Abstract
Background
Many patients with heart failure (HF) experience changes in left ventricular ejection fraction (LVEF) during follow‐up. We sought to evaluate the predictors and outcomes of different HF phenotypes according to longitudinal changes in EF.
Methods and Results
A total of 2104 patients with acute HF underwent echocardiography at baseline and follow‐up. Global longitudinal strain was measured at index admission. HF phenotypes were defined as persistent HF with reduced EF (persistent HFrEF, LVEF ≤40% at baseline and follow‐up), heart failure with improved ejection fraction (LVEF≤40% at baseline and improved to >40% at follow‐up), heart failure with declined ejection fraction (LVEF>40% at baseline and declined to ≤40% at follow up), and persistent HF with preserved EF (persistent HFpEF, LVEF>40% at baseline and follow‐up). Overall, 1130 patients had HFrEF at baseline; during follow‐up, 54.2% and 46.8% had persistent HFrEF and heart failure with improved ejection fraction, respectively. Among 975 patients with HFpEF at baseline, 89.5% and 10.5% had persistent HFpEF and heart failure with declined ejection fraction at follow‐up, respectively. The 5‐year all‐cause mortality rates were 43.1%, 33.1%, 24%, and 17% for heart failure with declined ejection fraction, persistent HFrEF, persistent HFpEF, and heart failure with improved ejection fraction, respectively (global log‐rank P<0.001). In multivariable analyses, each 1% increase in global longitudinal strain (greater contractility) was associated with 10% increased odds for heart failure with improved ejection fraction among patients with HFrEF at baseline and 7% reduced odds for heart failure with declined ejection fraction among patients with HFpEF at baseline.
Conclusions
LVEF changed during follow‐up. Each HF phenotype according to longitudinal LVEF changes has a distinct prognosis. Global longitudinal strain can be used to predict the HF phenotype.
REGISTRATION: URL: https://www.clinicaltrials.gov; Unique identifier: NCT03513653.
Keywords: ejection fraction change, heart failure, HFdEF, HFiEF, myocardial strain
Subject Categories: Heart Failure
Nonstandard Abbreviations and Acronyms
- AHF
acute heart failure
- GLS
global longitudinal strain
- HFdEF
heart failure with declined ejection fraction
- HFiEF
heart failure with improved ejection fraction
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- LVEF
left ventricular ejection fraction
Clinical Perspective.
What Is New?
We reclassified 2105 patients with heart failure according to the longitudinal changes in left ventricular ejection fraction during follow‐up.
Nearly half of patients with heart failure with reduced ejection fraction at the index admission developed heart failure with improved ejection fraction, and these patients had the best prognosis; whereas 11% of patients with heart failure with preserved ejection fraction at the index admission developed heart failure with declined ejection fraction, and these patients had the worst prognosis.
Global longitudinal strain was a significant predictor of both heart failure with improved ejection fraction and heart failure with declined ejection fraction, independent of left ventricular ejection fraction, and each heart failure phenotype according to longitudinal left ventricular ejection fraction changes has a distinct prognosis, and global longitudinal strain can be used to predict the HF phenotype.
What Are The Clinical Implications?
Because heart failure phenotypes according to the longitudinal left ventricular ejection fraction change have a distinct prognosis, repeated echocardiographic evaluations and re‐phenotyping should be considered in all patients with heart failure.
Currently, heart failure (HF) is categorized based on left ventricular ejection fraction (LVEF). Patients with EF <40 and ≥40% are defined as HF with reduced EF (HFrEF) and preserved EF (HFpEF), respectively.1 LVEF is not static, but changes over time. For example, many patients with HFrEF benefit from pharmacological and/or nonpharmacological therapies, and their LVEF improves during follow‐up.2, 3 Similarly, some patients with HFpEF experience a decline in LVEF.4
A few studies, including ours,3, 5, 6 showed that patients with improved EF (HFiEF) had better prognosis than those with persistent HFrEF and that those with declined EF (HFdEF) had worse outcomes than those with persistent HFpEF; patients with HFiEF as well as those with HFdEF have distinct prognosis and differential responses to medical therapy. Therefore, it is of clinical interest to identify predictors of longitudinal changes in LVEF in patients with HF.
Myocardial strain is based on the speckle‐tracking method and measures the contractility of the myocardium directly. It assesses reliably the systolic function,7 and predicts the outcomes of HF independently of LVEF.8, 9 Because the change in LVEF during follow‐up may be determined by the contractility of the myocardium, myocardial strain may have a fundamental value to predict the LVEF change.
We hypothesized that myocardial strain may predict the changes in LVEF in HF. The aims of this study were as follows: first, we evaluated the longitudinal changes in LVEF; secondly, we identified predictors of LVEF change, including the role of myocardial strain; and thirdly, we compared the outcomes of the HF phenotypes according to their LVEF change in a large cohort of patients with acute HF.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Patients
The STRATS‐AHF (Strain for Risk Assessment and Therapeutic Strategies in patients with Acute Heart Failure) registry (ClinicalTrials.gov/, NCT: 03513653) included 4312 patients who were hospitalized for acute HF in 3 tertiary university hospitals between January 2009 and December 2016. Detailed information and primary outcomes have been reported elsewhere.8 In line with the aim of the study, we included only patients who underwent echocardiographic examination at the index admission and at least 1 additional time during follow‐up. The vital statuses of all patients were collected from the National Death Records.
The study protocol was approved by the ethics committee at each hospital. Written informed consent was waived by the institutional review board. The study complied with the Declaration of Helsinki.
Echocardiography and Strain Analysis
Patients underwent echocardiography during the index admission (median time interval between admission and echocardiography: 1 day; interquartile range [IQR]: 0–2 days) and at least 1 additional time during follow‐up. In cases with multiple echocardiographic examinations, we took the last examination (median time interval between admission and follow‐up echocardiography: 406 days; IQR: 270–676 days). Standard techniques were used in accordance with the American Society of Echocardiography guidelines.10 LVEF was calculated by Simpson's biplane method. Detailed measurements of global longitudinal strain (GLS) have been provided elsewhere.8 In brief, echocardiographic images were obtained and analyzed using Image‐Arena (Tomtec Imaging System, Munich, Germany), a vendor‐independent program, at the strain core laboratory.11 Peak GLS was computed automatically. All strain measurements were performed by strain specialists blinded to other patient data.
Study Variables and Definitions
Based on echocardiographic findings at the index admission for acute HF, patients were categorized as having either HFrEF (LVEF ≤40%), or HFpEF (LVEF >40%).12 Among patients with HFrEF at the index admission, those whose LVEF improved to >40% or remained ≤40% were defined as having HFiEF or persistent HFrEF, respectively. Similarly, among patients with HFpEF at the index admission, HFdEF and persistent HFpEF were defined as those who had ≤40% and LVEF >40% at follow‐up, respectively.
Because GLS is a negative value, we used the absolute value |x| for a simpler interpretation. Therefore, in this study a higher positive value for absolute GLS is regarded as more myocardial contractility. Because different centers measured the levels of different types of natriuretic peptides (ie, B‐type natriuretic peptide versus N‐terminal‐pro B‐type natriuretic peptide), we transformed the natriuretic peptide level into natriuretic peptide percentiles to compare the natriuretic peptide levels throughout the centers.
The primary outcome was the changes in HF phenotypes during follow‐up. The secondary outcome included the 5‐year all‐cause mortality and hospitalization for HF according to the HF phenotypes.
Statistical Analysis
Data were presented as numbers and frequencies for categorical variables and as means±SD or medians with IQRs for continuous variables. For comparisons among groups, the χ2 test (or Fisher exact test when any expected count was <5 for a 2×2 table) was used for categorical variables, and the unpaired Student t test, 1‐way ANOVA, Mann Whitney U test, or Kruskal‐Wallis test was used for continuous variables.
Pearson's correlation was used to calculate the association between LVEF and GLS. The chronological trend of the outcomes was expressed as Kaplan‐Meier estimates and compared according to the HF phenotypes. A multivariable binary logistic regression was used to determine the independent predictors of HFiEF and HFdEF. We included all variables found to be statistically significant (P<0.05) in the univariate analysis as covariates in the multivariable analysis, excluding those with >10% of data that were missing or those having multicollinearity with other variables.
A 2‐sided P<0.05 was considered to indicate a statistically significant difference. Statistical tests were performed using SPSS, V.23 (IBM, Armonk, NY) and R programming version 3.3.0 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline Characteristics of the Study Population
Of 4312 patients who were included in the STRATS‐AHF Registry, 2104 patients had at least 2 echocardiography examinations. Overall, 1130 patients had HFrEF at the index admission; among these, 613 (54.2%) had persistent HFrEF and 517 (45.8%) had HFiEF. Likewise, of 974 patients with HFpEF at the index admission, 872 (89.5%) and 102 (10.5%) developed persistent HFpEF and HFdEF, respectively (Figure 1).
Figure 1. Study population.
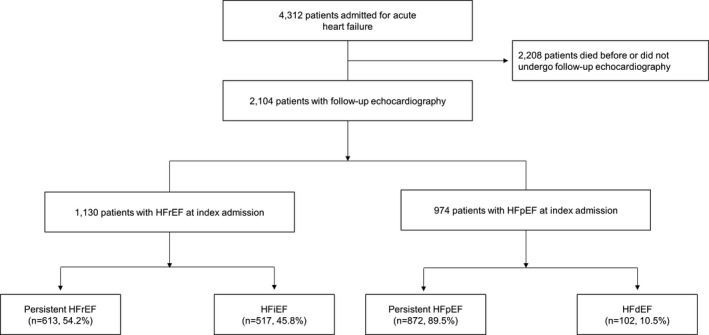
HFrEF, heart failure with reduced ejection fraction; HFiEF, heart failure with improved ejection fraction; HFpEF, heart failure with preserved ejection fraction; and HFdEF, heart failure with declined ejection fraction.
The baseline characteristics differed significantly between the groups. Among patients with HFrEF, patients with HFiEF were younger (67.0±13.7 years versus 63.3±14.2 years, P<0.001), less likely to be male (68.5% versus 58.6%, P=0.001), and less likely to have ischemic heart disease (34.6% versus 28.0%, P=0.019) than those with HFiEF. Natriuretic peptide levels did not differ between the groups. Regarding the echocardiographic parameters, patients with HFiEF had higher LVEF (26.7±7.0% versus 28.8±7.0%, P<0.001) and higher GLS (7.9±3.2% versus 8.7±3.4%, P<0.001) than those with persistent HFrEF. In addition, they received more β‐blockers at discharge from the index admission (68.4% versus 75.7%, P=0.006).
Regarding patients with HFpEF at the index admission, HFdEF had a higher natriuretic peptide level (40.3±26.9 percentiles versus 59.8±26.7 percentiles, P<0.001), lower LVEF (54.8±8.6% versus 49.0±6.8%, P<0.001), and lower GLS (14.1±4.6% versus 11.8±4.5%, P<0.001) than persistent HFpEF (Table 1).
Table 1.
Baseline Characteristics of the Patients at the Index Admission
| Persistent HFrEF (n=613) | HFiEF (n=517) | P Value | Persistent HFpEF (n=872) | HFdEF (n=102) | P Value | |
|---|---|---|---|---|---|---|
| Age, y | 67.0±13.7 | 63.3±14.2 | <0.001 | 71.1±12.6 | 73.2±12.6 | 0.110 |
| Men, % | 68.5% | 58.6% | 0.001 | 42.5% | 50.0% | 0.170 |
| Body mass index, kg/m | 23.4±4.1 | 24.0±4.9 | 0.033 | 24.2±4.1 | 22.9±3.3 | 0.001 |
| Past medical history | ||||||
| Hypertension | 52.2% | 51.6% | 0.852 | 60.4% | 65.7% | 0.297 |
| Diabetes mellitus | 36.7% | 32.5% | 0.139 | 32.5% | 34.3% | 0.717 |
| Ischemic heart disease | 34.6% | 28.0% | 0.019 | 30.5% | 37.3% | 0.162 |
| Atrial fibrillation | 23.5% | 26.9% | 0.192 | 35.5% | 34.3% | 0.819 |
| NYHA functional class | 0.638 | 0.701 | ||||
| I/II | 8.8% | 8.7% | 6.8% | 5.4% | ||
| III | 47.3% | 50.2% | 48.4% | 52.7% | ||
| IV | 43.9% | 41.1% | 44.8% | 41.9% | ||
| Physical examination | ||||||
| Systolic BP, mm Hg | 124.2±23.4 | 130.1±25.3 | <0.001 | 131.3±27.3 | 126.5±32.1 | 0.101 |
| Diastolic BP, mm Hg | 73.6±14.8 | 78.2±17.8 | <0.001 | 73.9±16.5 | 73.0±18.0 | 0.616 |
| Heart rate, beats/min | 89.3±23.3 | 96.6±24.9 | <0.001 | 83.7±24.6 | 83.8±25.3 | 0.987 |
| Laboratory findings | ||||||
| Hemoglobin, mg/dL | 12.7±2.1 | 12.8±2.5 | 0.856 | 12.0±2.3 | 11.6±2.3 | 0.109 |
| Sodium, mmol/L | 136.6±5.2 | 137.5±4.5 | 0.002 | 136.8±5.0 | 136.8±5.5 | 0.982 |
| Potassium, mmol/L | 4.2±0.7 | 4.2±0.6 | 0.762 | 4.2±0.7 | 4.1±0.8 | 0.339 |
| BUN, mg/dL | 25.5±16.3 | 24.7±16.3 | 0.406 | 24.5±15.3 | 27.1±15.0 | 0.107 |
| Creatinine, mg/dL | 1.5±1.9 | 1.6±1.9 | 0.521 | 1.5±1.7 | 1.8±2.0 | 0.093 |
| CKD, % | 43.3% | 37.3% | 0.052 | 44.0% | 57.7% | 0.011 |
| BNP, pg/mL | 1579±1296 | 1730±1976 | 0.496 | 976±1218 | 2450±3015 | 0.062 |
| NT‐proBNP, pg/mL | 9259±12819 | 8543±12328 | 0.452 | 6113±8356 | 11524±11207 | <0.001 |
| NP 100 percentiles | 53.0±26.4 | 50.8±26.8 | 0.202 | 40.3±26.9 | 59.8±26.7 | <0.001 |
| Echocardiographic parameters | ||||||
| LVEDD, mm | 60.7±9.1 | 57.0±8.1 | <0.001 | 49.2±7.2 | 51.7±7.6 | 0.001 |
| LA diameter, mm | 45.8±8.9 | 45.5±8.7 | 0.632 | 46.1±10.0 | 42.9±10.7 | 0.003 |
| LA volume index, mL/m2 | 61.9±38.9 | 55.1±24.0 | 0.001 | 64.4±48.4 | 46.1±10.0 | 0.043 |
| E‐wave, m/s | 0.9±0.5 | 0.9±0.3 | 0.154 | 0.9±0.4 | 0.8±0.4 | 0.095 |
| A‐wave, m/s | 1.0±4.6 | 1.7±12.6 | 0.348 | 0.8±0.3 | 0.8±0.3 | 0.367 |
| DT, ms | 158.8±61.8 | 159.3±66.4 | 0.906 | 206.6±102.4 | 202.7±91.9 | 0.740 |
| E/e’ | 21.0±13.5 | 19.8±9.4 | 0.116 | 17.0±9.8 | 16.1±8.1 | 0.419 |
| LVEF, % | 26.7±7.0 | 28.8±7.0 | <0.001 | 54.8±8.6 | 49.0±6.8 | <0.001 |
| GLS (%) | 7.9±3.2 | 8.7±3.4 | <0.001 | 14.1±4.6 | 11.8±4.5 | <0.001 |
| Medication at discharge | ||||||
| ACE inhibitor or ARB | 84.3% | 82.7% | 0.464 | 66.9% | 78.0% | 0.024 |
| β‐ blocker | 68.4% | 75.7% | 0.006 | 63.3% | 63.0% | 0.954 |
| MRA | 58.6% | 54.4% | 0.157 | 42.8% | 40.0% | 0.591 |
ACE indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin‐receptor‐blocker; BP, blood pressure; BUN, blood urea nitrogen; BNP, B‐type natriuretic peptide; CKD, chronic kidney disease; DT, deceleration time; E/e’, mitral E wave to mitral tissue Doppler e’ wave; GLS, global longitudinal strain; HF, heart failure; HFdEF, heart failure with declined ejection fraction; HFiEF, heart failure with improved ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LA, left atrium; LVEDD, left ventricular end‐diastolic dimension; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; NP, natriuretic peptide; and NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
Changes in LVEF During Follow‐Up
In all patients with HFrEF at the index admission, LVEF increased from 27.7±7.1% at the index admission to 39.9±14.0% at the follow‐up echocardiography (paired t test, P<0.001). In HFiEF, the LVEF improved from 28.8±7.0% to 52.8±7.8%. In 60.5% of the patients, LVEF improved to >50% regardless of the severity of baseline LVEF impairment (in patients with LVEF ≤20% at baseline, LVEF improved from 17.1±2.5% to 54.0±7.3% at follow‐up; 20%<LVEF≤30%, 25.8±6.3% to 52.8±7.6%; 30%<LVEF≤40%, 35.3±2.8% to 52.4±8.2%). By contrast, in persistent HFrEF, the LVEF changed only marginally from 26.7±7.0% to 29.0±7.2%. The change in LVEF according to baseline LVEF impairment was marginal as well (in patients with LVEF ≤20% at baseline: LVEF improved from 17.1±2.8% to 24.7±7.7% at follow‐up; 20%<LVEF≤30%, 25.6±2.9% to 28.6±6.3%; 30%<LVEF≤40%, 34.6±2.8% to 32.4±6.2%) (Figure 2A). The change in LVEF was similar in patients with and without ischemic heart disease, and in those with sinus rhythm and atrial fibrillation.
Figure 2. Longitudinal changes in ejection fraction according to heart failure phenotypes and baseline ejection fraction.
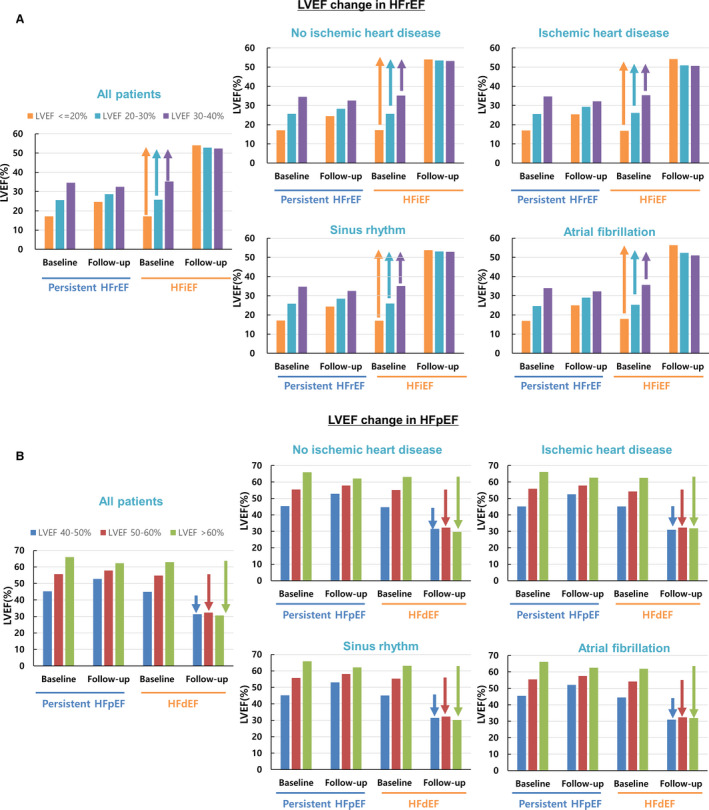
A, In patients with heart failure with improved ejection fraction (HFiEF), the left ventricular ejection fraction (LVEF) improved to >50% in mildly (LVEF: 30–40%), moderately (LVEF: 20–30%), and severely (LVEF ≤20%) depressed LVEF; whereas the change in LVEF was minimal among patients with persistent heart failure with reduced ejection fraction (HFrEF). B, In patients with heart failure with declined ejection fraction (HFdEF), the LVEF decreased to <35% in all 3 groups (LVEF: 40–50%, 50–60%, or >60%); whereas the change in LVEF was minimal among patients with persistent heart failure with preserved ejection fraction (HFpEF).
Among patients with HFpEF, the LVEF changed minimally from 54.2±8.6% at the index admission to 54.6±11.0% at the follow‐up (paired t test P<0.001). In HFdEF, LVEF declined from 49.0±6.8% to 31.5±6.6%, and 64.7% of the patients had a decline to <35% regardless of baseline LVEF (in patients with 40%<LVEF≤50% at baseline, LVEF declined from 44.9±2.9% to 31.3±7.3% at follow‐up; 50<LVEF≤60%, 54.8±2.9% to 32.3±5.6; LVEF >60%, 62.9±1.5% to 30.6±4.0%), whereas in patients with persistent HFpEF, the LVEF change was marginal (in patients with 40%<LVEF≤50% at baseline, LVEF changed from 45.3±2.9% to 52.7±7.3% at follow‐up; 50<LVEF≤60%, 55.6±2.8% to 57.9±6.7%; LVEF>60%, 66.0±4.0% to 62.2±6.5%) (Figure 2B).
Predictors of LVEF Change
The mean GLS and LVEF were 10.8±4.8% and 39.8±15.3%, respectively. There was a significant correlation between GLS and LVEF (r=0.67, P<0.001). When stratifying the patients according to GLS tertiles, the proportion of persistent HFrEF and that of HFdEF were highest in the lowest GLS tertile (Figure 3A).
Figure 3.
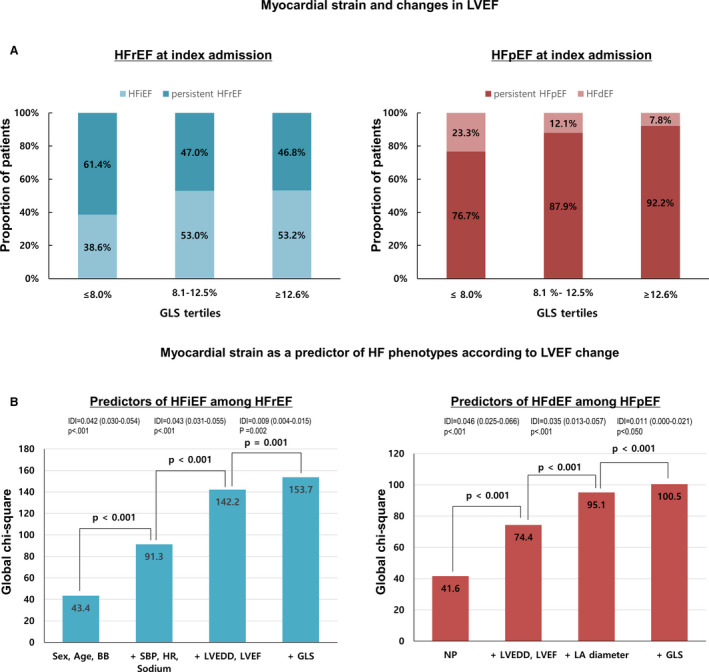
Myocardial strain and heart failure phenotypes according to changes in LVEF. A, Patients were stratified according to GLS tertiles. The proportion of persistent HFrEF and that of HFdEF was highest in the lowest GLS tertile. B, Incremental prognostic value of predictors by binary logistic regression model presented as global χ2 value. The addition of GLS offers a significant additional benefit over conventional parameters. BB indicates beta‐blockers; GLS, global longitudinal strain; HFdEF, heart failure with declined ejection fraction; HFiEF, heart failure with improved ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, heart rate; IDI, integrated discrimination index; LA, left atrium; LVEDD, left ventricular end diastolic diameter; LVEF, left ventricular ejection fraction; NP, natriuretic peptide; and SBP, systolic blood pressure.
In a multivariate analysis, each 1% increase in GLS was associated with 10% increased odds for HFiEF (odds ratio [OR], 1.10; 95% CI, 1.05–1.14; P<0.001) along with age, male sex, body mass index, heart rate, serum sodium level, and LV end‐diastolic diameter (Table 2). For easier interpretation, we categorized GLS using the median value. GLS above the median was associated with 49% increased odds for HFiEF (OR, 1.49; 95% CI, 1.10–2.01; P<0.001).
Table 2.
Predictors of Heart Failure Phenotypes
| Variables | Univariate | 95% CI | Multivariate | 95% CI | ||
|---|---|---|---|---|---|---|
| P Value | OR | P Value | OR | |||
| Predictors of HFiEF | ||||||
| Age (for each 1‐y increase) | <0.001 | 0.98 | 0.97–0.99 | <0.001 | 0.97 | 0.96–0.98 |
| Men (vs female) | 0.001 | 0.65 | 0.51–0.83 | 0.002 | 0.65 | 0.49–0.86 |
| Body mass index, kg/m | 0.035 | 1.03 | 1.00–1.06 | <0.001 | 1.01 | 1.01–1.02 |
| Ischemic heart disease | 0.019 | 0.74 | 0.57–0.95 | … | … | … |
| CKD | 0.052 | 0.78 | 0.61–1.00 | … | … | … |
| Systolic BP (for each 1 mm Hg increase) | <0.001 | 1.01 | 1.01–1.01 | … | … | … |
| Heart rate (for each 1 beat/min increase) | <0.001 | 1.01 | 1.01–1.02 | <0.001 | 1.01 | 1.01–1.02 |
| Sodium, mmol/L | 0.002 | 1.04 | 1.01–1.07 | 0.002 | 1.04 | 1.02–1.07 |
| LA volume index, mL/m2 | 0.002 | 0.99 | 0.99–1.00 | … | … | … |
| LVEDD, mm | <0.001 | 0.95 | 0.94–0.96 | <0.001 | 0.95 | 0.94–0.97 |
| LVEF, % | <0.001 | 1.04 | 1.03–1.06 | … | … | … |
| GLS, % | <0.001 | 1.08 | 1.04–1.12 | <0.001 | 1.10 | 1.05–1.14 |
| β‐Blocker | 0.006 | 1.44 | 1.11–1.88 | … | … | … |
| Predictors of HFdEF | ||||||
| Body mass index, kg/m | 0.003 | 0.92 | 0.87–0.97 | … | … | … |
| NP 100 percentiles | 0.000 | 1.03 | 1.02–1.04 | <0.001 | 1.02 | 1.01–1.03 |
| CKD | 0.011 | 1.74 | 1.13–2.66 | … | … | … |
| LVEDD, mm | 0.001 | 1.05 | 1.02–1.08 | 0.014 | 1.05 | 1.01–1.09 |
| LA diameter, mm | 0.003 | 0.97 | 0.94–0.99 | <0.001 | 0.93 | 0.90–0.96 |
| LA volume index, mL/m2 | 0.151 | 1.00 | 0.99–1.00 | … | … | … |
| LVEF, % | <0.001 | 0.91 | 0.88–0.94 | 0.001 | 0.93 | 0.89–0.97 |
| GLS, % | <0.001 | 0.89 | 0.85–0.94 | 0.038 | 0.93 | 0.87–1.00 |
| ACE inhibitor or ARB | 0.025 | 1.76 | 1.07–2.88 | … | … | … |
ACE indicates, angiotensin‐converting enzyme inhibitor; ARB, angiotensin‐receptor‐blocker; BP, blood pressure; CKD, chronic kidney disease; GLS, global longitudinal strain; HFdEF, heart failure with declined ejection fraction, HFiEF, heart failure with improved ejection fraction, LA, left atrium; LVEDD, left ventricular end‐diastolic dimension; LVEF, left ventricular ejection fraction; NP, natriuretic peptide; and OR, odds ratio.
Regarding HFdEF, high natriuretic peptide levels and left ventricular end‐diastolic dimension as well as low left atrium diameter, LVEF, and GLS were independent predictors of HFdEF. In multivariable analysis, GLS less than the median was associated with 2.12‐fold increased odds for HFiEF (OR, 2.12; 95% CI, 1.21–3.70, P=0.008).
GLS had an incremental value for predicting both HFiEF and HFdEF in addition to other predictors (Figure 3B).
Outcomes
The median follow‐up duration was 1304 days (IQR, 784–1835 days). At 5 years, 533 (25.3%) patients died. In the Kaplan‐Meier survival analysis, there was no difference in mortality between HFrEF and HFpEF (Figure 4A). However, under stratification by HF phenotypes, patients with HFiEF had the lowest, and those with HFdEF had the highest mortality. The 5‐year all‐cause mortality rates were 43.1, 33.1, 24, and 17% for HFdEF, persistent HFrEF, persistent HFpEF, and HFiEF, respectively (global log‐rank P<0.001, pairwise P value between all groups <0.05, except between persistent HFrEF and HFdEF with P=0.055) (Figure 4B). Similar findings were observed for the composite of all‐cause mortality and hospitalization for HF (Figure 4C). Regarding the timing of echocardiography follow‐up, 913 (43.4%) patients had follow‐up echocardiography ≤1 year from the index admission (median: 245 days, IQR 191–311 days) and 1191 (56.6%) patients had follow‐up echocardiography >1 year from the index admission (median 627 days, IQR 456–955 days). The changes in LVEF were similar in both groups (Figure S1).
Figure 4.
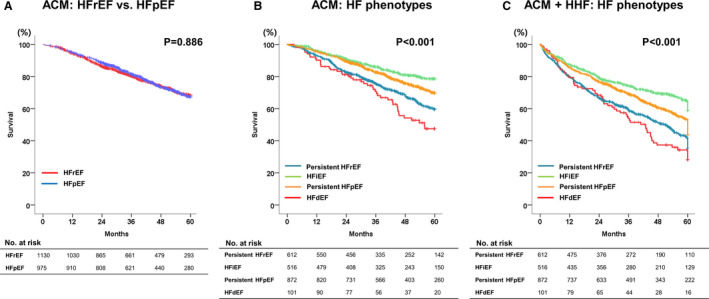
Five‐year ACM and its composite and hospitalization according to HF phenotypes. A, ACM in HFrEF vs HFpEF. B, ACM according to HF phenotypes. HFiEF, heart failure with improved ejection fraction; C, The composite of ACM and hospitalization for HF according to HF phenotypes. ACM indicates all‐cause mortality; HF, heart failure; HFdEF, heart failure with declined ejection fraction; HFiEF, heart failure with improved ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; and HHF, hospitalization for HF.
When applying the European Society of Cardiology (ESC classification (ie, HFrEF: EF <40%, HFmrEF: EF 40‐49, HFpEF: EF ≥50%), there was no difference in all‐cause mortality between the 3 groups. However, under reclassification of patients by the follow‐up LVEF, patients with LVEF ≥50% had the best, whereas those with LVEF <40% had the worst prognosis regardless of baseline LVEF (Figure S2).
Discussion
In this comprehensive analysis of HF, we investigated the longitudinal changes in LVEF in a large cohort of patients with acute HF, and showed that nearly half of patients with HFrEF at the index admission developed HFiEF, who had the best prognosis; whereas 11% of patients with HFpEF at the index admission developed HFdEF, who had the worst prognosis (Figure 5). We also showed that GLS was a significant predictor of both HFiEF and HFdEF, independent of LVEF. This study emphasized the importance of HF phenotyping according to LVEF change and illustrates the benefit of myocardial strain for the prediction of HF phenotypes for the first time.
Figure 5.
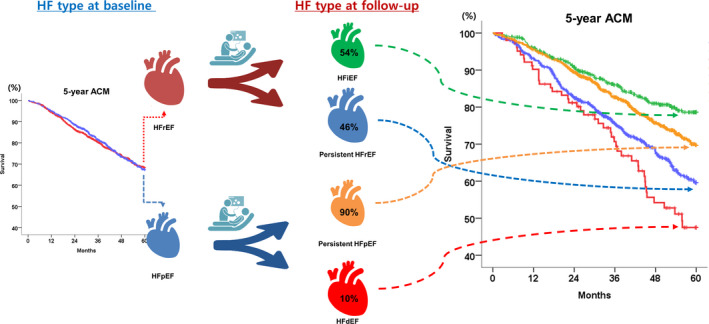
Longitudinal changes of HF phenotypes and outcomes. Patients with HFpEF and HFrEF have a similar prognosis according to classification at baseline. LVEF is not static but changes during follow‐up. Each HF phenotype according to the longitudinal LVEF change (ie, persistent HFrEF, HFiEF, persistent HFpEF, and HFdEF) has a distinct prognosis. HF indicates heart failure; HFdEF, heart failure with declined ejection fraction; HFiEF, heart failure with improved ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction, and LVEF, left ventricular ejection fraction.
LVEF Changes Occur According to Distinct Clinical Categories
In this study, 45% of the patients with HFrEF at the index admission had HFiEF during the follow‐up. Interestingly, when their LVEF improved, it increased to >50% and was almost normalized regardless of the severity of baseline LV systolic dysfunction. By contrast, those with persistent HFrEF showed only marginal changes in LVEF during the follow‐up. It appears that the improvement in LVEF occurs according to distinct clinical categories, and those whose LVEF improved (ie, HFiEF) versus those who did not (ie, persistent HFrEF) represented 2 very distinctive HF phenotypes. A similar phenomenon was observed in patients with HFpEF, but in the opposite direction; when patients with HFpEF experienced a decline in LVEF, the LVEF declined to <35% regardless of the patient's baseline LVEF. This phenomenon was also observed in patients with ischemic cause, atrial fibrillation, and short‐term follow‐up duration. Nonetheless, the reason for the phenomenon is unclear.
Previous studies have reported improvements of LVEF during follow‐up; nonetheless, those studies did not distinguish between patients whose LVEF improved versus those whose did not.13, 14 Our findings suggest that the longitudinal changes in LVEF occur according to distinct clinical categories, and the presence of patients with HF whose LVEF improves or declines. This new concept will need to be confirmed in other HF cohorts.
GLS as Predictors of LVEF Change
Identifying the predictors for LVEF change is clinically important. In this study, older age, male sex, and low body mass index were negative predictors of HFiEF. This is consistent with the findings from The Valsartan Heart Failure Trial substudy13 and CART 2 (Chronic Heart Failure Analysis and Registry in the Tohoku District‐2 Study).15 An important finding of this study was the predictive value of GLS for longitudinal LVEF change. In a small study with 166 patients with nonischemic cardiomyopathy, Swat et al showed that higher baseline absolute longitudinal strain was associated with recovered EF.16 In this large, confirmative study with 2105 patients, we showed that GLS was a significant predictor of both HFiEF and HFdEF: each 1% increase in GLS was associated with a 10% increased and 7% reduced odds for HFiEF and HFdEF, respectively. Interestingly, the baseline LVEF was not a predictor after adjustment.
GLS measures the contractility of the myocardium directly. There exists a significant correlation between LVEF and GLS; however, for a given LVEF, there was a wide distribution of GLS.8 This implies that patients with HFrEF have both preserved and reduced GLS. We hypothesized that some patients with HFrEF and preserved contractility (ie, high GLS) have the potential to improve their LVEF. The same is the case for patients with HFpEF, but in the opposite direction.
Regarding the medical treatment, neither β‐blockers nor renin‐angiotensin‐system inhibitors were associated with HFiEF after adjustment. This contradicts the findings from previous reports including ours.13 However, when excluding GLS from the multivariate analysis, the use of β‐blockers was associated with HFiEF (data not shown). There was also an interaction between β‐blocker use and GLS for the prediction of HFiEF (P for interaction=0.087), possibly explaining the discrepancy between the studies.
Different Outcomes According to HF Phenotypes
The current guideline for HF divides patients with HF into 2 categories, ie, HFrEF and HFpEF, based on baseline echocardiography.1 Although many patients experience changes in the LVEF during follow‐up and have different outcomes, this was not adequately addressed in the current guidelines.1, 12, 17 Our principal finding is related to clinical outcomes according to HF phenotypes. First, there was no difference in mortality between HFrEF and HFpEF, which was consistent with previous findings.18, 19 However, under stratification by the HF phenotypes according to LVEF change, the 5‐year mortality was 2‐fold higher in persistent HFrEF than HFiEF, which is in line with previous reports.20 Because the proportion of patients with HFiEF and persistent HFrEF were comparable, the resulting overall survival of HFrEF was placed in the middle of both curves. Regarding HFpEF, HFdEF had a 2‐fold higher mortality than persistent HFpEF. Nonetheless, the relatively small proportion of HFdEF (ie, 11%) among patients with HFpEF explains the low impact of HFdEF on the overall survival of HFpEF.
These results have important clinical implications. In contrast to the common assumption that HFrEF and HFpEF have similar outcomes, our study results emphasize the importance of reclassification of HF according to the longitudinal LVEF change, because the 4 HF phenotypes (ie, persistent HFrEF, HFiEF, persistent HFpEF, and HFdEF) have very distinctive prognoses. Therefore, an echocardiographic re‐evaluation of all patients with HF should be considered, instead of maintaining the initial diagnosis of index admission (ie, HFrEF versus HFpEF).
Strengths and Limitations of the Study
There have been several studies that investigated the changes of LVEF during follow‐up. Those studies investigated only HFrEF,2, 13, 21 whereas our study evaluated changes in LVEF across the entire spectrum of patients with HF including those with HFpEF. Therefore, this study is the first study that compared the outcomes of various HF phenotypes according to LVEF changes. In addition, we also demonstrated that the incremental value of GLS can be used to predict the LVEF change in HF.
Nevertheless, this study had some limitations. First, this study was an analysis of a cohort study; therefore, there could be unmeasured confounding factors. Second, because we selected only patients who had at least 2 echocardiographic examinations, patients who died before the follow‐up echocardiography were excluded. Although all Korean HF patients were encouraged to get a repeat echocardiogram, it is likely that compliance with this suggestion was greater for those with changed symptoms. This presents a significant selection bias, which is also reflected in the relatively low 5‐year all‐cause mortality of 26% in this substudy compared with the 5‐year mortality of 40% in the original STRATS‐AHF cohort. The 5‐year mortality rate of patients without follow‐up echo was 55.4%, 59.9%, and 50.2% for all, HFrEF, and HFpEF, respectively. Specifically, the estimates of 54.2% remaining HFrEF and only 10.5% becoming HFdEF should not be assumed to represent all patients who were initially HFrEF or HFpEF, respectively. We do not have information on the use of angiotensin‐receptor neprilysin‐inhibitor or ivabradine, nor data on the onset and reversible causes of HF, which may have different characteristics and outcomes. In addition, whether the phenomenon that LVEF changes according to distinct clinical categories is a mathematical artifact caused by classification or a real physiologic phenomenon needs validation in future studies. Finally, because we enrolled only East Asian patients admitted for acute HF with echocardiography and considering changes in echocardiographic parameters during acute HF,14 it is unknown whether our findings can be extrapolated to patients with chronic stable HF or with other ethnicities.
In conclusion, in patients with HF, LVEF is not static but changes during the follow‐up. Each HF phenotype according to the longitudinal LVEF change (ie, persistent HFrEF, HFiEF, persistent HFpEF, and HFdEF) has a distinct prognosis. These phenotypes may explain why HFrEF and HFpEF had a similar prognosis. GLS can be used to predict the HF phenotype. Therefore, we suggest repeated echocardiographic evaluations and measurements of GLS as the standard measurements in all patients with HF.
Sources of Funding
None.
Disclosures
None.
Supporting information
Figures S1–S2
(J Am Heart Assoc. 2020;9:e015009. DOI: 10.1161/JAHA.119.015009.)
Supplementary Materials for this article are available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.119.015009
For Sources of Funding and Disclosures, see page 11.
References
- 1. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, et al. 2013 accf/aha guideline for the management of heart failure: executive summary: a report of the american college of cardiology foundation/american heart association task force on practice guidelines. Circulation. 2013;128:1810–1852. [DOI] [PubMed] [Google Scholar]
- 2. Kalogeropoulos AP, Fonarow GC, Georgiopoulou V, Burkman G, Siwamogsatham S, Patel A, Li S, Papadimitriou L, Butler J. Characteristics and outcomes of adult outpatients with heart failure and improved or recovered ejection fraction. JAMA Cardiol. 2016;1:510–518. [DOI] [PubMed] [Google Scholar]
- 3. Basuray A, French B, Ky B, Vorovich E, Olt C, Sweitzer NK, Cappola TP, Fang JC. Heart failure with recovered ejection fraction: clinical description, biomarkers, and outcomes. Circulation. 2014;129:2380–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dunlay SM, Roger VL, Weston SA, Jiang R, Redfield MM. Longitudinal changes in ejection fraction in heart failure patients with preserved and reduced ejection fraction. Circ Heart Fail. 2012;5:720–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Park JJ, Park CS, Mebazaa A, Oh IY, Park HA, Cho HJ, Lee HY, Kim KH, Yoo BS, Kang SM, et al. Characteristics and outcomes of hfpef with declining ejection fraction. Clin Res Cardiol. 2020;109:225–234. [DOI] [PubMed] [Google Scholar]
- 6. Park CS, Park JJ, Mebazaa A, Oh IY, Park HA, Cho HJ, Lee HY, Kim KH, Yoo BS, Kang SM, et al. Characteristics, outcomes, and treatment of heart failure with improved ejection fraction. J Am Heart Assoc. 2019;8:e011077. doi: 10.1161/JAHA.118.011077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kovacs A, Olah A, Lux A, Matyas C, Nemeth BT, Kellermayer D, Ruppert M, Torok M, Szabo L, Meltzer A, et al. Strain and strain rate by speckle‐tracking echocardiography correlate with pressure‐volume loop‐derived contractility indices in a rat model of athlete's heart. Am J Physiol Heart Circ Physiol. 2015;308:H743–748. [DOI] [PubMed] [Google Scholar]
- 8. Park JJ, Park JB, Park JH, Cho GY. Global longitudinal strain to predict mortality in patients with acute heart failure. J Am Coll Cardiol. 2018;71:1947–1957. [DOI] [PubMed] [Google Scholar]
- 9. Marwick TH, Shah SJ, Thomas JD. Myocardial strain in the assessment of patients with heart failure: a review. JAMA Cardiol. 2019;4:287–294. [DOI] [PubMed] [Google Scholar]
- 10. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the american society of echocardiography and the european association of cardiovascular imaging. J Am Soc Echocardiogr. 2015;28(1–39):e14. [DOI] [PubMed] [Google Scholar]
- 11. Kraigher‐Krainer E, Shah AM, Gupta DK, Santos A, Claggett B, Pieske B, Zile MR, Voors AA, Lefkowitz MP, Packer M, et al. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63:447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, et al. 2016 esc guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the european society of cardiology (esc). Developed with the special contribution of the heart failure association (hfa) of the esc. Eur J Heart Fail 2016;18:891–975 [DOI] [PubMed] [Google Scholar]
- 13. Florea VG, Rector TS, Anand IS, Cohn JN. Heart failure with improved ejection fraction: clinical characteristics, correlates of recovery, and survival: results from the valsartan heart failure trial. Circ Heart Fail. 2016;9:e003123. [DOI] [PubMed] [Google Scholar]
- 14. Yamamoto M, Seo Y, Ishizu T, Nishi I, Hamada‐Harimura Y, Machino‐Ohtsuka T, Sato K, Sai S, Nakatsukasa T, Sugano A, et al. Different impact of changes in left ventricular ejection fraction between heart failure classifications in patients with acute decompensated heart failure. Circulation J. 2019;83:584–594. [DOI] [PubMed] [Google Scholar]
- 15. Tsuji K, Sakata Y, Nochioka K, Miura M, Yamauchi T, Onose T, Abe R, Oikawa T, Kasahara S, Sato M, et al. Characterization of heart failure patients with mid‐range left ventricular ejection fraction‐a report from the chart‐2 study. Eur J Heart Fail. 2017;19:1258–1269. [DOI] [PubMed] [Google Scholar]
- 16. Swat SA, Cohen D, Shah SJ, Lloyd‐Jones DM, Baldridge AS, Freed BH, Vorovich EE, Yancy CW, Jonnalagadda SR, Prenner S, et al. Baseline longitudinal strain predicts recovery of left ventricular ejection fraction in hospitalized patients with nonischemic cardiomyopathy. J Am Heart Assoc. 2018;7. doi: 10.1161/JAHA.118.009841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim MS, Lee JH, Kim EJ, Park DG, Park SJ, Park JJ, Shin MS, Yoo BS, Youn JC, Lee SE, et al. Korean guidelines for diagnosis and management of chronic heart failure. Korean Circul J. 2017;47:555–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. [DOI] [PubMed] [Google Scholar]
- 19. Kang SH, Park JJ, Choi DJ, Yoon CH, Oh IY, Kang SM, Yoo BS, Jeon ES, Kim JJ, Cho MC, et al. Prognostic value of nt‐probnp in heart failure with preserved versus reduced ef. Heart. 2015;101:1881–1888. [DOI] [PubMed] [Google Scholar]
- 20. Lupon J, Diez‐Lopez C, de Antonio M, Domingo M, Zamora E, Moliner P, Gonzalez B, Santesmases J, Troya MI, Bayes‐Genis A. Recovered heart failure with reduced ejection fraction and outcomes: a prospective study. Eur J Heart Fail. 2017;19:1615–1623. [DOI] [PubMed] [Google Scholar]
- 21. Halliday BP, Wassall R, Lota AS, Khalique Z, Gregson J, Newsome S, Jackson R, Rahneva T, Wage R, Smith G, et al. Withdrawal of pharmacological treatment for heart failure in patients with recovered dilated cardiomyopathy (tred‐hf): an open‐label, pilot, randomised trial. Lancet. 2019;393:61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1–S2


