Abstract
Polycystic ovary syndrome (PCOS), the most common endocrine disorder in women of reproductive age, is characterized by hyperandrogenemia, obesity, insulin resistance, and elevated blood pressure. However, few studies have focused on the consequences of pregnancy on postmenopausal cardiovascular disease and hypertension in PCOS women. In hyperandrogenemic female (HAF) rats, the hypothesis was tested that previous pregnancy protects against age-related hypertension. Rats were implanted with dihydrotestosterone (7.5 mg/90 days, beginning at 4 weeks and continued throughout life) or placebo pellets (controls), became pregnant at 10–15 weeks, and pups were weaned at postnatal day 21. Dams and virgins were then aged to 10 months (still estrous cycling) or 16 months (postcycling). Although numbers of offspring per litter were similar for HAF and control dams, birth weights were lower in HAF offspring. At 10 months of age, there were no differences in blood pressure, proteinuria, nitrate/nitrite excretion, or body composition in previously-pregnant HAF versus virgin HAF. However, by 16 months of age, despite no differences in DHT, fat mass/ or lean mass/body weight, previously-pregnant HAF had significantly lower blood presure and proteinuria, higher nitrate/nitrite excretion, with increased intrarenal mRNA expression of endothelin B receptor and eNOS, and decreased ACE, AT1aR and endothelin A receptor than virgin HAF. Thus pregnancy protects HAF rats against age-related hypertension, and the mechanism(s) may be due to differential regulation of the nitric oxide, endothelin and renin-angiotensin systems. These data suggest that PCOS women who have experienced uncomplicated pregnancy may be protected from postmenopausal hypertension.
Keywords: nitric oxide, menopause, endothelin, aging, renin-angiotensin system
Graphical Abstract
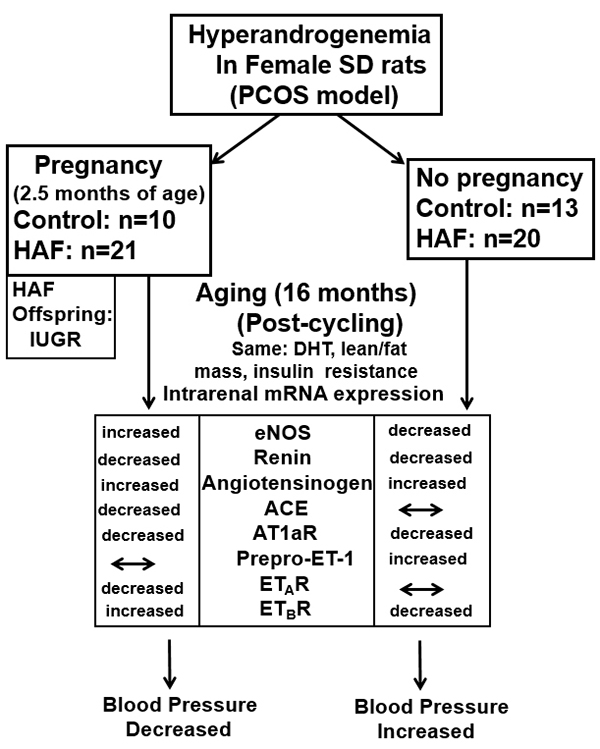
Summary
Previous pregnancy protects hyperandrogenemic female rats from age-related hypertension. The mechanisms for the attenuation of the hypertension are not due to different levels of androgens, body composition (fat mass, lean mass), insulin levels, or glucose metabolism, but rather are likely due to increased vasodilator systems (eNOS, ETBR, RAS) and attenuation of the RAS and endothelin vasoconstrictor systems.
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrine pathology in women of reproductive age, affecting 5–10% of the population, often beginning in adolescence 1–3. PCOS in young women is characterized by hyperandrogenemia, modest increases in blood pressure, insulin resistance, and increased inflammation 2–4. With aging, hyperandrogenemia does not abate, even following menopause 5–7. The clinical guidelines for PCOS diagnosis have only been in place since 2002–2004 1; thus there are few studies that have focused on the consequences of aging (i.e. post-menopause) on cardiovascular disease (CVD) risk in PCOS women, due to the lack of aging populations in which PCOS had been definitively diagnosed, i.e. with androgen measurements. Furthermore, to our knowledge, there are no studies in which the effect of prior pregnancy on postmenopausal CVD and hypertension in PCOS women has been studied.
PCOS women may have difficulty becoming pregnant and have a higher incidence of requiring assisted reproduction, such as in vitro fertilization 8, 9. Persson, et al., reported that Scandinavian PCOS women take longer to become pregnant and have fewer children than women without PCOS, but once pregnant, the probability of childbirth was similar in PCOS women versus controls 8. Hu and colleagues reported that PCOS women had elevated blood pressure during pregnancy, as measured by ambulatory recording, but there were no data on pre-pregnancy blood pressures 10, nor did the blood pressures reach guideline levels required for treatment of hypertension.
One may surmise that exposure to cardiovascular and metabolic risk factors throughout the entirety of their reproductive lives would predispose PCOS women to early CVD risk. However, this supposition is controversial 11–14. For example, recent reviews question whether women with PCOS have greater morbidity and mortality than the general population of postmenopausal women, and point out that data are scarce as to the postmenopausal health of PCOS women, and even suggest that PCOS women are not different from the general population or actually may be protected from CVD 11, 15–17. One study showed that PCOS women go through menopause four years later than age-matched controls 18, which may imply protection from CVD due to longer exposure to estradiol.
In recent years, we have studied the hyperandrogenemic female (HAF) rat that mimics many of the characteristics of women with PCOS 19. Female Sprague Dawley rats are given dihydrotestosterone (DHT), beginning shortly after weaning and continued throughout their lives 19; DHT is used since it cannot be converted to estradiol. The serum levels of androgens are similar to levels found in PCOS women and do not affect endogenous synthesis of testosterone and estradiol 19. By 14–16 weeks of age, HAF rats develop obesity, hyperlipidemia, insulin resistance, inflammation and elevated blood pressure 19. We found that the elevated blood pressure in the young HAF rat is mediated in part by increased intrarenal vascular 20-HETE 20, and increased sympathetic activation 21. We have also characterized the HAF rats as they stop estrous cycling, by 12–13 months of age 22. Blood pressure continues to increase with aging in HAF rats, and they become hypertensive 22.
Thus using the HAF rat model in the current study, we tested the hypothesis that pregnancy would protect against the hypertension that occurs in virgin HAF rats with aging (16 months of age), and evaluated the intrarenal mRNA expression of genes known to contribute to postmenopausal hypertension, such as the nitric oxide pathway, the renin-angiotensin system and the endothelin system. We also determined if differences in blood pressure occurred at 10 months of age prior to cessation of estrous cycling, and whether there were differences in body composition that occurred during pregnancy or with aging that could impact blood pressure in HAF rats.
Material and methods
The data from this work are available from the corresponding author upon reasonable request.
Animal model:
Female Sprague–Dawley (SD) rats were obtained at 3 weeks of age from the vendor (Envigo, Indianapolis, IN) and allowed to equilibrate in a temperature-controlled environment with 12-h:12-h light:dark cycle for 1 week. As shown in Figure S1A and S1B, rats were randomly selected to be implanted with either 5α-dihydrotestosterone (DHT) or placebo pellets to generate hyperandrogenemic females (HAF) or controls, respectively, as we previously described 19,22. All protocols followed the ARRIVE Guidelines, and were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center and complied with the Guidelines for the Care and Use of Laboratory Animals by the National Institutes of Health.
Estrous cycle and Pregnancy:
HAF rats exhibited a 6-days estrous cycle rather than the typical 4-days cycle as found in controls 19,22. In separate groups of rats and as shown in Figures S1A and SB, at 2 months of age, body composition (lean and fat mass) was measured in HAF and control rats (n=4–14/group) by Echo-MRI 24 (see Supplemental Methods), and then at 2.5–3 months of age, HAF and control rats were paired with SD males. Pregnancies occurred in approximately 60% of HAF rats and 99% of control rats. When pregnancy was detected, as denoted by increased body weight, males were removed from the cage. HAF and control dams were allowed to deliver, and within 12 hrs of delivery, the number of pups in each litter (both control and HAF) were counted (n = 16 – 25 litters/group), body weights were obtained, and anogenital distances were measured to differentiate males from females (~ 3 – 4 mm in males and ~ 1 – 2 mm in females). Pups were reweighed at 48 hrs. The weights of male and female pups were averaged per litter at both time points, and were then averaged as groups: male control, male HAF, female control, female HAF pups. All dams were allowed to suckle their pups, and pups were weaned at 21 days of age.
In a separate group of rats, body composition was measured prior to pregnancy in HAF rats and in age-matched controls (noted above), as shown in Figure S1A and S1B, and body composition was measured again by Echo-MRI 2 days postpartum in HAF and control rats and compared to age-matched virgin controls and HAF rats.
Aging protocols:
As shown in Figure S1A and S1B, following weaning of the pups, control and HAF previously-pregnant rats were allowed to age to 10 months or 16 months (n = 4 – 16/group). Age-matched HAF and control virgins were also allowed to age to 10 or 16 months. At 10 and 16 months of age, 24-hour urine collections were made for measurement of protein and nitrate/nitrite excretion in virgin and previously pregnant HAF rats, as we previously described 23, 24 (see Supplemental Methods). Body composition and oral glucose tolerance (OGTT) were also measured at 10 and 16 months of age in HAF virgins and previously pregnant rats, as previously described 24. Mean arterial pressure (MAP), systolic (SBP) and diastolic blood pressure (DBP) were measured by radiotelemetry for 4 days at 10 and 16 months of age in both virgin and previously pregnant HAF and control rats, as previously described (see Supplemental Methods).
Upon completion of MAP in 16 months old virgin and previously pregnant HAF rats (n=6/group), blood samples were taken for plasma DHT and insulin that were measured as we previously described 24 (see Supplemental Methods). In additional group of rats, kidneys from virgin and previously pregnant control and HAF rats removed and flash-frozen in liquid nitrogen for real-time qPCR measurement of mRNA.
Gene expression:
In order to determine mRNA expression of genes in systems that are known to play a role in blood pressure control, qPCR was performed in kidneys from virgin and previously pregnant control and HAF rats (n= 6–12/group), as we previously described 24 (see Supplemental Methods). The data for endothelial nitric oxide synthase (eNOS), renin, angiotensinogen, angiotensin converting enzyme (ACE), angiotensin 1a receptor (AT1aR), pre-pro-endothelin (ET-1), ETA receptor (R), and ETBR were factored for the geometric mean of four housekeeping genes (shown in Figure S2).
Statistical analyses:
All data are expressed as means ± SEM. Two-way ANOVA was used to determine the differences among groups in most studies. For the data in Tables 1A and B, two way ANOVA with repeated measures was used. Student’s T test was used to determine differences in number of pups per litter, plasma DHT and insulin levels, body weight gains pre and post-pregnancy. Uncorrected Fisher’s LSD test was used for post hoc tests when necessary. Values of p ≤ 0.05 were considered statistically significant. Statistical analyses were performed using GraphPad Prism software (GraphPad Software Inc. V6.0c, San Diego, CA, USA).
Table 1A:
Body weight (BW) and body composition in pregnant control and HAF rats prior to pregnancy and 48 hours after delivery.
| Parameter | Pregnant Control (n = 5) | Pregnant HAF (n = 3) | Interaction | Time | Androgens | |
|---|---|---|---|---|---|---|
| BW (g) | Pre-pregnancy | 197.8 ± 5.2 | 251.0 ± 3.0 * | p = 0.53 | p <0.01 | p < 0.01 |
| Post-delivery | 263.6 ± 12.3 † | 323.7 ± 7.1 * | ||||
| BW gain during pregnancy (g) | 65.8 ± 7.4 | 72.7 ± 4.3 | ||||
| Fat mass (g) | Pre-pregnancy | 16.5 ± 0.6 | 18.5 ± 1.0 | p = 0.86 | p =0.04 | p = 0.41 |
| Post-delivery | 21.7 ± 2.5 | 22.9 ± 1.6 | ||||
| Fat mass (% of BW) | Pre-pregnancy | 8.4 ± 0.4 | 7.4 ± 0.4 | p = 0.97 | p =0.67 | p = 0.07 |
| Post-delivery | 8.2 ± 0.6 | 7.1 ± 0.4 | ||||
| Lean mass (g) | Pre-pregnancy | 174.9 ± 6.1 | 227.4 ± 2.5 * | p = 0.54 | p <0.01 | p < 0.01 |
| Post-delivery | 232.2 ± 10.1 ‡ | 288.9 ± 6.6 *‡ | ||||
| Lean mass (% of BW) | Pre-pregnancy | 88.4 ± 0.8 | 90.6 ± 0.1 * | p = 0.45 | p =0.30 | p = 0.02 |
| Post-delivery | 88.1 ± 0.4 | 89.3 ± 0.2 | ||||
Values represent mean ± S.E.M. Fat and lean masses were determined by EchoMRI as described in Methods. Statistical analyses by ANOVA with repeated measures and uncorrected Fisher’s LSD; significance was defined as p ≤ 0.05.
, p<0.05, compared to pregnant controls, p ≤ 0.05;
, p<0.05, compared to pre-pregnancy of the same group. BW gain was compared by t-test.
Table 1B:
Body weight (BW) and body composition in pregnant and virgin HAF prior to pregnancy and 48 hours after delivery.
| Parameter | Age-matched Virgin HAF (n=4) | Pregnant HAF (n = 3) | Interaction | Time | Pregnancy | |
|---|---|---|---|---|---|---|
| BW (g) | Pre-pregnancy | 243.0 ± 13.2 | 251.0 ± 3.0 | p = 0.30 | p < 0.01 | p = 0.91 |
| Post-delivery | 327.3 ± 7.1 ‡ | 323.7 ± 7.1 ‡ | ||||
| BW gain during pregnancy (g) | 84.3 ± 7.9 | 72.7 ± 4.3 | ||||
| Fat mass (g) | Pre-pregnancy | 18.0 ± 1.6 | 18.5 ± 1.0 | p = 0.052 | p < 0.01 | p = 0.18 |
| Post-delivery | 32.8 ± 3.8 ‡ | 22.9 ± 1.6 † ‡ | ||||
| Fat mass (% of BW) | Pre-pregnancy | 7.4 ± 0.6 | 7.4 ± 0.4 | p = 0.047 | p = 0.09 | p = 0.09 |
| Post-delivery | 10.0 ± 0.8 ‡ | 7.1 ± 0.4 † | ||||
| Lean mass (g) | Pre-pregnancy | 219.7 ± 2.5 | 227.4 ± 2.5 | p = 0.92 | p < 0.01 | p = 0.71 |
| Post-delivery | 282.1 ± 17.8 ‡ | 288.9 ± 6.6 ‡ | ||||
| Lean mass (% of BW) | Pre-pregnancy | 90.4 ± 0.8 | 90.6 ± 0.1 | p < 0.01 | p < 0.01 | p = 0.12 |
| Post-delivery | 86.1 ± 0.8 ‡ | 89.3 ± 0.8 † ‡ | ||||
Values represent mean ± S.E.M. Fat and lean masses were determined by EchoMRI as described in Methods. Statistical analyses by ANOVA with repeated measures and uncorrected Fisher’s LSD; significance was defined as p ≤ 0.05.
, p<0.05 compared to virgin HAF;
, p<0.05, compared to pre-pregnancy of the same group. BW gain was compared by t-test.
Results
Characteristics of the offspring of HAF and control pregnancies:
As shown in Figure 1A, there were no differences in the number of offspring per litter in HAF and control pregnancies (control: 12 ± 1; HAF: 11 ± 1 pups/litter). Birth weights for both male and female HAF offspring were less than male or female offspring born to control dams (Figure 1B), and HAF offspring weights remained lower at 48 hours postnatally (Figure 1C). Female HAF offspring also weighed less than male HAF offspring at both ages (Figure 1B). These data support significant impacts of both androgens and sex on the differences, but no interaction was noted.
Figure 1: Number of pups per litter and body weights of HAF and control offspring.
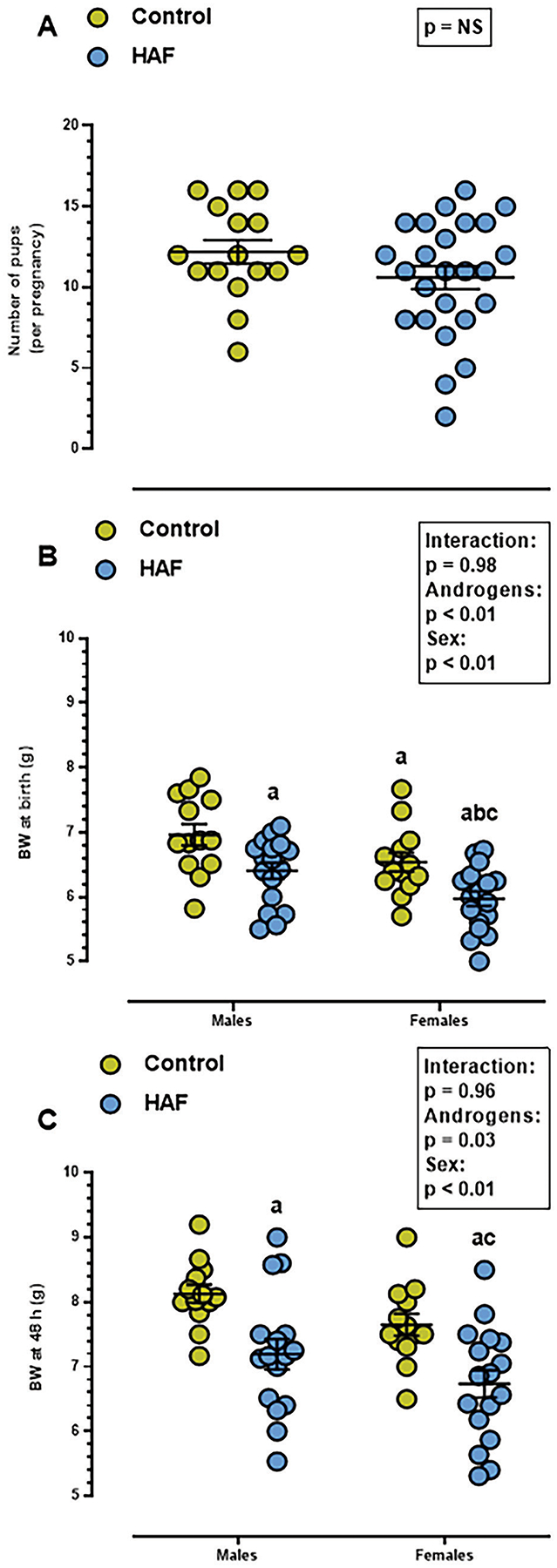
A) There were no differences in average numbers of pups per litter between control and HAF dams (p=NS). B) Birth weights were lower in male and female HAF offspring than control offspring, and female HAF birth weights were lower than male HAF. C) At postnatal day 2, body weights remained lower in male and female HAF offspring than controls. Statistical significance using Student’s t-test (A) or 2-way ANOVA (B and C) was defined as p ≤ 0.05. a, p ≤ 0.05 compared to control males; b, p ≤ 0.05 compared to HAF male offspring; c, p ≤ 0.05 compared to control female offspring. NS: non-significant.
Mean arterial pressure (MAP):
As shown in Figure 2A, at 16 months of age, MAP in virgin HAF rats was higher than in other groups, and previous pregnancy attenuated the hypertension in HAF rats. MAP in previously-pregnant HAF rats was slightly higher than in previously pregnant control rats. Thus there were significant impacts of both androgens and parity on MAP at 16 months, but no interactions noted.
Figure 2: Blood pressure in virgin and previously-pregnant HAF and/or control rats at 10 months or 16 months of age.
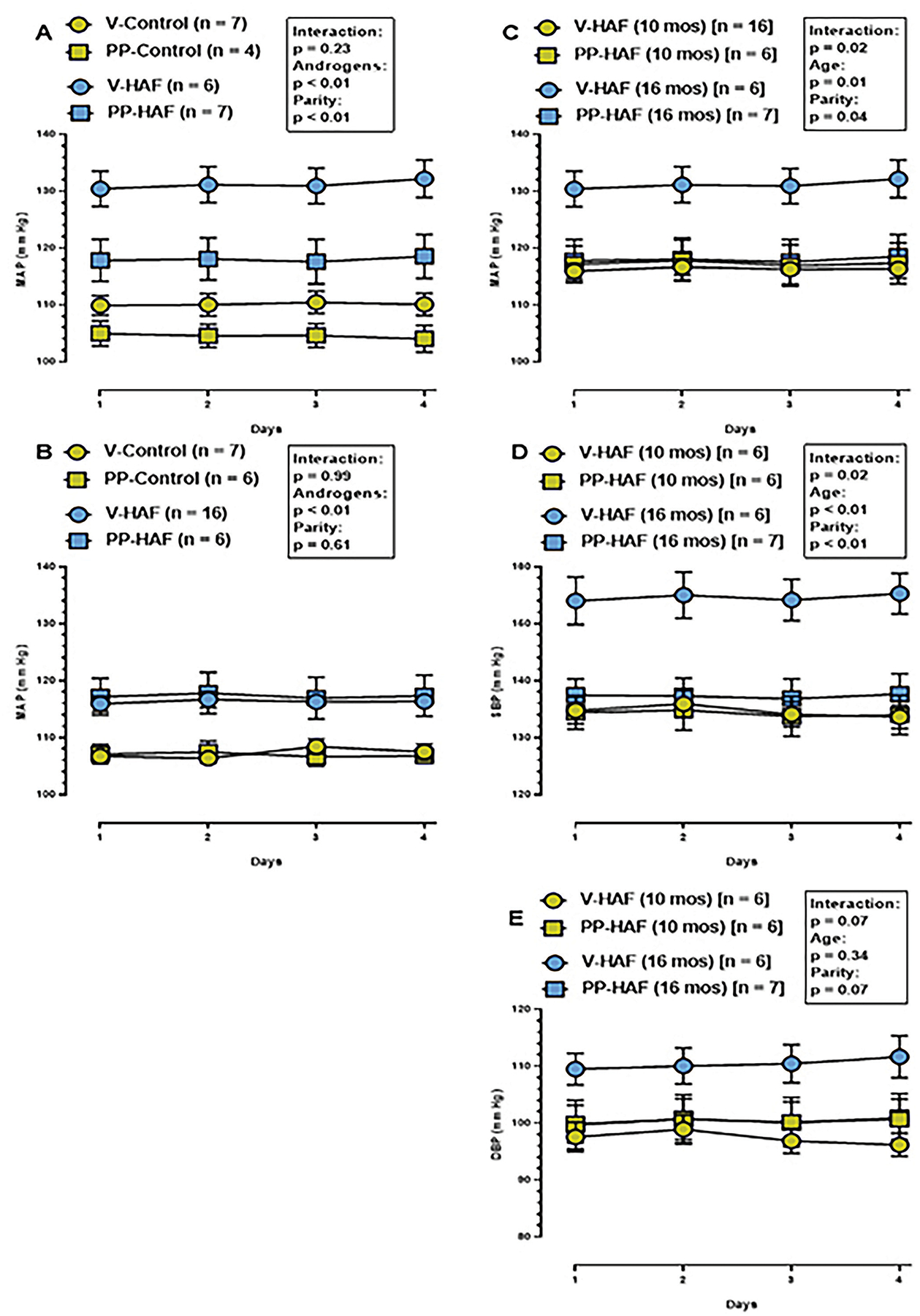
Statistical significance was determined by two-way ANOVA with repeated measures, and defined as p ≤ 0.05.
A) At 16 months of age, MAP was similar in previously-pregnant and virgin controls. MAP in previously-pregnant HAF rats was not different than virgin controls but was higher than previously-pregnant controls. MAP was higher in virgin HAF rats than all controls or previously pregnant HAF rats.
B) MAP at 10 months of age was higher in virgin and previously-pregnant HAF rats than in virgin or previously-pregnant controls. Pregnancy had no effect on MAP in either controls or HAF rats.
C) Comparison of MAP at 10 months and 16 months of age in virgin and previously pregnant HAF rats. MAP was not different in previously pregnant HAF rats at 16 months of age compared to either virgins or previously pregnant HAF rats at 10 months of age. However, MAP increased in virgin HAF rats between 10 months and 16 months of age.
D) Comparison of systolic blood pressure (SBP) at 10 months and 16 months of age in virgin and previously-pregnant HAF rats. SBP was not different in previously-pregnant HAF rats at 16 months of age compared to either virgins or previously-pregnant HAF rats at 10 months of age. However, SBP increased in virgin HAF rats between 10 months and 16 months of age, and there was significant interaction with age and parity.
E) Comparison of DBP at 10 months and 16 months of age in virgin and previously-pregnant HAF rats. DBP was not different in vigin or previously-pregnant control rats at 10 or 16 months of age. There was no effect of age or parity and there were no interactions.
In order to determine whether there were differences in MAP prior to cessation of estrous cycling, MAP was measured in virgin and previously-pregnant control and HAF rats, 10 months of age. As shown in Figure 2B, only androgens, not parity, impacted the hypertension in HAF vs control rats, and there was no interaction noted.
When blood pressures were compared at 10 and 16 months of age in HAF rats (Figure 2C), MAP was similar between virgin HAF at 10 months of age, previously-pregnant HAF at 10 months of age, and previously-pregnant HAF at 16 months of age. Importantly, MAP increased significantly in virgin HAF rats between 10 and 16 months of age. There was significant interaction between age and parity in these studies.
Interestingly, as shown in Figures 2D and 2E, changes in systolic blood pressure rather than diastolic blood pressure accounted for the increased blood pressure in virgin HAF at 16 months of age.
Potential mechanisms that could contribute to lower blood pressure in previously pregnant HAF rats, aged 16 months:
DHT levels:
We have shown previously that DHT levels are increased approximately 3-fold with DHT treatment compared to placebo controls 19,20,33,39. To determine if there were differences in DHT levels in control and previously pregnant HAF rats that could account for the differences in MAP at 16 months of age, plasma DHT was measured. However, DHT was not different between virgin and previously pregnant HAF rats (virgin HAF: 102.8 ± 22.9; previously-pregnant HAF (n=6): 101.9 ± 22.9 pg/mL; p=NS).
Body Composition, pre-pregnancy and post-delivery:
In order to determine whether pregnancy itself affected body composition in HAF rats that could impact later in life blood pressure, body weight, fat and lean masses and their ratios to body weight were measured pre-pregnancy or 48 hours post-delivery in control or HAF rats and compared with age-matched virgin HAF rats. As shown in Table 1A, prior to and after pregnancy, HAF rats had higher body weight than controls. Body weight increased similarly with time in both pregnant groups. Time and androgens provided the major differences between the groups, with no interactions noted. Changes in fat mass were affected only by time, not androgens. Fat mass factored for body weight were not affected by pregnancy or time. Lean mass was higher in HAF rats than control prior to and after pregnancy and both time and androgens were the major factors for the differences, with no interactions noted. When factor for body mass, only androgens were the main factor for differences between control and HAF pregnant rats with no interactions found. As shown in Table 1B comparing pregnant and virgin HAF rats, prior to pregnancy there were no differences in the rats. With time, both groups increased body weights to similar extent, and there was no difference in body weight gain. Fat mass did not increase to the same extent in pregnant HAF rats as in controls, but there was no interaction noted. When fat mass was factored for body weight there was interaction noted, however. Lean mass increased in both groups over the time of pregnancy with no interactions. However, when factored for body weight, there was interaction with time with pregnant groups increasing lean mass more than control virgin HAF.
Body composition and metabolic factors with aging:
In order to determine if there were differences in body composition with aging in HAF rats that may contribute to the differences in MAP, fat and lean masses were measured. As shown in Table 2, both age and pregnancy status significantly affected body weight with no significant interaction, with body weight being similar in virgin and previously pregnant HAF at 10 months, but was reduced in previously pregnant HAF at 16 months compared to virgins. Fat mass was higher in 16 months old groups, whereas lean mass was not different among the groups. Lean mass was not different among the groups, but lean mass/body weight ratios were significantly lower in HAF at 16 months than the other groups. Fat mass, fat mass/body weight ratios, and lean mass/body weight ratios were only affected by time, not parity, with no significant interactions.
Table 2:
Body weights (BW) and body composition of virgin (V) and previously-pregnant (PP) HAF rats at 10 and 16 months of age.
| HAF 10 mos | HAF 10 mos | HAF 16 mos | HAF 16 mos | ||||
|---|---|---|---|---|---|---|---|
| Parameter | Virgin | Previously- pregnant | Virgin | Previously- Pregnant | Interaction | Parity | Age |
| BW (g) | 399.5 ± 6.4 | 392.3 ± 5.2 | 427.8 ± 7.4 | 392.5 ± 16.7 | p = 0.16 | p = 0.02 | p = 0.02 |
| Fat mass (g) | 56.2 ± 3.8 | 54.7 ± 4.2 | 81.6 ± 8.7 | 80.5 ± 7.8 | p = 0.95 | p= 0.82 | p < 0.01 |
| Lean mass (g) | 317.2 ± 5.4 | 315.6 ± 5.6 | 321.1 ± 10.5 | 321.1 ± 10.5 | p =0.34 | p = 0.25 | p = 0.64 |
| Fat mass/BW ratio | 0.14 ± 0.01 | 0.14 ± 0.01 | 0.19 ± 0.02 | 0.20 ± 0.01 | p = 0.78 | p = 0.86 | p < 0.01 |
| Lean mass/BW ratio | 0.80 ± 0.01 | 0.80 ± 0.01 | 0.75 ± 0.02 | 0.75 ± 0.01 | p = 0.71 | p = 0.75 | p < 0.01 |
Values represent mean ± SEM. Fat and lean masses were determined by EchoMRI, as described in Methods. Statistical analyses were performed by two-way ANOVA; significance was defined as p<0.05.
To determine if there were differences in insulin resistance between virgin and previously-pregnant HAF rats, plasma insulin levels were measured, and oral glucose tolerance test was performed. Plasma insulin was not different between the HAF groups at 16 months of age (virgin HAF (n=14): 1.3 ± 0.2; previously-pregnant HAF (n=10): 1.1 ± 0.2 ng/ml; p=NS). Oral glucose tolerance, as shown in Figure S3, was not affected by previous pregnancy in HAF rats at either 10 or 16 months of age. Fasting blood glucose level (BGL) was also not elevated in either HAF groups at either age, showing rats were not diabetic.
Proteinuria and nitrate/nitrite excretion:
We determined if proteinuria increased with aging in HAF rats and whether pregnancy affected the levels. As shown in Figure 3A, both aging and parity impacted the level of proteinuria with no interactions noted. Proteinuria was higher in 16 month old HAF virgins than virgin or previously-pregnant HAF at 10 months, and previous-pregnancy attenuated proteinuria in HAF rats, 16 months of age.
Figure 3: Proteinuria (A) and nitrate/nitrite (UNOx) (B) excretion in virgin and previously-pregnant HAF rats at 10 and 16 months of age.
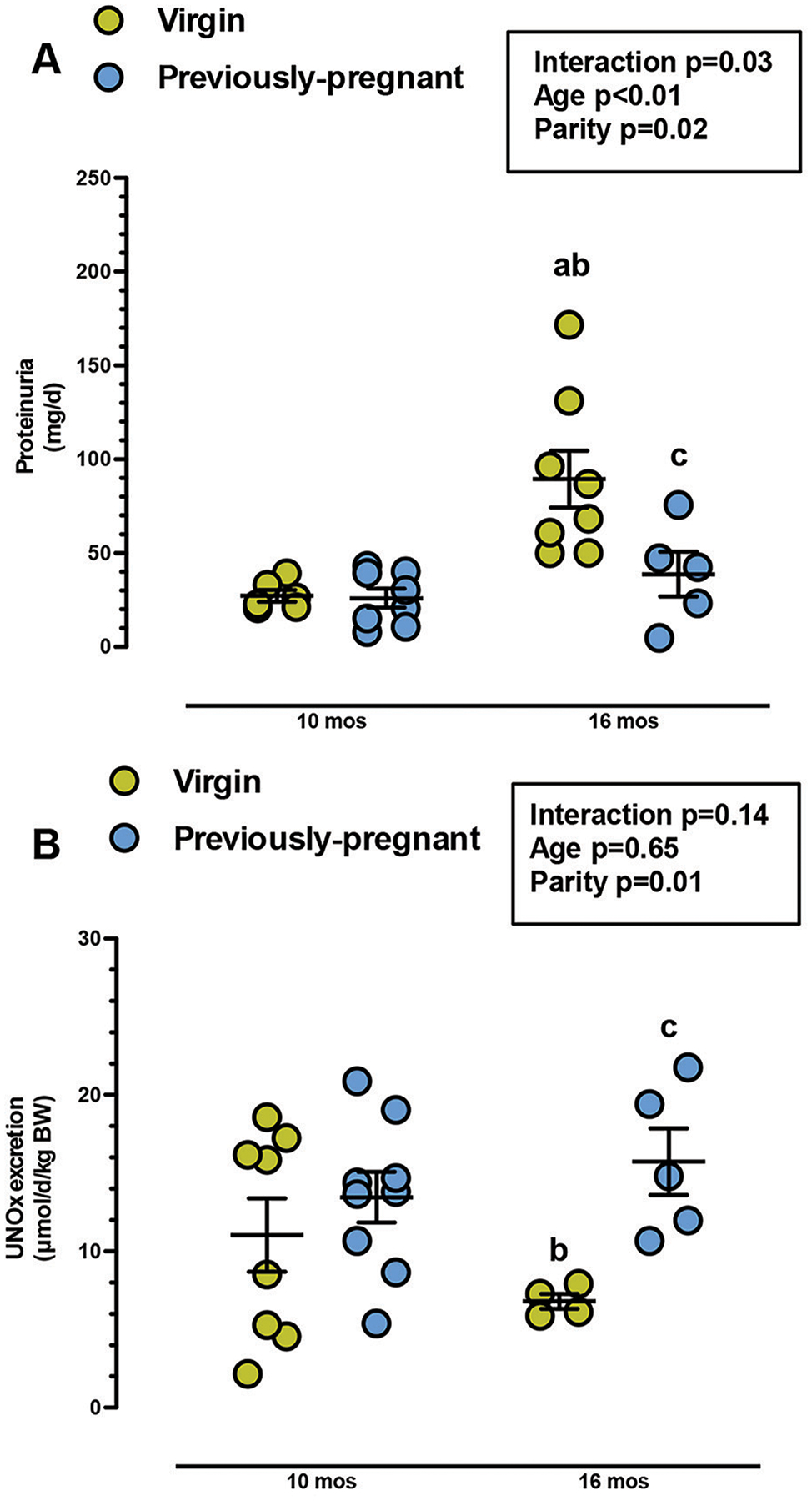
A) Protein excretion was not different between previously-pregnant and virgin HAF rats at 10 months of age. At 16 months, protein excretion was higher in virgin HAF compared to all HAF rats at 10 months. Proteinuria in previously pregnant HAF at 16 months was similar to HAF rats at 10 months, and was lower than age-matched virgin HAF rats. Statistical significance was determined by two-way ANOVA with Fisher’s LSD post hoc test, and defined as p ≤ 0.05.
B) Nitrate/nitrite excretion was not different in virgin and previously-pregnant HAF rats at 10 months of age, or virgin HAF rats at 16 months of age. However, nitrate/nitrate excretion was higher in previously-pregnant HAF, aged 16 months, than age-matched virgin HAF rats. Statistical significance was determined by two-way ANOVA with Fisher’s LSD post hoc test, and defined as p ≤ 0.05. a, p ≤ 0.05, compared to virgin HAF rats, 10 months; b, p ≤ 0.05, compared to previously-pregnant HAF, 10 months; c, p ≤ 0.05, compared to virgin HAF, 16 months.
As shown in Figure 3B, there was a significant interaction between age and parity on nitrate/nitrite excretion in virgin and previously-pregnant HAF rats, aged 10 and 16 months. Nitrate/nitrite excretion decreased in virgin HAF, aged 16 months, compared to virgin and previously pregnant HAF at 10 months. However, by 16 months of age, previous-pregnancy increased nitrate/nitrite excretion in HAF to levels similar to HAF groups at 10 months of age.
Intrarenal gene expression analyses:
In order to determine if there were differences in intrarenal gene expression that could contribute to differences in MAP among virgin and previously-pregnant control and HAF rats, 16 months of age, we evaluated mRNA expression of endothelial nitric oxide synthase (eNOS), and components of the renin-angiotensin system (RAS) and the endothelin (ET) system. As shown in Table 3, eNOS expression was decreased in virgin HAFs compared to controls, whereas pregnancy normalized eNOS in HAF. In components of the RAS, renin and AT1aR mRNA were lower and angiotensinogen mRNA was higher in both HAF groups. Pregnancy tended to further reduce AT1aR expression in HAF, and ACE was also lower in previously-pregnant HAF than virgin HAF. Intrarenal pre-pro-ET-1 mRNA was higher in virgin HAF than controls or previously-pregnant HAF, ETAR expression was lower in previously-pregnant HAF compared to virgin HAF, but ETBR expression was lower in virgin HAF than controls or previously-pregnant HAF.
Table 3:
Intrarenal mRNA expression of endothelial nitric oxide synthase, components of the renin-angiotensin system and endothelin system in virgin and previously-pregnant control rats, aged 16 months, compared to age-matched virgin and previously-pregnant HAF rats.
| Renal mRNA expression (ΔΔCT/geometric mean of housekeeping genes) | Control | HAF | ||
|---|---|---|---|---|
| Virgin (n = 6) |
Previously-pregnant (n = 7) |
Virgin (n = 12) |
Previously-pregnant (n = 9) |
|
| eNOS (NOS3) | 1.00 ± 0.05 | 0.98 ± 0.08 | 0.82 ± 0.04 *† | 1.03 ± 0.04 ‡ |
| Renin | 1.00 ± 0.13 | 0.62 ± 0.07 * | 0.48 ± 0.06 * | 0.57 ± 0.09 * |
| Angiotensinogen | 1.00 ± 0.13 | 0.87 ± 0.08 | 8.16 ± 1.30 *† | 9.31 ± 1.30 *† |
| ACE | 1.00 ± 0.08 | 0.97 ± 0.06 | 1.10 ± 0.08 | 0.82 ± 0.09 ‡ |
| AT1aR | 1.00 ± 0.05 | 0.93 ± 0.07 | 0.69 ± 0.05 *† | 0.52 ± 0.03 *† ‡ |
| Pre-proET-1 | 1.00 ± 0.07 | 1.05 ± 0.07 | 1.68 ± 0.20 *† | 1.45 ± 0.10 |
| ETAR | 1.00 ± 0.06 | 1.00 ± 0.08 | 1.08 ± 0.08 | 0.91 ± 0.06 ‡ |
| ETBR | 1.00 ± 0.05 | 0.96 ± 0.07 | 0.80 ± 0.02 *† | 0.94 ± 0.05 ‡ |
Key:eNOS, endothelial nitric oxide synthase; ACE, angiotensin I converting enzyme; pre-pro-ET-1, pre-pro-endothelin-1; ETAR, endothelin A receptor; ETBR, endothelin B receptor. Expression values were normalized to the geometric mean of 4 housekeeping genes, as described in Supplemental Methods. Values represent mean ± S.E.M. Statistical analyses were performed using two-way ANOVA followed by uncorrected Fisher`s LSD test; significance was defined as p ≤ 0.05.
, p ≤ 0.05, compared to virgin controls;
, p ≤ 0.05, compared to previously-pregnant controls;
, p ≤ 0.05, compared to virgin HAF rats.
Discussion
In this study we tested the hypothesis that previous pregnancy would protect HAF rats from age-related hypertension. In fact, we found that: 1) previous pregnancy in aging (16 months) HAF rats attenuated the hypertension found in age-matched virgins; 2) along with the reduction in blood pressure, previously-pregnant HAF exhibit upregulation of intrarenal eNOS and ETB receptor mRNA, along with downregulation of renin, ETAR, and AT1aR mRNA; 3) similarly, previously-pregnant HAF rats exhibited a reduction in proteinuria and an increase in nitrate/nitrite excretion compared to virgin HAF rats; 4) prior to cessation of estrous cycling (10 months of age), there were no differences in blood pressure, proteinuria, or nitrate/nitrite excretion in HAF rats regardless of pregnancy status; 5) male and female offspring born to HAF rats exhibited intrauterine growth restriction (IUGR) with lower birth weights, compared to offspring born to control females.
The HAF rat is a well-characterized model that mimics many of the symptoms of women with PCOS, including insulin resistance, hyperlipidemia, and elevated blood pressure as early as 14–16 weeks of age 19. However, this is the first study, to our knowledge, to address the cardiovascular-metabolic consequences of pregnancy using the HAF model. Common rodent models that seek to study the outcomes of hyperandrogenemic pregnancy on dams or the offspring inject dams with testosterone beginning on gestational days 15–17 only, and at doses that are significantly higher than the DHT doses we use for the HAF model (0.5 mg/kg/d testosterone vs 0.25–0.33 μg/kg/d DHT) 25, 26. Our HAF rats are treated with DHT beginning at 4 weeks of age (pre-pubertal) and throughout pregnancy and lactation. Thus the HAF model better mimics pregnancy in women with PCOS than the late gestation androgen model.
In studying the potential changes in gene expression that could contribute to hypertension in virgin HAF rats and protection seen in previously-pregnant HAF, we measured mRNA expression of both vasodilators and vasoconstrictors. We found that in previously-pregnant HAF rats, there was an increase in eNOS mRNA expression that was supported by an increase in nitrate/nitrite excretion, an index of nitric oxide (NO). Whether eNOS activity is increased in previously-pregnant rats HAF will need to be determined in future studies. However, we found previously that the components of the NADPH oxidase system are upregulated in young HAF rats 19. Since aging is associated with increased oxidative stress, it is also possible then that in aging virgin HAF rats, any NO being produced may be scavenged by superoxide, produced by NADPH oxidase, thus reducing the bioavailability of NO to cause vasodilation and contributing to their elevated blood pressure. Whether oxidative stress increases with aging in virgin HAF rats to a greater extent than in previously- pregnant HAF will also need to be determined to further identify the mechanisms responsible for their hypertension.
Young women with PCOS have increased plasma renin levels and activity 27, 28 and increased plasma prorenin 29. In contrast, in the present study in the aging HAF rats, we found that intrarenal renin mRNA expression was decreased in both virgin and previously-pregnant rats. Androgens are known to upregulate intrarenal angiotensinogen, and we have shown previously that angiotensinogen was elevated by 10 fold at 14–16 weeks of age in virgin HAF rats 19, 30. In the present study angiotensinogen was also increased by 8–9 fold, but was similar in both HAF groups. Renin is the rate-limiting step in the synthesis of angiotensin II (Ang II), but if renin is not working at Vmax, then the lower levels of renin may be offset by the significant increases in angiotensinogen substrate that would support higher Ang II levels in the HAF rats. In young PCOS women, angiotensin converting enzyme (ACE) gene polymorphism was associated with adverse metabolic comorbidities 31, 32. In the present study intrarenal ACE mRNA tended to be decreased in previously-pregnant HAF rats compared to virgins. Similarly, intrarenal AT1aR mRNA expression was decreased significantly in previously-pregnant HAF rats compared to virgins. Enalapril (ACE inhibitor) and telmisartan (AT1R antagonist) were shown by others to cause significant decreases in BP in HAF rats or PCOS women, respectively 33, 34. Taken together, these data support that the RAS may contribute to the elevated blood pressure in HAF rats, but whether virgin HAF have higher Ang II than previously-pregnant rats needs to be determined. Previously-pregnant HAF rats may also have higher levels of the vasodilator arm of the RAS, Ang(1–7) or AT2R, that could contribute to their attenuated blood pressure compared to virgins, and will also need to be determined.
Young women with PCOS and female to male transsexuals have elevated levels of endothelin 35, 36. There are no studies to our knowledge on the levels of ET-1 in postmenopausal PCOS women. Alexander and colleagues reported that Ang II stimulates intrarenal ET-1 production 37. Androgens then may directly stimulate endothelin synthesis or may stimulate the RAS to increase endothelin, thus leading to the expression of two powerful vasoconstrictors that could impact BP in women with PCOS 38. In the present study there were no differences in levels of plasma DHT between the virgin and previously-pregnant HAF rats, and pre-pro-ET-1 mRNA expression was upregulated to similar levels in both groups as well, suggesting that intrarenal ET-1 may be similar in the groups. However, intrarenal ETAR expression was lower and ETBR expression was higher in previously-pregnant HAF, both suggesting these differences could contribute to the higher blood pressure in virgin HAF. Interestingly, Usselman and colleagues reported that young PCOS women have elevated ET-1, that endothelial vasodilation via ETBR predominated, but is attenuated compared to controls, and that the effect was independent of NO, since L-NAME had no effect on vasodilatory response to ET-1 30, 36. Additional studies will be necessary to determine the full contribution of endothelin and endothelin receptors to the age-related hypertension in HAF rats.
One interesting finding for the present studies is that the difference in hypertension between previously-pregnant and virgin HAF rats does not occur until after cessation of estrous cycling and with advanced aging. At 10 months of age, when the rats are still cycling, the blood pressure, although elevated compared to controls, was not different between previously-pregnant and virgin HAF rats. We have shown previously that the HAF rat model ceases to cycle by 13 months of age 22. If loss of estrogens in the face of increased androgens was the mechanism by which blood pressure increased with aging in the HAF model, one would expect that blood pressure would also rise in previously-pregnant HAF rats that have also stopped estrous cycling. Thus it is not likely that estrogens played a role in the age-related protection against the increase in blood pressure in the previously-pregnant HAF rats.
Women with PCOS often have difficulty becoming pregnant and require assisted reproduction 40. Just as in PCOS women, our HAF rat model also has reproductive difficulties with only 60% of those placed with a male becoming pregnant, compared with >99% of control SD rats that become pregnant. Another consequence of hyperandrogenemia during pregnancy was the lower birth weights of both male and female offspring of the HAF rats. In women with PCOS, their children are born either small for gestational age 41, 42, as in our study, or large for gestational age 40. If PCOS women have elevated glucose associated with obesity and insulin resistance, their children may be born large for gestational age 40. Golopalakrishnan and colleagues reported that testosterone supplements given from day 15–19 of pregnancy in rats caused a reduction in both placental weights and fetal weights and was associated with increased expression of markers of hypoxia, such as hypoxia-inducible factor-1α, along with reductions in uterine blood flow 25. Thus the presence of elevated androgens in the HAF rats may have contributed to the lower birth weights in their offspring.
We have shown previously that our model of hyperandrogenemia has insulin resistance (elevated insulin levels) but no increase in fasting glucose both as young adults and following estrous cycling 19, 22, so the HAF rats are not diabetic. We have not measured insulin or glucose handling during pregnancy in the HAF model, but since the offspring are born small for gestational age, it is not likely the dams have gestational hyperglycemia. We also have not measured blood pressure during the pregnancy in our HAF rats. However, it is also not likely that pregnancy in these rats is associated with preeclampsia-like symptoms, otherwise the dams should have early CVD and hypertension, as is common for women with preeclampsia 43. In this regard the HAF rat model may be similar to the spontaneously-hypertensive rat (SHR) that is hypertensive prior to pregnancy but exhibits a reduction in blood pressure in late pregnancy 44, and multiple pregnancies have no adverse effect on their renal function or blood pressure 45. Similarly, Hu and colleagues performed ambulatory blood pressure measurements in PCOS and control women as they progressed throughout their pregnancies. They found that PCOS women had higher blood pressure in the first trimester (11–13 weeks gestation) than control women, but there was no gestational effect, i.e. pregnancy per se did not further increase blood pressure 10. In addition, the average blood pressures in the PCOS women did not reach the levels required for treatment at that time (Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure (JNC VII)) 46.
To our knowledge, there are no studies in which the amounts of fat mass and lean mass were measured in PCOS women, pre- and post-pregnancy. We performed these studies in our HAF dams in order to determine if the pregnancy itself reduced the amount of body fat mass compared to the virgin HAF controls. It was interesting that the levels of fat mass/body weight ratio increased and the lean mass/body ratio decreased so significantly during the time of pregnancy in the HAF and control rats. However, these differences in fat and lean masses observed when the rats were young were not present at 10 and 16 months of age. Thus the data do not support a role for improved body composition during pregnancy as mediating the lower blood pressure in previously pregnant HAF rats.
One caveat of the present study is that approximately 60% of the HAF rats, when placed with a male, became pregnant. Thus it is possible that the 60% had a more favorable cardiovascular/reproductive system that allowed them to become pregnant and thus with aging also exhibited lower blood pressure. However, there were no differences in the pre-pregnancy body weights or body composition, lean or fat mass, between the HAF rats, whether they were scheduled to be mated or remain virgins, that could account for the difficulty in becoming pregnant. Female rats can become pseudopregnant, but we have no evidence that this occurred in HAF rats, and only rats that became pregnant and delivered pups were included in the present study.
Perspectives
In the present study we tested the hypothesis that previous pregnancy would protect against age-related hypertension in our model of PCOS. Despite no differences in DHT levels, body composition, or insulin resistance, hypertension was indeed attenuated in previously- pregnant HAF rats compared to virgin HAF. The mechanism(s) responsible for the protection may be differential intrarenal regulation of the nitric oxide, endothelin and renin-angiotensin systems.
While some studies show PCOS women are at increased risk to develop preeclampsia during pregnancy and/or they may take longer to become pregnant, their pregnancy outcomes are not different than control women 47–49. Thus studies need to be performed in aging PCOS women who have been pregnant and compare them to PCOS women who have never been pregnant to determine their relative risks of developing postmenopausal hypertension and CVD compared to the general population. Now that PCOS diagnosis guidelines have been in place more than 15 years, these studies should be forthcoming, but in addition to verification of pregnancy, the new studies need to also verify that the women studied actually had PCOS as defined by increased androgens during their reproductive years.
Supplementary Material
Novelty and Significance:
What Is New:
This is the first study to evaluate the consequences of prior pregnancy on age-related hypertension in a rat model of hyperandrogenemia, that mimics many of the characteristics of women with polycystic ovary syndrome.
Previous pregnancy attenuated the hypertension in hyperandrogenemic female (HAF) rats, aged 16 months, compared to virgin controls.
Mechanisms responsible are likely differential regulation of intrarenal vasoconstrictors/vasodilators.
What Is Relevant:
There are no studies that have evaluated the consequences of pregnancy on cardiovascular disease and hypertension in aging, postmenopausal women with polycystic ovary syndrome, who have hyperandrogenemia.
Acknowledgements
The authors would like to acknowledge the excellent technical support of Huimin Zhang, Ruth M. Vinson, and Kacey Davenport for these studies.
Sources of Funding
This work was supported by NIH grants, R01HL135089 (JFR), P01HL051971 (JFR, DGR), P20GM121334 (JFR, ROM, DGR), P20GM104357 (JFR), American Heart Association Postdoctoral fellowship #20POST35150001 (NMS), and R21DK113500 (DGR).
Footnotes
Disclosures: Nothing to disclose other than funding.
References:
- 1.Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (pcos). Hum Reprod 2004;19:41–47 [DOI] [PubMed] [Google Scholar]
- 2.Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, Taylor AE, Witchel SF. Positions statement: Criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: An androgen excess society guideline. J Clin Endocrinol Metab 2006;91:4237–4245 [DOI] [PubMed] [Google Scholar]
- 3.Witchel SF. Puberty and polycystic ovary syndrome. Mol Cell Endocrinol 2006;254–255:146–153 [DOI] [PubMed] [Google Scholar]
- 4.Escobar-Morreale HF, San Millan JL. Abdominal adiposity and the polycystic ovary syndrome. Trends Endocrino. Metab, 2007;18:266–272 [DOI] [PubMed] [Google Scholar]
- 5.Diamanti-Kandarakis E, Papavassiliou AG, Kandarakis SA, Chrousos GP. Pathophysiology and types of dyslipidemia in pcos. Trends Endocrinol Metab 2007;18:280–285 [DOI] [PubMed] [Google Scholar]
- 6.Markopoulos MC, Rizos D, Valsamakis G, Deligeoroglou E, Grigoriou O, Chrousos GP, Creatsas G, Mastorakos G. Hyperandrogenism in women with polycystic ovary syndrome persists after menopause. J Clin Endocrinol Metab 2011;96:623–631 [DOI] [PubMed] [Google Scholar]
- 7.Puurunen J, Piltonen T, Morin-Papunen L, Perheentupa A, Järvelä I, Ruokonen A, Tapanainen JS. Unfavorable hormonal, metabolic, and inflammatory alterations persist after menopause in women with pcos. J Clin Endocrinol Metab 2011;96:1827–1834 [DOI] [PubMed] [Google Scholar]
- 8.Persson S, Elenis E, Turkmen S, Kramer MS, Yong EL, Sundstrom-Poromaa I. Fecundity among women with polycystic ovary syndrome (pcos)-a population-based study. Hum Reprod 2019;34:2052–2060 [DOI] [PubMed] [Google Scholar]
- 9.He Y, Lu Y, Zhu Q, Wang Y, Lindheim SR, Qi J, Li X, Ding Y, Shi Y, Wei D, Chen ZJ, Sun Y. Influence of metabolic syndrome on female fertility and in vitro fertilization outcomes in pcos women. Am J Obstet Gynecol 2019;221:138.e131–138.e112 [DOI] [PubMed] [Google Scholar]
- 10.Hu S, Leonard A, Seifalian A, Hardiman P. Vascular dysfunction during pregnancy in women with polycystic ovary syndrome. Hum Reprod 2007;22:1532–1539 [DOI] [PubMed] [Google Scholar]
- 11.Gunning MN, Fauser B. Are women with polycystic ovary syndrome at increased cardiovascular disease risk later in life? Climacteric 2017;20:222–227 [DOI] [PubMed] [Google Scholar]
- 12.Lambrinoudaki I Cardiovascular risk in postmenopausal women with the polycystic ovary syndrome. Maturitas 2011;68:13–16 [DOI] [PubMed] [Google Scholar]
- 13.Schmidt J, Landin-Wilhelmsen K, Brannstrom M, Dahlgren E. Cardiovascular disease and risk factors in pcos women of postmenopausal age: A 21-year controlled follow-up study. J Clin Endocrinol Metab 2011;96:3794–3803 [DOI] [PubMed] [Google Scholar]
- 14.Doroszewska K, Milewicz T, Mrozińska S, Janeczko J, Rokicki R, Janeczko M, Warzecha D, Marianowski P. Blood pressure in postmenopausal women with a history of polycystic ovary syndrome. Prz Menopauzalny 2019;18:94–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramezani Tehrani F, Amiri M, Behboudi-Gandevani S, Bidhendi-Yarandi R, Carmina E. Cardiovascular events among reproductive and menopausal age women with polycystic ovary syndrome: A systematic review and meta-analysis. Gynecol Endocrinol 2020;36:12–23 [DOI] [PubMed] [Google Scholar]
- 16.Alur-Gupta S, Dokras A. Polycystic ovary syndrome: Is the cardiometabolic risk increased after menopause? Menopause 2019;26:331–333 [DOI] [PubMed] [Google Scholar]
- 17.Armeni E, Lambrinoudaki I. Cardiovascular risk in postmenopausal women with polycystic ovary syndrome. Curr Vasc Pharmacol 2019;17:579–590 [DOI] [PubMed] [Google Scholar]
- 18.Forslund M, Landin-Wilhelmsen K, Schmidt J, Brannstrom M, Trimpou P, Dahlgren E. Higher menopausal age but no differences in parity in women with polycystic ovary syndrome compared with controls. Acta Ob Gynecol Scand 2019;98:320–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yanes LL, Romero DG, Moulana M, Lima R, Davis DD, Zhang H, Lockhart R, Racusen LC, Reckelhoff JF. Cardiovascular-renal and metabolic characterization of a rat model of polycystic ovary syndrome. Gend Med 2011;8:103–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dalmasso C, Maranon R, Patil C, Moulana M, Romero DG, Reckelhoff JF. 20-HETE and CYP4A2 omega-hydroxylase contribute to the elevated blood pressure in hyperandrogenemic female rats. Am J Physiol Renal Physiol 2016;311:F71–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maranon R, Lima R, Spradley FT, do Carmo JM, Zhang H, Smith AD, Bui E, Thomas RL, Moulana M, Hall JE, Granger JP, Reckelhoff JF. Roles for the sympathetic nervous system, renal nerves, and melanocortin-4 receptor in the elevated blood pressure in hyperandrogenemic female rats. Am J Physiol Regu, Integ, Comp Physiol 2015;308:R708–R713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dalmasso C, Maranon R, Patil C, Bui E, Moulana M, Zhang H, Smith A, Yanes Cardozo LL, Reckelhoff JF. Cardiometabolic effects of chronic hyperandrogenemia in a new model of postmenopausal polycystic ovary syndrome. Endocrinol 2016;157:2920–2927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reckelhoff JF, Kellum JA, Blanchard EJ, Bacon EE, Wesley AJ, Kruckeberg WC. Changes in nitric oxide precursor, l-arginine, and metabolites, nitrate and nitrite, with aging. Life Sci 1994;55:1895–1902 [DOI] [PubMed] [Google Scholar]
- 24.Yanes LL, Romero DG, Iles JW, Iliescu R, Gomez-Sanchez C, Reckelhoff JF. Sexual dimorphism in the renin-angiotensin system in aging spontaneously hypertensive rats. Am J Physiol Reg. Integr Comp Physiol 2006;291:R383–390 [DOI] [PubMed] [Google Scholar]
- 25.Gopalakrishnan K, Mishra JS, Chinnathambi V, Vincent KL, Patrikeev I, Motamedi M, Saade GR, Hankins GD, Sathishkumar K. Elevated testosterone reduces uterine blood flow, spiral artery elongation, and placental oxygenation in pregnant rats. Hypertension 2016;67:630–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gulan T, Yeernuer T, Sui S, Mayinuer N. A rat model of maternal polycystic ovary syndrome shows that exposure to androgens in utero results in dysbiosis of the intestinal microbiota and metabolic disorders of the newborn rat. Med Sci Monit 2019;25:9377–9391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alphan Z, Berberoglu Z, Gorar S, Candan Z, Aktas A, Aral Y, Ademoglu E. Increased total renin levels but not angiotensin-converting enzyme activity in obese patients with polycystic ovary syndrome. Med Prin Pract 2013;22:475–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uncu G, Sozer MC, Develioglu O, Cengiz C. The role of plasma renin activity in distinguishing patients with polycystic ovary syndrome (pcos) from oligomenorrheic patients without pcos. Gynecol Endocrinol 2002;16:447–452 [PubMed] [Google Scholar]
- 29.Morris RS, Wong IL, Hatch IE, Gentschein E, Paulson RJ, Lobo RA. Prorenin is elevated in polycystic ovary syndrome and may reflect hyperandrogenism. Fertil Steril 1995;64:1099–1103 [DOI] [PubMed] [Google Scholar]
- 30.Usselman CW, Taylor HS, Stachenfeld NS. Microvascular endothelial function in lean versus obese women with polycystic ovary syndrome: Role of the endothelin b receptor. FASEB J 2017;31:691.695–691.695 [Google Scholar]
- 31.Celik O, Yesilada E, Hascalik S, Celik N, Sahin I, Keskin L, Ozerol E. Angiotensin-converting enzyme gene polymorphism and risk of insulin resistance in PCOS. Reprod Biomed 2010;20:492–498 [DOI] [PubMed] [Google Scholar]
- 32.Ożegowska K, Bogacz A, Bartkowiak‑Wieczorek J, Seremak‑Mrozikiewicz A, Pawelczyk L. Association between the angiotensin converting enzyme gene insertion/deletion polymorphism and metabolic disturbances in women with polycystic ovary syndrome. Mol Med Rep 2016;14:5401–5407 [DOI] [PubMed] [Google Scholar]
- 33.Torres Fernandez ED, Huffman AM, Syed M, Romero DG, Yanes Cardozo LL. Effect of glp-1 receptor agonists in the cardiometabolic complications in a rat model of postmenopausal pcos. Endocrin 2019;160:2787–2799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jensterle M, Janez A, Vrtovec B, Meden-Vrtovec H, Pfeifer M, Prezelj J, Kocjan T. Decreased androgen levels and improved menstrual pattern after angiotensin II receptor antagonist telmisartan treatment in four hypertensive patients with polycystic ovary syndrome: Case series. Croat Med J 2007;48:864–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diamanti-Kandarakis E, Spina G, Kouli C, Migdalis I. Increased endothelin-1 levels in women with polycystic ovary syndrome and the beneficial effect of metformin therapy. J Clin Endocrinol Metab 2001;86:4666–4673 [DOI] [PubMed] [Google Scholar]
- 36.Usselman CW, Yarovinsky TO, Steele FE, Leone CA, Taylor HS, Bender JR, Stachenfeld NS. Androgens drive microvascular endothelial dysfunction in women with polycystic ovary syndrome: Role of the endothelin B receptor. J. Physiol 2019;597:2853–2865 [DOI] [PubMed] [Google Scholar]
- 37.Alexander BT, Cockrell KL, Rinewalt AN, Herrington JN, Granger JP. Enhanced renal expression of preproendothelin mrna during chronic angiotensin ii hypertension. Am. J Physiol Regu Integr Comp Physiol 2001;280:R1388–R1392 [DOI] [PubMed] [Google Scholar]
- 38.Reckelhoff JF. Polycystic ovary syndrome, androgens and hypertension. 2007; Hypertension 49:1220–1221 [DOI] [PubMed] [Google Scholar]
- 39.Patil CN, Racusen LC, Reckelhoff JF. Consequences of advanced aging on renal function in chronic hyperandrogenemic female rat model: Implications for aging women with polycystic ovary syndrome. Physiol Rep 2017; 5(20). pii: e13461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roos N, Kieler H, Sahlin L, Ekman-Ordeberg G, Falconer H, Stephansson O. Risk of adverse pregnancy outcomes in women with polycystic ovary syndrome: Population based cohort study. BMJ 2011;343:d6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kjerulff LE, Sanchez-Ramos L, Duffy D. Pregnancy outcomes in women with polycystic ovary syndrome: A metaanalysis. Am. J. Ob. Gyn 2011;204:558.e551–558.e556 [DOI] [PubMed] [Google Scholar]
- 42.Sir-Petermann T, Hitchsfeld C, Maliqueo M, Codner E, Echiburú Br, Gazitúa R, Recabarren S, Cassorla F. Birth weight in offspring of mothers with polycystic ovarian syndrome. Hum Reprod 2005;20:2122–2126 [DOI] [PubMed] [Google Scholar]
- 43.Intapad S, Alexander BT. Pregnancy complications and later development of hypertension. Curr Cardiovasc Risk Rep 2013;7:183–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peracoli JC, Rudge MV, Sartori MS, da Silva Franco RJ. Effects of hypertension on maternal adaptations to pregnancy: Experimental study on spontaneously hypertensive rats. Sao Paulo Med J 2001;119:54–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baylis C. Immediate and long-term effects of pregnancy on glomerular function in the SHR. Am J. Physiol 1989;257:F1140–1145 [DOI] [PubMed] [Google Scholar]
- 46.Wright JT Jr, Roccella EJ; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee: The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure: The JNC 7 Report. JAMA 2003;289 : 2560–2571 [DOI] [PubMed] [Google Scholar]
- 47.Persson S, Elenis E, Turkmen, Kramer MS, Yong EL, Sundström-Poromaa I. Fecundity among women with polycystic ovary syndrome (PCOS)-a population-based study. Hum Reprod 2019;pii: dez159. doi: 10.1093/humrep/dez159 [DOI] [PubMed] [Google Scholar]
- 48.Forslund M, Landin-Wilhelmsen K, Schmidt J, Brannstrom M, Trimpou P, Dahlgren E. Higher menopausal age but no differences in parity in women with polycystic ovary syndrome compared with controls. Acta Obstet Gynecol Scand 2019;98:320–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roos N, Kieler H, Sahlin L, Ekman-Ordeberg G, Falconer H, Steohansson O. Risk of adverse pregnancy outcomes in women with polycystic ovary syndrome: population based cohort study. BMJ 2011;13:343;d6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


