Abstract
Background.
Pulmonary decline in CF is heterogeneous, with socio-environmental factors contributing to this variability. Few studies have attempted to disentangle the effects of tobacco smoke exposure and socioeconomic factors on lung function deterioration in pediatric CF. The current study evaluates their contributions longitudinally across the entire U.S. CF care network population.
Methods.
Data from the CF Foundation Patient Registry were obtained on all individuals who at the end of 2016 were 6–18 years old. Lung function measures (ppFEV1) for each person were calculated at each attained age. Multivariable analyses used mixed modeling to assess the impact of smoke exposure and socioeconomic factors on initial lung function and change over time.
Results.
The sample included 10,895 individuals contributing 65,581 person years. At age 6, ppFEV1 of smoke-exposed children was 4.7% lower than among unexposed. The deficit persisted through age 18. In adjusted mixed models, smoke exposure and socioeconomic factors had independent, additive associations with lung function. Median ppFEV1 declined 2.4% with smoke exposure, 4.9% with lower paternal education, 0.3% with public insurance, and increased 0.2% with each $10,000 annual household income. The effect of smoke exposure on ppFEV1 was larger in disadvantaged children compared to privileged counterparts (3.2% vs 1.2%).
Conclusions.
Smoke exposure and socioeconomic factors are independent risk factors for decreased ppFEV1 in pediatric CF. Smoking cessation strategies should be emphasized at the time of CF diagnosis and reiterated during infancy and early childhood. Interventions may be prioritized in disadvantaged families, where the exposure has a disproportionately large effect.
BACKGROUND
Cystic fibrosis (CF), an autosomal recessive disorder caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) ion channel, is characterized by abnormal secretions in multiple organ systems and eventual respiratory failure. Significant variation in disease progression exists even among individuals with identical CFTR genotypes.1–2 While the impact of gene modifiers continues to be a subject of intense research, 3 non-genetic factors, such as socioeconomic and environmental exposures, also contribute to this variability,4 accounting for approximately 50% of the clinical variation in CF.1
An association between indicators of socioeconomic status (SES) and CF outcomes has been reported previously,5–9 observed as early as in infancy.10 Tobacco smoke exposure (TSE) has also been described as a risk factor for CF lung health.2,9,11 Because TSE is more prevalent among individuals of lower SES, both in the general population12–14 and among CF patients,15,16 it has been proposed as a primary mechanism by which SES affects CF lung health.2,17 Few studies have attempted to disentangle the effects of TSE and SES and define the contributions of these complementary yet distinct exposures on CF lung function trajectory. The most relevant ones came to separate conclusions,2,18 perhaps as a consequence of using different measures of SES and TSE and different populations derived from cohorts assembled for unrelated research (the CF Twin and Sibling Study and the EPIC Observational Study).
The current longitudinal study extends this line of research across the entire U.S. CF care network by using population-level data from the national CF Foundation Patient Registry.19 We evaluate the contributions of TSE and three distinct SES indicators – household income, paternal education, and health insurance type - on lung function decline from 6 to 18 years of age.
METHODS
The study cohort comprised all individuals in the CF Foundation Patient Registry (CFFPR) who were born between 1/1/1998 and 12/31/2010. These individuals were between 6 years old (initial age of reproducible spirometry reported to the Registry) and 18 years old at the end of 2016. The study period was limited to 2006-2016 because the CFFPR did not collect smoke exposure data prior to 2006. The study was approved by the Institutional Review Board of the University of Alabama at Birmingham (protocol 300002076).
Measures
Outcome variable.
Participants’ lung function measures (forced expiratory volume in 1 second, percent predicted [ppFEV1], calculated using the Global Lung Function Initiative [GLI] reference equations20) at each attained age from 6 to 18 years old were obtained from encounter-based data, which include multiple ppFEV1 records per year. For each individual, the median ppFEV1 values at each age were calculated; additionally, lung function peak (highest recorded ppFEV1, representative of optimal lung health) and lung function nadir (lowest recorded ppFEV1, representative of severity of exacerbations) at each age were obtained and used for sensitivity analyses.
Exposure variables.
Self-reported tobacco smoke exposure (Yes/No), measured at each age, was determined based on response to the questions, “Does anyone in the patient’s household smoke cigarettes?” (Yes/No) and “During the reporting year, how often was this patient exposed to second-hand smoke?” (Daily/Several times per week/Several times per month or less/Never). TSE was coded as ‘Yes’ if either the response to the first question was affirmative or the response to the second question was “Daily” or “Several times per week.” TSE was treated as a time-invariant measure: individuals were coded as smoke-exposed if the above was true at any age. Time-varying measures of smoke exposure were explored, but model fit statistics (AIC and BIC) indicated that “ever exposed to smoke” produced a better model fit of lung function than “currently exposed to smoke.” Annual household income (<$10,000 to >$90,000, in $10,000 increments) and type of health insurance (Private/Public/Both private and public/None) were measured at each age. Paternal education (Less than High school/High school/Some college/College degree) was recorded as the highest educational level of the father; if father’s data were not available, mother’s education was used. Father’s education was preferred over mother’s education because of previously reported stronger association with CF lung function.10,21
Covariates.
Covariates included historical confounders identified through a review of the literature and through our prior, single-center analyses. Socio-demographic covariates included age, sex (Male/Female), race/ethnicity (White/Hispanic any race/Other), and household size. Clinical covariates included mean body mass index (BMI) percentile at each age, genotype (F508del homozygous/F508del heterozygous/Other), CF newborn screening diagnosis (Yes/No), number of hospitalizations at each age (None/One/Two or more), number of pulmonary exacerbations at each age (None/One/Two or more), CFTR modulator use (None/Ivacaftor/Lumacaftor-Ivacaftor), P. aeruginosa at each age (Yes/No),22 mucoid P. aeruginosa ever (Yes/No), and B. cepacia complex ever (Yes/No).
Statistical analysis
To gain a thorough understanding of the effects of TSE and socioeconomic factors on lung function from age 6 to age 18, we used hierarchical linear mixed models, or growth curve models. They correct for the interdependency created by using multiple observations from the same person and allow us to estimate the variance associated with each of the components in the level 1 model (intercept, slope, change in slope, and individual error) while estimating the coefficients for the level 2 model.23 The slope, or change over time, is measured as age, centered at 6 years old to allow for interpretable intercept that represents expected lung function at age 6 accounting for the other covariates. The non-linear change in slope, typical for lung function, is measured as the quadratic of age. All analyses were performed separately for median ppFEV1 (primary outcome) and peak and nadir ppFEV1 (secondary outcomes) at each age.
To examine the impact of SES after accounting for the effect of TSE, and vice versa, we used nested models. Specifically, two restricted and one full model were estimated: Model 1 (restricted) with TSE only, not adjusted for SES indicators; Model 2 (restricted) with SES indicators only, not adjusted for TSE; and Model 3 (full) with both TSE and SES indicators, accounting for the impact of both. All three models were adjusted for the same covariates: sex, race/ethnicity, household size, BMI percentile, genotype, newborn screening, hospitalizations, pulmonary exacerbations, CFTR modulator use, P. aeruginosa, and B. cepacia. Additional models assessed whether the impact of TSE was disproportionately severe in low-SES children by interacting TSE with each SES indicator. The association between SES and lung function was also assessed separately in the subgroups of those exposed and unexposed to smoke while applying the same controls as above and including an interaction between TSE and each SES indicator. Finally, we estimated lung function by TSE and SES, comparing low SES (defined as household income ≤$20,000, paternal education <high school, and public health insurance: all three conditions met) to high SES (defined as household income ≥$100,000, paternal education college degree, and private health insurance: all three conditions met) while retaining the significant interactions obtained in previous models. All time-varying variables (income, health insurance, household size, BM, hospitalizations, exacerbations, CFTR modulator use, P. aeruginosa, and B. cepacia) were allowed to vary over time and were not confined to the value when they were first observed.
Missing data were addressed with multiple imputations (n=10 data sets) using Markov Chain Monte Carlo (MCMC) methods.24 Imputations were conducted on the wide version (one observation per individual) of the data. Following best practices, we did not impute values for those missing on the dependent variable.24 Due to high level of missingness on the income variable, several precautions were taken. We examined whether those with income data were different than those without income data (Supplementary Table 1S). Individuals who were not smoke-exposed and had only public or private insurance but not both were more likely to be missing on income. To reduce the likelihood of violation of the missing-at-random assumption, in the imputation phase we included an auxiliary measure of income that is a good approximation of the value of the missing income. Specifically, for each year of the survey data, we calculated zip-code income based on residential zip-codes in the CFFPR and publicly available zip-code income data from the IRS website. Additionally, measures of paternal education and health insurance were included in both the imputation and analysis models. Zip-code income, paternal education, and health insurance accounted for 45% of the variation in participant income in 2006, confirming that these variables perform well as imputation variables. We also conducted sensitivity analyses comparing non-imputed and imputed estimates, and found that the results are substantively the same. Given the high correlation between parental education, health insurance, and income, we examined whether there were multicollinearity issues and did not find any. Analyses were performed with Stata 16 (College Station, TX: StataCorp LLC).
RESULTS
The analytic sample included 10,895 individuals, who contributed a total of 65,581 person-years. Sociodemographic and clinical characteristics of the 2016 population are presented in Table 1. We present 2016 data because it is the most complete data for the sample, which comprises individuals born between 1/1/1998 and 12/31/2010. The sample was approximately half female and F508del homozygous. Mean annual household income was approximately $60,000, half had college-educated fathers, and 42% had only public health insurance. Tobacco smoke exposure (TSE) was reported for 27% of individuals. Among these, 80% never changed TSE status, 10% changed it once, 6% twice, and 4% three or more times. The most prevalent change was from exposure to no exposure.
Table 1.
Characteristics of the sample, 2016 (N=10,895)
| Unimputed | Imputed | % missing | |
|---|---|---|---|
| % or mean (SD) | |||
| SOCIODEMOGRAPHIC | |||
| Age | 12.5 (0.04) | .. | 0 |
| Female | 49.3 | .. | 0 |
| Race/ethnicity | 0 | ||
| White | 80.5 | .. | |
| Hispanic any race | 8.0 | .. | |
| Other | 11.5 | .. | |
| Household size | 4.3 (0.01) | 4.3 (0.01) | 13.8 |
| Health insurance | 3.4 | ||
| Private only | 47.2 | 47.7 | |
| Public only | 42.7 | 42.0 | |
| Both private and public | 9.2 | 9.3 | |
| Other/none | 0.9 | 1.0 | |
| Household income, $10K increments | 6.3 (0.04) | 6.4 (0.04) | 46.9 |
| Parental education | 16.3 | ||
| Less than high school | 5.1 | 4.7 | |
| High school | 26.1 | 25.7 | |
| Some college | 18.3 | 18.2 | |
| College | 50.5 | 51.4 | |
| Smoke exposure | 27.3 | 27.6 | 7.8 |
| CLINICAL | |||
| Lung function | 0 | ||
| Median ppFEV1 | 88.6 (0.19) | .. | |
| Peak ppFEV1 | 95.4 (0.19) | .. | |
| Nadir ppFEV1 | 80.2 (0.22) | .. | |
| BMI, percentile | 52.7 (0.26) | 52.7 (0.28) | 0.4 |
| Genotype | 0.6 | ||
| F508del homozygous | 47.0 | 47.1 | |
| F508del heterozygous | 38.8 | 38.8 | |
| Other | 14.2 | 14.1 | |
| Newborn screening | 29.0 | .. | 0 |
| P. aeruginosa | 27.7 | 27.7 | 3.4 |
| Mucoid | 4.7 | 4.7 | |
| B. cepacia | 2.0 | .. | 0 |
| Hospitalizations, 12 months | 0 | ||
| None | 63.8 | .. | |
| One | 19.9 | .. | |
| Two or more | 16.3 | .. | |
| Exacerbations, 12 months | 0 | ||
| None | 68.8 | .. | |
| One | 17.7 | .. | |
| Two or more | 13.5 | .. | |
| CFTR modulator use | 0 | ||
| None | 71.2 | .. | |
| Ivacaftor | 6.1 | .. | |
| Lumacaftor/Ivacaftor | 22.7 | .. | |
TSE varied by household income and paternal education. In 2016, the risk of smoke exposure was 2.5 times greater in households with annual income <$60,000 than in households with annual income ≥$60,000 (Risk Ratio 2.53 [95% CI 2.33, 2.75], p<0.001). Similarly, children whose fathers were not college-educated had more than 2 times higher risk of smoke exposure than those with college-educated fathers (RR 2.25 [2.08, 2.44], p<0.001).
TSE was associated with diminished lung function (Figure 1). At age 6, the median ppFEV1 of smoke-exposed children was 4.7% lower than in their unexposed counterparts (94.8 [SE 0.24] vs. 90.1 [SE 0.33], p<0.001). By age 18, the disparity in ppFEV1 between smoke-exposed and unexposed children reached 7.4% (81.0 [SE 0.89] vs 73.6 [SE 1.40], p<0.001). Peak and nadir ppFEV1 followed the same pattern (supplementary Figure 1S).
Figure 1.
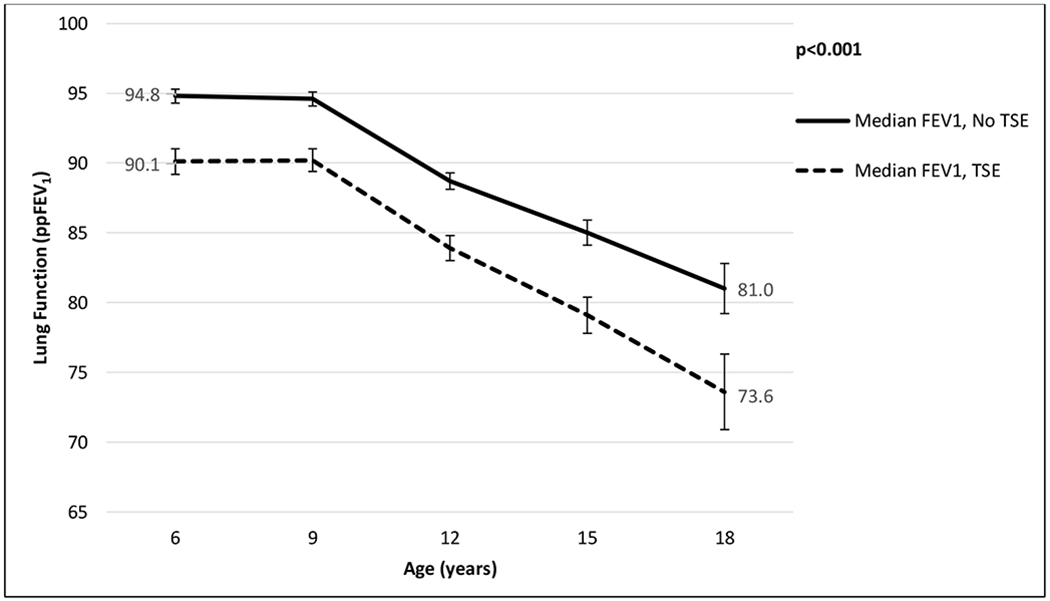
Median lung function for the CF population, age 6 to 18, by tobacco smoke exposure (TSE)
The adjusted trajectories of median ppFEV1 from growth curve models are presented in Figure 2 (adjusted trajectories of peak and nadir ppFEV1 are shown in supplementary Figure 2S). When controlling for SES indicators and all demographic and clinical covariates, at age 6 there remained a 2.4% deficit in median ppFEV1 attributable to smoke exposure.
Figure 2.
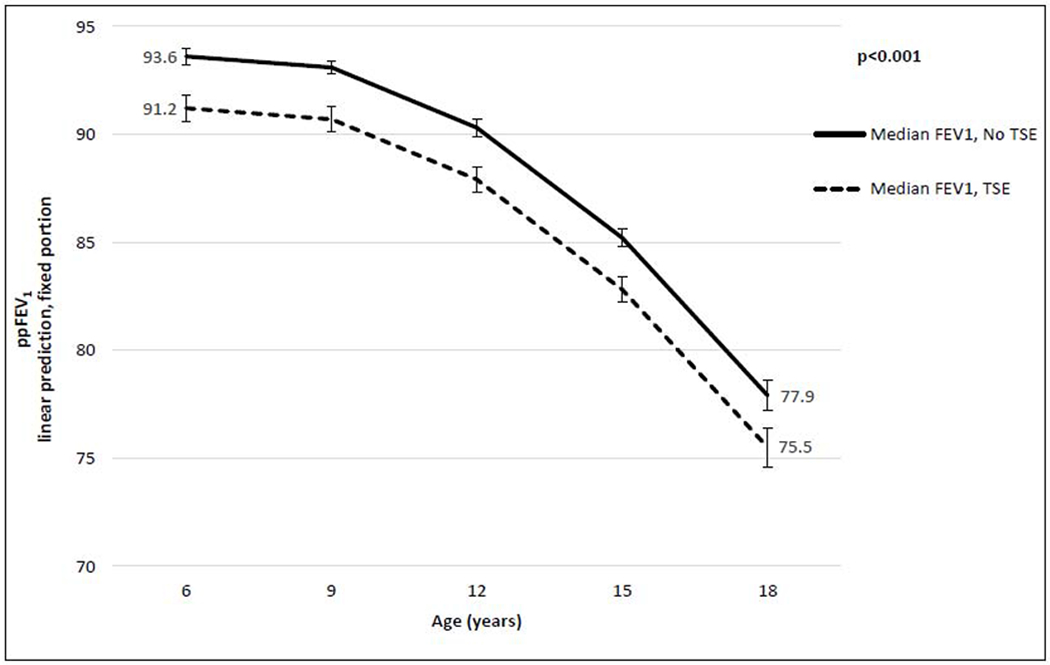
Multivariable growth curve models of median lung function, by tobacco smoke exposure (TSE): predictive margins with 95% confidence intervals*
To further test whether smoke exposure is associated with greater annual rate of ppFEV1 decline from 6 to 18 years of age, we included interactions between TSE and measures of change (age and age squared). These interactions were not significant for either ppFEV1 measure, confirming that the consequence of smoke exposure for lung function is fully manifested by age 6.
Table 2 shows results from nested multivariable growth curve models of median ppFEV1 adjusted for all demographic and clinical covariates. (Peak and nadir ppFEV1 are shown in the supplementary Table 2S.) The regression coefficients are an estimate of the independent or unique effect of each variable on ppFEV1 from 6 to 18 years of age. In the restricted Model 1 (TSE alone, without SES indicators), TSE was associated with nearly 4% decrease in ppFEV1 (β= −3.88 [−4.57, −3.19], p<0.001). In the restricted Model 2 (SES indicators alone, without TSE), each $10,000 income was associated with approximately 0.25% increase in median ppFEV1 (β=0.25 [0.17, 0.34], p<0.001), corresponding to a 1.5% increase for $60,000 income. Lack of paternal college education was negatively associated with median ppFEV1: decrease of 5.5% for less than high-school education, 3.2% for high-school education, and 1.7% for some college. Similarly, public health insurance was associated with 0.4% decrease in median ppFEV1 compared to private insurance. In the full Model 3 (TSE and SES indicators), both TSE and SES remained significant, indicating that they do not explain each other’s effect but contribute independently to lung function. TSE was associated with a 2.4% decrease in median ppFEV1; less-than-high-school, high-school, and some college education with 4.9%, 2.6%, and 1.3% decrease, respectively; and public insurance with 0.3% decrease. The magnitude of smoke exposure’s effect on lung function was comparable to that of newborn screening (2.3%) in the same model.
Table 2.
Multivariable regression: mixed models predicting median ppFEV1 (N=65,581 person years)
| Median ppFEV1 |
||||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||||
| P-value | p-value | p-value | ||||
| β (95% CI) | β (95% CI) | β (95% CI) | ||||
| Intercept (ppFEV1 at age 6) | 95.5 (94.1, 96.2) | <0.001 | 95.7 (95.0, 96.4) | <0.001 | 96.2 (95.5, 96.9) | <0.001 |
| Tobacco smoke exposure | −3.88 (−4.57, −3.19) | <0.001 | - | −2.41 (−3.16, −1.65) | <0.001 | |
| Household income, each $10,000 | - | 0.25 (0.17, 0.34) | <0.001 | 0.22 (0.14, 0.31) | <0.001 | |
| Parental education | ||||||
| Less than high school | - | −5.50 (−7.21, −3.78) | <0.001 | −4.90 (−6.65, −3.17) | <0.001 | |
| High school | - | −3.16 (−4.04, −2.27) | <0.001 | −2.56 (−3.47, −1.66) | <0.001 | |
| Some college | - | −1.71 (−2.57, −0.85) | <0.001 | −1.34 (−2.21, −0.47) | 0.003 | |
| College degree | - | 1.00 (reference category) | 1.00 (reference category) | |||
| Health insurance | ||||||
| Private only | - | 1.00 (reference category) | 1.00 (reference category) | |||
| Public only | - | −0.39 (−0.66, −0.13) | 0.004 | −0.33 (−0.60, −0.07) | 0.014 | |
| Both private and public | - | −0.04 (−0.34, 0.25) | 0.786 | −0.02 (−0.32, 0.27) | 0.885 | |
| None/other | - | −0.48 (−1.16, 0.21) | 0.172 | −0.45 (−1.14, 0.23) | 0.196 | |
| Annual rate of change | 0.22 (0.12, 0.33) | <0.001 | 0.21 (0.10, 0.31) | <0.001 | 0.21 (0.10, 0.32) | <0.001 |
| Annual rate of change, squared | −0.13 (−0.14, −0.12) | <0.001 | −0.13 (−0.14, −0.12) | <0.001 | −0.13 (−0.14, −0.12) | <0.001 |
| Clinical covariates | ||||||
| BMI percentile | 0.14 (0.14, 0.15) | <0.001 | 0.14 (0.14, 0.15) | <0.001 | 0.14 (0.14, 0.15) | <0.001 |
| Newborn screening | 2.14 (1.48, 2.80) | <0.001 | 2.46 (1.80, 3.11) | <0.001 | 2.34 (1.69, 3.00) | <0.001 |
| ≥2 hospitalizations in 12 months | −0.55 (−0.96, −0.15) | 0.009 | −0.55 (−0.95, −0.14) | 0.009 | −0.54 (−0.95, −0.14) | 0.009 |
| ≥2 exacerbations in 12 months | −4.94 (−5.39, −4.48) | <0.001 | −4.94 (−5.39, −4.48) | <0.001 | −4.93 (−5.39, −4.48) | <0.001 |
| Mucoid P. aeruginosa | −0.13 (−0.30, −0.05) | 0.157 | −0.13 (−0.31, 0.04) | 0.143 | −0.13 (−0.31, 0.04) | 0.141 |
| B. cepacia | 0.00 (−0.65, 0.66) | 0.991 | 0.01 (−0.64, 0.67) | 0.969 | 0.01 (−0.64, 0.67) | 0.971 |
All models adjusted for sex, race/ethnicity, household size, genotype, and CFTR modulator use.
Boldface indicates statistical significance: p<0.05, two-tailed tests.
SES accounted for the association between TSE and lung function to a greater degree than TSE accounted for the association between SES and lung function. This is demonstrated by the greater reduction in magnitude of the TSE coefficients (Table 2: Model 1 to Model 3) relative to the SES coefficients (Table 2: Model 2 to Model 3). Specifically, household income accounted for more than 38% of the association between TSE and ppFEV1, while TSE accounted for only 12% of the association between income and ppFEV1.
The independent effect of SES on median ppFEV1 over time was confirmed in multivariable growth curve models that examined the role of socioeconomic factors separately in the subsets of those exposed and unexposed to smoke (Table 3; peak and nadir ppFEV1 in supplementary Table 3S). In both smoke-exposed and unexposed children, lower household income and paternal high-school education were associated with worse ppFEV1. For smoke-exposed children, public and no/other health insurance were associated with worse ppFEV1.
Table 3.
Multivariable regression: mixed models predicting median ppFEV1, by tobacco smoke exposure (N=65,581 person-years)
| Median ppFEV1 |
||||
|---|---|---|---|---|
| Smoke-exposed | Unexposed | |||
| β (95% CI) | p-value | β (95% CI) | p-value | |
| Intercept (ppFEV1 at age 6) | 92.8 (91.1, 94.3) | <0.001 | 96.8 (96.0, 97.6) | <0.001 |
| Household income, each $10,000 | 0.31 (0.17, 0.46) | <0.001 | 0.17 (0.07, 0.27) | <0.001 |
| Paternal education | ||||
| Less than high school | −3.49 (−6.28, −0.71) | 0.014 | −5.71 (−7.88, −3.54) | <0.001 |
| High school | −2.48 (−4.08, −0.88) | 0.003 | −2.40 (−3.45, −1.36) | <0.001 |
| Some college | −0.38 (−2.28, 1.52) | 0.694 | −1.68 (−2.75, −0.61) | 0.003 |
| College degree | 1.00 (reference category) | 1.00 (reference category) | ||
| Health insurance | ||||
| Private only | 1.00 (reference category) | 1.00 (reference category) | ||
| Public only | −0.63 (−1.12, −0.13) | 0.013 | −0.21 (−0.54, 0.11) | 0.202 |
| Both private and public | −0.33 (−0.88, 0.23) | 0.250 | 0.08 (−0.26, 0.42) | 0.641 |
| None/other | −1.38 (−2.71, −0.06) | 0.041 | −0.04 (−0.93, 0.86) | 0.938 |
| Annual rate of change | 0.30 (0.09, 0.52) | 0.005 | 0.17 (0.04, 0.30) | 0.012 |
| Annual rate of change, squared | −0.14 (−0.16, −0.12) | <0.001 | −0.12 (−0.13, −0.11) | <0.001 |
All models adjusted for sex, race/ethnicity, household size, BMI, genotype, newborn screening, hospitalizations, pulmonary exacerbations, modulator use, P. aeruginosa, and B. cepacia.
Boldface indicates statistical significance: p<0.05, two-tailed tests.
Figure 3 shows adjusted peak ppFEV1 trajectories from growth curve models by SES and TSE, controlling for all demographic and clinical covariates. Low SES is defined as household income ≤$20,000, paternal education ≤high school, and public health insurance (all three conditions met). High SES is defined as household income ≥$100,000, paternal education college degree, and private health insurance (all three conditions met). The effect of smoke exposure, both at age 6 and over time through age 18, is larger among children of low SES than among those of high SES (3.2 vs 1.2%; interaction between TSE and income=0.24 [0.06, 0.42], p=0.009). Children of low SES who are not smoke-exposed have lower lung function than children of high SES who are smoke-exposed.
Figure 3. Multivariable growth curve models of peak lung function, by socioeconomic status (SES)* and tobacco smoke exposure (TSE): predictive margins with 95% confidence intervals.
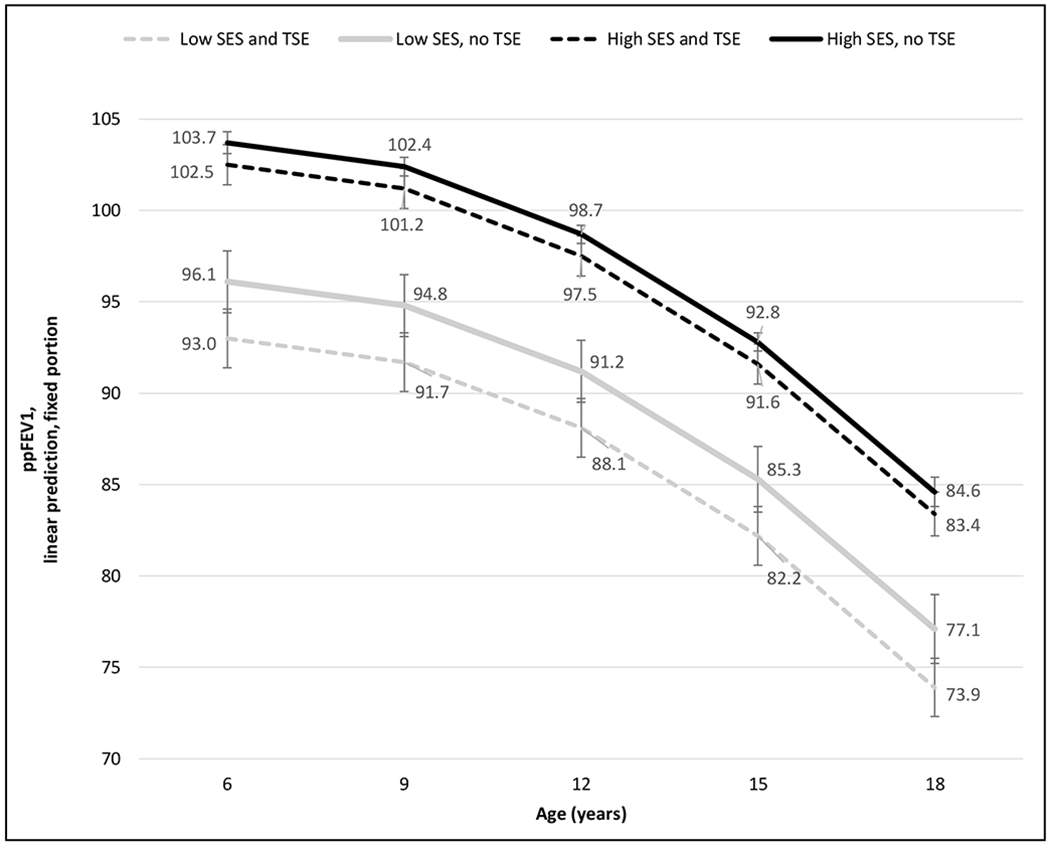
*Low SES: household income ≤$20,000, paternal education ≤high school, and public health insurance.
High SES: household income ≥$100,000, paternal education college degree, and private health insurance.
Adjusted for household income, paternal education, health insurance, household size, sex, race/ethnicity, BMI, newborn screening, genotype, hospitalizations, pulmonary exacerbations, CFTR modulator use, P.aeruginosa, and B.cepacia.
DISCUSSION
We conducted a longitudinal analysis of data from the CF Foundation Patient Registry (2006-2016) to evaluate the contributions of tobacco smoke exposure and socioeconomic factors on lung function impairment in pediatric patients with CF. This is the first study with national CF population-based data that quantifies the impact of smoke exposure and socioeconomic factors on initial spirometry at age 6 and change in ppFEV1 over time through age 18 years. The primary lung function measure was median ppFEV1, but we also assessed changes in peak (highest) and nadir (lowest) ppFEV1, and these led to similar conclusions. The results show significant and sustained impact of tobacco smoke exposure (TSE) on CF lung function. Further, by nesting the growth curve models, we were able to separate the effect of TSE from that of socioeconomic status (SES) measured by annual household income, paternal education, and health insurance. We found that while TSE and SES partially overlap, they make independent, additive contributions to ppFEV1.
Our results demonstrate that the damaging effect of TSE on CF lung function begins early. By age 6, ppFEV1 of smoke-exposed children was significantly lower than that of unexposed children: 4.7% lower in bivariate analysis (p<0.001) and 2.4% lower in adjusted multivariable growth curve models (p<0.001). These data emphasize the adverse consequences of smoke exposure for CF lung health from the onset of the life course.25–27 Therefore, attempts to interrupt smoke-induced lung disease should be introduced early – if not prenatally, then at least at initial diagnosis of CF, before disease trajectory has been established.
The lung function deficit between smoke-exposed and unexposed children at age 6 persisted over time. Additional analyses indicate that the impact of TSE was stable through age 18. In adjusted multivariable growth curve models, TSE was associated with a 2.4% decrement across all ppFEV1 measures. This effect is similar to the 2.6%–3.2% decrease in mean ppFEV1 over 4 years reported by Ong et al.,18 and in line with the predicted mean ppFEV1 at age 20 estimated by Collaco et al.2
These findings indicate that addressing exposure merits prioritization in CF care. The fact that 27% of U.S. pediatric CF patients are smoke-exposed per parental self-report, and 60% of the smoke-exposed are still exposed at age 18, highlights the need for revisiting current standards of care. Moving forward, the question is how to implement successful “smoke eradication” strategies similar to what has been accomplished through P. aemginosa eradication protocols. Our results suggest that smoking cessation and exposure prevention should be emphasized at the time of CF diagnosis and reiterated regularly during infancy and early childhood. Best practices to limit exposure also need to be developed. To date, no interventional research studies of smoking cessation in CF families have been published in the U.S., although the CF Foundation has supported learning collaboratives to facilitate the development and uptake of quality improvement approaches to target tobacco smoke exposure.28 Screening of pediatric CF patients for smoke exposure with objective measures, such as biomarkers of nicotine metabolites, is virtually unexplored.
We measured TSE as a non-time varying variable, so that if a child was ever exposed they were considered always exposed. This was done because ‘ever exposed’ models produced a better model fit than ‘currently exposed’ models. Additionally, TSE in the sample was relatively stable. Only 20% experienced change in TSE status, mostly from exposure to no exposure. Because we modeled TSE as time-invariant, we could not examine whether smoking cessation is associated with improved lung function and whether that effect declines with time or has a diminished return if initiated past a certain age. Future research should investigate the impact of smoking cessation on lung function trajectory, including optimal periods for cessation and whether this relationship varies by SES.
Importantly, our study reveals that SES has an association with lung function that is independent of and additive to TSE. Overall, SES accounted for 38% of the association between TSE and ppFEV1. Similar findings were reported with data from the EPIC observational cohort18 and challenge the assertion that TSE is the primary mechanism through which low SES negatively impacts respiratory health. Moreover, in a dose-response relationship, every additional $10,000 in annual income was associated with a 0.31-0.40% ppFEV1 increase among smoke-exposed children but with just a 0.17-0.21% ppFEV1 increase among unexposed children. We found that the adverse effect of TSE was amplified among children of low SES compared to their high-SES counterparts (3.2 vs 1.2%). These data indicate that TSE has a greater negative impact on patients with less financial resources, and that smoking cessation should be a major clinical priority in CF families from disadvantaged backgrounds.
By utilizing the population-based data available in the U.S. CF Foundation Patient Registry, our study provides a delineation of the independent, additive contributions of TSE and indicators of SES – household income, paternal education, and health insurance - to pulmonary decline in CF. The consequence of smoke exposure is manifested by the time of first spirometry at age 6. Its damage is buffered by access to financial (income), human (education), and healthcare (insurance) resources. Its detrimental impact is magnified in socioeconomically disadvantaged children with CF. This evidence necessitates targeting of smoking cessation and exposure prevention strategies to this vulnerable population.
The major limitation of this study is the self-reported nature of the smoke exposure data. For example, in our sample, approximately 15% of those who responded affirmatively to the question, “Does anyone in the patient’s household smoke cigarettes?”, also responded that their child is “Never” exposed to smoke. The difficulty in measuring the exposure led to treating TSE as a non-time varying variable (‘ever exposed’ rather than ‘currently exposed’), as this approach produced a better model fit. This may partially account for the fact that TSE did not impact the slope of lung function change from early childhood to late adolescence. Clearly, biomarkers of smoke exposure would be preferred and should be implemented to validate our conclusions. It should be noted that we only addressed the effect of second-hand smoke exposure, rather than active smoking. However, the proportion of smokers in the sample was extremely low: among individuals age ≥15, less than 0.5% reported smoking, of them n=24 (0.2%) regularly and n=36 (0.3%) occasionally.
We also acknowledge the self-reported nature and large proportion of missing income data. This limitation was addressed with multiple imputations and by including additional indicators of SES in the analyses. Furthermore, the imputed and non-imputed models produced substantively similar results. Our population-based sample and statistical modeling approach allowed us to break new ground in separating the independent contributions of TSE and SES for lung function decline in pediatric CF.
CONCLUSION
Tobacco smoke exposure and socioeconomic factors are independent risk factors of pulmonary decline in pediatric CF. Smoke exposure is a major contributor to diminished lung function at age 6, with sustained negative impact through age 18. Smoking cessation and exposure prevention should therefore be a therapeutic priority in pediatric CF care, introduced from the time of diagnosis. Smoke exposure is disproportionately prevalent in families with lower income and education, but it does not account for the effect of socioeconomic status: low income, low education, and public health insurance have considerable additive effect on lung function independent of smoke exposure. To address disparities in CF lung health, screening programs and cessation interventions should prioritize smoke-exposed low-income children, among whom the adverse effects of tobacco smoke exposure are disproportionately large.
Supplementary Material
Figure 1S. Peak and nadir lung function for the CF population, age 6 to 18, by tobacco smoke exposure (TSE)
Figure 2S. Multivariable growth curve models of peak and nadir lung function, by tobacco smoke exposure (TSE): predictive margins with 95% confidence intervals*
Highlights.
By age 6, smoke-exposed children with CF in the U.S. have lower FEV1% than unexposed children, and the deficit persists through age 18.
The effect of smoke exposure on lung function is amplified in disadvantaged children compared to privileged counterparts.
Tobacco smoke exposure and socioeconomic factors are independent risk factors for decreased ppFEV1 in pediatric CF.
To address disparities in CF lung health, interventions should prioritize smoke-exposed low-income children, among whom the adverse effects of tobacco smoke exposure are disproportionately large.
Acknowledgments:
The authors would like to thank the Cystic Fibrosis Foundation for the use of CF Foundation Patient Registry data to conduct this study. Additionally, we thank the patients, care providers, and clinic coordinators at CF centers throughout the United States for their contributions to the CF Foundation Patient Registry.
Funding: This study was supported by grants from the National Institutes of Health (P30DK72482, K08HL140190), the Agency for Healthcare Research and Quality (K12HS023009), and the CF Foundation (CC032-14).
Abbreviations:
- TSE
tobacco smoke exposure
- SES
socioeconomic status
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: The authors have no conflicts of interest.
References
- 1.Collaco JM, Blackman SM, McGready J, Naughton KM, Cutting GR. Quantification of the Relative Contribution of Environmental and Genetic Factors to Variation in Cystic Fibrosis Lung Function. J Pediatr. 2010;157(5):802–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collaco JM, Vanscoy L, Bremer L, et al. Interactions between Secondhand Smoke and Genes That Affect Cystic Fibrosis Lung Disease. JAMA. 2008;299(4):417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corvol H, Blackman SM, Boelle PY, et al. Genome-Wide Association Meta-Analysis Identifies Five Modifier Loci of Lung Disease Severity in Cystic Fibrosis. Nat Commun. 2015;6:8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolfenden LL, Schechter MS. Genetic and Non-Genetic Determinants of Outcomes in Cystic Fibrosis. Paediatr Respir Rev. 2009;10(1):32–36. [DOI] [PubMed] [Google Scholar]
- 5.Barr HL, Britton J, Smyth AR, Fogarty AW. Association between Socioeconomic Status, Sex, and Age at Death from Cystic Fibrosis in England and Wales (1959 to 2008): Cross-Sectional Study. Brit Med J. 2011;343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor-Robinson DC, Smyth RL, Diggle PJ, Whitehead M. The Effect of Social Deprivation on Clinical Outcomes and the Use of Treatments in the UK Cystic Fibrosis Population: A Longitudinal Study. The Lancet Respiratory Medicine. 2013;1(2):121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schechter MS, Shelton BJ, Margolis PA, FitzSimmons SC. The Association of Socioeconomic Status with Outcomes in Cystic Fibrosis Patients in the United States. Am J Respir Crit Care Med. 2001;163(6):1331–1337. [DOI] [PubMed] [Google Scholar]
- 8.Kovell LC, Wang J, Ishman SL, Zeitlin PL, Boss EF. Cystic Fibrosis and Sinusitis in Children: Outcomes and Socioeconomic Status. Otolaryngol Head Neck Surg. 2011;145(1):146–153. [DOI] [PubMed] [Google Scholar]
- 9.Sanders DB, Emerson J, Ren CL, et al. Early Childhood Risk Factors for Decreased FEV1 at Age Six to Seven Years in Young Children with Cystic Fibrosis. Ann Am Thorac Soc. 2015;12(8):1170–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Britton LJ, Oates GR, Oster RA, et al. Risk Stratification Model to Detect Early Pulmonary Disease in Infants with Cystic Fibrosis Diagnosed by Newborn Screening. Pediatr Pulmonol. 2016;51(11):1168–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell PW 3rd, Parker RA, Roberts BT, Krishnamani MR, Phillips JA 3rd. Association of Poor Clinical Status and Heavy Exposure to Tobacco Smoke in Patients with Cystic Fibrosis Who Are Homozygous for the F508 Deletion. J Pediatr. 1992;120(2 Pt 1):261–264. [DOI] [PubMed] [Google Scholar]
- 12.Benowitz NL, Nardone N, Jain S, et al. Comparison of Urine 4-(Methylnitrosamino)-1-(3)Pyridyl-1-Butanol and Cotinine for Assessment of Active and Passive Smoke Exposure in Urban Adolescents. Cancer Epidemiol Biomarkers Prev. 2018;27(3):254–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thun M, Peto R, Boreham J, Lopez AD. Stages of the Cigarette Epidemic on Entering Its Second Century. Tob Control. 2012;21(2):96–101. [DOI] [PubMed] [Google Scholar]
- 14.Kim JE, Tsoh JY. Cigarette Smoking among Socioeconomically Disadvantaged Young Adults in Association with Food Insecurity and Other Factors. Prev Chronic Dis. 2016;13:E08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schechter MS. Non-Genetic Influences on Cystic Fibrosis Lung Disease: The Role of Sociodemographic Characteristics, Environmental Exposures, and Healthcare Interventions. Sem Respir Crit Care Med. 2003;24(6):639–652. [DOI] [PubMed] [Google Scholar]
- 16.Schechter M, Emerson J, Rosenfeld M. The Relationship of Socioeconomic Status and Environmental Tobacco Smoke Exposure with Disease Outcomes in the Epic Observational Cohort. Pediatr Pulmonol. 2012;47(S35). [Google Scholar]
- 17.Schechter MS. Nongenetic Influences on Cystic Fibrosis Outcomes. Curr Opin Pulm Med. 2011;17(6):448–454. [DOI] [PubMed] [Google Scholar]
- 18.Ong T, Schechter M, Yang J, et al. Socioeconomic Status, Smoke Exposure, and Health Outcomes in Young Children with Cystic Fibrosis. Pediatrics. 2017;139(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knapp EA, Fink AK, Goss CH, et al. The Cystic Fibrosis Foundation Patient Registry. Design and Methods of a National Observational Disease Registry. Ann Am Thorac Soc. 2016;13(7):1173–1179. [DOI] [PubMed] [Google Scholar]
- 20.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-Ethnic Reference Values for Spirometry for the 3-95-Yr Age Range: The Global Lung Function 2012 Equations. Eur Respir J. 2012;40(6):1324–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oates GR, Stepanikova I, Gamble S, Gutierrez HH, Harris WT. Adherence to Airway Clearance Therapy in Pediatric Cystic Fibrosis: Socioeconomic Factors and Respiratory Outcomes. Pediatr Pulmonol. 2015;50(12):1244–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee TW, Brownlee KG, Conway SP, Denton M, Littlewood JM. Evaluation of a New Definition for Chronic Pseudomonas Aeruginosa Infection in Cystic Fibrosis Patients. J Cyst Fibros. 2003;2(1):29–34. [DOI] [PubMed] [Google Scholar]
- 23.Hedeker D, Gibbons RD. Longitudinal Data Analysis. Hoboken, NJ: Wiley-Interscience; 2006. [Google Scholar]
- 24.Allison P Missing Data: Quantitative Applications in the Social Sciences. Vol 136 Thousand Oaks, CA: Sage; 2002. [Google Scholar]
- 25.Kopp BT, Sarzynski L, Khalfoun S, et al. Detrimental Effects of Secondhand Smoke Exposure on Infants with Cystic Fibrosis. Pediatr Pulmonol. 2015;50(1):25–34. [DOI] [PubMed] [Google Scholar]
- 26.Lewis BW, Sultana R, Sharma R, et al. Early Postnatal Secondhand Smoke Exposure Disrupts Bacterial Clearance and Abolishes Immune Responses in Muco-Obstructive Lung Disease. J Immunol. 2017;199(3):1170–1183. [DOI] [PubMed] [Google Scholar]
- 27.Gibbs K, Collaco JM, McGrath-Morrow SA. Impact of Tobacco Smoke and Nicotine Exposure on Lung Development. Chest. 2016;149(2):552–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schechter MS, Margolis P. Improving Subspecialty Healthcare: Lessons from Cystic Fibrosis. J Pediatr. 2005;147(3):295–301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1S. Peak and nadir lung function for the CF population, age 6 to 18, by tobacco smoke exposure (TSE)
Figure 2S. Multivariable growth curve models of peak and nadir lung function, by tobacco smoke exposure (TSE): predictive margins with 95% confidence intervals*


