Abstract
Background
There are conflicting data regarding the benefit of primary prevention implantable cardioverter‐defibrillators (ICDs) in patients with diabetes mellitus and heart failure (HF) with reduced ejection fraction. We aimed to assess the comparative effectiveness of ICD placement in patients with diabetes mellitus and HF with reduced ejection fraction.
Methods and Results
Data were obtained from the Get With the Guidelines–Health Failure registry, linked with claims from the Centers for Medicare & Medicaid Services. We used a Cox proportional hazards model censored at 5 years with propensity score matching. Of the 17 186 patients with HF with reduced ejection fraction from the Centers for Medicare & Medicaid Services claims database (6540 with diabetes mellitus; 38%), 1677 (646 with diabetes mellitus; 39%) received an ICD during their index HF hospitalization or were prescribed an ICD at discharge. Patients with diabetes mellitus and an ICD (n=646), as compared with those without an ICD (n=1031), were more likely to be younger (74 versus 78 years of age) and have coronary artery disease (68% versus 60%). After propensity matching, ICD use among patients with diabetes mellitus, as compared with those without an ICD, was associated with a reduced risk of all‐cause mortality at 5 years after HF discharge (54% versus 59%; multivariable hazard ratio, 0.73; 95% CI, 0.64–0.82; P<0.0001). Ischemic heart disease did not modify the association between ICD use and all‐cause mortality (P=0.95 for interaction). Similar results were seen in patients without diabetes mellitus.
Conclusions
Primary prevention ICD use among older patients with HF with reduced ejection fraction and diabetes mellitus was associated with a reduced risk of all‐cause mortality. Our analysis supports current guideline recommendations for implantation of primary prevention ICDs among older patients with diabetes mellitus and HF with reduced ejection fraction.
Keywords: arrhythmia, diabetes mellitus, implantable cardioverter‐defibrillator, sudden cardiac death, sudden cardiac death
Subject Categories: Cardiomyopathy, Heart Failure, Treatment, Pacemaker
Nonstandard Abbreviations and Acronyms
- CMS
Centers for Medicare & Medicaid Services
- DCRI
Duke Clinical Research Institute
- EF
ejection fraction
- GWTG‐HF
Get With The Guidelines–Heart Failure
- HFrEF
heart failure with reduced ejection fraction
- ICD
implantable cardioverter‐defibrillator
Clinical Perspective
What Is New?
Using information from the Get With The Guidelines–Heart Failure registry–linked Centers for Medicare & Medicaid Services claims database, we evaluated the effectiveness of implantable cardioverter‐defibrillator (ICD) implantation versus no ICD implantation among patients with diabetes mellitus.
After propensity adjustment, ICD placement was associated with a reduced risk of all‐cause mortality at 5 years.
The presence of ischemic heart disease did not modify our results.
What Are the Clinical Implications?
Our analysis supports current guideline recommendations for implantation of primary prevention ICDs among older patients with diabetes mellitus and heart failure with reduced ejection fraction.
Strategies need to be evaluated to improve the use of ICD in this patient population.
Among patients with heart failure (HF) and a reduced ejection fraction (HFrEF), diabetes mellitus has emerged as one of the most common noncardiovascular comorbidities.1, 2, 3 Patients with diabetes mellitus plus heart failure, versus those without diabetes mellitus, have a higher risk of all‐cause and cardiovascular mortality.4 Among patients with diabetes mellitus, HF events, including HF death, form a significant burden of all‐cause mortality.5 There are different underlying pathophysiologic pathways involving inflammation and fibrosis, and these influence disease progression among patients with diabetes mellitus and HF compared with those without diabetes mellitus.6, 7 Patients with diabetes mellitus appear to have an increased risk of sudden death, which may be modified by the implantation of a primary prevention implantable cardioverter‐defibrillator (ICD).8 HF guidelines recommend use of primary prevention ICDs among eligible HFrEF patients with comorbidities, including diabetes mellitus.9, 10 One analysis suggested that, among those who received an ICD, compared with medical therapy alone, all‐cause mortality was not reduced among patients with diabetes mellitus and HFrEF.8 Furthermore, there is a high burden of competing risk among patients with HF, which may suggest that some patients derive less benefit from primary prevention ICDs.11, 12 A recent study has suggested that, among patients with nonischemic HFrEF, an ICD on top of medical therapy, compared with medical therapy alone, may not significantly reduce the risk of all‐cause mortality, particularly in patients >70 years of age.13 These results suggest that certain populations of patients with HFrEF may not achieve significant benefit from an ICD.
In this study we assessed the real‐world comparative effectiveness of ICD use among HFrEF patients ≥65 years of age with and without diabetes mellitus in the US GWTG‐HF (Get With The Guidelines–Heart Failure) registry.
Methods
Source of Data
Data for this analysis were obtained from the GWTG‐HF registry linked with claims data from the Centers for Medicare & Medicaid Services (CMS). Data used in this analysis will not be publicly available. Details regarding the GWTG‐HF registry have been described previously. Briefly, starting in 2000, the GWTG‐HF has been a voluntary US hospital–based quality improvement initiative.14 All institutions participating in the GWTG‐HF registry are required to comply with local regulatory guidelines and, if required, secure institutional review board approval. IQVIA (formerly Quintiles, Cambridge, MA) serves as the data collection and coordination center for the GWTG‐HF registry. The Duke Clinical Research Institute (DCRI; Durham, NC) is the data analysis center. Patients’ demographics and clinical characteristics, including comorbidities, therapies, and interventions, are collected prospectively through the GWTG‐HF registry. Data related to ICD therapy for each hospitalization included whether an ICD was present at admission, implanted during the index hospitalization, or planned after hospital discharge. Data on contraindications to ICD therapy, and any reason documented by a physician for not implanting or prescribing an ICD, are also collected. CMS data include Part A inpatient claims and the corresponding denominator files from 2005 through 2014. We linked the registry data to CMS claims data following a validated method that uses combinations of indirect identifiers.15
Study Population
For this analysis, the group of interest included patients with and without diabetes mellitus in the GWTG‐HF registry who were ≥65 years of age, linked to CMS data, and from sites without >25% missing medical history (n=130 167 from 432 sites). We excluded patients who died during hospital admission (n=4468), received comfort care only (n=5897), were not discharged home (n=4386), had missing left ventricular ejection fraction (EF) data (n=15 846), had a left ventricular EF >35% (n=61 852), already had ICD at admission (n=5031), or had a contraindication to ICD placement (ie, HF diagnosis not predating the current index admission, recent myocardial infarction within 40 days or coronary revascularization [percutaneous coronary intervention or coronary artery bypass graft within 90 days], class IV HF symptoms, or no reasonable expectation of survival to 1 year; n=5534), and those who received cardiac resynchronization therapy (n=4883). Records of subsequent hospitalizations were also excluded (n=716). Patients who received cardiac resynchronization therapy were excluded due to the challenges in distinguishing benefits from the use of ICDs. After these exclusions, the final study population included 17 186 patients (6683 with diabetes mellitus; 39%) from 410 hospital sites. Patients were considered to have an ICD if they either received the device during the index hospitalization or were prescribed one at discharge. Among the remaining CMS patients, 1677 patients received or were prescribed an ICD (646 had diabetes mellitus; 39%). This group made up the ICD population to whom non‐ICD patients were matched.
End Points
The end point of interest was all‐cause mortality as determined using the Medicare denominator file. Patients with no record of death in the denominator file were considered alive as of December 31, 2014 or the date at which the patients were no longer enrolled in Part A and Part B fee‐for‐service Medicare, whichever came first.
Statistical Analysis
Baseline characteristics, comorbidities, and laboratory data were assessed overall and by treatment group. Differences between groups were tested using a chi‐square or Fisher's exact test. We presented continuous variables as medians with 25th and 75th percentiles for continuous variables, and differences between groups were tested using the Wilcoxon rank sum test.
We used multivariable Cox proportional hazards models to compare the effectiveness of ICD versus no ICD on all‐cause mortality among patients with diabetes mellitus. A similar analysis was conducted among patients without diabetes mellitus. The variables selected are based or previous models derived from the GWTG‐HF registry.16, 17 We used a Cox proportional model with a propensity score matching approach to control for potential selection bias. First, a logistic regression model was used to assign a propensity of treatment selection to each patient based on the distribution of a defined set of covariates, including systolic/diastolic arterial pressure, age, presence of anemia, blood urea nitrogen, renal insufficiency, previous cerebrovascular accident/transient ischemic attack, race, hypertension, chronic obstructive pulmonary disease, atrial fibrillation/flutter, category of HF (preserved EF or depressed EF), renal function, drugs at discharge, composite performance measure (HF all‐or‐none measure), and hospital‐level variables. Cases and controls were matched at a 1:3 ratio, and balance of baseline characteristics before and after matching was checked. A caliper width of 0.25 × (standard deviation of the logit) was used. For a given ICD patient, all non‐ICD patients were considered whose logit differed from the ICD patient's logit by less than the caliper width; among these patients, the non‐ICD patients with the shortest Mahalanobis distance from the ICD patients were selected as the match. Variables used in calculating the Mahalanobis distance were all significant predictors from the propensity model. If there were no non‐ICD patients who could be matched within the caliper width, then the ICD patient was omitted. Next, the Cox proportional hazard regression was run and hazard ratios (HRs) of the two treatment groups reported, along with the corresponding P value and 95% CI. Multivariable adjustment of the covariates in the Cox proportional hazards model was conducted using standard patient‐level clinical covariates: systolic/diastolic arterial pressure and demographic features; category of HF (preserved EF or depressed EF); serum creatinine; drugs at discharge; composite performance measures (HF all‐or‐none measure); and hospital‐level variables. The impact of age on modifying the association between ICD and mortality was assessed in patients with and without diabetes mellitus through an interaction term between ICD and age. To describe the association of ICD use among patients with diabetes mellitus for the outcome of all‐cause mortality on the basis of age, the propensity‐matched population was split into two age groups (65–74 and ≥75 years of age). Among patients with diabetes mellitus, the association between ICD use and all‐cause mortality was assessed among these age categories. Death was censored at the earlier timepoint between 5 years after index HF discharge date and the end‐of‐study date (December 31, 2014).
Colinearity between the predictor variables in the final model was assessed using variance inflation factors. High variance inflation factor values (>5) between variables were examined. If there was evidence of a strong correlation between two covariates, one was dropped from the model. Multiple imputations were used for missing adjustment values (Table S1). Hospital characteristics were not imputed. If a patient had missing medical history, it was assumed that the medical condition did not occur. If variables had a missing rate of >50%, they were not included in the model. Differences were declared to be statistically significant at P<0.05, and all statistical tests were two‐sided. For all analyses, SAS version 9.2 (SAS Institute, Cary, NC) was used.
Results
The unmatched baseline characteristics of patients with diabetes mellitus and HFrEF (n=6540) who have received or were prescribed an ICD (n=646) compared with those without an ICD (n=5894) are presented in Table 1. Patients with an ICD, compared with those without an ICD, were younger (74.0 versus 78.0 years of age) and more likely to be male (66.3% versus 55.7%) and have a reduced burden of some comorbidities, including anemia (11.9% versus 18.3%), previous stroke or transient ischemic attack (14.9% versus 17.4%), depression (7.0% versus 10.3%), peripheral vascular disease (13.6% versus 16.8%), and renal insufficiency (serum creatinine, >2.0 mg/dL; 18.6% versus 23.6%). Patients with an ICD were more likely to have a history of coronary artery disease (67.8% versus 60.4%) and previous myocardial infarction (28.6% versus 24.1%). Patients with an ICD also had a lower left ventricular EF (25.0% versus 27.0%). Patients with an ICD were also more likely to be hospitalized at a teaching center (70.3% versus 58.0%). In the population before matching, among patients with diabetes mellitus who received an ICD, 44% (285 of 646) were prescribed an ICD at discharge. Similar trends are seen for patients without diabetes mellitus (Table 1). After propensity matching, differences between the two groups were balanced (Table 2, Figure 1). The absolute standardized difference on all variables was <10% in patients with and without diabetes mellitus.
Table 1.
Unmatched Baseline Characteristics
| Demographics | With Diabetes Mellitus | Without Diabetes Mellitus | ||||
|---|---|---|---|---|---|---|
| Overall (N=6540) | ICD (N=646) | No ICD (N=5894) | Overall (N=10 646) | ICD (N=1031) | No ICD (N=9615) | |
| Median age, y | 77.0 | 74.0 | 78.0 | 81.0 | 76.0 | 82.0 |
| Male, n (%) | 3712 (56.8) | 428 (66.3) | 3284 (55.7) | 5884 (55.3) | 704 (68.3) | 5180 (53.9) |
| Race, n (%) | ||||||
| Asian | 314 (4.8) | 29 (4.5) | 285 (4.8) | 437 (4.1) | 50 (4.8) | 387 (4.0) |
| Hispanic (any race) | 476 (7.3) | 47 (7.3) | 429 (7.3) | 427 (4.0) | 38 (3.7) | 389 (4.0) |
| Black | 925 (14.1) | 104 (16.1) | 8241 (13.9) | 1170 (11.0) | 109 (10.6) | 1061 (11.0) |
| White | 4713 (72.1) | 459 (71.1) | 4254 (72.2) | 8432 (79.2) | 823 (79.8) | 7609 (79.1) |
| Missing | 1.7 | 7 (1.1) | 105 (1.8) | 180 (1.7) | 11 (1.1) | 169 (1.8) |
| Median ejection fraction, % | 27.0 | 25.0 | 27.0 | 25.0 | 25.0 | 26.0 |
| Baseline medical history, n (%) | ||||||
| Anemia | 1155 (17.7) | 77 (11.9) | 1078 (18.3) | 1408 (13.2) | 90 (8.7) | 1318 (13.7) |
| Coronary disease | 3996 (61.1) | 438 (67.8) | 3558 (60.4) | 5434 (51.0) | 584 (56.6) | 4850 (50.4) |
| COPD or asthma | 1743 (26.7) | 167 (25.9) | 1576 (26.7) | 25 851 (24.2) | 241 (23.4) | 2340 (24.3) |
| CVA/TIA | 1123 (17.2) | 96 (14.9) | 1027 (17.4) | 1469 (13.8) | 126 (12.2) | 1343 (14.0) |
| Depression | 652 (10.0) | 45 (7.0) | 607 (10.3) | 810 (7.6) | 750 (7.8) | 750 (7.8) |
| Previous MI | 1604 (24.5) | 185 (28.6) | 1419 (24.1) | 2121 (19.9) | 289 (28.0) | 1832 (19.1) |
| Peripheral vascular disease | 1078 (16.5) | 88 (13.6) | 990 (16.8) | 1059 (9.9) | 84 (8.1) | 975 (10.1) |
| Prior heart failure | 3925 (60.0) | 395 (61.1) | 3530 (59.9) | 6046 (56.8) | 615 (59.7) | 5431 (56.5) |
| Hyperlipidemia | 3606 (55.1) | 395 (61.1) | 2311 (54.5) | 4317 (40.6) | 490 (47.5) | 3827 (39.8) |
| Hypertension | 5266 (80.5) | 517 (80.0) | 4749 (80.6) | 7307 (68.6) | 696 (67.5) | 6611 (68.8) |
| Renal insufficiency (SCr >2 mg/dL) | 1511 (23.1) | 120 (18.6) | 1391 (23.6) | 1626 (15.3) | 146 (14.2) | 1480 (15.4) |
| Patient laboratory values at admission | ||||||
| Median sodium, mEq/L | 138.0 | 138.0 | 138.0 | 138.0 | 139.0 | 138.0 |
| Median BUN, mg/dL | 27.0 | 25.0 | 28.0 | 24.0 | 23.0 | 24.0 |
| Median serum creatinine, mg/dL | 1.4 | 1.3 | 1.4 | 1.3 | 1.3 | 1.3 |
| Median BNP, pg/mL | 1130.0 | 967.5 | 1150.0 | 1290.0 | 1113.0 | 1306.0 |
| Median hemoglobin, g/dL | 11.9 | 12.5 | 11.9 | 12.4 | 12.9 | 12.3 |
| Medications at discharge, n (%) | ||||||
| ACE inhibitors | 3551 (54.3) | 395 (61.1) | 3156 (53.5) | 6010 (56.5) | 605 (58.7) | 5405 (56.2) |
| ASA | 3608 (55.2) | 375 (58.0) | 3233 (54.9) | 5387 (50.6) | 584 (56.6) | 4803 (50.0) |
| ARB | 1088 (16.6) | 132 (20.4) | 956 (16.2) | 9154 (86.0) | 189 (18.3) | 1342 (14.0) |
| β‐Blocker | 5723 (87.5) | 587 (90.9) | 5136 (87.1) | 9154 (86.0) | 925 (89.7) | 8229 (85.6) |
| Aldosterone antagonist | 1406 (21.5) | 172 (26.6) | 1234 (20.9) | 2251 (21.1) | 262 (25.4) | 1989 (20.7) |
| Hospital characteristics, n (%) | ||||||
| Hospital type (teaching) | 3871 (59.2) | 454 (70.3) | 3417 (58.0) | 6148 (57.7) | 738 (71.6) | 5410 (56.3) |
ACE indicates angiotensin‐converting enzyme; ARB, angiotensin receptor blockers; ASA, acetylsalicylic acid; BNP, brain natriuretic peptide; BUN, blood urea nitrogen; COPD, chronic obstructive pulmonary disorder; CVA, cerebrovascular accident; ICD, implantable cardioverter‐defibrillator; MI, myocardial infarction; SCr, serum creatinine; TIA, transient ischemic attack.
Table 2.
Baseline Characteristics After 1:3 Matching
| Demographics | With Diabetes Mellitus | Without Diabetes Mellitus | ||||
|---|---|---|---|---|---|---|
| Overall (N=2518) | ICD (N=644) | No ICD (N=1874) | Overall (N=3929) | ICD (N=1025) | No ICD (N=2904) | |
| Median age, y | 74.0 | 74.0 | 73.0 | 76.0 | 76.0 | 76.0 |
| Male, n (%) | 1660 (65.9) | 427 (66.3) | 1233 (65.8) | 2620 (66.7) | 698 (68.1) | 1922 (66.2) |
| Race, n (%) | ||||||
| Asian | 128 (5.1) | 29 (4.5) | 99 (5.3) | 173 (4.4) | 49 (4.8) | 124 (4.3) |
| Hispanic (any race) | 184 (7.3) | 47 (7.3) | 137 (7.3) | 173 (4.4) | 38 (3.7) | 135 (4.6) |
| Black | 415 (16.5) | 104 (16.1) | 311 (16.6) | 441 (11.2) | 109 (10.6) | 332 (11.4) |
| White | 1744 (69.3) | 457 (71.0) | 1287 (68.7) | 3076 (78.3) | 818 (79.8) | 2258 (77.8) |
| Missing | 47 (1.9) | 7 (1.1) | 40 (2.1) | 66 (1.7) | 11 (1.1) | 55 (1.9) |
| Median ejection fraction (%) | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 |
| Baseline medical history, n (%) | ||||||
| Anemia | 299 (11.9) | 76 (11.8) | 223 (11.9) | 338 (8.6) | 90 (8.8) | 248 (8.5) |
| Coronary disease | 1668 (66.2) | 436 (67.7) | 1232 (65.7) | 2250 (57.3) | 579 (56.6) | 1671 (57.5) |
| COPD or asthma | 656 (26.1) | 167 (25.9) | 489 (26.1) | 927 (23.6) | 241 (23.5) | 686 (23.6) |
| CVA/TIA | 354 (14.1) | 96 (14.9) | 258 (13.8) | 498 (12.7) | 125 (12.2) | 373 (12.8) |
| Depression | 226 (9.0) | 45 (7.0) | 181 (9.7) | 261 (6.6) | 60 (5.9_ | 201 (6.9) |
| Previous MI | 683 (27.1) | 185 (28.7) | 498 (26.6) | 920 (23.4) | 287 (28.0) | 633 (21.8) |
| Peripheral vascular disease | 389 (15.4) | 88 (13.7) | 301 (16.1) | 373 (9.5) | 83 (8.1) | 290 (10.0) |
| Previous heart failure | 1527 (60.6) | 394 (61.2) | 1133 (60.5) | 2312 (58.8) | 610 (59.5) | 1702 (58.6) |
| Hyperlipidemia | 1432 (56.9)_ | 392 (61.2) | 1038 (55.4) | 1736 (44.2) | 489 (47.7) | 1247 (42.9) |
| Hypertension | 2028 (80.5) | 516 (80.1) | 1512 (80.7) | 2676 (68.1) | 695 (67.8) | 1981 (68.2) |
| Renal insufficiency (SCr >2 mg/dL) | 479 (19.0) | 120 (18.6) | 359 (19.2) | 565 (14.4) | 144 (14.0) | 421 (14.5) |
| Patient laboratory values at admission | ||||||
| Median sodium, mEq/L | 138.0 | 138.0 | 138.0 | 139.0 | 139.0 | 139.0 |
| Median BUN, mg/dL | 26.0 | 25.0 | 26.0 | 23.0 | 23.0 | 22.5 |
| Median serum creatinine, mg/dL | 1.4 | 1.3 | 1.4 | 1.3 | 1.3 | 1.2 |
| Median BNP, pg/mL | 1073.0 | 967.5 | 1093.9 | 1200.0 | 1113.0 | 1230.0 |
| Median hemoglobin, g/dL | 12.2 | 12.5 | 12.0 | 12.7 | 12.9 | 12.6 |
| Medications at discharge, n (%) | ||||||
| ACE inhibitors | 1549 (61.5) | 392 (61.2) | 115 (61.6) | 2421 (61.6) | 600 (58.5) | 1821 (62.7) |
| ASA | 1448 (57.5) | 375 (58.2) | 1073 (57.3) | 2184 (55.6) | 582 (56.8) | 1602 (55.2) |
| ARB | 470 (18.7) | 131 (20.3) | 339 (18.1) | 580 (14.8) | 189 (18.4) | 391 (13.5) |
| β‐Blocker | 2305 (91.5) | 585 (90.8) | 1720 (91.8) | 3515 (89.5) | 919 (89.7) | 2596 (89.4) |
| Aldosterone antagonist | 596 (23.7) | 172 (26.7) | 424 (22.6) | 915 (23.3) | 261 (25.5) | 654 (22.5) |
| Hospital characteristics, n (%) | ||||||
| Hospital type (teaching) | 1736 (68.9) | 453 (70.3) | 1283 (68.5) | 2782 (70.8) | 736 (71.8) | 2046 (70.5) |
ACE indicates angiotensin‐converting enzyme; ARB, angiotensin receptor blockers; ASA, acetylsalicylic acid; BNP, brain natriuretic peptide; BUN, blood urea nitrogen; COPD, chronic obstructive pulmonary disorder; CVA, cerebrovascular accident; ICD, implantable cardioverter‐defibrillator; MI, myocardial infarction; TIA, transient ischemic attack; SCr, serum creatinine.
Figure 1. Standardized differences of patients' characteristics before and after propensity matching.
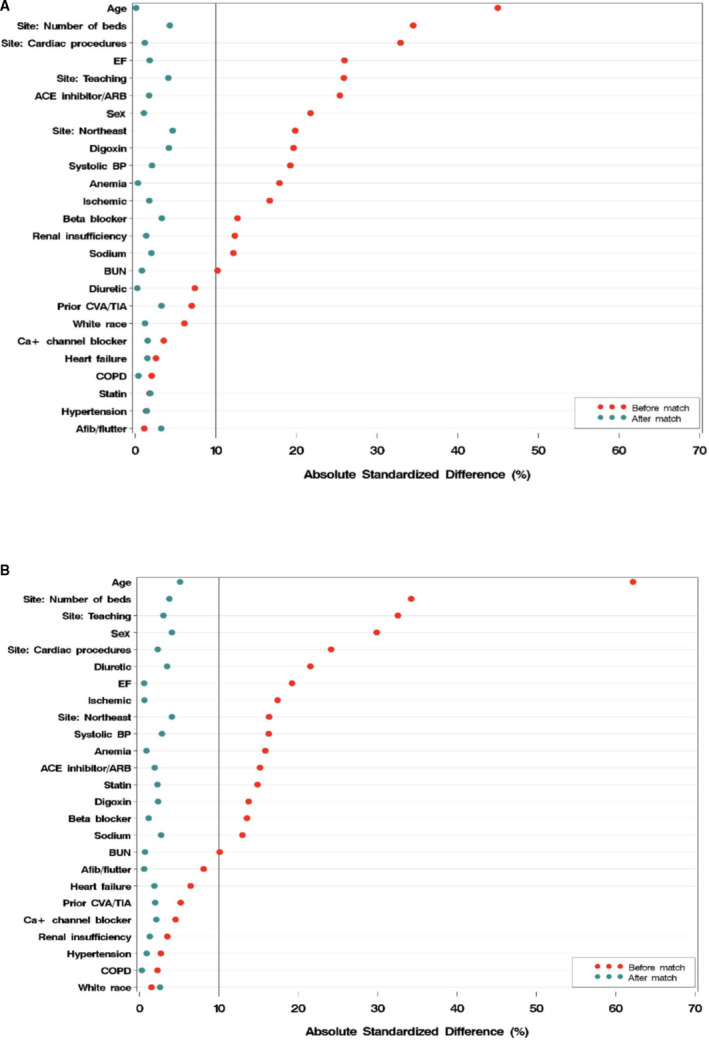
A, with diabetes mellitus; B, without diabetes mellitus. ACE indicates angiotensin‐converting enzyme; ARB, angiotensin receptor blockers; BUN, blood urea nitrogen; COPD, chronic obstructive pulmonary disorder; CVA, cerebrovascular accident; EF, ejection fraction; TIA, transient ischemic attack.
Association of ICD Use and Outcomes
Patients With Diabetes Mellitus
In the propensity‐matching sample, the median follow‐up time in our analysis among patients with diabetes mellitus with an ICD was 1.9 years and (among patients with diabetes mellitus) without an ICD was 1.4 years. The death rate at 5 years among patients with diabetes mellitus and an ICD was 53.9% (67.6% cumulative incidence rate). The death rate at 5 years among patients with diabetes mellitus who did not have an ICD was 59.3% (75.6% cumulative incidence rate). In the propensity‐matched population, diabetes mellitus was associated with a significant increase in the risk of all‐cause mortality (HR, 1.13; 95% CI, 1.05–1.20; P<0.0005). ICD implantation or prescription, compared with those without an ICD, was associated with a reduced risk of 5‐year all‐cause mortality (unadjusted HR, 0.75; 95% CI, 0.67–0.85; P<0.0001; Table 3). After multivariable adjustment the association remained unchanged (adjusted HR, 0.73; 95% CI, 0.64–0.82; P<0.0001; Figure 2A).
Table 3.
Risk of All‐Cause Mortality Associated With ICD Implantation or Prescription
| Patients With Diabetes Mellitus | |||||
|---|---|---|---|---|---|
| Raw Mortality Rate (%) | Cumulative Incidence Rate (%) | Unadjusted Hazard Ratio (95% CI) | Adjusted Hazard Ratio (95% CI) | ||
| ICD | No ICD | ICD | No ICD | ||
| 53.9 | 59.3 | 67.6 | 75.6 | 0.75 (0.67–0.85); P<0.0001 | 0.73 (0.64–0.82); P<0.0001 |
| Patients Without Diabetes Mellitus | |||||
|---|---|---|---|---|---|
| Raw Mortality Rate (%) | Cumulative Incidence Rate (%) | Unadjusted Hazard Ratio (95% CI) | Adjusted Hazard Ratio (95% CI) | ||
| ICD | No ICD | ICD | No ICD | ||
| 47.4 | 58.0 | 57.1 | 70.5 | 0.65 (0.59–0.72); P<0.0001 | 0.65 (0.59–0.72); P<0.0001 |
P=0.14 for adjusted interaction between diabetes mellitus and ICD implantation for all‐cause mortality. ICD indicates implantable cardioverter‐defibrillator.
Figure 2. Kaplan–Meier curves for incidence of mortality.
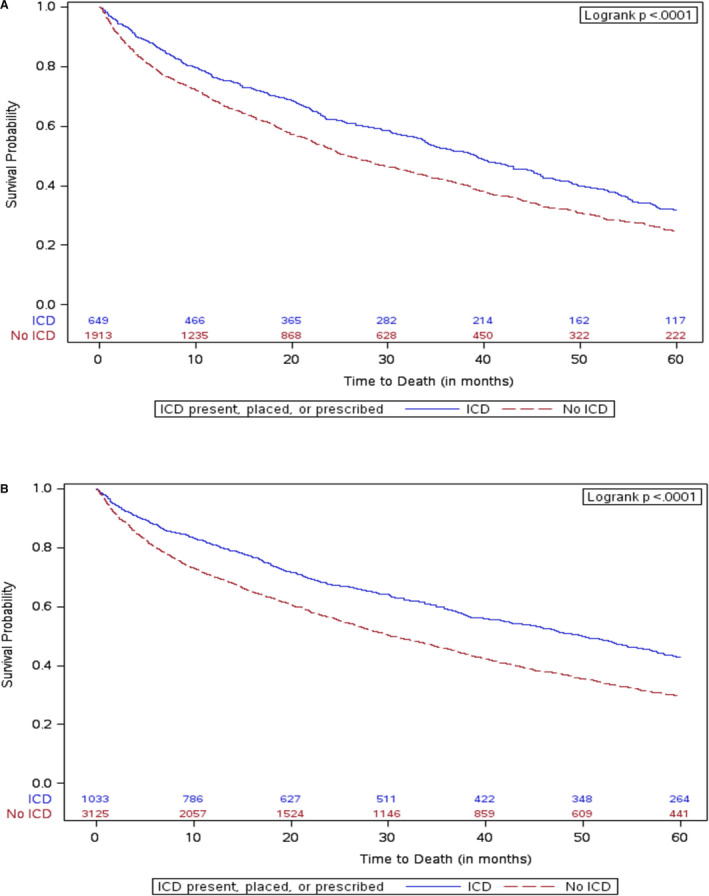
A, with diabetes mellitus; B, without diabetes mellitus. ICD indicates implantable cardioverter‐defibrillator.
Patients Without Diabetes Mellitus
In the propensity‐matching sample, the median follow‐up among patients without diabetes mellitus with an ICD was 4.1 years and without an ICD was 2.4 years. The death rate at 5 years among patients without diabetes mellitus and an ICD was 47.4% (57.1% cumulative incidence rate). The death rate at 5 years among patients without diabetes mellitus who did not have an ICD was 58.0% (70.5% cumulative incidence rate). ICD implantation or prescription among patients without diabetes mellitus was associated with a reduced risk of all‐cause mortality (unadjusted HR, 0.65; 95% CI, 0.59–0.72; P<0.0001; Table 3). After multivariable adjustment, the association remained unchanged (adjusted HR, 0.65; 95% CI, 0.59–0.72; P<0.0001; Figure 2B). An interaction analysis demonstrated that the relationship between an ICD and all‐cause mortality was not modified by the presence of diabetes mellitus (P=0.14).
Sensitivity Analysis
A sensitivity analysis was conducted where patients with an ICD were defined as only those who received an ICD during the index hospitalization. In the sensitivity analysis, 716 of the 1669 ICD patients (42.9%) were excluded for not having an in‐hospital ICD. Overall, use of an ICD, compared with nonuse, was associated with a reduced risk of all‐cause mortality (Table 4).
Table 4.
Sensitivity Analysis With ICD Defined as ICD Implanted During Index Hospitalization
| Patients With Diabetes Mellitus | |||||
|---|---|---|---|---|---|
| Raw Mortality Rate (%) | Cumulative Incidence Rate (%) | Unadjusted Hazard Ratio (95% CI) | Adjusted Hazard Ratio (95% CI) | ||
| ICD | No ICD | ICD | No ICD | ||
| 54.4 | 59.3 | 62.5 | 75.6 | HR, 0.65 (0.56–0.76); P<0.0001 | HR, 0.64 (0.55–0.74); P<0.0001 |
| Patients Without Diabetes Mellitus | |||||
|---|---|---|---|---|---|
| Raw Mortality Rate (%) | Cumulative Incidence Rate (%) | Unadjusted Hazard Ratio (95% CI) | Adjusted Hazard Ratio (95% CI) | ||
| ICD | No ICD | ICD | No ICD | ||
| 43.8 | 58 | 51.2 | 70.5 | HR, 0.53 (0.47–0.60); P<0.0001 | HR, 0.56 (0.49–0.64); P<0.0001 |
P=0.11 for adjusted interaction P value between diabetes mellitus and ICD implantation for all‐cause mortality. ICD indicates implantable cardioverter‐defibrillator; HR, hazard ratio.
Impact of Ischemia and Age on Association Between ICD Use and All‐Cause Mortality
A history of ischemic heart disease did not modify the association between an ICD use and all‐cause mortality in patients with diabetes mellitus (P=0.53) or patients without diabetes mellitus (P=0.97). Furthermore, we did not find the interaction effect between age and ICD to be significant in either group (P=0.95 in patients with diabetes mellitus versus P=0.64 in patients without diabetes mellitus).
In the propensity‐matched population, among patients with diabetes mellitus 65 to 74 years of age (n=1371), those with an ICD, compared with those without an ICD, had a reduced risk of all‐cause mortality (HR, 0.78; 95% CI, 0.65–0.93) (Figure S1). Among patients with diabetes mellitus and ≥75 years of age (n=1147), those with an ICD, versus without an ICD, had a reduced risk of all‐cause mortality (HR, 0.70; 95% CI, 0.59–0.83) (Figure S2). Among those without diabetes mellitus and 65 to 74 years of age (n=1711), those with an ICD, versus those without an ICD, had a reduced risk of all‐cause mortality (HR, 0.59; 95% CI, 0.49–0.71) (Figure S3). Among those without diabetes mellitus and ≥75 years of age (n=2218), patients with an ICD, versus those without an ICD, had a reduced risk of all‐cause mortality (HR, 0.67; 95% CI, 0.60–0.76) (Figure S4).
Discussion
There is limited information on outcomes after primary prevention ICD use in patients with HFrEF and diabetes mellitus. Using data from the GWTG‐HF registry, we assessed the association between primary prevention ICD implantation (defined as receiving an ICD during the index HF hospitalization or prescribed an ICD at discharge) and all‐cause mortality in patients with and without diabetes mellitus. Our analysis has multiple key findings. First, patients receiving a primary prevention ICD versus those without and ICD had a lower rate of all‐cause mortality regardless of history of diabetes mellitus. Second, this relationship was not modified by the presence of ischemic heart disease. Finally, use of ICDs among eligible patients with and without diabetes mellitus remains low, even in this very high‐risk population. Our results reinforce guideline recommendations to consider ICD implantation for primary prevention among indicated patients with HFrEF who have diabetes mellitus.
A previous patient‐level meta‐analysis from the Multicenter Automatic Defibrillator Implantation Trials I and II,18 Defibrillators in Non‐Ischemic Cardiomyopathy Treatment Evaluation trial,19 and SCD‐HeFT (Sudden Cardiac Death in Heart Failure Trial)20 evaluated outcomes after ICD among patients with and without diabetes mellitus.8 ICD use associated with a reduced risk of all‐cause mortality among patients without diabetes mellitus (HR, 0.56; 95% CI, 0.46–0.67), but not among those with diabetes mellitus (HR, 0.88; 95% CI, 0.7–1.12; P=0.015 for interaction between ICD and diabetes mellitus for the outcome of all‐cause mortality). Among patients with diabetes mellitus, ICD use was associated with a reduced risk of arrhythmic death (adjusted subdistribution HR, 0.51; 95% CI, 0.33–0.81; P=0.004); this was also observed in patients without diabetes mellitus (subdistribution HR, 0.27; 95% CI, 0.19–0.40; P=0.0001). Diabetes mellitus modified the interaction between ICD implantation and the risk of sudden death, indicating a reduced magnitude of benefit (P=0.036 for interaction between ICD treatment and diabetes mellitus in relation to arrhythmic death). One proposed explanation for these findings is that patients with diabetes mellitus have an increased risk of competing causes of death, which may not be modified by the presence of an ICD.5 There are limitations to that study finding as those trials were completed over a decade ago. Furthermore, medical management of HF and diabetes mellitus in those trials is not reflective of current standard of care. Our results suggest that eligible patients with diabetes mellitus who have an indication for a primary prevention ICD should receive this therapy.
In the DANISH (Danish Study to Assess the Efficacy of ICDs in Patients with Non‐Ischemic Systolic Heart Failure on Mortality) trial, prophylactic ICD implantation in patients with nonischemic HFrEF was not associated with a reduction in the risk of all‐cause mortality compared with usual clinical care, although there appeared to be an interaction by age and the majority of patients had a cardiac resynchronization therapy pacemaker device present at enrollment.13 Our results suggest that the presence or absence of a previous history of ischemic heart disease did not modify the relationship between ICD and all‐cause mortality in patients with or without diabetes mellitus. Furthermore, our findings suggest that age does not modify the relationship between ICD prescription/use and mortality. These findings are nonrandomized and derived from a population‐based cohort. The selection bias for individuals being prescribed or receiving an ICD compared with those those not selected for an ICD may contribute to the differences in our results compared with the clinical trials. However, more research will be needed to identify the populations of patients who could derive maximum benefit from ICD use to understand the reasons why our real‐world data differ from those of the clinical trials.
Another important finding in our analysis is the overall low use of ICDs in patients with and without diabetes mellitus who are at very high risk of death. Only 11% of eligible patients with diabetes mellitus received an ICD. Similar findings were obtained in a previous study of ICD use from the GWTG‐HF registry and other population‐based analyses.21 Several explanations may help to explain the low use of ICDs seen in our study. There are well‐documented gaps, variations, and disparities in the use of guideline‐directed medication and device therapies in eligible patients. In addition, our study population focused on in patients hospitalized with HF. The optimal timing of ICD use for such patients is not well established. Clinicians may potentially have opted to consider ICD use at another date after further duration or titration of medical therapy. Some patients may have had contraindications or other medical exceptions to ICD placement that were present but not documented.
Further studies are needed to increase the use of ICDs among eligible patients with HFrEF and diabetes mellitus given the high risk of sudden death among these patients. Although the causes of death could not be ascertained from our data set, the findings suggest that mortality rates are still modifiable in these patients through provision of guideline‐based care. The Kaplan–Meier curves in our analysis for patients with and without diabetes mellitus diverge for the first 12 months, but then stay nearly parallel. The risk of sudden death among patients postacute HF hospitalization appears to be high shortly after hospital discharge.22 ICD placement, especially those devices implanted during hospitalization or shortly thereafter, may have a greater magnitude of benefit early on.
The role of ICD implantation among patients with diabetes mellitus and HFrEF should also be considered in the context of emerging antihyperglycemic therapies. Trials of sodium glucose cotransporter‐2 inhibitors have demonstrated a reduction in risk of HF hospitalization among patients with type 2 diabetes mellitus who have cardiovascular disease or are at high risk of cardiovascular disease.23, 24, 25 The benefits of these therapies have been demonstrated in post‐hoc analyses of patients with HFrEF; however, many of these trials had a very low percentage of individuals with any form of HF.26 Dedicated studies are being conducted among patients with and those without diabetes mellitus who have HFrEF or HF with preserved EF. Among ambulatory patients with HFrEF who were enrolled in clinical trials, the rates of sudden death have declined substantially over time22; this was primarily thought to be the result of an increased use of evidence‐based medications for this cause of death. Similarly, as anti‐hyperglycemic medical therapies increase in use, the role of ICD use among patients with diabetes mellitus should be further evaluated.23
Limitations
Our analysis is subject to the limitation that it was an observational study and that treatments were not assigned randomly. Propensity matching and subsequent multivariable adjustment may not have completely accounted for residual confounding. Our analysis was limited to CMS patients hospitalized with HF within the GWTG‐HF registry. As a result, the findings may not be generalizable to a younger, healthier patient population or in those without medical insurance. The patients who did not receive an ICD may have had other considerations that could have precluded them from being eligible for ICD placement. For instance, they may have appeared too frail or too clinically unwell to have been prescribed an ICD during hospitalization or at discharge. Among patients with or without an ICD prescription, the subsequent implantation rate data were not available for the present study. The use of propensity matching to enable a comparison between the ICD and non‐ICD group may have also eliminated patients who are too dissimilar to match. Data on the duration of diabetes mellitus and glycemic control and cause‐specific mortality were also not available. Our analysis primarily evaluated all‐cause mortality and not cerebrovascular death or sudden death, which may have impacted our ability to see a relationship between ICD placement and outcomes. Finally, measures of frailty, such as grip strength and other functional measures (eg, 6‐minute walk test), were not available in our data.
Conclusions
Among older patients with diabetes mellitus who were admitted with HF, those with an EF who were implanted with a primary prevention ICD (or were prescribed an ICD upon discharge) had a lower risk of all‐cause mortality compared with those without an ICD. Our analysis was nonrandomized and observational, so there may be have been unmeasured confounders that influenced the results. However, our analysis has provided further evidence for guideline recommendations for placement of primary prevention ICDs in eligible patients with diabetes mellitus who have HF and reduced EF. Further studies are needed to identify strategies to increase the use of primary prevention ICDs among patients with diabetes mellitus.
Sources of Funding
The Get With The Guidelines®–Heart Failure (GWTG‐HF) program is provided by the American Heart Association. GWTG‐HF is sponsored, in part, by Novartis, Boehringer Ingelheim Lilly, Novo Nordisk, Sanofi, AstraZeneca and Bayer.
Disclosures
A.S. reports Junior 1 award support from the Fonds de Researche Sante Quebec; a grant from the AHA Strategically Focused Research Network―Heart Failure (16SFRN30180010); an Alberta Innovates Health Solution Clinician Scientist fellowship; a Young Investigator Award from the European Society of Cardiology; and received research support from Roche Diagnostics, Takeda and BMS‐Pfizer, and a Bayer Vascular award from the Canadian Cardiovascular Society. G.C.F. reports consulting work with Abbott, Amgen, Bayer, Janssen, Novartis, and Medtronic. JG reports grants from Boehringer Ingelheim and Sanofi/Lexicon, and consulting work from Boehringer Ingelheim/Lilly Alliance, AstraZeneca, and NovoNordisk. GMF reports research grants from NHLBI, American Heart Association, Amgen, Bayer, Merck, Cytokinetics, Myokardia, and Roche Diagnostics; he has acted as a consultant to Novartis, Amgen, BMS, Cytokinetics, Medtronic, Cardionomic, V‐Wave, Myokardia, Innolife, EBR Systems, Arena, Abbott, Roche Diagnostics, Alnylam, LivaNova, Rocket Pharma, Reprieve, and SC Pharma. The remaining authors have no disclosures to report.
Supporting information
Table S1 Figures S1–S4
(J Am Heart Assoc. 2020;9:e012405 DOI: 10.1161/JAHA.119.012405.)
For Sources of Funding and Disclosures, see page 11.
References
- 1. Sharma A, Zhao X, Hammill BG, Hernandez AF, Fonarow GC, Felker GM, Yancy CW, Heidenreich PA, Ezekowitz JA, DeVore AD. Trends in noncardiovascular comorbidities among patients hospitalized for heart failure. Circ Heart Fail. 2018;e004646. [DOI] [PubMed] [Google Scholar]
- 2. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corra U, Cosyns B, Deaton C, et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2016;2315–2381.27222591 [Google Scholar]
- 3. Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Chushman M, Dellin FN, Deo R, et al. Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation. 2018;E67–E492. [DOI] [PubMed] [Google Scholar]
- 4. Cavender MA, Steg PG, Smith SC, Eagle K, Ohman EM, Goto S, Kuder J, Im K, Wilson PW, Bhatt DL. Impact of diabetes mellitus on hospitalization for heart failure, cardiovascular events, and death: outcomes at 4 years from the Reduction of Atherothrombosis for Continued Health (REACH) Registry. Circulation. 2015;923–931. [DOI] [PubMed] [Google Scholar]
- 5. Sharma A, Green JB, Dunning A, Lokhnygina Y, Al‐Khatib SM, Lopes RD, Buse RD, Lachin JM, Van de Werf F, Armstrong PW, et al. Causes of death in a contemporary cohort of patients with type 2 diabetes and atherosclerotic cardiovascular disease: insights from the TECOS trial. Diabetes Care. 2017;1763–1770. [DOI] [PubMed] [Google Scholar]
- 6. Sharma A, Demissei BG, Tromp J, Hillege HL, Cleland JG, O'Connor CM, Metra M, Ponikowski P, Teerlink JR, Davidson BA, et al. A network analysis to compare biomarker profiles in patients with and without diabetes mellitus in acute heart failure. Eur J Heart Fail. 2017;1310–1320. [DOI] [PubMed] [Google Scholar]
- 7. Sharma A, Cooper LB, Fiuzat M, Mentz RJ, Ferreira JP, Butler J, Fitchett D, Moses AC, O'Connor C, Zannad F. Antihyperglycemic therapies to treat patients with heart failure and diabetes mellitus. JACC Heart Fail. 2018;813–822. [DOI] [PubMed] [Google Scholar]
- 8. Sharma A, Al‐Khatib SM, Ezekowitz JA, Cooper LB, Fordyce CB, Felker GM, Bardy GH, Poole JE, Thomas BJ, Buxton AE, et al. Implantable cardioverter‐defibrillators in heart failure patients with reduced ejection fraction and diabetes. Eur J Heart Fail. 2018;1031–1038. [DOI] [PubMed] [Google Scholar]
- 9. Al‐Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callands DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarrow GC, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: executive summary. Circulation. 2018;e210–e271. [DOI] [PubMed] [Google Scholar]
- 10. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;1810–1852. [DOI] [PubMed] [Google Scholar]
- 11. Bilchick KC, Wang Y, Cheng A, Curtis JP, Dharmarajan K, Stukenborg GJ, Shadman R, Anand I, Lund LH, Dahlstrom U, et al. Seattle heart failure and proportional risk models predict benefit from implantable cardioverter‐defibrillators. J Am Coll Cardiol. 2017;2606–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Elming MB, Nielsen JC, Haarbo J, Videbaek L, Korup E, Signorovitch J, Olesen LL, Hidebrandt P, Steffensen FH, Bruun NE, et al. Age and outcomes of primary prevention implantable cardioverter‐defibrillators in patients with nonischemic systolic heart failure. Circulation. 2017;1772–1780. [DOI] [PubMed] [Google Scholar]
- 13. Køber L, Thune JJ, Nielsen JC, Haarbo J, Vidbaek L, Korup E, Kensen G, Hildedrandt P, Steffensen FH, Bruun NE, et al. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med. 2016;1221–1230. [DOI] [PubMed] [Google Scholar]
- 14. Fonarow GC, Abraham WT, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, O'Connor CM, Pieper K, Sun JL, Yancy C, et al. Association between performance measures and clinical outcomes for patients hospitalized with heart failure. J Am Med Assoc. 2007;61–70. [DOI] [PubMed] [Google Scholar]
- 15. Hammill BG, Hernandez AF, Peterson ED, Fonarow GC, Schulman KA, Curtis LH. Linking inpatient clinical registry data to Medicare claims data using indirect identifiers. Am Heart J. 2009;995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Peterson PN, Rumsfeld JS, Liang L, Albert MM, Hernandez AF, Peterson ED, Fonarow GC, Masoudi FA. A validated risk score for in‐hospital mortality in patients with heart failure from the American Heart Association get with the guidelines program. Circ Cardiovasc Qual Outcomes. 2010;25–32. [DOI] [PubMed] [Google Scholar]
- 17. Eapen ZJ, Liang L, Fonarow GC, Heidenreich PA, Curtis LH, Peterson ED, Hernandez AF. Validated, electronic health record deployable prediction models for assessing patient risk of 30‐day rehospitalization and mortality in older heart failure patients. JACC Heart Fail. 2013;245–251. [DOI] [PubMed] [Google Scholar]
- 18. Hohnloser SH, Kuck KH, Dorian P, Roberts RS, Hampton JR, Hatala R, Fain E, Gent M, Connolly SJ. Prophylactic use of an implantable cardioverter‐defibrillator after acute myocardial infarction. N Engl J Med. 2004;2481–2488. [DOI] [PubMed] [Google Scholar]
- 19. Steinbeck G, Andresen D, Seidl K, Brachmann J, Hoffman E, Wojciechowski D, Kornacewixz‐Jach Z, Sredniawa B, Lupkovics G, Hofgartner F, et al. Defibrillator implantation early after myocardial infarction. N Engl J Med. 2009;1427–1436. [DOI] [PubMed] [Google Scholar]
- 20. Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, et al. Amiodarone or an implantable cardioverter–defibrillator for congestive heart failure. N Engl J Med. 2005;225–237. [DOI] [PubMed] [Google Scholar]
- 21. Zeitler EP, Hellkamp AS, Schulte PJ, Fonarow GC, Hernandez AF, Peterson ED, Sanders GD, Ynacy CW, Alk‐Khatib SM. Comparative effectiveness of implantable cardioverter defibrillators for primary prevention in women. Circ Heart Fail. 2016;e002630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vaduganathan M, Patel RB, Mentz RJ, Subacius H, Chatterjee NA, Greene SJ, Ambrosy AP, Maggioni AP, Udelson JE, Swedberg K, et al. Sudden death after hospitalization for heart failure with reduced ejection fraction (from the EVEREST trial). Am J Cardiol. 2018;255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mosleh W, Sharma A, Sidhu MS, Page B, Sharma UC, Farkouh ME. The role of SGLT‐2 inhibitors as part of optimal medical therapy in improving cardiovascular outcomes in patients with diabetes and coronary artery disease. Cardiovasc Drugs Ther. 2017;311–318. [DOI] [PubMed] [Google Scholar]
- 24. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;2117–2128. [DOI] [PubMed] [Google Scholar]
- 25. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;644–657. [DOI] [PubMed] [Google Scholar]
- 26. Fitchett D, Zinman B, Wanner C, Lachin JM, Hantel S, Salsali A, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA‐REG OUTCOME® trial. Eur Heart J. 2016;1526–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Figures S1–S4


