Abstract
Over 2 million mostly rural Americans are at risk of drinking water from private wells that contain arsenic (As) exceeding the U.S. Environmental Protection Agency (USEPA) Maximum Contaminant Level (MCL) of 10 μg/L. How well existing treatment technologies perform in real world situations, and to what extent they reduce health risks, are not well understood. This study evaluates the effectiveness of household As treatment systems in southern-central Maine (ME, n=156) and northern New Jersey (NJ, n=94) and ascertains how untreated well water chemistry and other factors influence As removal. Untreated and treated water samples, as well as a treatment questionnaire, were collected. Most ME households had point-of-use reverse-osmosis systems (POU RO), while in NJ, dual-tank point-of-entry (POE) whole house systems were popular. Arsenic treatment systems reduced well water arsenic concentrations ([As]) by up to two orders of magnitude, i.e. from a median of 71.7 to 0.8 μg/L and from a mean of 105 to 14.3 μg/L in ME, and from a median of 8.6 to 0.2 μg/L and a mean of 15.8 to 2.1 μg/L in NJ. More than half (53%) of the systems in ME reduced water [As] to below 1 μg/L, compared to 69% in NJ. The treatment system failure rates were 19% in ME (> USEPA MCL of 10 μg/L) and 16% in NJ (> NJ MCL of 5 μg/L). In both states, the higher the untreated well water [As] and the As(III)/As ratio, the higher the rate of treatment failure. POE systems failed less than POU systems, as did the treatment systems installed and maintained by vendors than those by homeowners. The 7-fold reduction of [As] in the treated water reduced skin cancer risk alone from 3,765 to 514 in 1 million in ME, and from 568 to 75 in 1 million in NJ.
Keywords: private well, arsenic removal, point-of-entry, point-of-use, exposure, health risk
Graphical abstract
1. Introduction
High arsenic (As) concentrations in drinking water have emerged as a worldwide public health concern, especially in rural areas where homeowners do not have access to public water supplies but rely on private wells. In the United States (U.S.), approximately 44.1 million, or 14% of the population are dependent on private well water (Ayotte et al., 2017), with 11% of these private wells having As concentrations ([As]) at risk of exceeding the U.S. Environmental Protection Agency (USEPA) Maximum Contaminant Level (MCL) of 10 μg/L for As nationwide (Ayotte et al., 2017; Focazio et al., 2006). It has been estimated that 2.1 million people living in the conterminous U.S. are at risk of drinking well water that exceeds the arsenic MCL (Ayotte et al, 2017). Compared to public water supply wells, private wells are not subject to monitoring or regulatory mandate to meet drinking water quality standards, thus homeowners take their own actions to ensure their water is of safe quality by switching to alternative water sources such as bottled water or installing As treatment systems (Zheng and Ayotte, 2015). Private well households must make decisions on treatment options, based on a range of commercially available technologies, or a combination of treatments offered by various local private water treatment companies in a typically unregulated market. Moreover, it is the responsibility of the homeowner for maintaining and monitoring the effectiveness of the treatment systems (Flanagan et al., 2015).
Prior studies on the performance of As treatment systems have documented various degrees of success or failure, and concerns remain regarding how effective household water treatment systems are in real world situations (Flanagan et al., 2015). Household well water treatment systems are categorized into, 1) point-of-entry (POE) whole house treatment systems which are typically installed in the basement after the pressure tank, and 2) point-of-use (POU) treatment systems which are typically installed for use through the kitchen faucet. Common treatment technologies for household arsenic systems used in the U.S. include reverse osmosis (RO), chlorination or manganese dioxide oxidation, activated alumina (AA), iron oxide adsorption and co-precipitation, and ion-exchange (Mohan and Pittman, 2007; USEPA, 2006). Earlier studies reported reduction in [As] but not always to below the USEPA MCL, especially when RO systems were used. A study in Churchill County in western-central Nevada found that half of the 134 households with water treatment (47% used RO or distillation) still had tap water [As] above 13 μg/L (Walker et al., 2005). Also in western-central Nevada, among 19 homes using POU RO units to treat for arsenic, 10 homes still had [As] higher than the USEPA MCL in treated water (George et al., 2006). Another study of 59 households in the Lahontan Valley in rural western-central Nevada found POU RO systems removed on average 80% of arsenic from well water but 18 households still had [As] exceeding 10 μg/L in treated water (Walker et al., 2008). A study done in southeastern Michigan found POU RO systems reduced [As] by 85.5% in 5 households, with all households meeting the USEPA MCL (Slotnick et al., 2006). A study in rural Arizona found that RO reduced arsenic levels by 81% (up to 99%) in 5 homes, whereas activated carbon (AC) only reduced [As] by 24% (up to 45%) in 5 homes (Lothrop et al., 2015). It is encouraging that recent studies have found improved performance of As removal systems. For example, in Hopewell, New Jersey (NJ), 8 POE and 4 POU systems all reduced well water [As] to below 3 μg/L, with the POE systems performing better than the POU systems (Spayd et al., 2015). A follow-up study in Hopewell also found that 51 out of 55, or 93% homes with POE treatment systems succeeded in treating [As] to below the NJ state MCL of 5 μg/L (Rockafellow-Baldoni et al., 2018). In North and South Dakota, 6 homes located in American Indian communities, all with newly installed POU adsorptive filters, worked well in reducing [As] to below 1 μg/L for at least 9 months (Powers et al., 2019).
Factors affecting the performance of household arsenic treatment systems include the natural, prevailing well water chemistry conditions and the oxidation state of the well water arsenic. The presence of competing anions also interferes with As treatment (Meng et al., 2000). Reducing groundwater tends to have more inorganic As(III) that is more difficult to treat than As(V) (Sharma and Sohn, 2009). Human behavioral factors related to installation and maintenance of treatment systems are suspected to play a role, although the evidence is generally lacking (Zheng, 2017). Therefore, this study seeks to evaluate the effectiveness of household As treatment systems in a large number of households in Maine (n=156) and New Jersey (n=94) and to ascertain to what extent the natural well water chemistry influences As removal. The reduction in exposure and associated health risks is also discussed. The study builds on over 15 years of collaborative efforts among Columbia University’s Superfund Research Program (CU SRP), Maine Water Science Center of the U.S. Geological Survey (USGS), Maine Geological Survey (MGS), New Jersey Department of Environmental Protection (NJDEP), New Jersey Department of Health (NJDOH), and the Centers for Disease Control and Prevention (CDC). This collaborative research has investigated the occurrence, distribution, and mobilization mechanisms of As in groundwater, as well as barriers to water testing and treatment for As in Maine and New Jersey through extensive interaction with private well households. The findings enable the households to make informed choices in selecting and maintaining arsenic treatment systems to reduce exposure.
2. Materials and Methods
2.1. Study area and sampling
2.1.1. Maine
About 50% households in Maine rely on private wells drilled into bedrock that have a high occurrence rate of As, with 18.4% exceeding the USEPA MCL of 10 μg/L (Ayotte et al., 1999; Ayotte et al., 2006; Nielsen et al., 2010). A 2013 survey of central Maine households, who were notified 3–7 years earlier that their well water contained As above 10 μg/L, found that 43% of households reported installing As treatment systems, whereas another 30% report taking other mitigation actions such as drinking bottled water, and the remaining 27% of households did not act (Flanagan et al., 2015).
2.1.1.1. USGS sampling in 2001–2002 and 2006–2007
Households with private wells included in this study were identified and prescreened prior to both sampling periods in 2001–2002 and 2006–2007 by project personnel associated with the U.S. Centers for Disease Control and Prevention (US CDC) and the Maine Center for Disease Control and Prevention (Maine CDC). Only households that had elevated [As] (>10 μg/L) in well water and had previously installed water-purification systems designed to remove arsenic were considered for this study. This resulted in 31 households in 2001–2002 sampling and 100 households in 2006–2007 sampling (Culbertson et al., 2020a; Culbertson et al., 2020b) (Fig. 1a).
Fig. 1.
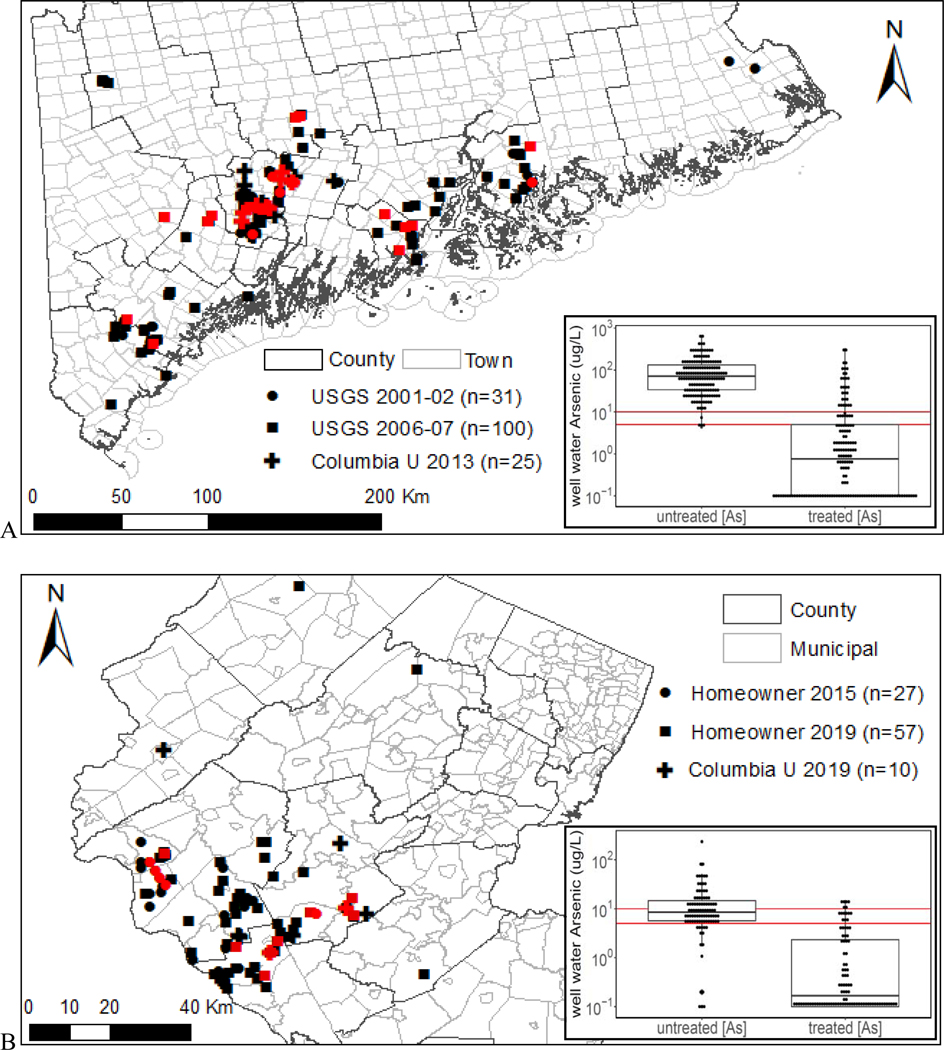
Sampled households with arsenic treatment systems in southern-central Maine (A) and northern New Jersey (B). All households were sampled only one time. Households with failed treatment, i.e. water arsenic > 10 μg/L in Maine and > 5 μg/L in New Jersey, are highlighted in red. Inserted are box plots of untreated and treated water arsenic, with the USEPA MCL of 10 μg/L and the NJ MCL of 5 μg/L highlighted as red horizontal lines.
Untreated well water samples were collected prior to the pressure tank and household water treatment system(s) by using the existing well water pump installed for household water supply. Before the collection of untreated well water samples, temperature, specific conductance (SPC), pH, and dissolved oxygen (DO) were monitored using a multi-parameter water quality monitor immersed in a flow-through chamber under a steady water flow rate of approximately 1–1.5 liters per minute. Untreated water samples were collected after water quality monitor readings had stabilized, which was generally 15–45 minutes. Treated water samples were collected from the kitchen faucet after running the faucet continuously for approximately 20 minutes for homes that did not have a POU unit attached to the kitchen faucet. The faucet aerator was removed to minimize sample aeration, and faucets were disinfected with alcohol (isopropanol) wipes prior to sample collection. For homes that had a POU unit on the faucet, treated water samples were collected from the POU faucet after running water through the kitchen faucet for approximately 20 minutes and through the POU unit for a few minutes (Culbertson et al., 2020a).
Untreated well water samples for unfiltered total arsenic, filtered dissolved arsenic (0.45-μm), dissolved As(III) and As(V), and other geochemical analyses were collected at the POE and processed onsite prior to laboratory analysis. Separation of As(III) and As(V) was done using anion-exchange chromatography columns packed with AG-1 anion exchange resin in the acetate form (50–100 mesh, Bio-Rad Laboratories, Hercules, CA). Water hardness was measured onsite by EDTA titration following Standard Method 2340B. All samples collected were chilled to 4 degrees Celsius and shipped to labs by overnight mail on the day of collection.
Homeowners reported the arsenic treatment system information including POE or POU, treatment technology, and other water treatment systems during the sampling visit.
2.1.1.2. CU-MGS sampling in 2013
Columbia University (CU) and Maine Geological Survey (MGS) had tested 1,428 private wells for arsenic in 17 towns of central Maine between 2006 and 2011 (Yang, 2010; Yang et al., 2012; Yang et al., 2009). A total of 25 households with As > 10 μg/L were re-visited by staff members of MGS between August and November 2013. Untreated and treated water samples were collected following a similar 6 protocol as the aforementioned USGS sampling, with the exception that samples for arsenic speciation were processed using a disposable arsenic speciation cartridge packed with highly selective adsorbent (Metalsoft Center, Highland Park, NJ) in the field to remove As(V) (Flanagan et al., 2015). Arsenic treatment system information was collected using questionnaire filled by homeowners.
2.1.2. New Jersey
New Jersey has a more protective state standard for As in drinking water of 5 μg/L for public and non-public water supplies including private wells, and has implemented the Private Well Testing Act (PWTA) since 2002 to enforce well water testing during real estate transactions. This has resulted in 49,404, or approximately 30% of private wells in the state, mostly in northern New Jersey, having been tested for arsenic by 2018. Approximately 8.3% and 2.8% of these wells had well water [As] exceeding the NJ MCL of 5 μg/L and the USEPA MCL of 10 μg/L, respectively (NJDEP, 2020). The percentage of private wells in the NJ Piedmont region exceeding the NJ MCL is 17.3%.
2.1.2.1. Homeowner mailed-in sampling in 2015 and 2019
A mailed household survey conducted in 2014 in 17 arsenic-affected towns in northern New Jersey included questions on well testing, treatment practices, basic demographic information and psychological factors that may influence water treatment and consumption behavior (Flanagan et al., 2016a). In 2015 and 2019, as a follow-up to the 2014 survey, selected households (Fig. 1b) were shipped sample bottles with sampling instructions, a questionnaire and a pre-paid return box to self-collect untreated well water samples at the basement pressure tank and treated water samples at the kitchen tap (Flanagan et al., 2016b). Upon the receiving of mailed-in samples from homeowners, water samples were acidified to a pH <2 with HNO3 (Optima grade), and stored at 4 °C for dissolution for at least a week before analysis. A total of 84 households (27 from the 2015 sampling period and 57 from the 2019 sampling period) that had arsenic treatment systems were included in this study. Most of these households had [As] in untreated well water that ranged between 5 and 50 μg/L.
2.1.2.2. CU sampling in 2019
Between July and October 2019, CU SRP collected samples from 10 households in northern New Jersey with 5 households having [As] higher than 50 μg/L in untreated well water based on prior testing results and homeowner’s reports of having installed an arsenic treatment system. A similar sampling protocol as that used for the 2013 Maine sampling (i.e. arsenic speciation samples using the arsenic speciation cartridge, and a questionnaire for well and water treatment information), was followed to collect both untreated well water from basement pressure tank and treated kitchen tap water. Samples were kept in a cooler with ice during the day, then shipped to the laboratory in the evening, where they were acidified to pH <2 using HNO3 (Optima grade) and stored at 4 °C before analysis.
2.2. Sample analysis
2.2.1. Maine water samples
During the 2006–07 USGS study, metal concentrations, including Fe, Mn, Pb, and U, and major anion concentrations, including fluoride, sulfate, nitrate, and nitrite, were measured in well water samples by Underwriters Laboratories Inc. (UL). During the earlier 2001–02 USGS study, Fe, Mn, phosphate and sulfate concentrations were only measured in the field upon sample collection (Culbertson et al., 2020a; Culbertson et al., 2020b). Arsenic, and arsenic species (As(III) and As(V), speciated in the field and measured as arsenic) were analyzed using Inductively Coupled Plasma Mass Spectrometry (ICP-MS) by the Health and Environmental Testing Laboratory (HETL) of the Maine Department of Human Services following US EPA Method 200.8. The reporting limit for arsenic was 0.5 μg/L (Culbertson et al., 2020a).
Maine samples collected in 2013 were analyzed by high resolution ICP-MS at the Lamont-Doherty Earth Observatory (LDEO) of Columbia University following a revised US EPA method 200.8 that included As and 33 other elements (Cheng et al., 2004). Repeated analyses of the standard solution NIST1643e with 60.5 μg/L As revealed an accuracy within 10% and a precision within 5%. The reporting limit for arsenic was 0.06 μg/L.
2.2.2. New Jersey water samples
All the water samples collected in New Jersey were analyzed for As and 33 other elements by the high resolution ICP-MS at the LDEO of CU following the same protocol described above.
2.3. Statistical analysis
The household As treatment failure rates (defined as treated water [As] > the USEPA MCL of 10 μg/L in ME or the state MCL in NJ of 5 μg/L) by different treatment technologies, by POE vs POU systems, by untreated well water [As] ranges, and in New Jersey vs Maine, were compared by nonparametric Chi-squared test (Yates, 1934) using R statistical software.
The hydrogeochemical parameters of untreated well water in successfully treated samples and treatment failed samples were compared by the nonparametric Mann-Whitney U test (Mann and Whitney, 1947) using R statistical software.
2.4. Health risk assessment
Cancer risk (R) was assessed based on inorganic As exposure according to the following equation:
| (1) |
where SF is the slope factor of 1.5 kg·d·mg−1 for adults as recommended by USEPA (USEPA, 2002), and EDIiAs is the estimated daily intake of inorganic As (iAs) in mg·kg−1·d−1. The estimated daily intake from drinking water was calculated according to the following equation:
| (2) |
where [iAs]w is the mean inorganic As concentration (μg L−1) of untreated or treated water in the sampled households, V is the daily consumption of water and is assumed to be 2 L, and BW is the body weight (kg) of an adult and is assumed to be 83.5 kg (USCDC, 2017).
3. Results and Discussion
3.1. Preference for POU RO in Maine and duel-tank POE in New Jersey
In Maine, the majority of homes sampled had point-of-use reverse-osmosis systems installed (POU RO), which might be due to state government recommendations and the wide availability of RO systems on the market in the 1990s and early 2000s (Boyle et al., 2010; Smith et al., 2016; USEPA, 2006). Specifically, out of 69 households who indicated they had installed POU only water treatment systems, 55 households or 80% had POU RO (Table 1). For the 52 households having both POE and POU systems, 36 of them or 69% had POU RO. The popularity of RO system extends to POE RO: 41% of households (11 out of 27) with POE only treatment system and 14% of households (7 out of 52) with both POE and POU systems installed POE RO. Overall, 109 RO systems (91 POU RO and 18 POE RO) were installed by 148 households who provided treatment system information (Table 1), equating to 74% of households having RO systems.
Table 1.
Summary statistics of household water treatment systems
| Study Area | Treatment System | Sediment filter | Softener | Arsenic removal |
|
|---|---|---|---|---|---|
| Reverse Osmosis | Oxidationo, adsorptiono, precipitation, ion exchange, others | ||||
| Southerncentral Maine | POE+ only (n=27) | 2 | 7 | 11 | 18 |
| POU+ only (n=69) | 5 | 2 | 55 | 15 | |
| POE and POU (n=52) | 24 | 21 | 43 | 31 | |
| all (n=148*) | 31 | 30 | 109 | 64 | |
| northern New Jersey | POE only (n=71) | 19 | 22 | 1 | 70# |
| POU only (n=10) | 0 | 0 | 4 | 4 | |
| POE and POU (n=13) | 3 | 6 | 8 | 11 | |
| all (n=94) | 22 | 28 | 13 | 85 | |
No treatment information was provided by 8 households in Maine.
Only 28 households provided arsenic removal technology information in the questionnaire.
POE: point-of entry; POU: point-of-use
Oxidation: chlorination, manganese dioxide; adsorption: activated alumina, iron/manganese oxides, iron oxyhydroxides.
Because of the recommendation to install POE treatment systems over POU by the NJDEP (NJDEP, 2007) and government “zero-percent interest loans” for the installation of private-well-water treatment systems, the “recommended” dual-tank POE arsenic removal systems (a worker tank followed by a safety tank) were widely adopted by the suppliers in NJ (Flanagan et al., 2016a). In a few cases with well water [As] > 200 μg/L, three-tank POE arsenic removal systems were installed. These dual-tank systems typically used iron oxide, titanium, or iron-impregnated resin adsorption technology. Of 94 households, 81 households or 86% installed POE systems; we assume most of these are dual-tank systems based on the high rate (6 out of 10 homes) observed during 2019 home visits. It is noteworthy that RO was used by those New Jersey households who installed POU-only systems (4 out of 10 households) or both POE and POU systems (8 out of 13 households). A total of 13 RO systems were installed by 94 New Jersey households.
3.2. Effectiveness of arsenic removal by household treatment system
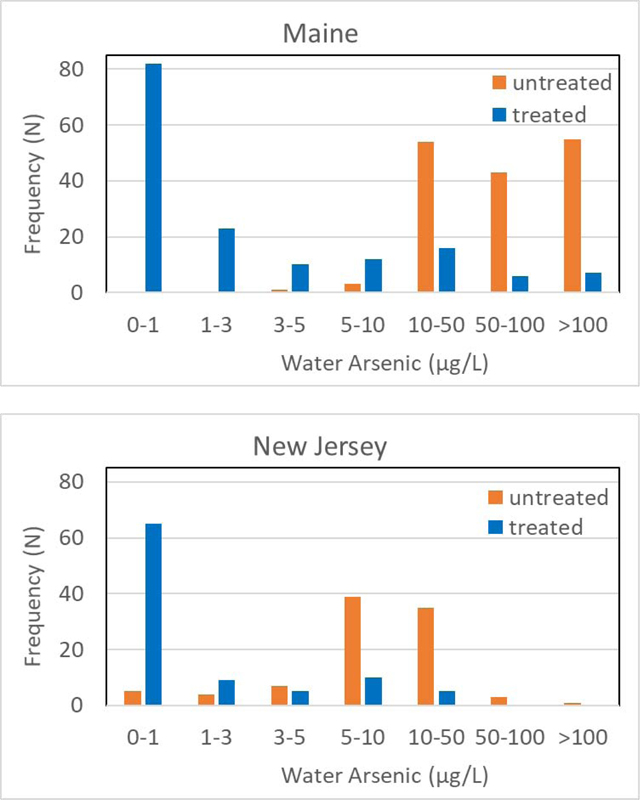
Despite the difference in preference for POE or POU and arsenic treatment technologies used in Maine and New Jersey, arsenic treatment systems reduced the well water [As] by up to two orders of magnitude (Table 2). In Maine, the untreated well water had a median [As] of 71.7 μg/L, whereas the 10 treated water had a median [As] of 0.8 μg/L. In New Jersey, private well water [As] was reduced from a median of 8.6 μg/L to a median of 0.2 μg/L after treatment. Furthermore, 53% (82/156) of Maine household As treatment systems reduced water [As] to below 1 μg/L, compared to 69% (65/94) in New Jersey (Fig. 3). It should be noted that in rare occasions, i.e. 1 case in Maine (untreated: 170 μg/L, treated: 330 μg/L) and 1 case in New Jersey (untreated: 4.4 μg/L, treated: 9.1 μg/L), the household treatment systems did not reduce water [As] but rather the treated water [As] was higher than the untreated well water. Although the reasons for this are unclear, it is possible that this increase might be due to fluctuation in groundwater As concentrations or the incomplete flushing of well borehole and water pipes before sampling (Slotnick et al., 2006). In the Maine case, the untreated water pH was 6.8 while the water after an unspecified POE treatment system and a RO POU unit displayed a pH of 7.1. The untreated water was highly reducing, with 2.58 mg/L of Fe(II) and 0.13 mg/L of Fe(III), and 140 μg/L As(III) and 30 μg/L of As(V). The very high level of phosphate at 3.2 mg/L may interfere with treatment.
Table 2.
Summary statistics of untreated and treated domestic well water arsenic in southern-central Maine and northern New Jersey
| Study Area | Sampling Year | Samples collected by | N | untreated [As] |
treated [As] |
||||
|---|---|---|---|---|---|---|---|---|---|
| median (μg/L) | n (%) > 10 μg/L | n (%) > 5 μg/L | median (μg/L) | n (%) > 10 μg/L | n (%) > 5 μg/L | ||||
| Southerncentral Maine | 2001 – 2002 | USGS | 31 | 130.0 | 30 (97%) | 30 (97%) | < 0.1 | 5 (16%) | 9 (29%) |
| 2006 – 2007 | USGS | 100 | 62.5 | 99 (99%) | 100 (100%) | 0.8 | 16 (16%) | 22 (22%) | |
| 2013 | Columbia Univ. | 25 | 72.5 | 23 (92%) | 25 (100%) | 3.9 | 8 (32%) | 10 (40%) | |
| All | 156 | 71.7 | 152 (97%) | 155 (99%) | 0.8 | 29 (19%) | 41 (26%) | ||
| Northern New Jersey | 2015 | Homeowner | 27 | 7.5 | 8 (30%) | 22 (81%) | 0.3 | 2 (7%) | 5 (19%) |
| 2019 | Homeowner | 57 | 8.5 | 24 (42%) | 46 (81%) | 0.1 | 2 (4%) | 8 (14%) | |
| 2019 Summer | Columbia Univ. | 10 | 23.6 | 7 (70%) | 10 (100%) | 0.2 | 1 (10%) | 2 (20%) | |
| All | 94 | 8.6 | 39 (41%) | 78 (83%) | 0.2 | 5 (5%) | 15 (16%) | ||
USGS: United States Geological Survey
Fig. 3.
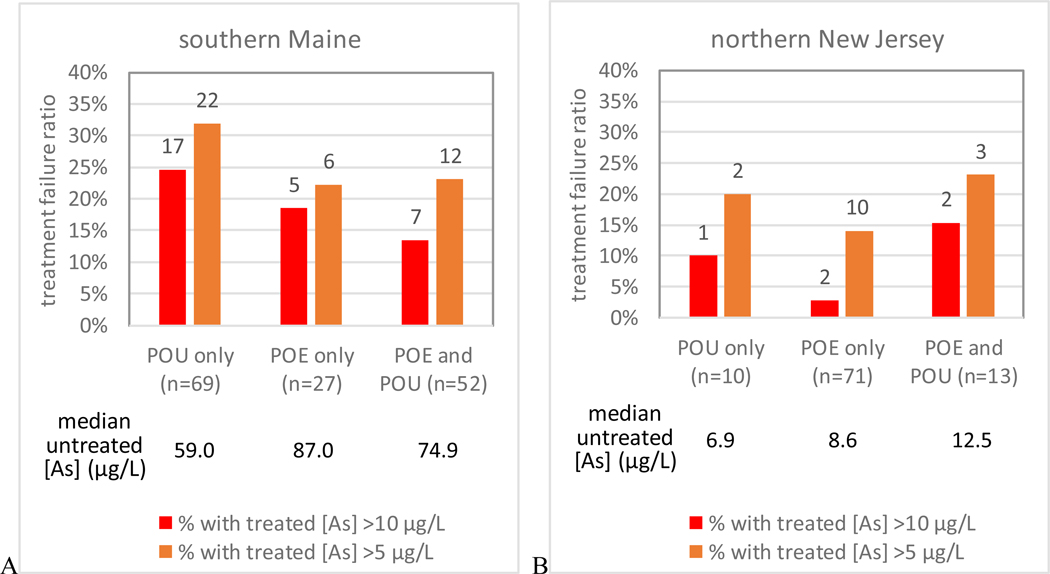
Arsenic removal performance by point-of entry (POE) and/or point-of-use (POU) treatment systems in southern-central Maine (A) and northern New Jersey (B)
In terms of compliance with the respective standards in Maine (10 μg/L) and New Jersey (5 μg/L), 29 out of 156 household treatment systems or 19% in Maine and 15 out of 94 household treatment systems or 16% in New Jersey failed (Table 2). Relative to the USEPA MCL, the As treatment failure in New Jersey is 5%, much lower than in Maine (p < 0.01). Relative to the New Jersey MCL, the As treatment failure in Maine is 26%, much higher than in New Jersey (p < 0.01).
3.3. Factors influencing arsenic removal
3.3.1. Untreated well water arsenic concentration
In both Maine and New Jersey, we found that higher untreated well water [As] led to higher rates of failure in treatment (Table 3). In Maine, 5 out of 54, or 9% of treatment units failed (As in treated water >10 μg/L) when untreated well water [As] ranged 10–50 μg/L, whereas this rate increased to 16% (7 out of 43) when untreated well water [As] ranged 50–100 μg/L, and further to 31% (17 out of 55) when untreated well water [As] was > 100 μg/L. Arsenic treatment failure rates were significantly higher (p=0.014) in Maine when untreated well water [As] was higher (Table 3). This is further supported by the statistically significant difference (p=0.01) in households with successful treatment systems and those with failed treatment systems in Maine (Table 4). In New Jersey (Table 3), 6 out of 39, or 15% of treatment units failed ([As] in treated water > 5 μg/L) when untreated well water [As] ranged 5–10 μg/L, while the failure rate increased to 23% (8 out of 35) when untreated well water [As] ranged 10–50 μg/L. Although this failure rate increased with untreated well water [As], it was not statistically significant (p=0.30).
Table 3.
Treatment success and failure according to untreated As concentration ranges
| Study area | [As] (μg/L) | < 1 | 1 – 3 | 3 – 5 | 5 – 10 | 10 – 50 | 50 – 100 | > 100 | all |
|---|---|---|---|---|---|---|---|---|---|
| Southerncentral Maine | n of untreated [As] | 0 | 0 | 1 | 3 | 54 | 43 | 55 | 156 |
| n of treated [As] > 10 | 0 | 0 | 5 | 7 | 17 | 29 | |||
| % of treated [As] > 10 | 9% | 16% | 31% | 19% | |||||
| n of treated [As] > 5 | 0 | 1 | 7 | 12 | 21 | 41 | |||
| % of treated [As] > 5 | 13% | 28% | 38% | 26% | |||||
|
|
|||||||||
| northern New Jersey | n of untreated [As] | 5 | 4 | 7 | 39 | 35 | 3 | 1 | 94 |
| n of treated [As] > 10 | 0 | 0 | 5 | 0 | 0 | 5 | |||
| % of treated [As] > 10 | 14% | 5% | |||||||
| n of treated [As] > 5 | 1 | 6 | 8 | 0 | 0 | 15 | |||
| % of treated [As] > 5 | 15% | 23% | 16% | ||||||
The failure rates in Maine and New Jersey in the same group with untreated well water [As] of 10–50 μg/L are not statistically significantly different (p=0.14).
Table 4.
Hydrogeochemistry characteristics of untreated well water by functioning and failed treatment systems in southern-central Maine
| Functioning (n=127) | Failed (n=29) | p value* | |
|---|---|---|---|
| well depth (m) | 69 (49 – 91) | 61 (49 – 81) | 0.55 |
| pH | 7.96 (7.53 – 8.50) | 8.13 (7.75 – 8.67) | 0.15 |
| DO# (mg/L) | 0.54 (0.09 – 3.13) | 0.34 (0.07 – 2.06) | 0.25 |
| SPC# (μS/cm) | 202 (151 – 270) | 214 (146 – 325) | 0.31 |
| Hardness (mg CaCO3/L) | 56 (42 – 76) | 47 (35 – 72) | 0.23 |
| Fe (μg/L) | 40 (20 – 108) | 49 (20 – 190) | 0.24 |
| Mn (μg/L) | 5.4 (<0.1 – 100.0) | 13.0 (<0.1 – 180.0) | 0.09 |
| As (μg/L) | 60.0 (32.5 – 121.3) | 100.0 (73.0 – 140.0) | 0.01 |
| As(III) (μg/L) | 1.6 (0.5 – 23.2) | 14.3 (4.0 – 72.3) | <0.01 |
| As(III)/As | 3% (0.7% – 38%) | 22% (6% – 80%) | 0.01 |
p value of Mann-Whitney U test comparing functioning vs failed groups
DO: dissolved oxygen; SPC: specific conductance
Other studies also have found that when [As] in untreated water was high, [As] in treated water could exceed the USEPA MCL even though treatment removed more than 95% of the arsenic (Walker et al., 2008). Further, although it has been reported that more households installed treatment units if their untreated well water [As] was high (Flanagan et al., 2015), the results here show that these systems are more likely to fail and therefore require closer monitoring.
3.3.2. Untreated well water arsenic speciation
Arsenic in natural waters occurs mainly in the oxidation states +III (arsenite) and +V (arsenate). Owing to its neutral charge in most groundwater, the removal of As(III) is more difficult than the removal of As(V) (Driehaus et al., 1995). Thus, many As treatment technologies now oxidize As(III) to As(V) prior to its removal. In this study, the untreated well water with failed treatment systems had a much higher (p<0.01) median As(III) of 14.3 μg/L than that of 1.6 μg/L in untreated well water with successful treatment systems. The As(III)/As ratios in untreated well water with failed treatment systems (median = 22%) were significantly higher (p=0.01) than in untreated well water with functioning treatment (median = 3%). Walker et al., (2008) showed a similar treatment failure rate among RO systems when As(III) was the dominant arsenic species (Walker et al., 2008).
3.3.3. Other hydrogeochemical parameters of untreated well water
Other hydrogeochemical parameters of untreated well water in households with failed treatment did not have statistically significant differences from those with successful treatment, except for untreated well water arsenic concentrations and its speciation (Table 4). Although not significantly different, well water with failed treatment had higher pH, lower DO, higher Fe and Mn, and lower hardness, i.e. Ca2+ and Mg2+, than well water with successful treatments, which may have contributed to less effective arsenic removal. Arsenic adsorption to iron hydroxides or other adsorbents is very sensitive to pH, with higher pH (>8) favoring As(V) desorption (Dixit and Hering, 2003), potentially causing treatment failure. The presence of Ca2+ and Mg2+ has been found to enhance the adsorption of As(V) but reduce As(III) removal by iron hydroxides at neutral to higher pH conditions (Meng et al., 2000). Lower DO and higher Fe and Mn might be associated with more-reducing well water, which also has greater As(III)/As ratios, resulting in a higher chance of treatment failure.
3.3.4. POE vs POU
In both Maine and New Jersey, households with only POU systems had higher failure rates than households with only POE systems (Fig. 3), although the difference is not statistically significant. This is consistent with a previous study (Spayd et al., 2015) that found whole house POE arsenic water treatment systems provided a more effective reduction of arsenic exposure from well water than that obtained by POU treatment. Furthermore, unlike POE treatment, POU-only treatment at a single faucet in the home can result in exposure from drinking water at untreated taps, and is not effective against potential dermal exposure, although this route of exposure is currently considered to be negligible compared to ingestion. The study of 59 households in Lahontan Valley, NV had an even higher failure rate of 31% from POU RO treatment systems (Walker et al., 2008) than we found in this study, possibly because the untreated [As] was higher at a median of 103 μg/L.
The households with only POU systems also had lower median values of untreated well water [As] (59.0 and 6.9 μg/L, in Maine and New Jersey, respectively) than those with only POE systems (87.0 and 8.6 μg/L, in Maine and New Jersey, respectively). In New Jersey, households with both POE and POU systems had higher median [As] in untreated well water (12.5 μg/L) than those with only POU systems (6.9 μg/L) (Fig. 3). This suggests that homeowners tended to, or were encouraged, by state and local government agencies or water treatment professionals, to install POE treatment systems if their well water [As] was higher, and to further “boost“ treatment with a back-up POU system. When the untreated water [As] is higher, there is a tendency for the households to install a POU system in the kitchen in addition to the POE system, especially in Maine. For example, the percentage of households that installed both POE and POU in Maine (52/148, or 35%) is higher than that in New Jersey (13/94, or 14%) where untreated well water [As] is an order of magnitude lower than in Maine.
3.3.5. Treatment system installation and maintenance
Out of the 93 households who provided treatment system information in the 2006–07 sampling in Maine, 75% (70/93) of treatment systems were installed by vendors, but only 30% (28/93) were maintained by a vendor (Table 5). The treatment systems installed by homeowners had a higher failure rate of 26% (6/23) than that of 13% (9/70) installed by vendors. The treatment systems maintained by homeowners also showed a higher failure rate of 20% (13/65) than that of 7% (2/28) by vendors. This suggests that installation and maintenance of the treatment systems by vendors is preferred to reduce the likelihood of both treatment failure and exposure. Maintenance by vendors might also reduce the risk associated with disposing of the used treatment system materials enriched with high concentration of As back to the environment whereby adjacent water sources could be contaminated (Sylvester et al., 2008).
Table 5.
Characteristics of failed vs. functioning treatment systems in southern-central Maine sampled during 2006–2007
| functioning systems (n=78) | failed systems (n=15) | |
|---|---|---|
| installed by homeowner (n=23) | 17 | 6 |
| installed by vendor (n=70) | 61 | 9 |
| maintained by homeowner (n=65) | 52 | 13 |
| maintained by vendor (n=28) | 26 | 2 |
1 household with failed system and 6 households with functioning system did not report installation and maintenance information.
3.3.6. Sediment filter and water softener
Although sediment filters and water softeners do not directly remove As, they are very common forms of water treatment in the study areas. Sediment filters are used by 21% and 23% of households in Maine and New Jersey, respectively, and water softeners by 20% and 30% in ME and NJ, respectively (Table 1).
Because arsenic, especially arsenate, is readily adsorbed onto and co-precipitated with iron hydroxides, manganese oxides, or other adsorbents in the well water, sediment filters might remove a fraction of As, particularly when the water Fe/As ratio is high (Berg et al., 2006). A previous study found sand filters could remove 63% of arsenic (Do et al., 2014). In this study, there was only 4% difference between filtered and unfiltered average [As] in Maine samples, and the treatment failure rate in households with installed sediment filters was almost identical to the failure rate in households without sediment filters, thus, sediment filters alone likely would not contribute substantially to As removal.
A study in southeastern Michigan found water softeners alone did not reduce [As] in well water (Slotnick et al., 2006). In this study, households that had installed softeners had higher As treatment failure rates than those without in both Maine (27% vs 17%) and New Jersey (21% vs 14%), although the differences were not statistically significant. Previous studies have found Ca2+ and Mg2+ can enhance the As(V) adsorption and removal by iron hydroxides at neutral to higher pH conditions (Meng et al., 2000). The removal of Ca2+ and Mg2+ and slight increases in pH by water softeners might lead to the reduction in As adsorption and removal. It’s notable that while water softeners can significantly reduce Ca and Mg concentrations in well water, they can significantly increase Na concentrations in the treated tap water. For example, in 28 NJ households with installed water softeners, the median [Ca] decreased from 50.3 to 0.6 mg/L and the median [Mg] decreased from 16.6 to 0.1 mg/L. However, the median [Na] increased from 24.4 to 77.0 mg/L in the treated water, with 17 or 61% households drinking water with [Na] > 50 mg/L. In this case households using water softeners are at risk of drinking water with Na concentrations higher than the NJ secondary standard of 50 mg/L (NJDEP, 2018) and the USEPA recommended level of 30–60 mg/L based on aesthetic effects (i.e., taste) (USEPA, 2018).
3.4. Health risks reduction by arsenic treatment
A 1 in 1 million cancer risk is considered the acceptable level of risk when MCLs are set for carcinogens in drinking water. However, the USEPA MCL for arsenic was set much higher due to the high cost of compliance based on cost and benefit analysis. At the USEPA MCL for arsenic of 10 μg/L, the skin cancer risk alone is ~ 500 per million population (USEPA, 2002) and the combined bladder and lung cancer risk is ~ 3,000 per million population. We calculated that by lowering the mean [As] from 105 μg/L before treatment to 14.3 μg/L after treatment in Maine, the corresponding skin cancer risk would be significantly lowered from 3,765 per million population to 514 per million population, a 7.3-fold reduction. Likewise, by lowering the mean [As] from 15.8 μg/L before treatment to 2.1 μg/L after treatment in New Jersey, the corresponding skin cancer risk would be lowered from 568 per million population to 75 per million population, or a 7.5-fold reduction, bringing it closer to the level of protection necessary for public health. Therefore, significant reductions of arsenic concentrations in treated water can lead to reduction in exposure and health risk to private well water users.
3.5. Limitation and public health implications
One limitation of this study is that sampling protocols likely varied between samples collected by homeowners and those collected by research staff, such as pumping time/volume to flush the well borehole water and household plumbing before sampling, which could have an influence on the sampled water [As]. Anecdotal evidence indicates that as many as one-third of the POU RO treatment systems in the Maine study failed within a few months to a year after installation, and failure was almost always associated with As(III) dominance, or high As in the source water (e.g. >200 μg/L). The specific mechanisms of and the time before treatment system failure are beyond the scope of this study.
The current study demonstrates that there are a number of factors to consider when choosing treatment systems to reliably remove arsenic from household drinking water and protect public health. First, the choice between using POU and/or POE systems to remove arsenic should be informed by the concentrations of As(III) and As(V) and the As(III)/As ratio, as well as measurements of other water quality parameters that are known to influence As removal, such as DO, pH, Fe, Mn, and competing anions. Second, if As removal is the goal, the water treatment system designed and certified to remove As should be recommended to the households for installation. Finally, having a vendor install and maintain an arsenic treatment system is more protective of health because these systems are less likely to fail that those installed and maintained by homeowners.
4. Conclusions
Private well households in southern-central Maine rely primarily on point-of-use (POU) reverse-osmosis (RO) systems to remove arsenic from drinking water, while in northern New Jersey, most homes installed dual-tank point-of-entry (POE) arsenic removal systems. Despite the differences in the treatment technology used in two states, household treatment systems reduced well water arsenic by as much as two orders of magnitude, with most of the treated water containing below 1 μg/L of arsenic. Whole house, or POE treatment systems performed slightly better than POU systems installed on kitchen faucets. However, the failure to bring arsenic in the treated water to below the respective standards in Maine and New Jersey remains an issue: nearly 20% of systems in this study failed to reduce arsenic to acceptable levels. Furthermore, treatment systems are more likely to fail with higher influent arsenic levels and higher As(III)/As ratios. Treatment systems installed and/or maintained by homeowners were also more likely to fail than those installed and/or maintained by vendors. Despite numerous challenges encountered in household water treatment for arsenic, the 7-fold reduction in arsenic concentrations in the treated water has resulted in significant reduction in exposure and lowered cancer risks by 7-fold among private well water users in southern-central Maine and northern New Jersey. This demonstrates the benefits of installing and maintaining household arsenic treatment systems, which can effectively reduce As in drinking water to acceptable levels using existing technologies.
Fig.2.
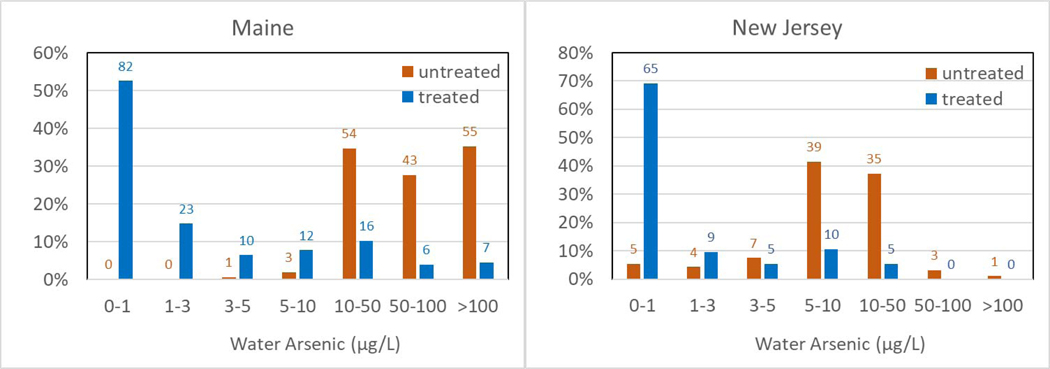
Arsenic distribution in untreated and treated water in Maine and New Jersey
* Labels on the bars are the numbers of wells falling into each arsenic range.
HIGHLIGHTS.
Arsenic treatment reduced well water [As] by up to two orders of magnitude;
Half to two thirds of household treatment systems lowered water [As] to < 1 μg/L;
The higher the [As] and As(III)/As, the higher likelihood of treatment failure;
Systems installed/maintained by vendors failed less than those by homeowners;
Seven-fold reduction in As concentration and cancer risk was achieved by treatment.
Acknowledgements
This work was supported by the U.S. National Institute of Environmental Health Sciences (NIEHS) Superfund Research Program (SRP) 3 P42 ES10349 and by the U.S. Centers for Disease Control and Prevention (USCDC) Cooperative Agreement Number NUE2EH001326–03. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the USCDC or the Department of Health and Human Services. The authors thank Rebecca Schwartz of New Jersey Geological and Water Survey for field sampling and editorial assistance, Tyler Ellis of Lamont-Doherty Earth Observatory for sample analysis on ICP-MS, and Joseph D Ayotte and Graham K Thomas of USGS for reviewing and comments. The authors also thank two anonymous reviewers from STOTEN for comprehensive reviews and suggestions. This is LDEO contribution #xxxx.
Footnotes
Declaration of conflict of interest
The authors declare that they do not have any conflict of financial interests to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ayotte JD, Medalie L, Qi SL, Backer LC and Nolan BT (2017) Estimating the High-Arsenic Domestic-Well Population in the Conterminous United States. Environmental Science & Technology 51, 12443–12454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayotte JD, Nielsen MG, Robinson GR and Moore RB (1999) Relation of Arsenic, Iron, and Manganese in Ground Water to Aquifer Type, Bedrock Lithogeochemistry, and Land Use in the New England Coastal Basins. U.S. Geological Survey, Water-Resources Investigations Report 99–4162., P70. [Google Scholar]
- Ayotte JD, Nolan BT, Nuckols JR, Cantor KP, Robinson GR, Baris D, Hayes L, Karagas M, Bress W, Silverman DT and Lubin JH (2006) Modeling the Probability of Arsenic in Groundwater in New England as a Tool for Exposure Assessment. Environ. Sci. Technol 40, 3578–3585. [DOI] [PubMed] [Google Scholar]
- Berg M, Luzi S, Trang PTK, Viet PH, Giger W. and Stüben D. (2006) Arsenic Removal from Groundwater by Household Sand Filters: Comparative Field Study, Model Calculations, and Health Benefits. Environmental Science & Technology 40, 5567–5573. [DOI] [PubMed] [Google Scholar]
- Boyle KJ, Kuminoff NV, Zhang C, Devanney M. and Bell KP (2010) Does a property-specific environmental health risk create a “neighborhood” housing price stigma? Arsenic in private well water. Water Resour. Res 46. [Google Scholar]
- Cheng Z, Zheng Y, Mortlock R. and van Geen A. (2004) Rapid multi-element analysis of groundwater by high-resolution inductively coupled plasma mass spectrometry. Anal. Bioanal. Chem 379, 512–518. [DOI] [PubMed] [Google Scholar]
- Culbertson CW, Caldwell JM, Schalk LF, Manassaram D, Backer LC and Smith AE (2020a) Methods of Data Collection and Quality Assessment of Arsenic in Well-Water Supplies in Maine, 2001–2 and 2006–7: U.S. Geological Survey DS XXX, xx p., https://doi.org/xxxxxx. [Google Scholar]
- Culbertson CW, Caldwell JM, Schalk LF and Medalie L. (2020b) Arsenic datasets and other physical and chemical measurements for selected domestic well-water supplies in Maine: 2001–2 and 2006–7: U.S. Geological Survey data release, 10.5066/P9X5HVDF. [DOI]
- Dixit S. and Hering JG (2003) Comparison of Arsenic(V) and Arsenic(III) Sorption onto Iron Oxide Minerals: Implications for Arsenic Mobility. Environmental Science & Technology 37, 4182–4189. [DOI] [PubMed] [Google Scholar]
- Do AT, Kuroda K, Hayashi T, Nga TTV, Oguma K. and Takizawa S. (2014) Household survey of installation and treatment efficiency of point-of-use water treatment systems in Hanoi, Vietnam. Journal of Water Supply Research and Technology-Aqua 63, 154–161. [Google Scholar]
- Driehaus W, Seith R. and Jekel M. (1995) Oxidation of arsenate(III) with manganese oxides in water treatment. Water Res. 29, 297–305. [Google Scholar]
- Flanagan SV, Marvinney RG, Johnston RA, Yang Q. and Zheng Y. (2015) Dissemination of well water arsenic results to homeowners in Central Maine: Influences on mitigation behavior and continued risks for exposure. Sci. Total Environ 505, 1282–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan SV, Spayd SE, Procopio NA, Chillrud SN, Braman S. and Zheng Y. (2016a) Arsenic in private well water part 1 of 3: Impact of the New Jersey Private Well Testing Act on household testing and mitigation behavior. Sci. Total Environ 562, 999–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan SV, Spayd SE, Procopio NA, Chillrud SN, Ross J, Braman S. and Zheng Y. (2016b) Arsenic in private well water part 2 of 3: Who benefits the most from traditional testing promotion? Sci. Total Environ 562, 1010–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focazio MJ, Tipton D, Shapiro SD and Geiger LH (2006) The Chemical Quality of Self-Supplied Domestic Well Water in the United States. Ground Water Monitoring & Remediation 26, 92–104. [Google Scholar]
- George CM, Smith AH, Kalman DA and Steinmaus CM (2006) Reverse osmosis filter use and high arsenic levels in private well water. Archives of environmental & occupational health 61, 171–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lothrop N, Wilkinson ST, Verhougstraete M, Sugeng A, Loh MM, Klimecki W. and Beamer PI (2015) Home Water Treatment Habits and Effectiveness in a Rural Arizona Community. Water 7, 1217–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann HB and Whitney DR (1947) On a test of whether one of two random variables is stochastically larger than the other. Annals of Mathematical Statistics 18, 50–60. [Google Scholar]
- Meng X, Bang S. and Korfiatis GP (2000) Effects of silicate, sulfate, and carbonate on arsenic removal by ferric chloride. Water Res. 34, 1255–1261. [Google Scholar]
- Mohan D. and Pittman CU (2007) Arsenic removal from water/wastewater using adsorbents—A critical review. J. Hazard. Mater 142, 1–53. [DOI] [PubMed] [Google Scholar]
- Nielsen MG, Lombard PJ and Schalk LF (2010) Assessment of Arsenic Concentrations in Domestic Well Water, by Town, in Maine, 2005–09. U.S Geological Survey Scientific Investigations Report 2010–5199, 68 p. (Also available at http://pubs.usgs.gov/sir/2010/5199.). [Google Scholar]
- NJDEP (2007) Arsenic Water Treatment for Residential Wells in New Jersey. https://www.nj.gov/dep/pwta/Arsenic_Treatment.pdf.
- NJDEP (2018) Drinking Water Standards by Constituent, by New Jersey Department of Environmental Protection. [Google Scholar]
- NJDEP (2020) NJ Private Well Testing Act Data Summary. (https://www.nj.gov/dep/dsr/pwta/). accessed on 02.18.2020
- Powers M, Yracheta J, Harve D, O’Leary M, Best LG, Bear AB, MacDonald L, Susan J, Hasan K, Thomas E, Morgan C, Olmedo P, Chen R, Rule A, Schwab K, Navas-Acien A. and George CM (2019) Arsenic in groundwater in private wells in rural North Dakota and South Dakota: Water quality assessment for an intervention trial. Environ. Res 168, 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockafellow-Baldoni M, Spayd SE, Hong JY, Meng Q, Ohman-Strickland P. and Robson MG (2018) Arsenic Exposure and Cancer Risk Reduction with Local Ordinance Requiring Whole-House Dual-Tank Water Treatment Systems. Hum Ecol Risk Assess 24, 1256–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma VK and Sohn M. (2009) Aquatic arsenic: Toxicity, speciation, transformations, and remediation. Environment International 35, 743–759. [DOI] [PubMed] [Google Scholar]
- Slotnick MJ, Meliker JR and Nriagu JO (2006) Effects of time and point-of-use devices on arsenic levels in Southeastern Michigan drinking water, USA. Science Of The Total Environment 369, 42–50. [DOI] [PubMed] [Google Scholar]
- Smith AE, Lincoln RA, Paulu C, Simones TL, Caldwell KL, Jones RL and Backer LC (2016) Assessing arsenic exposure in households using bottled water or point-of-use treatment systems to mitigate well water contamination. Sci. Total Environ 544, 701–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spayd SE, Robson MG and Buckley BT (2015) Whole-house arsenic water treatment provided more effective arsenic exposure reduction than point-of-use water treatment at New Jersey homes with arsenic in well water. Sci. Total Environ 505, 1361–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester P, Moller T. and Boyd O. (2008) The minimization of wastes produced during the treatment of arsenic contaminated drinking water, in: Zamorano M, Brebbia CA, Kungolos A, Popov V, Itoh H. (Eds.), Waste Management and the Environment Iv, pp. 719–727. [Google Scholar]
- USCDC (2017) Body Measurements by CDC/National Center for Health Statistics, https://www.cdc.gov/nchs/fastats/body-measurements.htm.
- USEPA (2002) Integrated Risk Information System (IRIS) for inorganic arsenic, U.S Environmental Protection Agency National Center for Environmental Assessment. [Google Scholar]
- USEPA (2006) Point-of-Use or Point-of-Entry Treatment Options for Small Drinking Water Systems. EPA 815-R-06–010. [Google Scholar]
- USEPA (2018) 2018 Edition of the Drinking Water Standards and Health Advisories, by U.S. Environmental Protection Agency. [Google Scholar]
- Walker M, Benson M. and Shaw WD (2005) Significance of private water supply wells in a rural Nevada area as a route of exposure to aqueous arsenic. Journal of water and health 3, 305–312. [DOI] [PubMed] [Google Scholar]
- Walker M, Seiler RL and Meinert M. (2008) Effectiveness of household reverse-osmosis systems in a Western US region with high arsenic in groundwater. Science of the Total Environment 389, 245–252. [DOI] [PubMed] [Google Scholar]
- Yang Q. (2010) Arsenic in fractured bedrock aquifers in Greater Augusta, Maine, USA. Doctoral Dissertation, Earth and Environmental Sciences. City University of New York, New York, NY, p. 143. [Google Scholar]
- Yang Q, Jung H-B, Culbertson CW, Marvinney RG and Zheng Y. (2012) Can arsenic occurrence rates in bedrock aquifers be predicted? Environmental Science & Technology 46, 2080–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Jung HB, Culbertson CW, Marvinney RG, Loiselle MC, Locke DB, Cheek H, Thibodeau H. and Zheng Y. (2009) Spatial Pattern of Groundwater Arsenic Occurrence and Association with Bedrock Geology in Greater Augusta, Maine. Environmental Science & Technology 43, 2714–2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates F. (1934) Contingency Tables Involving Small Numbers and the χ2 Test. Supplement to the Journal of the Royal Statistical Society 1, 217–235. [Google Scholar]
- Zheng Y. (2017) Lessons Learned from Arsenic Mitigation among Private Well Households. Current environmental health reports 4, 373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y. and Ayotte JD (2015) At the crossroads: Hazard assessment and reduction of health risks from arsenic in private well waters of the northeastern United States and Atlantic Canada. Sci. Total Environ 505, 1237–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]


