Abstract
The present study examined the extent to which leukocyte infiltration into the kidneys in angiotensin II (AngII)-induced hypertension is determined by elevation of renal perfusion pressure (RPP). Male Sprague-Dawley rats were instrumented with carotid and femoral arterial catheters for continuous monitoring of blood pressure (BP) and a femoral venous catheter for infusion. An inflatable aortic occluder cuff placed between the renal arteries with computer-driven servo-controller maintained RPP to the left kidney at control levels during 7-days of intravenous AngII (50 ng/kg/min) or vehicle (saline) infusion. Rats were fed a 0.4% NaCl diet throughout the study. AngII infused rats exhibited nearly a 50 mmHg increase of RPP (carotid catheter) to the right kidney while RPP to the left kidney (femoral catheter) was controlled at baseline pressure throughout the study. As determined at the end of the studies by flow cytometry, right kidneys exhibited significantly greater numbers of T cells, B cells, and monocytes/macrophages compared to the servo-controlled left kidneys and compared to vehicle treated rats. No difference was found between AngII servo-controlled left kidneys and vehicle treated kidneys. Immunostaining found that the density of glomeruli, cortical and outer medullary capillaries were significantly reduced in the right kidney of AngII infused rats compared to servo-controlled left kidney. We conclude that in this model of hypertension the elevation of RPP, not AngII nor dietary salt, leads to leukocyte infiltration in the kidney and to capillary rarefaction.
Keywords: Angiotensin II, Renal perfusion pressure, Immune system, Capillary rarefaction, Kidney injury, SD rats
Graphical Abstract
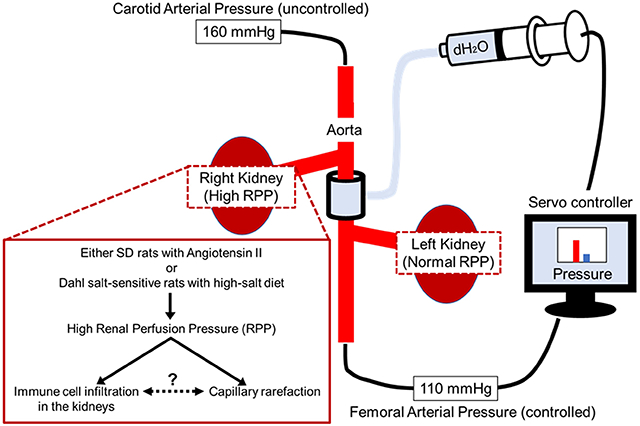
Introduction
The importance of renal infiltrating leukocytes in the development of hypertension and end-organ damage is now well recognized. Currently under investigation are mechanisms responsible for the entry of the leukocytes into the kidney that stimulate pathways of inflammation and oxidative stress. In the present study, the importance of elevated renal perfusion pressure (RPP) upon leukocyte infiltration in angiotensin II (AngII) induced hypertension in rats was examined. Considerations of the injurious effects of elevated RPP upon the integrity of the glomeruli (barotrauma) evolved from studies by Brenner and colleagues 1, 2 and advanced by Bidani and Griffin3–6 and studies in our laboratory7–12. Direct evidence and the quantitative relevance of the contribution of RPP to renal injury in hypertension was obtained as it became possible to control (servo-control) RPP to a single kidney while the contralateral kidney was exposed to hypertension. Using a custom designed computer-controlled servo-control system, several rat models of hypertension have been studied in this manner including the AngII + high salt model8, 11, the AngII + L-NAME model12, the norepinephrine model10, and the Dahl salt-sensitive rat (Dahl S)7, 9. The impact of RPP to influence renal injury is variable and dependent upon the model of hypertension that is studied.
As the importance of inflammation and immunity in hypertension was revealed using both immunosuppressive agents and genetic deletion approaches13–19, flow cytometry experiments demonstrated the numbers and types of immune cells that infiltrated into the kidney in various models of hypertension. Renal infiltration of nearly equal numbers of infiltrating T helper (CD4+), and cytotoxic T cells (CD8+) was found in hypertensive Dahl S rats fed a high salt diet20, 21 and it was found that prevention of a rise of RPP by servo-control methods attenuated renal infiltration of immune cells and the accompanying renal damage7. Given these observations, the question arose as to whether these changes were unique to the Dahl S model and whether they could meaningfully be extrapolated to other models of hypertension. The present study was therefore undertaken to determine the extent to which leukocyte infiltration into the kidney would be reduced by protecting the kidney from experimental hypertension in a genetically different strain of rat. In the present study, AngII induced hypertension was induced in Sprague-Dawley (SD) rats fed 0.4% NaCl throughout the study while RPP to the left kidney was servo-controlled to remain a normal control RPP and compared to the right uncontrolled kidneys. This internally paired comparison of the left and right kidneys eliminated inter-rat variations and provided a model in which both kidneys were perfused with same circulating arterial blood. The number of leukocytes infiltrating to kidneys were measured with a flow cytometric-based analysis and the renal glomerular and peritubular capillary density was determined by morphologic observations.
Methods
Detailed experimental methods and description of antibodies are available in the online-only Data Supplement.
Male SD rats (9 weeks of age) were purchased from Envigo (Indianapolis, IN) and housed in environmentally controlled rooms under a 12-h light/dark cycle. Rats had free access to 0.4% NaCl AIN-76A diet and water ad libitum. All protocols were approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee. At 10-11 weeks of age, chronic servo-controlling surgeries were done as previously described8 (Figure S1). After 7 days of recovery, baseline blood pressure was measured for 3 days, then the rats were infused with AngII (50 ng/kg/min) intravenously for 7 days to induce hypertension or saline as a vehicle control. We note that in previous manuscripts8, 11, we infused 25 ng/kg/min AngII to rats fed 4% NaCl diet, but that dose of AngII was not sufficient to induce hypertension in rats fed 0.4% NaCl so the dose was increased to 50 ng/kg/min. A computer-driven servo-control system was used to inflate the aortic cuff to maintain left RPP at baseline levels while right RPP was uncontrolled. Following 7 days of Ang II infusion, kidneys were flushed by saline and removed. The upper pole of each kidney was removed for flow cytometry. A thin slice, 2 mm around the papilla, was cut and fixed in neutral-buffered formalin for histological analyses. The bottom pole was divided into the cortex and medulla and snap frozen and stored in −80°C for RNA analyses. Flow cytometry7, 22, qPCR and immunohistochemistry were performed.
Histological analysis
More than 100 glomeruli in each slide were automatically and randomly selected throughout the entire renal cortex using a previously described program23. Glomeruli were scored as 0-4. Percent protein cast, α-smooth muscle actin (αSMA) positive area was semi-quantified using Metamorph analysis as we have described previously24. As measurement of micro vessel density is widely used for quantitative evaluation of the angiogenic or rarefaction response25, glomerular and peritubular capillary densities were determined by image analysis of anti-endothelial cell antibody (RECA1) staining using commercially available NiE microscope with Elements software (Nikon). Glomerular capillary density was calculated by dividing the RECA1 positive staining area with the total glomerular area in at least 50 glomeruli in each slide. The density of peritubular capillaries in the renal cortex and the capillary bundles in the outer medulla were calculated the same way after masking glomerulus and vessel area in 20 random frames in each slide.
Statistical analysis
Rats were randomly allocated to each group. All rats that were completed experimental period was analyzed. Sample size was chosen based on previously published experiments performed under similar conditions7, 9, 11. Analysis of tissue samples were performed blindly. Continuous values are presented as the means ± standard error of the means (SEM). Statistical comparisons were made using a t-test for two-group comparisons, and analysis of variance (ANOVA) followed by Holm-Sidak’s post-hoc test for multiple between-group comparisons. A p < 0.05 was considered significant.
Results
Chronic servo-control of renal perfusion pressure to the left kidney of conscious SD rats
RPP of the present study is shown in Figure 1. Carotid blood pressure, the surrogate of RPP in right kidney, rapidly increased from 117.6 ± 3.2 mmHg to 142.0 ± 3.2 mmHg during the first day following the start of AngII infusion (50 ng/kg/min) reaching a plateau elevation by day 4 of infusion and remained elevated at levels nearly 50 mmHg above control values throughout the 7 days of AngII infusion (Figure 1A). Femoral blood pressure, the surrogate of RPP in left kidney, was servo-controlled to remain within 5 mmHg of the levels of RPP measured during the three days preceding the infusion of AngII. RPP of vehicle treated rats, instrumented in the same manner but with no inflation of the balloon occluder, exhibited no changes in RPP throughout the experiment (Figure 1B) and no significant differences were observed between the left and right kidneys of vehicle treated rats. Of relevance, the RPP to the right and left kidneys in vehicle treated rats was not significantly different from the RPP of the servo-controlled kidneys of the AngII infused rats.
Figure 1. AngII infused servo-control model and immune cell infiltration.

(A) Blood pressure data of AngII infused group (n=9). (B) Blood pressure data of vehicle infused group (n=6, 1 carotid and 1 femoral catheters failed). ● indicates right RPP and ○ indicates left RPP. Data are presented as the means ± SEM of each day. *p < 0.05 versus baseline (Control 3), †p < 0.05 versus left RPP ; Holm-Sidak’s test.
(C) Flow cytometry data for T cell (CD3+), (D) B cell (CD45R+) and (E) Monocyte/Macrophage (CD11bc+). The data show individual values and means ± SEM. *p < 0.05 versus left servo-controlled kidney, †p < 0.05 versus vehicle infused group; Holm-Sidak’s test. AngII n=9, Vehicle n=6. L: Left kidney, R: Right kidney, RPP: Renal perfusion pressure
Servo-control of RPP to the left kidney prevented increased leukocyte infiltration observed in the right kidney that was exposed to elevated renal perfusion pressure during AngII induced hypertension.
The number of infiltrated leukocytes in each kidney was counted by flow cytometric analysis. CD3+ T cells and those subsets of helper (CD3+CD4+) T-cells, and cytotoxic (CD3+CD8+) T-cells were all significantly increased in the right kidney compared to the servo-controlled left kidney (Figure 1C). Similarly, both CD45R+ B cells and CD11bc+ monocytes or macrophages were also significantly higher in the right kidney (Figure 1D, E). No differences were observed in infiltrated leukocytes between the left and right kidneys of vehicle treated rats (Figure 1C, D, E). Importantly, the average numbers of leukocytes of the right and left kidneys in vehicle treated rats were similar to those observed in the left servo-controlled kidney of the AngII infused rats.
Increased renal perfusion pressure caused glomerular injury, but tubular damage was pressure independent.
Glomerular injury was quantified by scoring glomeruli on a scale of 0-4 (Figure S2). Glomerular injury of AngII infused rats was higher compared to either kidney of vehicle treated rats. The additional injurious effects of the elevated RPP to the glomeruli in the AngII infused rats was apparent. Glomerular scores were significantly higher in the AngII infused uncontrolled right kidneys than in the servo-controlled left kidneys (Figure 2A). In contrast, tubular casts did not differ significantly between the servo-controlled left and uncontrolled right kidney but were in both kidneys higher than observed in the vehicle treated rats (Figure 2B–D). Similar patterns were observed using another marker of kidney injury, Hepatitis A virus cellular receptor (Havcr)1 (also known as Kidney injury molecule1) as mRNA expression levels tended to increase in AngII infused rats compared to vehicle infused rats in both cortex and medulla, but no differences were detected between the right and servo-controlled left kidneys (Figure 2E, Figure S3A). Also assessed were renin mRNA expression levels which were suppressed in AngII infused uncontrolled right kidney exposed to the elevated perfusion pressure, but remained at levels similar to the vehicle infused rats in the servo-controlled left kidney (Figure 2F).
Figure 2. Histology.

(A) Glomerular score data. (B) Periodic acid–Schiff stained kidney. Scale bar 250 μm. Percent cast positive area (C) per cortex and (D) per outer medulla. mRNA expression level of (E) Havcr1 and (F) Ren in cortex. The data show individual values and means ± SEM. *p < 0.05 versus left servo-controlled kidney, †p < 0.05 versus vehicle infused group; Holm-Sidak’s test. AngII n=9, Vehicle n=6. L: Left kidney, R: Right kidney, RPP: Renal perfusion pressure
Elevated renal perfusion pressure decreased microvascular density.
Glomerular and peritubular capillary density was semi-quantified by the positive area staining of RECA1 to the rat endothelial cell antigen (mAb RECA-1)26 (Figure 3A). The yellow arrows denote a glomerular capillary and the red arrows denote a peritubular capillary. RECA1 positive area per glomerulus was significantly reduced in high RPP right kidney of AngII infused rats compared to servo-controlled left kidney (26.0% ± 2.9% vs 19.5% ± 2.3%) (Figure 3B). Similarly, the density of peritubular capillaries in the renal cortex and the capillary bundles in the outer medulla (Figure 3C, D) were significantly reduced in the right kidney compared to the servo-controlled left kidney (Cortex: 4.1% ± 0.5% vs 3.0% ± 0.4%, Medulla: 1.3% ± 0.2% vs 0.8% ± 0.2%). AngII tended to increase peritubular vascular density even in the servo-controlled left kidney compared to vehicle treated left kidney (Cortex: 4.1% ± 0.5% vs 2.9% ± 0.4%, Medulla: 1.3% ± 0.2% vs 0.6% ± 0.1%) although these did not reach statistical significance. Cortical tissue mRNA expression of Cd31, also an endothelial cell marker tended to be higher in the servo-controlled left kidney compared to the right kidney of AngII infused rats but did not reach significance (Figure S4A). The left kidney of the servo-controlled AngII infused rats was moderately but significantly higher than the vehicle left kidneys. The von Willebrand factor (Vwf) mRNA, made in endothelial cells was also tended to increase, but was not significantly different in either kidney of the AngII infused rats or the vehicle infused rats (Figure S4B). Cd31 and Vwf did not show statistically significant difference in medullary tissue mRNA expression (Figure S3B, C). Interestingly, Prostaglandin-Endoperoxide Synthase 2 (Cox2) mRNA expression in cortex was significantly elevated in the left kidney of AngII infused rats compared to vehicle (Figure S4C). It was also significantly lower in the right kidney reaching levels similar to vehicle infused rats. On the other hand, Cox2 mRNA expression in medulla was elevated in the right kidney of AngII infused rats (Figure S3D)
Figure 3. Microvascular density.
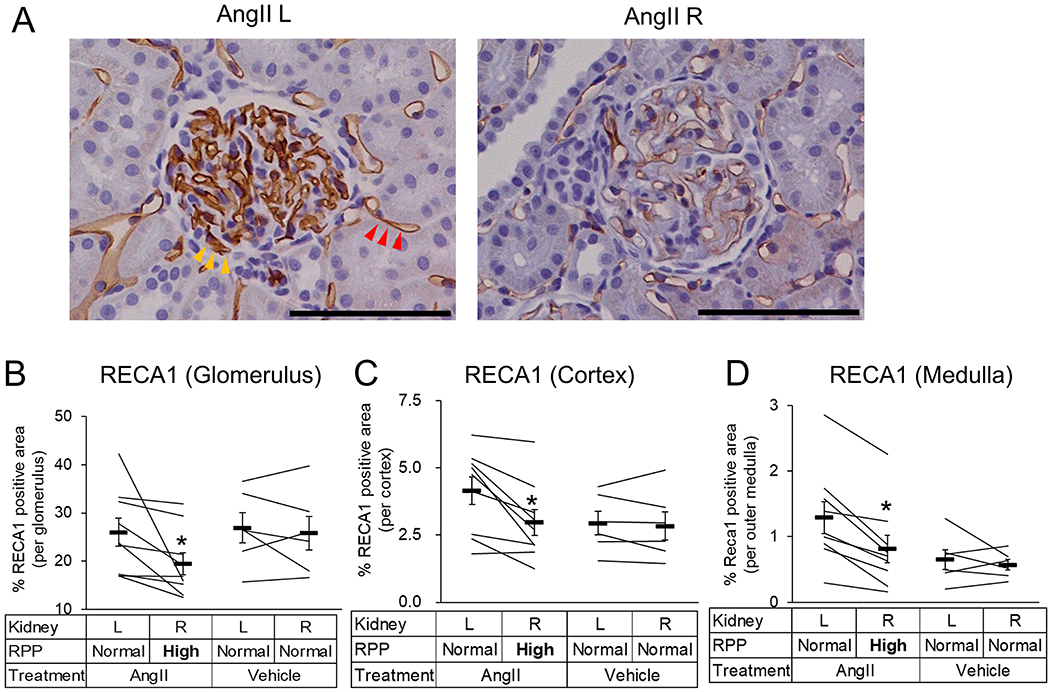
(A) RECA1 staining. Yellow arrows denote positive area in glomerulus and red arrows denote positive area in peritubular capillaries. Scale bar 100 μm. (B) RECA1 positive area per glomerulus. (C) RECA1 positive area per cortex and (D) outer medulla. The data show individual values and means ± SEM. *p < 0.05 versus left servo-controlled kidney, †p < 0.05 versus vehicle infused group; Holm-Sidak’s test. AngII n=9, Vehicle n=6. L: Left kidney, R: Right kidney, RPP: Renal perfusion pressure
Regional kidney injury.
Anti-CD68 antibody (ED1)+ macrophages were found around damaged glomeruli and some arteries in the right kidney of AngII infused rats but extensive infiltration throughout the kidney was not observed (Figure 4A). The marker of tissue fibrosis α-smooth muscle actin (αSMA) stained more densely in the AngII infused rats in both kidneys compared to vehicle infused rats but no significant differences were observed between uncontrolled right kidney and servo-controlled left kidney. (Figure 4B–C). Cortical tissue mRNA expression levels of αSma was significantly higher in the AngII infused uncontrolled right kidneys compared to the servo-controlled left kidneys (Figure 4D). αSma did not show statistically significant difference in medullary tissue mRNA expression (Figure S3E).
Figure 4. Immunohistochemistry.

(A) ED1 staining. Arrows denote positive area. Scale bar 100 μm. (B) αSMA staining. Arrows denote positive area. Scale bar 100 μm. (C) αSMA positive area per cortex. (D) mRNA expression level of αSma. The data show individual values and means ± SEM. *p < 0.05 versus left servo-controlled kidney, †p < 0.05 versus vehicle infused group; Holm-Sidak’s test. AngII n=9, Vehicle n=6. L: Left kidney, R: Right kidney, RPP: Renal perfusion pressure
Discussion
The present study determined the extent to which leukocyte infiltration into the kidneys would be reduced by protecting the kidney from hypertension in the AngII induced model of hypertension in SD rats. This study was prompted by our observations in the Dahl S rat demonstrating that there was a significantly higher abundance of T cells, B cells, and monocytes and macrophages in the hypertensive uncontrolled right kidney compared with the servo-controlled left kidney7. Remarkably, the present study that examined hypertension produced by AngII in a rat of a very different genetic background produced very similar results to that of the Dahl S rat fed high salt (Figure 5A). The robust level of hypertension (> 165 mmHg) induced by 7 days of AngII infusion resulted in significantly greater numbers of total infiltrated leukocytes, including T cells, B cells, and macrophages/monocytes, in the right kidney, which had elevated RPP, compared to the servo-controlled left kidney. This was not a consequence of a high salt diet which is often given with AngII to enhance the hypertension since the rats were maintained on a low-normal (0.4% NaCl) diet throughout the study. The data also demonstrates that the rise of arterial pressure must therefore precede the infiltration of lymphocytes and renal injury and that immune cell infiltration is not determined by elevations of AngII directly.
Figure 5. Comparison with the other model.

(A) Comparison of the ratios of T and B cell infiltration in the servo-controlled left kidneys (LK) to uncontrolled right kidneys (RK) of salt induced hypertension in Dahl Salt Sensitive (SS) rat (black) and AngII induced hypertension in SD rats (white). Nearly identical results were found in the two models. Comparison of (B) glomerular score and (C) cortical and (D) medullary protein cast salt induced hypertension in Dahl Salt Sensitive rats (SS + High Salt) and AngII induced hypertension in SD rats (SD + AngII). The data show individual values and means ± SEM. *p < 0.05 versus left servo-controlled kidney, †p < 0.05 versus SS + High Salt; Holm-Sidak’s test. SS+high salt n=6, SD+AngII n=9. L: Left kidney, R: Right kidney, RPP: Renal perfusion pressure
Data of salt induced hypertension in SS rat is adapted from our previous report7.
Varying patterns of renal injury related to specific models of hypertension.
Although a similar pattern of RPP dependent leukocyte and monocyte infiltration was observed comparing the Dahl S and present AngII model of hypertension, differences were observed in the nature of the associated renal injury. It is interesting that despite the remarkable similarities of the ratios for infiltrating immune cells between right and left kidney in both the Dahl S and AngII models, notable differences in the severity of renal injury were apparent between the two models. In both models, the glomerular injury score was higher in the uncontrolled right kidneys exposed to the elevated RPP although the overall glomerular damage was higher in Dahl S compared with AngII model (Figure 5B) despite similar levels of hypertension. Both AngII-infused and Dahl S rats exhibited greater levels of intersititial fibrosis in the uncontrolled right kidneys as indicated by αSMA staining but AngII-infused rats exhibited far less medullary tubular protein casts than the Dahl S rats (Figure 5C, D). It is interesting to speculate on the possible reasons for these differences. They could of course be ascribed to salt diet since the AngII infused rats of the present study were maintained on a low-normal salt diet. They could in part be attributed to strain differences of the rats. More likely, the differences were a consequence of the intrarenal vasoconstrictor actions of AngII. Polichnowski et al. reported that the renal injury with AngII chronic infusion is relatively mild despite the high RPP, and they suggested that this is related the vasoconstriction of preglomerular vasculature27. Whatever accounts for these differences, it is apparent that the effects of RPP upon renal injury can differ substantially between different forms of hypertension. Such differences of individuals to antihypertensive therapies and various models of hypertension has suggested by Bidani and Griffin28,29, and our own studies10–12.
Renin-angiotensin responses.
Renin mRNA expression was significantly reduced in the uncontrolled right kidneys as would be generally expected in face of high circulating levels of infused AngII and elevated RPP. However, suppression of renin was observed only in the kidney exposed to high RPP and not in the servo-controlled left kidney. If signaling for reduction of renin expression by high levels of circulating AngII was occuring via negative feedback this should have occurred in both kidneys30, 31 Such feedback may have been overridden by a reduction of juxtaglomerular wall tension of the preglomerular vasculature of the servo-controlled kidney27. However, recent studies in mice with conditional deletion of AngII-AT1 receptors on renin-producing cells found no evidence for existence of a direct negative feedback of AngII on the AT1 receptors32. This would indicate that suppression of renin in the right kidney would most likely be attributed to the elevated RPP. However, even in the absence of direct feedback AngII suppression in the servo-protected left kidney, high intrarenal AngII levels would be present in both kidneys due to the high levels of circulating AngII and thereby elevating vascular wall tone and perhaps reduced renal blood flow despite a constancy of RPP. This may explain why similar levels of tubular injury were observed in both right and left kidneys in AngII infused rats even though the RPP of these kidneys were different. The intrarenal actions of AngII such as preglomerular arteriolar vasoconstriction could also explain why differences in markers of renal injury were substantially less than was observed in the Dahl S servo-controlled model of hypertension.
Interestingly, an opposite expression pattern of Cox2 was observed in our study between the renal cortex and medulla. The mechanism for this is not apparent. Gonzalez et al. reported that Cox2 mRNA expression was elevated only in the renal medulla of SD rats infused for 7 days with AngII compared to control but not in the cortex 33. These differences and those observed in the present study between cortical and medullary expression of Cox2 may be a consequence of interactions between renin and Cox2. There is evidence that Cox2 expression is increased in high renin states with salt restriction, angiotensin converting enzyme inhibition and renovascular hypertension34 .
Glomerular and peritubular capillary density.
The loss of renal capillary density is considered one of the most important mechanisms in progression of kidney disease35. Both glomerular and peritubular capillary density were significantly reduced in the right kidneys exposed to the elevated RPP as revealed by RECA1 endothelial cell staining. The glomerular injury is consistent with the elevated glomerular injury score of non-servo-controlled right kidneys and the concept of “barotrauma” on glomerular injury and hyperfiltration36. Less readily explained by physical factors is the microvessel rarefaction observed in the cortical peritubular capillaries. Both red cell flux and peritubular capillary pressures of the superficial cortical microvessels have been found to be well autoregulated in the classic sense that they remain unchanged in response to acute step changes of RPP37. And yet, we and others have observed renal microvessel rarefaction in both the Dahl S and the AngII high salt models of hypertension in the cortex and especially prominent in the renal outer medulla38, 39. The mechanisms responsible for rarefaction of the cortical microvessels is unclear. It is possible that since AngII has been shown to reset autoregulation of renal blood flow to a lower level40, the post-glomerular microvessels may be subjected to higher levels of flow and pressure40. The more prominent capillary rarefaction in the renal outer medulla could be attributed to the well-recognized lack of blood flow autoregulation of blood flow to the outer medulla41–43.
Potential role of microvascular injury in leukocyte infiltration.
The mechanism(s) whereby elevations of RPP lead to increased infiltration of lymphocytes is poorly understood. However, it is relevant that in two very different models of hypertension, the Dahl S plus high salt diet (a low renin model) and the SD high AngII low salt model, the protection of the left kidney from the elevated RPP reduced the infiltration of all types of T-cells, B-cells and macrophages. This lack of selectivity clearly indicates that physical factors are responsible for enabling the infiltration of these cells, albeit to a different extent in two models. In a recent RNAseq analysis we have reported that increases of RPP result in the overexpression of the leukocyte transendothelial migration pathway in Dahl S rats fed a high salt diet44 indicative of endothelial damage in the kidney under high RPP.
Earlier studies have observed that peritubular microvascular rarefaction was present in both Dahl S rats fed a high salt diet, AngII infused SD rats, and in DOCA-salt hypertension38, 39, 45. The extent to which this was related to RPP could not be ascertained in those studies. Iwazu et al.45 found apoptosis and inhibition of proliferation of peritubular endothelial cells in the rat DOCA-salt model which was inhibited by spironolactone treatment indicating that rarefaction of microvessels in the kidneys was mediated via mineral corticoid receptor activation. However, the spironolactone also reduced hypertension in those studies which may have accounted for much of the protection from rarefaction.
In the present study, microvessel rarefaction and leukocyte infiltration were found only in the hypertensive right kidneys of AngII infused rats. The low RPP might directly protect from the mechanical pressures in the capillaries which lacked the autoregulation because of the high circulating AngII. Another possibility is that the higher expressed levels of renin in the servo-controlled kidney may have attenuated the rarefaction since high levels of both renin46 and COX247 have been found to stimulate angiogenesis. The close association of elevated RPP with microvessel rarefaction in our study indicates that mechanical factors were responsible for both the capillary loss and leukocyte infiltration although the molecular signaling mechanisms remain to be determined. It has been found that increased vascular wall tension can stimulate endothelial ROS production which is known to induce the endothelial dysfunction48,49. It has also been found that elevations of transmural vascular pressure can detach pericytes50, and cause capillary loss51, 52 and that endothelial damage induces an increase in vascular permeability53. Endothelial permeability is enhanced by oxidative injury, which increases entry of lipoproteins to the subendothelial space, where they are oxidized and enhance inflammation54. The results of the present study are consistent with the view that increased capillary permeability plays an important role in recruiting circulating leukocytes to the tissue55.
Supplementary Material
Perspectives.
In the study, we have used a chronic servo-control technique to identify the role of RPP in renal immune cell infiltration in AngII infused SD rats. The result was consistent in both Dahl S high-salt induced hypertension and SD AngII-induced hypertension, which indicates that the importance of RPP for recruitment of immune cells into the kidneys. We also suggested the relationship between capillary rarefaction and immune cell infiltration. Further study is necessary to elucidate the mechanism of the development of capillary rarefaction and vascular permeability.
As both Dahl S high-salt induced hypertension and Sprague Dawley AngII-induced hypertension are models exhibiting reduced kidney autoregulation, it would be interesting to carry out similar studies in a rat model with a greater autoregulatory capacity such as the Spontaneously Hypertensive Rat.
Novelty and Significance.
1). What Is New
The quantitative importance of renal perfusion pressure (RPP) related to the infiltration of leukocytes and related kidney injury in an angiotensin II induced model of hypertension was assessed by a custom-designed servo-control technique.
In this model of hypertension, the elevation of RPP, not AngII nor dietary salt, was responsible for leukocyte infiltration in the kidney.
The study shows that elevation of RPP must precede Immune cell infiltration.
Capillary density of the glomeruli, renal cortex, and the outer medulla was found to be highly dependent upon increased RPP.
2). What Is Relevant?
We previously reported that high RPP in Dahl S rats when fed a high salt diet is responsible for immune cell infiltration into the kidneys. In the present study we confirmed the importance of high RPP for the recruitment of immune cells into the kidneys in very different model of hypertension (e.g., AngII with a low salt diet) and in a different strain of rat (e.g., SD).
Capillary rarefaction has been reported to occur in several hypertensive models and is considered to be an important factor for leukocyte infiltration. The present results find that the rarefaction is highly dependent on RPP. Together, the findings emphasize the great importance of protecting of the kidneys from chronic elevations of arterial pressure and elucidate the consequences of failure to do so.
3). Summary
The present study finds that elevation of RPP determines leukocyte infiltration in AngII induced hypertension in SD rats in the presence of a low-normal level of salt intake. This infiltration parallels microvascular rarefaction of both renal glomerular and peritubular capillaries which we propose is importantly involved in the process of infiltration of leukocytes. We conclude that renal leukocyte infiltration and microvascular rarefaction is importantly determined by RPP and not directly by the effects of AngII nor dietary salt. Comparisons with the Dahl salt-sensitive rat model fed a high salt diet indicate that the impact of these physical forces can be quantitatively modified under varying conditions that determine intrarenal distributions of pressures and flows.
Acknowledgements
The authors are grateful to Children’s Hospital and Health System, Inc Research Institute Histology Core in Medical College of Wisconsin for immunostaining. We thank Glenn Slocum for assistance with microscopy.
Source(s) of Funding
This study was supported by grants for scientific research (P01 HL116264, RO1 HL137748)
Footnotes
Conflict(s) of Interest/Disclosure(s)
The authors declare no competing interests.
References
- 1.Brenner BM, Meyer TW and Hostetter TH. Dietary protein intake and the progressive nature of kidney disease: the role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease. N Engl J Med. 1982. September 9;307(11):652–659 [DOI] [PubMed] [Google Scholar]
- 2.Hostetter T, Olson J, Rennke H, Venkatachalam M and Brenner B. Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation. Am J Physiol. 1981. July;241(1):F85–F93. [DOI] [PubMed] [Google Scholar]
- 3.Griffin KA and Bidani AK. Hypertensive renal damage: insights from animal models and clinical relevance. Curr Hypertens Rep. 2004. April;6(2):145–153. [DOI] [PubMed] [Google Scholar]
- 4.Bidani AK and Griffin KA. Pathophysiology of hypertensive renal damage: implications for therapy. Hypertension. 2004. November;44(5):595–601. [DOI] [PubMed] [Google Scholar]
- 5.Loutzenhiser R, Griffin K, Williamson G and Bidani A. Renal autoregulation: new perspectives regarding the protective and regulatory roles of the underlying mechanisms. Am J Physiol Regul Integr Comp Physiol. 2006. May;290(5):R1153–R1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bidani AK and Griffin KA. Basic science: hypertensive target organ damage. J Am Soc Hypertens. 2015. March;9(3):235–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans LC, Petrova G, Kurth T, Yang C, Bukowy JD, Mattson DL and Cowley AW Jr. Increased perfusion pressure drives renal T-cell infiltration in the Dahl salt-sensitive rat. Hypertension. 2017. September;70(3):543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mori T and Cowley AW Jr. Role of pressure in angiotensin II-induced renal injury: chronic servo-control of renal perfusion pressure in rats. Hypertension. 2004. April;43(4):752–759 [DOI] [PubMed] [Google Scholar]
- 9.Mori T, Polichnowski A, Glocka P, Kaldunski M, Ohsaki Y, Liang M and Cowley AW Jr. High perfusion pressure accelerates renal injury in salt-sensitive hypertension. J Am Soc Nephrol. 2008. August;19(8):1472–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polichnowski AJ and Cowley AW Jr. Pressure-induced renal injury in angiotensin II versus norepinephrine-induced hypertensive rats. Hypertension. 2009. December;54(6):1269–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polichnowski AJ, Jin C, Yang C and Cowley AW Jr. Role of Renal Perfusion Pressure Versus Angiotensin II on Renal Oxidative Stress in Angiotensin II–Induced Hypertensive Rats. Hypertension. 2010. June;55(6):1425–1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polichnowski AJ, Lu L and Cowley AW Jr. Renal injury in angiotensin II+ l-NAME-induced hypertensive rats is independent of elevated blood pressure. Am J Physiol Renal Physiol. 2011. April;300(4):F1008–F1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pons H, Ferrebuz A, Quiroz Y, Romero-Vasquez F, Parra G, Johnson RJ and Rodriguez-Iturbe B. Immune reactivity to heat shock protein 70 expressed in the kidney is cause of salt-sensitive hypertension. Am J Physiol Renal Physiol. 2013. February 1;304(3):F289–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodríguez-Iturbe B, Pons H, Quiroz Y, Lanaspa MA and Johnson RJ. Autoimmunity in the pathogenesis of hypertension. Nat Rev Nephrol. 2014. January;10(1):56–62. [DOI] [PubMed] [Google Scholar]
- 15.Mattson DL. Infiltrating immune cells in the kidney in salt-sensitive hypertension and renal injury. Am J Physiol Renal Physiol. 2014. September 1;307(5):F499–F508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C and Harrison DG. Role of the T cell in the genesis of angiotensin II–induced hypertension and vascular dysfunction. J Exp Med. 2007. October 1;204(10):2449–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, Vinh A and Weyand CM. Inflammation, immunity, and hypertension. Hypertension. 2011. February;57(2):132–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mattson DL. Immune mechanisms of salt-sensitive hypertension and renal end-organ damage. Nat Rev Nephrol. 2019. May;15(5):290–300. [DOI] [PubMed] [Google Scholar]
- 19.Rudemiller N, Lund H, Jacob HJ, Geurts AM and Mattson DL. CD247 modulates blood pressure by altering T-lymphocyte infiltration in the kidney. Hypertension. 2014. March;63(3):559–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miguel CD, Guo C, Lund H, Feng D and Mattson DL. Infiltrating T lymphocytes in the kidney increase oxidative stress and participate in the development of hypertension and renal disease. Am J Physiol Renal Physiol. 2011. March;300(3):F734–F742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miguel CD, Das S, Lund H and Mattson DL. T lymphocytes mediate hypertension and kidney damage in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol. 2010. April;298(4):R1136–R1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alsheikh AJ, Lund H, Dasinger JH, Abais-Battad JM, Fehrenbach DJ and Mattson DL. Renal nerves and leukocyte infiltration in the kidney during salt-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol. 2019. July 1;317(1):R182–R189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bukowy JD, Dayton A, Cloutier D, Manis AD, Staruschenko A, Lombard JH, Woods LCS, Beard DA and Cowley AW. Region-based convolutional neural nets for localization of glomeruli in trichrome-stained whole kidney sections. J Am Soc Nephrol. 2018. August;29(8):2081–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor NE and Cowley AW Jr. Effect of renal medullary H2O2 on salt-induced hypertension and renal injury. Am J Physiol Regul Integr Comp Physiol. 2005. December;289(6):R1573–R1579. [DOI] [PubMed] [Google Scholar]
- 25.Hasan J, Shnyder S, Bibby M, Double J, Bicknel R and Jayson G. Quantitative angiogenesis assays in vivo–a review. Angiogenesis. 2004;7(1):1–16. [DOI] [PubMed] [Google Scholar]
- 26.Duijvestijn AM, van Goor H, Klatter F, Majoor GD, van Bussel E and van Breda Vriesman P. Antibodies defining rat endothelial cells: RECA-1, a pan-endothelial cell-specific monoclonal antibody. Lab Invest. 1992. April;66(4):459–466. [PubMed] [Google Scholar]
- 27.Polichnowski AJ, Griffin KA, Picken MM, Licea-Vargas H, Long J, Williamson GA and Bidani AK. Hemodynamic Basis for the Limited Renal Injury in Rats With Angiotensin II-induced Hypertension. Am J Physiol Renal Physiol. 2015. February 1;308(3):F252–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bidani AK, Griffin KA, Plott W and Schwartz MM. Genetic predisposition to hypertension and microvascular injury in the remnant kidney model. J Lab Clin Med. 1993. September;122(3):284–291. [PubMed] [Google Scholar]
- 29.Griffin KA, Abu-Amarah I, Picken M and Bidani AK. Renoprotection by ACE inhibition or aldosterone blockade is blood pressure–dependent. Hypertension. 2003. February;41(2):201–206. [DOI] [PubMed] [Google Scholar]
- 30.Johns DW, Peach MJ, Gomez RA, Inagami T and Carey RM. Angiotensin II regulates renin gene expression. Am J Physiol Renal Physiol. 1990. December;259(6 Pt 2):F882–887 [DOI] [PubMed] [Google Scholar]
- 31.Harrison-Bernard LM, El-Dahr SS, O’Leary DF and Navar LG. Regulation of angiotensin II type 1 receptor mRNA and protein in angiotensin II–induced hypertension. Hypertension. 1999. January;33(1 Pt 2):340–346. [DOI] [PubMed] [Google Scholar]
- 32.Neubauer B, Schrankl J, Steppan D, Neubauer K, Sequeira-Lopez ML, Pan L, Gomez RA, Coffman TM, Gross KW and Kurtz A. Angiotensin II short-loop feedback: is there a role of Ang II for the regulation of the renin system in vivo? Hypertension. 2018. June;71(6):1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonzalez AA, Green T, Luffman C, Bourgeois CR, Gabriel Navar L and Prieto MC. Renal medullary cyclooxygenase-2 and (pro) renin receptor expression during angiotensin II-dependent hypertension. Am J Physiol Renal Physiol. 2014. October 15;307(8):F962–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harris R Interactions between COX‐2 and the renin–angiotensin system in the kidney. Acta Physiol Scand. 2003. April;177(4):423–427 [DOI] [PubMed] [Google Scholar]
- 35.Sun IO, Santelli A, Abumoawad A, Eirin A, Ferguson CM, Woollard JR, Lerman A, Textor SC, Puranik AS and Lerman LO. Loss of renal peritubular capillaries in hypertensive patients is detectable by urinary endothelial microparticle levels. Hypertension. 2018. November;72(5):1180–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brenner BM, Lawler EV and Mackenzie HS. The hyperfiltration theory: a paradigm shift in nephrology. Kidney Int. 1996. June;49(6):1774–1777 [DOI] [PubMed] [Google Scholar]
- 37.Roman RJ and Cowley AW Jr. Characterization of a new model for the study of pressure-natriuresis in the rat. Am J Physiol. 1985. February;248(2 Pt 2):F190–F198. [DOI] [PubMed] [Google Scholar]
- 38.Johnson RJ, Gordon KL, Giachelli C, Kurth T, Skelton MM and Cowley AW Jr. Tubulointerstitial injury and loss of nitric oxide synthases parallel the development of hypertension in the Dahl-SS rat. J Hypertens. 2000. October;18(10):1497–1505. [DOI] [PubMed] [Google Scholar]
- 39.Lombardi D, Gordon KL, Polinsky P, Suga S, Schwartz SM and Johnson RJ. Salt-sensitive hypertension develops after short-term exposure to angiotensin II. Hypertension. 1999. April;33(4):1013–1019. [DOI] [PubMed] [Google Scholar]
- 40.Sorensen CM, Leyssac PP, Skott O and Holstein-Rathlou N-H. Role of the renin-angiotensin system in regulation and autoregulation of renal blood flow. Am J Physiol Regul Integr Comp Physiol. 2000. September;279(3):R1017–R1024. [DOI] [PubMed] [Google Scholar]
- 41.Cowley AW Jr. Role of the renal medulla in volume and arterial pressure regulation. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. Am J Physiol. 1997. July;273(1 Pt 2):R1–15. [DOI] [PubMed] [Google Scholar]
- 42.Cowley AW Jr, Mattson DL, Lu S and Roman RJ. The renal medulla and hypertension. Hypertension. 1995. April;25(4 Pt 2):663–73. [DOI] [PubMed] [Google Scholar]
- 43.Mattson DL, Lu S, Roman RJ and Cowley AW Jr. Relationship between renal perfusion pressure and blood flow in different regions of the kidney. Am J Physiol. 1993. March;264(3 Pt 2):R578–R583 [DOI] [PubMed] [Google Scholar]
- 44.Evans LC, Dayton A, Yang C, Liu P, Kurth T, Ahn KW, Komas S, Stingo FC, Laud PW and Vannucci M. Transcriptomic analysis reveals inflammatory and metabolic pathways that are regulated by renal perfusion pressure in the outer medulla of Dahl-S rats. Physiol Genomics. 2018. June 1;50(6):440–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iwazu Y, Muto S, Fujisawa G, Nakazawa E, Okada K, Ishibashi S and Kusano E. Spironolactone suppresses peritubular capillary loss and prevents deoxycorticosterone acetate/salt-induced tubulointerstitial fibrosis. Hypertension. 2008. March;51(3):749–754. [DOI] [PubMed] [Google Scholar]
- 46.Heffelfinger S The renin angiotensin system in the regulation of angiogenesis. Curr Pharm Des. 2007;13(12):1215–1229. [DOI] [PubMed] [Google Scholar]
- 47.Hu H, Han T, Zhuo M, Wu L-l, Yuan C, Wu L, Lei W, Jiao F and Wang L-W. Elevated COX-2 expression promotes angiogenesis through EGFR/p38-MAPK/Sp1-dependent signalling in pancreatic cancer. Sci Rep. 2017. March 28;7(1):470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vecchione C, Carnevale D, Di Pardo A, Gentile MT, Damato A, Cocozza G, Antenucci G, Mascio G, Bettarini U and Landolfi A. Pressure-induced vascular oxidative stress is mediated through activation of integrin-linked kinase 1/βPIX/Rac-1 pathway. Hypertension. 2009. November;54(5):1028–1034 [DOI] [PubMed] [Google Scholar]
- 49.Jin C, Hu C, Polichnowski A, Mori T, Skelton M, Ito S and Cowley AW Jr. Effects of renal perfusion pressure on renal medullary hydrogen peroxide and nitric oxide production. Hypertension. 2009. June;53(6):1048–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shimada S, Hirose T, Takahashi C, Sato E, Kinugasa S, Ohsaki Y, Kisu K, Sato H, Ito S and Mori T. Pathophysiological and molecular mechanisms involved in renal congestion in a novel rat model. Sci Rep. 2018. November 14;8(1):16808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kida Y and Duffield JS. Pivotal role of pericytes in kidney fibrosis. Clin Exp Pharmacol Physiol. 2011. July;38(7):467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kida Y, Tchao BN and Yamaguchi I. Peritubular capillary rarefaction: a new therapeutic target in chronic kidney disease. Pediatr Nephrol. 2014. March;29(3):333–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tran ED and Schmid-Schönbein GW. An in-vivo analysis of capillary stasis and endothelial apoptosis in a model of hypertension. Microcirculation. 2007. Nov-Dec;14(8):793–804. [DOI] [PubMed] [Google Scholar]
- 54.Sima AV, Stancu CS and Simionescu M. Vascular endothelium in atherosclerosis. Cell Tissue Res. 2009. January;335(1):191–203. [DOI] [PubMed] [Google Scholar]
- 55.Kreuger J and Phillipson M. Targeting vascular and leukocyte communication in angiogenesis, inflammation and fibrosis. Nat Rev Drug Discov. 2016. February;15(2):125–142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


