Abstract
Background
Data on the association between serum bilirubin and the risk of stroke are limited and inconclusive. We aimed to evaluate the association between serum bilirubin and the risk of first stroke and to examine any possible effect modifiers in hypertensive patients.
Methods and Results
Our study was a post hoc analysis of the CSPPT (China Stroke Primary Prevention Trial). A total of 19 906 hypertensive patients were included in the final analysis. Cox proportional hazards models were used to estimate the hazard ratios (HRs) and 95% CIs for the risk of first stroke associated with serum bilirubin levels. The median follow‐up period was 4.5 years. When serum total bilirubin was assessed as tertiles, the adjusted HR of first ischemic stroke for participants in tertile 3 (12.9–34.1 μmol/L) was 0.75 (95% CI, 0.59–0.96), compared with participants in tertile 1 (<9.3 μmol/L). When direct bilirubin was assessed as tertiles, a significantly lower risk of first ischemic stroke was also found in participants in tertile 3 (2.5–24.8 μmol/L) (adjusted HR, 0.77; 95% CI, 0.60–0.98), compared with those in tertile 1 (<1.6 μmol/L). However, there was no significant association between serum total bilirubin (tertile 3 versus 1: adjusted HR, 1.45; 95% CI, 0.89–2.35) or direct bilirubin (tertile 3 versus 1: adjusted HR, 1.27; 95% CI, 0.76–2.11) and first hemorrhagic stroke.
Conclusions
In this sample of Chinese hypertensive patients, there was a significant inverse association between serum total bilirubin or direct bilirubin and the risk of first ischemic stroke.
Keywords: direct bilirubin, first hemorrhagic stroke, first ischemic stroke, hypertension, total bilirubin
Subject Categories: Hypertension, Cerebrovascular Disease/Stroke, Ischemic Stroke
Nonstandard Abbreviations and Acronyms
- ALT
alanine aminotransferase
- BP
blood pressure
- CSPPT
China Stroke Primary Prevention Trial
- DBiL
direct bilirubin
- HR
hazard ratio
- SBP
systolic blood pressure
- TBiL
total bilirubin
- tHcy
total homocysteine
Clinical Perspective
What Is New?
Our study first demonstrates that, even after adjustments for multiple confounding factors, including blood pressure at baseline and during the treatment period, higher baseline serum total bilirubin or direct bilirubin levels were associated with decreased risk of first ischemic stroke in patients with hypertension.
What Are the Clinical Implications?
Serum bilirubin concentration can be measured easily in the clinical laboratory and applied in medical practice; therefore, if our findings are further confirmed by future studies, routine measurements of bilirubin could help identify those hypertensive patients at high risk of ischemic stroke.
Stroke is the leading cause of death in China.1 Previous studies indicate that traditional risk factors do not account for all strokes.2, 3 Therefore, there is continuous interest in identifying novel modifiable risk factors to further improve stroke primary prevention and reduce the related, enormous disease burden.
Bilirubin is an end metabolic product of heme degradation. More and more studies are demonstrating that even mildly elevated serum bilirubin has potent anti‐inflammatory, antioxidant, antiproliferative, and blood lipid‐modulating properties.4, 5, 6, 7, 8 Accordingly, recent studies have shown that serum bilirubin is inversely associated with myocardial infarction, coronary heart disease, chronic kidney disease, and all‐cause mortality.9, 10, 11, 12 However, although the inverse association between bilirubin and the prevalence of stroke was observed in previous cross‐sectional studies,13, 14 data from prospective studies on the association between serum bilirubin levels and incident stroke are limited and inconclusive.15, 16, 17, 18 Notably, few previous studies have comprehensively investigated potential modifiers on the association between bilirubin and first stroke risk.
Hypertension is one of the most important risk factors for stroke and cardiovascular diseases.19, 20 Our current study was motivated by the above gaps in knowledge and the opportunity to examine the prospective relationship of serum bilirubin with first stroke and its subtypes among hypertensive adults in the CSPPR (China Stroke Primary Prevention Trial),21 a randomized, double‐blind, clinical study.
Methods
The parent study (CSPPT: Clinical Trials.gov, NCT00794885) and the current study were approved by the Ethics Committee of the Institute of Biomedicine, Anhui Medical University, Hefei, China (Federal Wide Assurance [FWA] assurance No. FWA00001263). All participants provided written informed consent. The data that support the findings of this study will be available from the corresponding authors on request, after the request is submitted and formally reviewed and approved by the Ethics Committee of the Institute of Biomedicine, Anhui Medical University.
Study Participants and Design
The rationale, study design, and major results for the CSPPT have been described previously.21, 22, 23, 24, 25 Briefly, the CSPPT was a randomized, double‐blind, clinical trial conducted from May 19, 2008, to August 24, 2013, with 20 702 hypertensive adults in 32 communities in Jiangsu and Anhui provinces in China. It was designed to evaluate whether a combined therapy of enalapril plus folic acid is more effective than enalapril alone in reducing the risk of first stroke in hypertensive patients. Eligible participants were men and women, aged 45 to 75 years, who had hypertension, defined as seated, resting systolic blood pressure (SBP) of ≥140 mm Hg or diastolic blood pressure (BP) of ≥90 mm Hg at both the screening and recruitment visits or patients who were taking antihypertensive medication. The major exclusion criteria included a history of physician‐diagnosed stroke, myocardial infarction, heart failure, coronary revascularization, or congenital heart disease.
The present study was a post hoc analysis of the CSPPT. Among the initial 20 702 participants, those with missing data on the major variables (bilirubin or liver enzymes) (n=144) were excluded. Among the remaining 20 558 participants, the participants with potential Gilbert syndrome (total bilirubin [TBiL] >34.2 μmol/L [>2.0 mg/dL], alanine aminotransferase [ALT] <80 IU/L, aspartate aminotransferase <80 IU/L, γ‐glutamyl transpeptidase <80 IU/L, and no self‐reported history of hepatobiliary disease; n=96) or potential hepatobiliary disease (TBiL >34.2 μmol/L, ALT ≥80 IU/L, aspartate aminotransferase ≥80 IU/L, serum albumin <3.5 g/dL, or a positive, self‐reported history of hepatobiliary disease; n=556) were also excluded to avoid confounding.15 A total of 19 906 participants were included in the final analysis (Figure S1). Those participants excluded from the analysis did not differ substantially in baseline characteristics from those included in the final analysis (Table S1).
Laboratory Assays
Fasting serum TBiL, direct bilirubin (DBiL), ALT, aspartate aminotransferase, γ‐glutamyl transpeptidase, lipids, total homocysteine (tHcy), creatinine, and glucose were measured using automatic clinical analyzers (Beckman Coulter) at the core laboratory of the National Clinical Research Center for Kidney Disease, Guangzhou, China.
Diabetes mellitus was defined as fasting glucose ≥7.0 mmol/L, use of glucose‐lowering drugs, or self‐reported history of diabetes mellitus.
Outcomes
The study outcomes included first stroke and its subtypes (first ischemic and hemorrhagic stroke), excluding subarachnoid hemorrhage and silent stroke.
All outcomes were reviewed and adjudicated by an independent end‐point adjudication committee whose members were unaware of study group assignments.
Statistical Analysis
Means (SDs) or proportions were calculated for population characteristics by TBiL tertiles. The differences in population characteristics were compared using ANOVA tests, signed rank tests, or χ2 tests, accordingly.
Cox proportional hazards models were used to estimate the hazard ratios (HRs) and 95% CIs for the risk of first stroke associated with serum bilirubin levels without and with adjustments for treatment group (enalapril or enalapril+folic acid), age, sex, body mass index, smoking status, SBP and diastolic BP, total cholesterol, fasting glucose, triglycerides, ALT, γ‐glutamyl transpeptidase, tHcy, folate levels, antihypertensive drug use, lipid‐lowering drug use, glucose‐lowering drug use, and antiplatelet drug use at baseline, as well as time‐averaged SBP and diastolic BP during the treatment period. In addition, possible modifications of the association between serum bilirubin (per SD increment) and first ischemic stroke were also assessed for the following variables: age (<60 versus ≥60 years), sex, treatment group (enalapril versus enalapril+folic acid), body mass index (<25 versus ≥25 kg/m2), total cholesterol (<5.2 versus ≥5.2 mmol/L), current smoking (yes versus no), SBP (<160 versus ≥160 mm Hg), diabetes mellitus (absence versus presence), tHcy (median, <12.5 versus ≥12.5 μmol/L), folate (median, <8.1 versus ≥8.1 μmol/L), antihypertensive drug use (yes versus no), lipid‐lowering drug use (yes versus no), glucose‐lowering drug use (yes versus no), and antiplatelet drug use (yes versus no) at baseline, as well as time‐averaged SBP (<140 versus ≥140 mm Hg) during the treatment period. Heterogeneity across subgroups was assessed by Cox proportional hazards models, and interactions between subgroups and serum bilirubin concentrations were examined by likelihood ratio testing.
A 2‐tailed P<0.05 was considered to be statistically significant in all analyses. R software version 3.4.1 (http://www.R-project.org) was used for all statistical analyses. The “epicalc,” “forestplot,” and “survival” packages of R were used in our statistical analyses.
Results
Study Participants and Characteristics
General characteristics of study participants are presented by TBiL tertiles in Table 1. The mean and median of serum TBiL and DBiL levels were 12.0 (SD, 4.9) and 10.9 μmol/L and 2.3 (SD, 1.2) and 2.0 μmol/L, respectively. That is to say, most of the participants had normal or mildly elevated TBiL or DBiL levels.
Table 1.
Characteristics of the Study Participants, According to Baseline TBiL Concentrations
| Characteristics | TBiL, μmol/L | ||
|---|---|---|---|
| Tertile 1 (<9.3) | Tertile 2 (9.3–12.9) | Tertile 3 (12.9–34.1) | |
| No. of participants | 6621 | 6634 | 6651 |
| Age, y | 59.5 (7.6) | 60.1 (7.5) | 60.4 (7.5) |
| Men, N (%) | 1726 (26.1) | 2693 (40.6) | 3630 (54.6) |
| BMI, kg/m2 | 25.6 (3.8) | 25.0 (3.6) | 24.3 (3.5) |
| Enalapril‐folic acid, N (%) | 3344 (50.5) | 3294 (49.7) | 3345 (50.3) |
| Current smoker, N (%) | 1092 (16.5) | 1567 (23.6) | 1977 (29.7) |
| BP, mm Hg | |||
| Systolic BP at baseline | 168.0 (20.8) | 167.3 (20.5) | 165.5 (19.8) |
| Diastolic BP at baseline | 94.0 (11.8) | 94.2 (11.9) | 94.0 (12.1) |
| Time‐averaged systolic BP | 140.2 (11.7) | 139.6 (11.5) | 139.7 (11.2) |
| Time‐averaged diastolic BP | 83.1 (7.6) | 83.0 (7.6) | 83.0 (7.8) |
| Laboratory results | |||
| Fasting glucose, mmol/L | 6.0 (1.8) | 5.8 (1.6) | 5.6 (1.6) |
| Total cholesterol, mmol/L | 5.7 (1.2) | 5.6 (1.2) | 5.3 (1.2) |
| Triglycerides, mmol/L | 1.8 (1.5) | 1.6 (0.9) | 1.6 (0.9) |
| HDL‐C, mmol/L | 1.3 (0.3) | 1.4 (0.3) | 1.4 (0.4) |
| Alanine transaminase, IU/L | 12.9 (6.0) | 13.8 (6.8) | 15.4 (7.9) |
| Aspartate transaminase, IU/L | 23.0 (7.7) | 25.1 (8.6) | 28.2 (9.6) |
| γ‐Glutamyl transpeptidase, IU/L | 23.7 (22.4) | 26.1 (22.6) | 31.1 (34.5) |
| Total homocysteine, μmol/L | 13.9 (7.7) | 14.4 (8.3) | 15.1 (8.7) |
| Total bilirubin, μmol/L | 7.6 (1.2) | 11.0 (1.0) | 17.5 (4.3) |
| Direct bilirubin, μmol/L | 1.3 (0.5) | 2.0 (0.5) | 3.5 (1.1) |
| Medication use, N (%) | |||
| Antihypertensive drugs | 3339 (50.4) | 3043 (45.9) | 2817 (42.4) |
| Lipid‐lowering drugs | 58 (0.9) | 48 (0.7) | 55 (0.8) |
| Glucose‐lowering drugs | 134 (2.0) | 93 (1.4) | 77 (1.2) |
| Antiplatelet drugs | 238 (3.6) | 199 (3.0) | 150 (2.3) |
| Self‐reported history of disease | |||
| Coronary heart disease | 131 (2.0) | 96 (1.4) | 97 (1.5) |
| Arrhythmia | 70 (1.1) | 81 (1.2) | 63 (0.9) |
| Diabetes mellitus | 287 (4.3) | 189 (2.8) | 146 (2.2) |
Continuous variables are presented as mean (SD), and categorical variables are presented as number (percentage). BP indicates blood pressure; BMI, body mass index; HDL‐C, high‐density lipoprotein cholesterol; and TBiL, total bilirubin.
Serum TBiL levels were positively associated with age, male sex, current smoking, ALT, aspartate aminotransferase, γ‐glutamyl transpeptidase, tHcy, and high‐density lipoprotein cholesterol at baseline; and were inversely associated with body mass index, SBP, total cholesterol, triglycerides, fasting glucose, use of antihypertensive drugs, glucose‐lowering drugs, and antiplatelet drugs, and the self‐reported history of coronary heart disease and diabetes mellitus at baseline, as well as time‐averaged SBP during the treatment period (Table 1).
Relationship of Serum TBiL and DBiL With Study Outcomes
During a median follow‐up period of 4.5 years, there were 608 first strokes, including 489 ischemic strokes, 117 hemorrhagic strokes, and 2 uncertain types of stroke.
Overall, there was no significant association between TBiL (per SD increment; adjusted HR, 0.97; 95% CI, 0.88–1.06) or DBiL (adjusted HR, 0.91; 95% CI, 0.82–1.01) levels and first stroke (Tables 2 and 3). However, there was an inverse association between TBiL (Figure 1A; per SD increment; adjusted HR, 0.91; 95% CI, 0.82–1.02) or DBiL (Figure 1B; adjusted HR, 0.85; 95% CI, 0.75–0.95) levels and first ischemic stroke. Consistently, when TBiL was assessed as tertiles, the adjusted HR for first ischemic stroke for participants in tertile 3 (12.9–34.1 μmol/L) was 0.75 (95% CI, 0.59–0.96), compared with participants in tertile 1 (<9.3 μmol/L) (Table 2). When DBiL was assessed as tertiles, a significantly lower risk of first ischemic stroke was observed in participants in tertile 3 (2.5–24.8 μmol/L) (adjusted HR, 0.77; 95% CI, 0.60–0.98), compared with those in tertile 1 (<1.6 μmol/L) (Table 3). However, there was no significant relation of serum TBiL (tertile 3 versus 1; adjusted HR, 1.45; 95% CI, 0.89–2.35) or DBiL (tertile 3 versus 1; adjusted HR, 1.27; 95% CI, 0.76–2.11) with first hemorrhagic stroke. Consistently, there was also no significant association between serum TBiL (tertile 3 versus 1; adjusted HR, 0.87; 95% CI, 0.71–1.08) or DBiL (tertile 3 versus 1; adjusted HR, 0.87; 95% CI, 0.69–1.08) and first stroke (Tables 2 and 3).
Table 2.
Relationship of Baseline Serum TBiL Levels With First Stroke and Its Subtypes
| Total Bilirubin, μmol/L | Events/N (%) | HR (95% CI) | ||
|---|---|---|---|---|
| Unadjusted | Adjusteda | P Valuea | ||
| First stroke | ||||
| Per SD increment | 608 | 0.92 (0.85–1.00) | 0.97 (0.88–1.06) | 0.48 |
| Tertiles | ||||
| Tertile 1 (<9.3) | 225/6621 (3.4) | Reference | Reference | |
| Tertile 2 (9.3–12.9) | 192/6634 (2.9) | 0.84 (0.69–1.01) | 0.88 (0.72–1.08) | 0.22 |
| Tertile 3 (12.9–34.1) | 191/6651 (2.9) | 0.82 (0.68–0.99) | 0.87 (0.71–1.08) | 0.20 |
| P value for trend | … | 0.04 | 0.20 | … |
| First ischemic stroke | ||||
| Per SD increment | 489 | 0.87 (0.79–0.96) | 0.91 (0.82–1.02) | 0.10 |
| Tertiles | ||||
| Tertile 1 (<9.3) | 190/6621 (2.9) | Reference | Reference | |
| Tertile 2 (9.3–12.9) | 159/6634 (2.4) | 0.82 (0.66–1.01) | 0.85 (0.68–1.06) | 0.15 |
| Tertile 3 (12.9–34.1) | 140/6651 (2.1) | 0.71 (0.57–0.89) | 0.75 (0.59–0.96) | 0.02 |
| P value for trend | … | <0.01 | 0.02 | … |
| First hemorrhagic stroke | ||||
| Per SD increment | 117 | 1.14 (0.97–1.35) | 1.14 (0.93–1.37) | 0.16 |
| Tertiles | ||||
| Tertile 1 (<9.3) | 34/6621 (0.5) | Reference | Reference | |
| Tertile 2 (9.3–12.9) | 33/6634 (0.5) | 0.95 (0.59–1.53) | 1.03 (0.62–1.70) | 0.91 |
| Tertile 3 (12.9–34.1) | 50/6651 (0.8) | 1.42 (0.92–2.19) | 1.45 (0.89–2.35) | 0.14 |
| P value for trend | … | 0.10 | 0.12 | … |
HR indicates hazard ratio; and TBiL, total bilirubin.
Adjusted for age, sex, treatment group, body mass index, systolic blood pressure, diastolic blood pressure, smoking status, fasting glucose, total cholesterol, triglycerides, alanine aminotransferase, γ‐glutamyl transpeptidase, total homocysteine, folate levels, antihypertensive drug use, lipid‐lowering drug use, glucose‐lowering drug use, and antiplatelet drug use at baseline, as well as time‐averaged systolic blood pressure and diastolic blood pressure during the treatment period.
Table 3.
Relationship of Baseline Serum DBiL Levels With First Stroke and Its Subtypes
| Direct Bilirubin, μmol/L | Events/N (%) | HR (95% CI) | ||
|---|---|---|---|---|
| Unadjusted | Adjusteda | P Valuea | ||
| First stroke | ||||
| Per SD increment | 608 | 0.87 (0.80–0.95) | 0.91 (0.82–1.01) | 0.07 |
| Tertiles | ||||
| Tertile 1 (<1.6) | 223/6603 (3.4) | Reference | Reference | |
| Tertile 2 (1.6–2.5) | 201/6568 (3.1) | 0.89 (0.74–1.08) | 0.91 (0.75–1.11) | 0.37 |
| Tertile 3 (2.5–24.8) | 184/6735 (2.7) | 0.78 (0.65–0.95) | 0.87 (0.69–1.08) | 0.21 |
| P value for trend | … | 0.02 | 0.20 | … |
| First ischemic stroke | ||||
| Per SD increment | 489 | 0.80 (0.72–0.89) | 0.85 (0.75–0.95) | 0.01 |
| Tertiles | ||||
| Tertile 1 (<1.6) | 187/6603 (2.8) | Reference | Reference | |
| Tertile 2 (1.6–2.5) | 169/6568 (2.6) | 0.90 (0.73–1.10) | 0.91 (0.73–1.13) | 0.40 |
| Tertile 3 (2.5–24.8) | 133/6735 (2.0) | 0.68 (0.54–0.85) | 0.77 (0.60–0.98) | 0.04 |
| P value for trend | … | <0.01 | 0.04 | … |
| First hemorrhagic stroke | ||||
| Per SD increment | 117 | 1.13 (0.97–1.31) | 1.09 (0.90–1.32) | 0.37 |
| Tertiles | ||||
| Tertile 1 (<1.6) | 35/6603 (0.5) | Reference | Reference | |
| Tertile 2 (1.6–2.5) | 32/6568 (0.5) | 0.91 (0.56–1.47) | 0.96 (0.58–1.59) | 0.87 |
| Tertile 3 (2.5–24.8) | 50/6735 (0.7) | 1.36 (0.88–2.09) | 1.27 (0.76–2.11) | 0.36 |
| P value for trend | … | 0.15 | 0.34 | … |
DBiL indicates direct bilirubin; and HR, hazard ratio.
Adjusted for age, sex, treatment group, body mass index, systolic blood pressure, diastolic blood pressure, smoking status, fasting glucose, total cholesterol, triglycerides, alanine aminotransferase, γ‐glutamyl transpeptidase, total homocysteine, folate levels, antihypertensive drug use, lipid‐lowering drug use, glucose‐lowering drug use, and antiplatelet drug use at baseline, as well as time‐averaged systolic blood pressure and diastolic blood pressure during treatment.
Figure 1. Relationship of total bilirubin (TBiL) (A) and direct bilirubin (DBiL) (B) levels with first ischemic stroke in hypertensive patients.
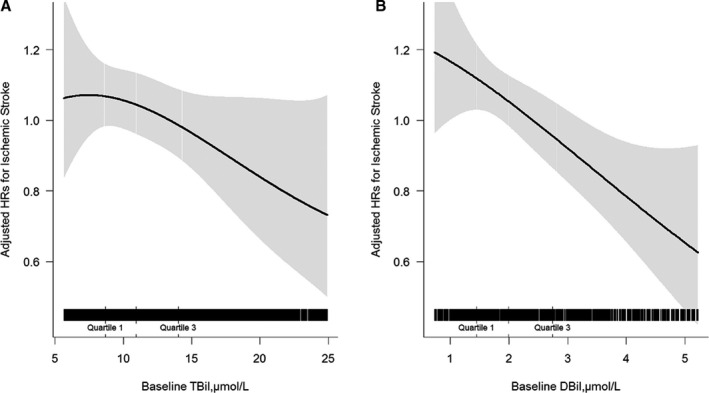
Adjusted for age, sex, treatment group, body mass index, systolic blood pressure, diastolic blood pressure, smoking status, fasting glucose, total cholesterol, triglycerides, alanine aminotransferase, γ‐glutamyl transpeptidase, total homocysteine, folate levels, antihypertensive drug use, lipid‐lowering drug use, glucose‐lowering drug use, and antiplatelet drug use at baseline, as well as time‐averaged systolic blood pressure and diastolic blood pressure during the treatment period. HR indicates hazard ratio.
Similar results were found in participants with normal levels of TBiL (3–≤17 μmol/L26) (Table S2) or normal levels of DBiL (≤6.8 μmol/L27) (Table S3). Further adjustment for the baseline serum albumin did not materially change the results (Table S4). Similar findings were also found for further adjustment for the history of diabetes mellitus, arrhythmia, and coronary heart disease at baseline (Table S5).
Furthermore, during the treatment period, participants with lower TBiL levels had a higher frequency use of glucose‐lowering drugs and diuretics and a lower frequency use of calcium channel blockers and antiplatelet drugs (Table S6). However, further adjustment for the use of medications (glucose‐lowering drugs, lipid‐lowering drugs, antiplatelet drugs, calcium channel blockers, and diuretics) during the treatment period did not substantially change the association between serum TBiL or DBiL and first ischemic stroke (Table S7).
Stratified Analysis by Potential Effect Modifiers
We further performed stratified analyses to assess the relationship of baseline serum DBiL (per SD increment) (Figure 2 and Table S8) and TBiL (per SD increment) (Table S9) with the risk of first ischemic stroke in various subgroups.
Figure 2. Interactions between serum direct bilirubin (per SD increment) and different variables on first ischemic stroke, examined by likelihood ratio testing.
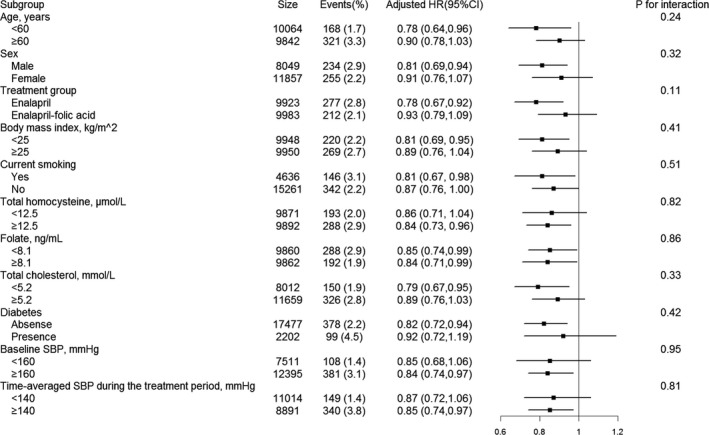
Adjusted for age, sex, treatment group, body mass index, systolic blood pressure (SBP), diastolic blood pressure, smoking status, fasting glucose, total cholesterol, triglycerides, alanine aminotransferase, γ‐glutamyl transpeptidase, total homocysteine, folate levels, antihypertensive drug use, lipid‐lowering drug use, glucose‐lowering drug use, and antiplatelet drug use at baseline, as well as time‐averaged SBP and diastolic blood pressure during the treatment period, if not stratified. Diabetes mellitus was defined as fasting glucose ≥7.0 mmol/L at baseline, use of glucose‐lowering drugs, or self‐reported history of diabetes mellitus. HR indicates hazard ratio.
None of the variables, including age (P‐interaction=0.24), sex (P‐interaction=0.32), treatment group (P‐interaction=0.11), body mass index (P‐interaction=0.41), current smoking (P‐interaction=0.51), tHcy (P‐interaction=0.82), folate (P‐interaction=0.86), total cholesterol (P‐interaction=0.33), diabetes mellitus (P‐interaction=0.42), SBP (P‐interaction=0.95), antihypertensive drug use (P‐interaction=0.20), lipid‐lowering drug use (P‐interaction=0.25), glucose‐lowering drug use (P‐interaction=0.70), and antiplatelet drug use (P‐interaction=0.73) at baseline, as well as time‐averaged SBP (P‐interaction=0.81) during the treatment period, significantly modified the association between DBiL and first ischemic stroke (Figure 2 and Table S8). Similar results were also observed for TBiL levels (Table S9).
Discussion
The current study demonstrates that, even after adjustments for multiple confounding factors, including BP at baseline and during the treatment period, higher baseline serum TBiL or DBiL levels were associated with decreased risk of first ischemic stroke in patients with hypertension.
The association between TBiL and the risk of stroke has been examined in several previous studies. Kimm et al15 found that higher serum TBiL levels showed a lower HR for ischemic stroke in men but not in women, in a cohort study of health examinees in Korea. However, a previous nested case‐control study by Ekblom et al17 showed that plasma TBiL was lower in stroke cases than in controls, but the difference reached significance only in women. Mahabadi et al18 reported that there was a significant inverse association between TBiL and stroke risk (only 95 stroke cases) in the general population without known liver disease. The PREVEND (Prevention of Renal and Vascular End‐Stage Disease) study16 demonstrated that there was no significant evidence of an association between TBiL and stroke risk (per SD increment; HR, 0.87; 95% CI, 0.72–1.04) in 7222 participants without a known history of cardiovascular disease. At the same time, using a mendelian randomization design, Lee et al28 suggested a noncausal association between TBiL and stroke risk. However, except for the inherent limitation of mendelian randomization design, this study did not perform a replication analysis in a powered second group, and could not confirm the exact recurrent stroke. Overall, these studies indicated that the association between blood TBiL and the risk of stroke remains uncertain. Of note, in the previous studies, only ≈10% to 50% of the participants had hypertension. More important, there was no information on BP levels during the follow‐up period in all of the previous studies. Therefore, the results could not be generalized to a hypertensive population. Our study provided an opportunity to assess the dose‐response relationship between serum bilirubin and first stroke in hypertensive patients receiving standard treatment, with comprehensive adjustments for a series of important confounders and a strict validation of study outcomes.21
Our current study found that both higher serum TBiL and DBiL were related to the lower risk of first ischemic stroke in hypertensive patients. Ischemic stroke is a complex process in which atherothrombosis plays an important role.29 Serum bilirubin is an endogenous antioxidative substance and is positively correlated with total antioxidant capacity.30 Previous studies reported that the antioxidative activity of bilirubin is independent of its forms (ie, unconjugated and conjugated bilirubins).31 It has been found that low serum bilirubin was associated with the oxidation of lipids and lipoproteins and the damage of endothelium, consequently leading to the formation of atherosclerotic plaques and arterial thrombosis.32, 33, 34 Accordingly, in a previous double‐blind, placebo‐controlled, crossover study,35 treatment with the bilirubin‐increasing drug atazanavir induced an increase in average bilirubin levels and improved plasma antioxidant capacity and endothelium‐dependent vasodilation in patients with type 2 diabetes mellitus. Moreover, Stein et al36 reported that higher levels of on‐treatment bilirubin (>10.1 versus ≤10.1 μmol/L) were associated with significantly slower progression of carotid artery intima‐media thickness. However, the findings and the detailed mechanisms still need to be confirmed in further studies.
The potential limitations of the current analyses should also be considered in interpreting the study results. First, post hoc analyses of randomized trials have inherent limitations, such as the possibility of residual imbalance in some unmeasured factors at baseline. Second, our study was conducted in Chinese hypertensive patients. Although the stratified analysis showed that BP at baseline and during the treatment period did not substantially change the findings, the generalizability of our results to nonhypertensive adults and other populations remains to be determined. Third, our study may be underpowered for evaluating the relation of bilirubin with the risk of hemorrhagic stroke. Fourth, the bilirubin levels of the current study population were mostly in the normal range. And the similar inverse association between serum TBiL or DBiL and first ischemic stroke was also found in those with normal TBiL or DBiL levels. Of note, our results just suggested the possible beneficial effect of relatively higher TBiL or DBiL levels within normal range on first ischemic stroke. Another limitation of the CSPPT is the lack of the classification of subtypes of ischemic stroke based on mechanisms. Finally, we did not have the direct measurements of indirect bilirubin. Because of these limitations, our results should be regarded as hypothesis generating. All findings need to be further investigated and confirmed in future studies.
In summary, our study suggests that a higher concentration of serum bilirubin is associated with a lower risk of first ischemic stroke. Serum bilirubin concentration can be measured easily in the clinical laboratory and applied in medical practice. If our findings are further confirmed by future studies, routine measurements of bilirubin could help identify those hypertensive patients at high risk of ischemic stroke.
Sources of Funding
The study was supported by funding from the following: the National Key Research and Development Program (2016YFE0205400, 2018ZX09739010, and 2018ZX09301034003); the National Natural Science Foundation of China (81730019 and 81973133); the Science and Technology Planning Project of Guangzhou, China (201707020010); the Science, Technology and Innovation Committee of Shenzhen (JSGG20170412155639040 and GJHS20170314114526143); and the Economic, Trade and Information Commission of Shenzhen Municipality (20170505161556110 and 20170505160926390).
Disclosures
Dr Xiping Xu reports grants from the National Key Research and Development Program (2016YFE0205400, 2018ZX09739010, and 2018ZX09301034003), the Science and Technology Planning Project of Guangzhou, China (201707020010), the Science, Technology and Innovation Committee of Shenzhen (JSGG20170412155639040 and GJHS20170314114526143), and the Economic, Trade and Information Commission of Shenzhen Municipality (20170505161556110 and 20170505160926390). Dr Xianhui Qin reports grants from the National Natural Science Foundation of China (81973133 and 81730019). The remaining authors have no disclosures to report.
Supporting information
Tables S1–S9 Figure S1
(J Am Heart Assoc. 2020;9:e015799. DOI: 10.1161/JAHA.119.015799.)
For Sources of Funding and Disclosures, see page 8.
Contributor Information
Xiping Xu, Email: pharmaqin@126.com.
Xianhui Qin, Email: xipingxu126@126.com.
References
- 1. Zhou M, Wang H, Zeng X, Yin P, Zhu J, Chen W, Li X, Wang L, Wang L, Liu Y, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;394:1145–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kim YD, Jung YH, Saposnik G. Traditional risk factors for stroke in East Asia. J Stroke. 2016;18:273–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. O'Donnell MJ, Chin SL, Rangarajan S, Xavier D, Liu L, Zhang H, Rao‐Melacini P, Zhang X, Pais P, Agapay S, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case‐control study. Lancet. 2016;388:761–775. [DOI] [PubMed] [Google Scholar]
- 4. Mancuso C. Bilirubin and brain: a pharmacological approach. Neuropharmacology. 2017;118:113–123. [DOI] [PubMed] [Google Scholar]
- 5. Zelenka J, Dvořák A, Alán L, Zadinová M, Haluzík M, Vítek L. Hyperbilirubinemia protects against aging‐associated inflammation and metabolic deterioration. Oxid Med Cell Longev. 2016;2016:6190609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Loboda A, Jazwa A, Grochot‐Przeczek A, Rutkowski AJ, Cisowski J, Agarwal A, Jozkowicz A, Dulak J. Heme oxygenase‐1 and the vascular bed: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2008;10:1767–1812. [DOI] [PubMed] [Google Scholar]
- 7. Maruhashi T, Soga J, Fujimura N, Idei N, Mikami S, Iwamoto Y, Kajikawa M, Matsumoto T, Kihara Y, Chayama K, et al. Hyperbilirubinemia, augmentation of endothelial function, and decrease in oxidative stress in Gilbert syndrome. Circulation. 2012;126:598–603. [DOI] [PubMed] [Google Scholar]
- 8. Idriss NK, Blann AD, Lip GY. Hemoxygenase‐1 in cardiovascular disease. J Am Coll Cardiol. 2008;52:971–978. [DOI] [PubMed] [Google Scholar]
- 9. Horsfall LJ, Nazareth I, Petersen I. Cardiovascular events as a function of serum bilirubin levels in a large, statin‐treated cohort. Circulation. 2012;126:2556–2564. [DOI] [PubMed] [Google Scholar]
- 10. Hopkins PN, Wu LL, Hunt SC, James BC, Vincent GM, Williams RR. Higher serum bilirubin is associated with decreased risk for early familial coronary artery disease. Arterioscler Thromb Vasc Biol. 1996;16:250–255. [DOI] [PubMed] [Google Scholar]
- 11. Ajja R, Lee DC, Sui X, Church TS, Steven NB. Usefulness of serum bilirubin and cardiorespiratory fitness as predictors of mortality in men. Am J Cardiol. 2011;108:1438–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang J, Wang B, Liang M, Wang G, Li J, Zhang Y, Huo Y, Cui Y, Xu X, Qin X. Independent and combined effect of bilirubin and smoking on the progression of chronic kidney disease. Clin Epidemiol. 2018;10:121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Perlstein TS, Pande RL, Creager MA, Weuve J, Beckman JA. Serum total bilirubin level, prevalent stroke, and stroke outcomes: NHANES 1999‐2004. Am J Med. 2008;121:781–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li RY, Cao ZG, Zhang JR, Li Y, Wang RT. Decreased serum bilirubin is associated with silent cerebral infarction. Arterioscler Thromb Vasc Biol. 2014;34:946–951. [DOI] [PubMed] [Google Scholar]
- 15. Kimm H, Yun JE, Jo J, Jee SH. Low serum bilirubin level as an independent predictor of stroke incidence: a prospective study in Korean men and women. Stroke. 2009;40:3422–3427. [DOI] [PubMed] [Google Scholar]
- 16. Kunutsor SK, Bakker SJ, Gansevoort RT, Chowdhury R, Dullaart RP. Circulating total bilirubin and risk of incident cardiovascular disease in the general population. Arterioscler Thromb Vasc Biol. 2015;35:716–724. [DOI] [PubMed] [Google Scholar]
- 17. Ekblom K, Marklund SL, Johansson L, Osterman P, Hallmans G, Weinehall L, Wiklund PG, Hultdin J. Bilirubin and UGT1A1*28 are not associated with lower risk for ischemic stroke in a prospective nested case‐referent setting. Cerebrovasc Dis. 2010;30:590–596. [DOI] [PubMed] [Google Scholar]
- 18. Mahabadi AA, Lehmann N, Möhlenkamp S, Kälsch H, Bauer M, Schulz R, Moebus S, Jöckel KH, Erbel R, Heusch G. Association of bilirubin with coronary artery calcification and cardiovascular events in the general population without known liver disease: the Heinz Nixdorf Recall study. Clin Res Cardiol. 2014;103:647–653. [DOI] [PubMed] [Google Scholar]
- 19. Fan F, Yuan Z, Qin X, Li J, Zhang Y, Li Y, Yu T, Ji M, Ge J, Zheng M, et al. Optimal systolic blood pressure levels for primary prevention of stroke in general hypertensive adults: findings from the CSPPT (China Stroke Primary Prevention Trial). Hypertension. 2017;69:697–704. [DOI] [PubMed] [Google Scholar]
- 20. He M, Qin X, Cui Y, Cai Y, Sun L, Xu X, Wang B, Tang G, Xing H, Wang X, et al. Prevalence of unrecognized lower extremity peripheral arterial disease and the associated factors in Chinese hypertensive adults. Am J Cardiol. 2012;110:1692–1698. [DOI] [PubMed] [Google Scholar]
- 21. Huo Y, Li J, Qin X, Huang Y, Wang X, Gottesman RF, Tang G, Wang B, Chen D, He M, et al. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: the CSPPT randomized clinical trial. JAMA. 2015;313:1325–1335. [DOI] [PubMed] [Google Scholar]
- 22. Qin X, Li Y, He M, Tang G, Yin D, Liang M, Wang B, Nie J, Huo Y, Xu X, et al. Folic acid therapy reduces serum uric acid in hypertensive patients: a substudy of the China Stroke Primary Prevention Trial (CSPPT). Am J Clin Nutr. 2017;105:882–889. [DOI] [PubMed] [Google Scholar]
- 23. Qin X, Li J, Zhang Y, Chen D, Wang B, He M, Fu J, Tang G, Cai Y, Shi X, et al. Effect of folic acid supplementation on risk of new‐onset diabetes in adults with hypertension in China: findings from the China Stroke Primary Prevention Trial (CSPPT). J Diabetes. 2016;8:286–294. [DOI] [PubMed] [Google Scholar]
- 24. Qin X, Li J, Spence JD, Zhang Y, Li Y, Wang X, Wang B, Sun N, Chen F, Guo J, et al. Folic acid therapy reduces the first stroke risk associated with hypercholesterolemia among hypertensive patients. Stroke. 2016;47:2805–2812. [DOI] [PubMed] [Google Scholar]
- 25. Huang X, Li Y, Li P, Li J, Bao H, Zhang Y, Wang B, Sun N, Wang J, He M, et al. Association between percent decline in serum total homocysteine and risk of first stroke. Neurology. 2017;89:2101–2107. [DOI] [PubMed] [Google Scholar]
- 26. Vítek L. Bilirubin and atherosclerotic diseases. Physiol Res. 2017;66(suppl 1):S11–S20. [DOI] [PubMed] [Google Scholar]
- 27. Yang Y, Jiang H, Tang A, Xiang Z. Reference intervals for serum bilirubin, urea, and uric acid in healthy Chinese geriatric population. J Clin Lab Anal. 2018;32:e22318 DOI: 10.1002/jcla.22318. Epub 2017 Sep 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee SJ, Jee YH, Jung KJ, Hong S, Shin ES, Jee SH. Bilirubin and stroke risk using a mendelian randomization design. Stroke. 2017;48:1154–1160. [DOI] [PubMed] [Google Scholar]
- 29. Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. [DOI] [PubMed] [Google Scholar]
- 30. Stocker R, Yamamoto Y, Mcdonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043. [DOI] [PubMed] [Google Scholar]
- 31. Wu TW, Fung KP, Wu J, Yang CC, Weisel RD. Antioxidation of human lowdensity lipoprotein by unconjugated and conjugated bilirubins. Biochem Pharmacol. 1996;51:859–862. [DOI] [PubMed] [Google Scholar]
- 32. Lapenna D, Ciofani G, Pierdomenico SD, Giamberardino MA, Ucchino S, Davì G. Association of serum bilirubin with oxidant damage of human atherosclerotic plaques and the severity of atherosclerosis. Clin Exp Med. 2018;18:119–124. [DOI] [PubMed] [Google Scholar]
- 33. True AL, Olive M, Boehm M, San H, Westrick RJ, Raghavachari N, Xu X, Lynn EG, Sack MN, Munson PJ, et al. Heme oxygenase‐1 deficiency accelerates formation of arterial thrombosis through oxidative damage to the endothelium, which is rescued by inhaled carbon monoxide. Circ Res. 2007;101:893–901. [DOI] [PubMed] [Google Scholar]
- 34. Ishizaka N, Ishizaka Y, Takahashi E, Yamakado M, Hashimoto H. High serum bilirubin level is inversely associated with the presence of carotid plaque. Stroke. 2001;32:580–583. [DOI] [PubMed] [Google Scholar]
- 35. Dekker D, Dorresteijn MJ, Pijnenburg M, Heemskerk S, Rasing‐Hoogveld A, Burger DM, Wagener FA, Smits P. The bilirubin‐increasing drug atazanavir improves endothelial function in patients with type 2 diabetes mellitus. Arterioscler Thromb Vasc Biol. 2011;31:458–463. [DOI] [PubMed] [Google Scholar]
- 36. Stein JH, Ribaudo HJ, Hodis HN, Brown TT, Tran TT, Yan M, Brodell EL, Kelesidis T, McComsey GA, Dube MP, et al. A prospective, randomized clinical trial of antiretroviral therapies on carotid wall thickness. AIDS. 2015;29:1775–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S9 Figure S1


