Abstract
Purpose
The COVID-19 pandemic has impacted early breast cancer (EBC) treatment worldwide. This study analyzed how Brazilian breast specialists are managing EBC.
Methods
An electronic survey was conducted with members of the Brazilian Society of Breast Cancer Specialists (SBM) between April 30 and May 11, 2020. Bivariate analysis was used to describe changes in how specialists managed EBC at the beginning and during the pandemic, according to breast cancer subtype and oncoplastic surgery.
Results
The response rate was 34.4% (503/1462 specialists). Most of the respondents (324; 64.4%) lived in a state capital city, were board-certified as breast specialists (395; 78.5%) and either worked in an academic institute or one associated with breast cancer treatment (390; 77.5%). The best response rate was from the southeast of the country (240; 47.7%) followed by the northeast (128; 25.4%). At the beginning of the pandemic, 43% changed their management approach. As the outbreak progressed, this proportion increased to 69.8% (p < 0.001). The southeast of the country (p = 0.005) and the state capital cities (p < 0.001) were associated with changes at the beginning of the pandemic, while being female (p = 0.001) was associated with changes during the pandemic. For hormone receptor-positive tumors with the best prognosis (Ki-67 < 20%), 47.9% and 17.7% of specialists would recommend neoadjuvant endocrine therapy for postmenopausal and premenopausal women, respectively. For tumors with poorer prognosis (Ki-67 > 30%), 34% and 10.9% would recommend it for postmenopausal and premenopausal women, respectively. Menopausal status significantly affected whether the specialists changed their approach (p < 0.00001). For tumors ≥ 1.0 cm, 42.9% of respondents would recommend neoadjuvant systemic therapy for triple-negative tumors and 39.6% for HER2 + tumors. Overall, 63.4% would recommend immediate total breast reconstruction, while only 3.4% would recommend autologous reconstruction. In breast-conserving surgery, 75% would recommend partial breast reconstruction; however, 54.1% would contraindicate mammoplasty. Furthermore, 84.9% of respondents would not recommend prophylactic mastectomy in cases of BRCA mutation.
Conclusions
Important changes occurred in EBC treatment, particularly for hormone receptor-positive tumors, as the outbreak progressed in each region. Systematic monitoring could assure appropriate breast cancer treatment, mitigating the impact of the pandemic.
Keywords: COVID-19, Early breast cancer, Hormone receptor-positive tumors, Breast surgery
Introduction
A novel coronavirus (SARS-COV-2) has led to a global health emergency, with the World Health Organization (WHO) ultimately declaring it a pandemic [1]. The transmission and progression of this disease (COVID-19) imply that a great number of infected individuals will need hospitalization and possibly admission to intensive care units [2, 3]. On May 11, over 168,000 individuals in Brazil had a confirmed COVID-19 infection and around 11,500 had died from the disease. The southeast and northeast were the regions most affected, with 43% and 34% of the accumulated cases, respectively [4]. Recognition of the seriousness of the situation resulted in the implementation of social distancing measures, with a consequently negative effect on the management of various different diseases [5].
The management of early breast cancer (EBC), a disease with well-established treatment protocols [6–8], also had to be adjusted in order to free up hospital beds and vital hospital supplies for individuals infected by COVID-19. Initial reports suggested that cancer patients might be at a greater risk of developing severe symptoms compared to the rest of the population, possibly due to their state of immunosuppression resulting from cancer treatment [9–11]. Expert groups have drawn up novel protocols for EBC during the pandemic [12–14]. The treatment of EBC with a low risk of progression, such as ductal carcinoma in situ or invasive hormone-positive tumors, could begin, for example, with neoadjuvant endocrine therapy (NET) [15]. Surgery would then be postponed with no negative effect on disease outcome [16–18]. Other suggestions include extending the use of neoadjuvant chemotherapy (NACT) to tumors of adverse biology [19, 20] and avoiding major or prophylactic surgeries.
Breast cancer treatment during the pandemic has been much debated in Brazil and worldwide [21–24]. Multimodal treatment, including upfront surgery associated with adjuvant therapy in most cases, improves prognosis [25]. This online survey aimed to evaluate how Brazilian breast specialists have managed EBC (stage I/II and clinically negative axilla) following publication of emergency treatment protocols [12–14, 23, 24], at the beginning and during the COVID-19 pandemic, according to EBC subtypes [26] and oncoplastic surgery.
Methods
Between April 30 and May 11, 2020, a survey was conducted by e-mailing a questionnaire to 1462 actively practicing physicians affiliated to the Brazilian Society of Breast Cancer Specialists (SBM). A previous pilot test involving 10 associates evaluated the time required to complete the questionnaire and the response rate, with any necessary changes then being incorporated. The recommendations of the American Association for Public Opinion Research were taken into consideration when constructing and applying the questionnaire and evaluating response. In view of the rapid changes triggered by the pandemic, the deadline for returning the questionnaires was short.
The questionnaire dealt with demographic aspects, changes in EBC management at the beginning and during the pandemic, and EBC management as a function of breast cancer subtypes and the oncoplastic surgery performed (see addendum). The first part of the questionnaire focused on the respondent’s demographic data including sex, age, board certification as a breast specialist (yes or no) and workplace (general hospital or cancer center). The following aspects of the respondent’s workplace were also evaluated: region of the country; type of city (state capital or other city/town); number of inhabitants; confirmed cases of COVID-19 and availability of dedicated COVID-free hospitals/wards for treating other diseases.
To evaluate changes in treatment plans, this survey dealt only with EBC (stages I and II), with clinically negative axilla, since these patients are normally submitted to primary surgery. Breast cancer subtype was based on immunohistochemical findings. Patients with HER2 3 + or HER2 2 + and FISH/SISH-positive tumors were classified as HER2 irrespective of hormone receptor (HR) status. Tumors that were HR-negative and HER2-negative were considered triple-negative (TN), while those expressing HR but that were HER2-negative were considered luminal. The Ki-67 proliferation index was used to sub-classify luminal tumors based on the 2015 St. Gallen Consensus, which suggested a cut-off point of between 20 and 30% [27]: a low proliferation index was considered luminal A (Ki-67 < 20%), while a Ki-67 index > 30% was classified as luminal B. Questions also dealt with menopausal status and its impact on EBC management. The final questions concerned oncoplastic and prophylactic surgery. The questionnaire ended by asking whether the individual had changed their management approach to EBC over the course of the pandemic, since the outbreak occurred at different times and at different degrees of intensity in the different geographical regions of the country, generating social restrictions that increased or decreased as a function of how the outbreak progressed and of the policies implemented in the different states.
The questions allowed a single answer to be selected. The SBM’s internal review board approved the study protocol prior to commencement and waived the requirement for informed consent, since the returned survey forms were unidentified.
The results were stratified by degree of priority in accordance with the COVID-19 Pandemic Breast Cancer Consortium: Priority A patients have a condition that is immediately life threatening and for whom a delay would alter prognosis, while Priority B patients are those who do not have an immediately life threatening condition, but for whom treatment should not be delayed until the end of the pandemic, and Priority C patients are those for whom treatment could be deferred [13]. Priority categories for surgical oncology were B1 (HER2 and TN patients), B3/C1 (T1/2 N0 luminal patients) and C3 (prophylactic surgery). According to the recommendations of the consortium, immediate breast reconstruction, partial or total, is not classified into degrees of priority, but should be limited to tissue expander or implant placement. Autologous reconstruction should be deferred. Here, this category is referred to as breast reconstruction.
The statistical analysis was conducted using the Statistical Package for the Social Sciences, version 24.0 (IBM SPSS). To analyze the responses, frequencies and percentages were calculated. The chi-square test was used in the bivariate analysis to evaluate possible management changes at the beginning and during the pandemic. Significance level was set at 5% with a 95% confidence interval (95% CI).
Results
The survey was sent to 1462 physicians affiliated with the SBM, with 503 (34.4%) returning a completed questionnaire. Of these, 271 (53.9%) were male and 229 (45.5%) were female. The most common age group was 31–40 years (172; 34.2%), followed by 41–50 years (157; 31.2%) and 51–60-years (110; 21.9%). Most (324; 64.4%) lived in a state capital city, were board-certified as breast specialists (395; 78.5%) and either worked in an academic institute or one associated with breast cancer treatment (390; 77.5%). The best response rate was from the southeast of the country (240; 47.7%), followed by the northeast (128; 25.4%), south (85; 16.9%), Midwest (35; 7%) and north (15; 3%). Most respondents (355; 70.6%) lived in large cities with over 500,000 inhabitants, while 12 respondents (2.4%) lived in towns with 50,000–100,000 inhabitants and 9 (1.8%) lived in towns with fewer than 50,000 inhabitants. Non-respondents were younger than respondents. Regarding their geographical distribution in the country, more respondents compared to non-respondents came from the northeast and fewer from the southeast. However, there is no statistically significant difference when the southeastern and northeastern regions, those most affected by COVID-19 in Brazil, are evaluated together (p = 0.746) (Table 1). No other statistically significant differences were found between the groups.
Table 1.
Characteristics of the members of the Brazilian Society of Breast Specialists
| Total N (%) | Respondents N (%) | Non-respondents N (%) | p-valuea | |
|---|---|---|---|---|
| Members | 1462 | 503 | 959 | |
| Sex | 0.080 | |||
| Men | 746 (51) | 271 (54) | 475 (49.5) | |
| Women | 716 (49) | 229 (46) | 487 (50.5) | |
| Data missing | – | 3 (0.6) | – | |
| Age | < 0.000 | |||
| < 40 years | 461 (31.6) | 178 (35.4) | 283 (29.5) | |
| 41–50 years | 393 (26.9) | 157 (31.2) | 236 (24.6) | |
| 51–60 years | 291 (19.9) | 110 (21.9) | 181 (18.9) | |
| > 60 years | 241 (16.5) | 58 (11.5) | 183 (19.1) | |
| Data missing | 76 (5.1) | 0 (0) | 76 (7.9) | |
| Region | 0.007 | |||
| Southeast | 756 (51.7) | 240 (47.7) | 516 (53.8) | |
| Northeast | 306 (20.9) | 128 (25.4) | 178 (18.5) | |
| South | 228 (15.6) | 85 (16.9) | 143 (14.9) | |
| Midwest | 127 (8.7) | 35 (7) | 92 (9.6) | |
| North | 45 (3) | 15 (3) | 30 (3.1) |
aChi-square test
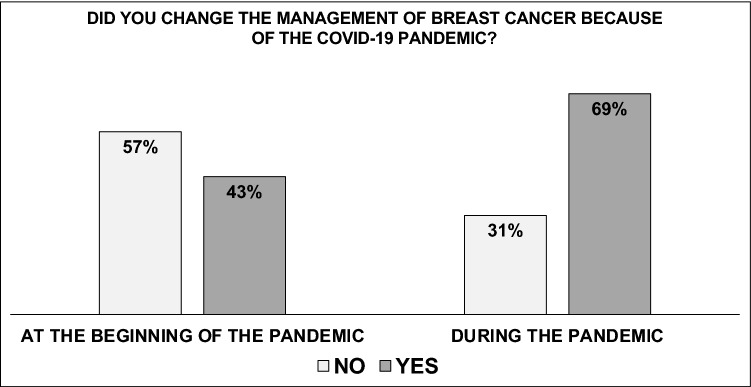
Overall, 498 respondents (99%) lived/worked in a city/town with confirmed cases of COVID-19 and 258 (51.3%) reported that there were COVID-free medical establishments in their cities. Overall, 217 (43%) changed their management approach at the beginning of the pandemic, while 351 (69.8%) made changes during the pandemic (p < 0.001) (Fig. 1).
Fig. 1.
Changes in the treatment of breast cancer after the COVID-19 pandemic in Brazil. *Chi-square test
The southeast of the country (p = 0.005) and the state capital cities (p < 0.001) were associated with changes at the beginning of the pandemic, while being female (p = 0.001) was associated with changes during the pandemic. Being board-certified, having COVID-free hospitals available and working in an academic institute associated with cancer treatment had no significant effect either at the beginning or during the pandemic (Table 2).
Table 2.
Bivariate analysis for the association between management change at the beginning of the pandemic and during its course according to the characteristics of the breast specialists
| Management change | Beginning of the pandemic | During the pandemic | ||||
|---|---|---|---|---|---|---|
| Total N = 503 respondents | Yes N (%) | No N (%) | p-value | Yes N (%) | No N (%) | p-value |
| Region | 0.005 | 0.020 | ||||
| Midwest | 14 (6.5) | 21 (7.4) | 20 (5.7) | 15 (10) | ||
| Southeast | 87 (40.1) | 153 (53.9) | 159 (45.3) | 81 (54) | ||
| South | 46 (21.2) | 38 (13.4) | 62 (17.7) | 23 (15.3) | ||
| North | 4 (1.8) | 11 (3.9) | 9 (2.6) | 6 (4) | ||
| Northeast | 66 (30.4) | 61 (21.5) | 101 (28.8) | 25 (16.7) | ||
| State capital city | < 0.001 | 0.002 | ||||
| Yes | 159 (73.3) | 163 (57.3) | 241 (68.7) | 81 (54.4) | ||
| No | 58 (26.7) | 120 (42.4) | 110 (31.3) | 68 (45.6) | ||
| Board-certified | 0.137 | 0.345 | ||||
| Yes | 177 (81.6) | 216 (76.1) | 280 (79.8) | 114 (76) | ||
| No | 40 (18.4) | 68 (23.9) | 71 (20.2) | 36 (24) | ||
| Cancer institution | 0.200 | 0.846 | ||||
| Yes | 174 (80.2) | 214 (75.4) | 271 (77.2) | 117 (78) | ||
| No | 43 (19.8) | 70 (24.6) | 80 (22.8) | 33 (22) | ||
Priority B1
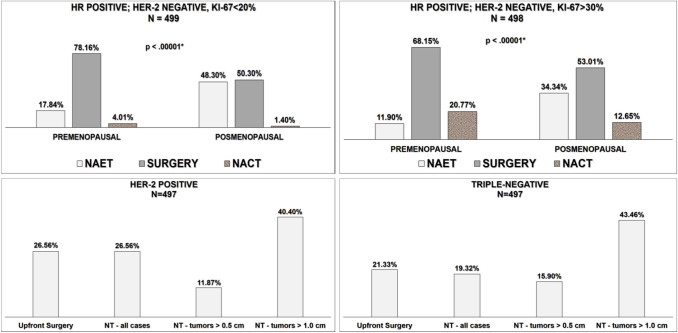
In HER2 breast cancer, 107 specialists (21.3%) would recommend neoadjuvant systemic therapy for all cases compared to 132 (26.2%) who would recommend upfront surgery. Overall, 199 specialists (39.6%) would recommend initial systemic therapy in cases of tumors ≥ 1.0 cm, while for 59 (11.7%) the cut-off point for neoadjuvant treatment would be ≥ 0.5 cm. In cases of triple-negative tumors, 96 (19.1%) would recommend NACT for all cases compared to 108 (21.5%) who would recommend upfront surgery. Overall, 216 (42.9%) specialists believed that NACT is the most appropriate option only for tumors ≥ 1.0 cm, while 77 (15.3%) would recommend neoadjuvant therapy in cases of tumors ≥ 0.5 cm (Fig. 2).
Fig. 2.
Decision making on treatment according to tumor biology. NET neoadjuvant endocrine therapy, NACT neoadjuvant chemotherapy, NT neoadjuvant therapy, N number of answers in the survey, HR hormone receptor; *Chi-square test
Priority B3/C1
In cases of HR-positive breast cancer tumors with the best prognosis (Ki-67 < 20%), 241 physicians (47.9%) would recommend NET for 3–6 months for postmenopausal patients compared to 251 (49.9%) who would recommend upfront surgery and 7 (1.4%) who would recommend NACT. While 89 respondents (17.7%) would recommend NET for premenopausal women, 390 (77.5%) would recommend upfront surgery and 20 (4%) would recommend NACT. In the case of HR-positive tumors with a higher proliferation index (Ki-67 > 30%), 171 specialists (34%) would recommend NET for postmenopausal women and 55 (10.9%) would recommend it for premenopausal women. For postmenopausal women, 63 (12.5%) specialists would recommend NACT and 264 (52.5%) would recommend surgery, while for premenopausal women, 103 (20.5%) would recommend NACT and 338 (67.2%) would recommend surgery. Menopausal status significantly affected specialists’ decision regarding whether to recommend NET in cases of EBC with Ki-67 < 20 and in tumors with a higher proliferation index (p < 0.00001) (Fig. 2).
Priority C3:
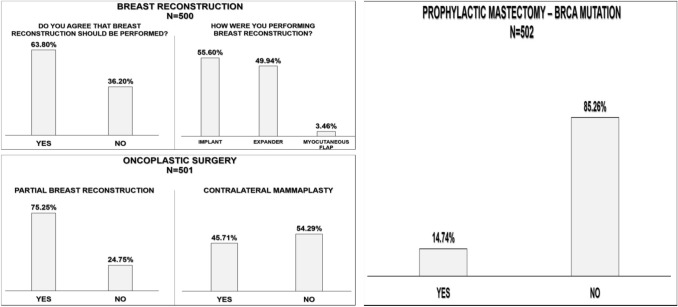
Overall, 427 respondents (84.9%) would not recommend risk-reducing mastectomy, even for patients with BRCA deleterious mutations.
Breast reconstruction
Overall, 319 (63.4%) participants would recommend immediate total breast reconstruction. A definitive implant would be the preferred method of reconstruction for 273 participants (54.3%), while 201 (40%) would recommend temporary tissue expanders and 17 (3.4%) would recommend reconstruction using autologous tissue. A total of 377 respondents (75%) would perform breast-conserving surgery with partial reconstruction, while 272 (54.1%) would contraindicate mammoplasty associated with breast-conserving surgery (Fig. 3).
Fig. 3.
Changes in the types of surgery performed due to the COVID-19 pandemic
Discussion
The immunohistochemical classification of breast cancer into subtypes [26–28] allows different treatment strategies to be adopted. In TN and HER2 tumors, NACT regimens and/or associated target therapy have contributed to minimizing surgical morbidity and to identifying patients with residual disease for additional adjuvant therapy [29–31]. On the other hand, the approach with luminal tumors is generally upfront surgery, with the need for chemotherapy being defined according to histopathology, immunohistochemistry and genomic assay [27]. In general, the use of NET has been restricted to exceptional cases [28].
Brazil is a country of continental dimensions, with a widely differing distribution of human and technical resources. The availability of hospital supplies and beds is greater in the southeast of the country where the concentration of COVID-19 cases is greater. The southeast is currently the most severely affected region, with over 840,000 cases (34%), followed by the northeast with 810,000 (33%), the north (393,000; 16%), the Midwest (230,000; 9%) and the south (205,000; 8%) [4, 32, 33]. The concentration of physicians affiliated to the SBM is also greater in the southeast, with the highest response rate in the present study being from that region (48%), followed by the northeast, the south, the Midwest and finally the north, with only 3%. In agreement with the SBM database, most respondents live in state capitals or in cities with more than 500,000 inhabitants, which are also those most affected by COVID-19 [34].
The onset of the COVID-19 epidemic in Brazil occurred relatively late compared to Europe and North America. Whereas the incidence of the disease was high in Italy and Spain in March, it was only beginning to appear in Brazil at that time, first in the city of São Paulo in the southeast and then spreading unevenly throughout the country [32]. This heterogenous pattern of spread may have resulted in poorer initial compliance by Brazilian breast specialists with the guidelines issued in those countries. Accordingly, 57% of participants did not change their management approach regarding EBC at the beginning of the pandemic, while 70% changed their management approach during the course of the pandemic. At the beginning of the pandemic, changes in management occurred similarly in both sexes and in the different age groups, and irrespective of board certification or workplace. The demographic characteristics most associated with a change in management at the beginning of the pandemic were living in the southeast of the country and in a state capital city, coinciding with the areas in which the incidence of COVID-19 infection was greatest, with an increasing demand for hospital beds and the implementation of restrictive measures by the government. On the other hand, during the pandemic, various state capital cities made an effort to reserve COVID-free hospitals or wards so as to guarantee the admission of elective patients, particularly cancer patients. These initiatives, however, had no effect on the results of this survey.
NET was more commonly indicated for postmenopausal women, both in the case of luminal A (48%) and luminal B tumors (34%). For premenopausal women, the specialists were more likely to recommend NET for cases of luminal A (18%) compared to luminal B tumors (11%). Menopausal status had a significant effect on how these specialists manage EBC. Conversely, although a considerable proportion of respondents suggested a different approach with respect to luminal tumors, upfront surgery remained the most common choice. For premenopausal women, 77.5% of respondents recommended upfront surgery for luminal A tumors and 67% for luminal B tumors. For postmenopausal patients, fewer respondents would recommend upfront surgery, either for luminal A tumors (50%) or for luminal B tumors (52.5%). The pandemic and the emergency recommendations for EBC treatment impacted on the management strategies of Brazilian breast specialists. According to these recommendations [13–15, 23, 35, 36], HR-positive EBC should preferentially be treated using NET. Some societies have suggested that recommendations for the treatment of EBC should be classified by degree of priority [13, 37]. According to the advice provided in the Ontario Health Pandemic Planning Clinical Guideline for Patients with Cancer [37], cases of luminal tumors were classified as Priority B, while the COVID-19 Pandemic Breast Cancer Consortium stratified such tumors as Priority B3 (T2 or N1) or C1 (T1N0) [13]. Another cancer organization also suggested that luminal A tumors should be treated initially with NET, while in the case of luminal B tumors, particularly those in which the axilla is positive or clinical stage II, the use of genomic assays could be useful in making this decision [36, 38, 39]. On the other hand, despite the partial compliance of Brazilian breast specialists with NET in the present study, particularly in cases in which the proliferation index is low, it is clear that a good proportion of those interviewed are still reluctant to use this strategy, even in exceptional conditions such as during a pandemic. However, although NET is a treatment that remains under debate, its use has increased in recent years with the publication of studies showing its safety [16–18, 40, 41].
In HER2 and TN tumors, considerable variations were found in the protocols from the different organizations. In one, TN and HER2 tumors are classified as Priority B1, suggesting NACT for tumors over 2 cm or with affected axillae [13]. Others also suggest NACT without specifying the cut-off point for treatment [32, 33]. The Brazilian Society of Clinical Oncology suggests NACT for tumors over 5 mm or in the case of positive axillae [23]. These differences were reflected in this survey, with 21% of participants recommending NACT for all cases of HER2 tumors and 19% recommending it for all TN tumors. Overall, 40% of respondents considered the cut-off point of 1 cm for an indication of NACT to be appropriate in the case of HER2 tumors, while 43% deemed it appropriate for TN tumors. Conversely, 12% considered a cut-off point of 0.5 cm to be appropriate for NACT in HER2 tumors and 15% for TN tumors. In these subtypes, compliance with the recommendations would appear to be greater, although it is impossible to affirm that there was indeed any change. The prediction of a better pathological response to cytotoxic drugs and targeted therapy, as well as the possibility of selecting cases of residual disease for additional adjuvant therapy, had already rendered neoadjuvant therapy the standard treatment in many cases before the pandemic [29, 30, 42]. Likewise, in our understanding, the fact that one-third of respondents opted for NACT in cases of tumors < 1 cm may represent overtreatment, since there are safe options of de-escalating treatment, as occurs in cases of HER2 tumors [43]. A recent single institution guideline recommended upfront surgery for T1N0 HER2 and TN tumors [44].
Breast reconstruction constitutes the basic principle in the present-day surgical treatment of breast cancer [45, 46]. Approximately 60% of the participants would recommend total immediate breast reconstruction, with the most commonly suggested technique being definitive implants followed by tissue expanders. For breast-conserving surgery, 75% would recommend partial reconstruction, whereas 54% would contraindicate mammoplasty. Finally, 85% of respondents would not recommend risk-reducing mastectomy for patients with BRCA deleterious mutations. These data agree with the recommendations to avoid or delay major surgery that could prolong hospitalization and increase complications or require further hospital admissions [47–53].
There are other surveys that deal with the management of breast cancer [54, 55]. An interesting European study evaluated the changes in EBC management during the pandemic [55]. Unlike the results of the present study, upfront surgery in that study increased in cases of T1 N0 TN and in HER2. Indeed, 67% of respondents considered that chemotherapy increases the risk of developing COVID-19-related complications. In luminal A tumors, 68% recommended NET compared to 48% in the present study (postmenopausal women). These differences may be explained by the improvement in local conditions since this survey was conducted in April.
There are some limitations associated with the present study. Since the data were obtained from a survey, it is impossible to affirm that the behavior encountered in these results would be completely applicable when treatment for actual patients is being recommended. In addition, there is no information on whether the respondents worked in the public or private sector. Another limitation refers to the deadline established for the responses to the questionnaires to be received, which was short; however, the dynamics of the progression of the pandemic could have affected the results if a longer time had been allowed. Nevertheless, the short deadline may have affected the response rate of 34.4%, increasing the likelihood of bias. On the other hand, no significant differences were found between the different geographical regions of the country, or between the SBM database and our sample population, leading us to believe that the sample was indeed representative.
Conclusions
The present findings highlight important changes in the management approach of these breast specialists at the beginning of the pandemic and throughout, particularly with respect to HR-positive tumors. These data may provide further information on EBC treatment in Brazil during the COVID-19 pandemic and may be useful in the perception of treatment and its consequences, permitting adaptation and a return to the conventional guidelines as the outbreak progresses in each region of the country.
Acknowledgements
The authors are grateful to Fernanda Pereira Alves and Jéssica Telles Bonavita for their secretary work in providing the relevant data on the SBM members.
Addendum 1: Treatment of early breast cancer (EBC) clinical stages I and II with clinically negative axillae
Electronic survey for breast specialists affiliated to the Brazilian Society of Breast Specialists (SBM)
- How old are you?
- ≤ 30 years
- 31–40 years
- 41–50 years
- 51–60 years
- 61–70 years
- > 70 years
- Sex
- Female
- Male
- Are you board-certified as a breast specialist?
- Yes
- No
- Do you currently work in an institute that is a reference center or that is exclusively dedicated to cancer treatment?
- Yes
- No
- In which geographical region of Brazil do you work (where you spend the greatest proportion of your time working as a breast specialist)?
- North
- Northeast
- Midwest
- Southeast
- South
- Do you live in a state capital city?
- Yes
- No
- What is the population of the town/city in which you work?
- < 50,000 inhabitants
- 50,000 to 100,000 inhabitants
- 100,000 to 500,000 inhabitants
- > 500,000 inhabitants
- Have there been confirmed cases of COVID-19 in your town/city?
- Yes
- No
- Are there dedicated COVID-19-free institutes in your town/city?
- Yes
- No
- In general, do you believe that you changed the way in which you manage EBC (stages I and II with clinically negative axillae) at the beginning of the pandemic?
- Yes
- No
- During the course of the pandemic, describe how you have managed premenopausal patients with stages I or II EBC, clinically negative axillae, hormone receptor-positive tumors and proliferation index < 20% (based on Ki-67).
- Surgery
- Neoadjuvant endocrine therapy for 3–6 months
- Neoadjuvant chemotherapy
- During the course of the pandemic, describe how you have managed postmenopausal patients with stages I or II EBC, clinically negative axillae, hormone receptor-positive tumors and proliferation index < 20% (based on Ki-67).
- Surgery
- Neoadjuvant endocrine therapy for 3–6 months
- Neoadjuvant chemotherapy
- During the course of the pandemic, describe how you have managed premenopausal patients with stages I or II EBC, clinically negative axillae, hormone receptor-positive tumors and proliferation index > 30% (based on Ki-67).
- Surgery
- Neoadjuvant endocrine therapy for 3–6 months
- Neoadjuvant chemotherapy
- During the course of the pandemic, describe how you have managed post-menopausal patients with stages I or II EBC, clinically negative axillae, hormone receptor-positive tumor and proliferation index > 30% (based on Ki-67).
- Surgery
- Neoadjuvant endocrine therapy for 3–6 months
- Neoadjuvant chemotherapy
- During the pandemic, describe how you have managed patients with stage I EBC and a triple-negative tumor.
- Upfront surgery
- Neoadjuvant chemotherapy in all cases
- Neoadjuvant chemotherapy only in cases of tumors ≥ 0.5 cm (surgery if < 0.5 cm)
- Neoadjuvant chemotherapy in cases of tumors ≥ 1.0 cm (surgery if < 1.0 cm)
- During the pandemic, describe how you have managed patients with stage I EBC and HER-positive tumors.
- Upfront surgery
- Chemotherapy and neoadjuvant anti-HER2 therapy in all cases
- Chemotherapy and neoadjuvant anti-HER2 therapy if the tumor is ≥ 0.5 cm (surgery if < 0.5 cm)
- Chemotherapy and neoadjuvant anti-HER2 therapy if tumor is ≥ 1.0 cm (surgery if < 1.0 cm)
- If mastectomy were necessary, would you recommend immediate reconstruction during the pandemic?
- Yes
- No
- If immediate reconstruction were recommended, what would your preference be?
- Definitive implant
- Temporary tissue expanders
- Reconstruction using autologous tissue
- Would you recommend partial breast reconstruction using oncoplastic techniques during the pandemic?
- Yes
- No
- Would you recommend mammoplasty associated with breast-conserving surgery during the pandemic?
- Yes
- No
- Would you recommend prophylactic mastectomy and immediate reconstruction in patients with BRCA deleterious mutation during the pandemic?
- Yes
- No
- Have you changed your approach to managing EBC over the course of the pandemic?
- Yes
- No
Funding
None.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This is a survey study. All of those who answered the questionnaire agreed to participate. The completed survey forms were maintained anonymous.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Francisco Pimentel Cavalcante, Email: fpimentelcavalcante@gmail.com.
Guilherme Garcia Novita, Email: gui.novita@gmail.com.
Eduardo Camargo Millen, Email: eduardomillen@gmail.com.
Felipe Pereira Zerwes, Email: zerwes@hotmail.com.
Vilmar Marques de Oliveira, Email: vilmarmarques@yahoo.com.br.
Ana Luiza Lima Sousa, Email: demmilima@gmail.com.
Ruffo Freitas Junior, Email: ruffojr@terra.com.br.
References
- 1.World Health Organization (2020) WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020. (Director-General Speeches). https://www.who.int Accessed 14 May 2020
- 2.Zhu N, Zhang D, Wang W, China Novel Coronavirus Investigating and Research Team et al. A novel coronavirus from patients with pneumonia in China. N Engl J Med. 2019;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paules CI, Marston HD, Fauci AS. Coronavirus infections: more than just the common cold. JAMA. 2020 doi: 10.1001/jama.2020.0757. [DOI] [PubMed] [Google Scholar]
- 4.Coronavirus Brasil. Painel geral. https://www.covid.saude.gov.br. Accessed 28 July 2020.
- 5.Fineberg HV. Ten weeks to crush the curve. N Engl J Med. 2020;382:e37. doi: 10.1056/NEJMe2007263. [DOI] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network (NCCN) (2020) NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Breast Cancer version 4.2020 – May 8, 2020. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed 14 May 2020
- 7.Burstein HJ, Curigliano G, Loibl S, Members of the St. Gallen International Consensus Panel on the Primary Therapy of Early Breast Cancer 2019 et al. Estimating the benefits of therapy for early-stage breast cancer: the St. Gallen International Consensus Guidelines for the primary therapy of early breast cancer 2019. Ann Oncol. 2019;30:1541–1557. doi: 10.1093/annonc/mdz235. [DOI] [PubMed] [Google Scholar]
- 8.Cardoso F, Kyriakides S, Ohno S, ESMO Guidelines Committee et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30:1194–1220. doi: 10.1093/annonc/mdz173. [DOI] [PubMed] [Google Scholar]
- 9.Shah UA. Cancer and coronavirus disease 2019 (COVID-19): facing the "C Words". JAMA Oncol. 2020 doi: 10.1001/jamaoncol.2020.1848. [DOI] [PubMed] [Google Scholar]
- 10.Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang L, Zhu F, Xie L, et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan. China Ann Oncol. 2020;S0923–7534(20):36383–36393. doi: 10.1016/j.annonc.2020.03.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Shamsi HO, Alhazzani W, Alhuraiji A, et al. A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID-19) pandemic: an international collaborative group. Oncologist. 2020 doi: 10.1634/theoncologist.2020-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dietz JR, Moran MS, Isakoff SJ, et al. Recommendations for prioritization, treatment, and triage of breast cancer patients during the COVID-19 pandemic. The COVID-19 Pandemic Breast Cancer Consortium. Breast Cancer Res Treat. 2020;181:487–497. doi: 10.1007/s10549-020-05644-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curigliano G, Cardoso MJ, Poortmans P, et al. Recommendations for triage, prioritization and treatment of breast cancer patients during the COVID-19 pandemic. Breast. 2020;52:8–16. doi: 10.1016/j.breast.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martí C, Sánchez-Méndez JI. Neoadjuvant endocrine therapy for luminal breast cancer treatment: a first-choice alternative in times of crisis such as the COVID-19 pandemic. Ecancermedicalscience. 2020;14:1027. doi: 10.3332/ecancer.2020.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Semiglazov VF, Semiglazov VV, Dashyan GA, et al. Phase 2 randomized trial of primary endocrine therapy versus chemotherapy in postmenopausal patients with estrogen receptor-positive breast cancer. Cancer. 2007;110:244–254. doi: 10.1002/cncr.22789. [DOI] [PubMed] [Google Scholar]
- 17.Palmieri C, Cleator S, Kilburn LS, et al. NEOCENT: a randomised feasibility and translational study comparing neoadjuvant endocrine therapy with chemotherapy in ER-rich postmenopausal primary breast cancer. Breast Cancer Res Treat. 2014;148:581–590. doi: 10.1007/s10549-014-3183-4. [DOI] [PubMed] [Google Scholar]
- 18.Alba E, Calvo L, Albanell J, et al. Chemotherapy (CT) and hormonotherapy (HT) as neoadjuvant treatment in luminal breast cancer patients: results from the GEICAM/2006-03, a multicenter, randomized, phase-II study. Ann Oncol. 2012;23:3069–3074. doi: 10.1093/annonc/mds132. [DOI] [PubMed] [Google Scholar]
- 19.Citron ML, Berry DA, Cirrincione C, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003;21:1431–1439. doi: 10.1200/JCO.2003.09.081. [DOI] [PubMed] [Google Scholar]
- 20.Hurvitz SA, Martin M, Jung KH, et al. Neoadjuvant trastuzumab emtansine and pertuzumab in human epidermal growth factor receptor 2-positive breast cancer: three-year outcomes from the phase III KRISTINE Study. J Clin Oncol. 2019;37:2206–2216. doi: 10.1200/JCO.19.00882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tasoulis MK, Roche N, MacNeill F. Rationalizing breast cancer surgery during the COVID-19 pandemic. Eur J Surg Oncol. 2020;46:1192–1193. doi: 10.1016/j.ejso.2020.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Rashdan A, Roumeliotis M, Quirk S, et al. Adapting radiation therapy treatments for patients with breast cancer during the COVID-19 pandemic: hypo-fractionation and accelerated partial breast irradiation to address World Health Organization recommendations. Adv Radiat Oncol. 2020 doi: 10.1016/j.adro.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amorim GLS, Assad DX, Ferrari BL, et al. Breast oncology and the COVID-19 pandemic: recommendations from the Brazilian Society of Clinical Oncology (SBOC) Braz J Oncol. 2020;16:1–7. doi: 10.5935/2526-8732.20190024. [DOI] [Google Scholar]
- 24.Facina G, Oliveira VM. Breast cancer care during the coronavirus pandemic. Mastology. 2020;30:e20200014. doi: 10.29289/2594539420202020200014. [DOI] [Google Scholar]
- 25.Pondé NF, Zardavas D, Piccart M. Progress in adjuvant systemic therapy for breast cancer. Nat Rev Clin Oncol. 2019;16:27–44. doi: 10.1038/s41571-018-0089-9. [DOI] [PubMed] [Google Scholar]
- 26.Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 27.Coates AS, Winer EP, Goldhirsch A, et al. Tailoring therapies: improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol. 2015;26:1533–1546. doi: 10.1093/annonc/mdv221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harbeck N, Penault-Llorca F, Cortes J, et al. Breast cancer. Nat Rev Dis Primers. 2019;5:66. doi: 10.1038/s41572-019-0111-2. [DOI] [PubMed] [Google Scholar]
- 29.Masuda N, Lee SJ, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376:2147–2159. doi: 10.1056/NEJMoa1612645. [DOI] [PubMed] [Google Scholar]
- 30.von Minckwitz G, Huang CS, Mano MS, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380:617–628. doi: 10.1056/NEJMoa1814017. [DOI] [PubMed] [Google Scholar]
- 31.Cavalcante FP, Millen EC, Zerwes FP, Novita GG. Role of axillary surgery after neoadjuvant chemotherapy. JCO Glob Oncol. 2020;6:238–241. doi: 10.1200/JGO.19.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Covid-19 Now: a view of the main indicators by Brazilian state]. www.covid19agora.com.br. Accessed 14 May 2020
- 33.Brazilian Ministry of Health (2020) [Panel for hospital beds and supplies]. https://covid-insumos.saude.gov.br. Accessed 14 May 2020
- 34.Brazilian Ministry of Health (2020) [The coronavirus panel]. https://covid.saude.gov.br. Accessed 20 May 2020
- 35.European Society for Medical Oncology (ESMO) (2020) ESMO clinical practice guidelines: breast cancer. https://www.esmo.org. Accessed 14 May 2020
- 36.Breast Oncology Center Dana-Farber/Brigham and Women’s Cancer Center (2020) Suggested treatment modifications in multidisciplinary breast cancer management in the setting of COVID-19. www.dana-farber.org/covidmd Accessed 14 May 2020
- 37.Ontario Health (2020) Pandemic planning clinical guideline for patients with cancer. https://www.accc-cancer.org/docs/documents/cancer-program-fundamentals/oh-cco-pandemicplanning-clinical-guideline_final_2020-03-10.pdf. Accessed 14 May 2020
- 38.Dowsett M, Turner N. Estimating risk of recurrence for early breast cancer: integrating clinical and genomic risk. J Clin Oncol. 2019;37:689–692. doi: 10.1200/JCO.18.01412. [DOI] [PubMed] [Google Scholar]
- 39.Sestak I, Buus R, Cuzick J, et al. Comparison of the performance of 6 prognostic signatures for estrogen receptor-positive breast cancer: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2018;4:545–553. doi: 10.1001/jamaoncol.2017.5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hwang ES, Hyslop T, Hendrix LH, et al. Phase II single arm study of preoperative letrozole for estrogen receptor-positive postmenopausal ductal carcinoma in situ CALGB 40903 (Alliance) J Clin Oncol. 2020;38:1284–1292. doi: 10.1200/JCO.19.00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abubakar M, Figueroa J, Ali HR, et al. Combined quantitative measures of ER, PR, HER2, and KI67 provide more prognostic information than categorical combinations in luminal breast cancer. Mod Pathol. 2019;32:1244–1256. doi: 10.1038/s41379-019-0270-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 43.Tolaney SM, Guo H, Pernas S, et al. Seven-year follow-up analysis of adjuvant paclitaxel and trastuzumab trial for node-negative, human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2019;37:1868–1875. doi: 10.1200/JCO.19.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sheng JY, Santa-Maria CA, Mangini N, et al. Management of breast cancer during the COVID-19 pandemic: a stage- and subtype-specific approach. JCO Oncol Pract. 2020 doi: 10.1200/OP.20.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siqueira HF, Teixeira JL, Lessa Filho RS, et al. Patient satisfaction and quality of life in breast reconstruction: assessment of outcomes of immediate, delayed, and nonreconstruction. BMC Res Notes. 2020;13:223. doi: 10.1186/s13104-020-05058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weber WP, Haug M, Kurzeder C, et al. Oncoplastic Breast Consortium consensus conference on nipple sparing mastectomy. Breast Cancer Res Treat. 2018;172:523–537. doi: 10.1007/s10549-018-4937-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bennett KG, Qi J, Kim HM, Hamill JB, Pusic AL, Wilkins EG. Comparison of 2-year complication rates among common techniques for postmastectomy breast reconstruction. JAMA Surg. 2018;153:901–908. doi: 10.1001/jamasurg.2018.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meresse T, Chaput B, Grolleau JL, Gangloff D. Complications of autologous breast reconstruction. Ann Chir Plast Esthet. 2019;64:594–619. doi: 10.1016/j.anplas.2019.07.015. [DOI] [PubMed] [Google Scholar]
- 49.Huo J, Smith BD, Giordano SH, Reece GP, Shih Y-CT. Post-mastectomy breast reconstruction and its subsequent complications: a comparison between obese and non-obese women with breast cancer. Breast Cancer Res Treat. 2016;157:373–383. doi: 10.1007/s10549-016-3832-x. [DOI] [PubMed] [Google Scholar]
- 50.Moberg IO, Bredal IS, Schneider MR, Tønseth KA, Schlichting E. Complications, risk factors, and patients-reported outcomes after skin-sparing mastectomy followed by breast reconstruction in women with BRCA mutations. J Plast Surg Hand Surg. 2018;52:234–239. doi: 10.1080/2000656X.2018.1470093. [DOI] [PubMed] [Google Scholar]
- 51.Gschwantler-Kaulich D, Leser C, Salama M, Singer CF. Direct-to-implant breast reconstruction: higher complication rate vs cosmetic benefits. Breast J. 2018;24:957–964. doi: 10.1111/tbj.13113. [DOI] [PubMed] [Google Scholar]
- 52.Massenburg BB, Sanati-Mehrizy P, Ingargiola MJ, Rosa JH, Taub PJ. Flap failure and wound complications in autologous breast reconstruction: a national perspective. Aesthet Plast Surg. 2015;39:902–909. doi: 10.1007/s00266-015-0575-8. [DOI] [PubMed] [Google Scholar]
- 53.Simpson AM, Donato DP, Kwok AC, Agarwal JP. Corrigendum to "Predictors of complications following breast reduction surgery: A National Surgical Quality Improvement Program study of 16,812 cases" [J Plast Reconstr Aesthet Surg 72 (2019) 43–51] J Plast Reconstr Aesthet Surg. 2019;72:1434–1435. doi: 10.1016/j.bjps.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 54.Poggio F, Tagliamento M, Di Maio M, et al. Assessing the impact of the COVID-19 outbreak on the attitudes and practice of Italian oncologists toward breast cancer care and related research activities. JCO Oncol Pract. 2020 doi: 10.1200/OP.20.00297. [DOI] [PubMed] [Google Scholar]
- 55.Gasparri ML, Gentilini OD, Lueftner D, Kuehn T, Kaidar-Person O, Poortmans P. Changes in breast cancer management during the Corona Virus Disease 19 pandemic: an international survey of the European Breast Cancer Research Association of Surgical Trialists (EUBREAST) Breast. 2020;52:110–115. doi: 10.1016/j.breast.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]