Abstract
Background
Laparoscopic sleeve gastrectomy (LSG) has become the preferred bariatric procedure in many countries. However, there is one shortcoming of LSG in the long-term follow-up, and this is the onset of gastro-esophageal reflux disease (GERD) and erosive esophagitis (EE). Conversion to Roux-en-Y gastric bypass (RYGB) is considered an option in patients unresponsive to medical therapy. Currently, there is no evidence of EE improvement or resolution after conversion surgery. In this study, we objectively evaluate the effectiveness of RYGB in management of EE with upper endoscopy (EGD) to identify the significant variables in patients with GERD symptoms post LSG refractory to medical therapy and require conversion surgery.
Methods
Over a period of 11 years (2008–2019) at Singapore General Hospital, we retrospectively reviewed a prospectively collected database of a cohort of patients whom had conversion surgery to RYGB for refractory GERD and EE after LSG. Patient’s endoscopic findings and demographic and anthropometric data were analyzed.
Results
We identified a total of 14 patients who underwent LSG to RYGB conversions for endoscopic proven erosive esophagitis in our unit during the study period. Eight patients (57.1%) had concurrent hiatal hernia repaired. Nine (64.3%) patients were females. The median age of patients in this cohort was 44 (range 30–61) years. Mean weight and BMI were 87.7 kg (± 19.2) and 32.8 (± 3.09) kg/m2, respectively, on the day of conversion surgery. The median time between LSG and revision to RYGB was 36 (range 6–68) months. Seven patients (50%) had complete resolution of GERD symptoms after conversion, and 6 patients (42.9%) had partial resolution. Six out of 7 patients had complete resolution of EE. There were 4 anastomotic strictures (28.6%). Older patients, Indian ethnicity, present of hiatal hernia and lower weight loss after initial LSG were more likely to undergo conversion surgery.
Conclusion
Conversion to RYGB after LSG is clinically relevant and may be a feasible solution if patients have ongoing GERD refractory to medical therapy. Ninety-three percent of our patients achieved complete resolution of their GERD symptoms and significant improvement of erosive esophagitis with significant weight loss after conversion. This study has important implications as LSG is increasingly being performed and a proportion of these will need revision surgery for various reasons, particularly GERD which is extremely prevalent.
Keywords: Revisional, Sleeve gastrectomy, Roux-e-Y gastric bypass, Erosive esophagitis, GERD, Endoscopy
Introduction
The twin epidemics of obesity and type 2 diabetes are on the rise. According to 2014 data from the World Health Organization (WHO), it is estimated that > 1.9 billion adults aged 18 years and older are overweight (body mass index, BMI ≥ 25 kg/m2), with > 600 million of these adults in the obese range (BMI ≥ 30 kg/m2) [1]. The impact of obesity on overall health is significant, with an associated 50 to 100% increased risk of premature death when compared with individuals of a healthy weight [2]. An estimated 300,000 deaths annually are attributed to obesity, with obesity-related comorbid medical conditions contributing substantially to preventable morbidity and mortality [3]. Bariatric surgery, in conjunction with intensive lifestyle interventions and medical treatments, has been shown to produce marked weight loss and improvement in many obesity-related comorbidities [4].
Laparoscopic Roux-en-Y gastric bypass (RYGB) has traditionally been the most widely performed bariatric surgery operation. In recent years, there has been a paradigm shift favoring laparoscopic sleeve gastrectomy (LSG), which has become the commonest bariatric surgical operation worldwide [5]. In 2016, IFSO reported 340,550 (53.6%) sleeve gastrectomies carried out worldwide compared with 191,326 (30.1%) Roux-en-Y gastric bypass operations. However, there is one shortcoming of LSG in the long-term follow-up, and this is the onset of gastro-esophageal reflux disease (GERD) and erosive esophagitis (EE). Our previous study showed 31.7% patients experienced new GERD symptoms after sleeve gastrectomy, and the prevalence of erosive esophagitis (EE) increased from 14.3 to 44.4% [6]. Those patients are unresponsive to optimal medical management, and in the absence of a correctable anatomic factor, conversion to RYGB is considered the reasonable approach. RYGB was indeed the commonest conversion choice for revisions after sleeve gastrectomy in a recent systematic review [7]. Most available studies have small sample sizes, and major disadvantage is the over reliance on subjective descriptions of patient’s symptoms. As a result, many studies which use this indicator in their study are prone to bias (Table 1). More importantly, our previous study has shown there was no correlation between GERD symptomology with endoscopic evidence of erosive esophagitis post sleeve gastrectomy [6]. Hence, it would be imperative to assess the EE resolution post conversion surgery with objective measurement such as endoscopic evaluation with accepted classification system. The aim of the present study was (1) to compare demographic and clinical variables between patients with optimal medical therapy for GERD symptoms versus patients with conversion surgery and (2) to evaluate the resolution of erosive esophagitis with endoscopic evaluation after conversion to RYGB.
Table 1.
Review of studies showing resolution of GERD after conversion surgery
| Study | N | Time (primary to conversion surgery) | Complete resolution of GERD symptoms | Partial resolution of GERD symptoms (needing PPI) |
|---|---|---|---|---|
| Abdemur et al. | 9 | NA | 7 | 2 |
| Gautier et al. | 6 | 28.1 months (mean) | 6 | 0 |
| Langer et al. | 3 | 39.3 months (mean) | 3 | 0 |
| Van Rutte et al. | 5 | NA | 3 | 2 |
| Hendricks | 4 | 30 months (mean) | 3 | 1 |
| Parmar et al. | 10 | 16 months (mean) | 8 | 2 |
| Iannelli et al. | 11 | 18.6 months (mean) | 11 | 0 |
| Amiki et al. | 9 | 2 months- 8 years 9 months | 6 | 3 |
| Yorke et al. | 12 | 41.8 months (mean) | 9 | 0 |
Methods
We carried out a retrospective analysis of our prospectively maintained database-REDCap (Research Electronic Data Capture) to identify all the patients who underwent conversion of VSG to RYGB in our unit between July 2008 and September 2019 for intractable gastro-esophageal reflux disease (GERD) despite optimal medical therapy. We excluded patients with primary vertical banded gastroplasty (VBG) or laparoscopic adjustable gastric band (lap band) and those who underwent revision surgery for weight regain (WR) or insufficient weight loss (IWL). Before conversion, all patients underwent an additional nutritional and psychiatric evaluation. Anatomic assessment of sleeve was performed with endoscopy and contrast study or computed tomography (CT) scan to exclude incisural stenosis. All cases were discussed, and decision for conversion surgery was determined by a multidisciplinary team consisting of dietitians, endocrinologists, physiotherapist, psychologist, and surgeons at the Singapore General Hospital weight management program.
We reviewed the records of all study patients for the following clinical characteristics: type of revision surgery; time between the primary surgery and revision surgery; clinical characteristics, including gender, age, ethnicity, body weight (BW), and body mass index (BMI) at the time of primary surgery; BW and BMI at the time of revision surgery; and percent excess weight loss (%EWL) and percent total weight loss (%TWL) at the time of revision surgery. Additionally, our unit standardized questionnaire, including assessment of reflux symptoms, usage of proton pump inhibitor, smoking, and alcohol drinking, was administered preoperatively and at subsequent postoperative follow-up visits (Table 2).
Table 2.
Standardized questionnaire for GERD in bariatric patient
| History | Never | Sometimes | Always |
|---|---|---|---|
| 1. Do you have burning sensation or burning pain in your stomach or behind your breastbone (heartburn)? | |||
| 2. Do you have stomach content moving upwards to your throat or mouth? | |||
| 3. Do you experience belching or bloating? | |||
| 4. Does it happen within first 2 h after eating? | |||
| 5. Does it happen at any time and there is no relation to eating? | |||
| 6. Does it happen only when you eat a lot or more than you are accustomed to? | |||
| 7. Does it happen only when you eat too fast? | |||
| 8. Does it improve with antacids or Omeprazole? | |||
| 9. Do you smoke? | |||
| 10. Do you drink alcohol? |
We also reviewed patients’ records for the following perioperative/postoperative outcomes: operation time, postoperative length of stay, complications, reoperation or need for endoscopic intervention, operative mortality, BW, BMI, and %EWL after the conversion surgery. We then compared clinical, demographical, and endoscopic variables between the groups treated with optimal medical therapy versus the group underwent conversion surgery. Institutional Review Board approval for data collection was obtained.
Endoscopic Evaluation of the Esophagus
All patients preoperatively underwent endoscopic evaluation of the esophagus. The esophagus, stomach, and duodenal bulb mucosa was carefully inspected, and findings were recorded in EndoPRO IQ software (Pentax Medical, Tokyo, Japan). Esophagitis, if present, in our patients was graded according to the Los Angeles (LA) classification [8]. Details of the classification system are shown in Fig. 1.
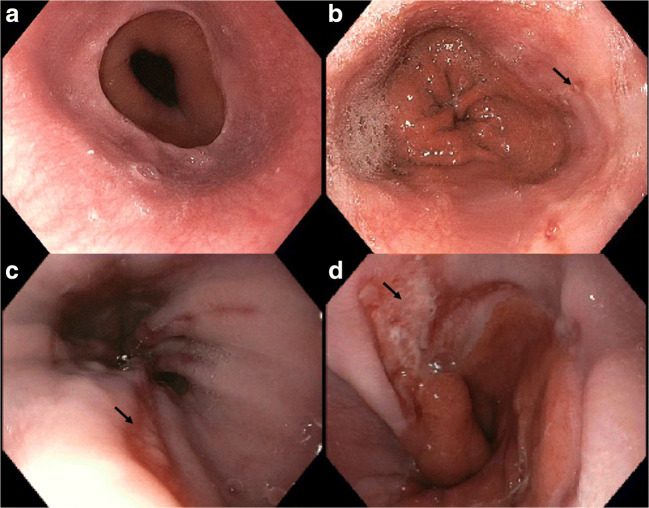
Fig. 1.
Los Angeles (LA) classification of erosive esophagitis. a Hiatal hernia, b grade A esophagitis, c grade B esophagitis, and d grade C esophagitis
A hiatal hernia diagnosis is made based on the presence of a diaphragmatic indentation of at least 2 cm distal to the squamocolumnar junction or Z line and the proximal margins of the gastric mucosal folds on endoscopic examination (Figs. 2 and 3).
Fig. 2.
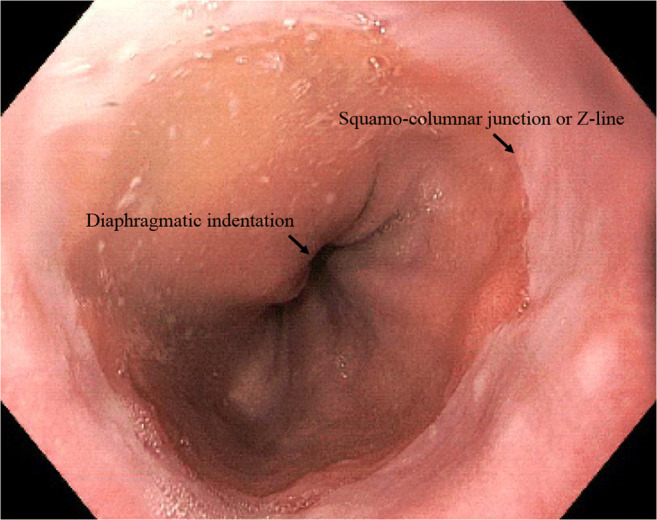
Hiatal hernia diagnosis is made based on the presence of a diaphragmatic indentation of at least 2 cm distal to the squamocolumnar junction or Z line
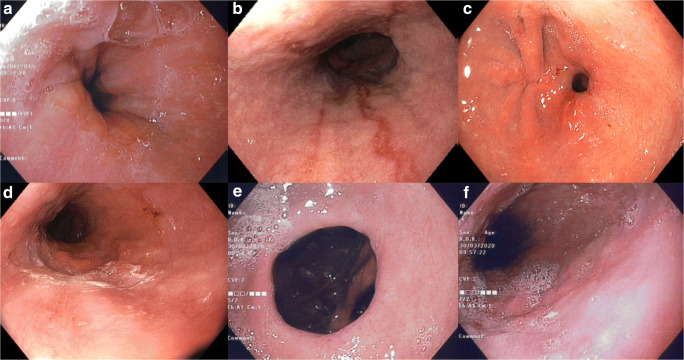
Fig. 3.
Endoscopic findings of a patient post conversion to RYGB complicated by gastrojejunostomy stenosis. a Preoperative endoscopy, b erosive esophagitis 3 months post LSG, c anastomotic stricture 1 month post conversion to RYGB, d. Resolution of erosive esophagitis 1 month post conversion despite anastomotic stricture, e Patent gastrojejunostomy 2 years post conversion to RYGB with f. No evidence of erosive esophagitis
Patients who developed GERD symptoms post LSG were evaluated with repeat upper endoscopy. Similar to preoperative examination, the mucosa of the esophagus, stomach, and duodenal bulb were evaluated in addition to the stapled line. All images and description of the findings were recorded in EndoPRO IQ software (Pentax Medical, Tokyo, Japan). Patients found to have erosive esophagitis on esophageal biopsy were treated with 40 mg of omeprazole (AstraZeneca) twice a day or dexlansoprazole (Takeda) 60 mg once daily and re-assessed in 6–8 weeks.
The standardized surgical technique of LSG adopted by all surgeons at our unit has been previously reported [6]. LSG was performed using five ports placed in upper abdomen through the anterior abdominal wall. The abdominal cavity was insufflated with CO2 and abdominal pressure maintained at 15 mmHg. Dissection was commenced at approximately 3 cm proximal to the pylorus; the omentum separated from the greater curvature by dividing the branches of the gastro-epiploic vessels and the short gastric vessels using a Harmonic scalpel (Ethicon Endosurgery, Cincinnati, OH). Special attention was paid in completely exposing the left crus of the diaphragm and ensuring complete clearance of the posterior aspect of the fundus. We routinely performed preoperative endoscopy for all bariatric procedures, and sliding hernia of > 2 cm will be repaired with posterior crural approximation using Endo-stich and 2–0 Surgidac (Medtronic, Minneapolis, MN). Prior to the formation of the gastric tube, a 36-Fr calibration tube was inserted per orally and the stomach tubularized with the applications of an endoscopic stapler (Echelon-Flex green, gold, and blue cartridges, Ethicon Endosurgery, Cincinnati, OH). After this was completed, the calibration tube was then removed and the staple line inspected for tissue continuation and the absence of bleeding. No drains were used in our procedure. The disconnected stomach was removed in an endoscopic bag (Endo Catch 15 mm, Medtronic, MN, USA) via the 15 mm opening at the umbilicus. All fascial closure was carried out with 2–0 Ethibond suture (Johnson & Johnson Medical N.V., Belgium).
Conversion from LSG to RYGB is similar to a primary laparoscopic RYGB with five ports. Closed pneumoperitoneum was established using Veress needle at left anterior subcostal port site (Palmer’s point) followed by optical insertion of a 12-mm port. One 15-mm and two 5-mm ports were used as working ports. A subxiphoid tract was created using a 5-mm port for insertion of Nathanson or Iron-intern liver retractor. A window is created in the “bare area” of the gastro-hepatic ligament immediately anterior to the caudate lobe of the liver. The Endo-GIA stapler (Medtronic, Minneapolis, MN) with a 60-mm white reload is used to divide the lesser omentum distal to first 2 branches of the left gastric artery. A small gastric pouch was created using Endo-GIA 60-mm purple or black cartridges to transect the existing gastric sleeve approximately 6–8 cm below the gastro-esophageal junction. Five of these patients also underwent complete crural dissection and posterior crural approximation for a hiatus hernia. A loop of small bowel was then brought ante colic/ante gastric to the gastric pouch. The jejunum was anastomosed 50–75 cm from duodenojejunal flexure to the posterior wall of the gastric pouch with Endo-GIA 60-mm purple reload utilizing only 2-cm cartridge length. Stapler entry enterotomies were closed with 2/0 Polysorb in two layers with Endo-Stitch device (Medtronic, Minneapolis, MN). The omega loop was divided just proximal to gastrojejunostomy with Endo-GIA stapler with a 60-mm white reload. A 100 cm of the Roux limb was then measured and anastomosed to the end of bilio-pancreatic limb in side-to-side fashion. Jejuno-jejunal mesenteric defect and Petersen’s defect were closed using the Endo-Stitch with 2–0 Surgidac (Medtronic, Minneapolis, MN). No drains were used.
In the first postoperative day, all patients were commenced on our post bariatric surgery protocol, which included small quantities of clear liquids, progressing to a full liquid diet by the afternoon. Patients were reviewed by our multidisciplinary team at 2 weeks postoperatively followed by review at 1 month, 3 months, 6 monthly, and subsequent annually. Discharged criteria included (1) able to drink 1.5 L of fluid per day and tolerating prescribed liquid diet, (2) pain adequately controlled with oral analgesia, (3) able to ambulate without assistance, and (4) understand and accept the written information sheets provided. We routinely perform endoscopy 1 year post bariatric surgery and surveillance endoscopy for LSG every 3–5 years.
Statistical Analysis
Descriptive results regarding categorical variables were given as percentages (%) of subjects affected. Normally distributed continuous variables were presented as the mean ± standard deviation (SD). Differences in continuous variables were analyzed by Student’s t test, and differences in categorical variables were analyzed by chi-square. P < 0.05 was taken to indicate statistical significance. All analyses were performed using GraphPad Prism version 7 software (GraphPad Software, Inc., La Jolla, CA).
Results
We performed 14 (2%) LSG to RYGB conversions for endoscopic proven erosive esophagitis in our unit between July 2008 and September 2019 out of a total of 708 LSG performed. Eight patients (57.1%) had concurrent hiatal hernia repaired (Table 2). Nine (64.3%) patients were females. The mean age of patients in this cohort was 43.6 (± 9.54) years. Mean weight and BMI was 87.7 kg (± 19.2) and 32.8 (± 3.09) kg/m2, respectively, on the day of conversion. The median time between LSG and revision to RYGB was 36 (range 6–68) months. The median operative time was 303.5 min (range 147–565), and the median postoperative hospital stay was 3 days (range 1–5). More than half of the procedures were performed by our surgical fellows under supervision, which may explain the longer operative time. The mean follow-up in this series was 16 months.
There were 4 anastomotic stricture (28.6%) presented within 1 month post conversion which were successfully treated with endoscopic balloon dilatation (CRE PRO Wire-guided Balloon Dilatation Catheter, Boston Scientific, MA). There were no leaks, conversions to open or mortality in our series. Seven patients (50%) had complete resolution of GERD symptoms after conversion with 6 patients (42.9%) still required proton pump inhibitor (PPI) on an as needed basis which was significantly improved from high dose twice daily before. Prior conversion surgery, all (100%) patients answered “always” for question 2. Do you have stomach content moving upwards to your throat or mouth? and question 4. Does it happen within first 2 h after eating? in the questionnaire. After conversion to RYGB, only 3 (21.4%) patients answered “sometime” and remaining 10 (71.4%) patients answered “never” for same questions.
Seven patients had follow-up upper endoscopy after conversion to RYGB, and 6 patients had complete resolution of erosive esophagitis (Table 3). Interestingly for the 4 patients who developed anastomotic stricture postoperative, their EE resolved despite regurgitation and vomiting. Three patients defaulted the routine follow-up endoscopy, and the remaining 4 patients had their procedures postponed due to the COVID-19 outbreak.
Table 3.
Baseline characteristics and endoscopic data of study population
| Optimal Medical Therapy N = 28 | Conversion to RYGB N = 14 | p | |
|---|---|---|---|
| Mean age (SD) | 36.71 ± 9.92 | 43.69 ± 9.54 | 0.040* |
| Gender | 0.515 | ||
| Male, n (%) | 14 (50) | 5 (35.7) | |
| Female, n (%) | 14 (50) | 9 (64.3) | |
| Race | 0.051 | ||
| Chinese, n (%) | 18 (64.3) | 5 (35.7) | |
| Malay, n (%) | 7 (25) | 3 (21.4) | |
| Indian, n (%) | 2 (7.1) | 6 (42.9) | |
| Other, n (%) | 1 (3.6) | 0 | |
| Height (cm) ± SD | 165.9 ± 11.22 | 162.9 ± 9.00 | 0.366 |
| Preoperative weight (kg) ± SD | 118.4 ± 5.34 | 110.9 ± 20.0 | 0.383 |
| Postoperative weight (kg) ± SD | 82.6 ± 20.9 | 87.7 ± 19.2 | 0.699 |
| Preoperative BMI (kg/m2) ± SD | 42.7 ± 1.89 | 41.71 ± 3.78 | 0.349 |
| Postoperative BMI (kg/m2) ± SD | 29.6 ± 1.55 | 32.8 ± 3.09 | 0.073 |
| Total weight loss (kg) | 36.9 ± 7.39 | 23.2 ± 14.78 | 0.003* |
| % excess weight loss (%) | 60.3 ± 14.4 | 45.3 ± 28.78 | 0.014* |
| Smoking, n (%) | 6 (21.4) | 2 (14.3) | 0.309 |
| Alcohol consumption, n (%) | 2 (7.1) | 2 (14.3) | 0.553 |
| EGD findings | |||
| Hiatus Hernia, n (%) | 6 (21.4) | 10 (71.4) | 0.002* |
| Erosive esophagitis (LA classification) | 0.503 | ||
| A | 15 (53.6) | 5 (35.7) | |
| B | 11 (39.3) | 7 (50) | |
| C | 2 (7.1) | 2 (14.3) | |
When we compared the clinical characteristics and endoscopic findings of patients with EE who underwent conversion surgery versus those on optimal medical treatment, patient’s age, total weight loss (TWL), percentage of excess weight loss (%EWL), and presence of hiatal hernia (Table 2) on preoperative endoscopic assessment were significant factors for conversion surgery. Older patients, Indian ethnicity, present of hiatal hernia and lower weight loss after initial surgery were more likely to undergo conversion surgery.
The percentage of excess weight loss after conversion from LSG to RYGB was 20.5% (range 0–48.5%) (Table 4). Before conversion, 7 patients had insufficient weight loss with %EWL of less than 50%. After conversion, only 1 patient had %EWL less than 50% calculated from initial weight prior to LSG.
Table 4.
Clinical and endoscopic outcomes after conversion of LSG to RYGB
| Los Angeles Classification | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case | Time (primary to conversion surgery) | Revision Surgery | Before LSG | Before conversion to RYGB | After conversion surgery | Hiatal hernia | % EWL post conversion | Resolution of GERD symptoms | PPI use post conversion | Complications |
| 1 | 26 | RYGB | N | A | N | Yes | 20.0 | Complete | No | Anastomotic stricture |
| 2 | 60 | RYGB | N | A | – | No | 40.8 | Partial | Yes | No |
| 3 | 68 | RYGB & HHR | N | B | N | Yes | 30.9 | Complete | No | Anastomotic stricture |
| 4 | 52 | RYGB & HHR | N | B | – | Yes | 10.9 | Complete | No | No |
| 5 | 60 | RYGB & HHR | N | B | – | Yes | 21.1 | Partial | Yes | No |
| 6 | 6 | RYGB | N | C | – | Yes | 25.0 | Partial | Yes | No |
| 7 | 45 | RYGB & HHR | N | B | N | Yes | 0 | No | Yes | No |
| 8 | 22 | RYGB & HHR | N | A | – | Yes | 31.9 | Partial | Yes | No |
| 9 | 10 | RYGB | N | B | N | Yes | 0 | Complete | No | Anastomotic stricture |
| 10 | 33 | RYGB & HHR | N | C | – | No | 8.3 | Complete | No | No |
| 11 | 38 | RYGB & HHR | N | B | N | Yes | 22.5 | Partial | Yes | No |
| 12 | 34 | RYGB | N | A | N | No | 48.5 | Complete | No | Anastomotic stricture |
| 13 | 16 | RYGB & HHR | B | B | – | Yes | 6.0 | Partial | Yes | No |
| 14 | 50 | RYGB | N | A | A | No | 21.5 | Complete | No | No |
Discussion
Laparoscopic sleeve gastrectomy (LSG) has come a long way since its inception, and it is now the commonest stand-alone bariatric procedure worldwide [5]. However, its reputation is marred by insufficient weight loss (IWL) or even weight regain (WR). Gastro-esophageal reflux disease (GERD) or erosive esophagitis (EE) unresponsive to medical management has also been frequently reported in these patients [6]. Treatments described in the literature include medical therapies and surgical conversion to a different bariatric procedure. However, many of these studies included a mix of indication for treatments and outcomes are reported as a group [9–11]. To complicate thing further, most studies included different revision procedures like re-sleeve, single-anastomosis gastric bypass, Roux-en-Y gastric bypass, and bilio-pancreatic diversion with duodenal switch [12, 13]. We found that this results in a conclusion that is often clouded and contradictory because patients with insufficient weight loss and gastro-esophageal disease are very different cohort of patients.
On-site anonymous survey during the Second International Consensus Summit for Sleeve Gastrectomy reported that the mean prevalence of postoperative GERD was 6.5%, ranging from 0 to 83% [14]. In our previous study, the prevalence of GERD symptoms increased from 31.7% to 47.6% after LSG. Thirty-two percent experienced new GERD symptoms, and 15.9% patients experienced worsening in preexisting GERD symptoms [6]. DuPree at el. reported similar findings with preexisting GERD in 44.5% of the LSG cohort and additional 8.6% new onset GERD post sleeve gastrectomy [15].
In our study, we focus solely on intractable GERD following post vertical sleeve gastrectomy. We only selected patients treated specifically with Roux-en-Y gastric bypass. All the other revisional procedures were excluded. Patients’ symptomology prior to and after revisional Roux-en-Y gastric bypass was collected as well as endoscopic evidence of the resolution of erosive esophagitis.
The diagnosis of gastro-esophageal reflux disease was carefully defined with an exhaustive workup including upper GI endoscopy, contrast study, or computerized tomography (CT) scan prior consideration of revisional surgery. These investigations are meant to demonstrate the correlation between subjective GERD symptoms reported by patients and objective erosive esophagitis on endoscopy. Preoperative workup is also intended to identify other anatomical anomalies such as incisural strictures which can be managed with endoscopic therapy or functional strictures like a twisted or kinked sleeved stomach. The gold standard of anti-reflux procedure in the patients post sleeve gastrectomy remains to be the standard Roux-en-Y gastric bypass.
After RYGB, we know from the literature and through our own experience to expect universal improvement in their symptoms however, very few studies show data on the endoscopic resolution of erosive esophagitis despite RYGB. We compared patient-reported GERD symptoms with endoscopic evidence and found that the classical symptom of “heartburn or regurgitation” did not correlate with the presence of erosive esophagitis at upper GI endoscopy after vertical sleeve gastrectomy [6]. Our current study showed complete resolution of erosive esophagitis in 6 out of 7 patients post conversion. This improvement can be explained by a small lesser curvature-based gastric pouch with an excluded antrum resulting in virtually no acid production. Therefore, despite experiencing regurgitation and vomiting from an anastomotic stricture, the severity of erosive esophagitis continues to improve after conversion to RYGB.
In addition to improvement of erosive esophagitis, revisional RYGB also showed that patients benefited from an estimated additional 20.5% weight loss. This is consistent with a study by Carmeli et al. and Homan et al. at 36 months follow-up [16, 17].
Conversion of sleeve gastrectomy to Roux-en-Y gastric bypass is considered safe; however in comparison with primary bariatric procedure, it is associated with higher perioperative complications with increased conversions to laparotomy rates [18–21]. Parmar series showed 2 patients (10%) developing marginal ulcer and 2 other patients experiencing persistent reflux requiring proton pump inhibitor (PPI) [22], while the Gautier series had 1 patient (5.5%) with small bowel injury and peritonitis [9]. There were 4 gastrojejunostomy strictures in our series which were successfully treated with endoscopic balloon dilatation. We believe the reason for the higher stricture rate was due to the creation of the gastrojejunostomy with existing narrow gastric sleeve making the anastomosis more challenging. We suspect that this is due to our institutional practice of fashioning tight sleeve with a 36 Fr calibration tube and utilization of linear stapler for anastomosis. To prevent high stricture rates, we adopted new technique of closing the gastrojejunostomy defect over a 40-Fr calibration tube passed through the mouth into the Roux limb.
An interesting finding from our study found that older patients of Indian ethnicity whom experienced lower percentage estimated weight loss post sleeve gastrectomy tended to require conversion surgery to treat GERD with erosive esophagitis. Another finding from our study revealed that the discovery of a hiatal hernia during endoscopy in the presence of insufficient weight loss also swayed the surgeons to perform conversion surgery in patients with GERD.
Currently there is a paucity of epidemiological data on the prevalence of GERD in Asians. A cross-sectional study by Wang et al. showed higher prevalence of GERD (22.2%) in a Southern Indian population [23]. There appears to be a significant association between pan masala chewing and GERD. Pan Masala is a mixture of coriander seeds, mint leaves, cardamom, lime, and catechu with or without tobacco. Indian cuisine by and large contains large amounts of paprika, vinegar, chili, and curry. Its acidic content makes volume reflux of such foods hard to control with proton pump inhibitors. Lee et al. showed that curry-induced acid reflux actually reported worse reflux symptoms than patients with reflux disease not caused by curry [24]. This may give us a clue to explain a higher incidence of conversion surgery in the Indian population.
Our results suggest that revision to Roux-en-Y gastric bypass after vertical sleeve gastrectomy is clinically relevant. It can be considered a viable solution to ongoing gastro-esophageal reflux disease which is resistant to medical therapy. Post RYGB, all of our patients achieved significant clinical resolution of their symptoms with endoscopic evidence of improvement in 6 of the 7 patients who underwent endoscopic surveillance. Endoscopic data is not available for the remaining 7 patients of whom 5 still required PPI on an as needed basis. It would be impossible to postulate since correlation between GERD symptoms and erosive esophagitis is poor and PPI usage in this study was solely on symptoms control. Almost all patients in this series achieved some degree of weight loss after conversion. This study has important implications as vertical sleeve gastrectomy is an increasingly popular option in the treatment of bariatric patients worldwide. Therefore, increased incidence of GERD with erosive esophagitis and its implications will be expected. Our study has shown that a decisive surgical treatment of resistant patients with erosive esophagitis should be Roux-en-Y gastric bypass.
Limitations to this study include the retrospective nature of the study as well as the small sample size. Although our unit questionnaire was not validated, it was more relevant to post sleeve gastrectomy subjects as it takes into account smoking and alcohol history, responses to acid suppression medication and differentiation of acid reflux versus volume reflux. Current validated questionnaires like GERD-Q or GERD-HRQL are an instrument for acid reflux but do not measure symptoms due to volume reflux like post-prandial regurgitation. Our work which is currently under review showed 88% of reported GERD symptoms happened only post-prandial which suggest that the reflux symptoms after LSG may be related to nonacid volume reflux instead of acid reflux. This is consistent with Althuwaini et al. which concluded 35.7% of regurgitation was nonacid food regurgitation [25]. Also there might be some unmeasured confounders, for example, smoking and alcohol consumption with GERD-Q or GERD-HRQL. Furthermore, there is no validated questionnaire in Mandarin, Malay, or Tamil version.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Approval Statement
For this type of retrospective study, formal consent is not required.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO Media Centre: obesity and overweight. Available at: http://www.who.int/mediacentre/factsheets/fs311/en/index.html. 2014. Accessed March, 2017.
- 2.National Institutes of Health (NIH), National Heart, Lung, and Blood Institute (NHLBI) Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Washington, DC: U.S. DHHS, Public Health Service (PHS); 1998.
- 3.Allison DB, Fontaine KR, Manson JE, Stevens J, VanItallie T. VanItallie TB annual deaths attributable to obesity in the United States. JAMA. 1999;282:1530–1538. doi: 10.1001/jama.282.16.1530. [DOI] [PubMed] [Google Scholar]
- 4.Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, Thomas S, Abood B, Nissen SE, Bhatt DL. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366(17):1567–1576. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angrisani L, Santonicola A, Iovino P, Vitiello A, Higa K, Himpens J, Buchwald H, Scopinaro N. IFSO worldwide survey 2016: primary, endoluminal, and revisional procedures. Obes Surg. 2018;28:3783–3794. doi: 10.1007/s11695-018-3450-2. [DOI] [PubMed] [Google Scholar]
- 6.Lim CH, Lee PC, Lim E, Tan J, Chan WH, Tan HC, Ganguly S, Tham KW, Eng A. Correlation between symptomatic gastro-esophageal reflux disease (GERD) and erosive esophagitis (EE) post vertical sleeve gastrectomy (VSG) Obes Surg. 2019;29:207–214. doi: 10.1007/s11695-018-3509-0. [DOI] [PubMed] [Google Scholar]
- 7.Cheung D, Switzer NJ, Gill RS, Shi X, Karmali S. Revisional bariatric surgery following failed primary laparoscopic sleeve gastrectomy: a systematic review. Obes Surg. 2014;24(10):1757–1763. doi: 10.1007/s11695-014-1332-9. [DOI] [PubMed] [Google Scholar]
- 8.Hendricks L, Alvarenga E, Dhanabalsamy N, Lo Menzo E, Szomstein S, Rosenthal R. Impact of sleeve gastrectomy on gastresophageal reflux disease in a morbidly obese population undergoing bariatric surgery. Surg Obes Relat Dis. 2016;12(3):511–517. doi: 10.1016/j.soard.2015.08.507. [DOI] [PubMed] [Google Scholar]
- 9.Abdemur A, Fendrich I, Rosenthal R. Laparoscopic conversion of laparoscopic sleeve gastrectomy to gastric bypass for intractable gastresophageal reflux disease. Surg Obes Relat Dis. 2012;8(5):654. doi: 10.1016/j.soard.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Gautier T, Sarcher T, Contival N, le Roux Y, Alves A. Indications and mid-term results of conversion from sleeve gastrectomy to roux-en-Y gastric bypass. Obes Surg. 2013;23(2):212–215. doi: 10.1007/s11695-012-0782-1. [DOI] [PubMed] [Google Scholar]
- 11.Langer FB, Bohdjalian A, Shakeri-Leidenmühler S, Schoppmann SF, Zacherl J, Prager G. Conversion from sleeve gastrectomy to Roux-en-Y gastric bypass- indications and outcome. Obes Surg. 2010;20(7):835–840. doi: 10.1007/s11695-010-0125-z. [DOI] [PubMed] [Google Scholar]
- 12.van Rutte PW, Smulders JF, de Zoete JP, et al. Indications and short term outcomes of revisional surgery after failed or complicated sleeve gastrectomy. Obes Surg. 2012;22(12):1903–1908. doi: 10.1007/s11695-012-0774-1. [DOI] [PubMed] [Google Scholar]
- 13.Amiki M, Seki Y, Kasama K, Hashimoto K, Kitagawa M, Umezawa A, Kurokawa Y. Revisional bariatric surgery for insufficient weight loss and gastroesophageal reflux disease: our 12-year experience. Obes Surg. 2020;30:1671–1678. doi: 10.1007/s11695-019-04374-6. [DOI] [PubMed] [Google Scholar]
- 14.Gagner M, Deitel M, Kalberer TL, Erickson AL, Crosby RD. The second international consensus summit for sleeve gastrectomy. Surg Obes Relat Dis. 2009;5(4):476–485. doi: 10.1016/j.soard.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 15.DuPree CE, Kelly B, Steele SR, et al. Laparoscopic sleeve gastrectomy in patients with pre-existing GERD- a National Analysis. JAMA Surg. 2014;149(4):328–334. doi: 10.1001/jamasurg.2013.4323. [DOI] [PubMed] [Google Scholar]
- 16.Carmeli I, Golomb I, Sadot E, Kashtan H, Keidar A. Laparoscopic conversion of sleeve gastrectomy to a biliopancreatic diversion with duodenal switch or a roux en Y gastric bypass due to weight loss failure: our algorithm. Surg Obes Relat Dis. 2015;11(1):79–85. doi: 10.1016/j.soard.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Homan J, Betzel B, Aarts EO, van Laarhoven KJHM, Janssen IMC, Berends FJ. Secondary surgery after sleeve gastrectomy: Roux-en-Y gastric bypass or biliopancreatic diversion with duodenal switch. Surg Obes Relat Dis. 2015;11(4):771–777. doi: 10.1016/j.soard.2014.09.029. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Tan WH, Chang R, Eagon JC. Perioperative risk and complications of revisional bariatric surgery compared to primary Roux-en-Y gastric bypass. Surg Endosc. 2015;29:1316–1320. doi: 10.1007/s00464-014-3848-4. [DOI] [PubMed] [Google Scholar]
- 19.Hallowell PT, Stellato TA, Yao DA, Robinson A, Schuster MM, Graf KN. Should bariatric revisional surgery be avoided secondary to increased morbidity and mortality? Am J Surg. 2009;197:391–396. doi: 10.1016/j.amjsurg.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 20.Himpens J, Dobbeleir J, Peeters G. Long-term results of laparoscopic sleeve gastrectomy for obesity. Ann Surg. 2010;252(2):319–324. doi: 10.1097/SLA.0b013e3181e90b31. [DOI] [PubMed] [Google Scholar]
- 21.Yorke E, Sheppard C, Switzer NJ, Kim D, de Gara C, Karmali S, Kanji A, Birch D. Revision of sleeve gastrectomy to roux-en-Y gastric bypass: a Canadian experience. Am J Surg. 2017;213:970–974. doi: 10.1016/j.amjsurg.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Parmar CD, Mahawar KK, Boyle M, Schroeder N, Balupuri S, Small PK. Conversion of sleeve gastrectomy to roux-en-Y gastric bypass is effective for gastro-oesophageal reflux disease but not for further weight loss. Obes Surg. 2017;27:1651–1658. doi: 10.1007/s11695-017-2542-8. [DOI] [PubMed] [Google Scholar]
- 23.Wang HY, Leene KB, Plymoth A, et al. Prevalence of gastro-esophageal reflux disease and its risk factors in a community based population in southern India. BMC Gastroenterol. 2016;16:36. doi: 10.1186/s12876-016-0452-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee GL, Tay HW, Ho KY. Curry induces acid reflux and symptoms in gastroesophageal reflux disease. Dig Dis Sci. 2011;56(12):3546–3550. doi: 10.1007/s10620-011-1799-3. [DOI] [PubMed] [Google Scholar]
- 25.Althuwaini S, Bamehriz F, Aldohayan A, Alshammari W, Alhaidar S, Alotaibi M, Alanazi A, Alsahabi H, Almadi MA. Prevalence and predictors of gastroesophageal reflux disease after laparoscopic sleeve gastrectomy. Obes Surg. 2018;28:916–922. doi: 10.1007/s11695-017-2971-4. [DOI] [PubMed] [Google Scholar]
- 26.Iannelli A, Debs T, Martini F, Benichou B, Ben Amor I, Gugenheim J. Laparoscopic conversion of sleeve gastrectomy to roux-en-Y gastric bypass: indications and preliminary results. Surg Obes Relat Dis. 2016;12:1533–1538. doi: 10.1016/j.soard.2016.04.008. [DOI] [PubMed] [Google Scholar]