Abstract
Afferent lesions of the arterial baroreflex occur in familial dysautonomia. This leads to excessive blood pressure variability with falls and frequent surges that damage the organs. These hypertensive surges are the result of excess peripheral catecholamine release and have no adequate treatment. Carbidopa is a selective DOPA-decarboxylase inhibitor that suppresses catecholamines production outside the brain. To learn whether carbidopa can inhibit catecholamine-induced hypertensive surges in patients with severe afferent baroreflex failure, we conducted a double-blind randomized crossover trial in which patients with familial dysautonomia received high dose carbidopa (600 mg/day), low dose carbidopa (300 mg/day) or matching placebo in three four-week treatment periods. Among the 22 patients enrolled (13 females/8 males), the median age was 26 (range 12 to 59 years). At enrollment, patients had hypertensive peaks to 164/116 (range 144/92 to 213/150 mmHg). 24-h urinary norepinephrine excretion, a marker of peripheral catecholamine release, was significantly suppressed on both high dose and low dose carbidopa, compared to placebo (p=0.0075). The two co-primary endpoints of the trial were met. The standard deviation of systolic BP variability was reduced at both carbidopa doses (low dose: 17±4, high dose: 18±5 mmHg) compared to placebo (23±7 mmHg, p=0.0013) and there was a significant reduction in the systolic BP peaks on active treatment (p=0.0015). High and low dose carbidopa were similarly effective and well tolerated. This study provides class Ib evidence that carbidopa can reduce BP variability in patients with congenital afferent baroreflex failure. Similar beneficial effects are observed in patients with acquired baroreflex lesions.
Keywords: Blood pressure variability, hypertension, norepinephrine, carbidopa, familial dysautonomia, baroreflex
Graphical Abstract
Introduction
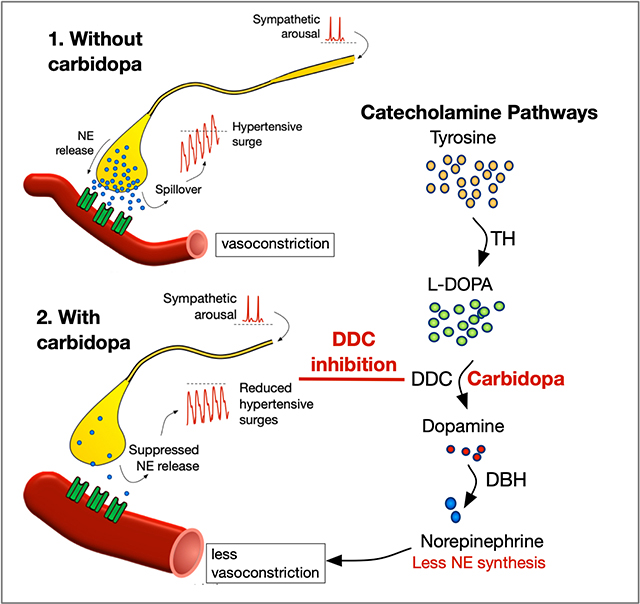
Familial dysautonomia is a congenital neuropathy that causes unstable blood pressure with orthostatic hypotension and transient bouts of arterial hypertension, tachycardia, and red skin blotching at times of catecholamine excess. 1, 2 It occurs because of a hereditary mutation in the ELP-1 gene, which impairs the development of sensory and autonomic fibers particularly afferent baroreceptor neurons in cranial nerves IX and X. 3–5 Without afferent inhibitory inflow from arterial baroreceptors, at times of arousal patients have uncontrolled spikes in sympathetic vasoconstrictor neurons with catecholamine release into the circulation. 6
From childhood, mild anxiety causes a rapid rise in blood pressure due to an increase in norepinephrine release. 1, 3 Reduced number of postganglionic sympathetic neurons results in enhanced vascular responsiveness to norepinephrine (i.e., denervation super-sensitivity). 7, 8 Patients have hypertensive surges with everyday activities that trigger central sympathetic activation. 9, 10 When the sympathetic stimulus is not removed, patients are prone to prolonged hypertensive crisis with nausea and vomiting as norepinephrine and dopamine are co-secreted into the circulation. 11 As teenagers, most patients will develop varying degrees of ongoing end-organ injury 12, 13 and biopsies show hypertensive nephrosclerosis and cardiotoxic effects induced by catecholamines. 14, 15
The goal of therapy is not to normalize blood pressure, but to decrease its variability by reducing the extreme ranges. 16 Although elevated blood pressure variability has been associated with early onset end-stage renal disease in patients with familial dysautonomia, options for treating the hypertensive surges in these patients are limited. 17 Anxiolytics like diazepam cause respiratory arrest, clonidine produces rebound hypertension, and the use of standard antihypertensives like calcium channel blockers is limited by the development of symptomatic hypotension or syncope. Based on the finding that dopamine levels were high during acute vomiting crisis, and the assumption that dopamine induces vomiting, we explored the approach of using peripheral decarboxylase inhibition with carbidopa (Lodosyn®) to treat these episodes. In a controlled clinical trial, familial dysautonomia patients had a 33% decrease in urinary dopamine and a significant improvement in nausea and vomiting.18 We also observed that carbidopa reduced urinary norepinephrine excretion and reduced blood pressure variability. If confirmed, carbidopa would be a novel way to control blood pressure instability in patients with labile hypertension caused by afferent baroreceptor lesions.
To test the hypothesis that peripheral decarboxylase inhibition decreases norepinephrine synthesis, blunts sympathetic activity, dampens hypertensive surges and lowers blood pressure variability, we performed a double-blind randomized placebo-controlled crossover study of carbidopa in patients with familial dysautonomia.
Methods
STUDY DESIGN AND OVERSIGHT
The data that support the findings of this study are available from the corresponding author upon reasonable request. Using a random order double-blind crossover trial design, patients received high dose carbidopa (600 mg/day), low dose carbidopa (300 mg/day) or matching placebo in three separate four-week treatment periods. Doses were divided into three times daily, administered six hours apart, with a four-day dose de-escalation and washout period between dose changes (Fig. 1). Total study duration was fourteen-weeks. Screening and baseline visits were conducted onsite. Telemedicine visits were used to follow-patients outside New York City. An independent data safety monitoring board was used to monitor trial safety and trial results. For all serious adverse events a report was generated within 48-hours. A quarterly report of all adverse events was sent to the study sponsor. A full report for the entire safety data set was sent every 6 months to the independent medical monitor. Details of the safety reporting are included in the protocol (Supplementary File). The study was sponsored and reviewed by the F.D.A.’s Office of Orphan Product Development under investigator-initiated IND #117435 and registered on ClinicalTrials.gov (NCT# 02553265). The F.D.A. had no role in the trial management, preparation of placebo and active treatments, or statistical analysis. As part of the federal funding-program, quarterly reports were submitted to monitor enrollment, dropouts and all adverse events. The protocol was approved by New York University (N.Y.U.) Institutional Review Board.
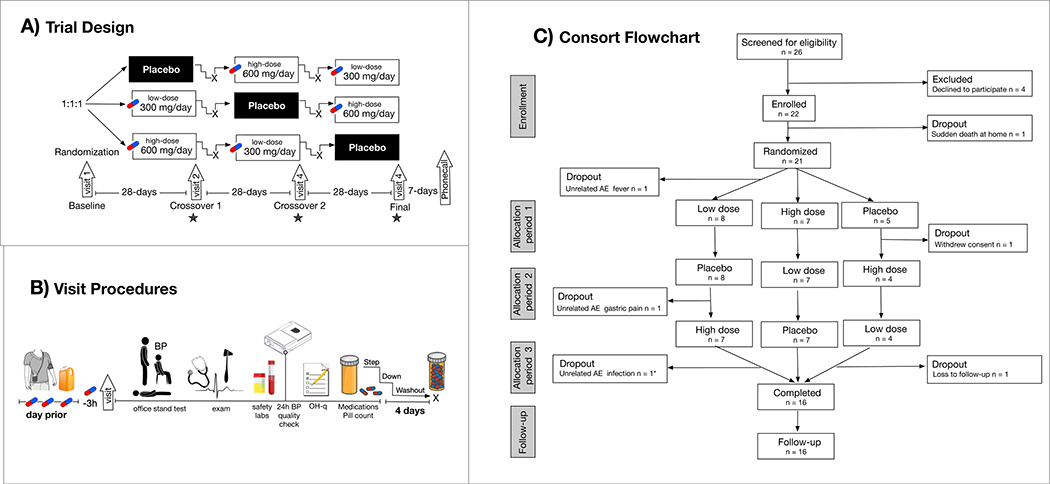
Figure 1: Trial design and participants.
A) Placebo-controlled crossover 14-week trial with 4 visits to test the effect of high dose and low dose carbidopa in patients with familial dysautonomia. Star denotes timing of primary efficacy outcome measures. B) Ambulatory blood pressure monitoring and urine catecholamines were measured and the end of each randomization block. Study medications were taken 3-h prior to visit; office measures included orthostatic vitals with active standing, physical/neurological exam, 12-lead electrocardiogram, urinalysis, metabolic panel, and complete blood count. Ambulatory recordings were repeated if the success rate was <80%. Orthostatic hypotension symptoms were scored on the OH-questionnaire (OH-q), concomitant medications and remaining study pills were checked. Doses were titrated down and patients were washed out prior to starting the next cross over. C) Sixteen patients completed the trial; * denotes only adverse event on carbidopa, all six drop outs were unrelated to carbidopa.
STUDY DESIGN AND OVERSIGHT
All participants were recruited from the outpatient clinic at the N.Y.U. Dysautonomia Center and were followed as part of a natural history study (NCT# 03920774). Afferent baroreflex failure was confirmed by autonomic testing 2. Participants were required to have a genetic diagnosis of the common founder intron-20 ELP-1 mutation on genetic testing and to meet the following main entry criteria: age 10 years old or older; with an unstable systolic blood pressure (defined as a standard deviation above 15 mmHg on ambulatory monitoring or documented hypertensive peaks >140 mmHg during an office visit), and the ability to provide written consent or ascent (for minors or those with a Montreal Cognitive Assessment score <25 points). Patients taking MAO-inhibitors, dopamine blockers (metoclopramide, domperidone, risperidone), tricyclic antidepressants or neuroleptics; with unstable angina, renal failure (creatinine >2.0 mg/dL), on supplementary oxygen, or pregnant were excluded.
RANDOMIZATION AND BLINDING
Identical capsules of 100 mg carbidopa, 200 mg carbidopa, and matching powder placebo were produced by a compounding pharmacy. At enrolment, eligible participants were randomly assigned to receive either carbidopa 100 mg three times daily (total daily dose of 300 mg), carbidopa 200 mg three times daily (total daily dose of 600 mg), or matching placebo using a computerized randomized 1:1:1 block. A thirty-day bottled supply of assigned capsules was distributed by the investigational pharmacy. Identical bottles were used in each randomization phase. Study drug was administered orally or via gastrostomy for patients with severe dysphagia. Patients using the gastrostomy were given instructions to open the capsules, dissolve the powder in 20 ml of water, administer through the gastrostomy, and to ensure no drug remained in the tube to flush with 30 ml of water. The blinding code was known only to the investigational pharmacist, kept in a secure location, and revealed only to the data safety monitoring board if requested by the principal investigator (H.K) in an emergency through a web response system. Participants and study personnel remained blinded to the treatment allocation until final analysis was complete.
PROCEDURES
There were four study visits (Fig. 1). At visit 1, patients were screened and demographic data was collected. A seven-day window was permitted to obtain baseline ambulatory blood pressure recordings and 24-h urine collections. After eligibility was confirmed, the patients were enrolled and the first batch of randomized medication bottles was dispensed. Subjects returned for follow-up assessment 28-days later (visit 2) and at the end of each subsequent crossover period (visit 3 and 4).
Safety measures were obtained at all visits including weight, temperature, office blood pressure reading after 5-minute supine, 3-minutes seated and 5-minute of active standing, complete blood count, comprehensive metabolic panel, physical and neurological examination, and a 12-lead ECG. Amendments to the protocol were submitted to allow remote telemedicine visits for follow-up with secure 2-way video-calls between the patient and the study investigators. In addition, patients were given prescriptions to obtain blood and urine samples with a local treating physician. Adverse events were monitored throughout with weekly phone calls and a daily symptom diary. The Orthostatic Hypotension questionnaire (OH-q)19 was administered at each visit to capture potential worsening of symptoms related to low blood pressure or further worsening of limitations to activities that involve standing and walking caused by exacerbating the hypotensive falls on standing.
Patients were instructed to remain on their baseline blood pressure medication regimes (table 1). Any medically necessary concurrent medication changes were followed through the trial. Medication compliance was discussed at all visits. All medication bottles and unused capsules were returned and accountability was checked by counting remaining study pills at the end of each treatment arm.
Table 1:
Subject characteristics at enrollment
| Case | Blood pressure (systolic/diastolic, mmHg) | |||||||
|---|---|---|---|---|---|---|---|---|
| Age (y)/sex | Baseline medication regime | Ambulatory monitoring | Office | History | ||||
| 24-h average | SD | Max | Min | Morning surge | Δ fall 3-min standing | Hypertensive crisis | ||
| 20/F | 2.5 mg midodrine prn, 0.05 mg fludrocortisone | 104/65 | 19/18 | 146/109 | 78/44 | +22/18 | −38/41 | Yes |
| 44/F | 5 mg diazepam prn | 154/102 | 32/29 | 213/150 | 90/46 | +101/104 | −34/47 | Yes |
| 31/F | 0.1 mg fludrocortisone, 0.1 mg clonidine prn, 10 mg diazepam prn | 117/69 | 18/17 | 156/105 | 97/50 | +36/34 | −15/23 | Yes |
| 15/F | 5 mg midodrine prn | 110/55 | 14/13 | 145/92 | 89/40 | +24/20 | −64/42 | No |
| 24/F3 | none | 106/67 | 18/22 | 147/122 | 77/40 | +27/23 | −21/10 | Yes |
| 18/M | 0.1 mg clonidine patch, 0.25 mg clonidine, 5 mg diazepam | 108/66 | 19/15 | 161/107 | 80/42 | +69/63 | −37/29 | Yes |
| 28/M | 0.1 mg clonidine, 0.1 mg clonazepam, 100 mg pregabalin | 119/78 | 24/24 | 184/145 | 79/40 | +16/6 | −16/28 | Yes |
| 30/M | 2.5 mg midodrine | 105/60 | 20/16 | 144/100 | 74/40 | +12/11 | −16/26 | No |
| 17/F | 7.5 mg midodrine, 0.1 mg fludrocortisone, 2 mg diazepam | 110/67 | 18/19 | 147/114 | 72/22 | +56/52 | −32/32 | Yes |
| 58/F | 0.1 mg clonazepam, amlodipine | 99/67 | 19/15 | 158/121 | 90/46 | +14/6 | −17/9 | No |
| 38/M | none | 112/70 | 25/27 | 178/133 | 82/39 | +30/28 | −14/42 | Yes |
| 12/F | 0.1 mg fludrocortisone | 112/74 | 19/23 | 155/105 | 82/82 | +26/32 | −43/42 | Yes |
| 26/F2 | 0.1 mg fludrocortisone, 5 mg diazepam prn | 108/69 | 19/17 | 164/97 | 66/43 | +42/39 | −53/27 | Yes |
| 32/M3 | none | 106/64 | 21/18 | 157/106 | 77/43 | +62/34 | −6/14 | Yes |
| 20/M | 2.5 mg midodrine prn, 2 mg diazepam prn, 0.1 mg clonidine prn | 115/71 | 26/24 | 170/130 | 78/41 | +48/48 | −38/41 | Yes |
| 24/F | 10 mg midodrine, 5 mg diazepam | 112/57 | 19/15 | 162/102 | 86/40 | +8/20 | −36/28 | Yes |
| 25/F2 | none | 123/72 | 25/21 | 161/110 | 91/42 | - | −47/37 | Yes |
| 23/M | 10 mg diazepam prn | 120/66 | 25/21 | 173/110 | 73/41 | +68/65 | −74/60 | Yes |
| 42/F | none | 118/79 | 24/26 | 174/128 | 77/42 | +35/46 | −24/42 | Yes |
| 36/F | 0.1 mg transdermal clonidine patch, Ramipril 1.25 mg | 119/72 | 19/19 | 180/126 | 86/40 | +19/18 | −4/2 | Yes |
| 34/M1 | 0.05 mg fludrocortisone, 5 mg midodrine prn, 10 mg diazepam prn | 145/103 | 20/19 | 185/134 | 95/52 | - | −9/26 | Yes |
| 21/F2 | 2.5 mg midodrine, 0.05 mg fludrocortisone | 120/77 | 26/24 | 167/118 | 75/40 | +56/52 | −33/22 | Yes |
SD = standard deviation, prn = as needed medication, F = female, M = male.
the patient that died prior to randomization
denotes the 3 discontinuations due to an adverse event.
denotes 2 subjects that withdrew consent or were lost to follow-up
Source documents were kept in designated study binders and data were captured in an online secure research database (RedCAP). Adverse events (AE) were grouped according to Medical Dictionary for Regulatory Activities (MedDRA) organ class. AE were defined as serious (SAE) when the outcome was death, or when the event was life-threatening (i.e., continued use of the study drug might have resulted in the death of the patient), resulted in initial or prolonged hospitalization, or resulted in persistent or significant disability or incapacity. Upon completion of the study, patients were followed up seven-days later by phone call. The protocol is available as supplementary information.
OUTCOME MEASUReS
There were two pre-defined co-primary endpoints: a) a reduction in daytime systolic blood pressure variability (SD) measured on 24-h ambulatory blood pressure monitoring; and b) a reduction in the maximum systolic blood pressure peak. Prior to each visit, patients were fitted with a validated ambulatory blood pressure monitor (90207, SpaceLabs, Washington, U.S.A.), programmed to take reading at 20-minute intervals during the day and 30-minute intervals overnight, and asked to fill a sleep/wake/activity diary. The same cuff size was used at each time point. Recordings were downloaded at study visits and repeated while the subject was still on study medication if the success rate was below 80%. Additional hemodynamic outcome measures included the average, standard deviation, coefficient of variation, maximum, and minimum blood pressures over the entire 24-h period as well as waking and sleeping hours were evaluated. The morning blood pressure surge was calculated as the peak blood pressure within the first 2-hours of awakening from sleep minus the average blood pressure during the hour that included the lowest blood pressure during sleep, as described 20.
To determine if carbidopa could suppress sympathetic activity, an additional outcome measure was the 24-hour urinary norepinephrine excretion (Fig. 1B). While undergoing ambulatory blood pressure monitoring, participants collected their urine in shaded bottles with acid preservative, which they kept refrigerated. Assays were run on 24-h urine using high-performance liquid chromatography to determine fractionated plasma catecholamine concentrations. Safety and tolerability outcomes included the incidence of treatment-emergent adverse events, changes in safety laboratory values, vital signs or alterations in physical and neurological examinations.
STATISTICAL ANALYSIS
All analysis was performed on the blinded data and without knowledge of the treatment assignment. Our main analysis compared high dose and low dose carbidopa to placebo with an intention to treat approach for all randomized participants. In addition, to account for potential covariates, such as age, gender, and disease severity, the analysis of (co)variance (ANOVA and ANCOVA) method we used to model the repeated measures data to compare both primary and all secondary endpoints. We adjusted the statistical methodology to account for two co-primary endpoints using the Bonferroni method. Subsequent analysis included comparisons between high dose and low dose carbidopa with nonparametric Wilcoxon test used robustness. Based on published data showing the association between the rate of decline in glomerular filtration rates and high blood pressure variability in patients with familial dysautonomia,17 we predicted that a 15% (2.9 mmHg) reduction in ambulatory blood pressure variability would lead to better renal outcomes. With a target enrollment of 20 subjects we predicted an 80% power to detect a minimum detectable difference of a 2.9 mmHg reduction in the primary outcome variable of the standard deviation in daytime blood pressure variability at a significance level of p<0.05. We estimated a dropout rate of 15%. All authors had full access to all the data and were responsible for the decision to submit for publication. The corresponding author had access to all the data in the study and had final responsibility for the decision to submit for publication. The p values reported for the co-primary endpoints were adjusted to account for multiple comparisons with a corrected alpha of 0.025 (95% confidence interval). Data are reported as mean±standard deviation, unless otherwise stated. Analysis was performed using Prism (GraphPad, version 8.4, U.S.A).
Results
STUDY PARTICIPANTS
A total of 26 subjects were screened and 22 patients were enrolled in the trial (see Fig. 1C for details). One patient died suddenly in the seven-day screening window prior to randomization, before receiving the study drug. Of the remaining patients, seven were initially randomized to high dose carbidopa, nine were first randomized to low dose, and five first were randomized to placebo. The groups were well matched at baseline and there were no significant differences in the groups that received carbidopa first or placebo first.
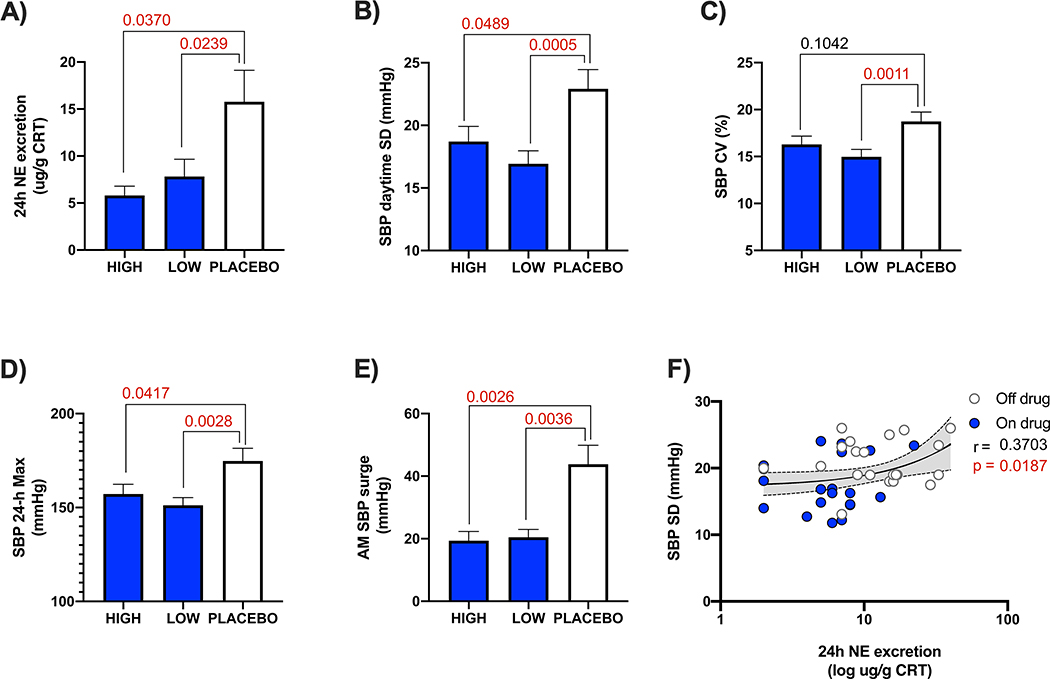
Baseline characteristics are presented in table 1. Baseline anti-hypertensive medications for autonomic crises included clonidine (n=5), diazepam (n=10), clonazepam (n=2), amlodipine (n=1) and ramipril (n=1). In addition, 8 patients took midodrine and 7 took fludrocortisone (table 1). All medications were kept stable during the trial. Average age of the subjects was 28-years (range 13 to 58 years). Fourteen were females. On average, mean 24-h systolic blood pressure was 115±12 mmHg and mean diastolic was 70±10 mmHg. The morning surge in blood pressure on waking from sleep was +39±24 mmHg. The standard deviation of the 24-h systolic blood pressure variability was 21±4 mmHg. Average norepinephrine excretion in urine on enrollment was 14 ug/g CRT (range 40 to 2 ug/g CRT). Overall, there was a significant positive correlation between norepinephrine excretion and systolic blood pressure variability (Fig. 2).
Figure 2: Efficacy endpoints carbidopa vs. placebo.
Significance tested with ANOVA with corrected p values for multiple co-primary endpoints comparisons to placebo. A) Lowering of norepinephrine excretion in 24-h urine when assigned to carbidopa treatment. B) Reduction in daytime systolic blood pressure variability (the primary end point). C) Lowering of the coefficient of variation in 24-h systolic blood pressure. D) Decrease in maximum systolic blood pressure spikes captured on ambulatory monitoring. E) Blunting of the morning surge in systolic blood pressure within 2-hours of waking from sleep. F) Shows relationship between norepinephrine excretion and systolic blood pressure variability modeled with pooled data. Shaded = on drug, clear = off drug. SBP = systolic blood pressure, NE = norepinephrine, CV = coefficient of variation, AM = morning, CRT = creatinine.
SAFETY AND TOLERABILITY
Carbidopa was well tolerated. Sixteen patients completed all three arms of the double-blind, randomized, placebo-controlled phase (Fig. 1C). In the double-blind cross over phase, there were a total of five participants who withdrew early. These included two participants who discontinued (one patient withdrew consent, one patient was lost to follow-up), two discontinuations related to SAE (one subject was hospitalized with fever in the week between baseline and randomization, before starting to take the study drug; and one subject was hospitalized for gastric pain on placebo); and one discontinuation related to an AE for moderate diarrhea post-antibiotic treatment of infection while on 600-mg dose). All 3 dropouts related to adverse events were judged to be expected in this specific cohort of patients, unrelated to the study drug, as the patients had hospitalizations for similar reasons in the past noted in their clinical chart. Non-serious AE in participants who were randomized included loose stools in 3, which self-resolved (n=2 while on 300-mg dose treatment, and n=1 while on placebo); and near-syncope with spontaneous resolution in 3 (n=2 while on placebo and n=1 while on 600-mg carbidopa). Electrocardiographic findings were unremarkable and there were no signs of renal or hepatotoxicity.
EFFECT ON BLOOD PRESSURE VARIABILTY AND HYPERTENSION
As shown in figure 2, there was less norepinephrine excreted in urine when patients were randomized to carbidopa compared to placebo. Table 2 shows the effect on hemodynamic variables. Both co-primary endpoints were met and the daytime systolic blood pressure variability (SD, p=0.0013) and maximum systolic blood pressure (p=0.0015) were significantly reduced on carbidopa compared to placebo. The coefficient of variation in systolic blood pressure (p=0.0047) as well as the morning surge on awakening from sleep (p=0.0007) were also lower on treatment with carbidopa. The number of hypertensive peaks captured in a 24-h period were also significantly lower when randomized to carbidopa. Carbidopa had similar effect in blunting the variability and surges in diastolic blood pressure. No treatment effect was observed on the nighttime blood pressure dipping profile. The effects on heart rate were small (table 2).
Table 2:
Treatment and dose effect on hemodynamic variables
| Autonomic Measures | Dose effect | Treatment effect | |||
|---|---|---|---|---|---|
| carbidopa 300 mg/day | carbidopa 600-mg/day | p value | placebo | p value | |
| Ambulatory blood pressure monitoring | |||||
| 24-h average systolic mmHg | 113±10 | 112±8 | 0.4863 | 120±14 | 0.0679 |
| 24-h average diastolic mmHg | 68±9 | 67±8 | 0.3882 | 74±12 | 0.0286 |
| 24-h average heart rate bpm | 73±6 | 75±7 | 0.0541 | 79±7 | 0.0017 |
| Daytime average systolic mmHg | 113±8 | 114±11 | 0.6138 | 122±17 | 0.0659 |
| Daytime average diastolic mmHg | 68±8 | 68±8 | 0.5665 | 75±15 | 0.0196 |
| Nighttime average systolic mmHg | 111±10 | 111±12 | 0.2617 | 114±8 | 0.7096 |
| Nighttime average diastolic mmHg | 65±11 | 67±12 | 0.0571 | 67±7 | 0.3390 |
| 24-h SD systolic mmHg | 18±4 | 17±4 | 0.4263 | 23±6 | 0.0009 |
| 24-h SD diastolic mmHg | 17±4 | 17±5 | 0.9515 | 21±8 | 0.0195 |
| 24-h SD heart rate bpm | 10±4 | 10±3 | 0.1909 | 11±3 | 0.1805 |
| 24-h CV systolic mmHg | 15±3 | 16±4 | 0.7609 | 19±4 | 0.0007 |
| 24-h CV diastolic mmHg | 25±5 | 25±6 | 0.9515 | 28±7 | 0.0683 |
| 24-h CV heart rate bpm | 13±4 | 14±5 | 0.2734 | 13±4 | 0.6169 |
| Systolic peaks in 24h n | 3±1 | 5±4 | 0.0984 | 7±2 | 0.0019 |
| Diastolic peaks in 24h n | 4±3 | 5±4 | 0.7224 | 8±3 | 0.0469 |
| Systolic hypertensive range %time | 8±6 | 14±12 | 0.1272 | 19±6 | 0.0547 |
| Diastolic in hypertensive range %time | 14±12 | 12±10 | 0.3054 | 23±12 | 0.0198 |
| Highest systolic mmHg | 157±20 | 150±17 | 0.1433 | 174±27 | 0.0015 |
| Highest diastolic mmHg | 106±18 | 109±17 | 0.7490 | 123±25 | 0.0097 |
| Highest heart rate bpm | 97±20 | 99±13 | 0.1932 | 105±13 | 0.1409 |
| Lowest systolic mmHg | 82±8 | 79±6 | 0.0818 | 79±9 | 0.5314 |
| Lowest diastolic mmHg | 43±6 | 41±2 | 0.1953 | 42±7 | 0.5058 |
| Lowest heart rate bpm | 55±9 | 59±7 | 0.1382 | 60±10 | 0.0999 |
| OH dizziness score item 1 points | 1.7±2.3 | 1.7±2.6 | 0.9999 | 0.7±1.3 | 0.2904 |
| OH activities daily activities score | 16±12 | 12±10 | 0.5303 | 9±11 | 0.3743 |
| Office readings | |||||
| Supine systolic mmHg | 127±18 | 127±21 | 0.7960 | 126±26 | 0.9959 |
| Supine diastolic mmHg | 73±19 | 75±22 | 0.9515 | 73±20 | 0.9501 |
| Supine heart rate bpm | 72±13 | 78±13 | 0.2345 | 76±11 | 0.3252 |
| 3 min standing systolic mmHg | 91±28 | 96±24 | 0.6698 | 96±28 | 0.6968 |
| 3 min standing diastolic mmHg | 44±18 | 51±26 | 0.6589 | 50±23 | 0.4567 |
| 3 min standing heart rate bpm | 72±12 | 78±13 | 0.0933 | 78±11 | 0.2136 |
OH = orthostatic hypotension, SD = standard deviation. P values for treatment effect adjusted to account for multiple comparisons using the Bonferroni method. Systolic peaks defined as >135 mmHg during the day and >120 mmHg during the night. Diastolic peaks defined as > 85 mmHg during the day and >70 mmHg during the night. p value comparing 300 versus 600 mg/day doses determined using nonparametric Wilcoxon test. Data are mean ± standard deviation.
EFFECT ON LOW blood pressure
Overall average 24-h diastolic blood pressure was 6 mmHg lower on carbidopa, but office blood pressure during supine rest in the office were not different (table 2). The lowest systolic and diastolic blood pressures captured on ambulatory monitoring were similar on carbidopa and placebo (table 2). There was also no measurable effect of carbidopa on blood pressures obtained in the office after three minutes of active standing (systolic: p=0.6968, diastolic: p=0.4567) and the orthostatic fall in blood pressure was also similar in all three treatment arms (systolic: p=0.8625, diastolic: p=0.7663). Orthostatic hypotension questionnaire scores for item one (dizziness/lightheadedness) were similar on placebo, high dose, and low dose carbidopa (p=0.2904). There was no effect of treatment with carbidopa on the activities of daily living scale that require standing or walking (p=0.3743).
HIGH DOSE VS. LOW DOSE
The patients tolerated well the transitions between low dose and high dose. Urinary norepinephrine levels were similarly suppressed on high dose carbidopa (5.8±3.1 ug/g CRT) and low dose (7.8±5.7 ug/g CRT). The 300 mg/day and the 600 mg/day doses had an equivalent effect on the primary endpoint, with a similar reduction in daytime systolic blood pressure variability achieved on both doses (figure 2, p=0.6387). The same reduction in the magnitude of the hypertensive peaks (p=0.1433) and morning blood pressure surge (p=0.4209) was seen with both doses. In-office blood pressure measurements at three minutes standing were similar on both doses (systolic p=0.6698 and diastolic p=0.6589) and there was no difference in dizziness/lightheadedness scores (p>0.9999) or activities of daily living (p=0.5303).
Discussion
In this clinical trial, patients with familial dysautonomia with unstable blood pressure due to a lesion in the afferent baroreflex neurons had less severe hypertensive surges when randomly assigned to the peripheral decarboxylase inhibitor carbidopa. The trial showed that carbidopa had a significant benefit on the primary endpoint and lowered the standard deviation of blood pressure variability on ambulatory monitoring. High dose and low dose carbidopa had similar effects, suggesting sufficient inhibition of norepinephrine synthesis can be achieved with 300 mg/day (100 mg three times per day). The results are a potential breakthrough in the treatment of patients with familial dysautonomia, as in contrast to standard anti-hypertensives, carbidopa was well tolerated.
Several clinical trials have shown that high blood pressure variability is an independent risk factor for the development of end-organ injury 21–23 and a higher morning blood pressure surge is associated with an increased risk of cardiovascular events. 20 This appears to also be the case in patients with familial dysautonomia, as higher blood pressure variability in childhood was associated with a faster progression to end-stage renal disease. 17 Aggressive treatment of the hypotension, did not reduce the prevalence of renal damage 12 and pathology findings suggest that it is the hypertensive surges that are most deleterious for the target organs in patients with familial dysautonomia. 14, 15
Carbidopa has several advantages for the long-term control of blood pressure as it does not cross the blood brain barrier and has no central sedative effects. In this small controlled trial, we did not see clinically significant worsening of hypotension on standing, nor did patients self-report more burdensome orthostatic symptoms or further limitations on standing or walking. The one-time near syncope reported as an adverse event on high dose carbidopa occurred in a patient on three additional medications to lower blood pressure. The reduction in diastolic blood pressure on 24-hour monitoring likely reflects the impact of less vasoconstrictor tone and blunting of the hypertensive peaks contributing to a lower overall mean. Early experiments in animals show that carbidopa causes a 40% suppression of norepinephrine synthesis in sympathetic neurons. 24 Presumably because carbidopa is a reversible competitive inhibitor of the DOPA-decarboxylase enzyme, it still allows some norepinephrine synthesis to occur and does not change resting blood pressure. 24, 25 This suggests that it is likely to be of major benefit in preventing the excess synthesis and release of norepinephrine when the sympathetic nervous system is overactivated. In line with this, resting blood pressure was unaffected and was similar in the supine position on and off treatment with carbidopa. Similar observations were noted in patients with autonomic failure where 200 mg of carbidopa was not found to change supine or standing blood pressure. 26 In our trial, the incidence of severe hypotension (<80 mmHg systolic) after 3 minutes of standing was the same in patients treated with placebo (40%, n = 6/16) and high dose carbidopa (35%, n = 6/17). Nevertheless, as a precaution against hypotension, in clinical practice we recommend starting at a very low dose of 25 mg three times daily and administer carbidopa in an upward titration of 50 mg increments with home monitoring of blood pressure.
Our trial has some important limitations. The sample size was small, but this is unavoidable as familial dysautonomia is a rare disease with only 350 known patients alive worldwide. 5 Carbidopa was given for one-month intervals and it is not known if these benefits are sustained, but this appears to indeed be the case in clinical practice. Norepinephrine in urine represents total body excretion and does not account for the levels directly secreted by sympathetic neurons or its re-uptake after release. Nevertheless, urine norepinephrine levels correlate well with systolic blood pressure variability over the same 24-hour period (figure 2). Finally, the trial was powered to detect an impact on hypertensive surges, but may be underpowered to detect worsening of orthostatic hypotension. It was not possible to systematically assess the impact of other medications, but from observations it is likely that symptomatic hypotension may occur when combined with multiple other anti-hypertensive drugs.
PERSPECTIVES
Blood pressure management in patients with afferent baroreceptor lesions is very challenging.16 This is the first clinical trial of a treatment to reduce the extreme hypertensive surges in afferent baroreflex failure. Using carbidopa to inhibit excessive peripheral catecholamine synthesis is a novel approach that may prove useful in treating patients with acquired lesions as well as other disorders of excessive catecholamine release.
Supplementary Material
Novelty and significance.
What is new?
This is the first clinical trial of a treatment to reduce the extreme hypertensive surges in patients with afferent baroreflex failure.
Carbidopa is a novel sympatholytic strategy for inhibiting catecholamine-driven hypertensive spikes.
What is relevant?
Unlike current treatments for blood pressure management in patients with familial dysautonomia, it was effective and well tolerated.
Summary
100 mg of carbidopa three times per day can successfully inhibit norepinephrine synthesis, reduce the extreme hypertensive surges, and lower blood pressure variability.
Acknowledgments
SOURCES OF FUNDING
Supported by the Food and Drug Administration’s Office of Orphan Product Development, including salary support for all 4 authors.
Footnotes
DISCLOSURES
None related to the matter of the article.
Bibliography
- 1.Riley CM, Day RA, Greeley DM, Landford WS. Central autonomic dysfunction with defective lacrimation: I. Report of five cases. Pediatrics. 1949;3:468–478 [PubMed] [Google Scholar]
- 2.Kaufmann H, Norcliffe-Kaufmann L, Palma JA. Baroreflex dysfunction. N Engl J Med. 2020;382:163–178 [DOI] [PubMed] [Google Scholar]
- 3.Norcliffe-Kaufmann L, Axelrod F, Kaufmann H. Afferent baroreflex failure in familial dysautonomia. Neurology. 2010;75:1904–1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slaugenhaupt SA, Blumenfeld A, Gill SP, Leyne M, Mull J, Cuajungco MP, Liebert CB, Chadwick B, Idelson M, Reznik L, Robbins C, Makalowska I, Brownstein M, Krappmann D, Scheidereit C, Maayan C, Axelrod FB, Gusella JF. Tissue-specific expression of a splicing mutation in the ikbkap gene causes familial dysautonomia. Am J Hum Genet. 2001;68:598–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norcliffe-Kaufmann L, Slaugenhaupt SA, Kaufmann H. Familial dysautonomia: History, genotype, phenotype and translational research. Prog Neurobiol. 2016 [DOI] [PubMed] [Google Scholar]
- 6.Macefield VG, Norcliffe-Kaufmann LJ, Axelrod F, Kaufmann H. Cardiac-locked bursts of muscle sympathetic nerve activity are absent in familial dysautonomia. J Physiol. 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pearson J, Brandeis L, Goldstein M. Tyrosine hydroxylase immunoreactivity in familial dysautonomia. Science. 1979;206:71–72 [DOI] [PubMed] [Google Scholar]
- 8.Smith AA, Dancis J. Exaggerated response to infused norepinephrine in familial dysautonomia. N Engl J Med. 1964;270:704–707 [DOI] [PubMed] [Google Scholar]
- 9.Fuente Mora C, Norcliffe-Kaufmann L, Palma JA, Kaufmann H. Chewing-induced hypertension in afferent baroreflex failure: A sympathetic response? Exp Physiol. 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Norcliffe-Kaufmann L, Palma JA, Kaufmann H. Mother-induced hypertension in familial dysautonomia. Clin Auton Res. 2016;26:79–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norcliffe-Kaufmann LJ, Axelrod FB, Kaufmann H. Cyclic vomiting associated with excessive dopamine in riley-day syndrome. J Clin Gastroenterol. 2013;47:136–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Norcliffe-Kaufmann L, Axelrod FB, Kaufmann H. Developmental abnormalities, blood pressure variability and renal disease in riley day syndrome. J Hum Hypertens. 2011;27:51–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elkayam L, Matalon A, Tseng CH, Axelrod F. Prevalence and severity of renal disease in familial dysautonomia. Am J Kidney Dis. 2006;48:780–786 [DOI] [PubMed] [Google Scholar]
- 14.Rekhtman Y, Bomback AS, Nash MA, Cohen SD, Matalon A, Jan DM, Kaufmann H, Axelrod FB, Radhakrishnan J, Appel GB. Renal transplantation in familial dysautonomia: Report of two cases and review of the literature. Clin J Am Soc Nephrol. 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reshef R, Aderka D, Suprun H, Manelis G, Manelis J. Myocardial infarction association with the riley-day syndrome. Am Heart J. 1977;94:486–490 [DOI] [PubMed] [Google Scholar]
- 16.Biaggioni I, Shibao CA, Diedrich A, Muldowney JAS 3rd, Laffer CL, Jordan J. Blood pressure management in afferent baroreflex failure: Jacc review topic of the week. J Am Coll Cardiol. 2019;74:2939–2947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palma JA, Norcliffe-Kaufmann L, Fuente-Mora C, Percival L, Mendoza-Santiesteban C, Kaufmann H. Current treatments in familial dysautonomia. Expert Opin Pharmacother. 2014;15:2653–2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Norcliffe-Kaufmann L, Martinez J, Axelrod F, Kaufmann H. Hyperdopaminergic crises in familial dysautonomia: A randomized trial of carbidopa. Neurology. 2013;80:1611–1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaufmann H, Malamut R, Norcliffe-Kaufmann L, Rosa K, Freeman R. The orthostatic hypotension questionnaire (ohq): Validation of a novel symptom assessment scale. Clin Auton Res. 2012;22:79–90 [DOI] [PubMed] [Google Scholar]
- 20.Kario K, Pickering TG, Umeda Y, Hoshide S, Hoshide Y, Morinari M, Murata M, Kuroda T, Schwartz JE, Shimada K. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: A prospective study. Circulation. 2003;107:1401–1406 [DOI] [PubMed] [Google Scholar]
- 21.Bae EH, Lim SY, Han KD, Oh TR, Choi HS, Kim CS, Ma SK, Kim SW. Association between systolic and diastolic blood pressure variability and the risk of end-stage renal disease. Hypertension. 2019;74:880–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mezue K, Goyal A, Pressman GS, Matthew R, Horrow JC, Rangaswami J. Blood pressure variability predicts adverse events and cardiovascular outcomes in sprint. J Clin Hypertens (Greenwich). 2018;20:1247–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossignol P, Girerd N, Gregory D, Massaro J, Konstam MA, Zannad F. Increased visit-to-visit blood pressure variability is associated with worse cardiovascular outcomes in low ejection fraction heart failure patients: Insights from the heaal study. Int J Cardiol. 2015;187:183–189 [DOI] [PubMed] [Google Scholar]
- 24.Wurtman RJ, Watkins CJ. Suppression of noradrenaline synthesis in sympathetic nerves by carbidopa, an inhibitor of peripheral dopa decarboxylase. Nature. 1977;265:79–80 [DOI] [PubMed] [Google Scholar]
- 25.Watanabe AM, Judy WV, Cardon PV. Effect of l-dopa on blood pressure and sympathetic nerve activity after decarboxylase inhibition in cats. J Pharmacol Exp Ther. 1974;188:107–113 [PubMed] [Google Scholar]
- 26.Kaufmann H, Saadia D, Voustianiouk A, Goldstein DS, Holmes C, Yahr MD, Nardin R, Freeman R. Norepinephrine precursor therapy in neurogenic orthostatic hypotension. Circulation. 2003;108:724–728 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.