Abstract
目的
探讨三维可视化3D打印在Bismuth-Corlette Ⅲ、Ⅳ型肝门部胆管癌个体化精准外科治疗中的应用价值。
方法
回顾性分析南方医科大学珠江医院肝胆外科2016年5月~2019年3月在三维可视化3D打印指导下10例肝门部胆管癌外科手术治疗患者。收集患者薄层CT数据,进行三维重建后打印3D模型,观察肿瘤与肝内胆管、肝动脉、门静脉和肝静脉系统的三维立体关系,进行术前模拟手术并制定手术方案,将3D打印模型带入手术室进行术中实时导航,指导手术治疗。
结果
10例患者均成功构建三维可视化打印3D模型,进行Bismuth-Corlette三维可视化分型:Ⅲa型4例、Ⅲb型4例、Ⅳ型2例,其中门静脉变异4例,肝动脉变异3例。门静脉“三分叉”变异2例;“工字型”变异1例;1例罕见的门静脉右前支缺如变异;2例既有门静脉变异又出现肝动脉变异。肝动脉变异3例,1例肝左动脉起自胃左动脉,2例肝右动脉起自肠系膜上动脉。其中Ⅲb型4例行左半肝切除术; Ⅲa型4例行右半肝切除;Ⅳ型1例行围肝门区域切除,1例行左半肝切除术。此组患者术前三维可视化3D打印模型及术前规划与术中情况均一致。手术时间452±75.12 min,术中出血量356±62.35 mL,术后住院时间15±4.61 d。术后出现胆漏1例, 少量胸腔积液3例,经通畅引流及内科治疗后康复出院,围手术期无肝功能衰竭及死亡病例。
结论
三维可视化联合3D打印对Bismuth-Corlette Ⅲ、Ⅳ肝门部胆管癌进行准确的术前评估、手术规划,优化手术方案,尤其在肝内血管变异情况下,有助于提高手术安全性,降低手术风险。
Keywords: 三维可视化, 3D打印, 肝门部胆管癌, 手术规划, 肝切除术
Abstract
Objective
To explore the application of 3D visualization and 3D printing in individualized precision surgical treatment of Bismuth-Corlette type Ⅲ and Ⅳ hilar cholangiocarcinoma.
Methods
We retrospectively analyzed the data of 10 patients with hilar cholangiocarcinoma undergoing surgeries under the guidance of 3D visualization and 3D printing in the Department of Hepatobiliary Surgery, Zhujiang Hospital from May 2016 to March 2019. Thin-section CT data of the patients were collected for 3D reconstruction and 3D printing, and the 3D printed models were used for observing the 3D relationship of tumor with the intrahepatic bile duct, hepatic artery, portal vein and hepatic vein system and for performing preoperative simulated surgery and surgical planning. The 3D printed models were subsequently used for real-time intraoperative navigation to guide surgeries in the operating room.
Results
3D visualization models were successfully reconstructed for all the 10 patients and printed into 3D models. The 3D visualization types in Bismuth-Corlette classification included type Ⅲa (4 cases), type Ⅲb (4 cases), and type Ⅳ (2 cases); 4 patients showed portal vein variation, 3 had hepatic artery variation, and 2 had both portal vein and hepatic artery variations. Two patients were found to have trifurcation type of portal vein variation, one had "I-shaped" variation, and one showed the absence of the right anterior branch of the portal vein; 3 patients had hepatic artery variations with the left hepatic artery originating from the left gastric artery (1 case) and the right hepatic artery originating from the superior mesenteric artery (2 cases). Four patients with type Ⅲb underwent left hepatectomy; 4 with type Ⅲa received right hepatectomy; 1 patient with of type Ⅳ received peripheral hepatic resection and another underwent left hepatectomy. The results of preoperative 3D reconstruction, 3D printed model and preoperative planning were consistent with the intraoperative findings. The operative time was 452±75.12 min with a mean intraoperative blood loss of 356±62.35 mL and a mean hospital stay of 15 ± 4.61 days in these cases. One patient had bile leakage and 3 patients had pleural effusion postoperatively, and they were discharged after drainage and medications. No liver failure or death occurred in these cases perioperatively.
Conclusion
3D visualization and 3D printing can facilitate accurate preoperative assessment, surgical planning and surgical procedure optimization for Bismuth-Corlette type Ⅲ and Ⅳ hilar cholangiocarcinoma to improve surgical safety and reduce surgical risks especially in cases of intrahepatic vascular variations.
Keywords: three-dimensional visualization, three-dimensional printing, hilar cholangiocarcinoma, surgical planning, hepatectomy
肝门部胆管癌(HCCA)起病隐匿、早期诊断困难、疾病进展迅速,整体预后不佳[1]。根治性切除术是首选的治疗方式,但由于肝门部胆管癌生长部位解剖结构复杂,胆管和血管变异率高,尤其是Bismuth-CorletteⅢ型、Ⅳ型患者,肿瘤已侵犯肝总管、左右肝管及二级胆管开口,且多伴有肝动脉、门静脉及肝内外胆管的侵犯及肝内管道的变异,如术前不能准确辨析及术前规划,导致根治性切除率较低,术后并发症发生率及死亡率较高[2]。如何进行精准的术前评估,优化手术方案,获得根治性切除(R0切除)是目前提高肝门部胆管癌患者长期生存率的关键[3-5]。
我科将三维可视化技术应用于肝胆胰外科已达10余年,取得了显著的效果[6]。查阅国内外文献关于三维可视化联合3D打印在肝门部胆管癌中的应用未见报道。近期我科将三维可视化联合3D打印技术应用于Bismuth-Corlette Ⅲ型、Ⅳ型肝门部胆管癌患者的术前诊断、手术规划及术中实时导航中取得了较满意的效果。
1. 资料和方法
1.1. 一般资料
回顾性分析南方医科大学珠江医院肝胆外科2016年5月~2019年3月10例Bismuth-Corlette Ⅲ、Ⅳ型肝门部胆管癌患者三维可视化3D打印指导下外科手术治疗的临床资料。其中男7例,女3例,年龄29~73(57.2± 5.61)岁。术前肝功能Child-Pugh A级8例,B级2例经治疗后达到A级;总胆红素197.2±43.71 μmol/L,直接胆红素102.4±15.01 μmol/L,谷丙转氨酶73.54 ±14.12 U/L;白蛋白35.15±7.36 g/L;CA-199(336±79.62)IU/mL;3例术前留置PTCD,2例肝吸虫抗体(+);1例HBAg(+)。所有患者均签署知情同意书,经南方医科大学珠江医院伦理委员会批准。
纳入标准:①术前肝功能评估Child-Pugh A~B级,能耐受大部分肝切除手术;②Bismuth-corlette Ⅲ、Ⅳ型患者;③无远处脏器及淋巴结转移。
排除标准:①Bismuth-corletteⅠ型、Ⅱ型患者;②合并其他严重心肺病变不能耐受手术者;③既往曾行手术治疗,术后复发患者;④肝功能Child-Pugh C级者。
1.2. 研究方法
采用256层螺旋CT进行上腹部肝、胆、胰、脾和腹腔血管增强扫描,采集患者平扫期、动脉期、门静脉期和静脉期的薄层(DICOM)数据。将各期DICOM数据导入本中心自行研发的腹部医学图像MI-3DVS软件系统中,构建三维模型。
将三维重建模型转换为标准模板库STL文件,并导入到快速成型软件ZEditTM3.21(3D systems)中,采用Spectrum ZTM 510(3D systems)打印机打印,等比例打印实体3D模型[7]。
1.3. 观察指标
(1)术前手术规划及实际手术中血管变异是否与术前三维重建3D打印模型相一致;(2)手术时间、术中出血量、术后住院时间、并发症和围手术期死亡率;(3)术后复查及随访所有患者术后随访1年,1~6月每月进行上腹部增强CT检查。无复发及死亡病例。
1.4. 统计分析
服从正态分布的计量资料进行独立样本t检验分析,对不服从正态分布的计量资料采用Wilcoxon秩和检验分析,采用卡方检验和fisher精确检验统计分析计数资料;以P<0.05为差异有统计学意义。
2. 结果
2.1. 三维重建及3D打印分析
10例患者均成功构建三维重建后打印3D模型,进行Bismuth-Corlette三维可视化分型:Ⅲa型4例、Ⅲb型4例、Ⅳ型2例,其中门静脉变异4例,肝动脉变异3例;2例既有门静脉变异又出现肝动脉变异。门静脉按Cheng氏分型[8]:“三分叉”变异2例(图 2),“工字型”变异1例;1例罕见右前支门静脉缺如,由门静脉左支发出分支支配Ⅴ、Ⅷ段肝脏(图 1)。肝动脉变异3例,肝左动脉来自胃左动脉1例;肝右动脉来自肠系膜上动脉2例[9](表 1)。
2.
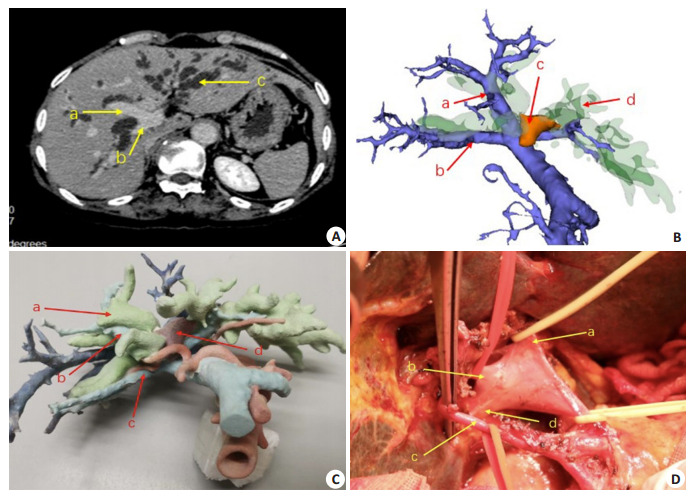
门静脉“三分叉”变异病例
A case showing trifurcation variation of the portal vein. A: Contrast-enhanced CT scan of the upper abdomen. a: The right anterior portal vein; b: The right posterior portal vein; B: 3D reconstructed model, a: The right anterior portal vein; b: The right posterior portal vein; c: The tumor; d: The dilated bile duct; C: Preoperative 3D printed model, a: The dilated bile duct; b: The right anterior portal vein; c: The right posterior portal vein; d: The tumor; D: Intraoperative dissociation of the first porta hepatis, a: The left portal vein; b: The right anterior portal vein; c: The right hepatic artery; d: The right posterior portal vein.
1.
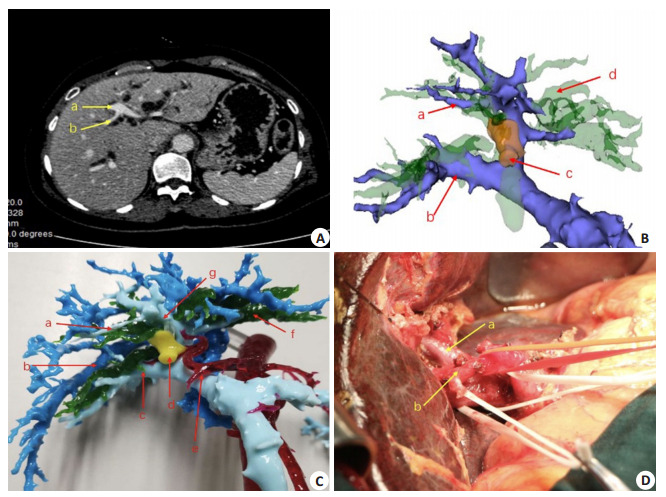
门静脉右前支来自门静脉左支变异病例
A variant case showing the right anterior portal vein derived from the left portal vein. A: Contrastenhanced CT scan of the upper abdomen a: The left portal vein; b: Portal vein of segment Ⅷ; B: 3D reconstructed model a: Portal vein of segment Ⅷ, arising from portal vein of segment Ⅳ; b: The right portal vein; c: The tumor; d: The dilated bile duct Preoperative 3D reconstructed model (portal vein of segment Ⅳ innervates segment Ⅷ); C: Preoperative 3D printed model a: Portal vein of segment Ⅷ, arising from portal vein of segment Ⅳ; b: The hepatic vein; c: The right portal vein; d: The tumor; e: The hepatic artery; f: The dilated bile duct; D: Intraoperative dissociation of the first porta hepatis a: The portal vein; b: The hepatic artery.
1.
手术资料
Surgical data of the patients (n=10)
| Case | Cheng's classification of the portal vein | Michels' classification of the the hepatic artery | Preoperative planning | Actual operation method (radical resection forhilar cholangiocarcinoma) | Pathology result | ||||||||||||
| CT | 3D | Intraoperative result | CT | 3D | Intraoperative result | CT | 3D | Intraoperative result | 3D planning | ||||||||
| 3D: 3D visualization; RAPV: The right anterior branch of the portal vein. | |||||||||||||||||
| 1 | Ⅳ | Ⅳ | Ⅳ | - | Ⅲ | Ⅲ | - | Ⅲ | Ⅲ | Perihilar hepatectomy | Perihilar hepatectomy | Moderately to poorly differentiated adenocarcinoma | |||||
| 2 | Ⅳ | Ⅳ | Ⅳ | - | Ⅰ | Ⅰ | - | Ⅰ | Ⅰ | Left hemihepatectomy | Left hemihepatectomy | Moderately differentiated cholangiocarcinoma | |||||
| 3 | Ⅳ | Ⅲb | Ⅲb | - | Ⅱ | Ⅱ | - | Ⅱ | Ⅱ | Left hemihepatectomy | Left hemihepatectomy | Moderately to poorly differentiated cholangiocarcinoma | |||||
| 4 | Ⅲa | Ⅲa | Ⅲa | - | Ⅰ | Ⅰ | - | Ⅰ | Ⅰ | Right hemihepatectomy | Right hemihepatectomy | Moderately to poorly differentiated cholangiocarcinoma | |||||
| 5 | Ⅲa | Ⅲa | Ⅲa | - | Ⅱ | Ⅱ | - | Ⅰ | Ⅰ | Right hemihepatectomy | Right hemihepatectomy | poorly differentiated adenocarcinoma | |||||
| 6 | Ⅲb | Ⅲb | Ⅲb | - | Ⅰ | Ⅰ | - | Ⅰ | Ⅰ | Left hemihepatectomy | Left hemihepatectomy | Moderately differentiated cholangiocarcinoma | |||||
| 7 | Ⅲb | Ⅲb | Ⅲb | - | RAPV absent | RAPV absent | - | Ⅰ | Ⅰ | Left hemihepatectomy | Left hemihepatectomy | Moderately differentiated cholangiocarcinoma | |||||
| 8* | Ⅲa | Ⅲa | Ⅲa | - | Ⅰ | Ⅰ | - | Ⅱ | Ⅱ | Right hemihepatectomy | Right hemihepatectomy | Moderately differentiated cholangiocarcinoma | |||||
| 9 | Ⅲb | Ⅲb | Ⅲb | - | Ⅰ | Ⅰ | - | Ⅰ | Ⅰ | Left hemihepatectomy | Left hemihepatectomy | Moderately differentiated cholangiocarcinoma | |||||
| 10 | Ⅲa | Ⅲa | Ⅲa | - | Ⅰ | Ⅰ | - | Ⅰ | Ⅰ | Right hemihepatectomy | Right hemihepatectomy | Moderately differentiated cholangiocarcinoma | |||||
2.2. 手术及围手术期情况
10例患者实际手术方式与术前3D规划完全一致,均在3D打印模型实时导航下进行肝门部胆管癌根治术。术中所见脉管变异及肿瘤浸润情况与术前3D打印模型相一致。其中Ⅲb型4例行左半肝切除术;Ⅲa型4例行右半肝切除;Ⅳ型1例行围肝门区域切除;1例行左半肝切除术。手术时间452±75.12 min,术中出血量356±62.35 mL,术后住院时间15±4.61 d。术后出现胆漏1例,少量胸腔积液3例,经通畅引流及内科治疗后康复出院,围手术期无出血、肝功能衰竭及死亡病例。
3. 讨论
3.1. 三维可视化、3D打印在肝脏外科中的应用
三维可视化技术在肝脏外科的应用已达十余年,取得了很好的社会效益[10]。但仍有一些不足,三维模型只能从二维的电脑屏幕上观看,如果术中需要调整角度观看会使解剖空间位置发生改变,缺少实体质感等。
近年来,3D打印技术临床应用在国内外迅猛发展[11-12]。该技术在肝脏外科也展现出了广泛的应用前景:Igami等[13]采用肝脏3D打印模型对术中B超未能发现的肝脏小病灶进行肝脏切除线的界定,完成了肝癌根治性切除术。Zein等[14]在活体肝移植中,采用3D肝脏物理模型对3个供体肝脏和3个受体的肝脏分别进行半透明3D打印,表明3D打印模型能准确评估肝体积,通过术中实时调整3D打印模型,可帮助关键部位的解剖定位、肝离断面的脉管处理,顺利完成肝切除术,减少手术时间和减少并发症。Takagi等[15]采用3D肝脏物理模型指导下顺利完成了肝内胆管癌患者行大部分肝切除术。国内方驰华等[7]开展三维可视化、3D打印联合3D腹腔镜应用于肝脏肿瘤外科,实现肝癌的精准切除。陈攀等[16]自主设计3 D打印截肝导板在肝癌切除手术中的应用,能够选择最佳手术入路、在保证切缘同时最大限度保护剩余肝组织,减少出血量和副损伤。
3D打印是三维可视化技术的进一步延伸发展,实现了三维虚拟图像向实体三维物理模型的跨越式转变,使得外科医生可以从多个维度清晰立体的观察胆管和血管的变异情况、肿瘤横向及纵向浸润情况、判断肿瘤是否侵犯血管以及肿瘤的可切除性,基于3D打印技术进行术前模拟手术,设计不同手术入路和切除范围,选择最佳的个体化的手术方案,达到精准肝切除的目的[17]。本组病例中术前构建三维模型3D打印,清楚显示肿瘤的位置及其与肝内各管道的关系,提供更全面的个体化信息,术前详细了解肝门部解剖信息及有无血管的变异,根据血管变异情况制定个体化的手术方案,避免术中副损伤,提高手术的安全性,最大限度的降低手术风险和并发症[10]。此组患者均成功进行手术治疗,围手术期无肝功能衰竭及死亡病例。
3.2. 3D打印在肝门部胆管癌肝内管道变异肝切除术中的意义
临床上腹腔动脉、门静脉、肝静脉变异十分常见[18],目前CT、MRI等传统影像学检查提供的二维图像信息并不直观,术前无法准确判断血管变异的类型。另外,肝门部解剖结构复杂,如术前对肝内管道变异无法准确了解,而贸然进行手术治疗,难免出现副损伤,导致严重并发症。因此术前准确辨析肝门部胆管癌有无肝内管道的变异,显得尤为重要。
3.2.1. 3D打印对肝动脉变异肝切除术中的意义
临床上在肝切除术中,肝动脉变异十分常见[19]。因此,术前对肝门部胆管癌手术治疗时,术前应用三维可视化3D打印模型辨析各型肝动脉的变异,防止术中副损伤十分重要。一旦肝动脉损伤常伴有肝脏缺血,导致术后肝功能异常;胆管血供障碍,导致缺血性胆病、胆肠吻合口缺血、胆漏,远期发生胆管狭窄等严重并发症[20]。临床上常见肝动脉变异类型有:肝左动脉起自胃左动脉;肝右动脉起自肠系膜上动脉;肝总动脉起自肠系膜上动脉。此组10例患者中有1例患者肝左动脉来自胃左动脉,因患者为Ⅲb型患者,则肝左动脉不在常规解剖位置走行,因此行左半肝切除术时,手术时应该沿胃左动脉位置走形寻找变异的肝左动脉,避免过多游离,防止副损伤。2例患者肝右动脉来自肠系膜上动脉,其中1例患者为Ⅲ b型患者,需行左半肝切除术,因此术中当游离第一肝门时,应注意不要过多游离,防止损伤变异的肝右动脉,防止副损伤。另外1例为Ⅳ型患者,术前手术规划拟行围肝门部区域切除术,因此当游离第一肝门时,应注意不要过多大范围游离,防止损伤变异的肝右动脉,防止副损伤[21]。
3.2.2. 3D打印对门静脉变异肝门部胆管癌肝切除术中的意义
临床上Ⅲ、Ⅳ型肝门部胆管癌患者,常伴有胆汁淤积性肝硬化,肿瘤侵犯门静脉,需要大范围肝切除术。因此术前准确辨析有无门静脉变异尤为重要。此组患者中门静脉“三分叉”变异2例。构建三维可视化3D打印模型,肿瘤位于门静脉左支上方,未侵犯门静脉主干及左支。肝切除极限点(P点)位置应向第一肝门前移,此时断肝切面距离门静脉右前支已非常临近,术中应避免损伤门静脉右前支。而术前通过3D打印模型可清晰定位胆管切除的极限点的部位,进行术前规划及体积计算,拟行左半肝切除术,术中保护门静脉右前支,避免损伤。术中情况与术前三维重建3D打印模型相一致,在肝外分离出门静脉右前支、右后支予置带保护,避免损伤门静脉右前支,继续向左侧解剖发现左支进入矢状裂,予置带牵引,均暂未离断;试夹门静脉左支,发现左半肝颜色明显变暗,右半肝颜色正常,于此处结扎切断门静脉左支、左肝动脉及肝左静脉,完成左半肝联合尾状叶切除、肝十二指肠韧带骨骼化清扫、右肝管成形、空肠Roux-Y吻合术。病理诊断:胆管细胞癌,肝脏切缘病理阴性(图 2)。
此组患者中见1例罕见门静脉右前支缺如,由门静脉左支发出分支配Ⅴ、Ⅷ段肝组织。患者为BismuthCorlette分型Ⅲb型,术前手术规划行左半肝切除术,术前考虑患者门静脉右前支缺如,行左半肝切除时,无法避免导致部分Ⅴ、Ⅷ段肝组织缺血,相当于行扩大左半肝切除术。术中行左侧门静脉临时控制后,在外周注射ICG,正常情况下会右肝呈强荧光而左肝未见强荧光。如果经外周注射ICG后右肝出现荧光显示后逐渐左肝也显示荧光,说明右侧门静脉有侧支支配左侧肝组织,因此行顺利行左半肝联合尾状叶切除、肝十二指肠韧带骨骼化清扫、右肝管成形、空肠Roux-Y吻合术。术后出现一过性转氨酶增高,经积极护肝后患者康复出院。病理诊断:胆管细胞癌,肝脏切缘病理阴性。徐安书[22]应用3D打印技术对肝脏巨大肿瘤肝内管道情况进行术前评估,对规范肝切除有很大的帮助。能降低并发症发生率,尤其是在出现肝内血管变异情况下,与此项研究结果相似。
三维可视化3D打印技术对复杂的Ⅲ、Ⅳ型肝门部胆管癌患者手术进行个体化手术规划,术中实时精准导航,尤其肝内管道变异的情况下,降低手术风险,避免副损伤,减少手术出血量[23],为肝门部胆管癌患者的精准外科治疗提供了一种新的方法。实现了“看得到、看得清、摸得着、切得准”的跨越式转变,有着广泛且光明的前景。但是目前还伴随有打印效率较低,费用较高等问题。在不久将来,这些问题得以解决,在临床中的应用会更深入广泛。目前3D生物打印活体肝组织也会在肝脏外科中发挥更大的作用,为医学的发展提供强大的助力。
Biography
曾宁,副主任医师,博士,E-mail: 867991031@qq.com
Funding Statement
“十三五”国家重点研发计划数字诊疗装备研发重点专项(2016YFC0106500);国家自然科学基金重大科研仪器研制项目(81627805);NSFC-广东联合基金项目(U1401254);广东省科技计划项目(2017ZC0110);广州市科技计划项目(201604020144)
Contributor Information
曾 宁 (Ning ZENG), Email: 867991031@qq.com.
方 驰华 (Chihua FANG), Email: fangch_dr@163.com.
References
- 1.Lee Y, Choi D, Han S, et al. Comparison analysis of left-side versus right-side resection in bismuth type Ⅲ hilar cholangiocarcinoma. Ann Hepatobiliary Pancreat Surg. 2018;22(4):350–8. doi: 10.14701/ahbps.2018.22.4.350. [Lee Y, Choi D, Han S, et al. Comparison analysis of left-side versus right-side resection in bismuth type Ⅲ hilar cholangiocarcinoma[J]. Ann Hepatobiliary Pancreat Surg, 2018, 22(4): 350-8.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu HJ, Jin YW, Shrestha A, et al. Predictive factors of early recurrence after R0 resection of hilar cholangiocarcinoma: a single institution experience in China. Cancer Med. 2019;8(4):1567–75. doi: 10.1002/cam4.2052. [Hu HJ, Jin YW, Shrestha A, et al. Predictive factors of early recurrence after R0 resection of hilar cholangiocarcinoma: a single institution experience in China[J]. Cancer Med, 2019, 8(4): 1567-75.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Y, Zhou ZY, Liu R, et al. Application of 3D visualization and 3D printing technology on ERCP for patients with hilar cholangiocarcinoma. http://europepmc.org/article/MED/29545843. Exp Ther Med. 2018;15(4):3259–64. doi: 10.3892/etm.2018.5831. [Yang Y, Zhou ZY, Liu R, et al. Application of 3D visualization and 3D printing technology on ERCP for patients with hilar cholangiocarcinoma[J]. Exp Ther Med, 2018, 15(4): 3259-64.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao HP, Zhang ZH, Zheng AH. Assessment of treatment outcomes in patients with advanced hilar cholangiocarcinoma (stages Ⅲ-Ⅳ): Clinical significance of interventional therapy. Medicine (Baltimore) 2018;97(39):e11550. doi: 10.1097/MD.0000000000011550. [Zhao HP, Zhang ZH, Zheng AH. Assessment of treatment outcomes in patients with advanced hilar cholangiocarcinoma (stages Ⅲ-Ⅳ): Clinical significance of interventional therapy[J]. Medicine (Baltimore), 2018, 97(39): e11550.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeng N, Tao HS, Fang CH, et al. Individualized preoperative planning using three-dimensional modeling for Bismuth and Corlette type Ⅲ hilar cholangiocarcinoma. World J Surg Oncol. 2016;14(1):44. doi: 10.1186/s12957-016-0794-8. [Zeng N, Tao HS, Fang CH, et al. Individualized preoperative planning using three-dimensional modeling for Bismuth and Corlette type Ⅲ hilar cholangiocarcinoma[J]. World J Surg Oncol, 2016, 14(1): 44.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.中华医学会数字医学分会, 中国研究型医院学会数字智能化外科专业委员会, 中华医学会外科学分会胆道外科学组, et al. 肝门部胆管癌三维可视化精准诊治中国专家共识(2019版) http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgsywkzz202003006. 中国实用外科杂志. 2020;40(3):260–6. [中华医学会数字医学分会, 中国研究型医院学会数字智能化外科专业委员会, 中华医学会外科学分会胆道外科学组, 等.肝门部胆管癌三维可视化精准诊治中国专家共识(2019版)[J].中国实用外科杂志, 2020, 40(3): 260-6.] [Google Scholar]
- 7.方 驰华, 方 兆山, 范 应方, et al. 三维可视化、3D打印及3D腹腔镜在肝肿瘤外科诊治中的应用. http://www.j-smu.com/oa/DArticle.aspx?type=view&id=201505639. 南方医科大学学报. 2015;35(5):639–45. [方驰华, 方兆山, 范应方, 等.三维可视化、3D打印及3D腹腔镜在肝肿瘤外科诊治中的应用[J].南方医科大学学报, 2015, 35(5): 639-45.] [PubMed] [Google Scholar]
- 8.Khamanarong K, Woraputtaporn W, Amarttayakong P, et al. Classification of portal vein tributaries in Thai cadavers including a new type Ⅴ. Surg Radiol Anat. 2016;38(6):735–9. doi: 10.1007/s00276-015-1592-7. [Khamanarong K, Woraputtaporn W, Amarttayakong P, et al. Classification of portal vein tributaries in Thai cadavers including a new type Ⅴ[J]. Surg Radiol Anat, 2016, 38(6): 735-9.] [DOI] [PubMed] [Google Scholar]
- 9.Michels NA. Newer anatomy of the liver and its variant blood supply and collateral circulation. Am J Surg. 1966;112(3):337–47. doi: 10.1016/0002-9610(66)90201-7. [Michels NA. Newer anatomy of the liver and its variant blood supply and collateral circulation[J]. Am J Surg, 1966, 112(3): 337- 47.] [DOI] [PubMed] [Google Scholar]
- 10.Chinese Society of Digital Medicine, Liver Cancer Committee of Chinese Medical Doctor Association, Clinical Precision Medicine Committee of Chinese Medical Doctor Association, Digital Intelligent Surgery Committee of Chinese Research Hospital Association Clinical practice guidelines for precision diagnosis and treatment of complex liver tumor guided by three-dimensional visualization technology (version 2019) http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=dyjydxxb202003001. J South Med Univ. 2020;40(3):297–307. doi: 10.12122/j.issn.1673-4254.2020.03.01. [Chinese Society of Digital Medicine; Liver Cancer Committee of Chinese Medical Doctor Association; Clinical Precision Medicine Committee of Chinese Medical Doctor Association; Digital Intelligent Surgery Committee of Chinese Research Hospital Association. Clinical practice guidelines for precision diagnosis and treatment of complex liver tumor guided by three-dimensional visualization technology (version 2019)[J]. J South Med Univ, 2020, 40(3): 297-307.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun ZH. 3D printing in medicine: current applications and future directions. Quant Imaging Med Surg. 2018;8(11):1069–77. doi: 10.21037/qims.2018.12.06. [Sun ZH. 3D printing in medicine: current applications and future directions[J]. Quant Imaging Med Surg, 2018, 8(11): 1069-77.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun Z, Liu D. A systematic review of clinical value of threedimensional printing in renal disease. http://europepmc.org/abstract/MED/29774184. Quant Imaging Med Surg. 2018;8(3):311–25. doi: 10.21037/qims.2018.03.09. [Sun, Z, Liu D. A systematic review of clinical value of threedimensional printing in renal disease[J]. Quant Imaging Med Surg, 2018, 8(3): 311-25.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Igami T, Nakamura Y, Hirose T, et al. Application of a threedimensional print of a liver in hepatectomy for small tumors invisible by intraoperative ultrasonography: preliminary experience. World J Surg. 2014;38(12):3163–6. doi: 10.1007/s00268-014-2740-7. [Igami T, Nakamura Y, Hirose T, et al.Application of a threedimensional print of a liver in hepatectomy for small tumors invisible by intraoperative ultrasonography: preliminary experience [J]. World J Surg, 2014. 38(12): 3163-6.] [DOI] [PubMed] [Google Scholar]
- 14.Zein NN, Hanouneh IA, Bishop PD, et al. Three-dimensional print of a liver for preoperative planning in living donor liver transplantation. Liver Transpl. 2013;19(12):1304–10. doi: 10.1002/lt.23729. [Zein NN. Hanouneh IA, Bishop PD, et al. Three-dimensional print of a liver for preoperative planning in living donor liver transplantation[J]. Liver Transpl, 2013. 19(12): 1304-10.] [DOI] [PubMed] [Google Scholar]
- 15.Takagi K, Nanashima A, Abo T, et al. Three-dimensional printing model of liver for operative simulation in perihilar cholangiocarcinoma. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=19d7a87e27e457166261ca03307743c9. Hepatogastroenterology. 2014;61(136):2315–6. [Takagi K, Nanashima A, Abo T, et al. Three-dimensional printing model of liver for operative simulation in perihilar cholangiocarcinoma[J]. Hepatogastroenterology, 2014, 61(136): 2315-6.] [PubMed] [Google Scholar]
- 16.陈 攀, 谢 威, 陈 杰, et al. 自主设计3D打印截肝导板在肝癌切除手术中的初步应用. 中华肝胆外科杂志. 2019;25(9):641–4. doi: 10.3760/cma.j.issn.1007-8118.2019.09.001. [陈攀, 谢威, 陈杰, 等.自主设计3D打印截肝导板在肝癌切除手术中的初步应用[J].中华肝胆外科杂志, 2019, 25(9): 641-4.] [DOI] [Google Scholar]
- 17.曾 思略, 曾 宁, 祝 文, et al. 三维可视化联合吲哚菁绿荧光影像技术在原发性肝癌诊治中的价值. http://www.j-smu.com/oa/DArticle.aspx?type=view&id=2019121402. 南方医科大学学报. 2019;39(12):1402–8. doi: 10.12122/j.issn.1673-4254.2019.12.03. [曾思略, 曾宁, 祝文, 等.三维可视化联合吲哚菁绿荧光影像技术在原发性肝癌诊治中的价值[J].南方医科大学学报, 2019, 39(12): 1402-8.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.季 顾惟, 王 科, 夏 永祥, et al. 合并解剖变异的进展期肝门部胆管癌治疗经验. 中华外科杂志. 2017;55(11):871–4. doi: 10.3760/cma.j.issn.0529-5815.2017.11.011. [季顾惟, 王科, 夏永祥, 等.合并解剖变异的进展期肝门部胆管癌治疗经验[J].中华外科杂志, 2017, 55(11): 871-4.] [DOI] [Google Scholar]
- 19.胡 立宝, 高 健, 刘 卓, et al. 肝动脉解剖变异的CTA与DSA对照研究. 中华普通外科杂志. 2019;34(2):122–4. doi: 10.3760/cma.j.issn.1007-631X.2019.02.007. doi: 10.3760/cma.j.issn.1007-631X.2019.02.007. [胡立宝, 高健, 刘卓, 等.肝动脉解剖变异的CTA与DSA对照研究[J].中华普通外科杂志, 2019, 34(2): 122-4.] [DOI] [Google Scholar]
- 20.Noussios G, Dimitriou I, Chatzis I, et al. The main anatomic variations of the hepatic artery and their importance in surgical practice: review of the literature. J Clin Med Res. 2017;9(4):248–52. doi: 10.14740/jocmr2902w. [Noussios G, Dimitriou I, Chatzis I, et al. The main anatomic variations of the hepatic artery and their importance in surgical practice: review of the literature[J]. J Clin Med Res, 2017, 9(4): 248-52.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.李 昂, 项 灿宏, 汤 睿, et al. 肝动脉右后支变异肝门部胆管癌的诊断与治疗. 中华消化外科杂志. 2019;18(5):506–8. doi: 10.3760/cma.j.issn.1673-9752.2019.05.018. [李昂, 项灿宏, 汤睿, 等.肝动脉右后支变异肝门部胆管癌的诊断与治疗[J].中华消化外科杂志, 2019, 18(5): 506-8.] [DOI] [Google Scholar]
- 22.徐 安书, 傅 朝春, 韦 萍, et al. 3D打印技术在精准切除治疗肝脏肿瘤中的应用. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgptwkzz201801005. 中国普通外科杂志. 2018;27(1):29–34. [徐安书, 傅朝春, 韦萍, 等. 3D打印技术在精准切除治疗肝脏肿瘤中的应用[J].中国普通外科杂志, 2018, 27(1): 29-34.] [Google Scholar]
- 23.刘 文瑛, 杨 剑, 欧阳 再兴, et al. 3D打印技术在复杂性肝癌精准肝切除术中的应用. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=gdywkzz201907005. 肝胆胰外科杂志. 2019;31(7):399–403. [刘文瑛, 杨剑, 欧阳再兴, 等. 3D打印技术在复杂性肝癌精准肝切除术中的应用[J].肝胆胰外科杂志, 2019, 31(7): 399-403.] [Google Scholar]


