Abstract
Massive craniofacial bone loss poses a clinical challenge to maxillofacial surgeons. Structural bone allografts are readily available at tissue banks, but are rarely used due to a high failure rate. Previous studies showed that intermittent administration of recombinant parathyroid hormone (rPTH) enhanced integration of allografts in a murine model of calvarial bone defect. To evaluate its translational potential, the hypothesis that rPTH would enhance healing of a mandibular allograft in a clinically relevant large animal model of mandibulectomy, was tested. Porcine bone allografts were implanted into a 5-cm-long continuous mandible bone defect in six adult Yucatan minipigs, which were randomized to daily intramuscular injections of rPTH (1.75 μg/kg) and placebo (n=3). Blood tests were performed on Day 56 pre-operation, Day 0 and on Day 56 post-op. Eight weeks post-surgery, bone healing was analyzed using high-resolution x-ray imaging (Faxitron and microCT) and three-point bending biomechanical testing. The results showed a significant 2.6-fold rPTH-induced increase in bone formation (p = 0.02). Biomechanically, the yield failure properties of the healed mandibles were significantly higher in the rPTH group (yield load: p = 0.03; energy to yield: p = 0.01), and the post-yield displacement and energy were higher in the placebo group (p < 0.05), suggesting increased mineralized integration of the allograft in the rPTH group. In contrast to similar rPTH therapy studies in dogs, no signs of hypercalcemia, hyperphosphatemia, or inflammation were detected. Taken together, we provide initial evidence that rPTH treatment enhances mandibular allograft healing in a clinically relevant large animal model.
Keywords: parathyroid hormone, structural allograft, mandible, reconstruction
Introduction
Mandibular bone regeneration following osteotomies or trauma continues to present a major clinical need. Mandible integrity is crucial for satisfactory mastication, swallowing, speech, and aesthetic facial contour. Due to its prominence, the mandible is the most frequently fractured facial bone in pediatric (Imahara, Hopper, Wang, Rivara, & Klein, 2008) and adult (Boffano et al., 2015) patients alike. Causes of these fractures vary from motor vehicle accidents to assaults and falls (Morris, Bebeau, Brockhoff, Tandon, & Tiwana, 2015). Massive bone loss in the mandible may also be the result of resection for oral malignancies—as many as 275,000 annual cases worldwide (Warnakulasuriya, 2009), with incidence rates steeply rising alongside the aging population (Xie, Semenciw, & Mery, 2015). Other causes of mandibular lesions include radiation necrosis (Tsai et al., 2013), congenital defects such as mandibular hypoplasia (Singh & Bartlett, 2005), and bisphosphonates-related osteonecrosis of the jaw (Ruggiero, Mehrotra, Rosenberg, & Engroff, 2004). Patients with mandibular fractures experience pain, infection, and trismus. These patients are often depressed, and may suffer from malnutrition, deformity, and permanent disability (Mathog, Toma, Clayman, & Wolf, 2000).
Current clinical approaches to repair bone following large mandibular resections are considerably limited. Autograft implantation of the patient’s own bone is widely considered the gold standard (Y. F. Liu, Xu, Zhu, & Liu, 2014; Mehta & Deschler, 2004; Rana et al., 2011). Autografts are often harvested from the iliac crest, ribs (Harashina, Nakajima, & Imai, 1978), or fibulae (Hidalgo, 1989), and contain both cells and growth factors that induce bone regeneration. Unfortunately, use of autografts is not universally ideal. They can be resorbed (Leipzig & Cummings, 1984) in 20% to 50% of cases (Emara, Diab, & Emara, 2015). In addition, comorbidity at the donor site may occur in as many as 50% of cases and may cause prolonged pain (Nassr et al., 2009), sensory changes, and infection (L. Wang, Liu, Shao, & Shang, 2015), as well as pneumothorax in the case of rib grafts (Skouteris & Sotereanos, 1989). When researchers measured the effect of autograft implantation on patients’ quality of life, they found that it did not significantly improve facial aesthetics, and only improved function in patients who underwent radiation therapy (Vu & Schmidt, 2008).
An alternative approach is the implantation of processed human bone from an allogeneic donor, an allograft, which is readily available at tissue banks but has very low osteogenic potential. Similar to autografts, allografts are often resorbed (Keith et al., 2006) but more frequently fail to integrate with the host bone (W. Wang & Yeung, 2017).
A third option lies in various biomaterials—some natural and some synthetic—which are composed of collagen, hydroxyapatite, β-tricalcium phosphate (Emara et al., 2015), or other substances such as polycaprolactone and other polyesters. Biomaterials can also be 3D-printed to a perfect fit to replace the missing segment of bone (Konopnicki et al., 2015; S. Lee, Choi, Shim, & Nam, 2020). These materials are mainly osteoconductive (Asahina, Watanabe, Sakurai, Mori, & Enomoto, 1997) and rely on the presence of pre-existing periosteum and bone, which are not always located in mandibular defects (Moore, Graves, & Bain, 2001). In recent years, the use of bone morphogenetic proteins (BMPs) has been suggested as a promising biological treatment to induce bone regeneration (Carter, Brar, Tolas, & Beirne, 2008; Oliveira, Gorla, Gabrielli, & Pereira-Filho, 2017). Nevertheless, use of BMPs has been associated with many adverse effects such as inflammation (K. B. Lee et al., 2012), inhibition of bone formation (Zara et al., 2011), bone cysts, and ectopic bone formation (Zara et al., 2011); and in some cases, related to spine surgery, it has led to neurological impairment (Wong, Kumar, Jatana, Ghiselli, & Wong, 2008).
Other therapeutic approaches have been proposed to address this clinical challenge. These include stem cell therapies such as the use of mesenchymal stem cells seeded on biodegradable scaffolds (Gómez-Barrena et al., 2015; Quarto et al., 2001), in vitro three-dimensional (3D) printing of personally fitted grafts (Alfotawei et al., 2014), gene therapy mediated by either cells (Steinhardt et al., 2008) or nonviral vectors (Kolk et al., 2016), and more. Yet, all these solutions are still in preclinical research, and it is predicted that a lengthy regulatory pathway lies ahead of them.
Previous research proposed an alternative approach that relies on available bone allografts and a U.S. Food and Drug Administration (FDA)–approved drug, such as teriparatide, which could provide an attractive method for mandible reconstruction that is also easily translatable to clinical use. Teriparatide is a recombinant parathyroid hormone (rPTH) composed of the hormone’s 1–34 amino acids. It has been approved by the FDA for use as an anabolic agent in the treatment of adults with severe osteoporosis to prevent fractures. In humans, the anabolic effects of teriparatide manifest as an increase in skeletal mass, markers of bone turnover, and bone strength (Boonen et al., 2006; Chen et al., 2007; Ebeling & Russell, 2003; Girotra, Rubin, & Bilezikian, 2006; Hodsman et al., 2005; Jiang et al., 2003; Lindsay et al., 2007; Rehman, Lang, Arnaud, Modin, & Lane, 2003; Tashjian & Gagel, 2006). Studies have shown that when rodents with either femoral or calvarial critical-sized bone defects were implanted with bone allografts and injected daily with rPTH, significant new bone regeneration occurred (compared to bone regeneration in placebo controls), leading to allograft integration with the host bone (Cohn Yakubovich et al., 2017; Dhillon et al., 2013; Nishitani et al., 2017; Reynolds et al., 2011; Sheyn et al., 2013; Takahata, Schwarz, Chen, O’Keefe, & Awad, 2012). These studies suggest that the inherent failure of allografts to integrate with host bone may be overcome by using rPTH. Theoretically, bone allografts can be cut and shaped to fit any size and geometry of the bone segment that was lost due to malignancy or trauma, unlike rib or fibula autografts, which can only provide poor restoration of the shape of the mandible.
The goal of this study was to test the scalability and reproducibility of published results on rPTH effects in rodent structural allograft models in a clinically relevant large animal model. The hypothesis was that intermittent rPTH administration would induce the osseous integration of structural mandibular bone allografts in a critical segmental mandibulectomy model, without the serious hypercalcemia observed in canine models (Nishitani et al., 2017). The minipig was the animal of choice, as its bones proved to be the most similar to human bones in composition and density in a comparison of seven vertebrates (Aerssens, Boonen, Lowet, & Dequeker, 1998). Specifically, the similarity mainly lies in the lamellar bone structure, the remodeling processes, and the regeneration rate (Pearce, Richards, Milz, Schneider, & Pearce, 2007). Importantly, the swine mandible is comparable to the human mandible in its bony and vascular anatomy, histology, physiology, biomechanical properties, and pathophysiology (Robertson & Smith, 1978; S. Wang, Liu, Fang, & Shi, 2007).
In the present study, processed allografts produced from minipig mandibles were implanted in minipigs that underwent critical-sized segmental mandibulectomy. The effects of intermittent rPTH administration on bone regeneration, osseous integration of the allograft, and blood chemistry values were compared to a placebo control.
Materials and Methods
All animal procedures were conducted according to protocols approved by the Institutional Animal Care and Use Committees at Cedars-Sinai Medical Center (IACUC approval number 6361), Covance Laboratories Inc. (IACUC approval number 2104) and the Hebrew University of Jerusalem (IACUC approval number MD-19-13780-4). Six skeletally mature female Yucatan minipigs (ages 3–7 years) purchased from S&S Farms (Ramona, CA, USA) were included in the segmental mandibulectomy study (Diagram 1). Mandibular allografts were generated from ten adolescent minipigs (ages 6–9 months) from unrelated studies following planned terminal procedures.
Diagram 1.
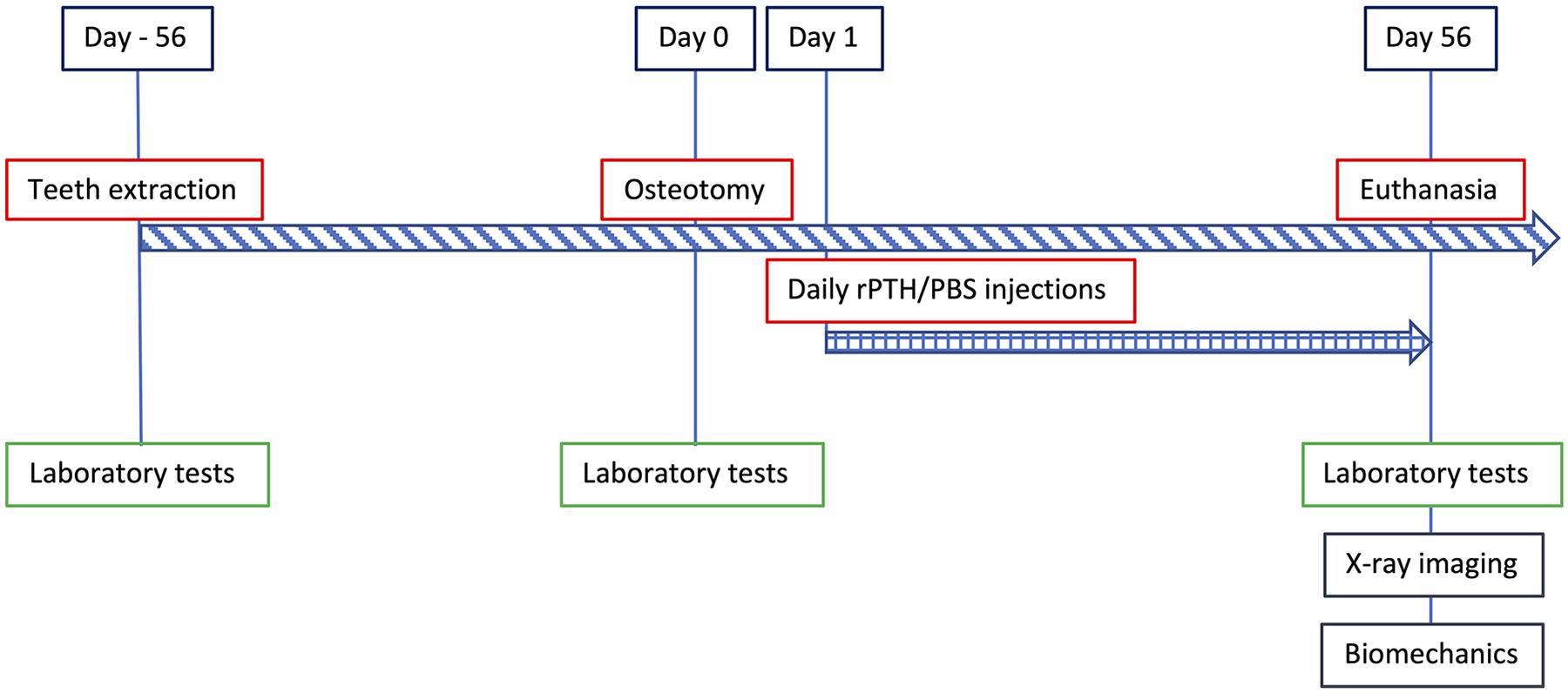
Experimental Design
Preparation of mandibular allografts
Twenty bone segments, each 5 cm in length, were aseptically harvested from the body of the mandible of adolescent minipigs. The segments were wrapped in sterile saline-soaked gauze and shipped to Veterinary Transplant Services, Inc. (Kent, WA, USA) on dry ice. At the VTS facility, dentition was removed at the alveolar margin and the procured segments were swab cultured for presence of microorganisms and then frozen. Segments with evidence of pathogenic organisms were not further processed. The acceptable mandibular segments (n=13) were thawed and processed for implantation in a laminar airflow clean room (ISO 6) and maintained in sterile water augmented with antibiotics (gentamicin and vancomycin) during the initial cleaning stage. All periosteum and soft tissue attachments were removed from the segments. Tooth roots, cementum, all soft tissues including that within the mandibular canal were removed. The majority of the cancellous bone was also removed leaving cleaned segments consisting of the ventral marginal bone and the buccal and lingual cortical plates (Fig. 1A and B). These were further cleaned and disinfected by sonication with Triton detergent, followed by brief exposure to isopropyl alcohol and hydrogen peroxide. The processed segments were rinsed with excess volumes of sterile water to reduce and remove residuals. Each segment was measured, cultured, wrapped in sterile gauze, and sealed in pouches with sterile saline and frozen until use. Final packaging microbial cultures were all negative after 14 days incubation and the mandibular segments were deemed suitable for implantation.
Figure 1: Mandible bone reconstruction using allografts in a minipig model.
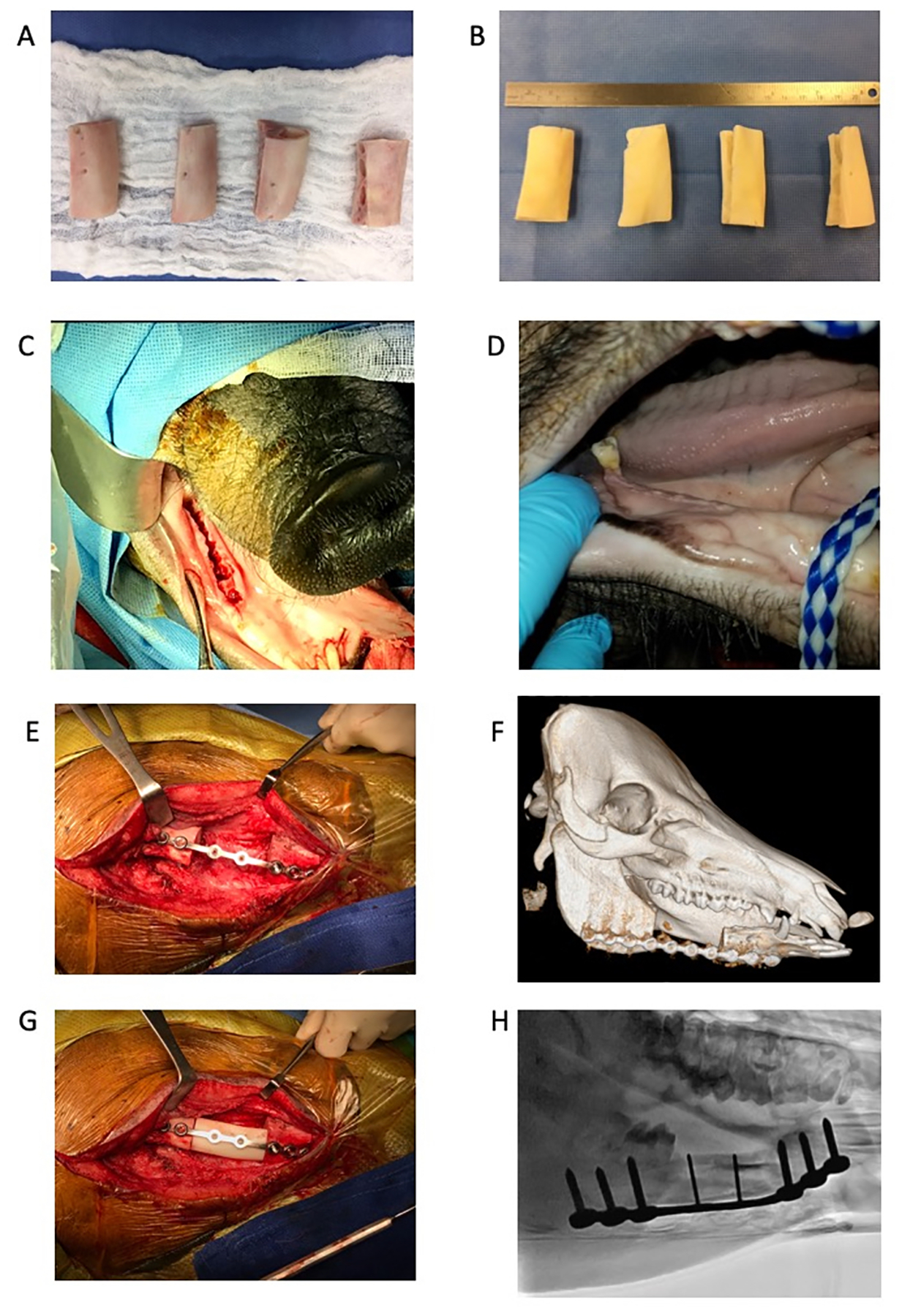
(A) Five-cm-long mandibular bone allografts harvested from minipigs. (B) Processed bone allografts. (C) Intraoral image of the right side of the mandible after teeth extraction. (D) Intraoral image of healed gingivae 8 weeks post-extractions. (E) Five-cm-long osteotomy in the right side of the mandible. Bone stabilized with a locking plate and screws. (F) 3D CT image of a minipig mandible post-osteotomy. (G) Mandible allograft placed within the defect site. The allograft is secured in its location by two locking screws. (H) Fluoroscopic image showing the allograft in place following surgery.
Preoperative care and anesthesia
Preoperative antibiotic care consisted of a 2-d treatment with intramuscular (IM) injection of EXCEDE® for Swine (ceftiofur crystalline free acid, Zoetis, Parsippany, NJ, USA) and amoxicillin/clavulanate potassium per os (15 mg/kg). Following an 18-hour preoperative fast, each pig was given 4.0 mg/kg carprofen and 0.02 mg/kg IM buprenorphine for analgesia. Anesthesia was induced via injection of 4.0 mg/kg Telazol (Zoetis, Parsippany, NJ, USA) and 0.02 mg/kg Atrophine through an ear vein catheter. The pigs were intubated and ventilated throughout the procedure. Inhaled gaseous isoflurane was used for maintenance of a deep anesthetic plane. Immobilization of the pigs was achieved by a succinylcholine injection into the muscle. Bupivacaine (0.5 %) was injected locally into all manipulated tissues including the skin, muscle, gingivae, and periosteum. Buprenorphine (0.01 mg/kg) was injected intravenously during the surgical procedure for analgesia.
In vivo surgery
To prevent infection at the osteotomy site from oral cavity pathogens, the bone segment was removed in two surgical sessions, as previously described (Ma, Pan, Tan, & Cui, 2009). First, the 1st and 2nd molar and premolar teeth were extracted while leaving the 3rd molar intact to maintain the bite (Fig. 1C). Extractions were made using standard dental tools and a dental drill. A digital dental x-ray camera was used to verify that no remnants of teeth roots were left in the alveolar bone. The gingivae were closed with absorbable sutures, and the animals were maintained on a soft diet. Eight weeks later, the animals were sedated and the gingivae were examined to assess whether there was adequate healing (Fig. 1D). If adequate healing was present, general anesthesia was induced, as described above, and a 5-cm continuous segmental bone defect was created in the body of the right mandible. Briefly, the skin was cut in parallel to the mandible’s lower margin line, and fat tissue and muscles were separated using blunt dissection and an electrosurgery pen until the mandible bone was fully exposed. A 5-cm-long segment was marked and resected using a high-speed dental drill mounted with a tungsten carbide burr (Drendel & Zwelling, Kalletal, Germany) (Fig. 1E and F). Care was taken not to penetrate the gingivae and the oral cavity. The periosteum was removed from the defect area and from adjacent bone stumps using a scalpel and electrocautery in order to prevent spontaneous bone formation as much as possible. The allograft was placed in the defect site, verifying a tight fit as much as possible, and a locking plate (Malleable locking plate 6×3.5mm & 2×2.0mm holes, 121.5mm, Veterinary Instrumentation, Sheffield, UK)) and eight locking screws were used to stabilize both allograft and mandible bone (Fig. 1G and H). Wound was closed in layers using absorbable sutures and then sealed with Dermabond (Ethicon, Somerville, NJ).
Postoperative care and treatment
Recovering animals were closely monitored until alert, responsive, and ambulatory before they were returned to their housing. The surgical sites were monitored for excessive bleeding an hour after the surgeries and daily thereafter. Food consumption and appetite were noted at every feeding, and the animals were weighed once per week. The pigs were housed individually. All enrichment was removed from their cages to prevent injury due to chewing and rubbing of the surgical site. The animals were fed a liquid diet consisting of Ensure Original Vanilla (at least 0.7 l/d [liquid form]) and Trophy Dyne supplement (0.9 l/d) for 2 days prior to the teeth extraction surgery and for 10 days after surgery. From Day 11 to Week 8 (2 days before the osteotomy surgery), food pellets were soaked in water to ease the mastication process. The pigs were also fed the same liquid diet for approximately 2 days prior to the osteotomy surgery and for 10 days afterward. From Day 11 until the last day of study, food pellets were soaked in water to ease the mastication process.
On Day 1 following the osteotomy surgery, the animals were arbitrarily randomized to two groups, based on the numbers they had been given at the pig farm. The first group (n=3) received daily intramuscular injections of rPTH (Forteo, Eli Lilly and Co. Indianapolis, IN, USA; 1.75 μg/kg) to the neck area for 8 weeks; this group is called the “rPTH” group. The second group (n=3) received injections of sterile phosphate-buffered saline (PBS), with an equivalent volume to the rPTH injection, and is called the “PBS” group.
Euthanasia was induced using general anesthesia, as described above, followed by intravenous administration of KCL or Euthasol (Virbac AH, Inc. Fort Worth, TX).
X-ray imaging
After induction of general anesthesia, as described above, the minipigs underwent in vivo imaging performed using computed tomography (CT, Dual energy scanner, Phillips, The Netherlands) and fluoroscopy (Infinix-i biplane system, Toshiba, Japan). The images confirmed stabilization of the defect with the locking plate (Fig. 1F), and the position of the allograft post-surgery (Fig. 1H).
Following euthanasia of the animals, the metal hardware was removed and the right hemimandibles were scanned using a high-resolution x-ray scanner (LX-60, Faxitron X-Ray, LLC Lincolnshire, IL, USA) (Fig. 2). Next, a bone saw was used to trim the specimen to a segment containing the defect site with 1 cm of bone extending on each side. Micro-CT scanning was performed using a VivaCT 40 scanner (Scanco Medical AG, Bassersdorf, Switzerland). Micro–tomographic slices were acquired at 2000 projections with a reconstructed voxel size of 18 μm and a 45 kVp potential. A constrained 3D Gaussian filter (sigma = 2.8 and support = 3.0) was used to partly suppress noise in the volumes, and a lower threshold of 400 was chosen to reflect mineral bone. A quantitative assessment of new bone volume based on microtomographic data sets was created using direct 3D morphometry, as previously described (Bez et al., 2017).
Figure 2: High resolution X-ray images of right hemimandibles.
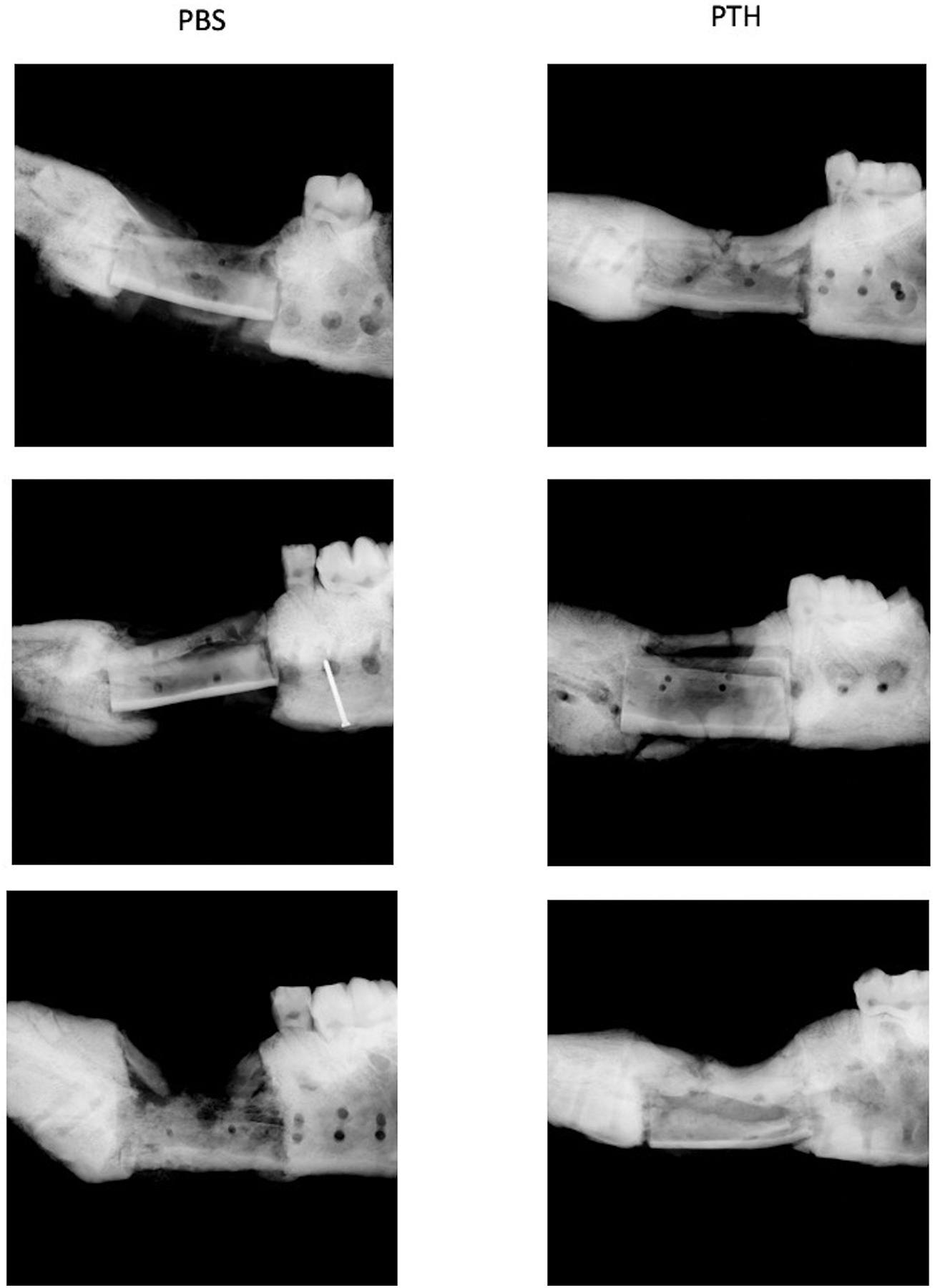
A Faxitron scanner was used to image surgically treated hemimandibles after the animals had been euthanized. Mandible allografts are seen at the defect site with variable amounts of new bone surrounding them.
Biomechanical testing
Trimmed mandible specimens were wrapped in saline-soaked gauze, frozen at −20°C, and shipped on dry ice for biomechanical testing to the Center for Musculoskeletal Research at the University of Rochester. A three-point bending test protocol was used based on a previously published work (W. Liu, Zhang, Tang, Wang, & Gui, 2011). Each sample was tested using an Instron ElectroPuls™ E10000 Material Testing System. Samples were centered over a 70-mm span encompassing the allograft and preloaded to a force of ~50 N to eliminate experimental variability due to soft tissues overlying the bone tissue. Each specimen was then loaded with a central loading rounded tip in displacement control at 5 mm/min, and data were collected at 10 data points per second. Values of yield/first break load, extension at yield, maximum load, extension at maximum load, and stiffness (load/extension in linear region N/mm) were calculated for each specimen from the force-displacement raw data.
Blood tests
Blood was drawn from the minipigs via an ear catheter, during the teeth extractions (Day 56 pre-op), osteotomy (Day 0) and at euthanasia (Day 56 post-op), to assess calcium, phosphate, percentage of neutrophils, and number of white blood cells as previously described (Nishitani et al., 2017).
Statistical analysis
GraphPad Prism 5.0f software (GraphPad Prism) was used to plot and analyze the quantifiable data. Results are presented as means ± standard deviations (SD). Data analysis was conducted using the Student t-test for the terminal microCT imaging and biomechanical testing. Two-way ANOVA with repeated measures and a Bonferroni post hoc test were used for statistical analysis of the longitudinal blood measurements. Both t-tests and ANOVA have demonstrated robustness even in the case of small sample sizes (de Winter, 2013). Additionally, F-tests were performed to confirm equal variances as necessary for testing. To assess significance, p < 0.05 was considered statistically significant.
Results
New bone formation
Qualitative evaluation of Faxitron (Fig. 2) and micro-CT (Fig. 3) images demonstrated new bone formation surrounding the bone allografts in both groups. Signs of bone remodeling were more evident in two allografts out of three in the rPTH group. Two of the allografts in the placebo group did not seem to be remodeled and the third was in a state of advanced resorption. Quantitative micro-CT analysis focused on the defect site showed that 132% more bone volume (new bone + allograft) was evident in the rPTH group versus the PBS group (p < 0.05; Fig. 3C). Histomorphometry of only new bone generated at the defect site indicated a 262% increase in the rPTH group (p < 0.05; Fig. 3D). Analysis of the entire bone tissue within the defects showed that bone mineral density was slightly higher in the PBS group, but this increase was not statistically significant (Fig. 3E; p = 0.054). Yet, when analyzing the mineral density of the new bone only, values were similar between groups (Fig. 3F). Furthermore, near complete new bridging bone that spanned the host-allograft-host junction, mostly circumferentially, was observed in two of the rPTH treated mandibles, while none of the allografted mandibles in the PBS showed any signs of bridging bone formation.
Figure 3: 3D micro-CT images of treated bone defects.
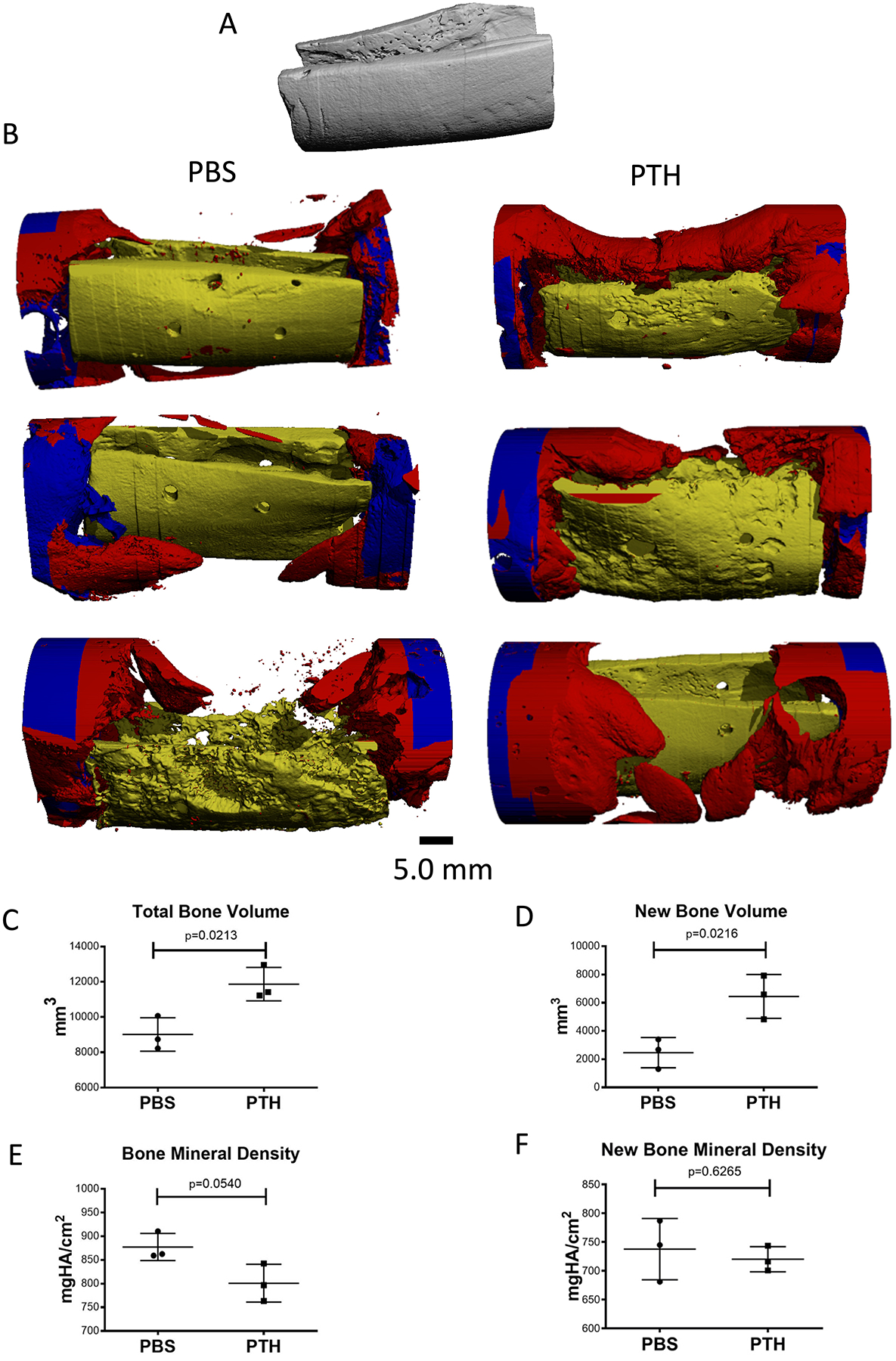
(A) 3D micro-CT scan of a mandibular allograft prior to implantation. (B) Hemimandibles were trimmed after Faxitron imaging and scanned using a micro-CT system. Allografts are highlighted in yellow, new bone in red, and host bone in blue. Note the signs of remodeling in two allografts in the PTH group and advanced resorption of one allograft in the PBS group. (C) Total bone volume at the defect site was quantified based on findings on micro-CT scans, showing greater bone volume in the PTH group than in the PTH group. (D) New bone volume at the defect site was manually contoured and quantified based on micro-CT scans, showing greater new bone volume in the PTH group than in the PTH group. (E) A higher level of bone mineral density of the total bone volume was calculated in the PBS group. (F) Similar level of bone mineral density was calculated in the new bone generated within the bone defects. (unpaired two-tailed t-test; graphs represent means and SEMs; n=3 per group).
Structural allograft osseous integration
Biomechanical testing results confirmed the improved structural allograft osseous integration with the host bone and the increased bridging in the rPTH group (Table 1). The yield load and energy to yield were 208% and 372% higher in the rPTH group compared to the PBS group (p < 0.05 and p < 0.01, respectively). There were no significant differences in the maximum force (p = 0.11) and energy to maximum force (p = 0.53), likely due to the soft tissue bearing the load beyond the yield point. The post-yield displacement, ratio of post-yield to maximum displacement, and energy were all significantly decreased in the rPTH group compared to the PBS group (p < 0.05), suggesting a less ductile mineralized tissue dominating the load bearing response in the rPTH group.
Table 1: Biomechanical test results.
Results of three-point bending tests performed on hemimandibles treated with bone allografts. (Values represent means and SDs; p-values were determined from unpaired two-tailed t-test).
| Properties | Group Allo+PBS | Group Allo+PTH | p-Values |
|---|---|---|---|
| Stiffness (N/mm) | 67.33±39.15 | 74±13.08 | 0.79 (n.s.) |
| Yield Load (N) | 225.6±84.72 | 468.43±89.54 | < 0.05* |
| Displacement at Yield (mm) | 3.52±2.23 | 7.04±0.63 | 0.06 (n.s.) |
| Maximum Load (N) | 382.27±153.19 | 594.47±92.2 | 0.11 (n.s.) |
| Displacement at Maximum Load (mm) | 10.74±4 | 10.43±1.58 | 0.91 (n.s.) |
| Energy to Yield (N*mm) | 451.09±274.06 | 1677.80±317.51 | < 0.01* |
| Energy to Maximum (N*mm) | 2822.49±1369.05 | 3461.71±836.66 | 0.53 (n.s.) |
| Post-Yield Displacement (mm) | 7.22±1.79 | 3.39±1.53 | <0.05* |
| Post-Yield Energy (N*mm) | 2371.40±1127.54 | 1783.91±799.35 | 0.50 (n.s.) |
| Post-Yield/Maximum Displacement (%) | 69.15±7.88 | 31.53±11.34 | <0.01* |
| Post-Yield/Maximum Energy (%) | 84.46±3.47 | 49.81±14.84 | <0.05* |
Systemic effects of rPTH treatment
Analysis of blood chemistry and hematology results showed that there were no changes in serum levels of calcium or phosphate in animals treated with rPTH compared to animals given placebo (Fig. 4A and B). In addition, no signs of systemic inflammation (Fig. 4C and D) or changes in liver function (not shown) were evident.
Figure 4: Quantitative results of laboratory tests.
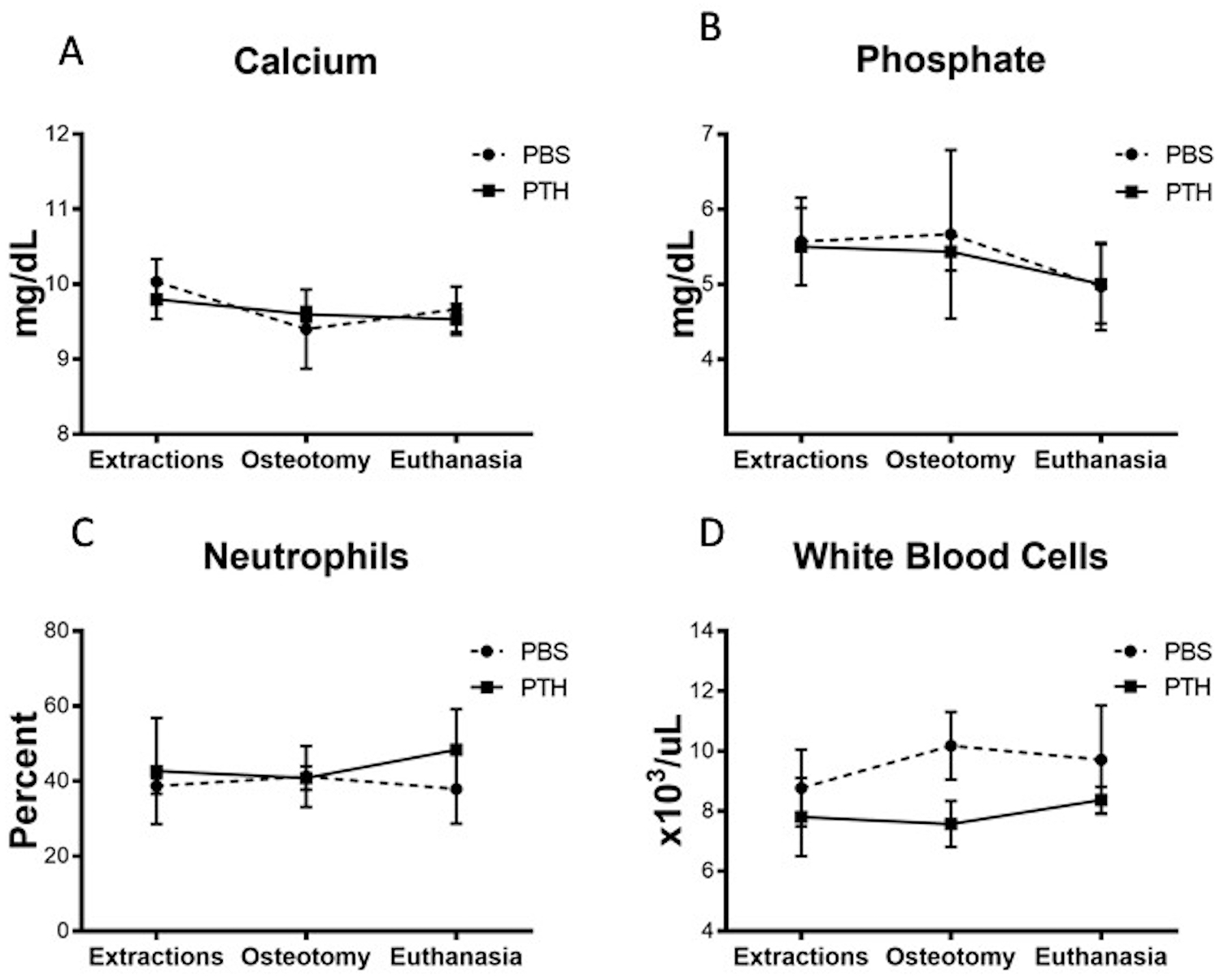
Blood was taken from the minipigs during the: extraction (Day 56 pre-op), osteotomy (Day 0) and at euthanasia (Day 56 post-op), to assess (A) calcium, (B) phosphate, (C) percentage of neutrophils, and (D) number of white blood cells. Note that no differences between groups were observed (two-way ANOVA with repeated measures; graphs represent means and SEMs).
Discussion
Craniofacial surgeons continue to search for novel tissue reconstruction solutions to treat massive bone loss. Structural allografts are a readily available source for bone grafts that are osteoconductive and biomechanically stable. Unlike vascularized bone grafts from the fibulae or ribs, they are not osteogenic or osteoinductive, but they can provide the 3D geometry and structural support necessary to reconstruct extensive segments of the mandible.
However, structural allografts are rarely used for jaw bone reconstruction, likely due to their known failure rate in long bone repair. In previous studies of mandible reconstruction, researchers attempted to revitalize bone allografts to enhance their osteogenic potential and level of integration with host bone. One strategy included the seeding of adult stem cells on autoclaved autografts (von Wilmowsky et al., 2010) or on bone xenografts (Bhumiratana et al., 2016); this was successful in reconstructing a limited segment and the ramus-condyle unit of pig mandibles, respectively. Runyan et al. investigated a different strategy in which mandibular allografts were filled with BMP-2 and adipose-derived stem cells, and implanted under the rib periosteum of live pigs (Runyan et al., 2014; Runyan et al., 2010). Although this strategy was not tested in a mandibular bone defect, it was determined that the retrieved grafts were equivalent to native bone in biomechanical properties and vasculature. In the present study, an alternative therapeutic approach was employed in which allografts were used for mandible reconstruction without the need for additional steps such as cell seeding and/or ectopic implantations. The use of rPTH could prevent allograft failure and enhance its integration with the remaining host bone, leading to successful reconstruction.
Several earlier studies showed that intermittent administration of rPTH enhances integration of structural allografts in a murine model of femoral bone defect (Dhillon et al., 2013; Reynolds et al., 2011; Takahata et al., 2012). The mechanism behind that effect was attributed to the prevention of scar formation around bone allografts by reducing mast cell accumulation and to the effect of enhanced new bone formation. It was proposed that rPTH alters arteriogenesis by inhibiting Ang-2 expression, leading to small blood vessel formation, which in turn limits mast cell penetration (Dhillon et al., 2013). Similar results were obtained when a murine calvarial bone model was treated with cranial allografts and rPTH (Cohn Yakubovich et al., 2017; Sheyn et al., 2013). In those studies, it was shown that rPTH downregulated the expression of TGF-β, a potent inducer of scar tissue; reduced the number of mast cells surrounding the cranial allografts; and shifted vascularization to small blood vessel formation. The effect of rPTH on blood vessels and mast cells was further corroborated in a calvarial defect model without the use of allografts (Zhang et al., 2017). It was also shown that rPTH enhanced the accumulation and osteogenic differentiation of endogenous mesenchymal progenitor cells at calvarial bone defects, leading to significantly more bone formation than that found in controls (Sheyn et al., 2013). However, although the same dose of rPTH was used in femoral and calvarial bone defect models, it was evident that the effect on new bone formation was more robust in the long bone model than in the craniofacial one. This could be explained by the difference in endogenous mesenchymal progenitor cells, as it was shown that maxillofacial-derived progenitors behave in a different manner than their counterparts from other sites in the body with respect to osteogenic differentiation and response to growth factors such as BMP (Akintoye, Giavis, Stefanik, Levin, & Mante, 2008; Akintoye et al., 2006; Osyczka, Damek-Poprawa, Wojtowicz, & Akintoye, 2009; Stefanik et al., 2008). Interestingly, porcine mandible bone marrow mesenchymal progenitors, isolated from 3- to 4-month-old pigs, displayed elevated expression of BMP-4 when compared to tibial mesenchymal progenitors, which could suggest a higher osteogenic potential (Lloyd et al., 2017). Indeed, when the segmental defect described in the current paper was generated in 6- to 9-month-old adolescent minipigs, significant spontaneous bone regeneration was observed even without administration of rPTH (data not shown), which could be the result from osteogenically potent resident progenitors.
Nishitani et al., attempted to translate findings from murine femoral bone studies to a large animal model (Nishitani et al., 2017). Twenty mongrel hounds received a 5-cm osteotomy in the femur, which was reconstructed with a bone allograft. The dogs were randomized to receive intermittent administration of rPTH or placebo for 8 weeks. The results showed that rPTH treatment significantly increased callus volume and cortical union, but there was no significant difference in biomechanical properties at the study’s end point. In addition, the dose of 5 μg/kg/d, which was used in the study, was not tolerated well by the dogs, which induced serious hypercalcemia, and had to be treated with intravenous saline. In the present study, similar results of increased new bone formation were evident in minipigs treated with rPTH, but without any evidence of hypercalcemia. In addition, several biomechanical parameters were significantly improved following rPTH treatment, which indicates increased host-induced bone formation and better graft integration. There are several differences between a long bone segmental defect and a similar defect in the mandible. Quadruped animals can distribute some weight to other limbs, reducing the load on the operated bone. But mastication loads are mostly bilateral, especially when there is little to no sensory feedback from the operated side of the mandible. In addition, the supporting plate of an operated femur can sustain some of the load along its long axis, whereas in the mandible loads are mostly perpendicular to the plate, generating substantial forces on the healing site. In light of these differences between the two models, it is remarkable that allograft integration was achieved in the mandibles of the animals treated with rPTH, with higher biomechanical properties compared to the PBS group.
The commonly used rPTH doses in rodent studies reach as high as 40 to50 μg/kg/d. Takahata et al. determined that only a high dose of 40 μg/kg/d led to a significant increase in bone formation when allografts were used to repair a murine femoral bone defect (Takahata et al., 2012). Human patients are treated with 20 μg of rPTH daily to prevent osteoporosis-related fractures (that is, 0.3 μg/kg/d assuming an average weight of 70 kg). In the present study, a daily dose of 1.75 μg/kg was used, which was shown to be tolerated by minipigs in a previous study (Sheyn et al., 2016). Here as well, there were no signs of hypercalcemia or any other changes in the blood chemistry tests. Although the dose was about one-third that of the dose used for the dog study, significant bone formation was seen. Hence, it is plausible that a short course of rPTH at a low dose can lead to allograft integration in human patients as well. It should be noted that the bone tissue within the mandible defects of animals treated with rPTH, appeared less mineralized compared to the bone in the control group. The difference was not noted in the new bone that was generated within the defects. A possible explanation is that in the rPTH group, remodeling of the allograft was more pronounced leading to loss of mineral. Albeit the difference in mineralization, biomechanical scores were higher in the rPTH group. This could be attributed to the biomechanical test used here that examined the level of allograft integration to host bone rather than the biomechanical properties of new bone formed within the defects.
There are several limitations to this study. First, the sample size in this study was small. However, given that significant differences in quantifiable functional outcomes were reported, we are confident that follow-up studies with a larger sample size will corroborate these preliminary findings. Longer periods of follow-up are also needed to determine if bone allografts can completely integrate with the host mandible. In addition, there is a need to determine if dental implants can be successfully osteointegrated into new bone formed over the allograft, in order to allow for normal bite and mastication. Current treatment with rPTH is given to patients with osteoporosis for up to 2 years. There is a need to determine the time required for sufficient allograft integration. In addition, a toxicology study should be undertaken to rule out risks such as osteosarcoma, which was detected in rats treated with rPTH (Vahle et al., 2002), if indeed higher doses are needed.
Conclusions
This pilot study shows that intermittent rPTH administration can enhance integration of mandibular allografts with host bone in a large animal model of mandibulectomy. Further studies should explore the potential of this therapeutic approach, which could provide a suitable solution for craniofacial bone reconstruction following trauma or treatment of malignancy. Since both rPTH and bone allografts are approved for clinical use, these results could be easily translated to the clinical arena.
Acknowledgments
The study was supported by grants from the National Institutes of Health (R01DE019902 to DG and EMS; and P30 AR069655 to EMS and HAA) and the Israel Ministry of Defense (1538-2015, DG). We thank Veterinary Transplant Services, Inc. for tissue banking and processing activities. We also thank Katherine Bresee, MS. for the statistical analyses and Dr. Melodie Metzger for the use of the Faxitron imaging system.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- Aerssens J, Boonen S, Lowet G, & Dequeker J (1998). Interspecies differences in bone composition, density, and quality: potential implications for in vivo bone research. Endocrinology, 139(2), 663–670. doi: 10.1210/endo.139.2.5751 [DOI] [PubMed] [Google Scholar]
- Akintoye SO, Giavis P, Stefanik D, Levin L, & Mante FK (2008). Comparative osteogenesis of maxilla and iliac crest human bone marrow stromal cells attached to oxidized titanium: a pilot study. Clin Oral Implants Res, 19(11), 1197–1201. doi: 10.1111/j.1600-0501.2008.01592.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akintoye SO, Lam T, Shi S, Brahim J, Collins MT, & Robey PG (2006). Skeletal site-specific characterization of orofacial and iliac crest human bone marrow stromal cells in same individuals. Bone, 38(6), 758–768. doi: 10.1016/j.bone.2005.10.027 [DOI] [PubMed] [Google Scholar]
- Alfotawei R, Naudi KB, Lappin D, Barbenel J, Di Silvio L, Hunter K, … Ayoub A (2014). The use of TriCalcium Phosphate (TCP) and stem cells for the regeneration of osteoperiosteal critical-size mandibular bony defects, an in vitro and preclinical study. J Craniomaxillofac Surg, 42(6), 863–869. doi: 10.1016/j.jcms.2013.12.006 [DOI] [PubMed] [Google Scholar]
- Asahina I, Watanabe M, Sakurai N, Mori M, & Enomoto S (1997). Repair of bone defect in primate mandible using a bone morphogenetic protein (BMP)-hydroxyapatite-collagen composite. J Med Dent Sci, 44(3), 63–70. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9608283 [PubMed] [Google Scholar]
- Bez M, Sheyn D, Tawackoli W, Avalos P, Shapiro G, Giaconi JC, … Gazit D (2017). In situ bone tissue engineering via ultrasound-mediated gene delivery to endogenous progenitor cells in mini-pigs. Sci Transl Med, 9(390). doi: 10.1126/scitranslmed.aal3128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhumiratana S, Bernhard JC, Alfi DM, Yeager K, Eton RE, Bova J, … Vunjak-Novakovic G (2016). Tissue-engineered autologous grafts for facial bone reconstruction. Sci Transl Med, 8(343), 343ra383. doi: 10.1126/scitranslmed.aad5904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boffano P, Roccia F, Zavattero E, Dediol E, Uglešić V, Kovačič Ž, … Forouzanfar T (2015). European Maxillofacial Trauma (EURMAT) project: a multicentre and prospective study. J Craniomaxillofac Surg, 43(1), 62–70. doi: 10.1016/j.jcms.2014.10.011 [DOI] [PubMed] [Google Scholar]
- Boonen S, Marin F, Mellstrom D, Xie L, Desaiah D, Krege JH, & Rosen CJ (2006). Safety and efficacy of teriparatide in elderly women with established osteoporosis: bone anabolic therapy from a geriatric perspective. J Am Geriatr Soc, 54(5), 782–789. doi:JGS695 [pii] 10.1111/j.1532-5415.2006.00695.x [DOI] [PubMed] [Google Scholar]
- Carter TG, Brar PS, Tolas A, & Beirne OR (2008). Off-label use of recombinant human bone morphogenetic protein-2 (rhBMP-2) for reconstruction of mandibular bone defects in humans. J Oral Maxillofac Surg, 66(7), 1417–1425. doi: 10.1016/j.joms.2008.01.058 [DOI] [PubMed] [Google Scholar]
- Chen P, Miller PD, Recker R, Resch H, Rana A, Pavo I, & Sipos AA (2007). Increases in BMD correlate with improvements in bone microarchitecture with teriparatide treatment in postmenopausal women with osteoporosis. J Bone Miner Res, 22(8), 1173–1180. doi: 10.1359/jbmr.070413 [DOI] [PubMed] [Google Scholar]
- Cohn Yakubovich D, Eliav U, Yalon E, Schary Y, Sheyn D, Cook-Wiens G, … Gazit Z (2017). Teriparatide attenuates scarring around murine cranial bone allograft via modulation of angiogenesis. Bone, 97, 192–200. doi: 10.1016/j.bone.2017.01.020 [DOI] [PubMed] [Google Scholar]
- de Winter JCF (2013). Using the Student’s t-test with extremely small sample sizes Practical Assessment, Research, and Evaluation, 18. doi: 10.7275/e4r6-dj05 [DOI] [Google Scholar]
- Dhillon RS, Xie C, Tyler W, Calvi LM, Awad HA, Zuscik MJ, … Schwarz EM (2013). PTH-enhanced structural allograft healing is associated with decreased angiopoietin-2-mediated arteriogenesis, mast cell accumulation, and fibrosis. J Bone Miner Res, 28(3), 586–597. doi: 10.1002/jbmr.1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebeling PR, & Russell RG (2003). Teriparatide (rhPTH 1–34) for the treatment of osteoporosis. Int J Clin Pract, 57(8), 710–718. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14627183 [PubMed] [Google Scholar]
- Emara KM, Diab RA, & Emara AK (2015). Recent biological trends in management of fracture non-union. World J Orthop, 6(8), 623–628. doi: 10.5312/wjo.v6.i8.623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girotra M, Rubin MR, & Bilezikian JP (2006). The use of parathyroid hormone in the treatment of osteoporosis. Rev Endocr Metab Disord, 7(1–2), 113–121. doi: 10.1007/s11154-006-9007-z [DOI] [PubMed] [Google Scholar]
- Gómez-Barrena E, Rosset P, Lozano D, Stanovici J, Ermthaller C, & Gerbhard F (2015). Bone fracture healing: cell therapy in delayed unions and nonunions. Bone, 70, 93–101. doi: 10.1016/j.bone.2014.07.033 [DOI] [PubMed] [Google Scholar]
- Harashina T, Nakajima H, & Imai T (1978). Reconstruction of mandibular defects with revascularized free rib grafts. Plast Reconstr Surg, 62(4), 514–522. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/358231 [DOI] [PubMed] [Google Scholar]
- Hidalgo DA (1989). Fibula free flap: a new method of mandible reconstruction. Plast Reconstr Surg, 84(1), 71–79. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/2734406 [PubMed] [Google Scholar]
- Hodsman AB, Bauer DC, Dempster DW, Dian L, Hanley DA, Harris ST, … Yuen CK (2005). Parathyroid hormone and teriparatide for the treatment of osteoporosis: a review of the evidence and suggested guidelines for its use. Endocr Rev, 26(5), 688–703. doi:er.2004–0006 [pii] 10.1210/er.2004-0006 [DOI] [PubMed] [Google Scholar]
- Imahara SD, Hopper RA, Wang J, Rivara FP, & Klein MB (2008). Patterns and outcomes of pediatric facial fractures in the United States: a survey of the National Trauma Data Bank. J Am Coll Surg, 207(5), 710–716. doi: 10.1016/j.jamcollsurg.2008.06.333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Zhao JJ, Mitlak BH, Wang O, Genant HK, & Eriksen EF (2003). Recombinant Human Parathyroid Hormone (1–34) [Teriparatide] Improves both Cortical and Cancellous Bone Structure. Journal of Bone and Mineral Research, 18(11), 1932–1941. [DOI] [PubMed] [Google Scholar]
- Keith JD, Petrungaro P, Leonetti JA, Elwell CW, Zeren KJ, Caputo C, … Warner MM (2006). Clinical and histologic evaluation of a mineralized block allograft: results from the developmental period (2001–2004). Int J Periodontics Restorative Dent, 26(4), 321–327. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/16939013 [PubMed] [Google Scholar]
- Kolk A, Tischer T, Koch C, Vogt S, Haller B, Smeets R, … Bissinger O (2016). A novel nonviral gene delivery tool of BMP-2 for the reconstitution of critical-size bone defects in rats. J Biomed Mater Res A, 104(10), 2441–2455. doi: 10.1002/jbm.a.35773 [DOI] [PubMed] [Google Scholar]
- Konopnicki S, Sharaf B, Resnick C, Patenaude A, Pogal-Sussman T, Hwang KG, … Troulis MJ (2015). Tissue-engineered bone with 3-dimensionally printed beta-tricalcium phosphate and polycaprolactone scaffolds and early implantation: an in vivo pilot study in a porcine mandible model. J Oral Maxillofac Surg, 73(5), 1016 e1011–1016 e1011. doi: 10.1016/j.joms.2015.01.021 [DOI] [PubMed] [Google Scholar]
- Lee KB, Taghavi CE, Murray SS, Song KJ, Keorochana G, & Wang JC (2012). BMP induced inflammation: a comparison of rhBMP-7 and rhBMP-2. J Orthop Res, 30(12), 1985–1994. doi: 10.1002/jor.22160 [DOI] [PubMed] [Google Scholar]
- Lee S, Choi D, Shim JH, & Nam W (2020). Efficacy of three-dimensionally printed polycaprolactone/beta tricalcium phosphate scaffold on mandibular reconstruction. Sci Rep, 10(1), 4979. doi: 10.1038/s41598-020-61944-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leipzig B, & Cummings CW (1984). The current status of mandibular reconstruction using autogenous frozen mandibular grafts. Head Neck Surg, 6(6), 992–997. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/6469659 [DOI] [PubMed] [Google Scholar]
- Lindsay R, Zhou H, Cosman F, Nieves J, Dempster DW, & Hodsman AB (2007). Effects of a one-month treatment with PTH(1–34) on bone formation on cancellous, endocortical, and periosteal surfaces of the human ilium. J Bone Miner Res, 22(4), 495–502. doi: 10.1359/jbmr.070104 [DOI] [PubMed] [Google Scholar]
- Liu W, Zhang ZY, Tang XJ, Wang JC, & Gui L (2011). Effect of outer mandibular cortex osteotomy on local morphology and biomechanics in young miniature pigs. J Craniomaxillofac Surg, 39(6), 425–430. doi: 10.1016/j.jcms.2010.10.013 [DOI] [PubMed] [Google Scholar]
- Liu YF, Xu LW, Zhu HY, & Liu SS (2014). Technical procedures for template-guided surgery for mandibular reconstruction based on digital design and manufacturing. Biomed Eng Online, 13, 63. doi: 10.1186/1475-925X-13-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd B, Tee BC, Headley C, Emam H, Mallery S, & Sun Z (2017). Similarities and differences between porcine mandibular and limb bone marrow mesenchymal stem cells. Arch Oral Biol, 77, 1–11. doi: 10.1016/j.archoralbio.2017.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JL, Pan JL, Tan BS, & Cui FZ (2009). Determination of critical size defect of minipig mandible. J Tissue Eng Regen Med, 3(8), 615–622. doi: 10.1002/term.203 [DOI] [PubMed] [Google Scholar]
- Mathog RH, Toma V, Clayman L, & Wolf S (2000). Nonunion of the mandible: an analysis of contributing factors. J Oral Maxillofac Surg, 58(7), 746–752; discussion 752–743. doi: 10.1053/joms.2000.7258 [DOI] [PubMed] [Google Scholar]
- Mehta RP, & Deschler DG (2004). Mandibular reconstruction in 2004: an analysis of different techniques. Curr Opin Otolaryngol Head Neck Surg, 12(4), 288–293. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/15252248 [DOI] [PubMed] [Google Scholar]
- Moore WR, Graves SE, & Bain GI (2001). Synthetic bone graft substitutes. ANZ J Surg, 71(6), 354–361. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/11409021 [PubMed] [Google Scholar]
- Morris C, Bebeau NP, Brockhoff H, Tandon R, & Tiwana P (2015). Mandibular fractures: an analysis of the epidemiology and patterns of injury in 4,143 fractures. J Oral Maxillofac Surg, 73(5), 951.e951–951.e912. doi: 10.1016/j.joms.2015.01.001 [DOI] [PubMed] [Google Scholar]
- Nassr A, Khan MH, Ali MH, Espiritu MT, Hanks SE, Lee JY, … Kang JD (2009). Donor-site complications of autogenous nonvascularized fibula strut graft harvest for anterior cervical corpectomy and fusion surgery: experience with 163 consecutive cases. Spine J, 9(11), 893–898. doi: 10.1016/j.spinee.2009.04.020 [DOI] [PubMed] [Google Scholar]
- Nishitani K, Mietus Z, Beck CA, Ito H, Matsuda S, Awad HA, … Schwarz EM (2017). High dose teriparatide (rPTH1–34) therapy increases callus volume and enhances radiographic healing at 8-weeks in a massive canine femoral allograft model. PLoS One, 12(10), e0185446. doi: 10.1371/journal.pone.0185446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira MR, Gorla LF, Gabrielli MA, & Pereira-Filho VA (2017). Off-Label Use of Bone Morphogenetic Protein 2 in the Reconstructions of Mandibular Continuity Defects. J Craniofac Surg, 28(1), 227–230. doi: 10.1097/SCS.0000000000003291 [DOI] [PubMed] [Google Scholar]
- Osyczka AM, Damek-Poprawa M, Wojtowicz A, & Akintoye SO (2009). Age and skeletal sites affect BMP-2 responsiveness of human bone marrow stromal cells. Connect Tissue Res, 50(4), 270–277. doi: 10.1080/03008200902846262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce AI, Richards RG, Milz S, Schneider E, & Pearce SG (2007). Animal models for implant biomaterial research in bone: a review. Eur Cell Mater, 13, 1–10. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/17334975 [DOI] [PubMed] [Google Scholar]
- Quarto R, Mastrogiacomo M, Cancedda R, Kutepov SM, Mukhachev V, Lavroukov A, … Marcacci M (2001). Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med, 344(5), 385–386. doi: 10.1056/NEJM200102013440516 [DOI] [PubMed] [Google Scholar]
- Rana M, Warraich R, Kokemüller H, Lemound J, Essig H, Tavassol F, … Gellrich NC (2011). Reconstruction of mandibular defects - clinical retrospective research over a 10-year period -. Head Neck Oncol, 3, 23. doi: 10.1186/1758-3284-3-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman Q, Lang TF, Arnaud CD, Modin GW, & Lane NE (2003). Daily treatment with parathyroid hormone is associated with an increase in vertebral cross-sectional area in postmenopausal women with glucocorticoid-induced osteoporosis. Osteoporos Int, 14(1), 77–81. doi: 10.1007/s00198-002-1312-0 [DOI] [PubMed] [Google Scholar]
- Reynolds DG, Takahata M, Lerner AL, O’Keefe RJ, Schwarz EM, & Awad HA (2011). Teriparatide therapy enhances devitalized femoral allograft osseointegration and biomechanics in a murine model. Bone, 48(3), 562–570. doi: 10.1016/j.bone.2010.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson DM, & Smith DC (1978). Compressive strength of mandibular bone as a function of microstructure and strain rate. J Biomech, 11(10–12), 455–471. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/730760 [DOI] [PubMed] [Google Scholar]
- Ruggiero SL, Mehrotra B, Rosenberg TJ, & Engroff SL (2004). Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg, 62(5), 527–534. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/15122554 [DOI] [PubMed] [Google Scholar]
- Runyan CM, Ali ST, Chen W, Calder BW, Rumburg AE, Billmire DA, & Taylor JA (2014). Bone tissue engineering by way of allograft revitalization: mechanistic and mechanical investigations using a porcine model. J Oral Maxillofac Surg, 72(5), 1000 e1001–1011. doi: 10.1016/j.joms.2014.01.017 [DOI] [PubMed] [Google Scholar]
- Runyan CM, Jones DC, Bove KE, Maercks RA, Simpson DS, & Taylor JA (2010). Porcine allograft mandible revitalization using autologous adipose-derived stem cells, bone morphogenetic protein-2, and periosteum. Plast Reconstr Surg, 125(5), 1372–1382. doi: 10.1097/PRS.0b013e3181d7032f [DOI] [PubMed] [Google Scholar]
- Sheyn D, Cohn Yakubovich D, Kallai I, Su S, Da X, Pelled G, … Gazit Z (2013). PTH promotes allograft integration in a calvarial bone defect. Mol Pharm, 10(12), 4462–4471. doi: 10.1021/mp400292p [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheyn D, Shapiro G, Tawackoli W, Jun DS, Koh Y, Kang KB, … Gazit D (2016). PTH Induces Systemically Administered Mesenchymal Stem Cells to Migrate to and Regenerate Spine Injuries. Mol Ther, 24(2), 318–330. doi: 10.1038/mt.2015.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh DJ, & Bartlett SP (2005). Congenital mandibular hypoplasia: analysis and classification. J Craniofac Surg, 16(2), 291–300. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/15750428 [DOI] [PubMed] [Google Scholar]
- Skouteris CA, & Sotereanos GC (1989). Donor site morbidity following harvesting of autogenous rib grafts. J Oral Maxillofac Surg, 47(8), 808–812. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/2746389 [DOI] [PubMed] [Google Scholar]
- Stefanik D, Sarin J, Lam T, Levin L, Leboy PS, & Akintoye SO (2008). Disparate osteogenic response of mandible and iliac crest bone marrow stromal cells to pamidronate. Oral Dis, 14(5), 465–471. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/18938273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhardt Y, Aslan H, Regev E, Zilberman Y, Kallai I, Gazit D, & Gazit Z (2008). Maxillofacial-derived stem cells regenerate critical mandibular bone defect. Tissue Eng Part A, 14(11), 1763–1773. doi: 10.1089/ten.tea.2008.0007 [DOI] [PubMed] [Google Scholar]
- Takahata M, Schwarz EM, Chen T, O’Keefe RJ, & Awad HA (2012). Delayed short-course treatment with teriparatide (PTH(1–34)) improves femoral allograft healing by enhancing intramembranous bone formation at the graft-host junction. J Bone Miner Res, 27(1), 26–37. doi: 10.1002/jbmr.518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashjian AH Jr., & Gagel RF (2006). Teriparatide [human PTH(1–34)]: 2.5 years of experience on the use and safety of the drug for the treatment of osteoporosis. J Bone Miner Res, 21(3), 354–365. doi: 10.1359/JBMR.051023 [DOI] [PubMed] [Google Scholar]
- Tsai CJ, Hofstede TM, Sturgis EM, Garden AS, Lindberg ME, Wei Q, … Dong L (2013). Osteoradionecrosis and radiation dose to the mandible in patients with oropharyngeal cancer. Int J Radiat Oncol Biol Phys, 85(2), 415–420. doi: 10.1016/j.ijrobp.2012.05.032 [DOI] [PubMed] [Google Scholar]
- Vahle JL, Sato M, Long GG, Young JK, Francis PC, Engelhardt JA, … Nold JB (2002). Skeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1–34) for 2 years and relevance to human safety. Toxicol Pathol, 30(3), 312–321. doi: 10.1080/01926230252929882 [DOI] [PubMed] [Google Scholar]
- von Wilmowsky C, Schwarz S, Kerl JM, Srour S, Lell M, Felszeghy E, & Schlegel KA (2010). Reconstruction of a mandibular defect with autogenous, autoclaved bone grafts and tissue engineering: An in vivo pilot study. J Biomed Mater Res A, 93(4), 1510–1518. doi: 10.1002/jbm.a.32635 [DOI] [PubMed] [Google Scholar]
- Vu DD, & Schmidt BL (2008). Quality of life evaluation for patients receiving vascularized versus nonvascularized bone graft reconstruction of segmental mandibular defects. J Oral Maxillofac Surg, 66(9), 1856–1863. doi: 10.1016/j.joms.2008.04.021 [DOI] [PubMed] [Google Scholar]
- Wang L, Liu K, Shao Z, & Shang ZJ (2015). Clinical experience with 80 microvascular couplers in 64 free osteomyocutaneous flap transfers for mandibular reconstruction. Int J Oral Maxillofac Surg, 44(10), 1231–1235. doi: 10.1016/j.ijom.2015.07.011 [DOI] [PubMed] [Google Scholar]
- Wang S, Liu Y, Fang D, & Shi S (2007). The miniature pig: a useful large animal model for dental and orofacial research. Oral Dis, 13(6), 530–537. doi: 10.1111/j.1601-0825.2006.01337.x [DOI] [PubMed] [Google Scholar]
- Wang W, & Yeung KWK (2017). Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact Mater, 2(4), 224–247. doi: 10.1016/j.bioactmat.2017.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnakulasuriya S (2009). Global epidemiology of oral and oropharyngeal cancer. Oral Oncol, 45(4–5), 309–316. doi: 10.1016/j.oraloncology.2008.06.002 [DOI] [PubMed] [Google Scholar]
- Wong DA, Kumar A, Jatana S, Ghiselli G, & Wong K (2008). Neurologic impairment from ectopic bone in the lumbar canal: a potential complication of off-label PLIF/TLIF use of bone morphogenetic protein-2 (BMP-2). Spine J, 8(6), 1011–1018. doi: 10.1016/j.spinee.2007.06.014 [DOI] [PubMed] [Google Scholar]
- Xie L, Semenciw R, & Mery L (2015). Cancer incidence in Canada: trends and projections (1983–2032). Health Promot Chronic Dis Prev Can, 35 Suppl 1, 2–186. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/26011811 [PubMed] [Google Scholar]
- Zara JN, Siu RK, Zhang X, Shen J, Ngo R, Lee M, … Soo C (2011). High doses of bone morphogenetic protein 2 induce structurally abnormal bone and inflammation in vivo. Tissue Eng Part A, 17(9–10), 1389–1399. doi: 10.1089/ten.TEA.2010.0555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Wang T, Chang M, Kaiser C, Kim JD, Wu T, … Schwarz EM (2017). Teriparatide Treatment Improves Bone Defect Healing Via Anabolic Effects on New Bone Formation and Non-Anabolic Effects on Inhibition of Mast Cells in a Murine Cranial Window Model. J Bone Miner Res, 32(9), 1870–1883. doi: 10.1002/jbmr.3178 [DOI] [PMC free article] [PubMed] [Google Scholar]


