Abstract
BACKGROUND
Patients with aortic stenosis are nearly twice as likely to have a diagnosis of gout compared with individuals without aortic valve disease.
METHODS
This retrospective study evaluated consecutive adults age ≥65 years with aortic stenosis between December 2012 and November 2016 who underwent at least 2 transthoracic echocardiograms (TTEs) separated by at least 1 year. Severe aortic stenosis was defined as any combination of an aortic valve peak velocity ≥4.0 m/sec, mean gradient ≥40 mm Hg, aortic valve area ≤1 cm2, or decrease in left ventricular ejection fraction as a result of aortic stenosis.
RESULTS
Of the 699 study patients, gout was present in 73 patients (10%) and not found in 626 patients (90%). Median follow-up was 903 days [552–1302] for patients with gout and 915 days [601–1303] for patients without gout (P = 0.60). The presence of severe aortic stenosis on follow-up transthoracic echocardiogram was more frequent in patients with gout compared to those without gout (74% vs 54%, P = 0.001; hazard ratio [HR] 1.45 [1.09–1.93]), even among the 502 patients without severe aortic stenosis at baseline (63% vs 39%, P = 0.003; hazard ratio 1.43 [1.07–1.91]). Gout remained associated with the development of severe aortic stenosis after multivariable adjustment (adjusted hazard ratio [aHR] 1.46 [1.03–2.08], P = 0.03). The annualized reduction in aortic valve area was numerically greater in the group with gout compared with the group without gout (−0.10 cm2/y [−0.18, −0.03] vs −0.08 cm2/y [−0.16, −0.01], P = 0.09); annualized change in peak velocity and mean gradient did not differ between groups.
CONCLUSIONS
Progression to severe aortic stenosis was more frequent in patients with gout compared with those without gout, supporting the hypothesis that gout is a risk factor for aortic stenosis.
Keywords: Aortic stenosis, Gout
INTRODUCTION
Calcific aortic stenosis is the most common form of valve disease in the Western world, with the population-based prevalence of moderate or severe aortic stenosis in the United States estimated to be as high as 4.6%.1 Aortic valve replacement accounts for at least 16% of all cardiac surgeries and the majority of all transcatheter valve procedures.2 Age and hereditary bicuspid valves are predictors of aortic stenosis, but the underlying pathogenesis and risk factors for aortic stenosis progression are incompletely understood.
Gout, a disease of urate metabolism characterized by inflammatory arthritis, is associated with a number of cardiovascular conditions, including coronary artery disease, heart failure, and peripheral vascular disease.3–4 Common underlying mechanisms associated with gout and cardiovascular disease include systemic inflammation, accumulation of reactive oxygen species, and loss of vasoactive mediators.5–7 A previous study by our group demonstrated that patients with aortic stenosis were nearly twice as likely to have a diagnosis of gout compared with individuals without aortic valve disease.8 The objective of the current study was to evaluate the association between gout and the progression of aortic stenosis over time.
METHODS
Study Design
This is a single-center, retrospective, cohort observational study. The New York University (NYU) School of Medicine Institutional Review Board granted a waiver of consent and provided regulatory approval of the study.
Study Population
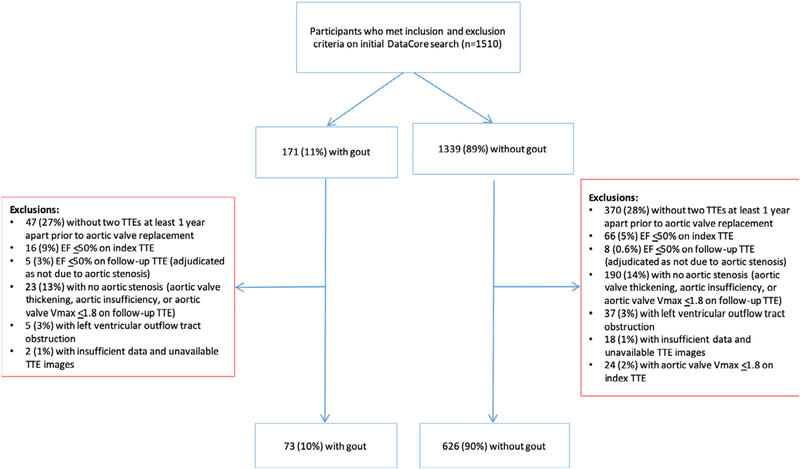
We retrospectively examined 1510 consecutive adults age ≥65 years who received care at NYU Langone Health between December 2012 and November 2016, had a diagnosis of aortic stenosis of any severity, and underwent at least 2 transthoracic echocardiograms (TTEs) separated by at least 1 year. A diagnosis of gout was identified according to the International Classifications of Disease-Ninth Revision (ICD-9) or the International Classifications of Disease-10th Revision (ICD-10) diagnosis codes (n = 106). Electronic medical records of patients with documented use of colchicine or urate-lowering therapy (ULT; allopurinol or febuxostat) but without a coded diagnosis of gout were reviewed to ensure accurate assignment by disease status (n = 104). Patients on colchicine or ULT for indications other than gout (eg, pericarditis, familial Mediterranean fever, asymptomatic hyperuricemia) were classified as not having gout. Patients were excluded from the analysis if there was an aortic valve prosthesis present at the time of the index TTE or the first eligible echocardiographic follow-up, or if the left ventricular ejection fraction (LVEF) was ≤50% on the index TTE. If LVEF was ≤50% on follow-up TTE, 3 board-certified cardiologists, blinded to the diagnosis of gout, adjudicated whether the decreased LVEF was attributable to hemodynamically significant aortic stenosis; patients with decreased LVEF not attributable to hemodynamically significant aortic stenosis were excluded from further analysis. Patients without aortic stenosis (cases of aortic valve thickening only, primary aortic insufficiency, or aortic valve peak velocity of ≤1.8 m/sec) and patients with concomitant dynamic left ventricular outflow tract obstruction were also excluded. Finally, patients with insufficient data or unavailable echocardiographic images were excluded. A diagram illustrating the study flow is shown in Figure 1.
Figure 1.
Study population.
TTE = transthoracic echocardiogram.
Endpoints
Severe aortic stenosis was defined as 1 or more of the following: aortic valve peak velocity ≥4.0 m/sec, mean gradient ≥40 mm Hg, aortic valve area ≤1 cm2, or decrease in LVEF as a result of aortic stenosis. Annualized changes in aortic valve peak velocity, aortic valve mean gradient, and aortic valve area were calculated as: [(Follow-up aortic valve parameter) − (Index aortic valve parameter)/ (Years between Index and Followup TTE)].
Statistical Analyses
Continuous variables are shown as median [inter-quartile range] and were compared between the gout and no gout groups using the nonparametric Mann-Whitney test. Categorical variables are shown as proportions and compared between the gout and no gout groups using Fisher exact test. Cox regression was performed to determine the association between gout and severe aortic stenosis on follow-up TTE in unadjusted models and after adjustment for the presence of aortic stenosis at the time of the index TTE, age, sex, body surface area, and clinical covariates that significantly differed between the 2 groups. Results of Cox regression analyses are presented as hazard ratios (HRs) with 95% confidence intervals. A sensitivity analysis was also performed to evaluate changes in aortic valve parameters after excluding patients who developed newly decreased LVEF attributed to the progression of aortic stenosis at follow-up TTE. Significance level was set at a 2-sided alpha level of 0.05.
RESULTS
Baseline Characteristics
Of the 699 patients who met study criteria, 73 patients (10%) had gout and 626 patients (90%) did not have gout. Patients with gout were more likely to be of male sex, have a larger body surface area, and have hypertension compared to patients without gout. Colchicine and at least 1 type of ULT were prescribed to 43% and 64% of gout patients, respectively. Patients with gout had lower glomerular filtration rates when compared to patients without gout. Baseline characteristics of the study population are shown in Table 1.
Table 1.
Baseline Characteristics
| Gout (n = 73) | No Gout (n = 626) | P Value | |
|---|---|---|---|
| Demographics | |||
| Age at index TTE, years | 82 [76–86] | 80 [74–86] | 0.38 |
| Male sex, % | 50 (69%) | 289 (46%) | <0.001 |
| Ever smoker, % | 36 (49%) | 296 (47%) | 0.81 |
| White race, % | 63 (86%) | 570 (91%) | 0.20 |
| Hispanic ethnicity, % | 2 (3.1%) | 19 (3.8%) | 0.99 |
| Body mass index, kg/m2 | 28.3 [24.9–32.3] | 27.1 [24.1–30.7] | 0.054 |
| Body surface area, m2 | 1.88 [1.73–2.02] | 1.80 [1.65–1.94] | 0.003 |
| Medical History, % | |||
| Hypertension | 68 (93%) | 514 (82%) | 0.01 |
| Hyperlipidemia | 60 (82%) | 446 (71%) | 0.053 |
| Diabetes | 24 (33%) | 191 (31%) | 0.69 |
| Coronary artery disease | 42 (58%) | 287 (46%) | 0.06 |
| Congestive heart failure | 17 (23%) | 113 (18%) | 0.27 |
| Peripheral vascular disease | 7 (10%) | 43 (7%) | 0.35 |
| Cancer | 24 (33%) | 197 (32%) | 0.79 |
| Osteoarthritis | 23 (32%) | 180 (29%) | 0.68 |
| Medications, % | |||
| Colchicine | 31 (43%) | 6 (1%) | <0.001 |
| Allopurinol | 46 (63%) | 12 (2%) | <0.001 |
| Febuxostat | 6 (8.2%) | 3 (0.5%) | <0.001 |
| Aspirin | 45 (62%) | 419 (67%) | 0.36 |
| Statin | 48 (66%) | 345 (55%) | 0.11 |
| Loop diuretic | 50 (69%) | 367 (59%) | 0.13 |
| Thiazide diuretic | 21 (29%) | 173 (28%) | 0.89 |
| Calcium channel blocker | 48 (66%) | 348 (56%) | 0.11 |
| Nonsteroidal anti-inflammatory drugs | 17 (23%) | 136 (22%) | 0.77 |
| Laboratory Data | |||
| Glomerular filtration rate, mL/min | 49 [36–65] | 64 [49–80] | <0.001 |
| Neutrophil/lymphocyte ratio | 3.2 [2.3–5.9] | 2.9 [2.0–4.3] | 0.12 |
| Index TTE | |||
| Left ventricular ejection fraction, % | 65 [60–70] | 65 [60–70] | 0.25 |
| Aortic valve peak velocity, m/sec | 2.8 [2.6–3.5] | 2.8 [2.4–3.3] | 0.14 |
| Aortic valve mean gradient, mm Hg | 17 [13–29] | 17 [12–25] | 0.42 |
| Aortic valve area, cm2 | 1.1 [1.0–1.4] | 1.2 [1.0–1.5] | 0.24 |
| Severe aortic stenosisa | 27 (37%) | 170 (27%) | 0.07 |
AVA = aortic valve area; LVOT = left ventricular outflow tract; TTE = transthoracic echocardiogram.
Continuous variables are shown as median [interquartile range] and compared between the gout and no gout groups using Mann-Whitney tests. Categorical variables are shown as frequency (proportion) and compared between the gout and no gout groups using Fisher exact test.
Defined as AVA ≤1.0 cm2, aortic valve peak velocity ≥4.0 m2, or aortic valve mean gradient ≥40 mm Hg.
On index TTE, the presence of severe aortic stenosis, defined as an aortic valve peak velocity ≥4.0 m/sec, mean gradient ≥40 mm Hg, or aortic valve area ≤1 cm2, did not differ between groups. Aortic valve peak velocity, aortic valve mean gradient, and aortic valve area also did not differ among patients with and without gout. (Table 1)
Aortic Valve Endpoints on Follow-Up TTE
Median follow-up was 903 days [552–1302] for patients with gout and 915 days [601–1303] for patients without gout (P = 0.60).
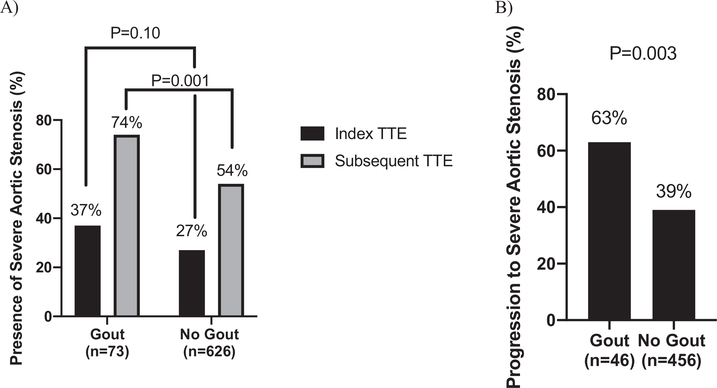
The presence of severe aortic stenosis on subsequent TTE was significantly more frequent in patients with gout compared with those without gout (74% vs 54%, P = 0.001; Figure 2A). Among patients without severe aortic stenosis at baseline (n = 46 gout, n = 456 no gout), the proportion of patients who progressed to severe aortic stenosis was also significantly greater in the group with gout compared with the group without gout (63% vs 39%, P = 0.003; Figure 2B). Gout was associated with the presence of severe aortic stenosis on follow-up TTE in unadjusted analyses (HR 1.45 [1.09–1.93], P = 0.01), after adjustment for the presence of severe aortic stenosis on index TTE (HR 1.43 [1.07–1.91], P = 0.02), and after additional adjustment for age, sex, body surface area, hypertension, and glomerular filtration rate (HR 1.46 [1.03–2.08], P = 0.03). (Table 2)
Figure 2.
(A) Presence of severe aortic stenosis on index and subsequent transthoracic echocardiogram (TTE) in patients with and without gout. (B) Proportion of patients without severe aortic stenosis on the index TTE who had progression to severe aortic stenosis on subsequent TTE.
Table 2.
Association Between Gout and the Presence of Severe Aortic Stenosis on Follow-Up Transthoracic Echocardiogram
| Hazard Ratio | 95% Confidence Interval | P Value | |
|---|---|---|---|
| Unadjusted | 1.45 | 1.09–1.93 | 0.01 |
| Adjusted for the presence of severe aortic stenosis on index TTE | 1.43 | 1.07–1.91 | 0.02 |
| Adjusted for the presence of severe aortic stenosis on index TTE, age, sex, body surface area, hypertension, and glomerular filtration rate | 1.46 | 1.03–2.08 | 0.03 |
TTE = transthoracic echocardiogram.
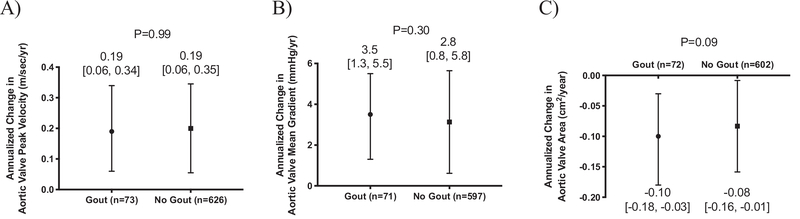
The annualized change in aortic valve peak velocity and mean gradient did not differ between patients with and without gout; however, the annualized reduction in aortic valve area was numerically greater in patients with gout compared to those without a diagnosis of gout (Figure 3A–C). Among patients without severe aortic stenosis at baseline (n = 46 gout, n = 456 no gout), the annualized change in peak velocity, mean gradient, and calculated valve area did not differ between the group with gout and the group without gout (Supplemental Table 1, available online).
Figure 3.
Annualized change in (A) aortic valve peak velocity, (B) aortic valve mean gradient, and (C) aortic valve between patients with and without gout.
Data shown as median [interquartile range] and compared using Mann-Whitney test.
Among patients with gout, 3 had a subsequent decrease in LVEF as a result of the progression of aortic stenosis, and 4 patients without gout had a subsequent decrease in LVEF as a result of aortic stenosis (4.1% vs 0.6%, P = 0.03). After excluding these 7 patients, gout remained associated with the presence of severe aortic stenosis on follow-up TTE in unadjusted analysis (HR 1.43 [1.06–1.92], P = 0.02) and after adjustment for presence of severe aortic stenosis on index TTE, age, sex, body surface area, hypertension, and glomerular filtration rate (HR 1.46 [1.02–2.09], P = 0.04).
DISCUSSION
To our knowledge, this is the first study to evaluate the progression over time of aortic stenosis in patients with gout compared with those without gout. First, we demonstrate that patients with gout were significantly more likely to have severe aortic stenosis on follow-up when compared with patients without gout. Second, among patients who did not have severe aortic stenosis on the index TTE, those with gout were more likely to progress over time to severe aortic stenosis than patients without gout. Third, patients with gout did not demonstrate a significantly more rapid progression of aortic stenosis by gradients or peak velocities when compared to patients without gout. However, there was a trend toward a more rapid decline over time in aortic valve area in patients with gout compared with those without gout.
Although the proportion of patients with a binary definition of severe aortic stenosis differed between groups at follow up, the change in individual aortic valve parameters over time did not differ significantly by gout status. One potential explanation for this seemingly contradictory finding could be baseline differences in valve severity by gout status. A more likely explanation is in the limited sensitivity of any individual aortic valve parameter alone to diagnose aortic stenosis, compared to the traditional integration of multiple aortic valve parameters to establish a diagnosis of aortic stenosis. Although we did not observe differences in the progression of individual parameters on an annualized basis between the 2 groups, there was a trend toward greater reductions in calculated aortic valve area among patients with gout compared with those without gout. Whether significant differences would have been observed in a larger cohort or over a longer observation period remains a matter of speculation. It is also unknown whether progression of aortic stenosis over time is linear. It is possible that once severe aortic stenosis develops, further acceleration of individual aortic valve parameters may be non-linear and may vary substantially between individuals.
The pathophysiologic relationship between aortic stenosis and gout is not yet defined. The initial pathologic insult in aortic stenosis appears to be similar to that of atherosclerosis, with valvular and endothelial injury, lipid deposition, subsequent inflammation, and calcification. Gout results in hyperuricemia that leads to the deposition of monosodium urate crystals throughout tissues. Hyperuricemia may also contribute to chronic inflammation, accumulation of reactive oxygen species, and loss of vasoactive mediators, particularly nitric oxide, all of which have been shown to promote endothelial dysfunction and vascular remodeling.9 These effects can ultimately lead to accelerated atherosclerosis as well as calcification.10 A hyperuricemic mouse model induced by a highadenine diet has been shown to accelerate aortic calcification, and serum urate concentration predicts progression of atherosclerosis and calcification.11,12 Another study by Demir et al showed a positive correlation between serum urate concentrations and severity of aortic stenosis.13 Although a clear mechanism by which hyperuricemia in the setting of gout may contribute to valvular calcification and progression of aortic stenosis remains to be elucidated, a study by Klausser et al demonstrated a higher frequency of monosodium urate deposits by dual-energy computed tomography among patients with gout compared with controls.14
This study demonstrates the tendency for patients with gout and aortic stenosis to progress more quickly to severe aortic stenosis than patients without gout. This analysis builds on a previous retrospective case-control study from our research group that reported that patients with aortic stenosis are almost twice as likely to have a diagnosis of gout compared with controls (21.4% vs 12.5%).8 In that study, a diagnosis of gout preceded that of aortic stenosis by a mean of 5.8 ± 1.6 years, suggesting a possible temporal relationship. The current study adds to these initial data, suggesting a trend toward faster progression to severe aortic stenosis in patients with compared with those without gout. Still, further studies with greater power are needed.
This study has several limitations. First, the retrospective review did not mandate regular follow-up intervals and patient data from outside the institutional electronic medical record system were not captured. Second, based on the relatively small cohort with gout, the study may be underpowered to identify differences in individual aortic valve parameters. Third, data on inflammatory markers such as C-reactive protein and serum urate levels were not measured in a large proportion of the study cohort and, therefore, not included here. Finally, echocardiographic images were not reviewed systematically by a core laboratory. Nonetheless, this is the first study to demonstrate a faster progression to severe aortic stenosis in the group with gout compared with the group without gout and further supports the hypothesis that gout is a risk factor for aortic stenosis.
Supplementary Material
CLINICAL SIGNIFICANCE.
Patients with aortic stenosis are nearly twice as likely to have a diagnosis of gout compared with individuals without aortic valve disease.
Patients with gout are more likely to develop severe aortic stenosis on follow-up than patients without gout.
Gout is associated with the development of severe aortic stenosis even after multivariable adjustment.
Patients with gout have a tendency to progress more quickly to severe aortic stenosis than patients without gout.
ACKNOWLEDGMENTS
We dedicate this manuscript to the memory of Steven Sedlis, MD, Professor of Medicine, consummate mentor, and dedicated physician who devoted his career to improving patient outcomes, educating the next generation of physicians, and advancing science. Dr. Sedlis played a pivotal role with his contributions to background of this study.
NYU Langone Health DataCore provided clinical data reporting services under the auspices of Medical Center Information Technology, Center for Translational Science Institute, and Department Population Health.
Funding: BS was supported in part by the Biomedical Laboratory Research & Development Service of the VA Office of Research and Development (iK2CX001074). NRS was supported in part by an NYU CTSA grant, UL1 TR001445 and KL2 TR001446, from the National Center for Advancing Translational Sciences, National Institutes of Health.
Conflicts of Interest: MHP has received investigator-initiated grants from Hikma Pharmaceuticals and Horizon Therapeutics and serves as a consultant for Sobi, Horizon, and Ampel Biosciences. AA, ACS, CTT, JDL, NRS, VCP, RD report none.
Footnotes
SUPPLEMENTARY DATA
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amjmed.2020.01.019.
References
- 1.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005–11. [DOI] [PubMed] [Google Scholar]
- 2.Society of Thoracic Surgeons. STS Adult Cardiac Surgery DatabaseExecutive Summary - 2017 Harvest 2. Available at: https://www.sts.org/sites/default/files/documents/ACSD_2017Harvest2_ExecutiveSummary.pdf. Accessed May 10, 2019.
- 3.Kuo C-F, Yu K-H, See L-C, et al. Risk of myocardial infarction among patients with gout: a nationwide population-based study. Rheumatology 2013;52:111–7. [DOI] [PubMed] [Google Scholar]
- 4.Krishnan E Gout and the risk for incident heart failure and systolic dysfunction. BMJ Open 2012;2(1):e000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi HK, Curhan G. Independent impact of gout on mortality and risk for coronary heart disease. Circulation 2007;116:894–900. [DOI] [PubMed] [Google Scholar]
- 6.Clarson LE, Hider SL, Belcher J, Heneghan C, Roddy E, Mallen CD. Increased risk of vascular disease associated with gout: a retrospective, matched cohort study in the UK Clinical Practice Research Datalink. Ann Rheum Dis 2015;74:642–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah A, Keenan RT. Gout, hyperuricemia, and the risk of cardiovascular disease: cause and effect? Curr Rheumatol Rep 2010;12:118–24. [DOI] [PubMed] [Google Scholar]
- 8.Chang K, Yokose C, Tenner C, et al. Association between gout and aortic stenosis. Amer J Med 2017;130:230.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med 2008;359:1811–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berezin AE, Kremzer AA. Serum uric acid as a marker of coronary calcification in patients with asymptomatic coronary artery disease with preserved left ventricular pump function. Cardiol Res Pract 2013;2013:129369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atar AI, Yilmaz OC, Akin K, et al. Serum uric acid level is an independent risk factor for presence of calcium in coronary arteries: an observational case-controlled study. Anadolu Kardiyol Derg 2013;13: 139–45. [DOI] [PubMed] [Google Scholar]
- 12.Liu Z, Chen T, Niu H, et al. The establishment and characteristics of rat model of atherosclerosis induced by hyperuricemia. Stem Cells Int 2016;2016:1365257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demir B, Caglar IM, Ugurlucan M, et al. The relationship between severity of calcific aortic stenosis and serum uric acid levels. Angiology 2012;63:603–8. [DOI] [PubMed] [Google Scholar]
- 14.Klauser AS, Halpern EJ, Strobl S, et al. Dual-energy computed tomography detection of cardiovascular monosodium urate deposits in patients with gout [e-pub ahead of print]. JAMA Cardiol 2019. September 11 10.1001/jamacardio.2019.3201. Accessed March 3, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.