Abstract
Focused ultrasound (FUS) combined with microbubbles is a non-invasive method for targeted, reversible disruption of the blood-brain barrier (FUS-BBB opening). This approach holds great promise for improving delivery of therapeutics to the brain. In order to achieve this clinically important goal, the approach necessarily breaks a protective barrier, temporarily, which plays a fundamental role in maintaining a homeostatic environment in the brain. Preclinical and clinical research has identified a set of treatment parameters under which this can be performed safely, whereby the BBB is disrupted to the point of being permeable to normally non-penetrant agents without causing significant acute damage to endothelial or neuronal cells. Much of the early work in this field focused on engineering questions around how to achieve optimal delivery of therapeutics via BBB disruption. However, there is increasing interest in addressing biological questions related to whether and how various aspects of neurophysiology might be affected when this fundamental protective barrier is compromised by the specific mechanisms of FUS-BBB opening. Improving our understanding of these secondary effects is becoming vital now that FUS-BBB opening treatments have entered clinical trials. Such information would help to safely expand FUS-BBB opening protocols into a wider range of drug delivery applications and may even lead to new types of treatments. In this paper, we will critically review our current knowledge of the secondary effects caused by FUS-BBB opening on brain physiology, identify areas that remain understudied, and discuss how a better understanding of these processes can be used to safely advance FUS-BBB opening into a wider range of clinical applications.
Keywords: Focused ultrasound, blood-brain barrier, inflammation, Alzheimer’s disease, neurovascular response, cerebral blood flow, cerebral spinal fluid flow
Introduction
Almost twenty years ago, it was demonstrated that focused ultrasound (FUS) combined with systemically circulating microbubbles could be used to disrupt the blood-brain barrier (BBB) in a non-invasive and transient fashion (1). It was immediately recognized that this technology could have profound implications for drug delivery to the brain, indeed that was the impetus for the original study. The BBB prevents many small molecules, and essentially all large molecules, from crossing from the blood stream into the brain. Existing methods for delivering therapeutics to the brain, such as osmotic/chemical disruption of the BBB, intrathecal injection or direct neurosurgical injection, all have limitations in terms of invasiveness, poor spatial distribution or low efficacy (2). FUS-BBB opening, while presenting a different set of recognized challenges, offers the promise of a safe, non-invasive and targeted approach.
As with any new medical technology, most early research on FUS-BBB opening focused on optimizing the procedure for efficacy and demonstrating safety. Preclinical studies investigated how BBB opening varied as a function of the FUS parameters that were applied (3–5), how long the BBB remained open after the procedure (6–8), what size of molecule could be delivered (9), and strategies to control the dose of drug to be delivered (10). Safety studies were performed to investigate potential short-term histologic evidence of post-treatment damage and adverse effects on behavioral readouts (11–13). As preclinical evidence accumulated indicating that the method was both effective and safe, researchers deployed FUS-BBB opening for drug delivery to treat a wide range of brain diseases (14–16). The success of these preclinical studies culminated in the first clinical trials in 2018 applying FUS-BBB opening for the treatment of brain tumors, Alzheimer’s disease, and amyotrophic lateral sclerosis (NCT03739905, NCT03119961, NCT03671889).
While the above-mentioned safety and efficacy research did not reveal any immediate concerns, it was nevertheless understood that FUS-BBB opening was altering a fundamental protective barrier in the body and could therefore lead to significant, and potentially harmful, short and long-term secondary effects. In fact, breakdown of the BBB is known to occur in several neurodegenerative diseases, and has been implicated in contributing to a negative feedback loop that exacerbates disease progression (17). Accordingly, the first applications of FUS-BBB opening targeted terminal brain disorders with no alternative cure. As long as the procedure could be implemented under a particular set of treatment parameters that cleared a bar of minimum safety, the risk-benefit analysis was seen to be in favor of the procedure. Some early studies investigated the primary mechanisms of BBB permeability (18–20), but for the most part questions related to biological effects on brain physiology were not pursued.
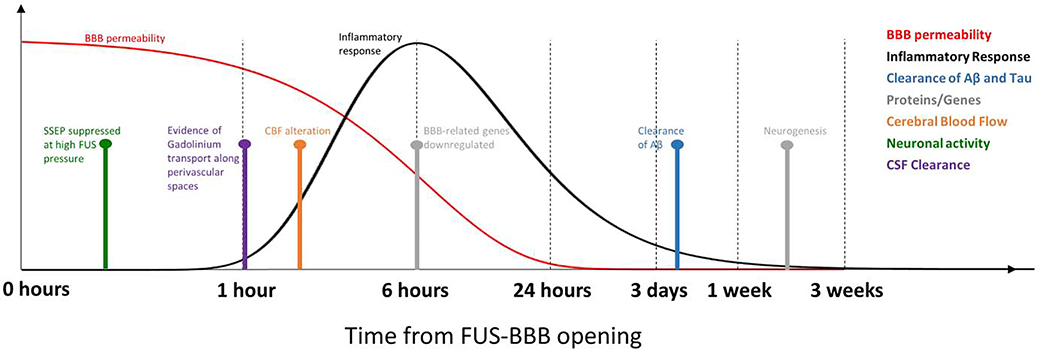
The past several years has seen a growing interest in research related to the secondary effects of FUSBBB opening. Specifically, after the BBB is permeated in this abrupt manner, what are the casual effects to brain physiology? The major secondary effects observed thus far include a generalized inflammatory response, reduction of amyloid β plaques and hyperphosphorylated tau proteins, changes in brain transcriptome and proteome profiles, alteration of cerebral blood flow, possible suppression of neuronal activity, and possible effects on clearance of metabolic waste products into the cerebral spinal fluid (CSF) (Figure 1). In most cases, the mechanisms underlying these effects and their dependence on FUS parameters remain unclear.
Figure 1.
Timing of secondary effects relative to FUS-BBB opening. Time courses for the extent of BBB permeability and the inflammatory response are based on observations in the literature from several different time points. Other effects are shown as single point observations taken from the literature. The x-axis is displayed using a log10 scale of time.
A better understanding of these effects is necessary to safely expand clinical indications of FUS-BBB opening. Expansion to new applications often requires more aggressive treatments parameters approaching a regime where the long-term consequences are unknown. Such indications may necessitate more aggressive BBB opening for delivery of larger therapeutics, BBB opening over a greater volume to treat diseases that affect the entire brain, BBB opening in disease states with an underlying neuroinflammatory component, delivery of therapies that are known to independently cause an inflammatory response themselves, and repeated BBB openings for treatments that require multiple dosing.
In this review, we begin with a brief overview of the known mechanisms by which FUS combined with systemically circulating microbubbles leads to permeabilization of the BBB. We then summarize the current state of knowledge about the different secondary effects on brain physiology that FUS-BBB opening induces. Next we consider CSF clearing, astrocytes and endothelial cells, three areas that are likely affected by FUS-BBB opening but are currently understudied in this regard. We conclude with an outlook for future studies needed to facilitate the wider use of FUS-BBB opening for drug delivery and possibly lead to new insights as to how physiological perturbations induced by this technology may be harnessed for new types of treatments.
1. The primary effect: FUS-induced disruption of the BBB
There are many excellent reviews on FUS-BBB opening that cover BBB breakdown mechanisms, FUS parameter optimization, safety studies, delivery of therapeutic agents and applications to treating neurological diseases. For example, see Aryal et al. (14), Burgess et al. (21), Timbie et al. (16), Meairs et al. (15) and Pandit et al. (22). Here we will only briefly review the key mechanisms implicated in BBB disruption necessary for understanding the secondary phenomena that are the focus of this review.
The BBB plays both a protective and a regulatory role. It is a highly selective physical barrier that prevents toxins from entering the brain from the bloodstream. Endothelial cells comprising blood vessel walls are connected by tight junction proteins that limit the diffusion of molecules along paracellular pathways. These tight junction complexes include claudins, occludins, zonula occludens (ZO) proteins and junctional adhesion molecules (JAMs) (22). In practice, the BBB is impermeable to many small molecules with a molecular weight under ~400 Da, depending on their charge and lipid-solubility, and essentially to all molecules whose molecular weight is greater than ~500 Da (23). In its regulatory capacity, the BBB acts to maintain ionic balance, selectively transport molecules to and from the central nervous system and support local immune surveillance (22). Molecular transport is achieved by both passive and active mechanisms, using active efflux as well as carrier-mediated and receptor-mediated transcytosis.
FUS-BBB opening disrupts this delicate regulatory system. The endothelial cells that primarily make up the BBB interact with pericytes, smooth muscle cells, astrocytes, neurons and microglia (Figure 2). Together with extracellular matrix components, these cells form the neurovascular unit (NVU). Multifactorial and reciprocal interactions between these cell types are needed for efficient regulation of systems that are key to brain homeostasis, such as cerebral blood flow. Disruptions to NVU functioning can affect cells locally and also have widespread consequences on the vasculature, neuronal networks and the CSF system.
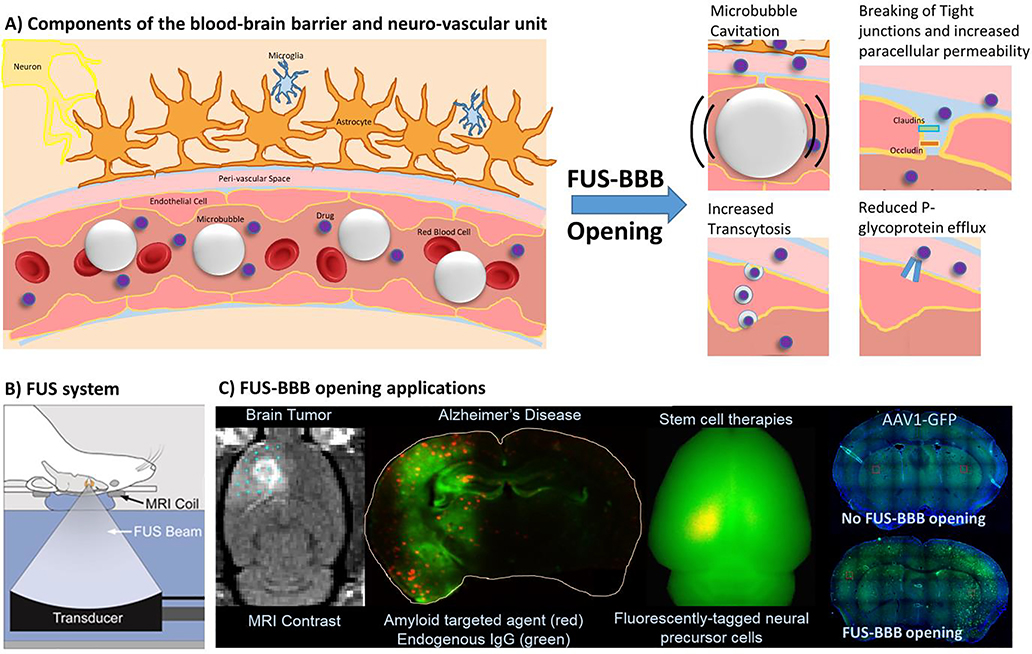
Figure 2.
Overview of FUS-BBB opening. A) Schematic drawing of the main components that form the blood-brain barrier and the neurovascular unit. Focused ultrasound applied in the presence of circulating microbubbles leads to several effects that increase the permeability of the BBB, including breaking of tight junctions between endothelial cells, increased transcytosis and a reduction in efflux mechanisms. B) A typical FUS system for preclinical studies performed on rodents. C) Several examples of FUSBBB opening applications for delivery of therapeutics to the brain. A version of the second image from the right in panel C) appeared in (27).
FUS-BBB opening is achieved through the combination of an ultrasound pressure field and gas-filled microbubbles circulating in the vasculature. The microbubbles are ~1 – 4 micrometers in diameter, typically have a lipid or albumin shell and are filled with a gas such as air, sulfur hexafluoride or perfluorocarbons. Three of the most common formulations are commercially available microbubbles that are FDA-approved as ultrasound contrast agents (Optison (GE Healthcare), SonoVue (branded as Lumason in the United States, Bracco) and Definity (Lantheus Medical Imaging)). When the microbubbles enter the focal zone of the ultrasound pressure field, they undergo a rapidly oscillating expansion and contraction in size known as cavitation and experience a radiation force in the direction of FUS propagation. These mechanical forces affect endothelial cells in several ways that have been observed in pre-clinical studies (Figure 2). This includes the immediate disruption of tight junctions as observed histologically by electron microscopy (18), a reduction in claudin 5, occludin and ZO-1 expression levels observed at the interendothelial cell clefts 1 hour post treatment (20), a decrease in drug efflux mechanisms (24–26), increased permeability of the cell plasma membrane and an increase in the number of transcytotic vesicles (19).
The relative contribution of these different factors to the disruption of the BBB is unknown. What is known is that immediately after FUS sonication the BBB becomes permeable to substances that normally do not cross this barrier. This includes contrast agents such as gadolinium (~500 Da), larger dyes such as Trypan blue (~67 kDa when bound to albumin), delivery vehicles such as gold nanoparticles (~ 20 nm), liposomes (~80 nm) or adeno-associated viral vectors (~25 nm), and a wide variety of therapeutics such as the chemotherapeutic agent Temozolomide (194 Da), brain-derived neurotrophic factor (~30 kDa), antibodies (~150 kDa) and even neural progenitor cells (~5–10 μm) (14). The BBB then gradually recloses with an exponential decay kinetics of several hours, and is typically fully closed by 24 hours post-sonication (6–8).
2. Secondary effects of FUS-BBB opening
2.1. Initiation of an inflammatory response
One of the principal secondary effects of FUS-BBB opening is inflammation. Recent studies in rats have observed hallmarks of an inflammatory response at the site of BBB disruption (28–31). Four of these studies observed signs of microglia activation by immunofluorescent staining, as evidenced by increased signal for the ionized calcium-binding adapter molecule 1 (Iba1) (28, 30–32). Microglia activation was observed at 1 hour, 6 hours and 24 hours post-BBB disruption, with some studies reporting persistent activation up to weeks after (28, 31). Three out of these four studies additionally observed evidence of astrocytic reactivity, as indicated by increased expression of glial fibrillary acidic protein (GFAP) (28, 31, 32). Astrocytic reactivity in these studies was paralleled by a strong inflammatory response.
Kovacs et al. proposed that mechanical forces induced by the FUS-BBB opening provoked the release of biomolecules known as damage-associated molecular patterns (DAMPs) from injured cells, which initiated and perpetuated a noninfectious inflammatory response commensurate with what is seen in other types of brain injury such as trauma or stroke (32–34). DAMP-initiated inflammatory response is evidenced by activation of NFκB and other pro-inflammatory pathways with subsequent upregulation of heat-shock protein 70, cytokines IL1α, IL1β, IL18, TNFα and IFNγ, as well as various other proinflammatory, anti-inflammatory, and trophic factors (32). Increases in the levels of inflammatory markers followed a kinetic similar to that of microglia activation, beginning less than 1 hour after FUSBBB opening and resolving 24 hours later. The intensity of the inflammatory response seems to correlate with the FUS microbubble dose. A study by McMahon et al. comparing different sonication schemes demonstrates that FUS-mediated BBB disruption can be achieved with a reduced inflammatory response when a lower dose of microbubbles combined with acoustic feedback control of the FUS pressure level is used (35). In addition to the microbubble dose level, about which there is some controversy (32, 36, 37), the volume of brain tissue over which the BBB has been opened is a possible factor that could explain the different levels of inflammatory response seen in the Kovacs (nine sonication targets) vs MacMahon (one sonication target) studies. However, no studies have been performed to explicitly test how the volume of BBB opening affects the inflammatory response. With respect to repetitive FUS-BBB opening, evidence of microhemorrhage scaling with the number of repetitions was found with MRI, although no significant increase in inflammation was seen using a PET marker [18F]-DPA714 (28, 31).
In a separate study McMahon et al. go into further details of the inflammatory response by gauging transcriptional changes in the microvasculature during the acute phase after FUS-BBB opening (38). Their data show an upregulation of a number of pro-inflammatory genes at 6 hours post-BBB opening, most of which return to their baseline levels by 24 hours. These include the astrocyte activation marker GFAP. The authors also report the downregulation of BBB transporter genes 6 hours post FUS-BBB opening, which also return to baseline levels at the 24-hour mark. Finally, the study reports the upregulation of genes associated with angiogenesis at both the 6-hour and 24-hour time points (38).
Acute inflammation can lead to a wide array of effects in the brain, some of which are protective. There are several reports of FUS-BBB opening appearing to stimulate neurogenesis. The hypothesis being that such an effect is downstream of the acute inflammation processes that have been observed. The earliest paper looked at the effect of FUS-BBB opening on cell survival signaling molecules and found increased activation of Akt signaling, an intracellular pathway involved in cell survival, growth and proliferation (39). Two further studies specifically observed that FUS-BBB opening by itself increased the number of proliferating cells and new neurons in the area of the mouse hippocampus targeted for BBB opening (40, 41). Importantly, neurogenesis was only observed under conditions where the FUS and microbubble parameters used successfully disrupted the BBB (40). Recently, Shin et al. documented the presence of hippocampal neurogenesis in a cholinergic degeneration model of dementia in the adult rat (42). These authors observed an increase in neurogenesis and higher expression levels of brainderived neurotrophic factor and of early growth response protein 1, which associated with improved behavioral performance in the Morris water maze test (42).
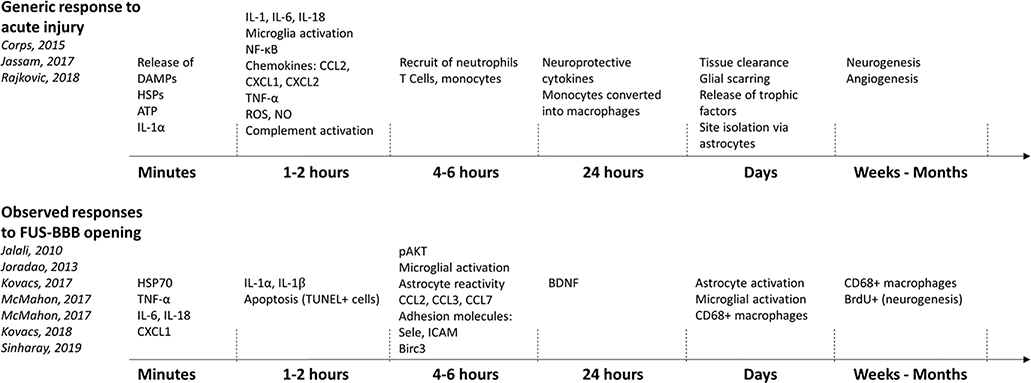
Figure 3 presents a time line of the inflammatory markers typically present after a mild traumatic brain injury (43–45) along with those observed in at least one of the different FUS-BBB opening studies. Note that some studies specifically did not see the presence of a particular inflammatory component cited in this figure, reflecting the dependence of the inflammatory response on the FUS parameters that were used.
Figure 3.
Time courses of inflammatory response for a typical acute brain injury (top) and what has been observed following FUS-BBB opening (bottom). Inflammatory components following FUS-BBB opening were included in the figure if they were observed in at least one study.
2.2. Clearance of the amyloid-β and Tau proteins
Persistent neuroinflammation can be harmful to the brain, and indeed is a hallmark of many neurodegenerative diseases (46). However, acute inflammatory and immune responses usually serve a positive protective purpose. Some of the most exciting recent work in the field of FUS-BBB opening is aimed at initiating an inflammatory response as a way to harness the body’s own immune system into fighting the underlying pathologies associated with neurodegenerative diseases (47, 48).
Extracellular amyloid-β (Aβ) plaques and hyperphosphorylated tau proteins that form intracellular neurofibrillary tangles are the two main histopathological hallmarks of Alzheimer’s disease. Preclinical studies successfully used FUS-BBB opening to deliver exogenous antibodies that would target and reduce Aβ plaques (27, 49). Interestingly, follow up experiments led to the unexpected finding that FUS-BBB opening alone (i.e., without delivery of exogenous antibodies) resulted in a reduction in Aβ plaques. Based on those findings and earlier studies showing that circulating immunoglobulin G (IgG) antibodies can enter the parenchyma after FUS-BBB opening (18, 27), it was hypothesized that FUSBBB opening could allow naturally occurring antibodies present in the blood to enter the brain, bind to Aβ plaques and facilitate their disaggregation (48).
Several following studies have now confirmed this hypothesis, with substantial evidence showing that FUS-BBB opening alone leads to reduction in Aβ plaque levels in targeted brain regions of Alzheimer’s disease mouse models (30, 48, 50). In these studies, endogenous antibodies IgG and immunoglobulin M (IgM) were both found colocalized with Aβ plaques (48), Aβ plaque size and surface were reduced in the targeted hemisphere (48), and the mean Aβ plaque number was reduced relative to the contralateral untreated hemisphere (50). In addition, a more aggressive “scanning ultrasound” approach that opens the BBB over the whole brain was also found to be effective in reducing the number of Aβ plaques compared to sham treated animals (30, 51).
The reduction in Aβ plaque burden appears to be driven by increased microglial and astrocytic phagocytosis, as indicated by postmortem analysis showing colocalization of Aβ plaques and activated microglia and astrocytes, and increased Aβ expression levels within glia (30, 48). Importantly, in addition to plaque reduction, several studies have also shown a behavioral effect in which the Alzheimer’s disease model mice treated with FUS-BBB opening exhibited significant improvement in memory-specific tasks (30, 42, 47). As a step towards translation, the approach was shown to be well tolerated in larger animals models (52, 53). Motivated by this preclinical work, a human clinical trial was carried out on five patients with early to moderate Alzheimer’s disease (54). All patients received one session of FUS-BBB opening in a 3×3 grid targeted to the dorsolateral prefrontal cortex, and four of the patients received a second treatment one month later. Although no difference between pre- and posttreatment amyloid levels was seen on [18F]-florbetaben PET scanning, no adverse events or radiologic signs of tissue damage were reported (54). There are currently three other on-going trials in the United States and Canada to use FUS-BBB opening alone to reduce Aβ plaque burden in Alzheimer’s disease patients (NCT03739905, NCT03119961, NCT03671889).
Recent research has focused on using a similar approach for the reduction of tau pathology. The initial results are promising, showing that both delivery of tau-targeting exogenous antibodies using FUS-BBB opening (55, 56) and FUS-BBB opening alone (57, 58) are effective in lowering the level of hyperphosphorylated tau proteins in mouse models of Alzheimer’s disease. Similar to the Aβ studies, this research has shown that microglia activation is a likely mechanism of action (58), and that reduction in tau levels leads to behavioral improvements (57).
2.3. Alteration of cerebral blood flow
There are several lines of evidence that FUS-BBB opening affects normal functioning of cerebral blood flow. Blood flows through the cerebral vascular network at a rate that is modulated by neurovascular coupling, a set of mechanisms by which the brain recruits additional blood flow to active areas. In 2008 Raymond et al. used in vivo optical imaging to directly observe that FUS-BBB opening induced vasospasm in mouse brain arteries and arterioles (59). In 14 out of the 16 mice rapid constriction of the vessel (an average of 60% reduction in diameter) was followed by recovery back to baseline within ~20 to 600 seconds. A separate study in rats observed the same effect of vessel constriction, but only in 25% of measured vessels and with a smaller constriction ranging from ~20% for 80 µm diameter vessels up to ~50% for 10 μm diameter vessels (60). We expect such arterial constriction to transiently reduce blood flow, although this was not directly measured in either study.
Another set of studies suggests that FUS-BBB opening disrupts neurovascular coupling and thereby reduces the brain’s ability to recruit additional blood flow to active regions. Chu et al. used electrophysiological recordings and functional MRI (fMRI) to study brain responses to forepaw stimulation following FUS-BBB opening (61). They saw a significant reduction in the evoked potential amplitude and latency but only when using high pressure FUS sonications (0.8 Mechanical Index, a parameter that accounts for both FUS pressure and frequency and has been shown to correlate with magnitude of BBB disruption). The fMRI blood oxygen level dependent (BOLD) signal was reduced at 1 hour post-BBB opening for both the high pressure group (0.8 Mechanical Index) and a moderate pressure group (0.55 Mechanical Index). The BOLD signal includes contributions from both cerebral blood flow (CBF) and the cerebral metabolic rate of oxygen (CMRO2), and therefore cannot isolate CBF changes alone. For the moderate pressure group, the BOLD signal returned to baseline at the 2- and 7-day follow ups. The high pressure parameters used were strong enough to cause microhemorrhage (61).
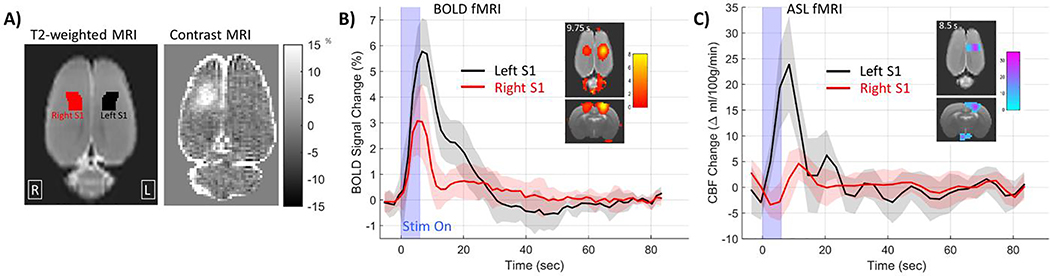
A recent study from our group used both fMRI BOLD measurements and an arterial spin labeling (ASL) MRI sequence to directly measure functional CBF at 1 hour after FUS-BBB opening (62). We also observed significantly reduced BOLD and CBF signal changes in response to hind paw stimulation in the region of BBB opening (Figure 4), which returned to baseline by 24 hours later. Measurements of CBF acquired without stimulation did not show any differences between the sonicated hemisphere and the non-targeted hemisphere, indicating that the FUS-BBB opening did not affect the baseline level of blood flow. This study used more moderate FUS-BBB opening parameters (0.41 Mechanical Index) and saw clear signs of BBB disruption on contrast MRI but no signs of microhemorrhage or other forms of tissue damage were visible on H&E stained histology slides (Figure 4) (62). It is not clear from either study what is the mechanistic link from BBB disruption to suppression of the neurovascular response. Possible mechanisms from the chain of events that make up neurovascular coupling include suppression of neuronal activity (discussed in Section 2.4), altered neurovascular signaling mechanisms, a compromised local response of the vasculature due to endothelial cell dysfunction, or disruption of signaling mechanisms along vessels that cause upstream arterioles to dilate (discussed in Section 3.3) (63). It is possible that the upregulation of inflammatory molecules could interfere with the neurovascular signaling mechanisms. If endothelial cell function has been compromised, this is known to affect neurovascular coupling (64, 65). It is also possible that the breaking of tight junctions between endothelial cells may interfere with retrograde signaling along vessels that is necessary for dilation of upstream vessels (66). It is also not known how these alterations to cerebral blood flow depend on FUSBBB opening parameters such as microbubble dose, volume of BBB disruption, or the effect of repeated BBB openings.
Figure 4.
Reduced changes in fMRI BOLD signal in response to hind paw stimulation following FUSBBB opening. A) Axial views of the rat cortex showing regions for left and right hind paw somatosensory cortex and an example of FUS-BBB opening targeted to the right hind paw somatosensory cortex. B) Group average results showing significantly reduced BOLD response to hind paw stimulation in the right somatosensory cortex targeted for BBB opening. C) Similar results from ASL imaging showing significantly reduced CBF response to hind paw stimulation.
Another piece of evidence comes from two fMRI-based studies that demonstrated FUS-BBB opening disrupts the resting state functional connectivity between brain regions. Resting state fMRI is a method to assess “functional connectivity” between brain regions based on temporal correlations in the BOLD data (67, 68). Our study demonstrated reduced functional connectivity in rats after FUS-BBB opening targeted to the right somatosensory cortex (69). Compared to sham controls, rats that underwent FUSBBB opening had reduced functional connectivity from the targeted right hind limb somatosensory cortex to several sensorimotor areas in the same hemisphere and to the left hind limb somatosensory cortex in the opposite hemisphere indicating disruption of large-scale neuronal network activity. Similar to the neurovascular response findings described above, the FUS-BBB opening protocol used did not result in any visible damage on H&E stained histology (69). Our findings were partially replicated by Meng et al. in a human study that also used resting state fMRI to assess metrics of functional connectivity in early stage Alzheimer’s disease patients who underwent FUS-BBB opening that was targeted to their right frontal lobe (70). Results of this study showed decreased connectivity in the ipsilateral right frontoparietal network, but not in the same network and brain regions of the non-targeted contralateral hemisphere. These changes were transient, with no significant differences in functional connectivity between BBB open and BBB closed cases at one-day and one-week after BBB opening (70). Additional studies still need to be performed to understand the underlying mechanisms and characterize the phenomenon in terms of FUS-BBB opening parameters.
2.4. Suppression of neuronal activity
Section 2.3 described a study by Chu et al. that demonstrated FUS-BBB opening performed with a high FUS pressure amplitude changes the characteristics of somatosensory evoked potential measurements, including a ~60% reduction in amplitude of the P1 peak and a ~50% increase in the latency of the P1 peak (61). These results raise the question of whether FUS-BBB opening could have a direct effect on neuronal activity. As evoked potentials are direct measures of neuronal electrical activity, it does appear that in this case FUS-BBB opening suppressed neuronal activity. However, the parameters used resulted in microhemorrhages seen on histology and suppression of both the evoked potential and BOLD measurements out to at least 7 days, indicating that the effect was likely due to local tissue damage. When a more moderate FUS pressure level was used, the BBB was still opened but the effects on evoked potential amplitude or latency were not significantly different from no-FUS controls (61). This implies that the BBB may be disrupted without having a significant impact on neuronal function. Although, interestingly, the study also considered three repeated BBB openings at the moderate FUS pressure level spaced by three days apart and found an effect on evoked potential amplitude and latency emerged after the second and third treatments. It is also possible that the effects of FUS-BBB opening are suppressing some types of inhibitory neurons, which have been shown in the stroke literature to be more sensitive to damage (71, 72).
Two more recent studies specifically looked at neuronal function after FUS-BBB opening and concluded that FUS-BBB opening did not affect neuronal excitability (73, 74). The first study used patch-clamp recordings from individual neurons in brain slices cut 2- and 24-hours after single-session whole-brain FUS-BBB opening and those cut one week and three months after six weekly whole-brain FUS-BBB opening sessions. No significant differences were seen in neuronal excitability parameters between FUS-treated and sham controls at any of the time points (73, 74). The study also evaluated neuronal morphology and found no signs of structural damage. The second study investigated the effects of repeated FUS-BBB opening in aged 12- and 18-month old mice. After six whole-brain FUS-BBB opening sessions, neuronal health was evaluated by electrophysiological recordings in brain slices and by Golgi staining. No significant differences were seen between FUS-treated and sham controls in terms of evoked excitatory post-synaptic currents, long-term potentiation, or neuronal morphology (73).
3. Understudied areas
There are a few brain systems and cell types associated with the BBB and/or the NVU that are relatively understudied in terms of how they are affected by FUS-BBB opening.
3.1. CSF clearance of waste products
One mechanism for clearance of waste products in the brain is convective flow of CSF through the interstitium to remove solutes, including proteins such as Aβ (75–77). One hypothesis for how this clearance works is that bulk flow of CSF travels along periarterial spaces, enters the brain interstitium partly through aquaporin-4 (AQP4) channels on astrocytic end-feet, is driven across the interstitial space by diffusion and advection while it mixes with interstitial fluid (ISF) and pulls along waste products and then the fluid and solutes drain via the peri-venous spaces (78). Research is ongoing to better understand both the directionality of the bulk flow and the extent to which AQP4 channels and astrocytes may be involved (79–81). The peri-arteriole and peri-venous spaces are directly adjacent to the BBB and therefore it is possible that the functioning of this waste clearance system is affected in some way by FUS-BBB opening.
To date, only one retrospective observational study investigated the impact of FUS-BBB opening on the CSF clearance system. Meng et al. examined MRI data acquired in eight Alzheimer’s disease patients and four amyotrophic lateral sclerosis patients acquired before, immediately after and one day after undergoing FUS-BBB opening procedures (82). In four of these patients they saw evidence of hyperintensity patterns in fluid-attenuated inversion recovery (FLAIR) images. This suggested the gadolinium contrast agent injected intravenously, gadobutrol, was exiting the vasculature at the area of increased BBB permeability and being transported through the interstitium to the peri-venous clearance pathway. Interestingly, an earlier paper evaluating the safety of FUS-BBB opening in non-human primates also observed similar patterns of gadolinium spread away from the targeted site but interpreted it as contrast agent leakage into a sulcus (12).
These two papers provide evidence that contrast agents let out of blood vessels by FUS-BBB opening might be transported along perivascular pathways in human and non-human primate brains. Data showing that FUS-BBB opening enhances intranasal delivery of agents to the brain is further indirect evidence that FUS-BBB opening affects the CSF clearance system (83–85). But there are still no studies on how the functioning of the CSF clearance system is affected by FUS-BBB opening. It remains to be seen whether FUS-BBB opening could be used to enhance CSF clearance properties, for example to boost the removal of disease pathology such as Aβ plaques. Or, if FUS-BBB opening somehow hinders the functioning of this system and actually slows the clearance of harmful waste products.
3.2. Astrocytes
Brain astrocytes are glial cells that are an integral part of the NVU. Astrocytes are physically adjacent to the vasculature, have endfeet in contact with the basement membranes surrounding the vasculature, and form synaptic connections with neurons. Although the full scope of the role astrocytes play in maintaining brain homeostasis and function is not fully known, these cells have been implicated in facilitating fluid exchange via AQP4 channels (76), helping to regulate the BBB (86), influencing neurotransmitter uptake and release (87), modulating neurovascular coupling (88, 89), and regulating neuroinflammation (90). It has been shown that FUS-BBB opening can lead to an increase in the number of activated astrocytes in the targeted area when more aggressive FUS parameters are used (28, 31, 48, 91). But it is not clear what this means for the many functional roles that astrocytes play.
There have been no studies that looked specifically at how FUS-BBB opening affects the functions of astrocytes. It is possible that astrocyte functionality could be directly affected by the mechanical forces of microbubble cavitation or by the inflammatory response that ensues. Changes to astrocytic behavior could be a component of several of the secondary effects of FUS-BBB opening that were previously discussed. For instance, activated astrocytes might alter neurovascular coupling and play a role in the attenuation of the neurovascular response described in Section 2.3. Also, if the expression of astrocytic AQP4 channels changes, it could modify the fluid flow properties of the CSF clearance system. Due to their proximity to blood vessels, activation of astrocytes could also have an effect on the pharmacodynamics and pharmacodistribution of the drugs that are released in the parenchyma after FUS-BBB opening.
3.3. Propagation of vasomotor responses along the vessel wall
Effects of FUS-BBB opening on individual endothelial cells has been more extensively studied to understand the mechanisms that contribute to BBB permeability. However, one area that remains unexplored is the impact of FUS-BBB opening on communication between endothelial cells and their role in regulating blood flow. To produce an efficient CBF increase throughout the depth of active cortical tissue, vessel dilation needs to occur not only locally but also in upstream arterioles and pial arteries. It has been hypothesized for some time that such a long distance signaling mechanism would need to be initiated at the site of neuronal activity and conducted along blood vessels all the way to feeding pial arteries (63). Both dilation and constriction signals can propagate along vascular walls via multiple routs depending on the nature of the signaling mechanism (92). A number of recent studies have demonstrated the role of endothelial cells in this process (93, 94). Chen et al. showed that a localized lesion in the endothelium of a single cortical pial arteriole prevented vasodilation from propagating past the lesion site (93). They further showed that more widespread endothelial lesions had a significant effect on the amplitude and time course of the hemodynamic response to somatosensory stimulation (93).
On the cellular level, the current hypothesis is that this rapid signaling involves activation of capillary endothelial cell inward-rectifier K+ (KIR2.1) channels (94) causing hyperpolarization that propagates along the blood vessel as an ionic current that travels through the endothelium via gap junctions (95, 96). It has been shown that FUS-BBB opening affects expression of tight junction proteins between endothelial cells, but effects on the connexin proteins that make up the gap junctions have not been studied. If this pathway for retrograde vessel dilation signaling is broken by FUS-BBB opening, that would help to explain the result of an attenuated neurovascular response seen by Chu et al. and Todd et al. (61, 62). All these questions warrant further investigations to both increase our knowledge of the basic science mechanisms behind these observations and identify novel means that could reduce the side-effects of this procedure and improve patient safety.
4. Future directions: clinical safety guidance and basic science studies
As a method for drug delivery to the brain, FUS-BBB opening has the potential to be applied to a broad range of therapy and disease combinations. It has already successfully reached the clinic for three applications: chemotherapy delivery for brain tumor patients (97) and FUS-BBB opening alone for
Alzheimer’s disease (54) and ALS (98) patients. These phase 1 clinical trials have provided the first evidence that the technology is safe in humans under the protocols used. Further trials are needed to establish its efficacy and gain regulatory approval as a new therapeutic modality. As the field moves forward, there are two compelling reasons for gaining a fuller understanding of the secondary effects FUS-BBB opening might have on brain physiology. First, understanding any potentially harmful effects is necessary in order to safely move the treatment into new applications. Second, as happened with the discovery that FUS-BBB opening alone helps to remove Aβ plaques, it is possible that other secondary mechanisms could be harnessed for therapeutic effects.
Many of the safety issues have to do with how the potentially harmful secondary effects of FUS-BBB opening vary as a function of treatment parameters. The currently published clinical data demonstrates that FUS-BBB opening can be performed safely with no adverse events in humans (54, 97, 98). However, it is possible that more aggressive treatments could tip some of these phenomena from benign and transient into posing more serious safety risks. Safety considerations include: 1) how does the volume of tissue over which the BBB is disrupted affect the potentially harmful effects of neuroinflammation and attenuation of the neurovascular response? Differences in BBB opening volume were potentially a factor in the different inflammatory responses observed by the Kovacs versus McMahon studies (35, 91). While Leinenga et al. showed promising results with no major side-effects after whole-brain BBB opening in rodents (30, 51), these results may not translate to the much larger human brain and hence still need to be evaluated. 2) How is the inflammatory response different when FUS-BBB opening is performed in patients that have persistent neuroinflammation as part of their disease profile or when delivering therapies such as gene therapy vectors that can induce an inflammatory response themselves (99)? 3) Do the alterations to cerebral blood flow become more severe with repeated BBB openings, larger microbubble dose, or greater volume of BBB opening? Answering these questions will allow the field to move forward into new types of drug delivery treatments with confidence.
Many of these secondary effects have only been discovered relatively recently and are still being investigated. In most cases the mechanistic links from FUS-BBB opening to the observed phenomena are not known. And in many cases the dependence on key FUS parameters has not yet been studied. Table 1 lists the secondary phenomena discussed here and how they have been shown to behave as a function of different treatment parameters, including if the effect has not been studied yet. As a more complete picture of cellular and molecular mechanisms and dependence on treatment parameters emerges, new ways to apply FUS-BBB opening beyond drug delivery may become apparent. Perhaps the most promising avenues relate to harnessing the positive aspects of the body’s inflammatory and immune responses, for both clearance of harmful waste products and promotion of neuroprotective elements.
Table 1.
Secondary effects observed and their dependence on FUS-BBB opening treatment parameters.
| Phenomenon Observed | Effect of FUS-BBB Opening Parameters on Phenomenon | ||||
|---|---|---|---|---|---|
| Time course of effect after BBB Opening | Stronger effect with larger volume of BBB Opening? | Stronger effect with higher microbubble dose? | Stronger effect with repeated BBB openings? | ||
| Inflammatory response | Activation of DAMPs leading to sterile inflammatory response mediated by NFκB pathway (Kovacs 2017) | First signs at ~1 hour; Peak at ~6 hours; Mostly return to baseline by 24 hrs (Kovacs 2017, McMahon 2018) | Possible (Kovacs 2017 vs McMahon 2018) | Yes (McMahon 2018) | Yes (Kovacs 2018) No (Sinharay 2019) |
| Clearance of Aβ and Tau | Reduced Aβ and tau burden following FUSBBB opening alone (Jordao 2013) | Seen as early as 4 days after treatment (Jordao 2013) | Aβ clearance seen for wholebrain BBB opening (Leinenga 2015), but effect not directly compared to smaller opening | Not Studied | No (O’Reilly 2017) |
| Gene/Protein expression | Downregulation of tight junction proteins and BBB transporter genes; upregulation of proinflammatory, angiogenesis and neurogenesis genes (McMahon 2017) | BBB-related gene changes seen at 6 and 24 hours (McMahon 2017); neurogenesis seen at 12 days (Mooney 2016) | Not Studied | Not Studied | Not Studied |
| Cerebral Blood Flow | Attenuation of hemodynamic response (Chu 2015; Todd 2019); breaking of functional connectivity (Todd 2018) | Effect seen at ~2 hours; Return to baseline by 24 hours (Todd 2019) | Not Studied | Not Studied | Not Studied |
| Neuronal activity | Suppression of evoked potentials at high FUS pressure (Chu 2015) | At high FUS pressure, effect persists out to 7 days (Chu 2015) | Not Studied | Not Studied | Yes (Chu 2015) No (Hatch 2016) |
| CSF clearance system | Transport of gadolinium along perivascular space after FUS-BBB opening (Meng 2019) | Evidence of gadolinium transport ~1 hour after treatment; resolved by 24 hours later (Meng 2019) | Not Studied | Not Studied | Not Studied |
| Astrocytes | Activated as part of inflammatory response (Jordao 2013, Kovacs 2017) | Activation seen within 1 hour (Kovacs 2017) and at 4 days (Jordao 2013) | Not Studied | Not Studied | Not Studied |
| Inter-endothelial cell signaling | Breaking of tight junctions between endothelial cells reduces vessel signaling mechanisms (hypothesized only, not directly observed) | Not Studied | Not Studied | Not Studied | Not Studied |
Highlights.
FUS-BBB opening is a promising tool for drug delivery to the brain.
Physical disruption of the BBB can lead to secondary effects on brain physiology.
This review summarizes current knowledge and key gaps in understanding these effects.
Known effects include inflammation, clearance of Aβ and changes to blood flow.
Better understanding of these effects will help to move treatments forward.
Acknowledgements
This manuscript was supported by The Hereditary Disease Foundation, The Brigham Research Institute, and NIH grant K01EB023983.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA (2001) Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology 220(3):640–6. [DOI] [PubMed] [Google Scholar]
- 2.Hersh DS, et al. (2016) Evolving Drug Delivery Strategies to Overcome the Blood Brain Barrier. Curr Pharm Des. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chopra R, Vykhodtseva N, Hynynen K (2010) Influence of exposure time and pressure amplitude on blood-brain-barrier opening using transcranial ultrasound exposures. ACS Chem Neurosci 1(5):391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDannold N, Vykhodtseva N, Hynynen K (2008) Effects of acoustic parameters and ultrasound contrast agent dose on focused-ultrasound induced blood-brain barrier disruption. Ultrasound Med Biol 34(6):930–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shin J, et al. (2018) Focused ultrasound-mediated noninvasive blood-brain barrier modulation: Preclinical examination of efficacy and safety in various sonication parameters. Neurosurg Focus. doi: 10.3171/2017.11.FOCUS17627. [DOI] [PubMed] [Google Scholar]
- 6.Conti A, Mériaux S, Larrat B (2019) About the Marty model of blood-brain barrier closure after its disruption using focused ultrasound. Phys Med Biol. doi: 10.1088/1361-6560/ab259d. [DOI] [PubMed] [Google Scholar]
- 7.Marty B, et al. (2012) Dynamic study of blood-brain barrier closure after its disruption using ultrasound: a quantitative analysis. J Cereb Blood Flow Metab 32(10):1948–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park J, Zhang Y, Vykhodtseva N, Jolesz FA, McDannold NJ (2012) The kinetics of blood brain barrier permeability and targeted doxorubicin delivery into brain induced by focused ultrasound. J Control Release 162(1):134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi JJ, Wang S, Tung YS, Morrison B, Konofagou EE (2010) Molecules of Various Pharmacologically-Relevant Sizes Can Cross the Ultrasound-Induced Blood-Brain Barrier Opening in vivo. Ultrasound Med Biol 36(1):58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun T, et al. (2017) Closed-loop control of targeted ultrasound drug delivery across the blood– brain/tumor barriers in a rat glioma model. Proc Natl Acad Sci 114(48):E10281–E10290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Downs ME, et al. (2015) Long-term safety of repeated blood-brain barrier opening via focused ultrasound with microbubbles in non-human primates performing a cognitive task. PLoS One 10(5):1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDannold N, Arvanitis CD, Vykhodtseva N, Livingstone MS (2012) Temporary disruption of the blood-brain barrier by use of ultrasound and microbubbles: Safety and efficacy evaluation in rhesus macaques. Cancer Res. doi: 10.1158/0008-5472.CAN-12-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weng J-C, Wu S-K, Lin W-L, Tseng W-YI (2011) Detecting blood-brain barrier disruption within minimal hemorrhage following transcranial focused ultrasound: a correlation study with contrast-enhanced MRI. Magn Reson Med 65(3):802–11. [DOI] [PubMed] [Google Scholar]
- 14.Aryal M, Arvanitis CD, Alexander PM, McDannold N (2014) Ultrasound-mediated blood-brain barrier disruption for targeted drug delivery in the central nervous system. Adv Drug Deliv Rev 72:94–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meairs S (2015) Facilitation of Drug Transport across the Blood-Brain Barrier with Ultrasound and Microbubbles. Pharmaceutics 7(3):275–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Timbie KF, Mead BP, Price RJ (2015) Drug and gene delivery across the blood-brain barrier with focused ultrasound. J Control Release. doi: 10.1016/j.jconrel.2015.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zlokovic BV (2008) The Blood-Brain Barrier in Health and Chronic Neurodegenerative Disorders. Neuron. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Sheikov N, McDannold N, Vykhodtseva N, Jolesz F, Hynynen K (2004) Cellular mechanisms of the blood-brain barrier opening induced by ultrasound in presence of microbubbles. Ultrasound Med Biol 30(7):979–989. [DOI] [PubMed] [Google Scholar]
- 19.Sheikov N, et al. (2006) Brain arterioles show more active vesicular transport of blood-borne tracer molecules than capillaries and venules after focused ultrasound-evoked opening of the blood-brain barrier. Ultrasound Med Biol. doi: 10.1016/j.ultrasmedbio.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 20.Sheikov N, McDannold N, Sharma S, Hynynen K (2008) Effect of Focused Ultrasound Applied With an Ultrasound Contrast Agent on the Tight Junctional Integrity of the Brain Microvascular Endothelium. Ultrasound Med Biol 34(7):1093–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burgess A, Shah K, Hough O, Hynynen K (2015) Focused ultrasound-mediated drug delivery through the blood-brain barrier. Expert Rev Neurother 15(5):477–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pandit R, Chen L, Götz J (2019) The blood-brain barrier: Physiology and strategies for drug delivery. Adv Drug Deliv Rev. doi: 10.1016/j.addr.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Pardridge WM (2005) The blood-brain barrier: Bottleneck in brain drug development. NeuroRx 2(1):3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho EE, Drazic J, Ganguly M, Stefanovic B, Hynynen K (2011) Two-Photon Fluorescence Microscopy Study of Cerebrovascular Dynamics in Ultrasound-Induced Blood—Brain Barrier Opening. J Cereb Blood Flow Metab 31(9):1852–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi HJ, Lee EH, Han M, An SH, Park J (2019) Diminished Expression of P-glycoprotein Using Focused Ultrasound Is Associated With JNK-Dependent Signaling Pathway in Cerebral Blood Vessels. Front Neurosci 13(December):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aryal M, et al. (2017) Effects on P-glycoprotein expression after blood-brain barrier disruption using focused ultrasound and microbubbles. PLoS One 12(1). doi: 10.1371/journal.pone.0166061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raymond SB, et al. (2008) Ultrasound enhanced delivery of molecular imaging and therapeutic agents in Alzheimer’s disease mouse models. PLoS One 3(5):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kovacs ZI, Burks SR, Frank JA (2018) Focused ultrasound with microbubbles induces sterile inflammatory response proportional to the blood brain barrier opening: Attention to experimental conditions. Theranostics. doi: 10.7150/thno.24181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kovacs ZI, et al. (2018) MRI and histological evaluation of pulsed focused ultrasound and microbubbles treatment effects in the brain. Theranostics 8(17):4837–4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leinenga G, Götz J (2015) Scanning ultrasound removes amyloid-b and restores memory in an Alzheimer’s disease mouse model. Sci Transl Med. doi: 10.1126/scitranslmed.aaa2512. [DOI] [PubMed] [Google Scholar]
- 31.Sinharay S, et al. (2019) In vivo imaging of sterile microglial activation in rat brain after disrupting the blood-brain barrier with pulsed focused ultrasound: [18F]DPA-714 PET study. J Neuroinflammation. doi: 10.1186/s12974-019-1543-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kovacs ZI, Burks SR, Frank JA (2017) Reply to Silburt et al.: Concerning sterile inflammation following focused ultrasound and microbubbles in the brain. Proc Natl Acad Sci U S A 114(33):E6737–E6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gadani SP, Walsh JT, Lukens JR, Kipnis J (2015) Dealing with Danger in the CNS: The Response of the Immune System to Injury. Neuron. doi: 10.1016/j.neuron.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen GY, Nuñez G (2010) Sterile inflammation: Sensing and reacting to damage. Nat Rev Immunol 10(12):826–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McMahon D, Hynynen K (2017) Acute inflammatory response following increased blood-brain barrier permeability induced by focused ultrasound is dependent on microbubble dose. Theranostics. doi: 10.7150/thno.21630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silburt J, Lipsman N, Aubert I (2017) Disrupting the blood–brain barrier with focused ultrasound: Perspectives on inflammation and regeneration. Proc Natl Acad Sci U S A 114(33):E6735–E6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McMahon D, Hynynen K (2018) Reply to Kovacs et al.: Concerning acute inflammatory response following focused ultrasound and microbubbles in the brain. Theranostics. doi: 10.7150/thno.25468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mcmahon D, Bendayan R, Hynynen K (2017) Acute effects of focused ultrasound-induced increases in blood-brain barrier permeability on rat microvascular transcriptome. Sci Rep 7(November 2016):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jalali S, Huang Y, Dumont DJ, Hynynen K (2010) Focused ultrasound-mediated bbb disruption is associated with an increase in activation of AKT: Experimental study in rats. BMC Neurol 10. doi: 10.1186/1471-2377-10-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mooney SJ, et al. (2016) Focused ultrasound-induced neurogenesis requires an increase in bloodbrain barrier permeability. PLoS One 11(7):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scarcelli T, et al. (2014) Stimulation of hippocampal neurogenesis by transcranial focused ultrasound and microbubbles in adult mice. Brain Stimul 7(2):304–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shin J, et al. (2019) Focused ultrasound-induced blood-brain barrier opening improves adult hippocampal neurogenesis and cognitive function in a cholinergic degeneration dementia rat model. Alzheimers Res Ther 11(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corps KN, Roth TL, McGavern DB (2015) Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol 72(3):355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jassam YN, Izzy S, Whalen M, McGavern DB, El Khoury J (2017) Neuroimmunology of Traumatic Brain Injury: Time for a Paradigm Shift. Neuron 95(6):1246–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rajkovic O, Potjewyd G, Pinteaux E (2018) Regenerative medicine therapies for targeting neuroinflammation after stroke. Front Neurol 9(SEP):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ransohoff RM (2016) How neuroinflammation contributes to neurodegeneration. Science (80- ). doi: 10.1126/science.aag2590. [DOI] [PubMed] [Google Scholar]
- 47.Burgess A, et al. (2014) Alzheimer disease in a mouse model: MR imaging-guided focused ultrasound targeted to the hippocampus opens the blood-brain barrier and improves pathologic abnormalities and behavior. Radiology. doi: 10.1148/radiol.14140245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jordão JF, et al. (2013) Amyloid-β plaque reduction, endogenous antibody delivery and glial activation by brain-targeted, transcranial focused ultrasound. Exp Neurol 248:16–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jordão JF, et al. (2010) Antibodies targeted to the brain with image-guided focused ultrasound reduces amyloid-β plaque load in the TgCRND8 mouse model of Alzheimer’s disease. PLoS One 5(5):4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burgess A, et al. (2014) Alzheimer disease in a mouse model: Mr imaging-guided focused ultrasound targeted to the hippocampus opens the blood-brain barrier and improves pathologic abnormalities and behavior. Radiology. doi: 10.1148/radiol.14140245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leinenga G, Götz J (2018) Safety and efficacy of scanning ultrasound treatment of aged APP23 mice. Front Neurosci 12(FEB):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pelekanos M, et al. (2018) Establishing sheep as an experimental species to validate ultrasoundmediated blood-brain barrier opening for potential therapeutic interventions. Theranostics 8(9):2583–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O’Reilly MA, et al. (2017) Investigation of the safety of focused ultrasound-induced blood-brain barrier opening in a natural canine model of aging. Theranostics 7(14):3573–3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lipsman N, et al. (2018) Blood–brain barrier opening in Alzheimer’s disease using MR-guided focused ultrasound. Nat Commun 9(1). doi: 10.1038/s41467-018-04529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Janowicz PW, Leinenga G, Götz J, Nisbet RM (2019) Ultrasound-mediated blood-brain barrier opening enhances delivery of therapeutically relevant formats of a tau-specific antibody. Sci Rep 9(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nisbet RM, et al. (2017) Combined effects of scanning ultrasound and a tau-specific single chain antibody in a tau transgenic mouse model. Brain 140(5):1220–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pandit R, Leinenga G, Götz J (2019) Repeated ultrasound treatment of tau transgenic mice clears neuronal tau by autophagy and improves behavioral functions. Theranostics 9(13):3754–3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Karakatsani ME, et al. (2019) Unilateral focused ultrasound-induced blood-brain barrier opening reduces phosphorylated Tau from the rTg4510 mouse model. Theranostics 9(18):5396–5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Raymond SB, Skoch J, Hynynen K, Bacskai BJ (2007) Multiphoton imaging of ultrasound/Optison mediated cerebrovascular effects in vivo. J Cereb Blood Flow Metab 27(2):393–403. [DOI] [PubMed] [Google Scholar]
- 60.Cho EE, Drazic J, Ganguly M, Stefanovic B, Hynynen K (2011) Two-photon fluorescence microscopy study of cerebrovascular dynamics in ultrasound-induced blood-brain barrier opening. J Cereb Blood Flow Metab 31(9):1852–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chu P-C, et al. (2015) Neuromodulation accompanying focused ultrasound-induced blood-brain barrier opening. Sci Rep 5:15477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Todd N, Zhang Y, Livingstone M, Borsook D, McDannold N (2019) The neurovascular response is attenuated by focused ultrasound-mediated disruption of the blood-brain barrier. Neuroimage. doi: 10.1016/j.neuroimage.2019.116010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Iadecola C (2017) The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron 96(1):17–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hu X, Michael De Silva T, Chen J, Faraci FM (2017) Cerebral Vascular Disease and Neurovascular Injury in Ischemic Stroke. Circ Res. doi: 10.1161/CIRCRESAHA.116.308427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang F, et al. (2018) Dysfunction of Cerebrovascular Endothelial Cells: Prelude to Vascular Dementia. Front Aging Neurosci. doi: 10.3389/fnagi.2018.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hillman EMC (2014) Coupling Mechanism and Significance of the BOLD Signal: A Status Report. Annu Rev Neurosci. doi: 10.1146/annurev-neuro-071013-014111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Biswal B, Zerrin Yetkin F, Haughton VM, Hyde JS (1995) Functional connectivity in the motor cortex of resting human brain using echo- planar mri. Magn Reson Med. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 68.van den Heuvel MP, Hulshoff Pol HE (2010) Exploring the brain network: A review on restingstate fMRI functional connectivity. Eur Neuropsychopharmacol. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 69.Todd N, et al. (2018) Focused ultrasound induced opening of the blood-brain barrier disrupts inter-hemispheric resting state functional connectivity in the rat brain. Neuroimage 178:414–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meng Y, et al. (2019) Resting state functional connectivity changes after MR-guided focused ultrasound mediated blood-brain barrier opening in patients with Alzheimer’s disease. Neuroimage. doi: 10.1016/j.neuroimage.2019.06.060. [DOI] [PubMed] [Google Scholar]
- 71.Kessler KR, Schnitzler A, Classen J, Benecke R (2002) Reduced inhibition within primary motor cortex in patients with poststroke focal motor seizures. Neurology. doi: 10.1212/WNL.59.7.1028. [DOI] [PubMed] [Google Scholar]
- 72.Oliviero A, et al. (2004) Brain sensorimotor hand area functionality in acute stroke: Insights from magnetoencephalography. Neuroimage. doi: 10.1016/j.neuroimage.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 73.Blackmore DG, et al. (2018) Multimodal analysis of aged wild-type mice exposed to repeated scanning ultrasound treatments demonstrates long-term safety. Theranostics 8(22):6233–6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hatch RJ, Leinenga G, Götz J (2016) Scanning ultrasound (SUS) causes no changes to neuronal excitability and prevents age-related reductions in hippocampal CA1 dendritic structure in wildtype mice. PLoS One 11(10):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Iliff JJ, et al. (2012) A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Benveniste H, Lee H, Volkow ND (2017) The Glymphatic Pathway: Waste Removal from the CNS via Cerebrospinal Fluid Transport. Neuroscientist 23(5):454–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xie L, et al. (2013) Sleep drives metabolite clearance from the adult brain. Science (80- ). doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Plog BA, Nedergaard M (2018) The Glymphatic System in Central Nervous System Health and Disease: Past, Present, and Future. Annu Rev Pathol Mech Dis. doi: 10.1146/annurev-pathol051217-111018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mestre H, et al. (2018) Aquaporin-4-dependent glymphatic solute transport in the rodent brain. Elife. doi: 10.7554/eLife.40070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Holter KE, et al. (2017) Interstitial solute transport in 3D reconstructed neuropil occurs by diffusion rather than bulk flow. Proc Natl Acad Sci U S A. doi: 10.1073/pnas.1706942114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Smith AJ, Yao X, Dix JA, Jin BJ, Verkman AS (2017) Test of the ‘glymphatic’ hypothesis demonstrates diffusive and aquaporin-4-independent solute transport in rodent brain parenchyma. Elife. doi: 10.7554/eLife.27679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Meng Y, et al. (2019) Glymphatics Visualization after Focused Ultrasound-Induced Blood–Brain Barrier Opening in Humans. Ann Neurol 86(6):975–980. [DOI] [PubMed] [Google Scholar]
- 83.Ye D, et al. (2018) Comparison of Focused Ultrasound-Mediated Intranasal Delivery and Focused Ultrasound-Induced Blood-Brain Barrier Disruption in the Delivery of Gold Nanoclusters to the Brainstem. IEEE International Ultrasonics Symposium, IUS doi: 10.1109/ULTSYM.2018.8580097. [DOI] [Google Scholar]
- 84.Ji R, et al. (2019) Focused ultrasound enhanced intranasal delivery of brain derived neurotrophic factor produces neurorestorative effects in a Parkinson’s disease mouse model. Sci Rep. doi: 10.1038/s41598-019-55294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ye D, et al. (2018) Focused ultrasound combined with microbubble-mediated intranasal delivery of gold nanoclusters to the brain. J Control Release. doi: 10.1016/j.jconrel.2018.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Abbott NJ, Rönnbäck L, Hansson E (2006) Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 7(1):41–53. [DOI] [PubMed] [Google Scholar]
- 87.Santello M, Volterra A (2009) Synaptic modulation by astrocytes via Ca2+-dependent glutamate release. Neuroscience 158(1):253–259. [DOI] [PubMed] [Google Scholar]
- 88.Gu X, et al. (2018) Synchronized Astrocytic Ca2+ Responses in Neurovascular Coupling during Somatosensory Stimulation and for the Resting State. Cell Rep 23(13):3878–3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang M, He Y, Sejnowski TJ, Yu X (2018) Brain-state dependent astrocytic Ca2+ signals are coupled to both positive and negative BOLD-fMRI signals. Proc Natl Acad Sci U S A 115(7):E1647–E1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Colombo E, Farina C (2016) Astrocytes: Key Regulators of Neuroinflammation. Trends Immunol 37(9):608–620. [DOI] [PubMed] [Google Scholar]
- 91.Kovacs ZI, et al. (2017) Disrupting the blood–brain barrier by focused ultrasound induces sterile inflammation. Proc Natl Acad Sci 114(1):E75–E84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jensen LJ, Holstein-Rathlou NH (2013) The vascular conducted response in cerebral blood flow regulation. J Cereb Blood Flow Metab. doi: 10.1038/jcbfm.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen BR, Kozberg MG, Bouchard MB, Shaik MA, Hillman EMC (2014) A critical role for the vascular endothelium in functional neurovascular coupling in the brain. J Am Heart Assoc. doi: 10.1161/JAHA.114.000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Longden TA, et al. (2017) Capillary K+-sensing initiates retrograde hyperpolarization to increase local cerebral blood flow. Nat Neurosci. doi: 10.1038/nn.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Figueroa XF, Duling BR (2009) Gap junctions in the control of vascular function. Antioxidants Redox Signal. doi: 10.1089/ars.2008.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Segal SS (2015) Integration and modulation of intercellular signaling underlying blood flow control. J Vasc Res. doi: 10.1159/000439112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mainprize T, Lipsman N, Huang Y, Meng Y, Bethune A (2019) Blood-Brain Barrier Opening in Primary Brain Tumors with Non- invasive MR-Guided Focused Ultrasound : A Clinical Safety and Feasibility Study. Sci Rep (September 2018):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Abrahao A, et al. (2019) First-in-human trial of blood–brain barrier opening in amyotrophic lateral sclerosis using MR-guided focused ultrasound. Nat Commun. doi: 10.1038/s41467-01912426-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Perez BA, Shutterly A, Chan YK, Byrne BJ, Corti M (2020) Management of Neuroinflammatory Responses to AAV-Mediated Gene Therapies for Neurodegenerative Diseases. Brain Sci 10(2):119. [DOI] [PMC free article] [PubMed] [Google Scholar]