Abstract
Background
Right ventricular systolic dysfunction (RVSD) is a known risk factor for adverse outcome in surgical aortic valve replacement. Transcatheter aortic valve replacement (TAVR), on the other hand, has been shown to be either beneficial or have no effect on right ventricular systolic function. However, the prognostic significance of RVSD on TAVR has not been clearly determined. We conducted a systematic review and meta‐analysis to define the impact of RVSD on outcomes in terms of 1‐year mortality in patients with severe aortic stenosis undergoing TAVR.
Methods and Results
An extensive literature review was performed, with an aim to identify clinical studies that focused on the prognosis and short‐term mortality of patients with severe symptomatic aortic stenosis who underwent TAVR. A total of 3166 patients from 8 selected studies were included. RVSD, as assessed with tricuspid annular plane systolic excursion, fractional area change or ejection fraction, was found to be a predictor of adverse procedural outcome after TAVR (hazard ratio, 1.31; 95% CI, 1.1–1.55; P=0.002). Overall, we found that RVSD did affect post‐TAVR prognosis in 1‐year mortality rate.
Conclusions
Patients with severe, symptomatic aortic stenosis and concomitant severe RVSD have a poor 1‐year post‐TAVR prognosis when compared with patients without RVSD. Right ventricular dilation and severe tricuspid regurgitation were associated with increased 1‐year morality post‐TAVR and should be considered as independent risk factors. Further evaluations of long‐term morbidity, mortality, as well as sustained improvement in functional class and symptoms need to be conducted to determine the long‐term effects.
Keywords: predictors, prognosis, quality of care, right ventricular dysfunction, transcatheter aortic valve implantation
Subject Categories: Aortic Valve Replacement/Transcather Aortic Valve Implantation, Meta Analysis, Percutaneous Coronary Intervention, Quality and Outcomes, Valvular Heart Disease
Nonstandard Abbreviations and Acronyms
- HR
hazard ratio
- RV
right ventricle
- RVSD
right ventricular systolic dysfunction
- RVSF
right ventricular systolic function
- TAPSE
tricuspid annular plane systolic excursion
- TAVR
transcatheter aortic valve replacement
- TR
tricuspid regurgitation
Clinical Perspective
What Is New?
This study adds to the evolving discussion of right ventricular systolic dysfunction as a prognostic marker for transcatheter aortic valve replacement, not only because of its large sample size but also its discovery of severe tricuspid regurgitation and right ventricular dilation as markers for adverse prognosis.
In patients with severe symptomatic aortic stenosis who undergo transcatheter aortic valve replacement, the presence of right ventricular systolic dysfunction, severe tricuspid regurgitation, and right ventricular dilation all portend increased risk of adverse outcomes.
What Are the Clinical Implications?
Clinicians should consider meticulous preoperative assessment of right ventricular systolic dysfunction, including evaluation of tricuspid regurgitation and right ventricular dilation in transcatheter aortic valve replacement candidates, and also strive for optimization of right ventricular function to improve outcomes.
Transcatheter aortic valve replacement (TAVR) continues to revolutionize the treatment of severe, symptomatic aortic stenosis.1, 2 Patients with severe aortic stenosis undergoing TAVR frequently present with markers of right‐sided heart dysfunction, including pulmonary hypertension, tricuspid insufficiency, and right ventricular systolic dysfunction (RVSD). By conservative estimates, pulmonary hypertension is noted in at least 30% of patients undergoing TAVR, whereas the presence of moderate to severe tricuspid regurgitation (TR) has been estimated to be about 15% to 20%.3, 4 RVSD is also often underappreciated and has been estimated to occur in ≥24% of patients undergoing TAVR.5 There are a plethora of contributors to the development of RVSD, including left ventricular systolic dysfunction, myocardial ischemia secondary to coronary artery disease, and uncontrolled pulmonary arterial pressures.4
Predictors of unfavorable outcomes after TAVR have been documented. They include oxygen‐dependent lung disease, renal dysfunction, poor functional capacity, and decreased baseline cognitive function.6, 7 Recent data suggest that even patients with advanced, severe left ventricular dysfunction who lack contractile reserve can tolerate TAVR reasonably well and may still garner some symptomatic benefit.8, 9, 10 But what about right ventricular (RV) systolic dysfunction? RVSD is considered to be a late marker of advanced heart failure. Currently, there are conflicting data on the impact of RVSD on clinical outcomes in patients with severe aortic stenosis treated with TAVR.11, 12, 13, 14, 15 A recent study indicated increased mortality post‐TAVR in patients with RVSD. However, that study did not use a standard definition of RVSD, relied on the highly variable echocardiography measures and cutoff values used by individual studies, and did not account for TR severity as an independent prognostic marker that can confound the association between RVSD and mortality.16 Given the conflicting data and lack of a consensus, we conducted a systematic review and meta‐analysis with a consolidated definition of RVSD (tricuspid annular plane systolic excursion [TAPSE] <16, fractional area change <35%, or decreased RV ejection fraction) to primarily analyze the impact of RVSD and, secondarily, the impact of TR and RV dilation on 1‐year mortality in patients with severe aortic stenosis undergoing TAVR.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request. This systematic review was performed in accordance with the statement on Preferred Reporting Items for Systematic Reviews and Meta‐Analyses. A comprehensive systematic search was performed on July 1, 2017 that included PubMed, Embase, Cochrane Library, ACP Journal Club, DARE, and Scopus. Both controlled vocabulary terms (ie, Medical Subject Headings) and key words were used to obtain relevant articles. The aim was to identify randomized and nonrandomized clinical studies that focused on the prognosis and short‐term mortality of patients with severe symptomatic aortic stenosis who underwent TAVR. Emphasis was placed on studies that noted a history of RVSD, RV dilation, and TR. This study was exempt from institutional review board approval as no protected health information was included.
Key words utilized in the initial PubMed (NLM) MEDLINE title search were “aortic valve” or “transcatheter aortic” or “aortic” or “valve replacement” or “TAVR” or “implantation” and “right ventricular” or “right ventricle” or “right systolic” or “ventricular dysfunction” or “right failure” or “diastolic dysfunction.”
Study Selection
Articles were considered eligible if they assessed right ventricular systolic function (RVSF) using TAPSE, percent RV fractional area change, or RV ejection fraction in patients pre‐ and post‐TAVR with a minimum follow‐up of 12 months post‐TAVR. Our prespecified criteria were that studies were published between January 1, 2002 and July 1, 2017, as the first human TAVR was performed in 2002, and only studies involving human subjects and those written in English were included. Articles were excluded if the patients had congenital cardiomyopathy, significant pulmonary or mitral stenosis, atrial or ventricular septal defects, infective endocarditis, or perivalvular fistula formation. Case reports, abstracts, editorials, and commentaries were also excluded. Three independent reviewers (S.G., M.I., and G.S.) independently selected articles based on the predefined search criteria as well as quality assessment. Any disagreement or discrepancies were resolved by majority consensus. Reference lists from previous studies were also perused for additional articles to be appraised.
Echocardiogram and RVSF Assessment
Although guidelines for the assessment of RVSF in adults have been described elsewhere,17 there remains a fair degree of variability in the clinical, qualitative, and quantitative assessment of RVSF. Furthermore, many of these measures may not have been routinely collected in various registries and trials, which limits retrospective data analysis. In this meta‐analysis, we identified studies that performed quantitative appraisal of RVSF with transthoracic echocardiography to measure TAPSE, fractional area change, and/or RV ejection fraction at baseline as well as post‐TAVR.
Statistical Analysis
Hazard ratios (HRs) with 95% CIs were used to compare pooled data from the included studies and respective treatment effects for binary end points. Continuous variable outcomes were compared with weighted mean differences. Der Simonian and Laird random‐effects models were used due to the anticipated heterogeneity between studies.11, 13, 14, 15, 18, 19, 20, 21, 22 Heterogeneity was examined using the Cochran Q test and I 2 statistics. P<0.10 and I 2>5% were considered significant for heterogeneity.
Results
An initial search of collective databases produced 1476 articles, with 763 articles remaining after the removal of 713 duplicates. A total of 752 articles were further excluded due to their lack of relevance to the scope of our study. The remaining 11 articles were appraised for eligibility using our inclusion and exclusion criteria, after which 8 articles remained for final inclusion in our meta‐analysis (Figure 1). The remaining 8 articles were comprised of 2 randomized, controlled clinical trials and 6 observational studies. All of the 8 studies selected for our meta‐analysis evaluated RVSF using transthoracic echocardiography to assess TAPSE, RV fractional area change and/or RV ejection fraction, and magnetic resonance imaging at baseline as well as post‐TAVR.
Figure 1. Flow diagram of Preferred Reporting Items for Systematic Reviews and Meta‐Analyses.
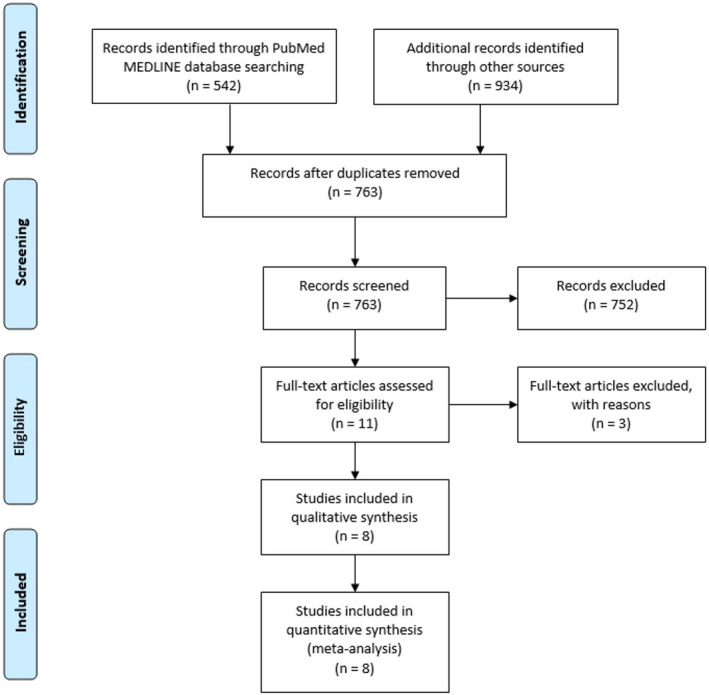
Keywords title search used to identify PubMed (NLM) MEDLINE articles: ((aortic valve[Title] OR transcatheter aortic[Title] OR aortic[Title] OR valve replacement[Title] OR TAVR[Title] OR implantation[Title])) AND (right ventricular[Title] OR right ventricle[Title] OR right systolic[Title] OR ventricular dysfunction[Title] OR right failure[Title] OR diastolic dysfunction[Title]) Filters: Humans, English, From 2002/01/01 to 2017/07/01. Retrieved articles: 542.
The studies included our meta‐analysis were chosen with the goal of evaluating outcomes in post‐TAVR patients with underlying RSVD, RV dilation, and/or TR, and compared with post‐TAVR patients with normal RV function. We defined our primary end point as the incidence of all‐cause mortality in patients with moderate to severe RVSD who underwent TAVR, with the secondary outcomes being the assessment of RV dilation or TR severity as a possible independent risk factor for 1‐year all‐cause mortality post‐TAVR.
The 8 selected studies were all single‐center studies published between 2007 and 2015, with an aggregate of 3166 patients across all studies. Mean age was 80.4±3.7 years. There was no difference in sex distribution. Baseline clinical and procedural characteristics are summarized in Table. The overall incidence of RVSD was 37.49%.8, 13, 14, 15, 16, 20, 23 Cumulative 1‐year mortality of patients with and without RVSD was 40.52% and 29.40%, respectively. In 4 of the 8 studies included, the 3‐year mortality of patients with RVSD was found to be 9.88%, compared with 14.2% in patients without RVSD.13, 14, 15, 23
Table 1.
Baseline Characteristics of Selected Studies Included in the Meta‐Analysis
| Author | Country, Year | Procedure | Type of Valve | Sample Size, n | Study Design | Male (%) | Age, y (Mean) | Mean STS Score (%) | RV Measurement (TAPSE, FAC, or S′) | Degree of TR | Pulmonary HTN | Duration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NRV RVD | NRV RVD | NRV RVD | NRV RVD | NRV RVD | NRV RVD | |||||||
| 1. Ito et al15 | USA, 2013 | TF‐TAVR, TA‐TAVR | Sapiens XT | 268 | Prospective |
50 72.1 |
80.5±8.1 80.1±7.6 |
9.5±4.8 10.8±5.6 |
No RV dilation: 200 | TR in no RV dilation: 21 | PASP in no RV dilation: 41.5±11.8 | 5 y |
| RV dilation: 68 | TR in RV dilation: 31 | PASP in RV dilation: 55.2±17.3 | ||||||||||
| 2. Koifman et al16 | USA, 2015 | TAVR | CoreValve | 606 | Prospective |
42 65 |
84±7 81±9 |
9.1±4.4 9.5±5.3 |
FAC >0.35: 146 | TR in no RV dilation, 19 | PASP in RVSD: 50±17 | 1 y |
| FAC <0.35: 460 | TR in no RV dilation: 38 | PASP in no RVSD: 44±16 | ||||||||||
| 3. Lindman et al20 | USA, 2012 | TAVR (from PARTNER II trial) | Sapiens, Sapiens XT | 488 | RCT |
49 56 |
84±8 86±10 |
10.1±5.3 11.5±6.0 |
Normal S′ :68 | No TR/mild TR: 372 | PASP for mild MR: 27; moderate: 31 | 1 y |
| Abnormal S′: 50 | Moderate TR: 117; severe TR: 18 | Severe: 28 | ||||||||||
| 4. Poliocikovaet al23 | UK, 2011 | TF‐TAVR, TA‐AVR, TS‐TAVR | Medtronic | 142 | Prospective |
61 48 |
79±4.7 82±6.3 |
Not reported | TAPSE <15: 18 | Mild TR: 97 (62.6%); moderate TR: 20 (12.9%) | Mean PAP for mild TR: 45 (29.0%) | 4 y |
| TAPSE >15: 124 | Severe TR: 13 (8.4%) | Moderate TR: 15 (9.7%); severe TR: 14 (9%) | ||||||||||
| 5. Schwartz et al14 | Israel, 2009 | TAVR | Not reported | 519 | Prospective |
43 46 |
82.4±6 84.8±5 |
Euroscore: 22.2±14 27.9±13 |
TAPSE in mild TR: 19.5±4 |
TR < moderate: normal, 37; mild: 46; moderate: 16; severe: 7 |
PASP in TR < moderate: 39.4±13 | 3 y |
|
TAPSE in moderate/severe TR: 18.4±5 |
TR > moderate: normal, 5; mild, 46; moderate, 47; severe, 12 |
PASP in TR > moderate: 61.2±13 | ||||||||||
| 6. Griese et al13 | Germany, 2014 | TF‐TAVR, TA‐TAVR | Sapiens XT, CoreValve | 702 | Prospective |
39.3 60 |
80±2 82±5 |
Euroscore: 30±15 17.±12 |
TAPSE >18: 462 | Not reported | Not reported | 5 y |
|
TAPSE 14–18: 190; TAPSE <14: 50 |
||||||||||||
| 7. Hutter et al5 | Germany, 2014 | TF‐TAVR, TA‐TAVR | Sapiens, CoreValve | 251 | Prospective |
47.9 45.5 |
80.8±6.4 80.5±7.4 |
5.7±3.4 7.1±4.3 |
Normal RV: 161 | Reported with MR | Mean PAP >60 mm Hg: 62 | 1 y |
| RV dysfunction: 45 | Mean PAP >60 mm Hg: 206 | |||||||||||
| 8. Lindsay et al21 | UK, 2016 | TF‐TAVR | Sapiens | 190 | Prospective |
49 51 |
79.7 81.4 |
Not reported |
Cardiac MRI RVEF >50: 145 |
Not reported | PASP RV <50 mm Hg: 45 | 2 y |
|
Cardiac MRI RVEF <50: 45 |
HTN indicates hypertension; MR, mitral regurgitation; MRI, magnetic resonance imaging; NRV, normal right ventricular function; PAP, pulmonary artery pressure; PASP (mm Hg), pulmonary artery systolic pressure (mm Hg); RVD, right ventricular dilation; RVEF, right ventricular ejection fraction; RVSP, right ventricle systolic pressure; S′, tricuspid lateral annular systolic velocity; STS, Society of Thoracic Surgeons operative mortality risk score; TA‐TAVR, transapical transcatheter aortic valve replacement; TF‐TAVR, transfemoral transcatheter aortic valve replacement; TR, tricuspid regurgitation; and TS‐TAVR, transseptal transcatheter aortic valve replacement.
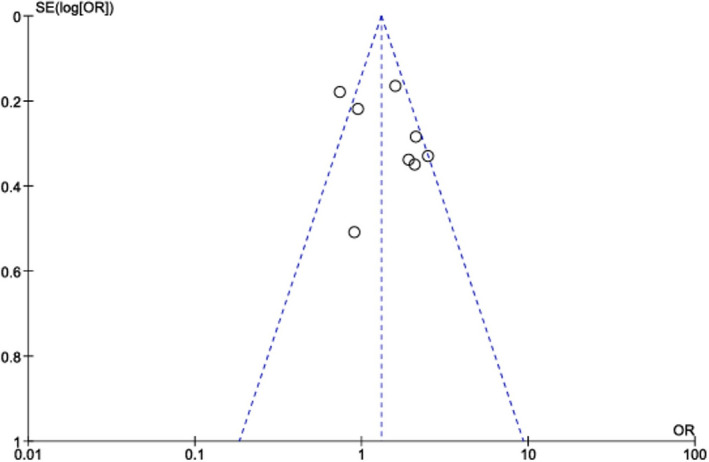
RVSD, as assessed with TAPSE, fractional area change, or ejection fraction, was found to be an independent predictor of adverse procedural outcome after TAVR (HR, 1.31; 95% CI, 1.1–1.55; P=0.002) (Figure 2). Overall, we found that RVSD did affect post‐TAVR prognosis in 1‐year mortality rate. In addition, the funnel plot revealed a similar outcomes distribution, thereby publication bias is unlikely (Figure 3).
Figure 2. Forest plot evaluating the impact of normal right ventricle function on all‐cause mortality in 1‐year vs RV dysfunction.
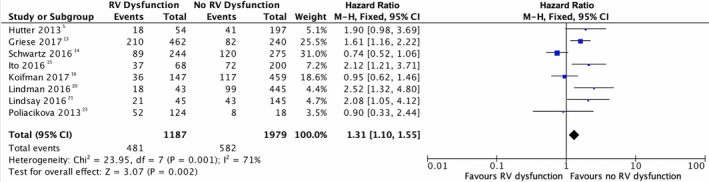
RV function is shown to be an independent predictor of adverse outcome after TAVR given the results did show a significant statistical difference (HR, 1.31; 95% CI, 1.10–1.55; P=0.002). HR indicates hazard ratio; TAVR, transcatheter aortic valve replacement; and RV, right ventricle.
Figure 3. Funnel plot showing no publication bias.
Discussion
Although earlier studies showed variable results, the present meta‐analysis of published studies assessing the effect of preoperative RVSD on 1‐year all‐cause mortality post‐TAVR has shown a statistically significant association between baseline RV dysfunction and mortality. Like several earlier single‐center studies, the current data support the finding that RVSD is an independent risk factor in short‐term all‐cause mortality in post‐TAVR patients. Three earlier retrospective studies have noted that RVSD, determined by low TAPSE values (<14 mm) on 2‐dimensional echocardiogram, is associated with increased short‐term all‐cause mortality in post‐TAVR patients. However, these particular studies were limited by their small sizes. Most included <100 patients, and had a limited number of patients with varying degrees of RVSD.11, 13, 15, 22, 23 Although all of the included studies specifically assessed RV function, the measures of RV function assessed varied between studies. Hutter et al3 noted the presence of RV dysfunction but did not specifically assess the outcomes‐based RV function. In reviewing their published data, the 1‐year mortality outcomes demonstrate 18 deaths over 54 people with moderate to severe TR (a surrogate for RV dysfunction) compared with 41 deaths over 197 people without moderate to severe TR for an HR of 1.9. In their study, the presence of RV dysfunction was noted to correlate and was significantly higher in those with moderate to severe TR than in those with mild to moderate TR: 10% versus 60%, respectively. Therefore, although this is a limitation, we used presence of severe TR as a surrogate marker of RV dysfunction. Our meta‐analysis supports the finding that RV dysfunction at baseline is an adverse marker of early mortality and adverse outcomes in TAVR patients.
Similar to our findings, a recent publication from the Swiss TAVR registry demonstrated that RVSD at baseline was associated with a >2‐fold increased risk of cardiovascular death at 1 year after TAVR. Furthermore, there was a gradient of risk according to recovery of RV dysfunction.24 It is important to note that RVSD cannot be analyzed in isolation. We must also take into account the company it keeps, namely TR and pulmonary hypertension, which often coexist. Multiple studies indicated that increased RV dilation and TR severity are independent predictors of post‐TAVR mortality.14, 19 Evaluation of the effects of TR severity and RV size in the inoperable cohort of the Placement of Aortic Trans‐Catheter Valves II trial indicated that both RV dilation and TR severity were independent risk factors for 1‐year mortality. After multivariable adjustment, there remained an increased risk of mortality in both severe TR (HR, 3.20; 95% CI, 1.50–6.82; P=0.003) and moderate TR (HR, 1.60; 95% CI, 1.02–2.52; P=0.042).19 Increased RV dilation remained borderline significant after adjustment for TR severity (P=0.058). Increased RV dilation may correlate with the chronicity and severity of RV pressure or volume overload rather than RV function, although further information is needed. When adjusted for confounders, RVSD alone was not predictive of overall mortality post‐TAVR.4, 14, 19
Historically, the presence of RVSD has been demonstrated to increase mortality in post–surgical aortic valve replacement patients.25, 26 Consequently, RVSD is considered an independent predictor of perioperative complications as well as late mortality after surgical aortic valve replacement.26, 27 The increased mortality may be multifactorial, including inadequate cardioprotection, high risk of right coronary air embolism, prolonged open heart surgery times, loss of atrioventricular synchrony, and reperfusion lung injury increasing pulmonary artery pressures.27 Each of these factors may contribute to the noncompensatory RV function secondary to postsurgical stress. However, these acute stressors cannot necessarily be extrapolated to post‐TAVR patients. TAVR is a minimally invasive procedure and therefore reduces the amount of stress on the heart and decreases the risk of postprocedure RVSD. Nonetheless, the presence of severe RVSD accompanied by TR may lead to poor outcomes in post‐TAVR patients.
During appraisal of data from the Placement of Aortic Trans‐Catheter Valves II trial, there was increased 1‐year mortality in patients with worsening TR: 16.9% for those with no or trace TR; 17.2% for mild TR; 32.6% for moderate TR; and 61.1% for severe TR (P<0.001).13, 19 The current data coincide with the fact that RVSD is an independent risk factor of poor outcomes post‐TAVR; the associations between TR as well as RV dilation and poor outcomes emphasize the importance of right heart assessment in preoperative work‐up. TAVR remains the preferred intervention for patients with severe, symptomatic aortic stenosis with RVSD. In addition, improved outcomes in 1‐year mortality may result from initial assessment of RV function and optimization.17 Perhaps no single quantitative measure of RVSD alone is sufficient for the assessment of right‐sided heart function in patients undergoing TAVR. Instead, a multimodal model incorporating various measures as well as the assessment of pulmonary hypertension and TR would be better predictive of outcomes and likelihood of functional recovery after TAVR. The ideal measures of RVSD for assessment in the TAVR population remain unknown. The first step would be to decide which measures carry some prognostic importance and are easily reproducible. Although some of these earlier studies were conducted in high‐risk or inoperable patients, it will also be important to identify whether the predictive validity remains in patients at lower risk as well. Thereafter, perhaps future predictive models can begin incorporating RVSD into estimates of post‐TAVR outcomes as well as likelihood of recovery of function.
Limitations
Our systematic review and meta‐analysis was limited by the availability of information and pertinent studies including data on RVSD in post‐TAVR assessment of all‐cause mortality. Our analysis is based on published and not patient‐level data, as we did not have available the patient‐level data for the included studies. In addition, as previously mentioned, there was significant heterogeneity in the methods of assessment of RVSF. Given there is no current “gold standard,” and the definition of RVSD was not uniform throughout the studies, the included studies used different measures of RV function. Furthermore, as noted earlier, for the Hutter et al study3 we used a surrogate marker of RV dysfunction, namely severe TR. There was limited follow‐up assessment of RVSD for determination of recovery of function. Most studies included retrospective analyses of RV function post‐TAVR, which may have introduced bias in the presentation of the results.
Conclusions
Patients with severe, symptomatic aortic stenosis and concomitant RVSD have worse 1‐year post‐TAVR prognosis compared with those without RVSD. RV dilation and severe TR were associated with increased 1‐year morality post‐TAVR and should be considered as independent risk factors.13 Given the significant heterogeneity of effects, RVSD in isolation should not be used to exclude patients from consideration for TAVR. Further evaluation of long‐term morbidity and mortality as well as sustained improvement in functional class and symptoms will be required to better elucidate the prognostic value of baseline RVSD in patients undergoing TAVR.
Sources of Funding
None.
Disclosures
None.
(J Am Heart Assoc. 2020;9:e014463. DOI: 10.1161/JAHA.119.014463.)
For Sources of Funding and Disclosures, see page 8.
References
- 1. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez‐Sarano M. Burden of valvular heart diseases: a population‐based study. Lancet. 2006;368:1005–1011. [DOI] [PubMed] [Google Scholar]
- 2. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, et al.; American College of Cardiology/American Heart Association Task Force on Practice G . 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:e57–e185. [DOI] [PubMed] [Google Scholar]
- 3. Hutter A, Bleiziffer S, Richter V, Opitz A, Hettich I, Mazzitelli D, Ruge H, Lange R. Transcatheter aortic valve implantation in patients with concomitant mitral and tricuspid regurgitation. Ann Thorac Surg. 2013;95:77–84. [DOI] [PubMed] [Google Scholar]
- 4. Barbanti M, Binder RK, Dvir D, Tan J, Freeman M, Thompson CR, Cheung A, Wood DA, Leipsic J, Webb JG. Prevalence and impact of preoperative moderate/severe tricuspid regurgitation on patients undergoing transcatheter aortic valve replacement. Catheter Cardiovasc Interv. 2015;85:677–684. [DOI] [PubMed] [Google Scholar]
- 5. Galli E, Guirette Y, Feneon D, Daudin M, Fournet M, Leguerrier A, Flecher E, Mabo P, Donal E. Prevalence and prognostic value of right ventricular dysfunction in severe aortic stenosis. Eur Heart J Cardiovasc Imaging. 2015;16:531–538. [DOI] [PubMed] [Google Scholar]
- 6. Arnold SV, Reynolds MR, Lei Y, Magnuson EA, Kirtane AJ, Kodali SK, Zajarias A, Thourani VH, Green P, Rodes‐Cabau J, et al.; Investigators P . Predictors of poor outcomes after transcatheter aortic valve replacement: results from the PARTNER (Placement of Aortic Transcatheter Valve) trial. Circulation. 2014;129:2682–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, et al.; Investigators PT . Transcatheter aortic‐valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. [DOI] [PubMed] [Google Scholar]
- 8. Lozano Granero VC, Fernandez Santos S, Fernandez‐Golfin C, Plaza Martin M, de la Hera Galarza JM, Faletra FF, Swaans MJ, Lopez‐Fernandez T, Mesa D, La Canna G, et al. Immediate improvement of left ventricular mechanics following transcatheter aortic valve replacement. Cardiol J. 2018;25:487–494. [DOI] [PubMed] [Google Scholar]
- 9. Kramer J, Biner S, Ghermezi M, Pressman GS, Shmueli H, Shimiaie J, Finkelstein A, Banai S, Steinvil A, Buffle E, et al. Impact of left ventricular filling parameters on outcome of patients undergoing trans‐catheter aortic valve replacement. Eur Heart J Cardiovasc Imaging. 2017;18:304–314. [DOI] [PubMed] [Google Scholar]
- 10. Fiedler AG, Bhambhani V, Laikhter E, Picard MH, Wasfy MM, Tolis G, Melnitchouk S, Sundt TM, Wasfy JH. Aortic valve replacement associated with survival in severe regurgitation and low ejection fraction. Heart. 2018;104:835–840. [DOI] [PubMed] [Google Scholar]
- 11. Griese DP, Kerber S, Barth S, Diegeler A, Babin‐Ebell J, Reents W. Impact of right and left ventricular systolic dysfunction on perioperative outcome and long‐term survival after transcatheter aortic valve replacement. J Interv Cardiol. 2017;30:217–225. [DOI] [PubMed] [Google Scholar]
- 12. Forsberg LM, Tamas E, Vanky F, Nielsen NE, Engvall J, Nylander E. Left and right ventricular function in aortic stenosis patients 8 weeks post‐transcatheter aortic valve implantation or surgical aortic valve replacement. Eur J Echocardiogr. 2011;12:603–611. [DOI] [PubMed] [Google Scholar]
- 13. Schwartz LA, Rozenbaum Z, Ghantous E, Kramarz J, Biner S, Ghermezi M, Shimiaie J, Finkelstein A, Banai S, Aviram G, et al. Impact of right ventricular dysfunction and tricuspid regurgitation on outcomes in patients undergoing transcatheter aortic valve replacement. J Am Soc Echocardiogr. 2017;30:36–46. [DOI] [PubMed] [Google Scholar]
- 14. Ito S, Pislaru SV, Soo WM, Huang R, Greason KL, Mathew V, Sandhu GS, Eleid MF, Suri RM, Oh JK, et al. Impact of right ventricular size and function on survival following transcatheter aortic valve replacement. Int J Cardiol. 2016;221:269–274. [DOI] [PubMed] [Google Scholar]
- 15. Koifman E, Didier R, Patel N, Jerusalem Z, Kiramijyan S, Ben‐Dor I, Negi SI, Wang Z, Goldstein SA, Lipinski MJ, et al. Impact of right ventricular function on outcome of severe aortic stenosis patients undergoing transcatheter aortic valve replacement. Am Heart J. 2017;184:141–147. [DOI] [PubMed] [Google Scholar]
- 16. Ren B, Spitzer E, Geleijnse ML, Zijlstra F, de Jaegere PPT, Van Mieghem NM, Tijssen JG. Right ventricular systolic function in patients undergoing transcatheter aortic valve implantation: a systematic review and meta‐analysis. Int J Cardiol. 2018;257:40–45. [DOI] [PubMed] [Google Scholar]
- 17. Zahaf M, Basu S, Bokowski J, Gasil S, Palacios I. Effect of transcatheter aortic valve replacement on right ventricular systolic function: systematic review and meta‐analyses. J Clin Exp Cardiol. 2016;7:447–452. [Google Scholar]
- 18. Abe T, Kamikubo Y, Taneichi T, Terada T, Sugiura J, Sakurai T, Tsuboi N, Sakurai H. Right heart failure secondary to compression of the right pulmonary artery by a large proximal aortic aneurysm. Circulation. 2013;128:1588–1589. [DOI] [PubMed] [Google Scholar]
- 19. Lindman BR, Maniar HS, Jaber WA, Lerakis S, Mack MJ, Suri RM, Thourani VH, Babaliaros V, Kereiakes DJ, Whisenant B, et al. Effect of tricuspid regurgitation and the right heart on survival after transcatheter aortic valve replacement: insights from the Placement of Aortic Transcatheter Valves II inoperable cohort. Circ Cardiovasc Interv. 2015;8 DOI: 10.1161/CIRCINTERVENTIONS.114.002073.PMID: 25855679; PMCID: PMC4438083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lindsay AC, Harron K, Jabbour RJ, Kanyal R, Snow TM, Sawhney P, Alpendurada F, Roughton M, Pennell DJ, Duncan A, et al. Prevalence and prognostic significance of right ventricular systolic dysfunction in patients undergoing transcatheter aortic valve implantation. Circ Cardiovasc Interv. 2016;9:e003486. [DOI] [PubMed] [Google Scholar]
- 21. Musa TA, Uddin A, Fairbairn TA, Dobson LE, Steadman CD, Kidambi A, Ripley DP, Swoboda PP, McDiarmid AK, Erhayiem B, et al. Right ventricular function following surgical aortic valve replacement and transcatheter aortic valve implantation: a cardiovascular MR study. Int J Cardiol. 2016;223:639–644. [DOI] [PubMed] [Google Scholar]
- 22. Poliacikova P, Cockburn J, Pareek N, James R, Lee L, Trivedi U, de Belder A, Hildick‐Smith D. Prognostic impact of pre‐existing right ventricular dysfunction on the outcome of transcatheter aortic valve implantation. J Invasive Cardiol. 2013;25:142–145. [PubMed] [Google Scholar]
- 23. Nilsson L, Appel CF, Hultkvist H, Vanky F. Evaluation of the valve academic research consortium‐2 criteria for myocardial infarction in transcatheter aortic valve implantation: a prospective observational study. PLoS One. 2015;10:e0130423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Asami M, Stortecky S, Praz F, Lanz J, Raber L, Franzone A, Piccolo R, Siontis GCM, Heg D, Valgimigli M, et al. Prognostic value of right ventricular dysfunction on clinical outcomes after transcatheter aortic valve replacement. JACC Cardiovasc Imaging. 2019;12:577–587. [DOI] [PubMed] [Google Scholar]
- 25. Kammerlander AA, Marzluf BA, Graf A, Bachmann A, Kocher A, Bonderman D, Mascherbauer J. Right ventricular dysfunction, but not tricuspid regurgitation, is associated with outcome late after left heart valve procedure. J Am Coll Cardiol. 2014;64:2633–2642. [DOI] [PubMed] [Google Scholar]
- 26. Wencker D, Borer JS, Hochreiter C, Devereux RB, Roman MJ, Kligfield P, Supino P, Krieger K, Isom OW. Preoperative predictors of late postoperative outcome among patients with nonischemic mitral regurgitation with ‘high risk’ descriptors and comparison with unoperated patients. Cardiology. 2000;93:37–42. [DOI] [PubMed] [Google Scholar]
- 27. Haddad F, Couture P, Tousignant C, Denault AY. The right ventricle in cardiac surgery, a perioperative perspective: II. Pathophysiology, clinical importance, and management. Anesth Analg. 2009;108:422–433. [DOI] [PubMed] [Google Scholar]


