating hospitals had more interventionists performing PCI (median [25th–75th percentile], participating, 3 [2–4] interventionists, versus nonparticipating, 2 [1–2] interventionists; P=0.01). After excluding 6 ineligible hospitals, we categorized the remaining 42 enrolling hospitals into 24 (57.2%) low‐volume, 8 (19.0%) medium‐volume, and 10 (23.8%) high‐volume hospitals. Of the 42 enrolling hospitals, 4 were public, with the remaining 38 being private. Public hospitals had high annual PPCI volumes and accounted for 29.3% of STEMI patients undergoing PPCI. Overall, the median (25th–75th percentile) annual hospital‐level PPCI volume was 237 (115–520). The median (25th–75th percentile) annual PPCI volume was 563 (283–640) and 200 (99–237) for public and private hospitals, respectively. Figure S1 depicts the histogram of institutional‐level annual PPCI volumes. Catheterization laboratories at low‐volume hospitals were performing PCI for a fewer number of years compared with medium‐ and high‐volume hospitals (low‐volume hospitals, 6.6±6.9 years; medium‐volume hospitals, 8.6±3.8 years; and high‐volume hospitals, 8.7±3.9 years; P<0.01). We reported the hospital‐level details in Table S1.
Patient Characteristics
Table 1 depicts the baseline patient characteristics. We included a total of 5560 patients in the final analysis, with 990 (17.8%), 915 (16.5%), and 3655 (65.7%) patients treated in low‐, medium‐, and high‐volume hospitals, respectively. These hospital groups differed considerably with regard to the clinical profile of patients subjected to PPCI. Overall, the mean age was 58.5±11.4 years, with women constituting 18.5% of the population. Patients classified as living in poverty represented 25%, 29.8%, and 40.7% of PPCI patients at low‐, medium‐, and high‐volume hospitals, respectively (P for trend <0.01). Public health insurance schemes funded a larger proportion of patients undergoing PPCI in high‐volume hospitals compared with other hospital groups (low‐volume hospitals, 5.9%; medium‐volume hospitals, 3.7%; and high‐volume hospitals, 36.3%; P for trend <0.01).
Table 1.
Baseline Characteristics Stratified Across Hospital Groups, Categorized According to Institutional‐Level Annual PPCI Volume
| Baseline Characteristics | Missing Data | Total Cohort | Hospitals Categorized According to Hospital‐Level Annual PPCI Volume | P Value | ||
|---|---|---|---|---|---|---|
| Low Volume | Medium Volume | High Volume | ||||
| No. of patients | … | 5560 | 990 | 915 | 3655 | |
| Age, mean (SD) y | 0 | 58.5 (11.4) | 58.7 (11.4) | 58.4 (11.6) | 58.4 (11.4) | 0.49 |
| <40 y, n (%) | 0 | 245 (4.4) | 43 (4.3) | 44 (4.8) | 158 (4.3) | 0.83 |
| ≥75 y, n (%) | 0 | 521 (9.4) | 96 (9.7) | 80 (8.7) | 345 (9.4) | 0.96 |
| Women, n (%) | 0 | 1026 (18.5) | 178 (18) | 145 (15.8) | 703 (19.2) | 0.14 |
| Below poverty level, n (%) | 0 | 2009 (36.1) | 248 (25) | 273 (29.8) | 1488 (40.7) | 0.01 |
| Self‐paid, n (%) | 1 | 3625 (65.2) | 778 (78.6) | 732 (80) | 2115 (57.9) | 0.01 |
| Anterior STEMI, n (%) | 0 | 2752 (49.5) | 502 (50.7) | 421 (46.0) | 1829 (50) | 0.05 |
| LBBB, n (%) | 10 | 118 (2.1) | 44 (4.4) | 20 (2.2) | 54 (1.5) | 0.01 |
| Cardiac biomarker positive, n (%) | 23 | 5537 (99.6) | 984 (99.4) | 913 (99.8) | 3640 (99.6) | 0.42 |
| Hypertension, n (%) | 0 | 2294 (41.3) | 445 (44.9) | 345 (37.7) | 1504 (41.2) | 0.15 |
| Diabetes mellitus, n (%) | 0 | 2383 (42.9) | 506 (51.1) | 377 (41.2) | 1500 (41) | 0.01 |
| Total cholesterol, mean (SD), mg/dL | 882 | 194 (59.6) | 193.2 (62.2) | 194.1 (50.9) | 194.3 (61.0) | 0.89 |
| LDL cholesterol, mean (SD), mg/dL | 1224 | 125.8 (47.8) | 128.3 (51.4) | 126 (43.6) | 124.9 (47.8) | 0.20 |
| HDL cholesterol, mean (SD), mg/dL | 1200 | 41.8 (11.7) | 41.4 (12.6) | 42.4 (12.5) | 41.7 (11.2) | 0.24 |
| Chronic kidney disease, n (%) | 0 | 160 (2.9) | 36 (3.6) | 34 (3.7) | 90 (2.5) | 0.02 |
| Body mass index, mean (SD), kg/m2 | 262 | 23.9 (3.1) | 24.1 (3.2) | 23.9 (3.3) | 23.9 (3.0) | 0.18 |
| Overweight, n (%) | 262 | 1674 (30.1) | 330 (26.3) | 241 (26.3) | 1103 (30.2) | 0.01 |
| Obesity, n (%) | 262 | 179 (3.2) | 39 (4.0) | 38 (4.2) | 102 (2.8) | 0.01 |
| Current smoker, n (%) | 0 | 1972 (35.5) | 285 (28.8) | 255 (27.9) | 1432 (39.2) | 0.01 |
| Family history of premature CAD, n (%) | 0 | 1100 (19.9) | 182 (18.4) | 188 (20.5) | 730 (20) | 0.37 |
| Cerebrovascular accident, n (%) | 0 | 116 (2.1) | 25 (2.5) | 10 (1.1) | 81 (2.2) | 0.96 |
| History of effort angina, n (%) | 0 | 863 (15.5) | 145 (14.6) | 76 (8.3) | 642 (17.6) | 0.01 |
| Prior MI, n (%) | 0 | 277 (5.0) | 56 (5.7) | 52 (5.7) | 169 (4.6) | 0.11 |
| Prior heart failure, n (%) | 0 | 44 (0.8) | 18 (1.8) | 4 (0.4) | 22 (0.6) | 0.01 |
| Aspirin use before MI, n (%) | 0 | 274 (4.9) | 75 (7.6) | 53 (5.8) | 146 (4.0) | 0.01 |
| Prior coronary revascularization, n (%) | 0 | 126 (2.3) | 24 (2.4) | 30 (3.3) | 72 (2) | 0.15 |
| TIMI risk score, median (25th–75th percentile) | 0 | 3 (2–5) | 3 (2–5) | 3 (2–5) | 3 (2–5) | 0.01 |
| Direct presentation to PCI‐capable hospital, n (%) | 0 | 2623 (47.2) | 629 (63.5) | 554 (60.5) | 1440 (39.4) | 0.01 |
| Arrival at PCI‐capable hospital by ambulance, n (%) | 0 | 2470 (44.4) | 291 (29.4) | 290 (31.7) | 1889 (51.7) | 0.01 |
| Ambulance use in direct presenting patients, n (%) | 0 | 2623 | 66 (10.5) | 57 (10.3) | 187 (13) | 0.02 |
| Cardiac arrest before admission, n (%) | 0 | 166 (3) | 42 (4.2) | 19 (2.1) | 105 (2.9) | 0.09 |
| Systolic blood pressure at presentation, mean (SD), mm Hg | 0 | 135 (30) | 137 (30) | 137 (28) | 134 (30) | 0.01 |
| Shock at admission, n (%) | 0 | 218 (3.9) | 37 (3.7) | 23 (2.5) | 158 (4.3) | 0.14 |
| Angiographic characteristics, n (%) | ||||||
| Single‐vessel coronary artery disease | 31 | 2861 (51.5) | 454 (45.9) | 446 (48.7) | 1961 (53.6) | 0.01 |
| Double‐vessel coronary artery disease | 31 | 1727 (31.1) | 335 (33.8) | 304 (33.2) | 1088 (29.8) | 0.01 |
| Triple‐vessel coronary artery disease | 31 | 938 (16.9) | 199 (20.1) | 156 (17.1) | 583 (16.0) | 0.01 |
| Left main disease | 0 | 136 (2.5) | 21 (2.1) | 17 (1.9) | 98 (2.7) | 0.27 |
| Proximal LAD artery culprit lesion | 117 | 2009 (36.1) | 352 (35.5) | 320 (35.0) | 1337 (36.5) | 0.01 |
CAD indicates coronary artery disease; HDL, high‐density lipoprotein; LAD, left anterior descending; LBBB, left bundle branch block; LDL, low‐density lipoprotein; MI, myocardial infarction; PCI, percutaneous coronary intervention; PPCI, primary PCI; STEMI, ST‐segment–elevation MI; and TIMI, Thrombolysis in Myocardial Infarction.
In terms of underlying patient risk, patients undergoing PPCI in low‐volume hospitals were more likely to have diabetes mellitus (low‐volume hospitals, 51.1%; medium‐volume hospitals, 41.2%; and high‐volume hospitals, 41%; P for trend <0.01) and history of heart failure (low‐volume hospitals, 1.8%; medium‐volume hospitals, 0.4%; and high‐volume hospitals, 0.6%; P for trend <0.01). Patients undergoing PPCI in low‐volume hospitals had a higher TIMI risk score (proportion of patients with TIMI risk score ≥4: low‐volume hospitals, 49.2%; medium‐volume hospitals, 43%; and high‐volume hospitals, 39.8%; P for trend <0.01) compared with medium‐ and high‐volume hospitals. However, the proportion of patients in shock on arrival (low‐volume hospitals, 3.7%; medium‐volume hospitals, 2.5%; and high‐volume hospitals, 4.3%; P for trend=0.14) and the proportion of patients with resuscitated cardiac arrest before admission were similar across the 3 groups (low‐volume hospitals, 4.2%; medium‐volume hospitals, 2.1%; and high‐volume hospitals, 2.9%; P for trend=0.09).
Quality Indicators
Table 2 lists quality indicators and outcomes. Key timeliness metrics, including the total ischemic time (median [25th–75th percentile]: low‐volume hospitals, 3.5 [2.4–5.5] hours; medium‐volume hospitals, 3.8 [2.6–6.1] hours; and high‐volume hospitals, 4.2 [2.8–6.3] hours; P for trend <0.01) and ECG‐to‐balloon time (median [25th–75th percentile]: low‐volume hospitals, 1.5 [1.0–2.4] hours; medium‐volume hospitals, 1.5 [0.8–2.8] hours; and high‐volume hospitals, 2 [1.2–3.1] hours; P for trend <0.01), differed significantly among hospital groups, with high‐volume hospitals experiencing the most delays. However, the door‐to‐balloon time (measured at the PCI center and not including transfer hospital) did not differ significantly between the hospitals. The symptom onset to first medical contact time also did not differ. Radial vascular access rates (low‐volume hospitals, 61.5%; medium‐volume hospitals, 71.3%; and high‐volume hospitals, 63.2%; P for trend=0.01) were higher at medium‐volume hospitals. The rates of nonculprit vessel PCI (low‐volume hospitals, 7.8%; medium‐volume hospitals, 10.8%; and high‐volume hospitals, 7.2%; P for trend=0.01) were also higher at medium‐volume hospitals. A total of 359 (6.5%) patients underwent nonculprit vessel PCI during the index hospitalization, and 81 patients underwent staged elective nonculprit vessel PCI after discharge. High‐volume hospitals had a significantly lower proportion of PPCI patients undergoing aspiration thrombectomy compared with low‐ and medium‐volume hospitals (low‐volume hospitals, 45.3%; medium‐volume hospitals, 37.5%; and high‐volume hospitals, 34.8%; P for trend <0.01). Discharge medication prescription patterns varied among hospital groups, with low‐volume hospitals having the highest rate of newer P2Y12‐inhibitor prescription (ticagrelor or prasugrel: low‐volume hospitals, 33.1%; medium‐volume hospitals, 18.4%; and high‐volume hospitals, 26.6%; P for trend <0.01) and high‐volume hospitals having more patients discharged on high‐intensity statins (low‐volume hospitals, 92.1%; medium‐volume hospitals, 91.8%; and high‐volume hospitals, 97.9%; P<0.01), angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers in those with left ventricular ejection fraction <40% (low‐volume hospitals, 37.7%; medium‐volume hospitals, 25.8%; and high‐volume hospitals, 62.1%; P for trend <0.01), and aldosterone blockers (low‐volume hospitals, 8.3%; medium‐volume hospitals, 12.4%; and high‐volume hospitals, 20.2%; P for trend <0.01). There were significant differences in baseline characteristics and quality‐of‐care metrics between high‐volume hospitals belonging to private and public sectors (Tables S2 and S3). Ninety (1.76%) patients underwent subsequent coronary artery bypass grafting, of which 25 (0.45%) were in hospital and the rest after discharge.
Table 2.
Quality Indicators and Outcomes
| Quality Indicators | Missing | Total, n (%) | Hospitals Categorized According to Hospital‐Level Annual PPCI Volume | P Value | ||
|---|---|---|---|---|---|---|
| Low Volume | Medium Volume | High Volume | ||||
| Timeliness | ||||||
| Total ischemic time, median (25th–75th percentile), h | 42 | 3.9 (2.7–6.1) | 3.5 (2.4–5.5) | 3.8 (2.6–6.1) | 4.2 (2.8–6.3) | 0.01 |
| ECG‐to‐balloon time, median (25th–75th percentile), h | 11 | 1.8 (1.1–3.0) | 1.5 (1.0–2.4) | 1.5 (0.8–2.8) | 2 (1.2–3.1) | 0.01 |
| Door‐to‐balloon time, median (25th–75th percentile), h | 7 | 1.2 (0.8–1.7) | 1.2 (0.9–1.7) | 1.16 (0.8–1.9) | 1.13 (0.8–1.6) | 0.60 |
| Symptom onset to first medical contact, median (25th–75th percentile), h | 0 | 1.5 (0.8–3.0) | 1.33 (0.7–3.0) | 1.5 (0.8–3.0) | 1.5 (0.9–3.0) | 0.96 |
| Procedural characteristics, n (%) | ||||||
| Radial access | 0 | 3572 (64.2) | 609 (61.5) | 652 (71.3) | 2311 (63.2) | 0.01 |
| Aspiration thrombectomy use | 0 | 2062 (37.1) | 448 (45.2) | 343 (37.5) | 1271 (34.8) | 0.01 |
| TIMI flow III final | 0 | 5190 (93.3) | 912 (92.1) | 853 (93.2) | 3425 (93.7) | 0.08 |
| Nonculprit vessel PCI during index admission | 0 | 359 (6.5) | 68 (6.9) | 91 (9.9) | 200 (5.5) | 0.01 |
| Discharge medications, n (%) | ||||||
| Aspirin | 8 | 5269 (98.5) | 918 (96.5) | 895 (99.4) | 3456 (98.8) | 0.01 |
| Clopidogrel | 7 | 3911 (73.1) | 630 (66.3) | 730 (81.1) | 2551 (72.9) | 0.01 |
| Ticagrelor | 8 | 847 (15.8) | 246 (25.9) | 109 (12.1) | 492 (14.1) | 0.01 |
| Prasugrel | 8 | 563 (10.5) | 68 (7.1) | 57 (6.3) | 438 (12.5) | 0.01 |
| High‐intensity statin | 8 | 5120 (95.7) | 875 (92.0) | 826 (91.8) | 3419 (97.7) | 0.01 |
| β Blocker | 9 | 3497 (65.4) | 649 (68.2) | 570 (63.3) | 2278 (65.1) | 0.16 |
| ACEI or ARB in patients with documented LV systolic dysfunction | 7 | 1321 (50.6) | 226 (37.7) | 109 (25.8) | 986 (62.1) | 0.01 |
| Aldactone | 213 | 825 (14.8) | 71 (7.2) | 111 (12.1) | 643 (17.6) | 0.01 |
| Eplerenone | 213 | 109 (2.0) | 11 (1.1) | 3 (0.3) | 95 (2.6) | 0.01 |
| Outcomes | ||||||
| Stent thrombosis, n (%) | 7 | 110 (2.0) | 16 (1.6) | 9 (1) | 85 (2.3) | 0.04 |
| Definite stent thrombosis, n (%) | 7 | 44 (0.8) | 9 (0.9) | 5 (0.5) | 30 (0.8) | 0.03 |
| TIMI major bleed, n (%) | 6 | 32 (0.6) | 2 (0.2) | 4 (0.4) | 26 (0.7) | 0.01 |
| LVEF, mean (SD), % | 270 | 39.2 (14.6) | 32.8 (13.9) | 39.6 (16.4) | 40.8 (13.8) | 0.01 |
| Cardiogenic shock, in hospital, n (%) | 7 | 363 (6.5) | 50 (5.1) | 30 (3.3) | 283 (7.7) | 0.01 |
| 30‐d Mortality, n (%) | 0 | 234 (4.2) | 44 (4.4) | 20 (2.2) | 170 (4.6) | 0.01 |
| 1‐y Mortality, n (%) | 0 | 410 (7.4) | 64 (6.5) | 31 (3.4) | 315 (8.6) | 0.01 |
ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, aldosterone receptor blocker; LV, left ventricular; LVEF, LV ejection fraction; PCI, percutaneous coronary intervention; PPCI, primary PCI; and TIMI, Thrombolysis in Myocardial Infarction.
Association Between Hospital Type and Outcomes
Short‐Term Outcomes
The 30‐day all‐cause mortality for the entire cohort was 4.2%, with significant variation across low‐, medium‐, and high‐volume hospitals (low‐volume hospitals, 4.4%; medium‐volume hospitals, 2.2%; and high‐volume hospitals, 4.6%; P for trend <0.01). Figure 2 depicts Kaplan‐Meier curves for the entire cohort, displaying the survival rates for low‐, medium‐, and high‐volume hospitals. After multivariable adjustment, a higher HR for 30‐day mortality was noted in both low‐volume (HR, 2.11; 95% CI, 1.13–3.93; P=0.01) and high‐volume hospitals (HR, 2.12; 95% CI, 1.26–3.57; P<0.01), compared with medium‐volume hospitals. However, adjusted mortality at 30 days was not significantly different between high‐ and low‐volume hospitals (HR, 1.01; 95% CI, 0.61–1.67; P=0.98). Figure 3 depicts the association between hospital‐level annual PPCI volumes and 30‐day mortality.
Figure 2. Kaplan‐Meier curves for the entire cohort (n=5560), displaying the unadjusted relationship between hospitals, categorized according to the hospital‐level annual primary percutaneous coronary intervention volume, and all‐cause mortality at 30 days (inset box) and 1 year with a comparison between groups using log‐rank test.
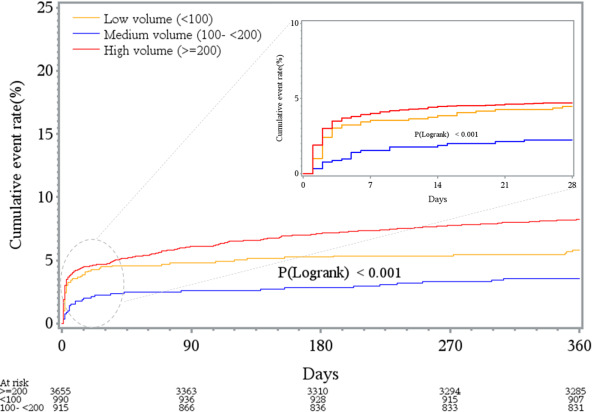
Figure 3. Hazard ratios (HRs) of 30‐day all‐cause mortality between hospital groups, categorized into low‐, medium‐, and high‐volume, according to the annual institutional primary percutaneous coronary intervention (PCI) volume.
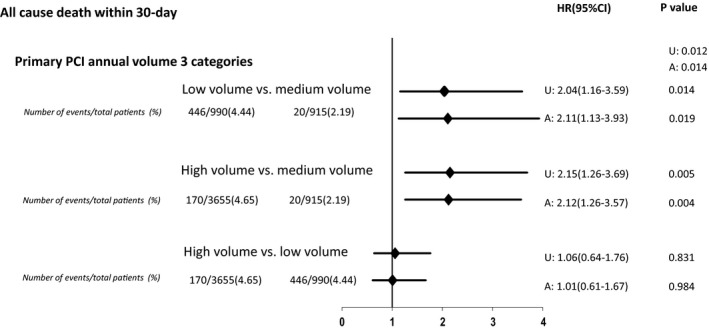
A indicates adjusted for sex, CI, confidence interval, TIMI (Thrombolysis in Myocardial Infarction) risk score, insurance coverage, living in poverty, family history, smoking status, culprit lesion in proximal left anterior descending artery, number of years catheterization laboratory was performing percutaneous coronary interventions, and year of enrollment. U, unadjusted.
Long‐Term Outcomes
The crude 1‐year all‐cause mortality for the entire cohort was 7.4%, with significant variation across low‐, medium‐, and high‐volume hospitals (low‐volume hospitals, 6.5%; medium‐volume hospitals, 3.4%; and high‐volume hospitals, 8.6%; P for trend <0.01). After multivariable adjustment, mortality within 1 year was higher among patients treated at high‐volume PPCI centers (HR, 2.53; 95% CI, 1.78–3.58; P<0.01) and for those treated at low‐volume centers (HR, 1.80; 95% CI, 1.12–2.90; P=0.01), compared with medium‐volume hospitals. Mortality within 1 year was not significantly different between high‐ and low‐volume hospitals (HR, 1.40; 95% CI, 0.91–2.17; P=0.13). We obtained consistent results in a sensitivity analysis performed after incorporating individual components of the TIMI STEMI risk score in the model as opposed to the risk score itself (Figure S2). We also performed this analysis with the exclusion of patients without insurance coverage treated at high‐volume hospitals. In this analysis, we found that the volume‐outcome relationship was maintained (high‐ versus medium‐volume hospitals: HR, 2.42; 95% CI, 1.7–3.4; P<0.01). Figure 4 depicts the association between hospital‐level annual PPCI volumes and the unadjusted and adjusted mortality within 1 year.
Figure 4. Hazard ratios (HRs) of 1‐year all‐cause mortality between hospital groups, categorized into llow‐, medium‐, and high‐volume, according to the annual institutional primary percutaneous coronary intervention (PCI) volume.
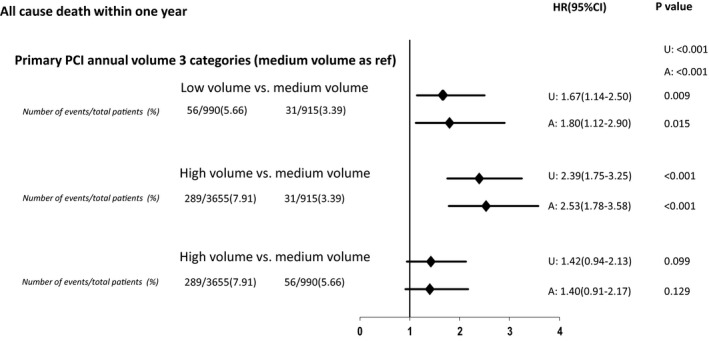
A indicates adjusted for sex, TIMI (Thrombolysis in Myocardial Infarction) risk score, insurance coverage, living in poverty, family history, smoking status, culprit lesion in proximal left anterior descending artery, number of years catheterization laboratory was performing percutaneous coronary interventions, and year of enrollment. U, unadjusted.
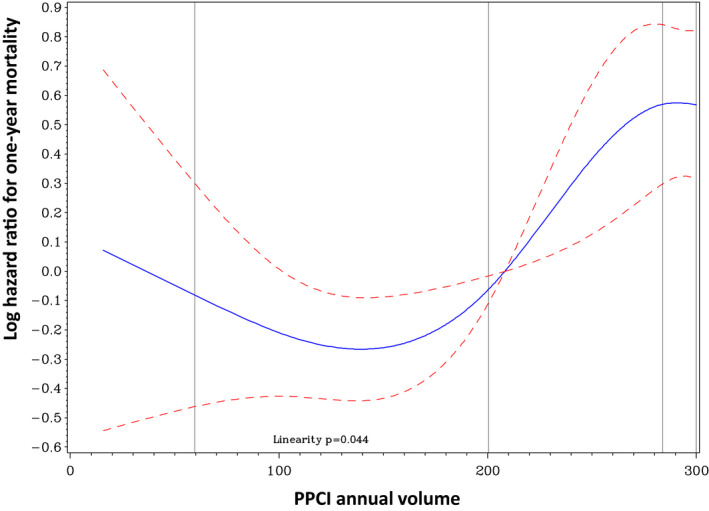
We then examined annual PPCI volume as a continuous variable. The relationship between the institutional annual PPCI volume and mortality within 1 year followed a nonlinear pattern, with low‐ and high‐volume hospitals having increased mortality rates compared with medium‐volume hospitals (Figure 5). Also, medium‐volume hospitals had the lowest rates of cardiogenic shock in hospital (low‐volume hospitals, 5.1%; medium‐volume hospitals, 3.3%; and high‐volume hospitals, 7.7%; P for trend=0.01) and stent thrombosis at 1 year (low‐volume hospitals, 1.6%; medium‐volume hospitals, 1.0%; and high‐volume hospitals, 2.3%; P for trend=0.04).
Figure 5. Relationship between institutional annual primary percutaneous coronary intervention (PPCI) volume as a continuous variable and risk of the primary outcome (1‐year mortality).
Discussion
Improving care pathways for acute myocardial infarction management in LMICs constitutes one of the most crucial but arduous tasks on the path to reducing global cardiovascular disease burden.2, 14 In India, STEMI represents the most frequent ACS presentation.5, 15 The southern Indian state of Kerala adopted a pragmatic approach to improving STEMI care by locally adapting quality improvement tool kits for ACS management16 and rapidly expanding the number of PPCI‐capable hospitals.4 However, several such hospitals have low PPCI volumes. Lower procedural volumes may be driving variability across hospitals in terms of quality of PPCI procedures, the use of guideline‐directed medical therapy, and patient outcomes.
In this large PPCI STEMI registry, including 55.2% of all PPCI‐capable hospitals in Kerala, several novel findings were observed. There were significant variations in the equity of access across hospital groups. Patients classified as living in poverty preferentially accessed high‐volume hospitals, many of which were offering government‐sponsored health insurance, possibly bypassing nearby low‐volume hospitals. However, patients who could afford to pay for health care preferentially accessed the more prevalent low‐ and medium‐volume hospitals. Such inequity in access resulted in the higher‐volume hospitals having longer total ischemic time despite the similar door‐to‐balloon time. There were significant procedural variations across hospital groups. Medium‐volume hospitals had higher rates of radial vascular access and nonculprit vessel PCI, compared with low‐ and high‐volume hospitals. Contrastingly, high‐volume hospitals had lower aspiration thrombectomy rates. Guideline‐directed medical therapy rates were also not uniform across hospital groups. Low‐volume hospitals discharged a higher proportion of patients on newer‐generation P2Y12 inhibitors (ticagrelor or prasugrel). High‐volume hospitals discharged a higher percentage of patients on high‐intensity statins, angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers, and aldosterone blockers. The level of affordability of the patient population, as well as the academic status of treating hospitals, likely impacted guideline‐directed medical therapy rates.
We identified a nonlinear relationship between hospital‐level annual PPCI volumes and 1‐year mortality among patients who underwent PPCI for STEMI in Kerala. Medium‐volume hospitals had lower rates of stent thrombosis, cardiogenic shock, and 30‐day and 1‐year mortality rates compared with low‐ and high‐volume hospitals. Several factors can explain the paradoxical PPCI volume‐outcome relationship in Kerala. First, the longer total ischemic time and ECG‐to‐balloon time documented at high‐volume hospitals is one such factor potentially contributing to the differential in outcomes. Second, there were significant differences in the procedural quality indicators between hospital groups, which could be impacting outcomes. Medium‐volume hospitals had significantly higher radial vascular access rates compared with low‐ and high‐volume hospitals. Besides, medium‐volume hospitals performed nonculprit vessel PCI in a higher proportion of patients compared with low‐ and high‐volume hospitals. The rates of nonculprit vessel PCI may be a surrogate marker of complete revascularization. In STEMI patients with multivessel coronary artery disease, complete revascularization reduces the risk of cardiovascular death and myocardial infarction.17 Similarly, low‐volume hospitals used aspiration thrombectomy in 45.2% of PPCIs, a rate much higher than in medium‐ and low‐volume hospitals. Third, all public hospitals were high‐volume hospitals. There were significant differences between private and public hospitals in terms of baseline patient characteristics, the clinical process‐of‐care metrics, and outcomes. Finally, there could be multiple unmeasured confounders influencing the PPCI volume‐outcome relationship in LMICs. Lower drug compliance among patients living in poverty may potentially explain the divergence in survival curves between low‐ and high‐volume hospitals after the first month. Although high‐volume hospitals had significantly higher rates of guideline‐directed medical therapy prescription at discharge compared with other hospital groups, compliance issues stemming from catastrophic healthcare expenditure could compromise this early advantage. Catastrophic healthcare expenditure and distress financing are typical after ACS in this geographic area.18 In our cohort, 65.2% of patients undergoing PPCI paid for their treatment expenses out of pocket, comparable to the 75.9% of patients lacking health insurance coverage in the Kerala ACS QUIK (Acute Coronary Syndrome Quality Improvement in Kerala) trial.16, 18 Furthermore, fellows or early‐career interventionists typically train at high‐volume hospitals. Lifetime operator‐level PCI volume may thus be a potential confounder affecting the volume‐outcome relationship.
The association between both hospital‐ and operator‐level volume benchmarks and cardiac interventional procedural quality would appear logical. Also, volume benchmarks have remained the cornerstone of surgical quality assurance programs. National cardiac societies and governmental agencies in many LMICs, including India, grapple with the need to adopt globally recognized institutional volume benchmarks to regulate the growth of cardiac catheterization laboratories and to mandate which hospitals can perform PPCI. Several international societies and regulatory bodies have issued institutional‐ and operator‐level volume benchmarks, with wide variance in the recommended volume cutoffs. The British Cardiovascular Intervention Society, for example, has endorsed that STEMI patients should undergo PPCI in hospitals performing a minimum of 100 PPCIs annually.8 However, the American College of Cardiology Foundation/American Heart Association/Society for Cardiovascular Angiography and Interventions guidelines recommended an annual institutional cutoff of at least 36 PPCIs and an operator‐level cutoff of 11 PPCIs.9, 10 The volume benchmarks are more stringent for hospitals without on‐site cardiac surgical support.9 Low‐volume hospitals, especially using low‐volume operators, may have worse outcomes6 and may require extra surveillance and operator backup.
Several concerns temper the enthusiasm to adapt globally recognized PCI volume benchmarks to STEMI care in LMICs. First, volume benchmarks may result in regionalization of care, creating further unintended hurdles to access to care. Impediments to the access of care may disproportionately impact lower socioeconomic strata patients residing in remote areas. Second, all structural and process metrics may not be equal. Quality improvement initiatives designed to improve total ischemic time may differently impact outcomes compared with efforts intended to strengthen structural parameters, like institutional PPCI volumes. LMICs may incur a high opportunity cost by basing quality improvement programs on benchmarks that are not methodically selected. Third, at least in some countries, PPCI as a procedure may be maturing, and the volume‐outcome relationship may have weakened.7, 19, 20 There are limited data to indicate where LMICs stand with regard to the maturity of PPCI procedures. Institutional‐level variations in process metrics may explain many of the differences in outcomes,6, 20 especially in LMICs.13
Our study has certain limitations. Our study involved only 55.2% of the PCI‐capable hospitals in this region. However, we have demonstrated that there are no significant differences between the participating and nonparticipating hospitals but for the number of interventionalists employed at each center. Also, 5.2% of patients were lost to follow‐up. Some of these patients could have died or switched healthcare providers. We did not measure the operator‐level procedural volume. Both lifetime and annual operator‐level procedure volumes may influence outcomes. Low‐volume operators performing PPCI at low‐volume hospitals may have worse results.6 We have not documented cardiac rehabilitation participation because most participating hospitals were not offering formal cardiac rehabilitation to STEMI patients during the study period. Nonetheless, our registry is the first multicenter PPCI STEMI registry from India and represents one of the earliest studies looking at long‐term outcomes following STEMI in India.
Conclusions
In conclusion, LMICs, like India, may have a unique nonlinear relationship between institutional‐level PPCI volumes and outcomes that appears to be partly driven by inequities in access to health care and institutional‐level variance of procedural quality metrics. STEMI quality improvement initiatives in Kerala should mainly focus on the relatively few high‐volume hospitals, especially those in the public sector. Process‐of‐care metrics, like timeliness of reperfusion and procedural characteristics, may be more vital than institutional‐level PPCI volumes in the quest to improve STEMI outcomes in India. Our data call into question the need for LMICs, like India, to regionally adapt global institutional‐level PPCI volume benchmarks.
Sources of Funding
The registry was funded by the Cardiological Society of India–Kerala chapter.
Disclosures
Dr Welsh received research grants or personal support from Astra Zeneca, Bayer, and Boehringer Ingelheim. The remaining authors have no disclosures to report.
Supporting information
Data S1 Tables S1–S3 Figures S1–S2
Acknowledgments
We acknowledge the contributions of Sunjidatul Islam and Wendimagegn Alemayehu of the Canadian VIGOUR Centre, University of Alberta, Edmonton, in managing the database. We acknowledge the contributions of Hisham Mohammed, John Paul, and Binoy Kurian in collecting data.
(J Am Heart Assoc. 2020;9:e014968 DOI: 10.1161/JAHA.119.014968.)
For Sources of Funding and Disclosures, see pages 11 and 12.
References
- 1. India State‐Level Disease Burden Initiative Collaborators . Nations within a nation: variations in epidemiological transition across the states of India, 1990–2016 in the Global Burden of Disease Study. Lancet. 2017;390:2437–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Prabhakaran D, Jeemon P, Roy A. Cardiovascular diseases in India: current epidemiology and future directions. Circulation. 2016;133:1605–1620. [DOI] [PubMed] [Google Scholar]
- 3. Roth GA, Johnson C, Abajobir A, Abd‐Allah F, Abera SF, Abya G, Ahmed M, Aksut B, Alam T, Alam K, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mathew A, Abdullakutty J, Sebastian P, Viswanathan S, Mathew C, Nair V, Mohanan PP, Geroge Koshy A. Population access to reperfusion services for ST‐segment elevation myocardial infarction in Kerala, India. Indian Heart J. 2017;69:S51–S56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mohanan PP, Mathew R, Harikrishnan S, Krishnan MN, Zachariah G, Joseph J, Eapen K, Abraham M, Menon J, Thomas M, et al. Presentation, management, and outcomes of 25 748 acute coronary syndrome admissions in Kerala, India: results from the Kerala ACS Registry. Eur Heart J. 2013;34:121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fanaroff AC, Zakroysky P, Dai D, Wojdyla D, Sherwood MW, Roe MT, Wang TY, Peterson ED, Gurm HS, Cohen MG, et al. Outcomes of PCI in relation to procedural characteristics and operator volumes in the United States. J Am Coll Cardiol. 2017;69:2913–2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kumbhani DJ, Cannon CP, Fonarow GC, Liang L, Askari AT, Peacock WF, Peterson ED, Bhatt DL. Association of hospital primary angioplasty volume in ST‐segment elevation myocardial infarction with quality and outcomes. JAMA. 2009;302:2207–2213. [DOI] [PubMed] [Google Scholar]
- 8. Banning AP, Baumbach A, Blackman D, Curzen N, Devadathan S, Fraser D, Ludman P, Norell M, Muir D, Nolan J, et al. Percutaneous coronary intervention in the UK: recommendations for good practice 2015. Heart. 2015;101(suppl):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Naidu SS, Aronow HD, Box LC, Duffy PL, Kolansky DM, Kupfer JM, Latif F, Mulukutla SR, Rao SV, Swaminathan RV, et al. SCAI expert consensus statement: 2016 best practices in the cardiac catheterization laboratory: (endorsed by the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia intervencionista; affirmation of value by the Canadian Association of Interventional Cardiology‐Association Canadienne de Cardiologie d'Intervention). Catheter Cardiovasc Interv. 2016;88:407–423. [DOI] [PubMed] [Google Scholar]
- 10. Harold JG, Bass TA, Bashore TM, Brindis RG, Brush JE Jr, Burke JA, Dehmer GJ, Deychak YA, Jneid H, Jollis JG, et al. ACCF/AHA/SCAI 2013 update of the clinical competence statement on coronary artery interventional procedures: a report of the American College of Cardiology Foundation/American Heart Association/American College of Physicians Task Force on Clinical Compete. Circulation. 2013;128:436–472. [DOI] [PubMed] [Google Scholar]
- 11. Patel A, Prabhakaran D, Berendsen M, Mohanan PP, Huffman MD. Pre‐hospital policies for the care of patients with acute coronary syndromes in India: a policy document analysis. Indian Heart J. 2017;69:S12–S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Patel A, Mohanan PP, Prabhakaran D, Huffman MD. Pre‐hospital acute coronary syndrome care in Kerala, India: a qualitative analysis. Indian Heart J. 2017;69:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huffman MD, Prabhakaran D, Abraham AK, Krishnan MN, Nambiar AC, Mohanan PP; Kerala Acute Coronary Syndrome Registry Investigators . Optimal in‐hospital and discharge medical therapy in acute coronary syndromes in Kerala: results from the Kerala acute coronary syndrome registry. Circ Cardiovasc Qual Outcomes. 2013;6:436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wilson H, Jollis J, Alexanderson E. Scaling STEMI care internationally: ACC's global STEMI quality improvement initiative. J Am Coll Cardiol. 2018;72:2528–2530. [DOI] [PubMed] [Google Scholar]
- 15. Xavier D, Pais P, Devereaux PJ, Xie C, Prabhakaran D, Reddy KS, Gupta R, Joshi P, Kerkar P, Thanikachalam S, et al. Treatment and outcomes of acute coronary syndromes in India (CREATE): a prospective analysis of registry data. Lancet. 2008;371:1435–1442. [DOI] [PubMed] [Google Scholar]
- 16. Huffman MD, Mohanan PP, Devarajan R, Baldridge AS, Kondal D, Zhao L, Ali M, Krishnan MN, Natesan S, Gopinath R, et al. Effect of a quality improvement intervention on clinical outcomes in patients in India with acute myocardial infarction: the ACS QUIK randomized clinical trial. JAMA. 2018;319:567–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mehta SR, Wood DA, Storey RF, Mehran R, Bainey KR, Nguyen H, Meeks B, Di Pasquale G, López‐Sendón J, Faxon DP, et al. Complete revascularization with multivessel PCI for myocardial infarction. N Engl J Med. 2019;381:1411–1421. [DOI] [PubMed] [Google Scholar]
- 18. Mohanan PP, Huffman MD, Baldridge AS, Devarajan R, Kondal D, Zhao L, Ali M, Joseph J, Eapen K, Krishnan MN, et al. Microeconomic costs, insurance, and catastrophic health spending among patients with acute myocardial infarction in India: substudy of a randomized clinical trial. JAMA Netw Open. 2019;2:e193831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. O'Neill D, Nicholas O, Gale CP, Ludman P, de Belder MA, Timmis A, Fox KA, Simpson IA, Redwood S, Ray SG. Total center percutaneous coronary intervention volume and 30‐day mortality: a contemporary national cohort study of 427 467 elective, urgent, and emergency cases. Circ Cardiovasc Qual Outcomes. 2017;10:e003186. [DOI] [PubMed] [Google Scholar]
- 20. Kumbhani DJ, Nallamothu BK. PCI volume benchmarks: still adequate for quality assessment in 2017? J Am Coll Cardiol. 2017;69:2925–2928. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 Tables S1–S3 Figures S1–S2


