Abstract
A 63-year-old man with a significantly high prostate-specific antigen level was diagnosed via pathology to have advanced prostate adenocarcinoma due to multiple lung metastases. He was then treated with androgen deprivation therapy (ADT) comprising bicalutamide and goserelin. Only after 6 months of stable disease, the cancer progressed and the drug was changed to abiraterone; however, no significant therapeutic effect was observed and the disease was considered as castration-resistant prostate cancer. The histopathologic analysis of the biopsied metastatic lymph node confirmed small-cell neuroendocrine carcinoma, and genetic testing revealed BRCA1 germ-line mutation. The oral PARP inhibitor olaparib was used and achieved a partial tumor response over a period of 2.5 months. Meanwhile, palliative radiotherapy was performed for pain control in the sacrococcygeal region with complete symptom relief. The combination chemotherapy strategy of etoposide and cisplatin was used after the failure of olaparib and achieved pain alleviation in the left leg. The patient received one cycle of this chemotherapy strategy and eventually died of a rapid tumor progression, respiratory failure, and heart failure on April 27, 2019.
Keywords: prostate cancer, castration-resistant, neuroendocrine transformation, BRCA1 germ-line mutation, PARP inhibitor, chemotherapy
Introduction
According to a recent study, although the incidence of prostate cancer is stable in most countries, worldwide its incidence is among the lowest in Asia. The incidence of prostate cancer increases by 2.6% every year in China.1 Moreover, the pathological types of prostate cancer vary; small-cell neuroendocrine carcinoma was one of the rarest pathological types and accounted for approximately 1% of prostate cancers.2 It is an aggressive subtype, which was usually discovered in patients with prostate adenocarcinoma who accepted androgen deprivation therapy (ADT).3 Breast cancer susceptibility gene 1 (BRCA1) was found in a 1993 Icelandic study and is located on the long arm of chromosome 17, composing of 24 exons, which is mainly involved in the DNA-repair process and was associated with increased risk of cancer development, including breast cancer, ovarian cancer, prostate cancer, pancreatic cancer and colorectal cancer.4,5 It was reported subsequently that the relative hazard of prostate cancer in male patients with BRCA1 mutation increased 2–3 fold and survival hazard reached 30%.6,7 In addition, BRCA1/2 mutation was associated with a higher Gleason score in patients with prostate cancer.8 In this report, we demonstrate a rare case diagnosed with prostate cancer, which underwent a transformation from adenocarcinoma to small-cell neuroendocrine carcinoma after ADT and harbored BRCA1 germ-line mutation. We also reviewed related literatures on the choice of treatment for this variant phenotype.
Case Description
A 63-year-old man with dysuria and urinary frequency was admitted to our hospital in April 2018. The value of serum prostate-specific antigen (PSA) was 55.13 ng/mL (normal value: 0–4 ng/mL). The pelvic enhanced magnetic resonance imaging (MRI) showed that prostate cancer had invaded the seminal vesicle and bladder and had metastasized to multiple pelvic lymph nodes and pelvic bones. Emission computed tomography (CT) further showed that the prostate cancer had metastasized to multiple bones including T1, T2, T12, L2 and L3, left superior femur, right 4th anterior rib, right 7th, 8th posterior rib, left superior humerus, right ilium, and the left sacroiliac joint. Transrectal ultrasound-guided biopsy revealed prostate adenocarcinoma after pathological examination, with a Gleason score of 8(4 + 4). The clinical stage was determined as stage IV (T4 N1 M1b) and the patient agreed to be treated with the ADT comprising bicalutamide combined with goserelin and monthly osteoclast inhibitors since April 2018. The patient showed a response to the aforementioned therapy; dysuria disappeared and serum PSA level decreased to 0.32 ng/mL (normal value: 0–4 ng/mL). Meanwhile, subsequent positron emission tomography and pelvic enhanced MRI suggested stable disease at the follow-up in June and August 2018.
After 6 months of ADT, dysuria reappeared and serum PSA was 0.53 ng/mL (normal value: 0–4 ng/mL). The pelvic enhanced MRI indicated that the primary prostate cancer was enlarged with multiple pelvic lymph nodes and bone metastasis in October 2018. It was concluded that the tumor progressed due to the failure of ADT. The therapeutic strategy was changed to a combination of abiraterone, prednisone and bicalutamide. After 1 month of this new therapeutic strategy, dysuria and sacrococcygeal pain occurred. The degree of pain was estimated as 5 score according to the numeric rating scales (NRS). The serum PSA level was 1.5 ng/mL (normal value: 0–4 ng/mL). The serum pro-gastrin-releasing peptide (ProGRP) level was 976.2 pg/mL (normal value: 0–54.8 pg/mL). The value of neuron-specific enolase (NSE) was 212.90 ng/mL (normal value: 0–17 ng/mL). Contrast pelvic enhanced CT in December 2018 showed that the prostate tumor had increased in size and new metastases were formed in the liver and bilateral lungs, which suggested a failure of the abiraterone combined with ADT and disease progression. The disease was then considered as castration-resistant prostate cancer. Tumor markers with ProGRP and NSE indicated that the tumor may have had neuroendocrine features. Therefore, a biopsy of a metastatic lymph node in the left neck was performed and showed small-cell neuroendocrine carcinoma. The immunohistochemical result contained characteristics of neuroendocrine carcinoma, which was positive for synaptophysin (Figure 1). BRCA1 germ-line mutation was detected by genetic testing of a blood sample. A PARP inhibitor for BRCA 1 mutation, olaparib, was used to control the tumor progression. To further relieve the patient’s pain, palliative radiotherapy to the pelvic tumors including the metastatic lymph nodes and the prostate tumor was performed with 30Gy/10 fractions. Sacrococcygeal pain and dysuria were quickly relieved during palliative radiotherapy. The tumor biomarkers decreased after radiotherapy and targeted therapy (NSE from 212.90 ng/mL to 22.12 ng/mL; ProGRP from 976.2 pg/mL to 383.7 pg/mL; whereas PSA increased from 1.5 to 2.86 ng/mL). Meanwhile, the patient displayed clinical improvement. In contrast to the CT demonstration in December 2018, the pelvic enhanced CT in January 2019 showed a nearly complete tumor response in the radiation field and a partial tumor response in the region that was not covered in the radiation field (Figure 2), which verified the therapeutic effect of olaparib.
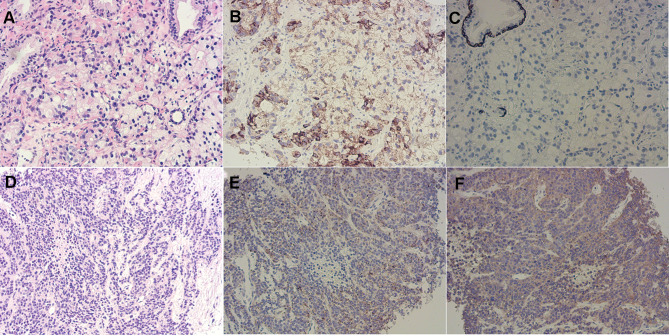
Figure 1.
Immunohistochemical result of prostate cancer. Immunohistochemical result of the initial biopsy before treatment showed moderately differentiated adenocarcinoma of the prostate (A) with Gleason score 8(4+4) and be positive for p504s (B) and CK5/6 (C) (original magnification X 200). Immunohistochemical result of the second biopsy after relapse showed small-cell carcinoma of the prostate (D) and be positive for chromogranin A (E) and synaptophysin (F) (original magnification X 200).
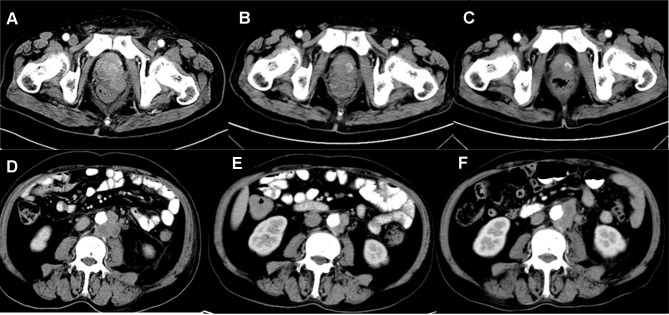
Figure 2.
Imaging material of prostate cancer. (A) Before radiotherapy combined with olaparib, computed tomography (CT) of the pelvis showed a primary prostate tumor, which invaded the bilateral seminal vesicle gland and closed to the rectum in December 2018. (B) The size of prostate tumor shrank significantly after radiotherapy combined with olaparib in January 2019. (C) Primary tumor remained stable after radiotherapy combined with olaparib in February 2019. (D) Before using olaparib, computed tomography (CT) of the pelvis showed enlarged lymph nodes adjacent to aorta abdominalis in December 2018, which was not included into the radiation field. (E) After using olaparib, lymph nodes adjacent to aorta abdominalis shrank significantly in January 2019, which was not included into the radiation field. (F) After 2.5 months with olaparib, lymph nodes adjacent to aorta abdominalis enlarged again in February 2019, which was not included into the radiation field.
However, in February 2019, the patient developed pain and numbness in the right leg and the NRS score was 6. Pelvic enhanced CT showed tumor progression (Figure 2) and with skull, sternum, bilateral upper humerus, bilateral scapula, multiple bilateral ribs, multiple spine, multiple pelvis, and bilateral femoral metastases.
Consequently, the patient was administered a combination chemotherapy of etoposide and cisplatinum (etoposide 0.1 g day 1–5 + cisplatin 40 mg day 1–3, once every 3 weeks). Moreover, olaparib was discontinued due to tumor progression. On the second day of chemotherapy, the patient felt intense pain in the neck when rotating his body, and the NRS score was estimated at 9. An emergency CT scan of the neck revealed a fracture of the second cervical vertebra. In view of the severity of the high cervical vertebra fracture and the severe pain experienced by the patient, an emergency surgery was performed in the department of orthopedics. After neck pain relief from the surgery, the previous chemotherapy strategy was re-initiated. Pain in the right leg was relieved after one cycle of chemotherapy. However, there was the repeated occurrence of severe myelosuppression, electrolyte disorder, flatulence and hypoproteinemia. The patient was then admitted to the ICU and eventually died of a rapid progression of the tumor, respiratory failure and heart failure on April 27, 2019.
Discussion
It is reported that the incidence of prostate cancer is stable in most countries; however, the incidence of prostate cancer rises by 2.6% every year in China.1 Small-cell neuroendocrine carcinoma of the prostate was one of the most uncommon pathological subtypes and accounted for approximately 1% of prostate cancers.2 Neuroendocrine transformation of prostate adenocarcinoma could be promoted by ADT and is related to the enhancer of zeste homolog 2.3 According to a previous report, the prognosis of patients with small-cell carcinoma was very poor and survival time was less than 1.5 years after diagnosis due to the rapid progression of tumor.9 Prostate cancer with neuroendocrine transformation had aggressive features and poor survival outcomes.10 The survival time of the patient in this study was only 1 year due to the rapid progression of the tumor caused by neuroendocrine transformation during ADT, in corroboration with the results of the previous study.9 Neuroendocrine cells could secret some special products which participate in the process of regulating the proliferation and differentiation of cells, such as ProGRP and NSE.11,12 Serum ProGRP could serve as a prognostic marker in patients with metastatic prostate cancer.13 In our study, the values of serum ProGRP and NSE were significantly higher than the normal level after the advent of neuroendocrine transformation and were associated with the disease state, which was consistent with some previous reports.13,14
The BRCA1 gene is located on the long arm of chromosome 17, comprising of 24 exons, which is mainly involved in the DNA-repair process.5 Moreover, BRCA 1 mutation may enhance the risk of breast cancer, ovarian cancer, prostate cancer, pancreatic cancer, and colorectal cancer.5 A recent study reported that colorectal cancer, melanoma, and pancreatic cancer play an important role in BRCA 1 pathogenic variant carriers.15 However, the father of the patient in our study had a history of lung cancer. The other family members did not have any history of cancers, including breast cancer or ovarian cancer. According to the report of Elena Castro et al, BRCA mutation in prostate cancer was associated with a higher hazard of nodal invasion, distant metastasis, and poor prognosis.16
It was indicated that the relative hazard increased 2–3 fold and the survival hazard reached 30% for patients with prostate cancer patients harboring the BRCA1 mutation.6,7 The Gleason score of the patient in this study was 8 score (4 + 4) and as reported in previous studies, patients with prostate cancer with BRCA1/2 mutations exhibited higher Gleason scores (average above 8), than those of patients with prostate cancer without BRCA1/2 mutation (average 5.9).8 In fact, a high Gleason score was a risk factor and associated with poor prognosis.17 Thus, the pathological subtype of small-cell neuroendocrine carcinoma, high Gleason score, and BRCA 1 mutation might have contributed to the poor prognosis of the patient in this study. Although a recent study showed that the outcomes of abiraterone and enzalutamide were better in patients with metastatic castration-resistant prostate cancer (mCRPC) harboring germ-line BRCA mutations than in those without BRCA mutation,18 these better outcomes were not observed in this study. Neuroendocrine carcinoma was usually discovered in patients with prostate adenocarcinoma who accepted ADT,19 as well as in our case.
Poly (ADP-ribose) polymerase (PARP) enzymes play an important role in repairing DNA single strands breaks (SSBs).20 PARP inhibitors lead to the death of tumor cells with BRCA mutations due to the accumulation of SSBs.21 On the basis of the results of Kaufman et al study, the PARP inhibitor, olaparib, was approved for advanced ovarian cancer harboring BRCA mutations by the US FDA in 2014.22 Olaparib was subsequently approved by the FDA, as a maintenance therapy for women with recurrent epithelial ovarian cancer in August 2017. Moreover, Mateo J et al reported that olaparib had a high response rate for patients with mCRPC and BRCA2 mutations in a clinical trial.23 On the basis of the excellent results of the PROfound clinical trial,24 olaparib has recently been approved for use in metastatic prostate cancer in the US. In our case, the primary tumor achieved partial response which was maintained for 2.5 months after the initiation of olaparib therapy. A previous study indicated that clinical resistance to olaparib was associated with secondary reversion mutations in the BRCA1/2 genes.25–27
According to the report of the STAMPEDE clinical trial,28 radiotherapy did not improve overall survival for patients with prostate cancer with high metastatic burden. However, local radiotherapy still played an important role in relieving pain and symptom control. In our case, radiotherapy was shown to control the sacrococcygeal pain of patient and improved the quality of life significantly.
According to previous reports,29,30 the combined chemotherapy strategy of etoposide and cisplatinum was effective for prostate neuroendocrine carcinoma. In addition, Papandreou et al reported that the response rate of the above-mentioned chemotherapy strategy was 61% and the median time to progression and overall survival time were 5.8 months and 10.5 months, respectively.31 However, severe side effects of this chemotherapy strategy in hematology were unavoidable (100%) and might influence patient survival. A previous study reported a Japanese patient diagnosed pathologically as metastatic adenocarcinoma of neuroendocrine carcinoma and harboring somatic and germ-line BRCA2 mutations, who subsequently received ADT and platinum-based doublet chemotherapy combined with etoposide and achieved partial remission of metastatic lesions.30 However, olaparib was not used in that case. Different from the Japanese study, we report a Chinese patient undergoing a small-cell neuroendocrine transformation of adenocarcinoma after ADT and harboring BRCA 1 germ-line mutation, who received olaparib therapy after the failure of ADT and displayed good therapeutic effects. The recent PROfound clinical trial verified the fact that olaparib can improve progression-free survival for men with mCRPC who had disease progression while receiving ADT and who had at least one alteration in BRCA1, BRCA2, or ATM.24 Regrettably, only one cycle of this strategy was conducted due to repeated myelosuppression in our case, though relieving the pain of the left leg. The eventual death of patient was caused by the rapid tumor progression, respiratory failure, and heart failure.
In conclusion, neuroendocrine transformation from prostate adenocarcinoma should be considered in the advent of castration-resistant during ADT, especially with increasing ProGRP and NSE levels. Targeted therapy with olaparib is a potential treatment method for patients with prostate cancer with BRAC1 mutation. Patients with prostate neuroendocrine carcinoma showed sensitivity to radiotherapy and chemotherapy strategy of etoposide combined with cisplatinum. The underlying relationship between neuroendocrine transformation and BRCA1 mutation for prostate cancer patients warrants further study.
Ethics Approval and Consent to Participate
This study was approved by the Ethics Committee of Shandong cancer hospital (Jinan, China), and written informed consent was acquired from the patient’s family for the publication of the case report and corresponding images.
Acknowledgments
This work was supported by the following grants: National Natural Science Foundation of China (Grant No. 81871895), Young Taishan Scholars and Academic Promotion Program of Shandong First Medical University (Grant No. 2019RC003).
Disclosure
All authors and their institutions have no conflicts of interest to disclose.
References
- 1.Culp MB, Soerjomataram I, Efstathiou JA, Bray F, Jemal A. Recent global patterns in prostate cancer incidence and mortality rates. Eur Urol. 2020;77(1):38–52. doi: 10.1016/j.eururo.2019.08.005 [DOI] [PubMed] [Google Scholar]
- 2.Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161(5):1215–1228. doi: 10.1016/j.cell.2015.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y, Zheng D, Zhou T, et al. Androgen deprivation promotes neuroendocrine differentiation and angiogenesis through CREB-EZH2-TSP1 pathway in prostate cancers. Nat Commun. 2018;9(1):4080. doi: 10.1038/s41467-018-06177-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arason A, Barkardottir RB, Egilsson V. Linkage analysis of chromosome 17q markers and breast-ovarian cancer in Icelandic families, and possible relationship to prostatic cancer. Am J Hum Genet. 1993;52(4):711–717. [PMC free article] [PubMed] [Google Scholar]
- 5.Faraoni I, Graziani G. Role of BRCA Mutations in Cancer Treatment with Poly(ADP-ribose) Polymerase (PARP) Inhibitors. Cancers. 2018;10(12):12. doi: 10.3390/cancers10120487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ford D, Easton DF, Bishop DT, Narod SA, Goldgar DE. Risks of cancer in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Lancet. 1994;343(8899):692–695. doi: 10.1016/S0140-6736(94)91578-4 [DOI] [PubMed] [Google Scholar]
- 7.Thompson D, Easton DF. Breast cancer linkage c. Cancer incidence in BRCA1 mutation carriers. J Natl Cancer Inst. 2002;94(18):1358–1365. doi: 10.1093/jnci/94.18.1358 [DOI] [PubMed] [Google Scholar]
- 8.Hubert A, Peretz T, Manor O, et al. The Jewish Ashkenazi founder mutations in the BRCA1/BRCA2 genes are not found at an increased frequency in Ashkenazi patients with prostate cancer. Am J Hum Genet. 1999;65(3):921–924. doi: 10.1086/302525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tetu B, Ro JY, Ayala AG, Johnson DE, Logothetis CJ, Ordonez NG. Small cell carcinoma of the prostate. Part I. A clinicopathologic study of 20 cases. Cancer. 1987;59(10):1803–1809. doi: [DOI] [PubMed] [Google Scholar]
- 10.Aggarwal R, Huang J, Alumkal JJ, et al. Clinical and genomic characterization of treatment-emergent small-cell neuroendocrine prostate cancer: a multi-institutional prospective study. J Clin Oncol. 2018;36(24):2492–2503. doi: 10.1200/JCO.2017.77.6880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Sant’Agnese PA, Cockett AT. The prostatic endocrine-paracrine (neuroendocrine) regulatory system and neuroendocrine differentiation in prostatic carcinoma: a review and future directions in basic research. J Urol. 1994;152(5 Pt 2):1927–1931. doi: 10.1016/S0022-5347(17)32417-5 [DOI] [PubMed] [Google Scholar]
- 12.Sciarra A, Mariotti G, Gentile V, et al. Neuroendocrine differentiation in human prostate tissue: is it detectable and treatable? BJU Int. 2003;91(5):438–445. doi: 10.1046/j.1464-410X.2003.03066.x [DOI] [PubMed] [Google Scholar]
- 13.Yashi M, Nukui A, Kurokawa S, et al. Elevated serum progastrin-releasing peptide (31-98) level is a predictor of short response duration after hormonal therapy in metastatic prostate cancer. Prostate. 2003;56(4):305–312. doi: 10.1002/pros.10260 [DOI] [PubMed] [Google Scholar]
- 14.Heck MM, Thaler MA, Schmid SC, et al. Chromogranin A and neurone-specific enolase serum levels as predictors of treatment outcome in patients with metastatic castration-resistant prostate cancer undergoing abiraterone therapy. BJU Int. 2017;119(1):30–37. doi: 10.1111/bju.13493 [DOI] [PubMed] [Google Scholar]
- 15.Silvestri V, Leslie G, Barnes DR, et al. Characterization of the cancer spectrum in men with germline BRCA1 and BRCA2 pathogenic variants: results from the consortium of investigators of modifiers of BRCA1/2 (CIMBA). JAMA Oncol. 2020. doi: 10.1001/jamaoncol.2020.2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castro E, Goh C, Olmos D, et al. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol. 2013;31(14):1748–1757. doi: 10.1200/JCO.2012.43.1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valero J, Peleteiro P, Henriquez I, et al. Age, Gleason Score, and PSA are important prognostic factors for survival in metastatic castration-resistant prostate cancer. Results of the Uroncor group (uro-oncological tumors) of the Spanish Society of Radiation Oncology (SEOR). Clin Transl Oncol. 2020;22(8):1378–1389. doi: 10.1007/s12094-019-02274-w [DOI] [PubMed] [Google Scholar]
- 18.Antonarakis ES, Lu C, Luber B, et al. Germline DNA-repair gene mutations and outcomes in men with metastatic castration-resistant prostate cancer receiving first-line abiraterone and enzalutamide. Eur Urol. 2018;74(2):218–225. doi: 10.1016/j.eururo.2018.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palmgren JS, Karavadia SS, Wakefield MR. Unusual and underappreciated: small cell carcinoma of the prostate. Semin Oncol. 2007;34(1):22–29. doi: 10.1053/j.seminoncol.2006.10.026 [DOI] [PubMed] [Google Scholar]
- 20.Schreiber V, Dantzer F, Ame JC, de Murcia G. Poly(ADP-ribose): novel functions for an old molecule. Nat Rev Mol Cell Biol. 2006;7(7):517–528. doi: 10.1038/nrm1963 [DOI] [PubMed] [Google Scholar]
- 21.Helleday T, Petermann E, Lundin C, Hodgson B, Sharma RA. DNA repair pathways as targets for cancer therapy. Nat Rev Cancer. 2008;8(3):193–204. doi: 10.1038/nrc2342 [DOI] [PubMed] [Google Scholar]
- 22.Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33(3):244–250. doi: 10.1200/JCO.2014.56.2728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mateo J, Carreira S, Sandhu S, et al. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373(18):1697–1708. doi: 10.1056/NEJMoa1506859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382(22):2091–2102. doi: 10.1056/NEJMoa1911440 [DOI] [PubMed] [Google Scholar]
- 25.Edwards SL, Brough R, Lord CJ, et al. Resistance to therapy caused by intragenic deletion in BRCA2. Nature. 2008;451(7182):1111–1115. doi: 10.1038/nature06548 [DOI] [PubMed] [Google Scholar]
- 26.Sakai W, Swisher EM, Karlan BY, et al. Secondary mutations as a mechanism of cisplatin resistance in BRCA2-mutated cancers. Nature. 2008;451(7182):1116–1120. doi: 10.1038/nature06633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christie EL, Fereday S, Doig K, Pattnaik S, Dawson SJ, Bowtell DDL. Reversion of BRCA1/2 germline mutations detected in circulating tumor DNA from patients with high-grade serous ovarian cancer. J Clin Oncol. 2017;35(12):1274–1280. doi: 10.1200/JCO.2016.70.4627 [DOI] [PubMed] [Google Scholar]
- 28.Parker CC, James ND, Brawley CD, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled Phase 3 trial. Lancet. 2018;392(10162):2353–2366. doi: 10.1016/S0140-6736(18)32486-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amato RJ, Logothetis CJ, Hallinan R, Ro JY, Sella A, Dexeus FH. Chemotherapy for small cell carcinoma of prostatic origin. J Urol. 1992;147(3 Pt 2):935–937. doi: 10.1016/S0022-5347(17)37427-X [DOI] [PubMed] [Google Scholar]
- 30.Kosaka T, Hongo H, Aimono E, et al. A first Japanese case of neuroendocrine prostate cancer accompanied by lung and brain metastasis with somatic and germline BRCA2 mutation. Pathol Int. 2019;69(12):715–720. doi: 10.1111/pin.12860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papandreou CN, Daliani DD, Thall PF, et al. Results of a Phase II study with doxorubicin, etoposide, and cisplatin in patients with fully characterized small-cell carcinoma of the prostate. J Clin Oncol. 2002;20(14):3072–3080. doi: 10.1200/JCO.2002.12.065 [DOI] [PubMed] [Google Scholar]