Abstract
Pancreatic ductal adenocarcinoma (PDAC) is currently the third leading cause of cancer-related deaths and has a 5-year survival rate of less than 10%, far below the ~70% national average for all cancers. This poor prognosis is driven by an extreme resistance to nearly all known cancer treatments, which has long been attributed to hypoxia driven interactions between tumor cells and the supporting stromal microenvironment. The cellular response to hypoxia is driven by the transcription factors known as the hypoxia inducible factors (HIFs), which have been hypothesized to play a role in the pathobiology of PDAC as well as a potential therapeutic target based on years of cell culture data. Attempts to validate the oncogenic role of HIF in PDAC through rigorous spontaneous tumor models have paradoxically shown that the HIFs may act as a tumor suppressor in epithelial cells. Here, we seek to resolve this paradox by discussing the roles of HIFs both in cancer cells and the supporting microenvironment and place them into context of current model systems that could be used to interrogate these interactions. We suggest that HIF may exert its oncogenic influences by modulating the form and function of the stroma rather than direct effects on cancer cells.
Keywords: Stroma, Tumor microenvironment, Hypoxia, CAFs, TAMs
Introduction
Oxygen is a highly reactive element that plays a vital role in all eukaryotic life. As such, our cellular systems have evolved various mechanisms to adapt to changing oxygen levels. The atmosphere of Earth contains ~21% oxygen, however most cellular tissues only encounter oxygen levels of 3–13% [1,2]. To deal with the constant fluctuations in oxygen, [3] our cells have evolved a conserved program driven by the hypoxia inducible factor (HIF) family of transcription factors. In oxygenated environments, EGLN prolyl hydroxylases are responsible for hydroxylating the highly conserved two prolyl residues on HIF to create a binding site for the Von Hippel-Lindau (VHL) protein. The VHL E3 ubiquitin ligase complex then degrades HIF via the proteasome. During hypoxia, however, EGLN proteins no longer have sufficient oxygen to drive HIF hydroxylation, which results in the rapid stabilization of the HIF alpha subunit, which is then free to bind to HIF1β/ARNT. Together, this heterodimer binds to cognate hypoxia response elements (HREs) within enhancer elements to increase the transcription of its target genes. The ultimate results of activation of these transcriptional networks are a wide range of biological processes associated with cell survival in a low oxygen environment including embryogenesis, angiogenesis, and erythropoiesis. Additionally, hypoxic cells are known to shift energy production toward glycolysis and away from oxidative metabolism through the induction of mitophagy to reduce mitochondrial numbers [4–6]. The HIF program can even promote survival during tremendous stresses such as radiation damage [7].
The tumor microenvironment is a dynamic milieu consisting of not only the malignant tumor cells themselves, but also blood vessels, immune cells, fibroblast, adipocytes, and extracellular matrix [8]. Interestingly, the complex nature of interactions that shape the microenvironment can both promote [9,10] and antagonize [11] tumor growth. Despite the wide presence of endothelial cells, tumors often lose the normal tissue architecture that supports oxygenation, which leads to pockets of hypoxia throughout the tumor. Although adaptation to hypoxia is important to normal tissues, many have postulated that low oxygen levels also contribute to cancer growth, survival, and treatment resistance. Pancreatic ductal adenocarcinoma (PDAC) has one of the worst survival rates of all cancers driven by a poor response to most cancer treatments. We hypothesis that the hypoxic tumor microenvironment plays a large role in maintaining therapeutic resistance. Surprisingly HIF can play a tumor suppressive role in PDAC. Therefore, in this review, we seek to resolve this paradox by focusing on the current literature addressing the role of HIF in pancreatic cancer, as well as potential strategies to address gaps in knowledge. We believe that a better understanding of the role of hypoxia in PDAC will help guide important therapeutic approaches.
Hypoxia is Central to the Pathobiology of Pancreatic Cancer
PDAC is known for its desmoplastic stroma composed of activated stellate cells that express a large amount of extracellular matrix (ECM) and alpha-smooth muscle actin (αSMA) [12]. Traditionally, the desmoplastic stroma is known to increase intratumoral pressure [13–15] and acts as a barrier to impede the delivery of therapeutic agents such as chemotherapy and radiation as well as nutrients, and oxygen. These effects are seen readily on triphasic CT scans of pancreatic tumors, which have a pathognomonic feature of hypoenhancement relative to the nearby normal parenchyma [16]. Direct measurement of the oxygen tension within tumors have consistently shown that pancreatic cancer is among the most hypoxic of known tumors [1,17,18]. This intratumoral hypoxia leads to therapeutic resistance against chemotherapy and radiotherapy, which often depend on oxygen for full cytotoxic effects [19].
However, the lack of chemical oxygen also provokes a biological response through the stabilization of the HIF proteins. As reviewed elsewhere, the EGLN family of prolyl hydroxylases are inhibited in low oxygen, preventing hydroxylation of the HIF alpha subunit and its subsequent degradation by proteasomes [20]. HIF1α and HIF2α are the two major isoforms in mammalian cells and are the primary drivers of hypoxia response. HIF1 is expressed ubiquitously and responds rapidly to changes in oxygen levels [21]. HIF2, on the other hand is expressed in select cell types including vascular endothelial, lung type II pneumocytes, liver parenchyma, interstitial cells in the kidney, and stem cells [22–27] and may be stabilized at higher concentrations of oxygen [7]. When HIF is expressed at high levels in tumors, many cancer cells become more resistant to chemotherapy [28,29] and radiation [30–32] and may exhibit enhanced proliferation and migration [33–36].
The role of HIF in pancreatic cancer remains unclear. The specific deletion of either HIF1α [37] or HIF2α in the pancreatic epithelia accelerated PDAC formation [38], suggesting a potential tumor suppressive role. Moreover the direct overexpression of HIF2 suppressed PDAC growth and improved survival [39]. It is important to note that the previously mentioned studies involved manipulation of HIF expression in only the epithelium. This is in contrast to the physiological response of HIFs which would occur in most if not all cell types in the tumor. The tumor suppressive role of HIF is not isolated to PDAC, but is further supported by preclinical models of lung cancer [40–42] and sarcoma [43] which also exhibited a growth suppressive phenotype for HIF. We note, however, that one tumor where HIF is oncogenic is clear cell renal carcinoma (ccRCC), where HIF2 expression enabled by VHL-loss drives changes in growth and fatty acid metabolism [44,45]. Specific inhibitors to HIF2α are now progressing through clinical trials to treat ccRCC [46,47], but whether HIF2 inhibition might be beneficial in other cancers is not known but is an area of active investigation.
Resolving the Paradox HIF in PDAC Microenvironment
The tumor suppressive role of HIF in preclinical pancreatic cancer models is at odds with studies correlating the poor outcomes in PDAC with its hypoxic nature. Although several possible explanations exist, including heterogeneity of hypoxia within the tumor [17,18,48] and physiologic differences between mouse and man [49], perhaps the most striking possibility may be that previous studies have not accounted for the direct actions of HIF signaling within the tumor microenvironment. The tumor microenvironment is now appreciated to play as critical a role in oncogenesis as the individual driver mutations [9,10]. Unfortunately, most studies have focused exclusively on HIF within cancer cells and have ignored the possibility that HIF might be acting through stromal components, like the cancer associated fibroblasts or immune cells.
A pancreatic tumor contains only a small fraction of adenocarcinoma cells [50]. The major cellular component of PDAC is actually the cancer-associated fibroblast (CAF), which may account for up to 80% of the tumor volume [51,52]. There is significant debate about the roles and subtypes of CAFs in pancreatic cancer. Recent work using single cell methodology has revealed that many different subtypes of fibroblasts promote tumor progression [53], some which express inflammatory cytokines [54] and some that provide metabolic support through delivery of non-essential amino acids [55] or lipids [56]. Hypoxia metabolically reprograms CAFs to take on a more catabolic phenotype [57] and promotes the secretion of pro and anti-angiogenic factors [58]. In regards to secretion, it is noteworthy that hypoxia induces lipid remodeling [59] and free cholesterol accumulation [60]. The importance of this is highlighted by recent work linking levels of cholesterol in the tumor microenvironment with antitumor activity [61]. Therefore, we speculate that the hypoxic tumor microenvironment induces the excretion of critical lipids from CAFs which contributes to therapeutic resistance by attenuating the host antitumor immune response.
Besides cancer cells and CAFs, pancreatic tumors possess microvessels lined by endothelial cells, which grow in response to vascular endothelial growth factor (VEGF), a known HIF target. HIF1α and HIF2α may have complementary functions in physiological and pathological angiogenesis. For instance, the loss of HIF1α in endothelial cells inhibits blood vessel growth and tumor size by decreasing VEGF expression [62], whereas endothelial HIF2α null mice have reduced capacity for metastases [63,64].
The emergence of immunotherapy to stimulate a patient’s immune system to destroy a solid tumor has transformed modern cancer care [65]. The ability for T-effector lymphocytes to infiltrate and recognize a tumor are critical for immunotherapy to work [66,67]. Even if an active CD8+ lymphocyte can enter a tumor, there may not be sufficient antigen quantity or quality to induce an immune response [68–70]. Moreover, nearby cells can reduce the activity of effector lymphocytes, such as immunosuppressive regulatory T (Treg) cells [71] and tumor-associated macrophages (TAMs) [72]. Interestingly, high levels of HIF2α was observed in some populations of TAMs, even in tumors that lack expression of HIF2 [73].
The function of these immune effectors in hypoxic tumors appears to be context dependent. For instance, hypoxia both limited cytotoxic T-cell infiltration [74] and enhances Treg infiltration [75] but may also inhibit Treg differentiation [76]. Stabilized HIF expression by knockout of the EGLN proteins was recently shown to reduce tumor colonization in the lung [77]. In macrophage populations, however, HIF1 enhances PD-L1 expression [78] while HIF2α knockout reduces TAM infiltration in different carcinoma models, which is associated with reduced tumor cell proliferation and progression [79].
Modeling the Hypoxic Interplay Between Cancer Cells and Stroma
One common method to study stromal effects are ex vivo systems using co-culture. These systems are relatively easy to use and have provided the framework for much of our current understanding of tumor-stroma crosstalk [80,81]. There are several ways to preserve and culture stromal tissue ex vivo, each having its own advantages and disadvantages. Enzymatic and/or mechanical methods can be used to dissociate stromal samples, which can subsequently be cultured in either a 2D monolayer or in a 3D spheroid culture [82]. 3D spheroid models of hypoxia exhibit distinct signaling patterns and drug response that can help better predict responses in vivo [83–85]. The organotypic matrix invasion assay is another mixed cell 3D model which generates a reproducible hypoxia gradient that is compatible with various immunohistochemical and fluorescence techniques [17], including the commonly used hypoxia marker pimonidazole [86]. To further resemble the in vivo condition as closely as possible, dissected samples can alternatively be implanted in immunodeficient mice to generate patient-derived xenograft models [82]. In order to keep tissue architecture intact, organotypic tissue slices can also be generated by careful sectioning of the specimen [82]. Additionally, in vitro “tumor/organ-on-a-chip” models rely on complex fluidics and engineered scaffolds [87,88] in order to replicate some of the complex interactions that occur within the tumor-stromal environment. The advantage of these microfluidic devices is that they are amendable to advanced microscopy techniques [88].
Models that utilize immunocompetent mice exploit tumors that are syngeneic to a particular host, such as KPC or LLC cells derived from C57BL/6 mice [89] or 4T1 cells derived from BALB/c strains. KPC mice contain a conditionally activated KRas allele (G12D) and a heterozygous Trp53 loss-of-function allele, driven by a pancreas- specific Cre (Ptf1a-Cre) [90]. To study the effects of the stroma, germline mutations can be induced to create null or hypomorphic expression of a particular protein in every tissue of the mouse. This is possible when the gene of interest does not cause embryonic or perinatal lethality, but unfortunately, the major genes in the hypoxia response—HIF1 [91], HIF2 [92] and EGLN1 [93] do not develop to adulthood, which limits this approach.
These limitations may be overcome with the use of a dual recombinase system that enables the independent manipulation of the tumor genetics and the stroma. We and others [94] have developed a system that that initiates pancreatic tumorigenesis in a Flp-dependent fashion using a Pdx1-Flp along with a coordinated activation of Kras (G12D) oncogene (FSF-Kras) and a p53 allele (Trp53 FRT/FRT) in a Flp-dependent fashion. We can also breed in floxed alleles of the EGLN proteins, HIF proteins, or other key regulators in order to knock out these genes in specific cell types without also deleting these same genes in the tumor. This allows complete spatial and temporal control of gene deletion in the stroma or tumor tissues. We believe that his powerful system could be used to interrogate each stromal component in a definitive fashion (Figure 1).
Figure 1. Dual recombinase system for pancreatic cancer.
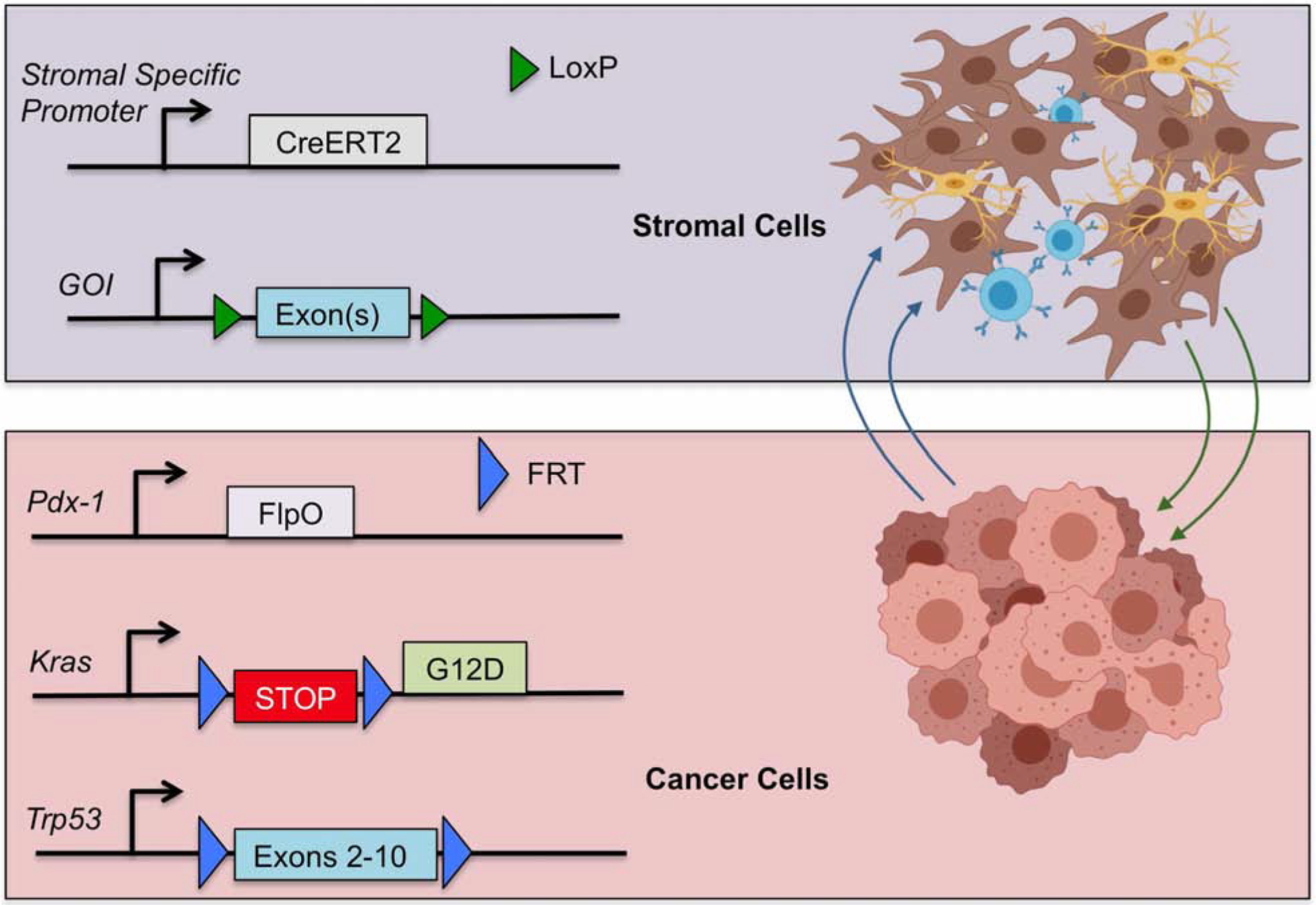
Flp recombinase directs tumorigenesis in the target tissue by a tissue-specific Flp (in this case, Pdx1-FlpO), which activates Kras and knocks out Trp53. This leaves stromal components available to be regulated by the Cre/lox system where a gene of interest (GOI) can be manipulated as desired with a tissue-specific Cre.
Conclusion and Future Directions
The function of HIF on the interplay between tumor and its stroma may be a key to unraveling the complex effects of hypoxia on cancer progression and treatment resistance. These data are of critical importance not only from a basic biology standpoint, but also because we now have the capability to target the function of the HIF transcription factors. For instance, the small molecule inhibitor PT2399 specifically binds to the HIF2α PAS B domain and causes tumor regression in ccRCC [47], which is depends on oncogenic expression of HIF2. It is unknown whether PT2399 or its derivatives may be useful in other cancer types that do not have canonical dependencies on HIF2. Similarly, the established tumor suppressive effects of epithelial HIF in PDAC suggest that selective targeting of stroma, or even specific stromal cell types is required for small molecule inhibition of HIF to induce tumor regression. Moreover, there still leaves the question of if HIF1 expression in CAFs have an effect on stromal formation or treatment resistance. The answers to these questions will provide essential insight into the role of HIF within the tumor microenvironment and should be fully understood before pursuing other HIF antagonists for the clinic.
Grant Support:
C.M.T. was supported by funding from National Institutes of Health under award number R01CA227517-01A1 and from the Cancer Prevention & Research Institute of Texas (CPRIT) grant RR140012, V Foundation (V2015-22), Sidney Kimmel Foundation, Sabin Family Foundation Fellowship, the Reaumond Family Foundation, and the McNair Family Foundation and generous philanthropic contributions to The University of Texas MD Anderson Moon Shots Program. This work was also supported by the NIH/NCI under award number P30CA016672 for use of the Small Animal Imaging Facility.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: C.M.T. is on the medical advisory board of Accuracy. The other authors report no relevant conflicts of interest.
Reference:
- [1].Koong AC, Mehta VK, Le QT, Fisher GA, Terris DJ, Brown JM, Bastidas AJ, Vierra M, Pancreatic tumors show high levels of hypoxia, Int. J. Radiat. Oncol. Biol. Phys 48 (2000) 919–922. 10.1016/S0360-3016(00)00803-8. [DOI] [PubMed] [Google Scholar]
- [2].Carreau A, El Hafny-Rahbi B, Matejuk A, Grillon C, Kieda C, Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia, J. Cell. Mol. Med 15 (2011) 1239–1253. 10.1111/j.1582-4934.2011.01258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Pugh CW, Ratcliffe PJ, Regulation of angiogenesis by hypoxia: Role of the HIF system, Nat. Med 9 (2003) 677–684. 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- [4].Semenza GL, Oxygen-dependent regulation of mitochondrial respiration by hypoxia-inducible factor 1, Biochem. J 405 (2007) 1–9. 10.1042/BJ20070389. [DOI] [PubMed] [Google Scholar]
- [5].Zhang H, Gao P, Fukuda R, Kumar G, Krishnamachary B, Zeller KI, Dang CVV, Semenza GL, HIF-1 Inhibits Mitochondrial Biogenesis and Cellular Respiration in VHL-Deficient Renal Cell Carcinoma by Repression of C-MYC Activity, Cancer Cell. 11 (2007) 407–420. 10.1016/j.ccr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- [6].Semenza GL, Roth PH, Fang HM, Wang GL, Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1, J. Biol. Chem 269 (1994) 23757–23763. [PubMed] [Google Scholar]
- [7].Taniguchi CM, Miao YR, Diep AN, Wu C, Rankin EB, Atwood TF, Xing L, Giaccia AJ, PHD inhibition mitigates and protects against radiation-induced gastrointestinal toxicity via HIF2, Sci. Transl. Med 6 (2014). 10.1126/scitranslmed.3008523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Balkwill FR, Capasso M, Hagemann T, The tumor microenvironment at a glance, J. Cell Sci 125 (2012) 5591–5596. 10.1242/jcs.116392. [DOI] [PubMed] [Google Scholar]
- [9].Hanahan D, Weinberg RA, Hallmarks of cancer: the next generation, Cell. 144 (2011) 646–674. 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- [10].Whiteside TL, The tumor microenvironment and its role in promoting tumor growth, Oncogene. 27 (2008) 5904–5912. 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA, Dekleva EN, Saunders T, Becerra CP, Tattersall IW, Westphalen CB, Kitajewski J, FernandezBarrena MG, Fernandez-Zapico ME, Iacobuzio-Donahue C, Olive KP, Stanger BZ, Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma, Cancer Cell. 25 (2014) 735–747. 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wang LM, Silva MA, D’Costa Z, Bockelmann R, Soonawalla Z, Liu S, O’Neill E, Mukherjee S, McKenna WG, Muschel R, Fokas E, The prognostic role of desmoplastic stroma in pancreatic ductal adenocarcinoma, Oncotarget. 7 (2016) 4183–4194. 10.18632/oncotarget.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Stylianopoulos T, Munn LL, Jain RK, Reengineering the Physical Microenvironment of Tumors to Improve Drug Delivery and Efficacy: From Mathematical Modeling to Bench to Bedside, Trends in Cancer. 4 (2018) 292–319. 10.1016/j.trecan.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jain RK, Tong RT, Munn LL, Effect of vascular normalization by antiangiogenic therapy on interstitial hypertension, peritumor edema, and lymphatic metastasis: Insights from a mathematical model, Cancer Res. 67 (2007) 2729–2735. 10.1158/00085472.CAN-06-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chauhan VP, Boucher Y, Ferrone CR, Roberge S, Martin JD, Stylianopoulos T, Bardeesy N, DePinho RA, Padera TP, Munn LL, Jain RK, Compression of Pancreatic Tumor Blood Vessels by Hyaluronan Is Caused by Solid Stress and Not Interstitial Fluid Pressure, Cancer Cell. 26 (2014) 14–15. 10.1016/j.ccr.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Koay EJ, Lee Y, Cristini V, Lowengrub JS, Kang Y, Anthony San Lucas F, Hobbs BP, Ye R, Elganainy D, Almahariq M, Amer AM, Chatterjee D, Yan H, Park PC, Rios Perez MV, Li D, Garg N, Reiss KA, Yu S, Chauhan A, Zaid M, Nikzad N, Wolff RA, Javle M, Varadhachary GR, Shroff RT, Das P, Lee JE, Ferrari M, Maitra A, Taniguchi CM, Kim MP, Crane CH, Katz MH, Wang H, Bhosale P, Tamm EP, Fleming JB, A visually apparent and quantifiable CT imaging feature identifies biophysical subtypes of pancreatic ductal adenocarcinoma, Clin. Cancer Res 24 (2018) 5883–5894. 10.1158/10780432.CCR-17-3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Conway JRW, Warren SC, Herrmann D, Murphy KJ, Cazet AS, Vennin C, Shearer RF, Killen MJ, Magenau A, Mélénec P, Pinese M, Nobis M, Zaratzian A, Boulghourjian A, Da Silva AM, del Monte-Nieto G, Adam ASA, Harvey RP, Haigh JJ, Wang Y, Croucher DR, Sansom OJ, Pajic M, Caldon CE, Morton JP, Timpson P, Intravital Imaging to Monitor Therapeutic Response in Moving Hypoxic Regions Resistant to PI3K Pathway Targeting in Pancreatic Cancer, Cell Rep. 23 (2018) 3312–3326. 10.1016/j.celrep.2018.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lohse I, Lourenco C, Ibrahimov E, Pintilie M, Tsao MS, Hedley DW, Assessment of hypoxia in the stroma of patient-derived pancreatic tumor xenografts, Cancers (Basel). 6 (2014) 459–471. 10.3390/cancers6010459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Riley PA, Free radicals in biology: Oxidative stress and the effects of ionizing radiation, Int. J. Radiat. Biol 65 (1994) 27–33. 10.1080/09553009414550041. [DOI] [PubMed] [Google Scholar]
- [20].Huang Y, Lin D, Taniguchi CM, Hypoxia inducible factor (HIF) in the tumor microenvironment: friend or foe?, Sci. China Life Sci 60 (2017) 1114–1124. 10.1007/s11427-017-9178-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Salceda S, Caro J, Hypoxia-inducible factor 1α (HIF-1α) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes, J. Biol. Chem 272 (1997) 22642–22647. 10.1074/jbc.272.36.22642. [DOI] [PubMed] [Google Scholar]
- [22].Covello KL, Kehler J, Yu H, Gordan JD, Arsham AM, Hu CJ, Labosky PA, Simon MC, Keith B, HIF-2α regulates Oct-4: Effects of hypoxia on stem cell function, embryonic development, and tumor growth, Genes Dev. 20 (2006) 557–570. 10.1101/gad.1399906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ema M, Taya S, Yokotani N, Sogawa K, Matsuda Y, Fujii-Kuriyama Y, A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1α regulates the VEGF expression and is potentially involved in lung and vascular development, Proc. Natl. Acad. Sci. U. S. A 94 (1997) 4273–4278. 10.1073/pnas.94.9.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Flamme I, Fröhlich T, von Reutern M, Kappel A, Damert A, Risau W, HRF, a putative basic helix-loop-helix-PAS-domain transcription factor is closely related to hypoxia-inducible factor-1 alpha and developmentally expressed in blood vessels., Mech. Dev 63 (1997) 51–60. 10.1016/s0925-4773(97)00674-6. [DOI] [PubMed] [Google Scholar]
- [25].Tian H, McKnight SL, Russell DW, Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells, Genes Dev. 11 (1997) 72–82. 10.1101/gad.11.1.72. [DOI] [PubMed] [Google Scholar]
- [26].Compernolle V, Brusselmans K, Acker T, Hoet P, Tjwa M, Beck H, Plaisance S, Dor Y, Keshet E, Lupu F, Nemery B, Dewerchin M, Van Veldhoven P, Plate K, Moons L, Collen D, Carmeliet P, Loss of HIF-2α and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice, Nat. Med 8 (2002) 702–710. 10.1038/nm721. [DOI] [PubMed] [Google Scholar]
- [27].Wiesener MS, Jürgensen JS, Rosenberger C, Scholze CK, Hörstrup JH, Warnecke C, Mandriota S, Bechmann I, Frei UA, Pugh CW, Ratcliffe PJ, Bachmann S, Maxwell PH, Eckardt KU, Widespread hypoxia-inducible expression of HIF-2alpha in distinct cell populations of different organs., FASEB J. 17 (2003) 271–273. 10.1096/fj.02-0445fje. [DOI] [PubMed] [Google Scholar]
- [28].Warfel NA, El-Deiry WS, HIF-1 Signaling in Drug Resistance to Chemotherapy, Curr. Med. Chem 21 (2014) 3021–3028. 10.2174/0929867321666140414101056. [DOI] [PubMed] [Google Scholar]
- [29].Samanta D, Gilkesa DM, Chaturvedia P, Xiang L, Semenza GL, Hypoxia-inducible factors are required for chemotherapy resistance of breast cancer stem cells, Proc. Natl. Acad. Sci. U. S. A 111 (2014) E5429–E5438. 10.1073/pnas.1421438111. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [30].Zhong R, Xu H, Chen G, Zhao G, Gao Y, Liu X, Ma S, Dong L, The role of hypoxiainducible factor-1α in radiation-induced autophagic cell death in breast cancer cells, Tumor Biol 36 (2015) 7077–7083. 10.1007/s13277-015-3425-z. [DOI] [PubMed] [Google Scholar]
- [31].Harada H, Inoue M, Itasaka S, Hirota K, Morinibu A, Shinomiya K, Zeng L, Ou G, Zhu Y, Yoshimura M, McKenna WG, Muschel RJ, Hiraoka M, Cancer cells that survive radiation therapy acquire HIF-1 activity and translocate towards tumour blood vessels, Nat. Commun 3 (2012). 10.1038/ncomms1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Moeller BJ, Cao Y, Li CY, Dewhirst MW, Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: Role of reoxygenation, free radicals, and stress granules, Cancer Cell. 5 (2004) 429–441. 10.1016/S1535-6108(04)00115-1. [DOI] [PubMed] [Google Scholar]
- [33].Gordan JD, Bertout JA, Hu CJ, Diehl JA, Simon MC, HIF-2α Promotes Hypoxic Cell Proliferation by Enhancing c-Myc Transcriptional Activity, Cancer Cell. 11 (2007) 335–347. 10.1016/j.ccr.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Chen S, Zhang M, Xing L, Wang Y, Xiao Y, Wu Y, HIF-1α contributes to proliferation and invasiveness of neuroblastoma cells via SHH signaling, PLoS One. 10 (2015). 10.1371/journal.pone.0121115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Semenza GL, Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics, Oncogene. 29 (2010) 625–634. 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Liao D, Johnson RS, Hypoxia: A key regulator of angiogenesis in cancer, Cancer Metastasis Rev. 26 (2007) 281–290. 10.1007/s10555-007-9066-y. [DOI] [PubMed] [Google Scholar]
- [37].Lee KE, Spata M, Bayne LJ, Buza EL, Durham AC, Allman D, Vonderheide RH, Simon MC, Hif1a deletion reveals pro-neoplastic function of B cells in pancreatic neoplasia, Cancer Discov. 6 (2016) 256–269. 10.1158/2159-8290.CD-15-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Criscimanna A, Duan LJ, Rhodes JA, Fendrich V, Wickline E, Hartman DJ, Monga SPS, Lotze MT, Gittes GK, Fong GH, Esni F, PanIN-specific regulation of Wnt signaling by HIF2a during early pancreatic tumorigenesis, Cancer Res. 73 (2013) 4781–4790. 10.1158/0008-5472.CAN-13-0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Fujimoto TN, Colbert LE, Huang Y, Molkentine JM, Deorukhkar A, Baseler L, De B Cruz B. La, Yu M, Lin D, Gupta S, Cabeceiras PK, Kingsley CV, Tailor RC, Sawakuchi GO, Koay EJ, Piwnica-Worms H, Maitra A, Taniguchi CM, Selective EGLN inhibition enables ablative radiotherapy and improves survival in unresectable pancreatic cancer, Cancer Res. 79 (2019) 2327–2338. 10.1158/0008-5472.CAN-18-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Mazumdar J, Hickey MM, Pant DK, Durham AC, Sweet-Cordero A, Vachani A, Jacks T, Chodosh LA, Kissil JL, Simon MC, Keith B, HIF-2α deletion promotes Kras-driven lung tumor development, Proc. Natl. Acad. Sci. U. S. A 107 (2010) 14182–14187. 10.1073/pnas.1001296107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Jiang HL, Xu CX, Kim YK, Arote R, Jere D, Lim HT, Cho MH, Cho CS, The suppression of lung tumorigenesis by aerosol-delivered folate-chitosan-graftpolyethylenimine/Akt1 shRNA complexes through the Akt signaling pathway, Biomaterials. 30 (2009) 5844–5852. 10.1016/j.biomaterials.2009.07.017. [DOI] [PubMed] [Google Scholar]
- [42].Kim WY, Perera S, Zhou B, Carretero J, Jen JY, Heathcote SA, Jackson AL, Nikolinakos P, Ospina B, Naumov G, Brandstetter KA, Weigman VJ, Zaghlul S, Hayes DN, Padera RF, Heymach JV, Kung AL, Sharpless NE, Kaelin WG, Wong KK, HIF2α cooperates with RAS to promote lung tumorigenesis in mice, J. Clin. Invest 119 (2009) 2160–2170. 10.1172/JCI38443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Nakazawa MS, Eisinger-Mathason TSK, Sadri N, Ochocki JD, Gade TPF, Amin RK, Simon MC, Epigenetic re-expression of HIF-2α suppresses soft tissue sarcoma growth, Nat. Commun 7 (2016) 10539 10.1038/ncomms10539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kondo K, Klco J, Nakamura E, Lechpammer M, Kaelin WG, Inhibition of HIF is necessary for tumor suppression by the von Hippel-Lindau protein, Cancer Cell. 1 (2002) 237–246. 10.1016/S1535-6108(02)00043-0. [DOI] [PubMed] [Google Scholar]
- [45].Du W, Zhang L, Brett-Morris A, Aguila B, Kerner J, Hoppel CL, Puchowicz M, Serra D, Herrero L, Rini BI, Campbell S, Welford SM, HIF drives lipid deposition and cancer in ccRCC via repression of fatty acid metabolism, Nat. Commun 8 (2017) 1769 10.1038/s41467-017-01965-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Cho H, Du X, Rizzi JP, Liberzon E, Chakraborty AA, Gao W, Carvo I, Signoretti S, Bruick RK, Josey JA, Wallace EM, Kaelin WG, On-target efficacy of a HIF-2α antagonist in preclinical kidney cancer models, Nature. 539 (2016) 107–111. 10.1038/nature19795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Chen W, Hill H, Christie A, Kim MS, Holloman E, Pavia-Jimenez A, Homayoun F, Ma Y, Patel N, Yell P, Hao G, Yousuf Q, Joyce A, Pedrosa I, Geiger H, Zhang H, Chang J, Gardner KH, Bruick RK, Reeves C, Hwang TH, Courtney K, Frenkel E, Sun X, Zojwalla N, Wong T, Rizzi JP, Wallace EM, Josey JA, Xie Y, Xie XJ, Kapur P, McKay RM, Brugarolas J, Targeting renal cell carcinoma with a HIF-2 antagonist, Nature. 539 (2016) 112–117. 10.1038/nature19796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Dhani NC, Serra S, Pintilie M, Schwock J, Xu J, Gallinger S, Hill RP, Hedley DW, Analysis of the intra- and intertumoral heterogeneity of hypoxia in pancreatic cancer patients receiving the nitroimidazole tracer pimonidazole, Br. J. Cancer 113 (2015) 864–871. 10.1038/bjc.2015.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Perlman RL, Mouse models of human disease: An evolutionary perspective., Evol. Med. Public Heal 2016 (2016) 170–6. 10.1093/emph/eow014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Fasano E, Serini S, Piccioni E, Toesca A, Monego G, Cittadini AR, Ranelletti FO, Calviello G, DHA induces apoptosis by altering the expression and cellular location of GRP78 in colon cancer cell lines, Biochim Biophys Acta. 1822 (2012) 1762–1772. 10.1016/j.bbadis.2012.08.003. [DOI] [PubMed] [Google Scholar]
- [51].Erkan M, Hausmann S, Michalski CW, Fingerle AA, Dobritz M, Kleeff J, Friess H, The role of stroma in pancreatic cancer: Diagnostic and therapeutic implications, Nat. Rev. Gastroenterol. Hepatol 9 (2012) 454–467. 10.1038/nrgastro.2012.115. [DOI] [PubMed] [Google Scholar]
- [52].Erkan M, Michalski CW, Rieder S, Reiser-Erkan C, Abiatari I, Kolb A, Giese NA, Esposito I, Friess H, Kleeff J, The Activated Stroma Index Is a Novel and Independent Prognostic Marker in Pancreatic Ductal Adenocarcinoma, Clin. Gastroenterol. Hepatol 6 (2008) 1155–1161. 10.1016/j.cgh.2008.05.006. [DOI] [PubMed] [Google Scholar]
- [53].Hwang RF, Moore T, Arumugam T, Ramachandran V, Amos KD, Rivera A, Ji B, Evans DB, Logsdon CD, Cancer-associated stromal fibroblasts promote pancreatic tumor progression, Cancer Res 68 (2008) 918–926. 10.1158/0008-5472.CAN-07-5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Öhlund D, Handly-Santana A, Biffi G, Elyada E, Almeida AS, Ponz-Sarvise M, Corbo V, Oni TE, Hearn SA, Lee EJ, Chio IIC, Il Hwang C, Tiriac H, Baker LA, Engle DD, Feig C, Kultti A, Egeblad M, Fearon DT, Crawford JM, Clevers H, Park Y, Tuveson DA, Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer, J. Exp. Med 214 (2017) 579–596. 10.1084/jem.20162024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Sousa CM, Biancur DE, Wang X, Halbrook CJ, Sherman MH, Zhang L, Kremer D, Hwang RF, Witkiewicz AK, Ying H, Asara JM, Evans RM, Cantley LC, Lyssiotis CA, Kimmelman AC, Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion, Nature. 536 (2016) 479–483. 10.1038/nature19084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Auciello FR, Bulusu V, Oon C, Tait-Mulder J, Berry M, Bhattacharyya S, Tumanov S, Allen-Petersen BL, Link J, Kendsersky ND, Vringer E, Schug M, Novo D, Hwang RF, Evans RM, Nixon C, Dorrell C, Morton JP, Norman JC, Sears RC, Kamphorst JJ, Sherman MH, A stromal lysolipid–autotaxin signaling axis promotes pancreatic tumor progression, Cancer Discov. 9 (2019) 617–627. 10.1158/2159-8290.CD-18-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Avagliano A, Granato G, Ruocco MR, Romano V, Belviso I, Carfora A, Montagnani S, Arcucci A, Metabolic Reprogramming of Cancer Associated Fibroblasts: The Slavery of Stromal Fibroblasts., Biomed Res. Int 2018 (2018) 6075403 10.1155/2018/6075403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Kugeratski FG, Atkinson SJ, Neilson LJ, Lilla S, Knight JRP, Serneels J, Juin A, Ismail S, Bryant DM, Markert EK, Machesky LM, Mazzone M, Sansom OJ, Zanivan S, Hypoxic cancer-associated fibroblasts increase NCBP2-AS2/HIAR to promote endothelial sprouting through enhanced VEGF signaling, Sci. Signal 12 (2019). 10.1126/scisignal.aan8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Botto L, Beretta E, Bulbarelli A, Rivolta I, Lettiero B, Leone BE, Miserocchi G, Palestini P, Hypoxia-induced modifications in plasma membranes and lipid microdomains in A549 cells and primary human alveolar cells, J. Cell. Biochem 105 (2008) 503–513. 10.1002/jcb.21850. [DOI] [PubMed] [Google Scholar]
- [60].Cao R, Zhao X, Li S, Zhou H, Chen W, Ren L, Zhou X, Zhang H, Shi R, Hypoxia induces dysregulation of lipid metabolism in HepG2 cells via activation of HIF-2α, Cell. Physiol. Biochem 34 (2014) 1427–1441. 10.1159/000366348. [DOI] [PubMed] [Google Scholar]
- [61].Ma X, Bi E, Lu Y, Su P, Huang C, Liu L, Wang Q, Yang M, Kalady MF, Qian J, Zhang A, Gupte AA, Hamilton DJ, Zheng C, Yi Q, Cholesterol Induces CD8+ T Cell Exhaustion in the Tumor Microenvironment, Cell Metab. 30 (2019) 143–156.e5. 10.1016/j.cmet.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Tang N, Wang L, Esko J, Giordano FJ, Huang Y, Gerber HP, Ferrara N, Johnson RS, Loss of HIF-1α in endothelial cells disrupts a hypoxia-driven VEGF autocrine loop necessary for tumorigenesis, Cancer Cell. 6 (2004) 485–495. 10.1016/j.ccr.2004.09.026. [DOI] [PubMed] [Google Scholar]
- [63].Skuli N, Liu L, Runge A, Wang T, Yuan L, Patel S, Iruela-Arispe L, Simon MC, Keith B, Endothelial deletion of hypoxia-inducible factor-2α (HIF-2α) alters vascular function and tumor angiogenesis, Blood. 114 (2009) 469–477. 10.1182/blood-2008-12-193581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Skuli N, Majmundar AJ, Krock BL, Mesquita RC, Mathew LK, Quinn ZL, Runge A, Liu L, Kim MN, Liang J, Schenkel S, Yodh AG, Keith B, Simon MC, Endothelial HIF-2α regulates murine pathological angiogenesis and revascularization processes, J. Clin. Invest 122 (2012) 1427–1443. 10.1172/JCI57322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Wei SC, Duffy CR, Allison JP, Fundamental mechanisms of immune checkpoint blockade therapy, Cancer Discov. 8 (2018) 1069–1086. 10.1158/2159-8290.CD-18-0367. [DOI] [PubMed] [Google Scholar]
- [66].Li J, Byrne KT, Yan F, Yamazoe T, Chen Z, Baslan T, Richman LP, Lin JH, Sun YH, Rech AJ, Balli D, Hay CA, Sela Y, Merrell AJ, Liudahl SM, Gordon N, Norgard RJ, Yuan S, Yu S, Chao T, Ye S, Eisinger-Mathason TSK, Faryabi RB, Tobias JW, Lowe SW, Coussens LM, Wherry EJ, Vonderheide RH, Stanger BZ, Tumor Cell-Intrinsic Factors Underlie Heterogeneity of Immune Cell Infiltration and Response to Immunotherapy, Immunity. 49 (2018) 178–193.e7. 10.1016/j.immuni.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Hou YC, Chao YJ, Hsieh MH, Tung HL, Wang HC, Shan YS, Low cd8+ t cell infiltration and high pd-l1 expression are associated with level of cd44+/cd133+ cancer stem cells and predict an unfavorable prognosis in pancreatic cancer, Cancers (Basel). 11 (2019). 10.3390/cancers11040541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Yarchoan M, Hopkins A, Jaffee EM, Tumor mutational burden and response rate to PD-1 inhibition, N. Engl. J. Med 377 (2017) 2500–2501. 10.1056/NEJMc1713444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Chan TA, Yarchoan M, Jaffee E, Swanton C, Quezada SA, Stenzinger A, Peters S, Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic., Ann. Oncol. Off. J. Eur. Soc. Med. Oncol 30 (2019) 44–56. 10.1093/annonc/mdy495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Nejati R, Goldstein JB, Wang H, Halperin DM, Wang H, Hejazi N, Rashid A, Katz MH, Lee JE, Fleming JB, Rodriguez-Canales J, Blando J, Wistuba II, Maitra A, Wolff RA, Varadhachary GR, Prognostic Significance of Tumor-Infiltrating Lymphocytes in Patients with Pancreatic Ductal Adenocarcinoma Treated with Neoadjuvant Chemotherapy, Pancreas. 46 (2017) 1180–1187. 10.1097/MPA.0000000000000914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Kamada T, Togashi Y, Tay C, Ha D, Sasaki A, Nakamura Y, Sato E, Fukuoka S, Tada Y, Tanaka A, Morikawa H, Kawazoe A, Kinoshita T, Shitara K, Sakaguchi S, Nishikawa H, PD-1+ regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer, Proc. Natl. Acad. Sci. U. S. A 116 (2019) 9999–10008. 10.1073/pnas.1822001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].DeNardo DG, Ruffell B, Macrophages as regulators of tumour immunity and immunotherapy, Nat. Rev. Immunol 19 (2019) 369–382. 10.1038/s41577-019-0127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Talks KL, Turley H, Gatter KC, Maxwell PH, Pugh CW, Ratcliffe PJ, Harris AL, The expression and distribution of the hypoxia-inducible factors HIF-1α and HIF-2α in normal human tissues, cancers, and tumor-associated macrophages, Am. J. Pathol 157 (2000) 411–421. 10.1016/S0002-9440(10)64554-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Jayaprakash P, Ai M, Liu A, Budhani P, Bartkowiak T, Sheng J, Ager C, Nicholas C, Jaiswal AR, Sun Y, Shah K, Balasubramanyam S, Li N, Wang G, Ning J, Zal A, Zal T, Curran MA, Targeted hypoxia reduction restores T cell infiltration and sensitizes prostate cancer to immunotherapy, J. Clin. Invest 128 (2018) 5137–5149. 10.1172/JCI96268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang LP, Gimotty PA, Gilks CB, Lal P, Zhang L, Coukos G, Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T reg cells, Nature. 475 (2011) 226–230. 10.1038/nature10169. [DOI] [PubMed] [Google Scholar]
- [76].Dang EV, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y, Bordman Z, Fu J, Kim Y, Yen HR, Luo W, Zeller K, Shimoda L, Topalian SL, Semenza GL, Dang CV, Pardoll DM, Pan F, Control of TH17/Treg balance by hypoxia-inducible factor 1, Cell. 146 (2011) 772–784. 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Clever D, Roychoudhuri R, Constantinides MG, Askenase MH, Sukumar M, Klebanoff CA, Eil RL, Hickman HD, Yu Z, Pan JH, Palmer DC, Phan AT, Goulding J, Gattinoni L, Goldrath AW, Belkaid Y, Restifo NP, Oxygen Sensing by T Cells Establishes an Immunologically Tolerant Metastatic Niche, Cell. 166 (2016) 1117–1131.e14. 10.1016/j.cell.2016.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, Bronte V, Chouaib S, PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced: MDSCmediated T cell activation, J. Exp. Med 211 (2014) 781–790. 10.1084/jem.20131916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Imtiyaz HZ, Williams EP, Hickey MM, Patel SA, Durham AC, Yuan LJ, Hammond R, Gimotty PA, Keith B, Simon MC, Hypoxia-inducible factor 2α regulates macrophage function in mouse models of acute and tumor inflammation, J. Clin. Invest 120 (2010) 2699–2714. 10.1172/JCI39506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Lee JH, Kim SK, Khawar IA, Jeong SY, Chung S, Kuh HJ, Microfluidic co-culture of pancreatic tumor spheroids with stellate cells as a novel 3D model for investigation of stromamediated cell motility and drug resistance, J. Exp. Clin. Cancer Res 37 (2018) 4 10.1186/s13046-017-0654-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Hickman JA, Graeser R, de Hoogt R, Vidic S, Brito C, Gutekunst M, van der Kuip H, Imi Predect consortium, Three-dimensional models of cancer for pharmacology and cancer cell biology: Capturing tumor complexity in vitro/ex vivo, Biotechnol. J 9 (2014) 1115–1128. 10.1002/biot.201300492. [DOI] [PubMed] [Google Scholar]
- [82].Meijer TG, Naipal KA, Jager A, Van Gent DC, Ex vivo tumor culture systems for functional drug testing and therapy response prediction, Futur. Sci. OA 3 (2017) FSO190 10.4155/fsoa-2017-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Riedl A, Schlederer M, Pudelko K, Stadler M, Walter S, Unterleuthner D, Unger C, Kramer N, Hengstschläger M, Kenner L, Pfeiffer D, Krupitza G, Dolznig H, Comparison of cancer cells in 2D vs 3D culture reveals differences in AKT–mTOR–S6K signaling and drug responses, J. Cell Sci 130 (2017) 203–218. 10.1242/jcs.188102. [DOI] [PubMed] [Google Scholar]
- [84].Bielecka ZF, Malinowska A, Brodaczewska KK, Klemba A, Kieda C, Krasowski P, Grzesiuk E, Piwowarski J, Czarnecka AM, Szczylik C, Hypoxic 3D in vitro culture models reveal distinct resistance processes to TKIs in renal cancer cells, Cell Biosci 7 (2017). 10.1186/s13578-017-0197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Klutzny S, Lesche R, Keck M, Kaulfuss S, Schlicker A, Christian S, Sperl C, Neuhaus R, Mowat J, Steckel M, Riefke B, Prechtl S, Parczyk K, Steigemann P, Functional inhibition of acid sphingomyelinase by Fluphenazine triggers hypoxia-specific tumor cell death, Cell Death Dis. 8 (2017) e2709 10.1038/cddis.2017.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Aguilera K, Brekken R, Hypoxia Studies with Pimonidazole in vivo, BIO-PROTOCOL. 4 (2014). 10.21769/bioprotoc.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Shik Mun K, Arora K, Huang Y, Yang F, Yarlagadda S, Ramananda Y, Abu-El-Haija M, Palermo JJ, Appakalai BN, Nathan JD, Naren AP, Patient-derived pancreas-on-a-chip to model cystic fibrosis-related disorders, Nat. Commun 10 (2019) 3124 10.1038/s41467-019-11178-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Kumar V, Varghese S, Ex Vivo Tumor-on-a-Chip Platforms to Study Intercellular Interactions within the Tumor Microenvironment, Adv. Healthc. Mater 8 (2019) e1801198 10.1002/adhm.201801198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Yu M, Nguyen ND, Huang Y, Lin D, Fujimoto TN, Molkentine JM, Deorukhkar A, Kang Y, San Lucas FA, Fernandes CJ, Koay EJ, Gupta S, Ying H, Koong AC, Herman JM, Fleming JB, Maitra A, Taniguchi CM, Mitochondrial fusion exploits a therapeutic vulnerability of pancreatic cancer, JCI Insight. 4 (2019). 10.1172/jci.insight.126915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Ying H, Kimmelman AC, Lyssiotis CA, Hua S, Chu GC, Fletcher-Sananikone E, Locasale JW, Son J, Zhang H, Coloff JL, Yan H, Wang W, Chen S, Viale A, Zheng H, Paik JH, Lim C, Guimaraes AR, Martin ES, Chang J, Hezel AF, Perry SR, Hu J, Gan B, Xiao Y, Asara JM, Weissleder R, Wang YA, Chin L, Cantley LC, DePinho RA, Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism, Cell. 149 (2012) 656–670. 10.1016/j.cell.2012.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Kotch LE, Iyer NV, Laughner E, Semenza GL, Defective vascularization of HIF-1α-null embryos is not associated with VEGF deficiency but with mesenchymal cell death, Dev. Biol 209 (1999) 254–267. 10.1006/dbio.1999.9253. [DOI] [PubMed] [Google Scholar]
- [92].Patel SA, Simon MC, Biology of hypoxia-inducible factor-2α in development and disease, Cell Death Differ. 15 (2008) 628–634. 10.1038/cdd.2008.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Minamishima YA, Moslehi J, Bardeesy N, Cullen D, Bronson RT, Kaelin WG, Somatic inactivation of the PHD2 prolyl hydroxylase causes polycythemia and congestive heart failure, Blood. 111 (2008) 3236–3244. 10.1182/blood-2007-10-117812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Schönhuber N, Seidler B, Schuck K, Veltkamp C, Schachtler C, Zukowska M, Eser S, Feyerabend TB, Paul MC, Eser P, Klein S, Lowy AM, Banerjee R, Yang F, Lee CL, Moding EJ, Kirsch DG, Scheideler A, Alessi DR, Varela I, Bradley A, Kind A, Schnieke AE, Rodewald HR, Rad R, Schmid RM, Schneider G, Saur D, A next-generation dualrecombinase system for time- and host-specific targeting of pancreatic cancer, Nat. Med 20 (2014) 1340–1347. 10.1038/nm.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]


