Abstract
Functional polymer coatings have become ubiquitous in biological applications, ranging from biomaterials and drug delivery to manufacturing-scale separation of biomolecules using functional membranes. Recent advances in the technology of chemical vapor deposition (CVD) have enabled precise control of the polymer chemistry, coating thickness, and conformality. That comprehensive control of surface properties has been used to elicit desirable interactions at the interface between synthetic materials and living organisms, making vapor-deposited functional polymers uniquely suitable for biological applications. This review captures the recent technological development in vapor-deposited functional polymer coatings, highlighting their biological applications, including membrane-based bio-separations, biosensing and bio-MEMS, drug delivery, and tissue engineering. The conformal nature of vapor-deposited coatings ensures uniform coverage over micro- and nano-structured surfaces, allowing the independent optimization of surface and bulk properties. The substrate-independence of CVD techniques enables facile transfer of surface characteristics among different applications. The vapor-deposited functional polymer thin films tend to be biocompatible because they are free of remnant toxic solvents and precursor molecules, potentially lowering the barrier to clinical success.
Keywords: Chemical vapor deposition, membranes, drug delivery, biosensors, bio-MEMS, tissue engineering
Graphical Abstract
vapor-deposited polymer thin films empower the next-generation biological applications including bio-separations, biosensors & bio-mems, drug delivery and tissue engineering.
1. Introduction
Polymers have touched almost every aspect of modern life because of their unique physicochemical properties such as light weight, selective permeability, and resistance to degradation, many of which stem from the versatile polymer chemistry. To take advantage of the rich organic functionalities afforded by polymers without sacrificing desirable bulk properties (such as mechanical strength), functional polymers have been made into thin film coatings, usually less than 800-nm thick,1 to decouple the surface and bulk characteristics.2 Functional polymer coatings have been used broadly to define the surface properties of various devices, transforming the fields ranging from energy and sustainability3–6 to healthcare7,8.
Biological applications challenge the design of functional materials in unprecedented aspects – biocompatibility and fouling resistance are often a must, in addition to the properties required by specific applications (e.g. regulating permeability). Multifunctional polymer coatings are uniquely suitable for addressing that challenge, especially the ones that can be applied in a substrate-independent manner to allow the individual optimization of surface and bulk properties.2 Indeed, functional polymer thin films have demonstrated unprecedented capability of reducing the undesirable tissue reactions9 (such as the foreign-body reaction that occurs on virtually any bioimplants10) and of resisting the nonspecific attachment of biomolecules and microorganisms,11 while preserving/improving device performance. Furthermore, the breadth of available chemistry for polymer synthesis and derivatization, joined with the evolving technologies for coating processing, have ushered in a new era of innovation in biomedical solutions, revolutionizing our ways of bio-separations, medical diagnosis,12 disease monitoring and delivery of therapeutics,13 and tissue engineering.14
Although solution-based techniques, such as layer-by-layer (LbL) assembly,15–19 inkjet printing,20 and spin/dip coating,21–24 remain the common approaches to form polymer coatings in biological applications, vapor-deposited coatings have emerged as an attractive alternative. Chemical vapor deposition (CVD) is the mainstay approach for solvent-free synthesis of polymer thin films.25 During a CVD process, reactive precursors (e.g. monomer) are metered into a vacuum reactor where polymerization and film formation occur simultaneously.26 CVD methods eliminate the need for solvents, and thus avoid the undesirable surface tension effects,25 a major source of coating defects like dewetting from the substrates, formation of aggregates27 and other types of non-uniformity.28,29 The solvent-free nature also makes CVD techniques benign, enabling the non-destructive coating of flexible, fragile, and/or non-wetting substrates (which is often the case in biomedical applications),25,30–32 and avoiding toxicity of remnant solvents toward tissues and mammalian cells.33 Moreover, unlike solution-based processing, vapor deposition techniques can be scaled up without extensive process re-design,32 thanks to the well-established fundamentals of heat and mass transport, fluid dynamics, and reaction kinetics. Manufacturing-scale CVD processes have already been implemented in many fields such as membrane technology,34 photovoltaics,35 and lithography36.
Over the last few decades, CVD polymer coatings have been successfully implemented in many biological applications, creating the need for a systematic review. It is our intention to provide a biology-centric sampling of CVD techniques in this review, instead of an exhaustive list of new CVD techniques. For the latter, readers are referred to the recent reviews on the topic of CVD polymers.11,28,37–39
We will first introduce and compare different CVD processes and their synthetic capabilities, and then review the various biological applications enabled by the CVD technologies, spanning bio-separation membranes, biosensors and microfluidic systems, drug delivery systems, and tissue engineering platforms.
2. Functional Polymer Thin Films via CVD
CVD systems typically consist of 3 parts (Figure 1): 1) a gas inlet, through which the vaporized precursors (e.g. monomer) are delivered into the deposition chamber; 2) deposition chamber, where the precursors are chemically activated to enable subsequent polymerization reactions on or near the substrate surface; and 3) gas exhaust, which removes the reactive species and maintains low- to medium- vacuum for various deposition mechanisms.40 CVD processes are commonly named after the energy sources (e.g. plasma-enhanced CVD) or reaction mechanisms (e.g. initiated CVD, photo-initiated CVD).26
Figure 1. Generalized schematic of CVD techniques.
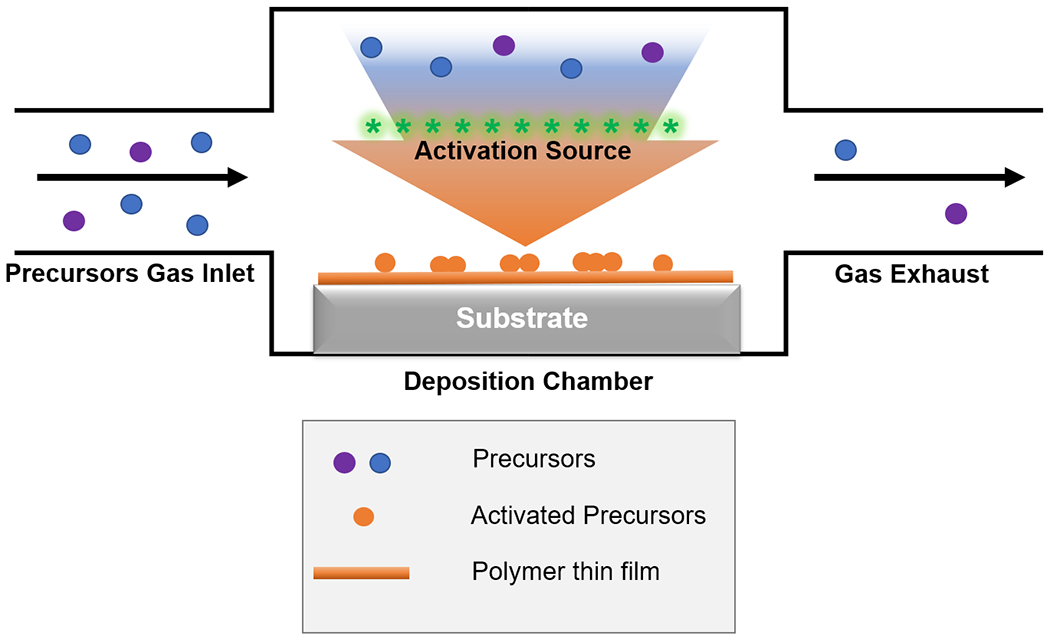
A CVD reactor typically consists of 3 main parts: precursors gas inlet, deposition chamber and gas exhaust. Blue and purple circles represent precursors in the vapor phase, orange circles activated/reactive species on the substrate surface, and orange line the as-deposited polymer thin film.
Using the CVD techniques, functional polymer thin films can be deposited via the chain-growth or the step-growth polymerization mechanisms. Techniques like initiated CVD (iCVD)41,42 and plasma-enhanced CVD (PECVD)43 employ the chain-growth mechanism, where monomers are added to a growing chain end; whereas in the step-growth mechanism, reaction could happen between any two adjacent molecules bearing appropriate moieties. The latter is commonly seen in oxidative CVD (oCVD)44,45 and poly(p-xylylene) (i.e. parylene) CVD.46 The aforementioned techniques – iCVD, parylene CVD, PECVD, and oCVD – are among the most commonly used ones to form functional polymer thin films in biological applications, and will thus be the focus of discussion.
Other CVD techniques, such as atomic layer deposition (ALD), molecular layer deposition [MLD, also been called alternating vapor deposition polymerization (AVDP) or vapor deposition polymerization (VDP)], are often used to deposit inorganic or organic/inorganic composite coatings, with biological applications largely limited to antifouling/antimicrobial surfaces. We refer the readers to existing reviews on those CVD approaches.11,47
2.1. Initiated Chemical Vapor Deposition (iCVD) and Photoinitiated CVD (piCVD)
In iCVD, a volatile initiator (e.g. tert-butyl peroxide) is introduced in the vapor phase along with the monomer(s). While monomers physiosorb onto the substrate to be coated, the initiator is activated thermally, by passing through a heating zone (created by resistively heating an array of metal filaments to 200 to 400 ºC, depending on the initiator used).41 Polymerization occurs when the radicals in the vapor phase collide with the monomers adsorbed on the substrate. Temperature of the substrate is kept at around 20-40 ºC to promote monomer physisorption and to preserve potentially heat-sensitive substrates.48,49 Therefore, iCVD polymerization mimics the radical chain-growth mechanism in solution synthesis, which entails the reaction steps of initiation, propagation, and termination. Furthermore, the kinetic rate constants of propagation and termination in iCVD closely match those of solution-based radical polymerization,50 enabling estimation of deposition kinetics by capitalizing on the existing knowledgebase of solution polymerization.
Compared to other vapor deposition techniques, iCVD affords a richer library of functional groups and more precise control of the polymer chemistry. A variety of chemically labile functional moieties, such as the glycidyl group51 and the pentafluorophenyl group,52 have been deposited using iCVD with high surface density of those moieties, owing to the low process temperature. In fact, iCVD polymers typically achieve 100% retention rate of the functional moieties borne by monomers (except the vinyl groups).53 Copolymer thin films can be achieved by co-flowing two monomers into the vacuum chamber, and the copolymer composition can be readily adjusted by changing the feed ratio of the monomers. Hence cross-linked polymers, which are typically insoluble and unlikely to be made into coatings using conventional methods, can be achieved with tunable cross-linking density, simply by including a feed stream of cross-linker [e.g. ethylene glycol diacrylate (EGDA)54 and divinylbenzene (DVB)55]. The ability to control cross-linking density is important in biological applications because greater cross-linking generally leads to lower swelling ratio54 and greater stiffness,55 both of which are known to affect in vitro cell growth in tissue engineering.56 The high retention rate of functional moieties and the ability to control copolymer composition offered facile tuning of surface energy54 and stimuli-responsive behavior,57 unlocking new possibilities for smart biomaterials (e.g. triggerable drug release and implantable actuators). The primary constraint on the polymer chemistry that is compatible with the iCVD process comes from the monomer volatility, because monomers need to be vaporized for delivery into the vacuum chamber.25 Nevertheless, that constraint has been overcome by using bubblers and carrier gas.58
In addition to surface chemistry, other properties of the iCVD films (e.g. coating thickness, molecular weight, conformality over micro-55,59–61 and nanostructures62,63) could also be controlled precisely, based on the well-established transport and reaction kinetic theories. 41,50,64 The precise and simultaneous control over polymer chemistry and thin film architecture is vital to meeting the multifaceted demands of biological applications, ranging from membrane separations to tissue engineering (see Section 3).
Similar chain-growth polymerization reactions can be initiated by ultraviolet (UV) radiation (instead of thermal activation of initiators), giving rise to a variation of iCVD called photoinitiated CVD (piCVD).65 Unlike iCVD, where thermally labile initiators are indispensable, piCVD allows the direct activation of certain methacrylate monomers [e.g. 2-hydroxyethyl methacrylate (HEMA)] via UV-decomposition of carbonyl species,66 and thus polymerization in the absence of an initiator. piCVD is also compatible with known gas-phase photoinitiators [e.g. 2,2’-azobis(2-methylpropane)],67 where the reaction mechanism is almost identical to that of iCVD. Comparatively, the main advantage of piCVD originates from its photoactivation mechanism, which eliminates high-temperature sources (e.g. heated filament arrays or plasma).25 As such, it is possible to achieve more precise temperature control of the reactor and the substrate to be coated, thereby enabling depositions on biomaterials with extreme temperature sensitivity. In addition, with Type II photoinitiators (e.g. benzophenone), piCVD can activate surface moieties on the substrate and turn them into highly reactive radical species, thereby allowing the synthesis and grafting of a polymer thin films in a single step.68 Nevertheless, piCVD suffers from a lack of a photointiator that provides high photolytic efficiency at UV intensities and wavelengths that are benign enough to preserve delicate substrates.25 More importantly, only a handful of monomers species have been demonstrated to photo-polymerize, possibly due to rapid decarboxylation of the radicalized monomers.69 Resolution of these two technical challenges of piCVD will considerably promote its applications in biotechnology and biomedicine.
2.2. Chemical Vapor Deposition of Poly(p-xylylene)
CVD of poly(p-xylylenes), also called parylene CVD or chemical vapor polymerization (CVP), is one of the most common techniques used in the interface engineering for biological applications.
The deposition process typically entails the free-radical ring-opening copolymerization using [2.2]paracyclophanes.70–74 During a deposition, [2.2]paracyclophanes are sublimated and delivered into a furnace, where [2.2]paracyclophanes are pyrolyzed to form a radical species, para-xylylene. P-xylylene is subsequently delivered into a reaction chamber, typically maintained at ambient temperature and medium vacuum (<1 Torr), where it undergoes step-growth polymerization to form a polymer thin film.
Parylene C [poly(p-xylylene) with one chlorine group per repeat unit] has demonstrated many desirable properties (e.g. anticorrosive, biocompatible, antibacterial, and barrier properties) and thus been widely used as an encapsulation coating in biological applications. It has been tested in vivo on various implants (stents, knee and hip implants, and heart valves75–77) and in vitro during the fabrication of biological microelectromechanical systems (BioMEMS).78,79
The library of functional moieties that are compatible with the CVP approach has been enriched in recent years by decorating the precursors, [2.2]paracyclophanes,74 or post-deposition derivatization.80 Those approaches allow the surface chemistry to be further tailored to specific applications, opening up new possibilities for applying multifunctional poly(p-xylylene) coatings in biological settings.
2.3. Plasma-Enhanced Chemical Vapor Deposition (PECVD)
PECVD is a relatively well-known mechanism for polymer thin film formation. It has been widely used to fabricate anti-reflective (AR) coatings for devices like solar cells and infrared reflectors.81 During PECVD, organic precursors (e.g. tetrafluoroethylene) are fragmented by high-energy plasma [created using radio frequency (RF) or direct current (DC) discharge] to form free radicals in a vacuumed chamber.82,83 The radical species subsequently undergo polymerization on the substrate surface, giving rise to a functional polymer thin film.
Due to the high-energy plasma, PECVD can deposit inorganic-organic composite films in a single chamber with rapid growth kinetics, which has been used to promote osteoblast adhesion on titanium-based implants.84 During a deposition, plasma etching and film deposition occur simultaneously, leading to in situ formation of surface nanostructures.85 That distinct surface morphology has been shown to influence cell growth on a PECVD-treated surface,86 and thus could be used as a control mechanism to tune cell adhesion. Nevertheless, the presence of high-energy plasma could limit PECVD in several aspects. Retention of organic functional moieties during PECVD could be lower than other CVD processes due to the chemical degradation by plasma. The simultaneous etching and film deposition could render PECVD coatings less conformal (i.e. how uniform the coating is over micro- and nanostructures) than other CVD techniques. Perhaps most importantly, the high-energy plasma could limit application of PECVD treatment on fragile substrates such as soft tissue scaffolds.87
To curb the damaging effects of plasma while still utilizing its reactive power, pulsed plasma enhanced CVD (PPECVD) has been developed, which offers more benign reaction conditions to preserve fragile substrates and improve retention of functional moieties.25 In PPECVD, plasma is supplied in short pulses in place of the continuous exposure used in PECVD, where the intervals allow excited molecules to return to their ground state, reducing undesirable molecule dissociation and other side reactions. Greater retention of functional moieties has been achieved using the PPECVD approach.88 Alternatively, by introducing a plasma-labile initiator (e.g. TBPO) to supplement the initiation mechanism of conventional PECVD, initiated PECVD (iPECVD) permits formation of radical species at lower plasma power density, thus improving the retention of functional moieties as well as deposition rates.89,90 Although PPECVD and iPECVD have not been used much in biological applications so far, it has great potential in areas of composite materials for bone implants and tissue engineering and drug delivery in general.
2.4. Chemical Vapor Deposition of Conductive Polymers
The two CVD techniques reviewed in this section, oxidative CVD (oCVD) and an approach named vapor phase polymerization [VPP, also called vapor deposition polymerization (VDP)], are distinct from the aforementioned methods because they could produce conjugated and thus conductive polymers, such as poly(3,4-ethylenedioxythiophene) (pEDOT).91 In fact, oCVD and VPP are most commonly used for device fabrication in energy applications (e.g. photovoltaics, batteries and other energy storage devices).92 Nevertheless, those flexible yet conductive coatings could enable novel designs of bioelectronics.
Monomers used in oCVD typically contain aromatic heterocyclic rings, where electrons of the heteroatom could participate in the conjugated system in the resulting polymers, giving rise to the electron conductivity. During oCVD, monomer(s) is vaporized and activated by an oxidant species (e.g. iron (III) chloride, iron (III) tosylate), which is commonly non-volatile and thus often sputtered onto the substrate. The oxidation reactions create radical cations, which then promote polymerization via the step-growth mechanism. The substrate is commonly maintained at ambient temperature during depositions, but that substrate temperature could be tuned to control the conductivity of an oCVD thin film.93 Due to the benign conditions of the oCVD process, the resulting polymer thin films often have high retention of monomer functional moieties.25 That high functional retention combined with their conductive nature make oCVD polymer coatings an attractive component for the next-generation biochemical and biological sensors, miniaturized pseudo capacitor-based power supplies, and other bioelectronics.94
VPP relies on pre-application of a layer of oxidant onto the substrate surface using solution-coating methods like spin-coating or inkjet-printing. The layer of oxidant then initiates polymerization when the coated substrate is exposed to monomer vapors inside a vacuum chamber.95,96 Using this methods, pEDOT coatings with the conductivity of ~60 S/cm to 2000 S/cm have been synthesized,95,96 and conductive pEDOT coatings have been applied onto cotton fabrics and nanofibers during device fabrication for photovoltaics, capacitors, and light-emitting apparatus.97–99 Notwithstanding the potential toxicity of the oxidants, a similar approach could be adopted in biological applications to fabricate sensing devices or to monitor electron transfer between biological systems.100
2.5. CVD Techniques: Powerful Toolbox for Biological Applications
At the interface between material surfaces and biological environments, seemingly small changes can sometimes trigger a cascade of biological responses, eventually resulting in notable impacts on the biological systems. Therefore, in order to push for deeper understanding of such responses, eliciting desirable interaction, and achieving rational design of devices for bioapplications, highly precise control of polymer thin film properties is essential. By enabling facile engineering of versatile polymer properties with high precision, CVD techniques provide a powerful toolbox for addressing fundamental questions and advancing various applications pertaining to bio-interfaces.
Among the polymer properties, functional moiety composition and arrangement (e.g. random or block copolymer), hydrophobicity/hydrophilicity, mechanical strength, morphology, electrical properties, biocompatibility, spatiotemporal display of bioactive compounds have shown remarkable impact on biological responses spanning multiples length scales.10,44,54,56,101–104 Those responses range from adhesion of biomolecules and cells on that interface, to regulation of cellular responses and functions, to orchestrating multicellular behavior such as biofilm formation and tissue differentiation. Equally important is stability of the polymer physicochemical properties over time, including stable adhesion to substrates and suppression of aging, even after extended exposure to biological environments – without these the long-term efficacy of coated devices may be compromised.
In Table 1 we systematically evaluated the main CVD techniques for synthesizing polymer thin films with respect to important features for bioapplications. As indicated in Table 1, each of the CVD techniques affords a set of advantages, making some more suited for specific bioapplications than their counterparts; nevertheless, to date none of the CVD techniques alone can provide all the desired features for bioapplications (Table 1). Thus, selecting an appropriate CVD technique based on the specific requirements of the intended bioapplications is crucial for obtaining successful outcomes. Table 1 provides a framework based on existing knowledge to facilitate such an informed selection. In addition, as readers proceed in Section 3, Table 1 may help reveal the rationale behind the selection of particular CVD techniques for various bioapplications.
Table 1.
Systematic evaluation of the main CVD techniques for synthesizing polymer thin films with respect to features important for various bioapplications.a
| Feature | Importance for Bioapplications | iCVD b | Parylene CVD | PECVD | oCVD |
|---|---|---|---|---|---|
| Rich monomer library | Diverse functional moieties attainable to meet the demands of various biological applications (e.g. antifouling, stimuli-responsive, antimicrobial, biocompatible, etc.) | Y [37] |
Y [191] |
Y [192] |
|
| Functional group retention | Ensuring high retention of functional groups and avoiding side reactions to achieve chemically tailored bio-interfaces | Y [44] |
Y [193] |
N c [42,44,65] |
Y [27,194] |
| Biofunctionalizability | Incorporation of bioactive molecules; Enabling more specific ligand-receptor binding | Y [52,104] |
Y [195] |
Y [196] |
|
| Electroconductivity | Often required for bioelectronics and biosensors | Y [87] |
Y [87,118] |
||
| Conformality over micro-/nano- structures | Engineering surface chemistry and/or surface mechanical properties of the substrates while preserving their nano- to microscale features | Y [62,197] |
Y [198] |
Y [199] |
|
| Thickness control with µm to nm precision | Controlling mechanical properties, mass transfer across coatings, swelling/actuation behavior | Y [41,50] |
Y [200] |
Y [201] |
Y [202] |
| Grafting | Long-term stability of the resulting thin films in complex biological environments | Y [114,203,204] |
Y [205,206] |
Y [207] |
Y [208] |
| Benign reaction conditions | Allowing for coating various sensitive (bio)materials | Y [165] |
Y [166] |
Y [209] |
Y [38] |
“Y” in a blue cell denotes that the feature (indicated by the row identity) has been demonstrated in the literature by using the CVD technique (indicated by column identity); “N” in a red cell denotes that existing literature support the lack of that feature; an empty grey cell denotes that the feature has not been demonstrated using the corresponding CVD technique. Examples are provided in “[ ]” for the marked cells.
Activated by heat or photons.
Variants of PECVD such as pulsed PECVD (PPECVD), initiated PECVD (iPECVD) have allowed higher monomer functionality retention and lower frequency of side reaction.89 Introducing monomers downstream of the plasma zone can also improve the retention of functional moieties.
3. Biological Applications Enabled by CVD Polymer Thin Films
The CVD functional polymer thin films have been applied to engineer the properties of bio-interfaces, where the living organisms and synthetic materials meet, interact, and exchange materials and information.11 Below, we review the bio-interfaces created by CVD polymers and their applications in bio-separations, biosensing and bio-MEMS, drug delivery, and tissue engineering.
3.1. Functional Coatings for Membrane-based Bio-separation Processes
In biological application, separation of molecules (e.g. proteins, DNA, polysaccharides) is commonly achieved using membranes, as a result of the sensitivity of biomolecules to heat and organic solvents. Membrane separation is ubiquitous in the manufacturing of biomolecules (e.g. pharmaceuticals105) because it is efficient, versatile, and performed at ambient temperature. Effective separation of molecules is achieved by differentiating their rates of diffusion through a membrane (in fact, selectivity of a membrane is defined as the permeability ratio of the two molecules to be separated).106 That difference in the rate of diffusion could be achieved via a porous membrane (e.g. based on the size-exclusion mechanism) or a non-porous membrane (e.g. based on the solution-diffusion mechanism).107
Functional polymer coatings could introduce major improvements to the membrane performance. In bio-separation processes, that improvement is often to render the membranes fouling resistant (Sections 3.1.1, 3.1.2, and 3.1.3), because proteins and polysaccharides have a strong tendency to attach to the surface of an unmodified membrane and reduce its throughput and/or selectivity. Recent advances in functional polymer coatings have also enabled the fabrication of ‘smart membranes’ – ones that could change their selectivity and/or permeability based on environmental cues – via surface modification (Section 3.1.4). The substrate-independent nature of the CVD techniques allows the antifouling or stimuli-responsive surface chemistry to be broadly applied to virtually any membranes. Table 2 provides a summary of the functional CVD polymers used for membrane-based bio-separation processes discussed in this review, along with their properties, the resulting functions/performance, and the CVD techniques employed.
Table 2.
Functional CVD polymers for membrane-based bio-separation processes.
| Polymer | Acronym | Property / Performance a | CVD Technique |
|---|---|---|---|
| Poly(2-hydroxyethyl methacrylate) | pHEMA | Hydrophilic / Antifouling | iCVD42 |
| Poly(1-vinyl-2-pyrrolidone) | pVP | Hydrophilic / Antifouling | iCVD42,108 |
| Poly(hydroxypropyl methacrylate) | pHPMA | Hydrophilic / Antifouling | iCVD111 |
| Poly(1-vinyl-2-pyrrolidone-co-ethylene glycol diacrylate) | pVP-co-EGDA | Hydrophilic / Antifouling | iCVD112 |
| Poly(2-dimethylamino ethyl methacrylate-co-ethylene glycol dimethacrylate) | pDMAEMA-co-EGDMA | Hydrophilic / Antifouling | iCVD114 |
| Poly(2-hydroxyethyl methacrylate-co-perfluorodecylacrylate) | pHEMA-co-PFDA | Amphiphilic / Antifouling | iCVD116 |
| Poly(3,4-ethylene dioxythiophene) | pEDOT | Conductive, Hydrophobic / Antifouling | VDP96, oCVD118 |
| Poly(methyl acrylic acid-co-ethylene glycol dimethacrylate) | pMAA-co-EGDMA | pH Responsive, Hydrophilic /Tunable Membrane Permeability | iCVD120 |
| Poly(maleic anhydride-co-dimethylacrylamide-co-diethylene glycol divinyl ether] | pMaDD | pH Responsive / Antifouling, Tunable Membrane Permeability | iCVD121 |
| Poly(N-isopropyl acrylamide-co-ethylene glycol dimethacrylate) | pNIPAAm-co-EGDMA | Temperature Responsive, Hydrophilic / Tunable Membrane Permeability | iCVD123 |
| Poly(N,N-dimethyl aminoethyl methacrylate-co-ethylene glycol diacrylate) | pDMAEMA-co-EGDA | Temperature Responsive, Hydrophilic / Antifouling, Tunable Membrane Permeability | iCVD57 |
For porous membranes, the conformal nature of the CVD techniques is key to achieving complete and uniform coverage of the membrane pores. Those pores usually have large aspect ratios and thus uniform surface modification without pore blockage could be challenging using solution-based methods.87 For non-porous membranes, the selective layer, i.e. the layer responsible for membrane selectivity, is usually made thin [e.g. ~100 nm in some reverse osmosis (RO) membranes107] to maximize membrane throughput. That thin selective layer is thus commonly fragile, sensitive to heat and solvent, and the surface modification of which necessitates benign and solvent-free methods like CVD.108
Although this section focuses on the functional polymer coatings that have been applied to bio-separation processes, the capability of CVD techniques to improve membrane performance goes beyond that scope. For example, hydrophobic coatings, which are challenging to fabricate using solution-based methods due to their poor solubility, have been applied conformally to porous membranes using iCVD and improved the performance of membrane distillation.31,109,110 Readers are referred to other comprehensive reviews on this topic.37
3.1.1. Hydrophilic Polymer Coatings
Many hydrophilic coatings can resist non-specific protein or cell adhesion, and thus have been deposited on RO and nanofiltration (NF) membranes to reduce fouling. Among the CVD methods employed, iCVD is the most common technique, likely due to its high retention of functional moieties, which ensures strong hydrophilicity and thus fouling resistance. Furthermore, its benign reaction conditions allow the fragile membrane substrates to be surface modified in a non-destructive manner.
The iCVD technique has produced poly(2-hydroxyethyl methacrylate) (pHEMA) and poly(1-vinyl-2-pyrrolidone) (pVP) coatings with water contact angles (WCA) of 17° and 11° respectively, although their fouling resistance was not quantified.42 Also using iCVD, coatings of poly(hydroxypropyl methacrylate) (pHPMA) have been deposited onto poly(vinylidene fluoride) (PVDF) hollow fiber membranes for the purification of aerobically digested effluent from palm oil mills. With the WCA of 23.6° and reduced membrane surface roughness, the coated membranes exhibited as much as 80% improvement in flux and selectivity compared to the uncoated membranes. The tests were performed with synthetic aerobic digestates that are rich in organic foulants like lignin, tannic acid, and bovine serum albumin (BSA).111 Compared to the hydrophilic homopolymers discussed above, hydrophilic copolymers can be advantageous as they allow for further tuning of coating structures by adjusting the degree of cross-linking. For instance, hydrophilic poly(1-vinyl-2-pyrrolidone-co-ethylene glycol diacrylate) (pVP-co-EGDA) coatings have been synthesized as antifouling coatings for PVDF membranes via iCVD.112 Lowering the degree of cross-linking (by reducing the proportion of EGDA) in the copolymer resulted in a monotonic increase in surface hydrophilicity, with the least cross-linked copolymer achieving WCA = 33°; nonetheless, a higher degree of cross-linking favors more robust coatings. To resolve this dilemma, the authors introduced to graded copolymer architecture– with a hydrophilic pVP-co-EGDA topcoat directly grafted from a highly-cross-linked pEGDA basal layer – via a sequential iCVD deposition scheme. The resultant thin film composite has proved to be both robust and hydrophilic, capable of warding off both BSA (92% reduction) and E. coli cells. Further research into optimizing both compositional and architectural design of CVD copolymer thin films will likely bring about coatings with more well-rounded performance.
Zwitterionic polymers, with covalently linked cationic and anionic groups but neutral charge overall, are commonly employed in antifouling studies.113 Their fouling resistance is rooted in the strongly bound hydration layer around the zwitterionic moieties, imposing a large enthalpic penalty for the potential replacement of this layer by biofoulants.11 Ultrathin (~30 nm) zwitterionic polymer coatings have been synthesized in iCVD using a two-step procedure [Figure 2 (A), (C) and (E)]. In the first step, poly(2-dimethylamino ethyl methacrylate-co-ethylene glycol dimethacrylate) (pDMAEMA-co-EGDMA) was deposited and grafted onto commercially available RO membranes. In the second step, zwitterionic sulfobetaine structures were obtained by treating the pDMAEMA-co-EGDMA coating with a vapor of 1,3-propanesultone.114 The iCVD zwitterionic coatings prevented the attachment by Escherichia coli from a concentration bacteria culture and under static conditions, without impairing the membrane selectivity or permeability.114 The zwitterionic polymers were later re-designed to bear pyridine-based sulfobetaine side groups to improve the chemical stability of the antifouling coating.108 The pyridine-based zwitterionic polymer chemistry remained unchanged after soaking in a concentration chlorine solution. The zwitterionic coating mitigated biofouling by a marine bacterium, Vibrio cyclotrophicus, and the fouling resistance can be further boosted by chlorine at low concentration (5 ppm).108
Figure 2. Antifouling CVD thin films for separation membranes.
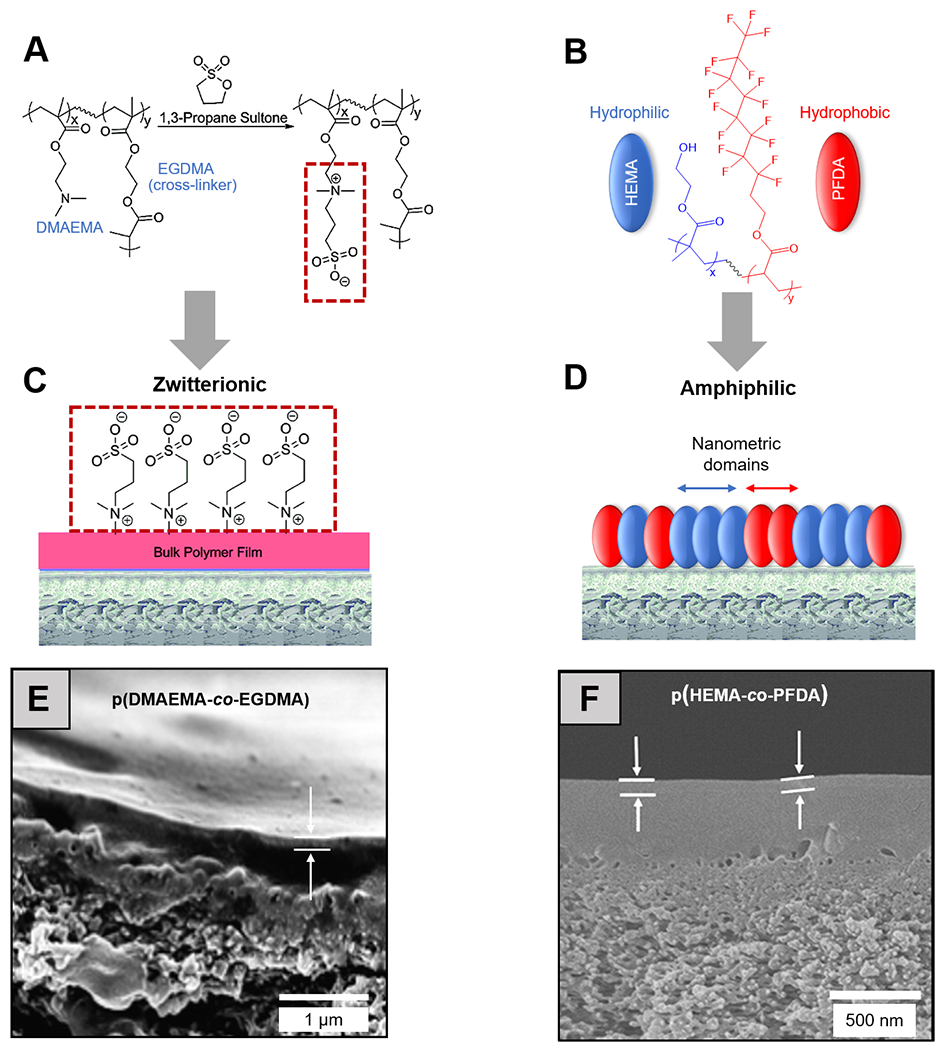
Molecular structures of (A) a zwitterionic polymer, synthesized by vapor-phased derivatization of an iCVD pDMAEMA-co-EGDMA thin film, and (B) an amphiphilic polymer, pHEMA-co-PFDA, with the hydrophilic and hydrophobic functional groups colored in blue and red respectively. Schematic representations of (C) zwitterionic and (D) amphiphilic antifouling coatings deposited on off-the-shelf reverse osmosis membranes.106 Reprinted with permission from R. Yang, J. Xu, G. Ozaydin-Ince, S. Y. Wong and K. K. Gleason, Chem. Mater., 2011, 23, 1263-1272. Copyright 2011 American Chemical Society. Cross-section SEM images of the membranes coated with (E) zwitterionic and (F) amphiphilic thin films; white arrows indicate the thickness of the iCVD antifouling coatings.106,108 Reprinted from Surface modification of reverse osmosis desalination membranes by thin-film coatings deposited by initiated chemical vapor deposition, 539, Gozde Ozaydin-Ince, Asif Martin, Zafarullah Khan, S.M. Javaid Zaidi, Karen K. Gleason, 181-187, 2013, with permission from Elsevier.
3.1.2. Amphiphilic Polymer Coatings
Amphiphilic copolymers are comprised of both hydrophilic and hydrophobic repeat units. They have demonstrated great potential as an antibiofouling chemistry, because their characteristic molecular-scale heterogeneity in surface energy is believed to create an entropic barrier for protein and cell adhesion.65,115
The CVD techniques have unique advantages for the synthesis of amphiphilic copolymers because of their solvent-free nature. Solution-based synthesis of amphiphilic chemistry usually involves lengthy processes of frequent solvent switches, in order to obtain the covalently linked hydrophilic and hydrophobic domains; whereas in all-dry synthesis processes like CVD, the monomers with no common solvents could be simply vaporized and co-delivered into a vacuum chamber and polymerize into a random copolymer on a substrate surface. In an attempt to render RO membranes antifouling, perfluorodecylacrylate (PFDA) and HEMA were copolymerized using iCVD to form an amphiphilic coating, which reduced the surface adhesion of BSA and E. coli cells [Figure 2 (B), (D) and (F)].116
3.1.3. Conductive Polymer Coatings
Conductive polymer coatings could be applied to membranes as a means of monitoring membrane integrity, and/or improving separation of charged particles, and/or boosting membrane fouling resistance.117 The aforementioned approaches (see Section 2.4), namely oCVD and VDP, have both been used to deposit pEDOT and its derivatives on membranes.
Using VDP, pEDOT coatings with the conductivity of 2000 S/cm have been deposited on anodic aluminum oxide (AAO) membranes conformally, giving rise to high-aspect-ratio pEDOT nanotubes after dissolution of the AAO membrane template.96 The conductive coatings could be used to as electrodes in bioelectronics or to control the transport of charged biomolecules for membrane separations. However, the conductive coatings also must resist the non-specific adhesion of biomolecules, i.e. fouling resistance, to remain effective in the long term. To achieve that goal, pEDOT coatings have been further derivatized to form zwitterionic moieties.118 pEDOT thin films deposited using oCVD have been exposed to 1,3-propanesultone to render pEDOT zwitterionic. Interestingly, that chemical modification of the pEDOT structure did not reduce the conductivity of the material – in fact, it increased slightly from 538 to 668 S/cm.118 Nonetheless, the zwitterionic pEDOT moieties did not outperform conventional pEDOT in a fouling propensity assessment performed using molecular force probe, and both had slightly greater interactions with various model foulants than the pyridine-based zwitterionic thin films synthesized using iCVD.108,118
3.1.4. Stimuli-responsive Polymer Coatings
Stimuli-responsive polymers can change material properties (e.g. charge, molecular assembly, hydrophilicity, etc.) in response to environmental cues (e.g. temperature, pH, electromagnetic field, chemical potential, etc.).119 Capitalizing on that stimuli-responsiveness, ‘smart membranes’ have been fabricated using the CVD techniques, which could adjust selectivity and/or permeability in response to changes in pH or temperature.
Using iCVD, thin films of poly(methylacrylic acid-co-ethylene glycol dimethacrylate) (pMAA-co-EGDMA) have been deposited onto AAO membranes.120 The iCVD technique provided conformal coating coverage of the pore walls, rendering the membrane permeability responsive to changes in the pH of the feed as shown in Figure 3(A) and (C). The coatings swell at pH levels above the pKa of pMAA (~ 4.8), due to the electrostatic repulsion among the deprotonated carboxylic acid groups, shrinking the pore sizes and thus reducing the membrane permeability; whereas at pH levels below the pKa, the carboxylic aid groups become neutral and the coatings thus collapse to allow greater permeability. That pH responsiveness was verified by demonstrating a greater permeability of water, or polystyrene nanoparticles, or dissolved polyacrylic acid (with molecular weight of ~100 kDa) at the pH of 3 than that at the pH of 9.120 Also using iCVD, a pH-responsive membrane that separates small molecule (glucose) and protein (BSA) has been fabricated,121 where thin films of poly(maleic anhydride-co-dimethylacrylamide-co-diethylene glycol divinyl ether) (pMaDD) were deposited onto AAO membranes. In aqueous environments, the maleic anhydride repeat-units readily hydrolyze to produce two carboxylic acid groups, giving rise to pH-dependent swelling of the coating and thus regulation of membrane permeability.
Figure 3. Stimuli-responsive polymer thin films for the design of smart membranes.
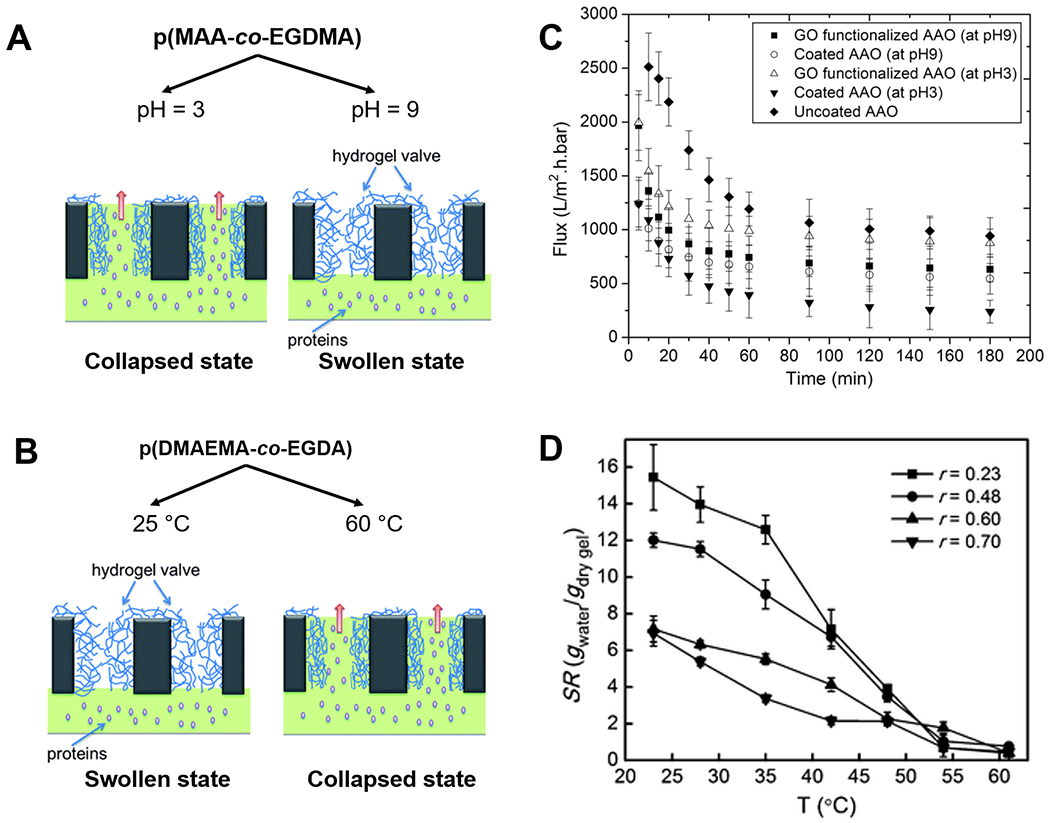
Mechanisms of membrane flux regulation in response to (A) pH and (B) temperature changes, enabled by thin films of pMAA-co-EGDMA and pDMAEMA-co-EGDA respectively. (C) pH-responsive flux changes of BSA through filtration membranes coated with pMAA-co-EGDMA.112 Reprinted from Smart membranes with pH-responsive control of macromolecule permeability, 537, Ali Tufani, Gozde Ozaydin-Ince, 255-262, 2017, with permission from Elsevier. (D) Swelling ratios of pDMAEMA-co-EGDA hydrogels with different compositions (r indicates the molar ratio of EGDA to DMAEMA) under different temperatures.60 Reproduced from Ref. 60 with permission from The Royal Society of Chemistry.
Temperature-responsiveness has been achieved with functional polymer coatings containing poly(N-isopropylacrylamide) (pNIPAAm) and/or poly(N,N-dimethyl aminoethyl methacrylate) (pDMAEMA) films [Figure 3(B)].57,122,123 A membrane, whose permeability can be regulated by temperature, has been fabricated by depositing poly(N,N-dimethyl aminoethyl methacrylate-co-ethylene glycol diacrylate) (pDMAEMA-co-EGDA) coatings (with thicknesses of 50 nm) onto nanoporous polycarbonate membranes using iCVD.57 In Figure 3(D), as temperature increases into the range of 32-50 ºC [i.e. above the lower critical solution temperature (LCST) of pDMAEMA-co-EGDA], the hydrophilic side chains on the DMAEMA repeat-units start to dehydrate, transitioning from hydrogen-bonding-dominated state to intramolecular bonding-dominated state and leading to collapsed and thus thinner coatings and larger pores. At 60 ºC, flux of BSA through the porous membranes was proven to be 10 times that at 25 ºC.
In summary, stimuli-responsive polymer thin films have enabled countless design of smart membranes, which could regulate their performance in response to environmental cues. Furthermore, some of those polymer coatings have been applied in biosensors (Section 3.2), drug delivery (Section 3.3), and tissue engineering (Section 3.4).
3.2. Biosensing and Bio-MEMS
Interface engineering is critical to biosensing and bio-MEMS, as surface properties (e.g. wetting properties, binding to biomolecules, etc.) are often the key for such devices to execute their functions. As a result, the fabrication and/or performance enhancement of biosensing apparatus and microfluidic devices commonly require the surface modification by functional polymer coatings.
The CVD techniques are often the preferred coating approach because of their conformality. To enable sensitive detection and accurate manipulation at the bio-interface, biosensors and bio-MEMS devices are often fabricated with surface micro- and nanostructures, boosting their surface-to-volume ratio. The CVD techniques allow conformal coverage of those surface structures by functional polymer thin films, enabling the modification of surface chemistry without altering the underlying structures.124,125 Table 3 summarizes the functional CVD polymers used for the biosensors and bio-MEMs applications highlighted in this review.
Table 3.
Functional CVD polymers for biosensing and bio-MEMS.
| Polymer | Acronym | Property / Performance | CVD Technique |
|---|---|---|---|
| Poly(2-hydroxyethyl methacrylate) | pHEMA | Hydrophilic, Biocompatible / Antifouling | piCVD66,iCVD134 |
| Poly(trivinyl trimethyl cyclotrisiloxane–hexavinyl disiloxane) | pV3D3–HVDS | Silicone-based, Hydrophobic / Insulating, Antifouling | iCVD128 |
| Poly(p-xylylene) | pPX | Hydrophobic / Biofunctionalizable, Adhesive | CVP129,138,139 |
| Poly(glycidyl methacrylate) | pGMA | Hydrophobic / Adhesive | iCVD135,137 |
| Poly(allylamine) | pAAm | Hydrophobic / Adhesive | iCVD135 |
| Poly(amino styrene) | pAS | Hydrophobic / Adhesive | iCVD136 |
3.2.1. Biosensors
Thin films of pHEMA are commonly deposited on biosensing devices because of their fouling resistance and biocompatibility.126 pHEMA has been shown to cause less inflammation and thrombosis than other polymers.66,127 In bioelectric sensing, poly(trivinyl trimethyl cyclotrisiloxane–hexavinyl disiloxane) (pV3D3–HVDS) has been used for its insulating nature and long-term stability.128 Poly(p-xylylene) (pPX) coatings have been used to boost the sensitivity of fluorescent imaging129 and to fabricate sensor arrays used in label-free molecular detection.130 Furthermore, the CVD techniques have been used to fabricate stand-alone polymer-based biosensors, which have been proven effective and long-lasting in vivo.131
A sodium-sensing optode has been coated with lightly cross-linked pHEMA films using piCVD.66 To ensure that the response time and sensitivity of the sensors remain unchanged, thickness of the coating was controlled to be ~100 nm and mesh size of the cross-linked network to be over 2 nm. The pHEMA coating effectively reduced the non-specific protein binding to the detection surface; X-ray photoelectron spectroscopy (XPS) indicated that the level of which was one eighth that on a pristine sensor. The piCVD technique has also been extended to coat particles without agglomeration, for potential application in particle-based sensors.66 The coated sensor with prolonged lifetime could be used in patients susceptible to hypernatremia.132 Thin films of pHEMA has also been deposited using iCVD onto the microelectrodes used in impedance sensors, improving contact at the electrode-electrolyte interface in biosensors while resisting biofouling.133 The pHEMA coatings improved the signal reproducibility and device stability, enhancing the performance of electrochemical impedance spectroscopy that is frequently used in biosensing.
To fabricate a probe to detect neural activities, a silicone-based polymer pV3D3–HVDS has been deposited using iCVD as an insulating coating.128 The coating improved biocompatibility of the coated probe compared to the uncoated stainless-steel probes, as demonstrated in vitro by the reduced surface adsorption of fibronectin and presence microglia cells in the vicinity of the probe. Astrocyte reactivity near the pV3D3–HVDS-coated probes was also lower than that on the other surfaces, indicating lower propensity to glial scar formation near the neural implants.
Biosensing frequently resorts to fluorescent imaging for the visualization of cells or other living organisms in situ at high spatial resolution. The inadequate signal-to-noise ratio, which is inherent to some biological systems, has been overcome using poly(p-xylylene) coatings.129 Leveraging fluorescence interference, the coating thickness was precisely controlled using the CVD technique, so that the test area was made with the thickness that enhanced fluorescence signals and the reference area with the thickness that diminishes them. Furthermore, the versatile functional moieties offered by CVD polymer thin films promised facile biofunctionalization for specific binding with target molecules (Figure 4). These CVD-modified imaging substrates demonstrated ultrasensitive detection of target biomolecules in situ.
Figure 4. Multifunctional CVD polymer thin films for biological sensing.
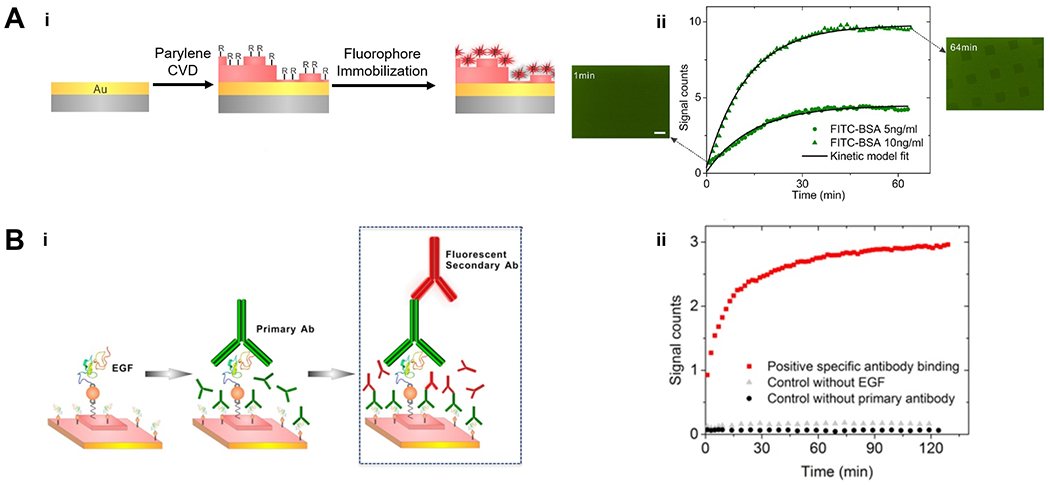
(A) Fabrication (i) and application (ii) of the substrates used to amplify fluorescent signals. Gold-coated glass was functionalized with poly(p-xylylene) thin films via CVD polymerization, which allowed the subsequently immobilization of fluorophores via physical adsorption or covalent bonds. The functionalized substrate enabled real‐time monitoring of FITC‐BSA adsorption. (B) Fabrication (i) and application (ii) of the substrates used to monitor antibody binding kinetics. Functionalized poly(p-xylylene) thin films allowed the immobilization of Epidermal Growth Factor (EGF) and subsequently a primary antibody (anti-EGF), enabling the monitor of binding kinetics of a secondary antibody conjugated with the Alexa 647 (a fluorophore) to the primary antibody.121 Copyright 2016, reproduced with permission from John Wiley and Sons.
Stand-alone polymer biosensors, which do not rely on pre-fabricated sensing devices have been enabled by the CVD techniques, and proven effective in vivo for the detection of physiological changes in test subjects in real time.131 To fabricate the polymer biosensors suitable for subcutaneous application and prolonged local retention time, pHEMA thin films were deposited conformally onto AAO-membrane templates using the iCVD technique, giving rise to pHEMA polymer nanotubes (Figure 5). The polymer nanotubes were subsequently filled with liquid optode, and their ends sealed with another layer of iCVD pHEMA (~50-nm thick). The tubular shape gave rise to a large hydrodynamic radius and thus prolonged retention time at the site of interest, which was demonstrated with subcutaneous injection in a mice model. The polymer biosensors achieved a reduction of 60% of the diffusion away from the injection site, while providing rapid response to sodium level changes in the surrounding tissue. The response dynamics of those polymer biosensors could be tuned by adjusting the cross-linking density of the pHEMA coating, which has been achieved by simply tuning the feed composition during iCVD.134
Figure 5. CVD polymer coatings for in vivo biomedical sensing.
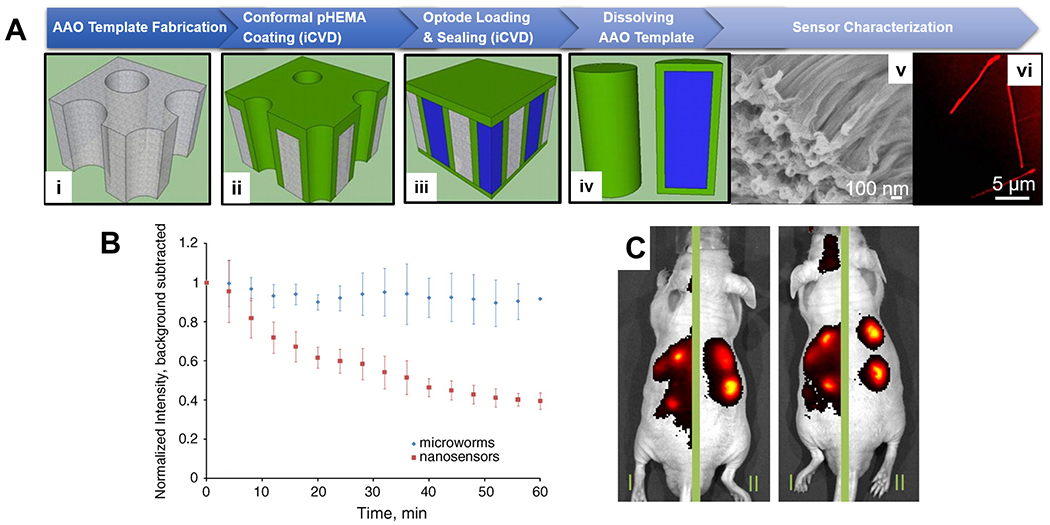
(A) Fabrication (i-iv) and imaging (v-vi) of in vivo sodium sensors, termed microworms, enabled by the iCVD technique. Thin layers of pHEMA was deposited conformally onto AAO membranes, the pores of which were then filled with a sodium-responsive optode solution (liquid in blue) and sealed via a subsequent pHEMA deposition. Shape of the fluorophore-encapsulating microworms was confirmed with SEM and confocal microscopy. (B) Decay of the normalized fluorescent intensity over time at the site of subcutaneously injection of the in vivo sensors. The microworms (blue diamonds) exhibited longer local retention time. (C) IVIS images of animals that received regular nanosensors on the left-side of their body and the microworms on the right side, showing greater localization and retention of the microworms after injection.123
3.2.2. Bio-MEMS
Bio-MEMS, i.e. biomedical microelectromechanical systems, have become a staple technology used in the fundamental investigation of cell biology and in applied sciences like organs-on-a chip. The ability control surface chemistry, which plays a pivotal role in both device fabrication and application (e.g. modulating the interactions at the bio-interface), could be realized using the CVD techniques. The high coating conformality and consistency offered by CVD techniques render them uniquely suitable for the surface modification of microfluidic channels, which typically have high aspect ratios and sometimes surface microstructures.
Coupling reactions enabled by CVD polymer thin films allowed robust bonding of bio-MEMS device components under benign reaction conditions, preventing liquid leakage while avoiding the harsh reaction conditions commonly used during bonding that could impair device performance. For example, the reaction between amine and glycidyl groups have been achieved by depositing poly(glycidyl methacrylate) (pGMA) on one part of the device and poly(allylamine) (pAAm) or poly(amino styrene) (pAS) on the other, and then pressing the two parts together to form strong covalent bonding.135,136 That approach has led to a device burst pressure of over 150 psi. Remarkably, that strong bonding remained unchanged after 3 months of storage. The burst pressure has been further improved to over 300 psi, by coupling two pGMA-coated surface using ethylenediamine (Figure 6).137
Figure 6. Functional polymer thin films for bio-MEMS.
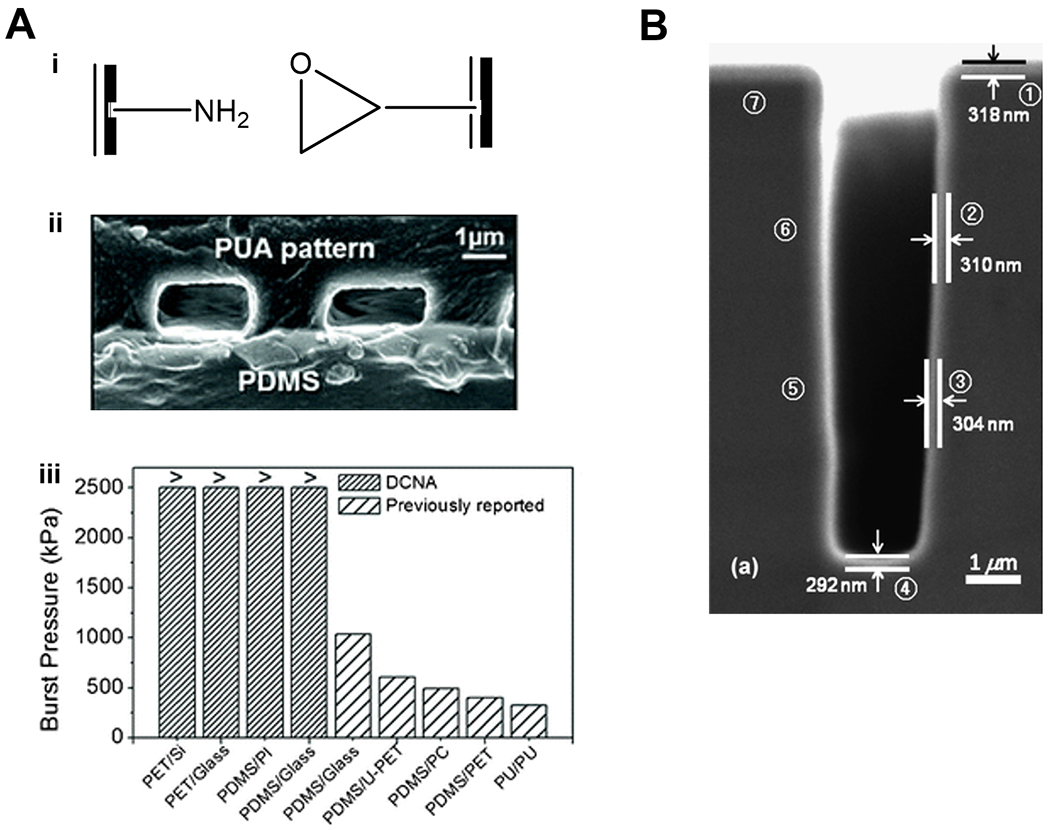
(A) Binding mechanism (i), imaging (ii), and performance (iii) of MEMS devices enabled by CVD polymers. The two compartments of the MEMS device were coated with polymer thin films bearing amine moieties and glycidyl moieties respectively for the facile and strong binding at the surface of contact. Successful binding was confirmed with cross-sectional SEM image and burst pressure measurements. Reproduced from Ref. 129 with permission from The Royal Society of Chemistry. (B) A cross-sectional SEM image of pGMA coating deposited conformally on a micro-trench (2 µm × 8 µm) etched into a silicon wafer. Reproduced from Ref. 127 with permission from The Royal Society of Chemistry.
Bio-MEMS devices are often fabricated with polydimethylsiloxane (PDMS), and surface functionalization of the microfluidic channels is thus required to control cell-surface interactions or the adhesion strength of biomolecules. The main CVD approach for that purpose has been the CVD of poly(p-xylylene) coatings. Poly(p-xylylene carboxylic acid pentafluorophenol ester-co-p-xylylene) has been deposited onto the luminal surface of microfluidic channels.138 The pentafluorophenol groups borne by the coating were rapidly substituted by biotin ligands (within seconds) in a second step, providing specific interactions with biomolecules and/or cell receptors. Using that device, the effect of echistatin and disintegrin on cell adhesion was illustrated. Building upon that proven fabrication procedure, thin films of poly(4-benzoyl-p-xylylene-co-p-xylylene) were deposited onto the luminal surface of a microfluidic device. The benzoyl groups were activated to form free radicals at the wavelengths of ~340 nm, allowing the subsequent surface patterning and immobilization of biomolecules.139
3.3. Drug Delivery
The development of functional and biocompatible polymers has been the foundation of drug delivery research, enabling targeted delivery of therapeutics to specific organs, programmed release upon external triggers, and sustained dosing over the course of treatment. Notwithstanding the ubiquitous application of solution-synthesized polymers in drug delivery, CVD techniques have started to demonstrate advantages that could improve the design of delivery vehicles. Unlike solution-based formulation approaches, where therapeutics are continuously dispersed throughout the polymer matrix, CVD polymer coatings could form a distinct encapsulation layer (i.e. without dispersed therapeutics). Moreover, CVD techniques commonly offer control of the coating thickness to the precision of tens of nanometers, allowing accurate tuning of the release kinetics (Section 3.3.1). Furthermore, stimuli-responsive CVD polymer coatings could be used as the encapsulation layer, enabling precise control of the response rate and magnitude (Section 3.3.2). Table 4 summarizes the functional CVD polymers that have played a key role in the drug delivery applications discussed in this section.
Table 4.
Functional CVD polymers for drug delivery.
| Polymer | Acronym | Property / Performance a | CVD Technique |
|---|---|---|---|
| Poly(2-hydroxyethyl methacrylate) | pHEMA | Hydrophilic, Swelling behavior / Controlled drug release | iCVD140,141,149,210 |
| Poly(methacrylic acid) | pMAA | pH-responsive, Hydrophilic / Triggerable drug release | iCVD140,141,149 |
| Poly (perfluorodecylacrylate) | pPFDA | Hydrophobic / Controlled drug release | iCVD141 |
| Poly(N,N-dimethyl aminoethyl methacrylate -co-ethylene glycol diacrylate) | pDMAEMA-co-EGDA | Hydrophilic / Controlled drug release | iCVD142 |
| Poly(4-vinylpyridine-co-ethylene glycol dimethacrylate) | p4VP-co-EGDMA | pH-responsive, Amphiphilic / Triggerable drug release | iCVD143 |
| Poly(methacrylic acid-co-ethylene dimethacrylate) | pMAA-co-EDMA | pH-responsive, Hydrophilic / Triggerable drug release | iCVD144,147 |
| Poly(methacrylic anhydride-co-methacrylic acid) | pMAH-co-MAA | pH-responsive, Amphiphilic / Triggerable drug release | iCVD148 |
| Poly(N-isopropylacrylamide) | pNIPAAm | Temperature-responsive, Hydrophilic / Triggerable drug release | iCVD149 |
Controlled drug release and triggerable drug release are different: the latter requires that the release of drug can be triggered by one or multiple environmental stimuli (e.g. pH and/or temperature); whereas the former emphasizes the capability to modulate the kinetics of drug release, without specifying any control over the onset of the release process.
3.3.1. Engineering Release Kinetics
CVD polymer coatings have been applied as an encapsulation layer to prevent burst release,140 to accelerate the controlled release of poorly soluble drugs,141 or to tune the kinetics of sustained release in general.142 Most applications employ the iCVD technique because it is free of high-energy plasma or high temperature, preventing degradation of the therapeutics during the coating process.
Using iCVD, polymer chemistry has been engineered to control drug release kinetics. To accelerate the release of clotrimazole, a drug commonly used to treat fungal infections, pHEMA, pMAA, and pPFDA have been deposited to provide sustained release while preventing the formation of slow-dissolving clotrimazole crystals.141 Although pPFDA was proven ineffective, pHEMA and pMAA encapsulation layers both reduced the crystallization of clotrimazole during storage. The release kinetics of clotrimazole, before and after encapsulation by 500-nm pHEMA or pMAA, were compared in a subsequent study, where clotrimazole was first loaded onto fabrics prior to the encapsulation via iCVD.140 The pHEMA and pMAA coatings did not meaningfully change the release profile of clotrimazole loaded onto cotton fibers. With the polycaprolactone fabric and poly(ethylene terephthalate) fabric, the release profile was close to zeroth-order release for the ones coated with pMAA, and between zeroth-order and burst release for the ones coated with pHEMA, although the molecular origin was unclear. The coated and clotrimazole-containing fabrics could be used as wound dressing.
Coating thickness and cross-linking density could also be controlled precisely to achieve the desired release kinetics. Using iCVD, the aforementioned poly(N,N-dimethyl aminoethyl methacrylate -co-ethylene glycol diacrylate) (pDMAEMA-co-EGDA) coating (see Section 3.1.1) has been applied on top of a layer of sirolimus (an antiproliferative drug) and atorvastatin (a cholesterol-lowering drug) that were drop-casted onto stainless steel substrates.142 At the cross-linker composition of 56% and coating thickness of 300 nm, atorvastatin followed zeroth-order release over the course of 30 days, whereas lower cross-linking densities led to varying degrees of burst release and release plateau, so did reducing coating thickness to 100 nm. The substrate-independent nature of the iCVD technique makes it possible to apply the encapsulation approach to fabricate drug-eluting coronary stents.
The drug release kinetics of nano-carriers could be controlled by depositing a stimuli-responsive coating. Electrospun poly(vinyl alcohol) (pVA) nanofibers loaded with chemotherapeutic agent Rose Bengal (RB) were coated with a pH-sensitive polymer poly(4-vinylpyridine-co-ethylene glycol dimethacrylate) (p4VP-co-EGDMA) by iCVD for brain cancer treatment.143 In this study, RB release from the coated nanofibers was investigated at different pH values to evaluate their stability and anti-cancer activity in in vitro cell culture experiments. The stability tests showed that uncoated nanofibers dissolved completely in <2 h at all pH values tested (4 to 9), while coated pVA-RB nanofibers showed high stability at neutral and basic pH values over an incubation period of 72 h. The drug release experiment demonstrated 80% release in 1 h for uncoated nanofibers at all pH, while coated pVA-RB nanofibers showed slower release kinetics at lower pH values and less than 60% release in the first hour for all nanofibers tested. The coating was proved to be biocompatible using glioblastoma multiforme cancer cells (U87MG). In summary, the iCVD coatings could render drug delivery vehicles responsive to the pH of the biological environment. Nevertheless, its applicability in cancer treatment needs to be further validated using appropriate animal models.
3.3.2. Enabling Triggerable Drug Release
Using stimuli-responsive polymers as drug delivery vehicles could enable the triggerable release of therapeutics upon external signals (e.g. light, pH, temperature, etc.). Tissue pH and temperature are the two most commonly used triggers.
Polymers containing carboxylic acid groups are often used to fabricate delivery systems that are triggerable by changes in pH. The iCVD technique has been used to make a pH-responsive delivery vehicle by depositing copolymers of MAA and ethylene dimethacrylate (EDMA) onto ibuprofen microcrystals (<100 µm in size).144 The pMAA-co-EDMA thin films remained collapsed at pH values lower than the pKa of pMAA of ~4.8 (see Section 3.1.4), minimizing drug release in the stomach; whereas the copolymer thin films swell at pH values greater than 4.8, triggering enteric drug release. The system could thus be used to deliver enteric therapeutics that may cause irritation to the stomach.145,146 The pMAA-co-EDMA coatings have also been used to functionalize biodegradable porous silicon, giving rise to a pH-responsive drug-delivery nano-composite.147 Camptothecin (CPT), an anticancer natural product, was loaded into porous silicon nanodevices via adsorption from a drug solution, and the nanodevice was subsequently encapsulated with 350-nm pMAA-co-EDMA using iCVD. At the pH values of 1.8 and 7.4, the released drug amount from the coated devices was ~10.7% and ~44.5% that from uncoated devices, respectively. Furthermore, by replacing EDMA with a hydrolyzable cross-linker, methacrylic anhydride (MAH), onset of the pH-triggered release could be controlled.148 The triggerable release of rifampicin (an antibiotic) was realized by loading the drug into polylactide membranes, followed by encapsulation with pMAH-co-MAA. The onset of release was 8, 2, and 0.5 hours respectively, when the membranes were soaked in solutions with the pH values of 1, 7.4, and 10, demonstrating faster release under alkaline conditions.
As a proof-of-principle for using temperature as a trigger for drug release, fluorescein was loaded into the pHEMA inner layer at elevated temperature (80°C), which swelled and induced stress on the shape-memory outer layer. Cooling of the polymer nanotubes to room temperature froze the swollen temporary shape and preserved the fluorescein; whereas bringing the fluorescein-loaded nanotubes back to 80°C led to burse release. The high temperature required to trigger drug release might be reduced by further molecular design of the shape-memory polymer. Similar temperature-triggered release behavior has been achieved with CVD pNIPAAm, also in a coaxial polymer nanotube device using phloroglucinol dye as a proof-of-principle.149 pNIPAAm is a material commonly used in drug delivery because its LCST is around body temperature, enabling effective triggerable drug release upon in vivo application.150,151
3.4. Tissue Engineering
Tissue engineering, where chemicals and materials are employed to enhance mammalian cell growth and to direct cell differentiation and/or functions, relies on the precisely controlled and well-defined surface structures and chemistry.152 Solution-based coating methods often fall short in coating structurally complex substrates, whereas the CVD techniques offer an alternative that provides conformal coverage over nano- and micro-structures. They have been used to create non-toxic bio-interfaces on micro-structured 3D scaffolds, which were proven beneficial for cell growth and harvest.25
A wide selection of functional moieties is compatible with the CVD techniques, some of which allow further surface conjugation with bioactive molecules. The mechanisms for bio-functionalization and types of biomaterials discussed in this section are summarized in Figure 7. The recent progress in tissue engineering fueled by CVD polymer thin films is reviewed under two categories: controlling cell adhesion and proliferation (3.4.1) and directing cell differentiation and cellular functions (3.4.2). Table 5 summarizes the CVD polymers discussed in this section, which have been applied to tissue engineering applications.
Figure 7.
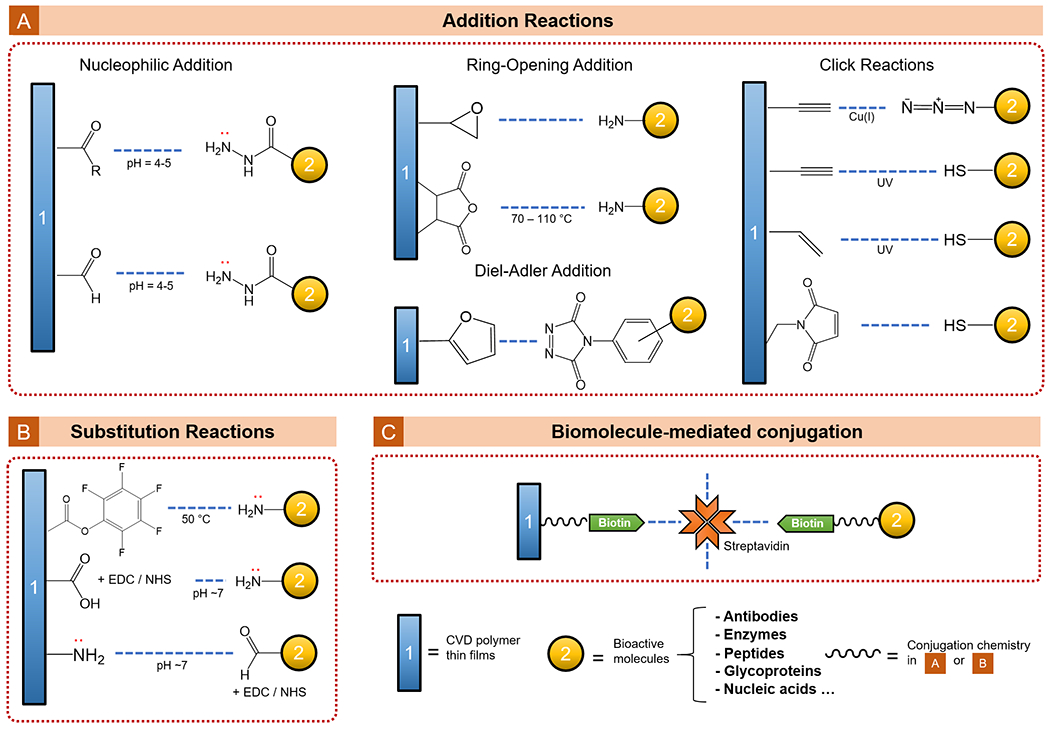
CVD functional polymer thin films that enabled bio-functionalization for tissue engineering.
Table 5.
Functional CVD polymers for tissue engineering.
| Polymer | Acronym | Property / Performance | CVD technique |
|---|---|---|---|
| Poly(acrylic acid) | pAA | Hydrophilic, Biofunctionalizable / Controlling cell adhesion and proliferation | PECVD154 |
| Poly(2-hydroxyethyl methacrylate) | pHEMA | Hydrophilic / Controlling cell adhesion and proliferationx | iCVD54,155, PECVD156,piCVD159 |
| Poly(allylamine) | pAAm | Hydrophilic, Biofunctionalizable / Controlling cell adhesion and proliferation | PECVD158 |
| Poly(2-hydroxyethyl methacrylate-co-ethylene glycol diacrylate-co-pentafluorophenyl methacrylate) | pHEMA-co-EGDA-co-PFM | Hydrophilic with swelling ability, Biofunctionalizable / Controlling cell adhesion and proliferation | iCVD52 |
| Poly(p-xylylene) | pPX | Hydrophobic, Biofunctionalizable / Controlling cell adhesion and proliferation, Directing cell differentiation | CVP161–164 |
| Poly(N-isopropylacrylamide) | pNIPAAm | Temperature-responsive, Hydrophilic, Biofunctionalizable / Controlling cell adhesion and proliferation | iCVD157 |
| Poly(perfluorodecylacrylate) | pPFDA | Hydrophobic / Directing cell differentiation | iCVD165 |
| Poly(glycidyl methacrylate) | pGMA | Biocompatible, Mechanically stable, Hydrophobic, Biofunctionalizable / Directing cell differentiation | iCVD165,167 |
3.4.1. Controlling Cell Adhesion and Proliferation
Conventionally, plasma-deposited polymer thin films have been widely applied to control the adhesion of cells on surfaces of implantable devices ranging from vascular prosthesis, intraocular lens, to implantable electrode and catheters.153 Acrylic acid (AA) and isopropyl alcohol are often used as precursors in the PECVD process, which could promote the controlled adhesion of human fibroblast cells while reducing non-specific protein binding and thrombosis.154 CVD-deposited pHEMA has also been commonly used to improve the controlled cell adhesion and surface proliferation because of its known biocompatibility.155,156 More recently, further development of the library of CVD polymers has led to new functional moieties, allowing the immobilization of bioactive molecules for specific ligand-receptor interactions (e.g. peptides that promote cell adhesion, proteins that induce cellular functions etc.) and stimuli-responsive tissue scaffolds and implants.157
Thin films of pAAm synthesized using PECVD promoted the adhesion of 3T3 fibroblasts in vitro (compared to a plasma-polymerized hexane surface), an effect believed to be a result of the greater surface affinity of pAAm for fibronectin than that for albumin.158 In comparison, vapor-deposited pHEMA has been a much more common choice of surface coating in tissue engineering.54,155 Thin films of iCVD pHEMA was shown to be free of remnant small-molecule chemicals, and thus supported the surface adhesion and proliferation of human dermal fibroblasts over the course of a week.155 Using Caco-2 cells (a heterogeneous human epithelia colorectal adenocarcinoma cell line), cross-linked pHEMA films (~3-µm thick) synthesized using piCVD demonstrated comparable cell adhesion and viability to that obtained on standard polystyrene tissue-culture apparatus.159
The surface adhesion and proliferation of cells could be further controlled, both spatially and temporally, via surface-grafting of bioactive molecules and stimuli-responsive coatings enabled by the growing library of CVD polymer chemistries. Hydrogel thin films, with surface-conjugated Arg-Gly-Asp (RGD) peptides to control cell adhesion, have been synthesized by copolymerizing HEMA, EGDA, and pentafluorophenyl methacrylate (PFM) via iCVD and subsequent bio-functionalization.52 The immobilization of RGD peptides occurred via nucleophilic substitution of the pentafluorophenyl group,160 which promoted the adhesion and growth of Human Umbilical Vein Endothelial Cells (HUVEC).52
The surface grafted RGD peptides were later used to improve biocompatibility of intraocular lenses. By depositing poly(vinyl-p-xylylene) on droplets of non-volatile liquids (e.g. silicone oil, ionic liquids), intraocular lenses have been fabricated.161 The vinyl functional groups afforded by the polymer coatings allowed subsequent grafting of (i) poly(ethylene glycol) (PEG) over the lens optic, to retain clarity by preventing cell adhesion; (ii) RGD peptides (Arg-Glu-Asp-Try-Try-Cys) over the lens haptic, to improve comfort by enhancing cell adhesion. Photo-activated thiol-ene click reactions were used for the surface grafting. The intraocular lenses successfully controlled cell adhesion spatially, as demonstrated using human lens epithelial cells, human corneal epithelial cells, and mouse embryonic fibroblasts.
The temporal control of cell adhesion and growth was achieved by using stimuli-responsive CVD polymers. The timed cell retrieval was achieved via microgrooves (a type of template used to grow longitudinal tissue constructs), conformally coated by iCVD pNIPAAm.157 The coated microgrooves guided the formation of NIH-3T3 fibroblasts tissue constructs at 37 ºC, and enabled the detachment of those constructs at room temperature (24 ºC) due to the great degree swelling of pNIPAAm at temperatures below its LCST (~32 ºC). The study provided an approach to form and retrieve tissue constructs with well-defined geometry on cue.
3.4.2. Directing Cell Differentiation and Cellular Functions
The ability to control cell differentiation using biomaterials, such as osteoinductive materials in bone regeneration, is a critical aspect of tissue engineering. Unlike systemically administered inductive compounds (e.g. DNA or proteins delivered via viral particles), CVD polymer coatings offer the precise placement of the inductive signals at the material-cell interface, avoiding systematic side effects and non-specific targeting.162
To enhance the in vitro osteoblast differentiation of bone marrow stromal cells, adenovirus that expresses runt-related transcription factor 2 (Runx2, a master regulator of osteoblast differentiation) has been conjugated to anti-adenovirus antibody immobilized on a vapor-deposited polymer thin film.162 The polymer thin film, poly(p-xylylene carboxylic acid pentafluorophenol ester-co-p-xylylene), was deposited on a frequently used scaffold material, poly(3-caprolactone), which is known for its biodegradability and biocompatibility. The bone marrow stromal cells incubated on the functionalized surfaces exhibited heightened alkaline phosphatase activity and matrix mineralization compared to that on uncoated surfaces, suggesting successful induction of cell differentiation. Similarly, vapor-deposited poly(N-hydroxy succinimide ester-p-xylylene-co-p-xylylene) films, which allowed the immobilization of bone morphogenetic protein 2 (BMP-2), have led to osteogenesis of porcine adipose stem cells.163 Furthermore, surface arrangement of the bioactive molecules could be controlled precisely by applying lithography to the CVD polymer thin films. A poly(maleimide-p-xylylene-co-p-xylylene) film has been patterned using microcontact imprinting and UV photopatterning to enable the spatially selective surface conjugation of fibroblast growth factor 2 and BMP-2.164 Osteogenesis activities of the murine preosteoblast cells were detected only in the regions with BMP-2 coverage, confirming that the osteogenic differentiation was spatially confined.
The substrate-independent nature of the CVD techniques allows those bioactive surface chemistries to be transferred, with high fidelity, to virtually any substrates, thus broadening the scope of application of tissue engineering. In one example, a paper-based tissue scaffold was fabricated using iCVD, which promoted in vivo bone regeneration in mice with calvarial bone defects.165 Papers (weighing paper, chromatography paper, and wiping tissue), serially coated with pPFDA (400 nm) and pGMA (200 nm) films using iCVD, were used as substrates to promote osteogenic differentiation in vitro among human adipose-derived stem cells (hADSCs). Implants made of stacked hADSCs-seeded papers and human-endothelial-cells-seeded papers repaired bone defects in vivo, highlighting the potential of this inexpensive device to treat tissue defects. Furthermore, the CVD techniques have enabled application of functional polymer thin films onto biotic substrates, such as decellularized porcine small intestine, creating parylene-C-coated intestinal replica with the original structures, such as crypts and villi, fully preserved.166 The coated intestine replica supported the adhesion and growth of Caco-2 cells, and led to a 2.3-fold increase in the alkaline phosphatase activities compared to that on PDMS, which was fabricated with biomimetic topographical features.
The substrate-independence also facilitated fundamental investigations on the decoupled effect of surface chemistry and surface topography on directed cell differentiation. In one study, polyurethane acrylate (PUA) substrates with nanoscale corrugated or dot-shaped topography were conformally coated with pGMA film (~50 nm) using iCVD, followed by immobilization of BMP-2 via the ring-opening coupling.167 The study revealed that surface nano-topography and biochemical signaling both took part in directing the osteogenic differentiation of human mesenchymal stem cells; and that osteogenic differentiation could be achieved in the absence of dissolved inducing factors by combining the effect of surface topography and BMP-2.
An emergent application of CVD polymer thin films in tissue engineering is to modulate the function of individual cells or to orchestrate the behavior of a community of cells. Many important cellular behavior, such as cell migration168 and structure formation169 are guided by the spatiotemporal distribution of signaling molecules (e.g. DNA or proteins), which could be controlled using vapor-deposited functional polymer coatings. A proof-of-concept has been demonstrated using vapor-deposited poly(p-xylylene) films.170 Through a counter-flow step up, gradients of aldehyde and amine moieties were simultaneously established on a surface, which were later functionalized orthogonally with adenovirus via a virus-biotin-avidin-biotin-material immobilization scheme. When cultured on the adenovirus gradients, the human gingival fibroblasts exhibited asymmetric transduction, confluent with the concentration gradient of viral particles.
4. Conclusions and Outlook
Vapor-deposited functional polymer thin films have experienced rapid growth in the last decade, with more deposition techniques developed every year and a rapidly expanding library of functional moieties. Some CVD techniques have been adopted at the manufacturing scale, where batch operations allow the surface modification of large apparatus (e.g. automotive tires) and the semi-continuous roll-to-roll operations enable the efficient manufacturing of flexible functional materials like membranes and fabrics.
Application of the vapor-deposited polymer thin films has penetrated virtually every area of research and development – consumer products, energy generation and storage, sustainability, and healthcare. With the advancement of nanofabrication techniques and single-cell characterization platforms, CVD polymers have garnered much attention in biological applications. That increasing ubiquity is a result of the distinct characteristics of CVD techniques – they are solvent-free and thus not limited by adverse surface tension effects, operated under vacuum, and in some cases under ambient temperature. As a result, CVD polymer coatings tend to have high conformality over structured surfaces. That uniform coverage results from the arrival of reactants to the surface by non-line-of-sight vapor phase diffusion (i.e. Knudsen diffusion) under modest vacuum conditions and limited sticking probability during a single surface collision. The high conformality enables engineering of surface chemistry without changing the underlying surface structure. Preservation of the latter has been proven important in tissue engineering (e.g. implants with hierarchical micro- and nanostructures to mimic human bones and intestines).171 That simultaneous yet independent control of surface chemistry and surface structure –with micro- to nanoscale resolution – will also provide a powerful platform for seeking answers to the fundamental questions regarding bio-interfaces: for instance, the decoupled roles of surface chemistry and surface structure in biological processes, ranging from modulating protein adsorption and conditioning film formation, chemotaxis and mechanosensing, cellular signal transduction and gene regulation, cell adhesion and differentiation, all the way to the development of community-level behaviors within tissues and biofilms.
Most CVD techniques have implemented in situ real-time monitoring of the growth of functional polymer films, through interferometer or quartz crystal microbalance. That precise control of coating thickness is particularly important for applications where the rate of heat and mass transfer needs to be tuned (such as for antifouling coatings applied in bio-separation membranes). In particular, future research may push toward a deeper understanding of the CVD deposition kinetics under nano-confinement (characteristic length ~102 nm or smaller), on ultrahigh-aspect-ratio structures (e.g. pores with aspect ratio >102)31,62,126, inside complex 3D geometric shapes (e.g. hourglass-shaped pores)172 and/or on novel templates (e.g. liquid crystals)74 – all of which are playing an increasingly important role in creating the state-of-the-art bio-interfaces, such as the ones covered in Section 3. Thickness could also affect the mechanical properties of a polymer coating, which in turn could affect cellular phenotypes (e.g. biofilm formation) 101,173–175 – a relatively unexplored area that could potentially offer an additional dimension for modulating cell-substrate interactions.
Although this review has focused predominantly on thin films, it is noteworthy that CVD methods are also capable of generating thicker films (up to ~102 µm). Free standing pHEMA films of thickness up to ~350 µm have been synthesized using iCVD.176 The mechanical stability afforded by the thick pHEMA films, coupled with their resistance to non-specific protein binding, makes thick pHEMA films intriguing candidates for synthetic scaffolds. Free-standing porous membranes with thickness up to 2 mm have been synthesized using iCVD by polymerizing the structured solid-state condensate of monomers, which were formed via a gas-to-solid phase transition on a substrate cooled below 0 ºC.177 That innovative deposition regime of iCVD has enabled synthesis of robust porous materials for a variety of novel applications including asymmetric membranes, microfluidic sensing devices, and formation of giant lipid vesicles as a model system for cell membranes.177–179 Moreover, thicker films, upon swelling, can trigger more significant actuation both in terms of magnitude of the resulting force and degree of expansion. Such properties are useful in applications where drastic substrate transformation is desirable (e.g. dynamic topography for repeated foul release180). Nevertheless, the marginal gain of vapor-deposition beyond certain thickness may diminish quickly as the required deposition time can get excessively long, especially if there exists a solution-based approach that could achieve the same thickness much faster. On the contrary, vapor-based approach could be the only viable option, despite the long deposition time, if the substrate has low surface energy, which often leads to dewetting upon solution-based coating application.49 Therefore, it is important to conduct a comprehensive comparison of the synthetic approaches to choose the more appropriate methods.
Needless to say, the surface chemistry is controlled precisely, in some cases to the molecular-level precision, in CVD processes. The benign reaction conditions employed by most CVD processes and the absence of solvents allow the synthesis and processing of polymers that are insoluble or thermosetting (e.g. from heavy cross-linking) into a thin film in a single step. Monomers that do not share common solvents could be copolymerized without phase separation, thus making CVD processes versatile tools for creating novel amphiphilic copolymer films, ones made of comonomers with contrasting surface energy. Future research in CVD copolymer compositional optimization may be further accelerated with machine learning algorithms (e.g. Bayesian optimization181), which have proven useful in assisting the choice of experiments, such that optimization of the target material performance in biological environments (e.g. antifouling) can be achieved with as few experiments as possible.
Development of novel CVD polymerization mechanisms will significantly bolster the synthesis toolbox for developing novel polymer thin films for bioapplications. Most CVD techniques rely on free-radical polymerization or step polymerization, which gives rise to a broad polydispersity that might lead to heterogeneities in, for instance, the rate of biodegradation upon in vivo application. Recent realization of cationic polymerization using CVD182–184 has demonstrated the possibility of mirroring other solution-based polymerization mechanisms at the vapor-solid interfaces.87 Future research into performing all-dry living polymerization using CVD could bring major breakthroughs in the development of vapor-deposited biomaterials.
Most CVD processes are substrate-independent, i.e. the ability to achieve the precise and simultaneous control of coating chemistry, thickness, and conformality does not depend upon the characteristics of the substrate. Though this statement holds true in general, recent research has uncovered intriguing effects of microscopic substrate properties (e.g. molecular patterns) on the local molecular architecture of vapor-deposited polymer films;185 such microscopic topography may inspire deeper understanding of how biomolecules (e.g. membrane proteins) interact with hierarchical structures spanning multiple length scales. Recent studies have also broadened the CVD-compatible substrates to include liquids, such as ionic liquids186 and silicone oils187. These innovative modes of operation have shown potential in synthesizing polymer nanoparticles (e.g. core-shell particles with independently controlled dimension for each layer)188 and structured polymer films189, which could find applications in drug delivery, sensing, and bio-separation. Those features render CVD processes and the resulting polymers uniquely suitable to address the challenges in bio-interface engineering, where the comprehensive control of surface characteristics could lead to regulated biological behaviors at the interface.
Translation of benchtop success to bio-interfaces useful in healthcare and sustainability requires the thin films to remain both chemically stable and mechanically reliable even under long-term exposure to complex biological environments. One important indicator of mechanical reliability is the work of adhesion between CVD polymer thin films and substrates. Recent advancement in characterizing work of adhesion by using water vapor condensation blistering has enabled in situ measurement with micrometer spatial resolution,190 which could help further the understanding of the physical and chemical properties critical to robust thin film-substrate adhesion.
This review provides an overview of the recent application of CVD functional polymers in biological applications ranging from fundamental investigation into the surface cell adhesion and differentiation to reducing the cost of industrial-scale separation of biomolecules. Nevertheless, the development of this combination between CVD and living organisms is still in its early stage. The precise and decoupled control over surface chemistry, structure, and coating thickness may prove powerful in the development of drug delivery vehicles, for the facile tuning of therapeutic release kinetics, or in the design of implants, where the tissue reaction could be modulated via surface interactions to achieve the optimal therapeutic outcome. The solvent-free nature of all CVD processes could lower the barrier to clinical success for the vapor-deposited polymer coatings, as remnant solvents and other small molecules are not a concern.
Acknowledgements
This work was financially supported by NIHDC016644 to R.Y.
Footnotes
Conflicts of interest
There are no conflicts to declare.
References
- 1.Ramanathan M and Darling SB, Prog. Polym. Sci, 2011, 36, 793–812. [Google Scholar]
- 2.Lau WJ, Ismail AF, Misdan N and Kassim MA, Desalination, 2012, 287, 190–199. [Google Scholar]
- 3.Lee J, Panzer MJ, He Y, Lodge TP and Frisbie CD, J. Am. Chem. Soc, 2007, 129, 4532–4533. [DOI] [PubMed] [Google Scholar]
- 4.Noh YY, Zhao N, Caironi M and Sirringhaus H, Nat. Nanotechnol, 2007, 2, 784–789. [DOI] [PubMed] [Google Scholar]
- 5.Street RA, Northrup JE and Salleo A, Phys. Rev. B - Condens. Matter Mater. Phys, 2005, 71, 165202. [Google Scholar]
- 6.Yan S, Deng J, Bae C, He Y, Asta A and Xiao X, Polym. Test, 2018, 72, 46–54. [Google Scholar]
- 7.Picart J-C, Caruso, F C. and Voegel, Layer-by-layer films for biomedical applications, Wiley-VCH Verlag, Weinheim, Germany, Germany, 2015. [Google Scholar]
- 8.Harsanyi G, Polymer Films in Sensor Applications, CRC Press, Milton, 2017. [Google Scholar]
- 9.Vendra VK, Wu L and Krishnan S, in Nanotechnologies for the Life Sciences, Wiley-VCH Verlag GmbH & Co, 2011. [Google Scholar]
- 10.Zhang L, Cao Z, Bai T, Carr L, Ella-Menye JR, Irvin C, Ratner BD and Jiang S, Nat. Biotechnol, 2013, 31, 553–556. [DOI] [PubMed] [Google Scholar]
- 11.Donadt TB and Yang R, ACS Biomater. Sci. Eng, 2020, 6, 182–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dersch R, Steinhart M, Boudriot U, Greiner A and Wendorff JH, Polym. Adv. Technol, 2005, 16, 276–282. [Google Scholar]
- 13.Lynn DM, Soft Matter, 2006, 2, 269–273. [DOI] [PubMed] [Google Scholar]
- 14.Khademhosseini A and Peppas NA, Adv. Healthc. Mater, 2013, 2, 10–12. [DOI] [PubMed] [Google Scholar]
- 15.Hammond PT, AIChE J, 2011, 57, 2928–2940. [Google Scholar]
- 16.Wood KC, Boedicker JQ, Lynn DM and Hammond PT, Langmuir, 2005, 21, 1603–1609. [DOI] [PubMed] [Google Scholar]
- 17.Hammond PT, Mater. Today, 2012, 15, 196–206. [Google Scholar]
- 18.Caruso F, Modern Techniques for Nano- and Microreactors/-reactions, Springer-Verlag, Berlin, 2010. [Google Scholar]
- 19.Richardson JJ, Björnmalm M and Caruso F, Science (80-. )., , DOI: 10.1126/science.aaa2491. [DOI] [PubMed] [Google Scholar]
- 20.Kawase T, Shimoda T, Newsome C, Sirringhaus H and Friend RH, in Thin Solid Films, 2003, pp. 279–287. [Google Scholar]
- 21.Sukanek PC, imaging Technol J., 1985, 11, 184–190. [Google Scholar]
- 22.Yimsiri P and MacKley MR, Chem. Eng. Sci, 2006, 61, 3496–3505. [Google Scholar]
- 23.Li X, Prukop SL, Biswal SL and Verduzco R, Macromolecules, 2012, 45, 7118–7127. [Google Scholar]
- 24.Ma C, Zhou H, Wu B and Zhang G, ACS Appl. Mater. Interfaces, 2011, 3, 455–461. [DOI] [PubMed] [Google Scholar]
- 25.Gleason KK, CVD polymers: fabrication of organic surfaces and devices, Wiley-VCH Verlag GmbH & Co, Weinheim, Germany, 2015. [Google Scholar]
- 26.Choy K-L, Chemical vapour deposition (CVD): advances, technology and applications, CRC Press, Boca Raton, 2019. [Google Scholar]
- 27.Asatekin A, Barr MC, Baxamusa SH, Lau KKS, Tenhaeff W, Xu J and Gleason KK, Mater. Today, 2010, 13, 26–33. [Google Scholar]
- 28.Moni P, Al-Obeidi A and Gleason KK, Beilstein J. Nanotechnol, 2017, 8, 723–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.González JPP, Lamure A and Senocq F, Surf. Coatings Technol, 2007, 201, 9437–9441. [Google Scholar]
- 30.Nagar P, Chauhan I and Yasir M, Drug Invent. Today, 2011, 3, 280–289. [Google Scholar]
- 31.Servi AT, Guillen-Burrieza E, Warsinger DM, Livernois W, Notarangelo K, Kharraz J, Lienhard V JH, Arafat HA and Gleason KK, J. Memb. Sci, 2017, 523, 470–479. [Google Scholar]
- 32.Pryce Lewis HG, Bansal NP, White AJ and Handy ES, Thin Solid Films, 2009, 517, 3551–3554. [Google Scholar]
- 33.Lahann J, Balcells M, Rodon T, Lee J, Choi IS, Jensen KF and Langer R, Langmuir, 2002, 18, 3632–3638. [Google Scholar]
- 34.Gopalakrishnan S and Diniz da Costa JC, J. Memb. Sci, 2008, 323, 144–147. [Google Scholar]
- 35.Masi M, Cavallotti C, Boccalari D and Castellana F, Cryst. Res. Technol, 2014, 49, 614–619. [Google Scholar]
- 36.Houweling ZS, Verlaan V, ten Grotenhuis GT and Schropp REI, Thin Solid Films, 2009, 517, 3566–3569. [Google Scholar]
- 37.Gleason KK, J. Vac. Sci. Technol. A, 2020, 38, 020801. [Google Scholar]
- 38.Chen N, Kim DH, Kovacik P, Sojoudi H, Wang M and Gleason KK, Annu. Rev. Chem. Biomol. Eng, 2016, 7, 373–393. [DOI] [PubMed] [Google Scholar]
- 39.Koenig M and Lahann J, Beilstein J. Nanotechnol, 2017, 8, 1250–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pierson HO, Handbook of chemical vapor deposition (CVD): principles, technology and applications, Noyes publications, Park Ridge, NJ, USA, 1999. [Google Scholar]
- 41.Lau KKS and Gleason KK, Macromolecules, 2006, 39, 3688–3694. [Google Scholar]
- 42.Martin TP, Lau KKS, Chan K, Mao Y, Gupta M, Shannan O’Shaughnessy W and Gleason KK, Surf. Coatings Technol, 2007, 201, 9400–9405. [Google Scholar]
- 43.D’Agostino R, Cramarossa F and Illuzzi F, J. Appl. Phys, 1987, 61, 2754–2762. [Google Scholar]
- 44.Tenhaeff WE and Gleason KK, Adv. Funct. Mater, 2008, 18, 979–992. [Google Scholar]
- 45.Winther-Jensen B and West K, Macromolecules, 2004, 37, 4538–4543. [Google Scholar]
- 46.Sutomo W, Wang X, Bullen D, Braden SK and Liu C, in Proceedings of the IEEE Micro Electro Mechanical Systems (MEMS), 2003, pp. 598–601. [Google Scholar]
- 47.George SM, Chem. Rev, 2010, 110, 111–131. [DOI] [PubMed] [Google Scholar]
- 48.De Luna MM, Karandikar P and Gupta M, Mol. Syst. Des. Eng, 2020, 5, 15–21. [Google Scholar]
- 49.Gleason KK, in Surface Modification of Polymers, Wiley-VCH Verlag GmbH & Co, 2019, pp. 107–134. [Google Scholar]
- 50.Lau KKS and Gleason KK, Macromolecules, 2006, 39, 3695–3703. [Google Scholar]
- 51.Bakker R, Verlaan V, van der Werf CHM, Rath JK, Gleason KK and Schropp REI, Surf. Coatings Technol, 2007, 201, 9422–9425. [Google Scholar]
- 52.Marí-Buyé N, O’Shaughnessy S, Colominas C, Semino CE, Gleason KK and Borrós S, Adv. Funct. Mater, 2009, 1276–1286, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coclite AM, Howden RM, Borrelli DC, Petruczok CD, Yang R, Yagüe JL, Ugur A, Chen N, Lee S, Jo WJ, Liu A, Wang X and Gleason KK, Adv. Mater, 2013, 25, 5392–5423. [DOI] [PubMed] [Google Scholar]
- 54.Chan K and Gleason KK, Langmuir, 2005, 21, 8930–8939. [DOI] [PubMed] [Google Scholar]
- 55.Petruczok CD, Yang R and Gleason KK, Macromolecules, 2013, 46, 1832–1840. [Google Scholar]
- 56.Caliari SR and Burdick JA, Nat. Methods, 2016, 13, 405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ye Y and Mao Y, J. Mater. Chem, 2011, 21, 7946–7952. [Google Scholar]
- 58.Xu J and Gleason KK, ACS Appl. Mater. Interfaces, 2011, 3, 2410–2416. [DOI] [PubMed] [Google Scholar]
- 59.Ozaydin-Ince G and Gleason KK, Chem. Vap. Depos, 2010, 16, 100–105. [Google Scholar]
- 60.Baxamusa SH and Gleason KK, Chem. Vap. Depos, 2008, 14, 313–318. [Google Scholar]
- 61.Baxamusa SH and Gleason KK, Thin Solid Films, 2009, 517, 3536–3538. [Google Scholar]
- 62.Asatekin A and Gleason KK, Nano Lett, 2011, 11, 677–686. [DOI] [PubMed] [Google Scholar]
- 63.Ozaydin-Ince G and Gleason KK, J. Vac. Sci. Technol. A Vacuum, Surfaces, Film, 2009, 27, 1135–1143. [Google Scholar]
- 64.Bonnet L, Altemus B, Scarazzini R, Veillerot M, D’Agosto F, Faguet J and Jousseaume V, Macromol. Mater. Eng, 2017, 302, 1–9. [Google Scholar]
- 65.Baxamusa SH and Gleason KK, Adv. Funct. Mater, 2009, 19, 3489–3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baxamusa SH, Montero L, Dubach JM, Clark HA, Borros S and Gleason KK, Biomacromolecules, 2008, 9, 2857–2862. [DOI] [PubMed] [Google Scholar]
- 67.Chan K and Gleason KK, Langmuir, 2005, 21, 11773–11779. [DOI] [PubMed] [Google Scholar]
- 68.Martin TP, Sedransk KL, Chan K, Baxamusa SH and Gleason KK, Macromolecules, 2007, 40, 4586–4591. [Google Scholar]
- 69.Rosenfeld RN and Weiner BR, J. Am. Chem. Soc, 1983, 105, 6233–6236. [Google Scholar]
- 70.Deng X and Lahann J, J. Appl. Polym. Sci, 2014, 131, 40315. [Google Scholar]
- 71.Gorham WF, J. Polym. Sci. Part A-1 Polym. Chem, 1966, 4, 3027–3039. [Google Scholar]
- 72.Bally-Le Gall F, Hussal C, Kramer J, Cheng K, Kumar R, Eyster T, Baek A, Trouillet V, Nieger M, Bräse S and Lahann J, Chem. - A Eur. J, 2017, 23, 13342–13350. [DOI] [PubMed] [Google Scholar]
- 73.Xie F, Deng X, Kratzer D, Cheng KCK, Friedmann C, Qi S, Solorio L and Lahann J, Angew. Chemie - Int. Ed, 2017, 56, 203–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cheng KCK, Bedolla-Pantoja MA, Kim YK, Gregory JV, Xie F, De France A, Hussal C, Sun K, Abbott NL and Lahann J, Science (80-. )., 2018, 362, 804–808. [DOI] [PubMed] [Google Scholar]
- 75.Xie X, Rieth L, Caldwell R, Diwekar M, Tathireddy P, Sharma R and Solzbacher F, IEEE Trans. Biomed. Eng, 2013, 60, 2943–2951. [DOI] [PubMed] [Google Scholar]
- 76.da S. Franciozi CE, Vangsness CT, Tibone JE, Martinez JC, Rodger D, Chou TC, Tai YC, Brant R, Wu L, Abdalla RJ, Han B, Evseenko D and Humayun M, Biomed. Microdevices, , DOI: 10.1007/s10544-017-0170-7. [DOI] [PubMed] [Google Scholar]
- 77.Chang TY, Yadav VG, De Leo S, Mohedas A, Rajalingam B, Chen CL, Selvarasah S, Dokmeci MR and Khademhosseini A, Langmuir, 2007, 23, 11718–11725. [DOI] [PubMed] [Google Scholar]
- 78.Kim BJ and Meng E, Polym. Adv. Technol, 2016, 27, 564–576. [Google Scholar]
- 79.Ortigoza-Diaz J, Scholten K, Larson C, Cobo A, Hudson T, Yoo J, Baldwin A, Hirschberg AW and Meng E, Micromachines, 2018, 9, 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lecomte A, Lecestre A, Bourrier D, Blatché MC, Jalabert L, Descamps E and Bergaud C, Microelectron. Eng, 2017, 177, 70–73. [Google Scholar]
- 81.Fuerst TF, Reese MO and Wolden CA, Plasma Process. Polym, 2016, 13, 184–190. [Google Scholar]
- 82.Biederman H and Slavínská D, Surf. Coatings Technol, 2000, 125, 371–376. [Google Scholar]
- 83.Böke F, Giner I, Keller A, Grundmeier G and Fischer H, ACS Appl. Mater. Interfaces, 2016, 8, 17805–17816. [DOI] [PubMed] [Google Scholar]
- 84.Lu M, Shao D, Wang P, Chen D, Zhang Y, Li M, Zhao J and Zhou Y, RSC Adv, 2016, 6, 82688–82697. [Google Scholar]
- 85.Bo Z, Ma W, Wang P, Wu E, Yang W, Yu K, Zhang X, Yan J and Cen K, Phys. Status Solidi Basic Res, 2014, 251, 155–161. [Google Scholar]
- 86.Bitar R, Cools P, De Geyter N and Morent R, Appl. Surf. Sci, 2018, 448, 168–185. [Google Scholar]
- 87.Alf ME, Asatekin A, Barr MC, Baxamusa SH, Chelawat H, Ozaydin-lnce G, Petruczok CD, Sreenivasan R, Tenhaeff WE, Trujillo NJ, Vaddiraju S, Xu J and Gleason KK, Adv. Mater, 2010, 22, 1993–2027. [DOI] [PubMed] [Google Scholar]
- 88.Jiang ZJ and Jiang ZJ, RSC Adv, 2012, 2, 2743–2747. [Google Scholar]
- 89.Coclite AM and Gleason KK, Plasma Process. Polym, 2012, 9, 425–434. [Google Scholar]
- 90.Gürsoy M, Uçar T, Tosun Z and Karaman M, Plasma Process. Polym, 2016, 13, 438–446. [Google Scholar]
- 91.Wang X, Zhang X, Sun L, Lee D, Lee S, Wang M, Zhao J, Shao-Horn Y, Dincǎ M, Palacios T and Gleason KK, Sci. Adv, , DOI: 10.1126/sciadv.aat5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Reeja-Jayan B, Kovacik P, Yang R, Sojoudi H, Ugur A, Kim DH, Petruczok CD, Wang X, Liu A and Gleason KK, Adv. Mater. Interfaces, 2014, 1, 1400117. [Google Scholar]
- 93.Smith PM, Su L, Gong W, Nakamura N, Reeja-Jayan B and Shen S, RSC Adv, 2018, 8, 19348–19352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brunetti F, Operamolla A, Castro-Hermosa S, Lucarelli G, Manca V, Farinola GM and Brown TM, Adv. Funct. Mater, 2019, 29, 1806798. [Google Scholar]
- 95.Laforgue A and Robitaille L, Macromolecules, 2010, 43, 4194–4200. [Google Scholar]
- 96.Back JW, Lee S, Hwang CR, Chi CS and Kim JY, Macromol. Res, 2011, 19, 33–37. [Google Scholar]
- 97.Ala O, Hu B, Li D, Yang CL, Calvert P and Fan Q, ACS Appl. Mater. Interfaces, 2017, 9, 29038–29046. [DOI] [PubMed] [Google Scholar]
- 98.Wang J, Cai K, Yin J and Shen S, Synth. Met, 2017, 224, 27–32. [Google Scholar]
- 99.Cho J, Shin KH and Jang J, Synth. Met, 2010, 160, 1119–1125. [Google Scholar]
- 100.Martin DC and Malliaras GG, ChemElectroChem, 2016, 3, 686–688. [Google Scholar]
- 101.Kolewe KW, Peyton SR and Schiffman JD, ACS Appl. Mater. Interfaces, 2015, 7, 19562–19569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mahapatro A, Mater. Sci. Eng. C, 2015, 55, 227–251. [DOI] [PubMed] [Google Scholar]
- 103.Lutolf MP and Hubbell JA, Nat. Biotechnol, 2005, 23, 47–55. [DOI] [PubMed] [Google Scholar]
- 104.Kim MJ, Lee B, Yang K, Park J, Jeon S, Um SH, Kim DI, Im SG and Cho SW, Biomaterials, 2013, 34, 7236–7246. [DOI] [PubMed] [Google Scholar]
- 105.van Reis R and Zydney A, J. Memb. Sci, 2007, 297, 16–50. [Google Scholar]
- 106.Robeson LM, J. Memb. Sci, 2008, 320, 390–400. [Google Scholar]
- 107.Park HB, Kamcev J, Robeson LM, Elimelech M and Freeman BD, Science (80-. )., 2017, 356, 1138–1148. [DOI] [PubMed] [Google Scholar]
- 108.Yang R, Jang H, Stocker R and Gleason KK, Adv. Mater, 2014, 26, 1711–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Servi AT, Kharraz J, Klee D, Notarangelo K, Eyob B, Guillen-Burrieza E, Liu A, Arafat HA and Gleason KK, J. Memb. Sci, 2016, 520, 850–859. [Google Scholar]
- 110.Guillen-Burrieza E, Servi A, Lalia BS and Arafat HA, J. Memb. Sci, 2015, 483, 94–103. [Google Scholar]
- 111.Subramaniam MNN, Goh PSS, Sevgili E, Karaman M, Lau WJJ and Ismail AFF, Eur. Polym. J, 2020, 122, 109360. [Google Scholar]
- 112.Sun M, Wu Q, Xu J, He F, Brown AP and Ye Y, J. Mater. Chem. B, 2016, 4, 2669–2678. [DOI] [PubMed] [Google Scholar]
- 113.Shao Q and Jiang S, Adv. Mater, 2015, 27, 15–26. [DOI] [PubMed] [Google Scholar]
- 114.Yang R, Xu J, Ozaydin-Ince G, Wong SY and Gleason KK, Chem. Mater, 2011, 23, 1263–1272. [Google Scholar]
- 115.Krishnan S, Weinman CJ and Ober CK, J. Mater. Chem, 2008, 18, 3405–3413. [Google Scholar]
- 116.Ozaydin-Ince G, Matin A, Khan Z, Zaidi SMJ and Gleason KK, Thin Solid Films, 2013, 539, 181–187. [Google Scholar]
- 117.de Lannoy C, Jassby D, Davis DD and Wiesner MR, J. Memb. Sci, 2012, 415–416, 718–724. [Google Scholar]
- 118.Wang M, Kovacik P and Gleason KK, Langmuir, 2017, 33, 10623–10631. [DOI] [PubMed] [Google Scholar]
- 119.Wandera D, Wickramasinghe SR and Husson SM, J. Memb. Sci, 2010, 357, 6–35. [Google Scholar]
- 120.Tufani A and Ozaydin Ince G, J. Memb. Sci, 2017, 537, 255–262. [Google Scholar]
- 121.Tenhaeff WE and Gleason KK, Chem. Mater, 2009, 21, 4323–4331. [Google Scholar]
- 122.Jeon G, Yang SY and Kim JK, J. Mater. Chem, 2012, 22, 14814–14834. [Google Scholar]
- 123.Alf ME, Hatton TA and Gleason KK, Polymer (Guildf)., 2011, 52, 4429–4434. [Google Scholar]
- 124.Alrifaiy A, Lindahl OA and Ramser K, Polymers (Basel)., 2012, 4, 1349–1398. [Google Scholar]
- 125.Teles FRR and Fonseca LP, Mater. Sci. Eng. C, 2008, 28, 1530–1543. [Google Scholar]
- 126.Ozaydin Ince G, Demirel G, Gleason KK and Demirel MC, Soft Matter, 2010, 6, 1635–1639. [Google Scholar]
- 127.Bajpai AK and Kankane S, J. Mater. Sci. Mater. Med, 2008, 19, 1921–1933. [DOI] [PubMed] [Google Scholar]
- 128.Achyuta AKH, Polikov VS, White AJ, Pryce Lewis HG and Murthy SK, Macromol. Biosci, 2010, 10, 872–880. [DOI] [PubMed] [Google Scholar]
- 129.Deng X, He S, Xie F, Friedmann C, Hess H and Lahann J, Adv. Mater, 2016, 28, 2367–2373. [DOI] [PubMed] [Google Scholar]
- 130.Wondimu SF, Hippler M, Hussal C, Hofmann A, Krämmer S, Lahann J, Kalt H, Freude W and Koos C, Opt. Express, 2018, 26, 3161. [DOI] [PubMed] [Google Scholar]
- 131.Ozaydin-Ince G, Dubach JM, Gleason KK and Clark HA, Proc. Natl. Acad. Sci. U. S. A, 2011, 108, 2656–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Adrogué HJ and Madias NE, N. Engl. J. Med, 2000, 342, 1493–1499. [DOI] [PubMed] [Google Scholar]
- 133.Montero L, Gabriel G, Guimerà A, Villa R, Gleason KK and Borrós S, Vacuum, 2012, 86, 2102–2104. [Google Scholar]
- 134.Yagüe JL and Gleason KK, Soft Matter, 2012, 8, 2890–2894. [Google Scholar]
- 135.Im SG, Bong KW, Lee CH, Doyle PS and Gleason KK, Lab Chip, 2009, 9, 411–416. [DOI] [PubMed] [Google Scholar]
- 136.Xu J and Gleason KK, Chem. Mater, 2010, 22, 1732–1738. [Google Scholar]
- 137.You JB, Min KI, Lee B, Kim DP and Im SG, Lab Chip, 2013, 13, 1266–1272. [DOI] [PubMed] [Google Scholar]
- 138.Lahann J, Balcells M, Lu H, Rodon T, Jensen KF and Langer R, Anal. Chem, 2003, 75, 2117–2122. [DOI] [PubMed] [Google Scholar]
- 139.Chen HY and Lahann J, Anal. Chem, 2005, 77, 6909–6914. [DOI] [PubMed] [Google Scholar]
- 140.Ghasemi-Mobarakeh L, Werzer O, Keimel R, Kolahreez D, Hadley P and Coclite AM, J. Appl. Polym. Sci, 2019, 136, 47858. [Google Scholar]
- 141.Christian P, Ehmann HMA, Coclite AM and Werzer O, ACS Appl. Mater. Interfaces, 2016, 8, 21177–21184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhi B and Mao Y, ACS Appl. Bio Mater, 2020, 3, 1088–1096. [DOI] [PubMed] [Google Scholar]
- 143.Sayin S, Tufani A, Emanet M, Genchi GG, Sen O, Shemshad S, Ozdemir E, Ciofani G and Ozaydin Ince G, Front. Bioeng. Biotechnol, 2019, 7, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lau KKS and Gleason KK, Macromol. Biosci, 2007, 7, 429–434. [DOI] [PubMed] [Google Scholar]
- 145.Rainsford KD, Ibuprofen: Pharmacology, therapeutics and side effects, 2012.
- 146.Yannuzzi LA, Rohrer KT, Tindel LJ, Sobel RS, Costanza MA, Shields W and Zang E, Ophthalmology, 1986, 93, 611–617. [DOI] [PubMed] [Google Scholar]
- 147.McInnes SJP, Szili EJ, Al-Bataineh SA, Xu J, Alf ME, Gleason KK, Short RD and Voelcker NH, ACS Appl. Mater. Interfaces, 2012, 4, 3566–3574. [DOI] [PubMed] [Google Scholar]
- 148.Shi X, Ye Y, Wang H, Liu F and Wang Z, ACS Appl. Mater. Interfaces, 2018, 10, 38449–38458. [DOI] [PubMed] [Google Scholar]
- 149.Armagan E and Ozaydin Ince G, Soft Matter, 2015, 11, 8069–8075. [DOI] [PubMed] [Google Scholar]
- 150.Shimomoto H, Shimizu K, Takeda C, Kikuchi M, Kudo T, Mukai H, Itoh T, Ihara E, Hoshikawa N, Koiwai A and Hasegawa N, Polym. Chem, 2015, 6, 8124–8131. [Google Scholar]
- 151.Schild HG, Prog. Polym. Sci, 1992, 17, 163–249. [Google Scholar]
- 152.Akter F, in Tissue Engineering Made Easy, 2016, pp. 3–16. [Google Scholar]
- 153.Ratner BD, Chilkoti A and Lopez GP, Plasma Deposition, Treatment, and Etching of Polymers, 1990.
- 154.Förch R, Chifen AN, Bousquet A, Khor HL, Jungblut M, Chu LQ, Zhang Z, Osey-Mensah I, Sinner EK and Knoll W, Chem. Vap. Depos, 2007, 13, 280–294. [Google Scholar]
- 155.Bose RK and Lau KKS, Chem. Vap. Depos, 2009, 15, 150–155. [Google Scholar]
- 156.Pfluger CA, Burkey DD, Wang L, Sun B, Ziemer KS and Carrier RL, Biomacromolecules, 2010, 11, 1579–1584. [DOI] [PubMed] [Google Scholar]
- 157.Tekin H, Ozaydin-Ince G, Tsinman T, Gleason KK, Langer R, Khademhosseini A and Demirel MC, Langmuir, 2011, 27, 5671–5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zelzer M, Albutt D, Alexander MR and Russell NA, Plasma Process. Polym, 2012, 9, 149–156. [Google Scholar]
- 159.McMahon BJ, Pfluger CA, Sun B, Ziemer KS, Burkey DD and Carrier RL, J. Biomed. Mater. Res. - Part A, 2014, 102, 2375–2382. [DOI] [PubMed] [Google Scholar]
- 160.O’Shaughnessy WS, Marí-Buyé N, Borrós S and Gleason KK, Macromol. Rapid Commun, 2007, 28, 1877–1882. [Google Scholar]
- 161.Wu J, Wu C, Fan S, Hsieh C and Hou Y, Chem. Mater, 2015, 27, 7028–7033. [Google Scholar]
- 162.Zhang Y, Deng X, Scheller EL, Kwon TG, Lahann J, Franceschi RT and Krebsbach PH, Biomaterials, 2010, 31, 3231–3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wu CY, Huang CW, Guan ZY, Wu JT, Yeh SY, Su CT, Chang CH, Ding ST and Chen HY, Mater. Sci. Eng. C, 2016, 69, 283–291. [DOI] [PubMed] [Google Scholar]
- 164.Tsai YT, Wu CY, Guan ZY, Sun HY, Cheng NC, Yeh SY and Chen HY, Langmuir, 2017, 33, 8943–8949. [DOI] [PubMed] [Google Scholar]
- 165.Park HJ, Yu SJ, Yang K, Jin Y, Cho AN, Kim J, Lee B, Yang HS, Im SG and Cho SW, Biomaterials, 2014, 35, 9811–9823. [DOI] [PubMed] [Google Scholar]
- 166.Koppes AN, Kamath M, Pfluger CA, Burkey DD, Dokmeci M, Wang L and Carrier RL, Biofabrication, 2016, 8, 035011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kim MJ, Lee B, Yang K, Park J, Jeon S, Um SH, Kim DI, Im SG and Cho SW, Biomaterials, 2013, 34, 7236–7246. [DOI] [PubMed] [Google Scholar]
- 168.Gunawan RC, Silvestre J, Gaskins HR, Kenis PJA and Leckband DE, Langmuir, 2006, 22, 4250–4258. [DOI] [PubMed] [Google Scholar]
- 169.Dertinger SKW, Jiang X, Li Z, Murthy VN and Whitesides GM, Proc. Natl. Acad. Sci. USA, 2002, 99, 12542–12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Elkasabi YM, Lahann J and Krebsbach PH, Biomaterials, 2011, 32, 1809–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ingber Donald E., FASEB J, 2006, 20, 811–827. [DOI] [PubMed] [Google Scholar]
- 172.Gravelle S, Joly L, Detcheverry F, Ybert C, Cottin-Bizonne C and Bocquet L, Proc. Natl. Acad. Sci. U. S. A, 2013, 110, 16367–16372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Song F, Wang H, Sauer K and Ren D, Front. Microbiol, 2018, 9, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Xu J, Asatekin A and Gleason KK, Adv. Mater, 2012, 24, 3692–3696. [DOI] [PubMed] [Google Scholar]
- 175.Lin P and Yan F, Adv. Mater, 2012, 24, 34–51. [DOI] [PubMed] [Google Scholar]
- 176.Bose RK and Lau KKS, Biomacromolecules, 2010, 11, 2116–2122. [DOI] [PubMed] [Google Scholar]
- 177.Dianat G and Gupta M, Polymer (Guildf)., 2017, 126, 463–469. [Google Scholar]
- 178.Dianat G, Movsesian N and Gupta M, ACS Appl. Polym. Mater, 2020, 2, 98–104. [Google Scholar]
- 179.Movsesian N, Tittensor M, Dianat G, Gupta M and Malmstadt N, Langmuir, 2018, 34, 9025–9035. [DOI] [PubMed] [Google Scholar]
- 180.Gu H, Lee SW, Buffington SL, Henderson JH and Ren D, ACS Appl. Mater. Interfaces, 2016, 8, 21140–21144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lookman T, Alexander F and Rajan K, Information Science for Materials Discovery and Design, Springer, 2016, vol. Volume 225. [Google Scholar]
- 182.Gao Y, Cole B and Tenhaeff WE, Macromol. Mater. Eng, 2018, 303, 1–6. [Google Scholar]
- 183.Bose RK, Nejati S, Stufflet DR and Lau KKS, Macromolecules, 2012, 45, 6915–6922. [Google Scholar]
- 184.Giambra DJ and Tenhaeff WE, 2019, MD.8.
- 185.Laird ED, Bose RK, Wang W, Lau KKS and Li CY, Macromol. Rapid Commun, 2013, 34, 251–256. [DOI] [PubMed] [Google Scholar]
- 186.Frank-Finney RJ, Bradley LC and Gupta M, Macromolecules, 2013, 46, 6852–6857. [Google Scholar]
- 187.Haller PD, Bradley LC and Gupta M, Langmuir, 2013, 29, 11640–11645. [DOI] [PubMed] [Google Scholar]
- 188.Frank-Finney RJ and Gupta M, Langmuir, 2016, 32, 11014–11020. [DOI] [PubMed] [Google Scholar]
- 189.Bradley LC and Gupta M, Langmuir, 2015, 31, 7999–8005. [DOI] [PubMed] [Google Scholar]
- 190.Ma J, Cahill DG and Miljkovic N, Nano Lett, 2020, 20, 3918–3924. [DOI] [PubMed] [Google Scholar]
- 191.Lahann J, Polym. Int, 2006, 55, 1361–1370. [Google Scholar]
- 192.Vasudev MC, Anderson KD, Bunning TJ, Tsukruk VV and Naik RR, ACS Appl. Mater. Interfaces, 2013, 5, 3983–3994. [DOI] [PubMed] [Google Scholar]
- 193.Fortin JB and Lu T-M, in Chemical Vapor Deposition Polymerization:The Growth and Properties of Parylene Thin Films, SPRINGER SCIENCE+BUSINESS MEDIA, LLC, Troy, 2004. [Google Scholar]
- 194.Trujillo NJ, Barr MC, Im SG and Gleason KK, J. Mater. Chem, 2010, 20, 3968–3972. [Google Scholar]
- 195.Nandivada H, Jiang X and Lahann J, Adv. Mater, 2007, 19, 2197–2208. [Google Scholar]
- 196.Slocik JM, Beckel ER, Jiang H, Enlow JO, Zabinski JS, Bunning TJ and Naik RR, Adv. Mater, 2006, 18, 2095–2100. [Google Scholar]
- 197.Moni P, Suh HS, Dolejsi M, Kim DH, Mohr AC, Nealey PF and Gleason KK, Langmuir, 2018, 34, 4494–4502. [DOI] [PubMed] [Google Scholar]
- 198.Carrow BP, Bakhru H, Wang PI, Chen Y and Senkevich JJ, Chem. Vap. Depos, 2006, 12, 239–244. [Google Scholar]
- 199.Nejati S, Minford TE, Smolin YY and Lau KKS, ACS Nano, 2014, 8, 5413–5422. [DOI] [PubMed] [Google Scholar]
- 200.Fortin JB and Lu TM, Chem. Mater, 2002, 14, 1945–1949. [Google Scholar]
- 201.Loyer F, Bulou S, Choquet P and Boscher ND, Plasma Process. Polym, 2018, 15, 1800121. [Google Scholar]
- 202.Drewelow G, Wook Song H, Jiang ZT and Lee S, Appl. Surf. Sci, 2020, 501, 144105. [Google Scholar]
- 203.Trujillo NJ, Baxamusa SH and Gleason KK, Chem. Mater, 2009, 21, 742–750. [Google Scholar]
- 204.Yang R, Buonassisi T and Gleason KK, Adv. Mater, 2013, 25, 2078–2083. [DOI] [PubMed] [Google Scholar]
- 205.US3600216A, United States Pat. Olfice, 1971.
- 206.Zeniieh D, Ledernez L and Urban G, Procedia Eng, 2014, 87, 1398–1401. [Google Scholar]
- 207.Ershov S, Khelifa F, Dubois P and Snyders R, ACS Appl. Mater. Interfaces, 2013, 5, 4216–4223. [DOI] [PubMed] [Google Scholar]
- 208.Im SG, Yoo PJ, Hammond PT and Gleason KK, Adv. Mater, 2007, 19, 2863–2867. [Google Scholar]
- 209.Lewis HGP, Edell DJ and Gleason KK, Chem. Mater, 2000, 12, 3488–3494. [Google Scholar]
- 210.Ozaydin-Ince G, Gleason KK and Demirel MC, Soft Matter, 2011, 7, 638–643. [Google Scholar]


