Abstract
Background
Evidence suggests familial aggregation and intergenerational associations for individual cardiovascular health (CVH) metrics. Over a 53‐year life course, we examined trends and association of CVH between parents and their offspring at similar mean ages.
Methods and Results
We conducted a series of cross‐sectional analyses of the FHS (Framingham Heart Study). Parent‐offspring pairs were assessed at exams where their mean age distributions were similar. Ideal CVH was defined using 5 CVH metrics: blood pressure (<120/<80 mm Hg), fasting blood glucose (<100 mg/dL), blood cholesterol (<200 mg/dL), body mass index (<25 kg/m2), and non‐smoking. Joinpoint regression and Chi‐squared test were used to assess linear trend; proportional‐odds regression was used to examine the association between parents and offspring CVH. A total of 2637 parents were paired with 3119 biological offspring throughout 6 exam cycles. Similar patterns of declining ideal CVH with advancing age were observed in parents and offspring. Small proportions of parents (4%) and offspring (17%) achieved 5 CVH metrics at ideal levels (P‐trend <0.001). Offspring of parents with poor CVH had more than twice the odds of having poor CVH (pooled odds ratio, 2.59; 95% CI, 1.98–3.40). Over time, elevated glucose levels and obesity doubled among the offspring and were the main drivers for declining ideal CVH trends.
Conclusions
Parental CVH was positively associated with offspring CVH. However, intergenerational CVH gains from declining smoking rates, cholesterol, and blood pressure were offset by rising offspring obesity and elevated glucose levels. This suggests an intergenerational phenotypic shift of risk factors and the need for a family‐centered approach to cardiovascular care.
Keywords: familial clustering, ideal cardiovascular health, offspring, parents, trends
Subject Categories: Cardiovascular Disease, Epidemiology, Primary Prevention, Risk Factors, Lifestyle
Nonstandard Abbreviations and Acronyms
- AHA
American Heart Association
- CVD
cardiovascular disease
- CVH
cardiovascular health
- FHS
Framingham Heart Study
- OR
odds ratio
Clinical Perspective
What Is New?
Parental cardiovascular health was positively associated with that of their offspring at similar age periods over the life course, indicating familial clustering and intergenerational transfer of cardiovascular health.
Over a period of 53‐years of follow‐up, intergenerational cardiovascular health gains from decreasing levels of smoking, cholesterol, and hypertension were offset by increasing obesity and elevated glucose levels among the offspring.
What Are the Clinical Implications?
Findings underscore the need for family‐centered approaches to cardiovascular risk assessment and promotion of cardiovascular health starting early in life, especially for high‐risk families and populations to reduce intergenerational cardiovascular disease morbidity and mortality.
Familial transmission of ideal cardiovascular health (CVH) from parents to children through behavioral‐lifestyle factors can positively impact the intergenerational prevention of cardiovascular disease (CVD).1, 2 In 2010, the American Heart Association (AHA) launched a campaign emphasizing the concept of ideal CVH. The AHA defined ideal CVH as the simultaneous presence of 4 ideal health behaviors (non‐smoking, body mass index [BMI] <25 kg/m2, physical activity, and dietary pattern consistent with current guidelines) and 3 ideal health factors (total cholesterol <200 mg/dL, blood pressure <120/<80 mm Hg, fasting glucose <100 mg/dL).3
Maintaining ideal CVH has been associated with substantially lower lifetime CVD risk,3 better cognition and quality of life4 and lower healthcare costs.5 Multiple genetic studies suggest that modifiable factors and behaviors are major contributors to ideal CVH because only 15% of ideal CVH is heritable.6, 7 However, a small fraction of the American population (<5%) has ideal CVH using the 7 CVH metrics criteria.3, 8
Clustering of shared exposure to diseases within families is attributable to interactions among genetic and shared environmental, behavioral factors and socioeconomic status.7, 9 Most studies on familial aggregation suggest the existence of positive parent‐offspring associations of individual CVD risk factors such as elevated BMI, physical inactivity and poor diet10, 11, 12; however, little is known about parent‐offspring clustering and the association of ideal CVH metrics over the life course. Thus, using data of 2 intergenerational cohorts in the FHS (Framingham Heart Study), we examined the life course trends and clustering of ideal CVH over the period of 1948 to 2001, spanning over 53 years, as well as associations of ideal CVH between parents and their adult offspring at exam cycles with similar distribution in ages.
Methods
Data Sharing
This study used data from the FHS (original and offspring cohorts). Because of the sensitive nature of the data used for this study, requests to access the data set from qualified researchers can be requested through the Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC) of the National Heart, Lung, and Blood Institute (https://biolincc.nhlbi.nih.gov/home/).
Study Population
The FHS is comprised of community dwelling individuals selected using systematic random sampling of residents of Framingham, MA. The FHS consists of several intergenerational cohorts. In 1948, a sample of 5209 Original Cohort participants aged 28 to 62 years was recruited.13, 14 The offspring cohort was enrolled in 1971 with a total of 5124 participants aged 6 to 70 years and comprised of children from the original cohort.14 The present analysis focused on the parent‐offspring pairs. All the participants gave informed written consent. This study was approved by the Institutional Review Board at the University of Massachusetts Boston.
The FHS is among the longest running multi‐generational epidemiological studies focusing on the epidemiology of CVD. The design of FHS is described in detail elsewhere.13, 14 In the present analysis, the original cohort (parents) and the offspring cohort (offspring) were selected and paired at exam cycles where the age distributions of parents and their offspring were approximately similar (Table S1). This approximate similarity in age distributions was justification for between cohort comparison of linear trends in CVH across the life course. Each offspring was linked to their available biological parent(s) using a unique family identifier in the database. Participants were included if they were aged ≥20 years at the first paired exam cycle. We did not require offspring to have both parents in the database or live together for the entire offspring's childhood. We estimated that a sample size of at least 863 parent‐offspring pairs would achieve a statistical power of 95% and allow us to detect a small effect size of 0.02 of association between the parents’ and offspring CVH at a significance level of 0.05.
Parents’ and offspring CVH scores were the main independent and dependent variables, respectively. CVH scores are ordinarily calculated using the following 7 metrics defined by AHA criteria: smoking status, BMI, total cholesterol, blood pressure, blood glucose, physical activity, and diet.8 Physical activity and dietary data, however, were not available at the selected study exam cycles, and thus used 5 of the 7 CVH metrics. For these 5 CVH metrics, ideal CVH was defined as: blood pressure (<120/<80 mm Hg), fasting blood glucose (<100 mg/dL), blood cholesterol (<200 mg/dL), BMI (<25 kg/m2), and having never smoked or quit in the past 12 months.8 Other clinical thresholds for intermediate and poor CVH are provided in Table S2. Each participant's 5‐metric CVH score was defined on a continuous scale ranging from 0 to 10, with the highest score indicating a perfect CVH score. Similar to others,15, 16 the CVH score categories were computed as an ordinal variable using the counts of CVH metrics attained at ideal levels (0–2 metrics=poor CVH, 3–4 metrics=intermediate CVH, and >4 metrics=ideal CVH).
Statistical Analysis
We computed descriptive statistics and graphical displays of CVH metrics for parents and offspring using a 2‐sample t test for continuous and the Chi‐square test for categorical variables. Linear associations between CVH scores were assessed using the Pearson correlation coefficient. We determined the linear trends of correlations over time and showed whether parent‐offspring CVH score correlations became more robust or attenuated with progressing follow‐up time. Linear trend analysis of proportions of parents and offspring for paired CVH categories (poor, intermediate, ideal) was determined using Chi‐squared (χ2) for linear trend (1 degrees of freedom). This test was used to determine whether the CVH proportions were different between cohorts. Linear trends for percent change (PC) of CVH categories for paired exam cycles were also performed using Joinpoint regression, where PC from 1 exam to the next was computed to determine the rate of change over the selected exam cycles. Joinpoint regression is used to analyze time trends data by performing several permutation tests to calculate percentage rate of change and computes P values using Monte Carlo methods while maintaining a nominal overall familywise significance level of 0.05 through a Bonferroni adjustment.17, 18
To examine the association between parents’ and offspring CVH at the paired exam cycles, we fitted exam‐specific cross‐sectional linear regression models for the continuous CVH score and proportional‐odds cumulative logit model for the categorical CVH score (ideal CVH, intermediate CVH, and poor CVH; coded as 0, 1, and 2, respectively). The offspring CVH score was analyzed as the dependent variable and the parents’ CVH score as the independent variable. Informed by studies that maternal and paternal CVH metrics have similar or additive effects on their offspring,19, 20 parents’ CVH score was derived from an average of the offspring's mother and father's CVH score. However, to ensure the congruency of the parents’ CVH score, we performed dyadic sensitivity analyses for mother‐offspring and father‐offspring CVH score for each paired exam cycle. Proportional‐odds models were fitted with a 3‐level ordinal outcome and predictor variables, namely, offspring CVH and parents’ CVH score, respectively. Since a proportional‐odds model assumes equal slopes among the ordered categories, before fitting the model, we used the Brant test to confirm the veracity of the proportionality of odds assumption.21 Odds ratios (ORs) obtained from a proportional‐odds model are interpreted considering the possible pairwise log‐odds comparisons such as “ideal CVH versus intermediate and poor CVH or, ideal and intermediate CVH versus poor CVH”.21
Contrary to polytomous logistic regression, coding of CVH in pairwise comparison using proportional‐odds model reflects and preserves the ordinal nature of the outcome21 such that intermediate CVH is coded between poor and ideal CVH. For a hypothetical example on interpreting the ORs (offspring CVH and parents CVH being dependent and independent variables, respectively), if the parents’ ORs for poor versus ideal CVH was 3.0 and the OR for intermediate versus ideal CVH was 2.0, then we would say: (1) offspring of parents with poor CVH were 3 times as likely to have poor CVH compared with having intermediate and ideal CVH, and (2) offspring of parents with intermediate CVH were twice as likely to have intermediate CVH compared with having ideal and poor CVH. In brief, offspring of parents with poor CVH or intermediate CVH were more than twice as likely to have less favorable CVH (lower CVH) than offspring of parents with ideal CVH. All regression models were adjusted for potential confounding from age, sex, and education attainment of the offspring. In addition, the regression models were adjusted for dependencies attributable to familial clustering using the family identifier as the cluster variable. Analyses were conducted using Stata version 14.2 (StataCorp LLC, College Station, Texas, USA), Joinpoint Regression Software, version 4.7.0.0 (National Cancer Institute, USA). A P value of <0.05 was used as the criteria for statistical significance.
Results
A total of 2637 parents and 3119 offspring in the original (parents) and offspring cohorts of the Framingham Heart Study were linked using the family identifier in the database. The mean ages between parents’ and offspring cohorts were comparable at the first exam (43.1 years, SD ±8.3 versus 43.1, SD ±10.2; respectively, P=0.291). However, parents were older (62.0±7.8 years) than their offspring (60.2±9.9 years) in the last paired exam cycle (P<0.001). There was a similar proportion of men and women in both cohorts (Table 1). Relatively more men than women had college degrees and/or a graduate education in both cohorts, however, the proportion with college degrees was higher among offspring (P<0.001). A significantly higher proportion of men than women had professional jobs, and offspring had a higher proportion of professional jobs than the parents (Table 1; P<0.001). A larger proportion of women in both cohorts self‐reported as housewives (parents 81.8% versus offspring 38.5%).
Table 1.
Sample Characteristics of Original and Offspring Cohorts by Sex in Baseline Exam Cycles
| FHS Original Cohort n=2637 | Offspring Cohort n=3119 | ||||
|---|---|---|---|---|---|
| Characteristic | Men, n% | Women, n% | Men, n% | Women, n% | P Value† |
| Mean age, y | 44 (8.4) | 43.0 (8.2) | 42.8 (10.3) | 43.3 (10.2) | <0.001 |
| Sex | 1290 (48.9) | 1347 (51.1) | 1491 (47.8) | 1628 (52.2) | 0.399 |
| Marital status | |||||
| Married | 1282 (99.4) | 1279 (95.0) | 967 (77.6) | 1017 (74.2) | <0.001 |
| Single | 0 (0.0) | 1 (0.7) | 158 (12.7) | 161 (11.7) | |
| Widowed | 5 (0.4) | 51 (3.8) | 12 (1.0) | 49 (3.6) | |
| Divorced/separated | 3 (0.3) | 16 (1.2) | 109 (8.8) | 144 (10.5) | |
| Education level | |||||
| <High school | 585 (47.3) | 547 (41.9) | 87 (7.0) | 85 (6.2) | <0.001 |
| High school | 307 (24.8) | 423 (32.4) | 368 (29.6) | 507 (37.0) | |
| Some college | 99 (8.0) | 105 (8.0) | 292 (23.5) | 433 (31.6) | |
| ≥College grad | 246 (19.9) | 231 (17.7) | 495 (39.9) | 344 (25.1) | |
| Type of work | |||||
| Professional | 104 (10.0) | 18 (1.5) | 267 (22.6) | 219 (16.5) | <0.001 |
| Executive | 21 (2.0) | 0 (0.0) | 23 (2.0) | 4 (0.3) | |
| Supervisory | 273 (26.4) | 19 (1.6) | 152 (12.9) | 36 (2.7) | |
| Technical | 124 (12.0) | 6 (0.5) | 124 (10.5) | 51 (3.9) | |
| Laborer | 400 (38.6) | 121 (10.3) | 468 (39.6) | 126 (9.5) | |
| Clerical | 37 (3.5) | 37 (3.2) | 42 (3.6) | 359 (27.1) | |
| Sales | 77 (7.4) | 12 (1.0) | 103 (8.7) | 22 (1.7) | |
| Housewife | 0 (0.0) | 959 (81.8) | 0 (0.0) | 510 (38.5) | |
First exam overall *mean age for parents=43.6 (8.3); offspring 43.1 (10.2); P=0.291; last paired exam, parents=62.0 (7.8); offspring 60.2 (9.9); P<0.001; (*mean ages, SDs, and P values are computed for between cohort mean age differences, not stratified by sex; † P values computed for between cohort differences stratified by sex). FHS indicates Framingham Heart Study.
Table 2 presents linear trends and prevalence of the 5 CVH metrics over the period of 1948 to 2001. Offspring had a significantly higher prevalence of obesity (BMI ≥30 kg/m2; P<0.001) than the parents in all selected exams. The prevalence of obesity among the offspring more than doubled (110% increase) from 14.5% in 1979 to 1983 to 30.4% in 1998 to 2001 (Table 2). Parents had statistically higher systolic and/or diastolic blood pressure (SBP ≥140 or diastolic blood pressure ≥90 mm Hg) than the offspring at all paired exam cycles (P<0.001) with the prevalence of elevated blood pressure increasing from 37.9% in 1948 to 1952 to 52.3% in 1969 to 1971, compared with a prevalence of 17.6% to 24.1% among the offspring from 1979 to 1983 to 1998 to 2001. A similar pattern was observed for total cholesterol ≥240 mg/dL (highest of 57.4% in 1961–1964). The prevalence of smoking for parents was highest in 1948 to 1952 (58.5%) and lowest in 1969 to 1971 (36.1%) exam cycles. The prevalence of offspring with diabetes mellitus or fasting blood glucose ≥126 mg/dL increased with increasing age and was significantly higher than parents in all exam periods (3.4% in 1979–1983 versus 0.7% in 1948–1952 and 8.8% in 1998–2001 versus 5.1% in 1969–1971 in offspring and parents, respectively, P<0.001).
Table 2.
Trends and Prevalence of 5‐CVH Metrics for Parents and Offspring in Paired Exam Cycles
| Parents | Offspring | |||
|---|---|---|---|---|
| Exam Date | CVH Metric, n (%) | Exam Date | CVH Metric, n (%) | P Value |
| BMI ≥30 kg/m2 | BMI ≥30 kg/m2 | |||
| 1948–1952 | 371 (14.1) | 1979–1983 | 380 (14.5) | 0.002 |
| 1953–1956 | 272 (12.9) | 1983–1987 | 693 (18.0) | <0.001 |
| 1956–1960 | 302 (13.7) | 1987–1991 | 464 (21.3) | <0.001 |
| 1961–1964 | 323 (14.1) | 1991–1995 | 568 (24.6) | <0.001 |
| 1965–1968 | 312 (14.0) | 1995–1998 | 622 (29.3) | <0.001 |
| 1969–1971 | 289 (16.2) | 1998–2001 | 684 (30.4) | <0.001 |
| High BP | High BP | |||
| 1948–1952 | 998 (37.9) | 1979–1983 | 460 (17.6) | <0.001 |
| 1953–1956 | 662 (28.3) | 1983–1987 | 532 (20.2) | <0.001 |
| 1956–1960 | 841 (35.6) | 1987–1991 | 656 (24.2) | <0.001 |
| 1961–1964 | 1030 (45.3) | 1991–1995 | 502 (19.7) | <0.001 |
| 1965–1968 | 980 (45.9) | 1995–1998 | 527 (22.3) | <0.001 |
| 1969–1971 | 887 (52.3) | 1998–2001 | 580 (24.1) | <0.001 |
| Cholesterol ≥240 mg/d | Cholesterol ≥240 mg/dL | |||
| 1948–1952 | 852 (33.2) | 1979–1983 | 380 (14.6) | <0.001 |
| 1953–1956 | 894 (38.7) | 1983–1987 | 552 (21.7) | <0.001 |
| 1956–1960 | 1104 (47.3) | 1987–1991 | 502 (19.2) | <0.001 |
| 1961–1964 | 1293 (57.4) | 1991–1995 | 402 (15.9) | <0.001 |
| 1965–1968 | 1019 (48.1) | 1995–1998 | 438 (18.9) | <0.001 |
| 1969–1971 | 697 (41.7) | 1998–2001 | 320 (14.0) | <0.001 |
| Smoking | Smoking | |||
| 1948–1952 | 1533 (58.5) | 1979–1983 | 1033 (39.5) | <0.001 |
| 1953–1956 | 1216 (54.9) | 1983–1987 | 789 (30.0) | <0.001 |
| 1956–1960 | 1293 (54.8) | 1987–1991 | 670 (25.0) | <0.001 |
| 1961–1964 | 1134 (50.8) | 1991–1995 | 495 (19.4) | <0.001 |
| 1965–1968 | 839 (39.3) | 1995–1998 | 339 (14.4) | <0.001 |
| 1969–1971 | 610 (36.1) | 1998–2001 | 317 (13.2) | <0.001 |
| FBG ≥126 mg/dL | FBG ≥126 mg/dL | |||
| 1948–1952 | 18 (0.7) | 1979–1983 | 84 (3.4) | <0.001 |
| 1953–1956 | 19 (0.8) | 1983–1987 | 80 (3.3) | <0.001 |
| 1956–1960 | 19 (0.8) | 1987–1991 | 106 (4.3) | <0.001 |
| 1961–1964 | 18 (0.8) | 1991–1995 | 149 (6.0) | <0.001 |
| 1965–1968 | 29 (1.4) | 1995–1998 | 183 (8.0) | <0.001 |
| 1969–1971 | 85 (5.1) | 1998–2001 | 195 (8.8) | <0.001 |
High blood pressure=%, systolic blood pressure ≥l40, or diastolic blood pressure ≥90 mm Hg; smoking=current smokers/or quit within 12 months. BMI indicates body mass index; BP, blood pressure; CVH, cardiovascular health; and FBG, fasting blood glucose.
Trends and distribution of 5‐metric CVH score for parents’ and offspring are presented in Figure. Similar patterns of declining ideal CVH over time periods was observed for parents and offspring (parents 1948–1971, offspring 1979–2001; Figure). The trend test for CVH score proportions (parent‐offspring: “poor versus poor”, “intermediate versus intermediate” and “ideal versus ideal” CVH) was not statistically significant. In other words, CVH score proportions (poor, intermediate, ideal) for parents’ and offspring were similar at each paired exam period over the course of 53 years of observation. However, rate of PC from 1 exam cycle to the next using Joinpoint regression for trend showed a statistically significant increase in proportions of offspring intermediate CVH (PC, 0.6%; 95% CI, 0.4%–0.8%) with no corresponding statistically significant PC for parents’ intermediate CVH. Even though both cohorts had a similar declining pattern in ideal CVH (Figure S1), parents had a significantly higher rate of percent decline in ideal CVH than the offspring (PC, −3.4%; 95% CI −6.3% to −0.5% versus PC −1.3%; 95% CI −2.4% to −0.1%, respectively). The highest prevalence of ideal CVH for parents’ and offspring was 7.6% in 1953 to 1956 and 22.6% in 1983 to 1987, respectively, with proportions with ideal CVH declining with the aging of both cohorts (Figure). Ideal CVH reduced with increasing age for both parents’ and offspring. Parents who attained all 5 CVH metrics at ideal levels declined from 6.9% to 3.7% between period 1 (1948–1952) and period 6 (1969–1971), while that of offspring declined from 21.8% to 16.9% between period 1 (1979–1983) to period 6 (1998–2001).
Figure 1. Prevalence and trends of cardiovascular health ("poor", "intermediate", "ideal") for parents and offspring at each paired exam.
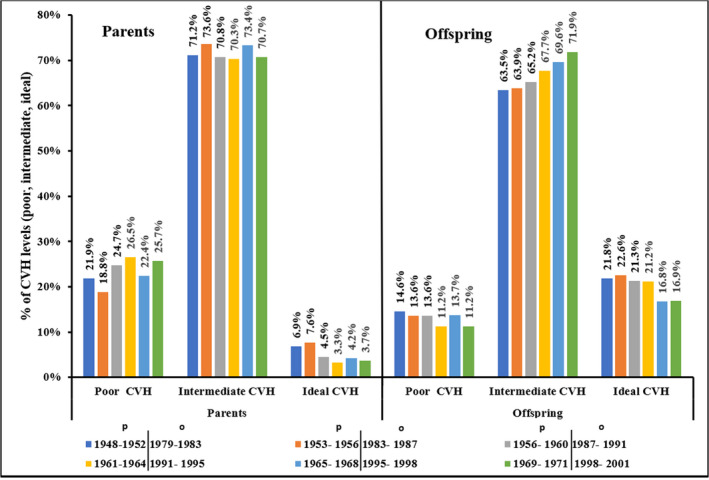
Each bar color coding represents the exam‐cycle year period for both parents and offspring. P trend <0.05 for percent change of parent/offspring ideal cardiovascular health and offspring intermediate cardiovascular health. CVH indicates cardiovascular health.
Parents’ CVH score was positively correlated with that of their offspring at similar mean age along their life course (r=0.2–0.4, P<0.001), and no substantial differences were found in this correlation when stratified by offspring mothers or fathers. In the proportional‐odds cumulative logit model, offspring of parents with poor CVH had nearly 3 times the odds of having poor CVH, after adjusting offspring age, sex, and education level (Table 3). A similar strength in the CVH association was found between parents and offspring with ideal CVH (data not shown). The ORs from our sensitivity regression analyses for mother‐offspring and father‐offspring CVH associations were comparable at each paired exam cycle (Table S3). Odds of having poor CVH declined according to increasing age and a lower education level (data not shown). Adjusting for parents’ CVH, offspring age, and education level, offspring men were twice as likely to have poor CVH as women (P<0.001) at every paired exam cycle.
Table 3.
Proportional Odds Regression Model Predicting Offspring CVH From Parents’ CVH at Each Paired Exam Cycle
| Paired Exams | Parent's CVH | OR | 95% CI |
|---|---|---|---|
| Exam 1, n=2429 | Referent: ideal CVH | ||
| Intermediate CVH | 1.67a | 1.18–2.39 | |
| Poor CVH | 2.87a | 1.97–4.18 | |
| Exam 2, n=1876 | Referent: ideal CVH | ||
| Intermediate CVH | 1.50a | 1.05–2.15 | |
| Poor CVH | 2.20a | 1.47–3.30 | |
| Exam 3, n=1996 | Referent: ideal CVH | ||
| Intermediate CVH | 1.05 | 0.68–1.60 | |
| Poor CVH | 1.86a | 1.18–2.93 | |
| Exam 4, n=1922 | Referent: ideal CVH | ||
| Intermediate CVH | 1.63a | 1.02–2.62 | |
| Poor CVH | 2.93a | 1.81–4.73 | |
| Exam 5, n=1726 | Referent: ideal CVH | ||
| Intermediate CVH | 1.87a | 1.25–2.81 | |
| Poor CVH | 3.61a | 2.36–5.52 | |
| Exam 6, n=1460 | Referent: Ideal CVH | ||
| Intermediate CVH | 1.61 | 0.98–2.64 | |
| Poor CVH | 2.73a | 1.61–4.61 | |
| Pooled model (All 6 exams) | Referent: Ideal CVH | ||
| Intermediate CVH | 1.78a | 1.39–2.28 | |
| Poor CVH | 2.59a | 1.98–3.40 | |
This table shows results from a proportional‐odds cumulative logit model, estimating the odds of predicting offspring cardiovascular health status using parents cardiovascular health status. Parents’ cardiovascular health variable was derived from an average of offspring's mother and father's cardiovascular health score. Dependent variable is offspring cardiovascular health (3‐level ordinal variable, coded 0, 1, 2: ideal, intermediate, and poor cardiovascular health, respectively), independent variable is parents’ cardiovascular health (coded 0, 1, 2 as well). All models were adjusted for offspring age, sex, and education. Note in this model, offspring were paired with their biological parents so that it was possible to estimate the cardiovascular health odds of offspring of parents with ideal, intermediate, and poor cardiovascular health. See details of proportional‐odds model's interpretation in the Methods section. CVH indicates cardiovascular health; and OR, odds ratio.
Statistically significant at P<0.05.
Discussion
The main findings from this study are: (1) Over a 53‐year life course, trends of ideal CVH were similar between parents and adult offspring, (2) The prevalence of obesity among the offspring nearly doubled that of the parents at the last paired exam cycle (110% percentage increase), (3) A small proportion of parents and offspring achieved CVH at ideal levels for all 5 CVH metrics, (4) Parents’ CVH score was linearly positively associated with that of their offspring at similar mean ages along their life course, with no difference by parents’ sex, and (5) Despite the increased odds of having poor CVH for offspring of parents with poor CVH, metrics driving declining ideal CVH were related to elevated BMI and glucose levels, rather than smoking, cholesterol, or hypertension. Our results underscore the need to incorporate targeted CVH metrics related to both parents and children in the individual assessment of cardiovascular risk.
To our knowledge, this is the first longitudinal (53‐year) study that examined intergenerational CVH trends of parents and offspring at similar mean age distribution. This study analyzed 5 of the 7 AHA CVH metrics (blood pressure, blood cholesterol, blood glucose, smoking, and BMI). Although FHS original and offspring cohort participants were born several decades apart, our results in terms of ideal CVH are similar to current ideal CVH among the general US population. Of concern, however, is that only a small proportion of parents and children achieved an ideal CVH on all 5 CVH metrics. Proportions of CVH scores for parents and offspring followed similar patterns at each exam period over the life course, indicating evidence of familial clustering and concordance of identical CVH scores between parents and offspring (Figure, Figure S1). The distribution of individual CVH factors and behaviors for parents and offspring, however, were markedly different. The trade‐off between declining smoking, cholesterol levels, and better blood pressure control was buffered by higher BMI and increasing blood glucose in varying magnitudes where offspring obesity and elevated blood glucose doubled that of the parents over the 6 paired exam cycles. Consequently, the rate of PC for offspring intermediate CVH increased gradually from 1979 to 2001, while ideal CVH for both parents and offspring declined for the selected time periods. Our findings related to increased BMI and glucose levels are important and highlight the disturbing and growing trend in these risk factors.
In our study, the highest achieved ideal CVH for parents and offspring was 8% and 22% (1953–1956; 1983–1987), respectively, at any point in the selected exam cycles (Figure). Similarly, a study using the Atherosclerosis Risk in Communities Study cohort (n=12 744), aged 45 to 65 years, reported that only 4% to 18% had 5 ideal CVH metrics and 0.1% had 7.22 In 2013 to 2014, only 13% of the US adults met ideal CVH for 5‐metric criteria, and only <1% had all 7‐metric criteria.8 Similar to our study, findings from other investigators showed that ideal CVH among US adults achieving 5 or 7 metrics is declining.23 However, 1 study enrolling employees of a large healthcare organization in South Florida (n=34 746) between 2011 and 2014 reported a marginal increase in ideal CVH from 0.3% in 2011 to 0.6% in 2014, with an improvement in diet, physical activity, and blood pressure, and a decline in BMI, total cholesterol, and blood glucose in 2014.24 The increase in CVH reported in the South Florida study could partly be attributable to the uniqueness of the sample being employees of a healthcare organization, and therefore not be representative of the general population.
Even though at similar ages we found a similar trend in the distribution of the different CVH categories between parents and offspring, as noted above, there was a marked difference in the presence of certain CVH factors and behaviors. Offspring had significantly higher prevalence of obesity (BMI ≥30 kg/m2) than the parents. Parents had statistically higher prevalence of hypertension (SBP ≥l40 or diastolic blood pressure ≥90 mm Hg) than the offspring at all paired exam cycles with the prevalence increasing from 37.9% to 52.3% (1948–1952; 1969–1971, respectively). Similar trends in high prevalence of total blood cholesterol and smoking among the parents were observed. These results should be interpreted in the context of the historical CVD prevention efforts, especially in terms of cigarette smoking trends. Since the 1960s, BMI has been increasing steadily for all age groups25, 26 with a 26% increase in obesity between 1960 and 2016 among individuals aged >18 years.27 Similarly, the highest prevalence of obesity (30.4%) was recorded in the offspring cohort in 1998 to 2001, which was the last exam cycle for our study, and it is expected that the obesity will continue to rise.28
As highlighted by different reports, the consequences related to increased BMI and obesity in the population has led to a public health crisis. Pathophysiological processes and complications of obesity are most often related to accumulation of lipids and fatty acids in the vascular endothelium, often culminating in the development of vascular inflammation and atheroscerlosis.27, 28 Obesity increases the proclivity for future adverse cardiovascular events, such as myocardial infarction.28, 29 Results of a recent systematic review and meta‐analysis of mendelian randomization studies also underscore the association of obesity with another risk factor: type 2 diabetes mellitus.30 This cumulative evidence led to the development of guidelines that target several CVD risk factors. Consequently, the 2019 American College of Cardiology/AHA and European Society of Cardiology guidelines emphasize screening for type 2 diabetes mellitus, promotion of weight management and weight loss for patients with obesity or overweight.31, 32 Both risk factors are now recognized as the “contemporary” drivers for CVD.
Recent genome‐wide association studies have identified several genetic variants or loci associated with chronic diseases such as CVD, though the heritability because of these genetic loci remains low at 15%.33, 34 This has fueled research on the role of gene‐environment interaction for multifactorial diseases such as CVD. A study examining gene‐environment interaction, the combined effects of environmental/lifestyle factors (hypertension, smoking, drinking, lack of physical activity, and being overweight/obese) and genetic factors on the risk of stroke showed that hypertensive subjects with combined at‐risk alleles (VKORC1 rs2359612C and Chr.9p21.3 rs10757274G) were at higher risk of stroke.35 Our study did not account for gene‐environment interaction, nevertheless, we found major variations in the proportions of SBP/diastolic blood pressure among parents and offspring. Phenotypic differences or shifts between parents and their offspring can occur because of genetic changes over generations (within‐generational phenotypic plasticity)36, 37 or because of environmental signals from the parents without involving the genetic change, a response referred to as transgenerational plasticity.37
Our results showed that parents had significantly higher prevalence of hypertension (SBP ≥140 or diastolic blood pressure ≥90 mm Hg) and SBP mean differences up to 20 mm Hg, a possible case of transgenerational plasticity. These findings are not surprising since early 1950s data from the FHS was an important driver for increased screening, diagnosis, and treatment of hypertension.38 Consequently, we expected that the original cohort (parents) of the FHS would have higher blood pressure than their offspring. Unsurprisingly as well, the proportion of parents who smoked was higher than the offspring in all exam cycles, with the highest prevalence recorded in the 1948 to 1960s for parents (up to 58%). Other studies have reported similar high prevalence of smoking (>50%) among US adults in the 1950s and 1960s.39, 40 Our data indicate that the prevalence of smoking among the parents’ cohort declined gradually from 58.5% to 36.1% (1948–1952; 1969–1971, respectively), which mirrors similar trends in the United States.39 While there is no proven direct causal relationship between smoking and hypertension, evidence suggests that smoking potentiates early vascular dysfunction and atherogenesis.41 More importantly, hypertensive smokers have accelerated atherosclerosis with increased risks for malignant hypertension and myocardial infaction.41, 42 Smoking cessation, therefore, is strongly recommended for all ages to reduce adverse atherosclerotic events and premature mortality.31 In addition, we also found a decline in total cholesterol levels, which may in part be attributable to the seminal mid‐20th century FHS findings revealing the association between dyslipidemia and CVD events.43
Our data indicate a low, but positive correlation (r=0.2–0.4) between parents’ and offspring 5‐metric CVH scores for the paired exam cycles over the life course, with no differences found in CVH correlations between mother‐offspring versus father‐offspring. These observations are consistent with linear correlations observed in other studies which reported parent‐offspring correlations of individual CVH metrics such as, physical activity (r=0.18–0.24)44 and healthy dietary components (including fruit/vegetables, snacks and sweets; r=0.20–0.52).45, 46, 47 Emerging evidence suggests some differences in mother/father and son/daughter CVH correlations. A study to examine parent‐children pedometer‐assessed physical activity reported modest or weak mother‐offspring correlations (r=0.18–0.24) but no significant correlations with father and son/daughter.44 In our study, we did not examine specific mother‐daughter/son versus father‐son/daughter effects on CVH metrics; however, based on the results of Jacobi et al,44 future studies should consider these comparisons.
Limitations
This study examined CVH metrics of parents and their offspring over a defined period of time over the life course. Since this was a comparative design, using different time periods with marked differences in CVD treatment, prevention, and technology, possibly adds to age‐period‐cohort effects that could not be statistically accounted for. Even though our approach was to match parents and offspring at selected exam cycles during which the distribution of age was approximately similar, this study cannot be classified as an age‐matched study since we did not match the parent's age to that of their offspring. By achieving similarities in the grouped distributions in age at each selected exam cycle, the similarities may mask the actual differences in age between parents and offspring in the same family. Data on physical activity and diet were not available for the selected exam cycles, which together with other CVH metrics, are important factors implicated for gene‐environment expressions. Another significant limitation was that FHS is conducted at just a single center with principally middle‐class, white participants; hence, it was impossible to examine racial differences and therefore our findings are not generalizable to the overall US population. However, findings from FHS have been validated and successfully contributed to cutting edge science in cardiovascular disease treatment and risk assessment and applied in several other multiethnic cohorts.14
Conclusions
We found a strong positive association between parental and offspring CVH. However, intergenerational CVH gains from declining smoking rates, cholesterol, and blood pressure were offset by increasing obesity and elevated glucose levels among the offspring. This suggests an intergenerational phenotypic shift, commonly referred to as transgenerational plasticity, of some CVD risk factors along the life course and the need to integrate peculiarity of the shifting familial trends of each CVH metric into an individual's cardiovascular risk assessment. Therefore, when obtaining CVD history, especially earlier in the life course of children, health professionals need to address CVH metrics in both children and parents. This calls for family‐centered approach to care and family‐centered heart‐healthy interventions which are likely to be most effective in achieving intergenerational transfer of cardiovascular health benefits.
Sources of Funding
This study was funded in part by a research grant received from the Sigma Theta Tau International, Theta Alpha Chapter.
Disclosures
None.
Supporting information
Tables S1–S3 Figure S1
Acknowledgments
This article was prepared using FRAMCOHORT, FRAMOFFSPRING Research Materials obtained from the National Heart, Lung, and Blood Institute Biologic Specimen and Data Repository Information Coordinating Center and does not necessarily reflect the opinions or views of the FRAMCOHORT, FRAMOFFSPRING, or the National Heart, Lung, and Blood Institute.
(J Am Heart Assoc. 2020;9:e016292 DOI: 10.1161/JAHA.120.016292.)
Supplementary Materials for this article are available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.016292
For Sources of Funding and Disclosures, see page 9.
References
- 1. Benschop L, Schalekamp‐Timmermans S, Roeters van Lennep J, Jaddoe VWV, Steegers EAP, Ikram MK. Cardiovascular risk factors track from mother to child. J Am Heart Assoc. 2018;7:e009536 DOI: 10.1161/JAHA.118.009536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Daniels SR, Pratt CA, Hollister EB, Labarthe D, Cohen DA, Walker JR, Beech BM, Balagopal PB, Beebe DW, Gillman MW, et al. Promoting cardiovascular health in early childhood and transitions in childhood through adolescence: a workshop report. J Pediatr. 2019;209:240–251. [DOI] [PubMed] [Google Scholar]
- 3. Lloyd‐Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 4. Allen NB, Badon S, Greenlund KJ, Huffman M, Hong Y, Lloyd‐Jones DM. The association between cardiovascular health and health‐related quality of life and health status measures among U.S. adults: a cross‐sectional study of the National Health and Nutrition Examination Surveys, 2001–2010. Health Qual Life Outcomes. 2015;13:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Osondu CU, Aneni EC, Valero‐Elizondo J, Salami JA, Rouseff M, Das S, Guzman H, Younus A, Ogunmoroti O, Feldman T, et al. Favorable cardiovascular health is associated with lower health care expenditures and resource utilization in a large US employee population: the Baptist Health South Florida Employee Study. Mayo Clin Proc. 2017;92:512–524. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 6. Allen NB, Lloyd‐Jones D, Hwang S‐J, Rasmussen‐Torvik L, Fornage M, Morrison AC, Baldridge AS, Boerwinkle E, Levy D, Cupples LA, et al. Genetic loci associated with ideal cardiovascular health: a meta‐analysis of genome‐wide association studies. Am Heart J. 2016;175:112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Muñoz M, Pong‐Wong R, Canela‐Xandri O, Rawlik K, Haley CS, Tenesa A. Evaluating the contribution of genetic and familial shared environment to common disease using the UK Biobank. Nat Genet. 2016;48:980–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. American Heart Association . 2019 heart disease & stroke statistical update fact sheet cardiovascular health. 2019.
- 9. Khera AV, Emdin CA, Drake I, Natarajan P, Bick AG, Cook NR, Chasman DI, Baber U, Mehran R, Rader DJ, et al. Genetic risk, adherence to a healthy lifestyle, and coronary disease. N Engl J Med. 2016;375:2349–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fuemmeler BF, Anderson CB, Mâsse LC. Parent‐child relationship of directly measured physical activity. Int J Behav Nutr Phys Act. 2011;8:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Massarani FA, Cunha DB, Muraro AP, Souza Bda S, Sichieri R, Yokoo EM. Familial aggregation and dietary patterns in the Brazilian population. Cad Saude Publica. 2015;31:2535–2545. [DOI] [PubMed] [Google Scholar]
- 12. Johnson PCD, Logue J, McConnachie A, Abu‐Rmeileh NM, Hart C, Upton MN, Lean M, Sattar N, Watt G. Intergenerational change and familial aggregation of body mass index. Eur J Epidemiol. 2012;27:53–61. [DOI] [PubMed] [Google Scholar]
- 13. Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study. Design and preliminary data. Prev Med. 1975;4:518–525. [DOI] [PubMed] [Google Scholar]
- 14. Tsao CW, Vasan RS. Cohort profile: the Framingham Heart Study (FHS): overview of milestones in cardiovascular epidemiology. Int J Epidemiol. 2015;44:1800–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Effoe VS, Carnethon MR, Echouffo‐Tcheugui JB, Chen H, Joseph JJ, Norwood AF, Bertoni AG. The American Heart Association ideal cardiovascular health and incident type 2 diabetes mellitus among blacks: the Jackson Heart Study. J Am Heart Assoc. 2017;6:e005008 DOI: 10.1161/JAHA.116.005008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dong C, Rundek T, Wright CB, Anwar Z, Elkind MSV, Sacco RL. Ideal cardiovascular health predicts lower risks of myocardial infarction, stroke, and vascular death across whites, blacks, and hispanics: the northern Manhattan study. Circulation. 2012;125:2975–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. [DOI] [PubMed] [Google Scholar]
- 18. National Cancer Institute . Joinpoint trend analysis software. 2020.
- 19. Vik KL, Romundstad P, Carslake D, Smith GD, Nilsen TI. Comparison of father‐offspring and mother‐offspring associations of cardiovascular risk factors: family linkage within the population‐based HUNT Study, Norway. Int J Epidemiol. 2014;43:760–771. [DOI] [PubMed] [Google Scholar]
- 20. Zheng J, Alves‐Wagner AB, Stanford KI, Prince NB, So K, Mul JD, Dirice E, Hirshman MF, Kulkarni RN, Goodyear LJ. Maternal and paternal exercise regulate offspring metabolic health and beta cell phenotype. BMJ Open Diabetes Res Care. 2020;8:e000890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Williams R. Understanding and interpreting generalized ordered logit models. J Math Sociol. 2016;40:7–20. [Google Scholar]
- 22. Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD; ARIC Study Investigators . Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol. 2011;57:1690–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang Q, Cogswell ME, Flanders WD, Hong Y, Zhang Z, Loustalot F, Gillespie C, Merritt R, Hu FB. Trends in cardiovascular health metrics and associations with all‐cause and CVD mortality among US adults. JAMA. 2012;307:1273–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ogunmoroti O, Utuama O, Spatz ES, Rouseff M, Parris D, Das S, Younus A, Guzman H, Tran T, Agatston A, et al. Trends in ideal cardiovascular health metrics among employees of a large healthcare organization (from the Baptist Health South Florida Employee Study). Am J Cardiol. 2016;117:787–793. [DOI] [PubMed] [Google Scholar]
- 25. Centers for Disease Prevention and Control . Prevalence of overweight, obesity, and extreme obesity among adults aged 20 and over: United States, 1960–1962 through 2013–2014. 2016.
- 26. von Hippel PT, Nahhas RW. Extending the history of child obesity in the United States: the Fels Longitudinal Study, birth years 1930–1993. Obesity (Silver Spring). 2013;21:2153–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009;6:399–409. [DOI] [PubMed] [Google Scholar]
- 28. Csige I, Ujvárosy D, Szabó Z, Lőrincz I, Paragh G, Harangi M, Somodi S. The impact of obesity on the cardiovascular system. J Diabetes Res. 2018;2018:3407306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ridker PM. C‐reactive protein and the prediction of cardiovascular events among those at intermediate risk: moving an inflammatory hypothesis toward consensus. J Am Coll Cardiol. 2007;49:2129–2138. [DOI] [PubMed] [Google Scholar]
- 30. Riaz H, Khan MS, Siddiqi TJ, Usman MS, Shah N, Goyal A, Khan SS, Mookadam F, Krasuski RA, Ahmed H. Association between obesity and cardiovascular outcomes: a systematic review and meta‐analysis of Mendelian randomization studies. JAMA Netw Open. 2018;1:e183788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd‐Jones D, McEvoy JW, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596–e646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, Federici M, Filippatos G, Grobbee DE, Hansen TB, et al. 2019 ESC guidelines on diabetes, pre‐diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255–323. [DOI] [PubMed] [Google Scholar]
- 33. CARDIoGRAMplusC4D Consortium , Deloukas P, Kanoni S, Willenborg C, Farrall M, Assimes TL, Thompson JR, Ingelsson E, Saleheen D, Erdmann J, Goldstein BA, et al. Large‐scale association analysis identifies new risk loci for coronary artery disease. Nat Genet. 2013;45:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Elosua R. Road to unravel gene–environment interactions on cardiovascular complex diseases. Circ Genom Precis Med. 2018;11:e002040. [DOI] [PubMed] [Google Scholar]
- 35. Feng C, Yang Y, Yang S, Tu X, Wang Y, Song Y, Hui R, Zhang W. Effect of gene‐gene and gene‐environment interaction on the risk of first‐ever stroke and poststroke death. Mol Genet Genomic Med. 2019;7:e846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bonduriansky R, Crean AJ, Day T. The implications of nongenetic inheritance for evolution in changing environments. Evol Appl. 2012;5:192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Luquet E, Tariel J. Offspring reaction norms shaped by parental environment: interaction between within‐ and trans‐generational plasticity of inducible defenses. BMC Evol Biol. 2016;16:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dawber TR, Meadors GF, Moore FE. Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951;41:279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Centers for Disease Prevention and Control . Achievements in public health, 1900–1999: tobacco use ‐United States, 1900–1999. 1999.
- 40. Escobedo LG, Peddicord JP. Smoking prevalence in US birth cohorts: the influence of gender and education. Am J Public Health. 1996;86:231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Messner B, Bernhard D. Smoking and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2014;34:509–515. [DOI] [PubMed] [Google Scholar]
- 42. Virdis A, Giannarelli C, Neves MF, Taddei S, Ghiadoni L. Cigarette smoking and hypertension. Curr Pharm Des. 2010;16:2518–2525. [DOI] [PubMed] [Google Scholar]
- 43. Mensah GA, Wei GS, Sorlie PD, Fine LJ, Rosenberg Y, Kaufmann PG, Mussolino ME, Hsu LL, Addou E, Engelgau MM, et al. Decline in cardiovascular mortality: possible causes and implications. Circ Res. 2017;120:366–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jacobi D, Caille A, Borys J‐M, Lommez A, Couet C, Charles MA, Oppert JM; FLVS Study Group . Parent‐offspring correlations in pedometer‐assessed physical activity. PLoS One. 2011;6:e29195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McGowan L, Cooke LJ, Gardner B, Beeken RJ, Croker H, Wardle J. Healthy feeding habits: efficacy results from a cluster‐randomized, controlled exploratory trial of a novel, habit‐based intervention with parents. Am J Clin Nutr. 2013;98:769–777. [DOI] [PubMed] [Google Scholar]
- 46. Wang Y, Beydoun MA, Li J, Liu Y, Moreno LA. Do children and their parents eat a similar diet? Resemblance in child and parental dietary intake: systematic review and meta‐analysis. J Epidemiol Community Health. 2011;65:177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yee AZH, Lwin MO, Ho SS. The influence of parental practices on child promotive and preventive food consumption behaviors: a systematic review and meta‐analysis. Int J Behav Nutr Phys Act. 2017;14:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3 Figure S1


