Abstract
Background
Approximately 60% of women have Stage B heart failure 1 year after a preeclamptic delivery. Emerging evidence suggests that the profibrotic growth factor activin A, which has been shown to induce cardiac fibrosis and hypertrophy, is elevated in preeclampsia and may be inhibited by aspirin therapy. We hypothesized that preeclamptic women receiving aspirin would have lower activin A levels and reduced global longitudinal strain (GLS), a sensitive measure of cardiac dysfunction, than women who do not receive aspirin. To test our hypothesis, we performed a cohort study of women with preeclampsia or superimposed preeclampsia and compared activin A levels and GLS in parturients who did or did not receive aspirin.
Methods and Results
Ninety‐two parturients were enrolled, of whom 25 (27%) received aspirin (81 mg/day) therapy. GLS, plasma activin A, and follistatin, which inactivates activin A, were measured. Women receiving aspirin therapy had lower median (interquartile range) levels of activin A (8.17 [3.70, 10.36] versus 12.77 [8.37, 31.25] ng/mL; P=0.001) and lower activin/follistatin ratio (0.59 [0.31, 0.93] versus 1.01 [0.64, 2.60] P=0.002) than women who did not receive aspirin, which also remained significant after multivariable analysis. Furthermore, GLS was worse in patients who did not receive aspirin (−19.84±2.50 versus −17.77±2.60%; P=0.03) despite no differences in blood pressure between groups.
Conclusions
Our study suggests that antepartum aspirin therapy reduced serum activin A levels and improved GLS in preeclamptic patients, suggesting that aspirin may mitigate the postpartum cardiac dysfunction seen in women with preeclampsia.
Keywords: activin A, aspirin, cardiac dysfunction, global longitudinal strain, pregnancy
Subject Categories: Biomarkers, Preeclampsia, Pregnancy, Women
Nonstandard Abbreviations and Acronyms
- ACOG
American College of Obstetricians and Gynecologists
- ADOPTe
Angiogenic Dysfunction Of Pregnancy and Transthoracic echocardiogram
- FSTL3
follistatin‐like 3
- GLS
global longitudinal strain
Clinical Perspective
What Is New?
This is the first study that demonstrates that aspirin therapy is associated with reduced activin A levels and improved global longitudinal strain in pregnant patients with preeclampsia.
In our exploratory observational analysis, receiving aspirin was associated with a decreased global longitudinal strain when compared with women who had not received aspirin therapy.
The biomarker activin A, which is elevated and pathogenic in preeclampsia, also results in cardiac dysfunction both predelivery and at 1 year postpartum in women with hypertensive disorders of pregnancy.
What Are the Clinical Implications?
Our study suggests that antepartum aspirin therapy reduces plasma activin A levels and improves global longitudinal strain in pregnant patients with preeclampsia.
Further studies need to be done to confirm these findings and to evaluate the possibility that antepartum aspirin may mitigate postpartum cardiac dysfunction seen in women with preeclampsia.
Preeclampsia is a hypertensive disorder that affects up to 5% of pregnancies and is the most common medical complication of gestation. Although preeclampsia was previously considered a self‐limited peripartum disease,1 women with preeclampsia are now recognized to be at increased risk for the subsequent development of cardiovascular disease.2 In a large epidemiological study using the National Readmission Database, women diagnosed with hypertensive disorders of pregnancy, such as preeclampsia and superimposed preeclampsia, were twice as likely to be admitted with new‐onset heart failure within 90 days of delivery than women with normotensive pregnancies.3 In addition, 60% of women with preeclampsia exhibit Stage B (subclinical) heart failure at 1 year postpartum. 12 , 2, 4 Stage B cardiac failure, which typically progresses to symptomatic heart failure, can be diagnosed by the measurement of global longitudinal strain (GLS).5 In fact, abnormal GLS in the second trimester of pregnancy predicts preeclampsia later in gestation.6
Despite the high incidence of serious morbidity and mortality due to postpartum cardiac dysfunction following preeclamptic pregnancy, there is a substantial gap in our knowledge about the mechanisms of sustained cardiac dysfunction in this disease or how to prevent it. A growing body of evidence suggests that activin A, a member of the transforming growth factor‐beta superfamily, may be pathogenic in the development of both preeclampsia and subsequent cardiovascular dysfunction.7, 8 Activin A is a profibrotic polypeptide produced by the placenta, macrophages, and other cell types and is bioneutralized by follistatin, which during healthy pregnancy circulates in excess of activin A. In women with preeclampsia, elevated levels of angiotensin II type 1 receptor antibodies are thought to stimulate the release of nicotinamide adenine dinucleotide phosphate oxidase, which leads to oxidative stress that increases placental and endothelial secretion of activin A.9 Clinically, activin A levels in preeclamptic women are 10‐fold higher than in women with a healthy pregnancy.10 In addition to a pathogenic role in preeclampsia, activin A signaling also causes cardiac fibrosis and hypertrophy via increased activin receptor‐like kinase 4 activity and p38 mitogen‐activated protein kinase and extracellular regulated kinase pathways.11 Indeed, women with hypertensive disorders of pregnancy have elevated levels of antepartum activin A that correlates with contemporaneously measured GLS and also with reduced GLS at 1‐year postpartum;12 however, the underlying mechanisms remain unknown. Furthermore, in vivo and in vitro data suggest that aspirin inhibits the expression of extracellular regulated kinase and also blocks the mitogen‐activated protein kinase pathway,13 2 potential mechanisms linking activin A and cardiac fibrosis and hypertrophy. However, the impact of aspirin therapy on activin A levels and cardiac dysfunction in preeclamptic women is unknown.
We hypothesized that preeclamptic women receiving aspirin therapy would have lower plasma activin A levels and improved GLS compared with women who are not receiving aspirin. To test our hypothesis, we performed a cohort study of women with preeclampsia or superimposed preeclampsia and compared plasma activin A and GLS in parturients who did and did not receive aspirin. We also measured activin A/follistatin and activin A/follistatin‐like 3 (FSTL3) ratios, as follistatin is a coregulatory factor of activin A and follistatin/activin A binding may affect activin A levels.
Methods
Study Design and Oversight
We conducted a cohort study of 92 women with preeclampsia or superimposed preeclampsia who had enrolled in a large observational cohort study (ADOPTe [Angiogenic Dysfunction Of Pregnancy and Transthoracic echocardiogram]). ADOPTe is an institutional review board approved ongoing observational study on the evaluation of biomarkers among women with hypertensive disorders of pregnancy (Institutional Review Board No. 14–0977).14 All participants gave written informed consent before initiation of study procedures. All procedures followed were in accordance with institutional guidelines. Complete information about this prospective observational study has been previously described.14 Pregnant women with preeclampsia and superimposed preeclampsia who received prenatal care and delivered at the University of Chicago between May 2017 and November 2019 were enrolled for this study. Patients in the ADOPTe study who had a blood sample drawn and a final diagnosis of preeclampsia/superimposed preeclampsia were included in this analysis. All data and supporting materials have been provided with the published article.
Study Cohort
Women were at least 18 years of age with a singleton gestation of <41 weeks. Patients were excluded if they had a preexisting diagnosis of cardiomyopathy, ischemic or valvular heart disease, pulmonary disease, or diabetes mellitus or were in labor. Enrollment in the ADOPTe study occurred when patients were admitted to labor and delivery or the antepartum floor. All clinical data were abstracted from medical records by technicians unaware of the results of assays or echocardiograms.
The diagnoses of preeclampsia and superimposed preeclampsia were based on modified American College of Obstetricians and Gynecologists (ACOG) criteria.15 An obstetrician (S.R.) unaware of study results confirmed the clinical diagnosis for all patients. Preeclampsia was defined as systolic blood pressure ≥140 mm Hg and/or diastolic blood pressure ≥90 mm Hg occurring after 20 weeks of gestation, combined with proteinuria or, in the absence of proteinuria, any other new‐onset sign of preeclampsia‐associated organ dysfunction (as defined by ACOG). Blood pressure readings were documented on at least 2 occasions 4 hours to 2 weeks apart. Proteinuria was defined as urinary excretion of ≥0.3 grams of protein in a 24‐hour urine specimen or urine protein (mg/dL)/creatinine (mg/dL) ratio of ≥0.3. In the absence of proteinuria, preeclampsia with severe features was defined per ACOG.15 Superimposed preeclampsia was defined as a patient with chronic hypertension who developed one of the following features of new‐onset proteinuria (as described previously), sudden increase in proteinuria if already present in early gestation, sudden increase in hypertension, or development of HELLP (hemolysis, elevated liver enzymes, low platelet count) syndrome.
Measurement of Circulating Activin A, Follistatin, and FSTL3 Levels
Venous blood samples were collected before delivery during the delivery admission. Blood samples were centrifuged for 8 minutes at −4°C and the plasma was then aliquoted, labeled with a study ID, and stored at −80°C. The regulatory hormone follistatin binds to and inactivates activin A; thus we measured follistatin levels in all patients. Additionally, because the biologically active form of activin A is unbound, activin A/follistatin ratios weree used as an indirect measure of free activin A levels.16 A single operator (J.D.) blinded to clinical information performed activin A, follistatin and FSTL3 ELISA assays on each plasma sample in duplicate using commercially available kits (Ansh Labs, Webster, TX). The coefficient of variation for intraassay and interassay variability was 4.23±0.06 and 2.29±0.02 ng/mL, respectively.
Echocardiography
Echocardiograms were performed as part of routine prenatal care on the basis of ACOG recommendations17 in the second trimester for pregnant patients with chronic hypertension or another indication. All echoes were performed by a certified sonographer in an Intersocietal Commission for the Accreditation of Echocardiography Laboratories Certified Cardiology Echocardiography Laboratory at the University of Chicago. Images were reported according to American Society of Echocardiography guidelines. Strain analysis was performed by the senior author, who is a certified echocardiographer. Importantly, all echocardiographic indices reported in the study were assessed using standardized software that uses a computer learning algorithm to facilitate endocardial border detection. This process thus reduces potential variability and results in reliable methods that can be replicated regardless of the person measuring the indices.6, 12, 18 Data from echocardiograms with complete two dimensional and color flow Doppler were abstracted from the medical record. Ejection fraction was calculated using the Simpson's biplane disc method. GLS measurement was performed using fully automated Tomtec software (AutoStrain, Tomtec Image Arena 1.2, Unterschleissheim, Germany), a vendor independent software that uses a computer learning algorithm to facilitate endocardial border detection. This software has previously been validated with good correlation and agreement between manual and automated strain measurements.19 All echocardiograms were analyzed in a blinded fashion.
Statistical Analysis
Our primary analysis evaluated the relationship between aspirin therapy and activin A levels. Secondary analyses tested the relationship between aspirin therapy and activin/follistatin and activin/FSTL3 ratios. In an exploratory analysis we also evaluated the relationship between aspirin therapy and GLS.
Data were assessed with the use of a t test or Wilcoxon Rank Sum test as appropriate and are presented as mean (±SD) or median (interquartile range) depending on the data distribution. Normality was assessed with the Shapiro‐Wilk test. Categorical variables are reported as frequencies and proportions and assessed with a chi‐square or Fisher's exact test. To evaluate whether the observed differences persisted, we performed multivariable linear regression. To meet model assumptions logarithmic transformations were performed, as necessary. Variables included in the adjusted model were selected on the basis of their biological plausibility. Statistical analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC). Two‐tailed P values of <0.05 were considered statistically significant for all analyses.
Results
Demographics
A total of 92 parturients with preeclampsia or superimposed preeclampsia were included in the analysis. Baseline characteristics are shown in Table 1. Of the 92 analyzed patients, 25 (27%) patients received aspirin therapy. No statistically significant difference was observed in ethnicity, body mass index, race, or antihypertensive medication use between patients who did and did not receive aspirin antepartum (all P>0.09). Although a higher proportion of patients who received aspirin had superimposed preeclampsia as compared with patients who did not receive aspirin (56% versus 42%), this difference was not significant (P=0.22).
Table 1.
Clinical Characteristics of Patients
| Asprin (N=25) | No Aspirin (N=67) | P Value | |
|---|---|---|---|
| Demographics | |||
| Race (%) | 0.09 | ||
| White | 0 (0) | 12 (17.91) | |
| Black | 22 (88.00) | 48 (71.64) | |
| Asian | 1 (4.00) | 1 (1.49) | |
| Multiracial | 1 (4.00) | 3 (4.48) | |
| Unknown | 1 (4.00) | 3 (4.48) | |
| Ethnicity (%) | 0.87 | ||
| Hispanic | 1 (4.00) | 4 (5.97) | |
| Non‐Hispanic | 22 (88.00) | 59 (88.06) | |
| Unknown | 2 (8.00) | 4 (5.97) | |
| Age, y | 34.29±5.44 | 28.63±7.26 | 0.001 |
| Prepregnancy body mass index (kg/m2) | 30.86±9.22 | 33.91±8.77 | 0.15 |
| Number of prenatal visits | 10 (8, 14) | 4 (1, 10) | 0.001 |
| Risk factors and comorbidities | |||
| Smoking status (%) | 0.71 | ||
| Never | 14 (56.00) | 45 (67.16) | |
| Past/quit before pregnancy | 7 (28.00) | 14 (20.90) | |
| Past/quit early in pregnancy | 1 (4.00) | 2 (2.99) | |
| Currently smokes | 2 (8.00) | 5 (7.46) | |
| Unknown | 1 (4.00) | 1 (1.49) | |
| Substance abuse (%) | 0 (0) | 9 (13.43) | 0.10 |
| Diabetes mellitus (%) | 8 (32.00) | 8 (11.94) | 0.02 |
| Previous history of preeclampsia (%) | 10 (40.00) | 9 (13.43) | 0.01 |
| Chronic hypertension (%) | 16 (64.00) | 26 (38.81) | 0.03 |
| History of cardiac disease (%) | 1 (4.00) | 2 (2.99) | 0.81 |
| Multiparity (%) | 24 (96.00) | 37 (55.22) | 0.0002 |
| Renal disease (%) | 2 (8.00) | 3 (4.48) | 0.51 |
| Autoimmune disease (%) | 1 (4.00) | 2 (2.99) | 0.81 |
| Diagnosis | |||
| Preeclampsia (%) | 11 (44.00) | 39 (58.21) | 0.22 |
| Superimposed preeclampsia (%) | 14 (56.00) | 28 (41.79) | |
| Hypertension | |||
| Hypertensive agents (%) | |||
| No agent | 4 (16.67) | 11 (16.42) | 0.95 |
| Single agent | 5 (20.83) | 16 (23.88) | |
| Multiple agent | 15 (62.50) | 40 (59.70) | |
| Delivery characteristics | |||
| Highest systolic blood pressure, mm Hg | 156 (137, 182) | 164 (153, 175) | 0.20 |
| Highest diastolic blood pressure, mm Hg | 87 (74, 96) | 94 (86, 107) | 0.02 |
| Mean arterial blood pressure, mm Hg | 110 (94, 123) | 118 (108, 127) | 0.04 |
| Gestational age, wk | 35.00 (33.50, 36.86) | 37.00 (34.86, 38.57) | 0.01 |
| Quantitative blood loss, mL | 427.5 (242.5, 1145.0) | 564.0 (269.0, 925.0) | 0.81 |
| Small for gestational age (%) | 6 (25.00) | 10 (15.63) | 0.31 |
Data are presented as mean±SD, median (quartile 1, quartile 3), or n (%) depending on variable type. These results are assessed between groups with a t test, Wilcoxon Rank Sum, or chi‐square test, respectively.
Patients receiving aspirin were more likely to be older (34.29±5.44 versus 28.63±7.26 years; P=0.001) and have a higher number of prenatal visits (10 [8, 14] versus 4 [1, 10]; P=0.001) as compared with women who did not receive aspirin. In patients who received aspirin, the mean gestational age at which aspirin was started was 18.96±7.47 weeks.
Activin A, Follistatin, and FSTL3 Measurements
Women receiving aspirin therapy had lower median (interquartile range) levels of activin A when compared with women who did not receive aspirin (Figure—Panel A; 8.17 [3.70, 10.36] versus 12.77 [8.37, 31.25] ng/mL; P=0.001). Similarly, activin A/follistatin and activin A/FSTL3 ratios were lower in patients receiving aspirin compared with no aspirin therapy (Figure—Panels D and E; 0.59 [0.31, 0.93] versus 1.01 [0.64, 2.60], P=0.002 and 0.22 [0.14, 0.28] versus 0.30 [0.20, 0.51], P=0.002, respectively). Follistatin and FSTL3 levels did not differ significantly between the 2 groups, respectively (Figure—Panels B and C; P values 0.80 and 0.18, respectively; Table 2). After adjusting for potential differences in baseline characteristics including chronic hypertension, diabetes mellitus, age, previous history of preeclampsia, and parity, the association between aspirin and activin persisted (P=0.005).
Figure 1. Biomarker levels stratified by antepartum aspirin use.
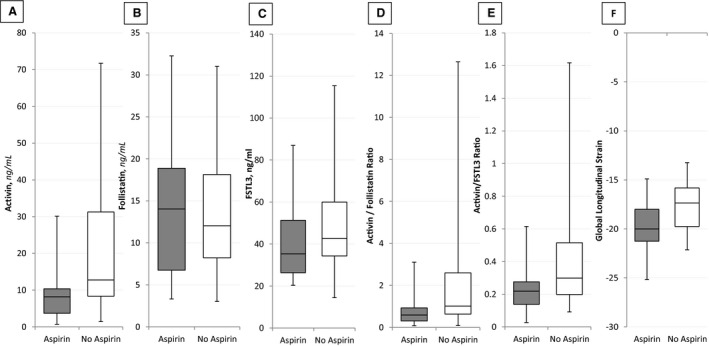
Biomarker levels for (A) activin, (B) follistatin, (C) FSTL3, (D) activin/follistatin ratio, (E) activin/FSTL3 ratio, and (F) global longitudinal strain of patients stratified by aspirin use during the antepartum period. Boxplots in grey indicate patients who received aspirin whereas white indicates patients who did not receive aspirin. The boxplot displays the minimum value (whisker), lower quartile (bottom of the box), median (middle line in box), upper quartile (top of box), and maximum value (whisker) within each group.
Table 2.
Angiogenic Biomarkers
| Asprin (N=25) | No Aspirin (N=67) | P Value | |
|---|---|---|---|
| Angiogenic factors | |||
| Activin, ng/mL | 8.17 (3.70, 10.36) | 12.77 (8.37, 31.25) | 0.001 |
| Follistatin, ng/mL | 14.04 (6.75, 18.88) | 12.02 (8.21, 18.15) | 0.80 |
| Follistatin‐like 3, ng/mL | 35.31 (26.34, 51.31) | 42.72 (34.34, 60.04) | 0.18 |
| Activin/follistatin | 0.59 (0.31, 0.93) | 1.01 (0.64, 2.60) | 0.002 |
| Activin/Follistatin‐like 3 | 0.22 (0.14, 0.28) | 0.30 (0.20, 0.51) | 0.002 |
Data is presented as median (quartile 1, quartile 3) or n (%) depending on variable type and assessed using a Wilcoxon Rank Sum or chi‐square test, respectively.
When classified by type of hypertensive disorder (preeclampsia or superimposed preeclampsia), women with preeclampsia alone who received aspirin therapy had significantly lower activin A levels, activin/follistatin ratios, and activin/FSTL3 (all P≤0.01) when compared with women not receiving aspirin (Table S1).
Echocardiography
When compared with patients not on aspirin therapy, a higher proportion of patients who received aspirin also underwent an echocardiogram as part of their routine medical care (27% versus 68%, P=0.0003). The mean gestational age at the time of the echocardiogram did not differ between groups (gestational weeks, 23.43±7.43 aspirin versus 27.6±9.66 no aspirin; P=0.15). The clinical indications for echo among patients who received them were chronic hypertension (23/35, 66%), diabetes mellitus (6/35, 17%), to rule out cardiac disease (3/35, 9%), obesity (1/35, 3%) and other unspecified reasons (2/35, 6%). No statistically significant difference was observed in the proportion of patients with chronic hypertension between those who did and did not receive aspirin (65% versus 72%, respectively; P=0.63).
In our exploratory analysis patients on aspirin had an increased GLS (Triplane, 3P) when compared with women without aspirin therapy (Figure—Panel F; −19.84±2.50 versus −17.77±2.60%, P=0.03). No differences were detected in other echocardiographic indices including ejection fraction, stroke volume, and end diastolic or systolic volume (Table 3). Similarly, no differences were observed in mean arterial blood pressure between those who did and did not receive aspirin therapy (95.56±13.86 versus 98.24±14.23 mm Hg, P=0.58). After adjusting for chronic hypertension, the association between aspirin and GLS persisted (P=0.03). Given the small sample size of this analysis, these results should be interpreted with caution.
Table 3.
Echocardiographic Indices and Blood Pressure
| Asprin (N=25) | No Aspirin (N=67) | P Value | |
|---|---|---|---|
| ECHO performed (%) | 17 (68.00) | 18 (26.87) | 0.0003 |
| Echocardiographic indices | |||
| End‐diastolic volume (ml) | 155.93±33.19 | 156.03±39.97 | 0.99 |
| Ejection fraction (%) | 52.32±5.91 | 48.97±7.63 | 0.19 |
| End‐systolic volume (ml) | 72.40 (55.78, 84.84) | 75.44 (62.96, 94.11) | 0.70 |
| Stroke volume (ml) | 80.68±13.38 | 75.70±19.93 | 0.42 |
| Global longitudinal strain (3P) (%) | −19.84±2.50 | −17.77±2.60 | 0.03 |
| Global longitudinal strain (4CH) (%) | −20.91±3.92 | −19.06±2.58 | 0.12 |
| Global longitudinal strain (2CH) (%) | −20.70±2.82 | −19.60±2.48 | 0.24 |
| Global longitudinal strain (3CH) (%) | −18.96±2.75 | −16.48±3.02 | 0.02 |
| Gestational age | |||
| At ECHO (weeks) | 23.43±7.43 | 27.6. ±9.66 | 0.15 |
| Aspirin characteristics | |||
| Total weeks administered | 16 (5.5, 22.5) | ··· | ··· |
| Total weeks administered at ECHO | 6.5 (2.3, 17.5) | ··· | ··· |
| Blood pressure | |||
| Systolic blood pressure at ECHO (mm Hg) | 133.94±16.62 | 132.89±20.72 | 0.87 |
| Diastolic blood pressure at ECHO (mm Hg) | 76.44±14.50 | 80.94±12.20 | 0.33 |
| Mean arterial blood pressure at ECHO (mm Hg) | 95.56±13.86 | 98.24±14.23 | 0.58 |
Data are presented as mean±SD, median (quartile 1, quartile 3), or n (%) depending on variable type. These results are assessed between groups with a t test, Wilcoxon Rank Sum, or chi‐square test, respectively. Results for echocardiographic indices and measurements at the time of echo are only reported for patients who ever underwent an echo during pregnancy. ECHO indicates echocardiogram. 3P(Triplane, Average GLS), 4CH(4 chamber), 2CH(2 chamber), 3CH(3 chamber).
Aspirin Compliance, Complications, and Fetal Outcomes
Compliance was defined as clinician documentation of aspirin use at every prenatal visit in the medical record. For analysis purposes noncompliant patients were instead considered nonaspirin users. We found that among all the patients who were prescribed aspirin (n=30), 5 patients (17%) were noncompliant. The remaining 25 patients (83%) were compliant on the aspirin. Thirteen percent of the patients included in our data were on aspirin at delivery. The median time from the cessation of aspirin to delivery was 1 week in our cohort. No statistically significant increase was observed in the risk of postpartum hemorrhage, as the median quantitative blood loss in women who received aspirin was 427.5 mL (interquartile range 242.5, 1145.0) as compared with 564.0 mL (269.0, 925.0) in women who did not receive aspirin (P=0.81). Similarly, no difference in bleeding was observed among women using aspirin within the last week of pregnancy compared with those who stopped aspirin before (median [interquartile range] 585.5 [234.0, 1080.0] versus 299.5 [242.5, 920.0]; P=0.67). Further, no statistically significant difference was observed in the proportion of births that were small for gestational age between the aspirin and no aspirin groups (25% versus 16%, respectively; P=0.32). We found no differences in proportion of chronic hypertension, diabetes mellitus, history of cardiac disease, history of preeclampsia, multiparity, autoimmune disease, among patients who were compliant versus noncompliant on aspirin (P values 0.47, 0.73, 0.54, 0.41, 0.25, and 0.45, respectively).
Discussion
In this cohort study of women with preeclampsia and superimposed preeclampsia, we found that women receiving aspirin therapy during pregnancy had lower activin A levels, activin A/follistatin ratios, and activin A/FSTL3 ratios at the time of delivery when compared with women not receiving aspirin. In addition, in our exploratory analysis patients receiving aspirin had a decreased (better) GLS when compared with women who had not received aspirin therapy. No difference in follistatin or FSTL3 levels was identified between the 2 groups.
Our data are consistent with prior levels (median 22.03 ng/mL [16.69, 25.98]) of activin A that are observed in women with preeclampsia.10 Existing data suggest that elevated activin A levels may be pathogenic in the development of preeclampsia and cardiac dysfunction and that activin A blockade in a murine model alleviates preeclampsia and improves cardiac function.8, 20 We now present evidence that aspirin therapy correlates with lower activin A levels and activin/follistatin ratios and that GLS is improved in preeclamptic women on aspirin when compared with women not on aspirin. Given that the mean gestational age of aspirin start in our cohort was >16 weeks it is possible that we are describing the effect of aspirin on activin levels and cardiac function rather than the effect of aspirin on placentation to reduce preeclampsia.
Early onset of subclinical heart failure in preeclampsia can be measured using GLS,18, 21, 22 which detects early heart failure through differentiating between active from passive contraction. In a prospective study of 207 women with hypertensive disorders of pregnancy and nonhypertensive controls, preeclamptic women had impaired GLS without a corresponding decrease in ejection fraction.18 Furthermore, worsened GLS correlated with increased circulating activin A in women with preeclampsia in the third trimester of pregnancy.12 GLS is also significantly worse in women with preeclampsia than in women with chronic hypertension in pregnancy, suggesting that hypertension alone does not cause cardiac dysfunction in preeclampsia.23 The current study not only corroborates previous findings but also suggests that aspirin therapy may decrease GLS in this population.
Our data have several implications for clinical practice. Thromboxane A2 contributes to the onset of preeclampsia; thus low‐dose aspirin (81 mg/day) is currently recommended in the 2018 ACOG guidelines to mitigate the risk of preeclampsia in women at high risk during pregnancy starting at 12 to 16 weeks of gestation.24 The guidelines recommend stopping aspirin at 36 weeks or at the time of delivery. Here we postulate that perhaps aspirin may also decrease the development of peripartum cardiac dysfunction. If so, postpartum aspirin may be a new therapeutic agent to reduce postpartum cardiac dysfunction and improve cardiovascular health among these high‐risk women.
Currently, aspirin use in patients with preeclampsia may be limited by concerns over an increased risk of peripartum bleeding.25 However, a 2018 ACOG risk analysis found no increased risk of placental abruption, postpartum hemorrhage, or mean blood loss.24 In a 2015 multicenter, randomized trial of 1776 pregnant women receiving aspirin at 150 mg/day, the incidence of preterm preeclampsia, pregnancy complications, and fetal or neonatal outcomes did not differ between those who did and did not receive aspirin.26 Although fetal effects of aspirin are understudied, an 81 mg/day dose acetylsalicylic acid (aspirin) transfer into milk is virtually undetectable by liquid chromatography.27 In our cohort we saw no difference in bleeding or fetal outcomes between patients receiving and not receiving aspirin therapy, although the sample size is severely limited to draw these conclusions.
Mechanistically, aspirin inhibits activin A induced expression of extracellular regulated kinase and also blocks the mitogen‐activated protein kinase pathway. In preeclampsia, inhibition of these pathways decreases both inflammation and oxidative stress.28, 29 Further in cell culture models, inhibition of these pathways suppresses inflammation, inhibits apoptosis, and decreases the secretion of soluble FMS‐like tyrosine kinase‐1 and soluble endoglin considered pathogenic in preeclampsia.30 Taken together these data suggest that aspirin use could prevent both preterm preeclampsia and postpartum cardiac dysfunction after a preeclamptic delivery.
Compliance with aspirin therapy plays a major role to prevent preterm preeclampsia. Previous work by Rolnik et al demonstrated that the group who was screened positive for high‐risk preeclampsia who were randomized to low‐dose aspirin had a significant lower risk of early‐onset preeclampsia if they were compliant, as compared with noncompliant pregnant women. Interestingly, the women who were screened as high risk, but were randomized to placebo, also had a significant lower risk of early‐onset preeclampsia in this study if they were compliant (with placebo tablets), as compared with noncompliant women. This indicates that “compliant” women are different (ie, healthier) from those not following the prescribed drugs. We found no differences among any comorbidities among compliant versus noncompliant women in our cohort, thus limiting the possibility of selection bias in our cohort.
Although we present promising new data here, our study has some limitations. First, because of the limited sample size, it is possible that differences in patient characteristics have affected our results. Additionally, the observational nature of this study does not allow us to prove that aspirin is responsible for the differences we observed but rather serves as a means for generating new hypotheses. Second, because our study was observational and the decision to prescribe aspirin was made according to the treating providers’ discretion, it is not possible to ascertain whether specific patient factors necessitated aspirin therapy and thus potentially affected GLS or angiogenic levels. Similarly, because this was a retrospective review of the chart, we did not ascertain aspirin compliance by pill counts; however, each encounter for each patient was carefully reviewed and the documented medication compliance and duration of aspirin carefully noted. Other studies have shown that aspirin compliance may play an important role; however, given the small sample size we are unable to ascertain how this may have affected our results. It is possible that differences in activin A levels we observed may be because of factors other than aspirin. Similarly, differences in GLS we observed between groups may have also been because of factors besides aspirin use. In our study echocardiograms were performed as part of routine clinical care and thus not all performed by the same person. Although statistically not significant timing between echocardiograms may have contributed to the observed differences in GLS. We are also unable to comment on the time of the preeclampsia diagnosis and whether this differed between the aspirin groups. Lastly, given that our data comes from a single institution, it is possible that differences in aspirin therapy administration practices may vary between institutions, thereby making our results less generalizable. Nevertheless, our data suggest that aspirin may reduce activin A levels when given during the antepartum period and lend support to clinical trials of aspirin for prevention of heart failure in postpartum women with preeclampsia.
Perspectives
In summary, antepartum aspirin therapy was associated with a reduction in activin A levels and improves GLS in pregnant patients with preeclampsia. Further work is needed to clarify the potential effect of aspirin on the postpartum development of heart failure in women with preeclampsia. Moreover, preclinical studies in animal models of preeclampsia are urgently needed to determine the importance of activin A in the development of subclinical cardiac dysfunction in preeclamptic women, as well as understand the mechanisms whereby aspirin therapy mitigates its onset and potentially the progression of postpartum cardiac dysfunction.
Sources of Funding
This project was funded through the Department of Obstetrics and Gynecology at the University of Chicago and NIH/NHLBI ‐1R21HL148488‐01.
Disclosures
None.
Supporting information
Table S1
(J Am Heart Assoc. 2020;9:e015997 DOI: 10.1161/JAHA.119.015997.)
For Sources of Funding and Disclosures, see page 8.
References
- 1. Rana S, Powe CE, Salahuddin S, Verlohren S, Perschel FH, Levine RJ, Lim KH, Wenger JB, Thadhani R, Karumanchi SA. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation. 2012;125:911–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Murphy MS, Smith GN. Pre‐eclampsia and cardiovascular disease risk assessment in women. Am J Perinatol. 2016;33:723–731. [DOI] [PubMed] [Google Scholar]
- 3. Nizamuddin J, Gupta A, Patel V, Minhaj M, Nizamuddin SL, Mueller AL, Naseem H, Tung A, Rana S, Shahul S. Hypertensive diseases of pregnancy increase risk of readmission with heart failure: a National Readmissions Database Study. Mayo Clin Proc. 2019;94:811–819. [DOI] [PubMed] [Google Scholar]
- 4. Breetveld NM, Ghossein‐Doha C, van Kuijk SM, van Dijk AP, van der Vlugt MJ, Heidema WM, van Neer J, van Empel V, Brunner‐La Rocca HP, Scholten RR, et al. Prevalence of asymptomatic heart failure in formerly pre‐eclamptic women: a cohort study. Ultrasound Obstet Gynecol. 2017;49:134–142. [DOI] [PubMed] [Google Scholar]
- 5. Yang H, Negishi K, Wang Y, Nolan M, Saito M, Marwick TH. Echocardiographic screening for non‐ischaemic stage B heart failure in the community. Eur J Heart Fail. 2016;18:1331–1339. [DOI] [PubMed] [Google Scholar]
- 6. Shahul S, Ramadan H, Mueller A, Nizamuddin J, Nasim R, Lopes Perdigao J, Chinthala S, Tung A, Rana S. Abnormal mid‐trimester cardiac strain in women with chronic hypertension predates superimposed preeclampsia. Pregnancy Hypertens. 2017;10:251–255. [DOI] [PubMed] [Google Scholar]
- 7. Hobson SR, Acharya R, Lim R, Chan ST, Mockler J, Wallace EM. Role of activin A in the pathogenesis of endothelial cell dysfunction in preeclampsia. Pregnancy Hypertens. 2016;6:130–133. [DOI] [PubMed] [Google Scholar]
- 8. Roh JD, Hobson R, Chaudhari V, Quintero P, Yeri A, Benson M, Xiao C, Zlotoff D, Bezzerides V, Houstis N, et al. Activin type II receptor signaling in cardiac aging and heart failure. Sci Transl Med. 2019;11:482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vaka VR, Cunningham MW Jr, Deer E, Franks M, Ibrahim T, Amaral LM, Usry N, Cornelius DC, Dechend R, Wallukat G, et al. Blockade of endogenous angiotensin II type I receptor agonistic autoantibody activity improves mitochondria reactive oxygen species and hypertension in a rat model of preeclampsia. Am J Physiol Regul Integr Comp Physiol. 2019;318:R256–R262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reddy A, Suri S, Sargent IL, Redman CW, Muttukrishna S. Maternal circulating levels of activin A, inhibin A, sFlt‐1 and endoglin at parturition in normal pregnancy and pre‐eclampsia. PLoS One. 2009;4:e4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hu J, Wang X, Wei SM, Tang YH, Zhou Q, Huang CX. Activin A stimulates the proliferation and differentiation of cardiac fibroblasts via the ERK1/2 and p38‐MAPK pathways. Eur J Pharmacol. 2016;789:319–327. [DOI] [PubMed] [Google Scholar]
- 12. Shahul S, Ramadan H, Nizamuddin J, Mueller A, Patel V, Dreixler J, Tung A, Lang RM, Weinert L, Nasim R, et al. Activin A and late postpartum cardiac dysfunction among women with hypertensive disorders of pregnancy. Hypertension. 2018;72:188–193. [DOI] [PubMed] [Google Scholar]
- 13. Li X, Wang G, QiLi M, Liang H, Li T, E X, Feng Y, Zhang Y, Liu X, Qian M, et al. Aspirin reduces cardiac interstitial fibrosis by inhibiting Erk1/2‐Serpine2 and P‐Akt signalling pathways. Cell Physiol Biochem. 2018;45:1955–1965. [DOI] [PubMed] [Google Scholar]
- 14. Lopes Perdigao J, Chinthala S, Mueller A, Minhas R, Ramadan H, Nasim R, Naseem H, Young D, Shahul S, Chan SL, et al. Angiogenic factor estimation as a warning sign of preeclampsia‐related peripartum morbidity among hospitalized patients. Hypertension. 2019;73:868–877. [DOI] [PubMed] [Google Scholar]
- 15. ACOG Practice Bulletin No. 202: gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133:e1–e25. [DOI] [PubMed] [Google Scholar]
- 16. Hedger MP, de Kretser DM. The activins and their binding protein, follistatin‐diagnostic and therapeutic targets in inflammatory disease and fibrosis. Cytokine Growth Factor Rev. 2013;24:285–295. [DOI] [PubMed] [Google Scholar]
- 17. ACOG Practice Bulletin No. 203: chronic hypertension in pregnancy. Obstet Gynecol. 2019;133:e26–e50. [DOI] [PubMed] [Google Scholar]
- 18. Shahul S, Medvedofsky D, Wenger JB, Nizamuddin J, Brown SM, Bajracharya S, Salahuddin S, Thadhani R, Mueller A, Tung A, et al. Circulating antiangiogenic factors and myocardial dysfunction in hypertensive disorders of pregnancy. Hypertension. 2016;67:1273–1280. [DOI] [PubMed] [Google Scholar]
- 19. Knackstedt C, Bekkers SC, Schummers G, Schreckenberg M, Muraru D, Badano LP, Franke A, Bavishi C, Omar AM, Sengupta PP. Fully automated versus standard tracking of left ventricular ejection fraction and longitudinal strain: the FAST‐EFs Multicenter Study. J Am Coll Cardiol. 2015;66:1456–1466. [DOI] [PubMed] [Google Scholar]
- 20. Wallace EM, Schneider‐Kolsky ME, Edwards A, Baker L, Jenkin G. Maternal serum activin A levels in association with intrauterine fetal growth restriction. BJOG. 2003;110:306–310. [PubMed] [Google Scholar]
- 21. Ali NA, Gutteridge D, Shahul S, Checkley W, Sevransky J, Martin GS. Critical illness outcome study: an observational study on protocols and mortality in intensive care units. Open Access J Clin Trials. 2011;2011:55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shahul S, Gulati G, Hacker MR, Mahmood F, Canelli R, Nizamuddin J, Mahmood B, Mueller A, Simon BA, Novack V, et al. Detection of myocardial dysfunction in septic shock: a speckle‐tracking echocardiography study. Anesth Analg. 2015;121:1547–1554. [DOI] [PubMed] [Google Scholar]
- 23. Shahul S, Rhee J, Hacker MR, Gulati G, Mitchell JD, Hess P, Mahmood F, Arany Z, Rana S, Talmor D. Subclinical left ventricular dysfunction in preeclamptic women with preserved left ventricular ejection fraction: a 2D speckle‐tracking imaging study. Circ Cardiovasc Imaging. 2012;5:734–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. ACOG Committee Opinion No. 743 summary: low‐dose aspirin use during pregnancy. Obstet Gynecol. 2018;132:254–256. [DOI] [PubMed] [Google Scholar]
- 25. Duley L, Henderson‐Smart DJ, Knight M, King JF. Antiplatelet agents for preventing pre‐eclampsia and its complications. Cochrane Database Syst Rev. 2019;10:CD004659. [DOI] [PubMed] [Google Scholar]
- 26. Rolnik DL, Wright D, Poon LC, O'Gorman N, Syngelaki A, de Paco Matallana C, Akolekar R, Cicero S, Janga D, Singh M, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med. 2017;377:613–622. [DOI] [PubMed] [Google Scholar]
- 27. Datta P, Rewers‐Felkins K, Kallem RR, Baker T, Hale TW. Transfer of low dose aspirin into human milk. J Hum Lact. 2017;33:296–299. [DOI] [PubMed] [Google Scholar]
- 28. Feng YL, Yin YX, Ding J, Yuan H, Yang L, Xu JJ, Hu LQ. Alpha‐1‐antitrypsin suppresses oxidative stress in preeclampsia by inhibiting the p38MAPK signaling pathway: an in vivo and in vitro study. PLoS One. 2017;12:e0173711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhao J, Peng W, Ran Y, Ge H, Zhang C, Zou H, Ding Y, Qi H. Dysregulated expression of ACTN4 contributes to endothelial cell injury via the activation of the p38‐MAPK/p53 apoptosis pathway in preeclampsia. J Physiol Biochem. 2019;75:475–487. [DOI] [PubMed] [Google Scholar]
- 30. Luo X, Yao ZW, Qi HB, Liu DD, Chen GQ, Huang S, Li QS. Gadd45alpha as an upstream signaling molecule of p38 MAPK triggers oxidative stress‐induced sFlt‐1 and sEng upregulation in preeclampsia. Cell Tissue Res. 2011;344:551–565. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


