Abstract
Older patients with advanced hematologic malignancies are increasingly considered for allogeneic hematopoietic cell transplantation (allo-HCT) yet their survival outcomes remain suboptimal. We and others have previously shown that pre-HCT multi-morbidity and functional limitation and post-HCT geriatric syndromes significantly impact outcomes. Sarcopenia, an accelerated loss of muscle mass and function, has been increasingly recognized in older cancer patients. We identified 146 lymphoma patients 50 years or older who were allografted from 2008 to 2018 at our institution and found that before allo-HCT, 80 (55%) patients were sarcopenic. Pre-HCT sarcopenia was significantly associated with overall survival, progression-free survival, and non-relapse mortality independent of multi-morbidity and functional limitation. In 6-month landmark analysis, post-HCT sarcopenia remained significantly associated with survival. Our findings illustrate the high prevalence and profound impact of sarcopenia on survival. While requiring prospective confirmation, preemptive, longitudinal, and multidisciplinary interventions for sarcopenia are warranted to improve HCT outcomes for older patients.
Keywords: Sarcopenia, geriatric assessment, lymphoma, allogeneic hematopoietic cell transplantation
INTRODUCTION
Allogeneic hematopoietic cell transplantation (allo-HCT) has been increasingly utilized in older adults with hematologic malignancies due to advances in supportive care measures, the development of reduced-intensity conditioning (RIC) regimens, and the improved patient selection 1–4. Biological age, rather than the chronological age, is now routinely considered for allo-HCT eligibility 5,6. Despite these advances, the mortality and morbidity of allo-HCT has remained high in older patients, with long-term overall survival (OS) in the 30–40% range and 2-year non-relapse mortality (NRM) of 20–30% 7–9. These suboptimal outcomes suggest that there are additional, age-related aspects of allo-HCT that require investigation and optimization.
Geriatric syndromes are multifactorial clinical conditions that occur when accumulated effects of impairments in multiple organ systems render an older person vulnerable to situational challenges 10. While clinically heterogeneous, these conditions often have multiple risk factors, involve multiple organ systems, and can complicate therapy and contribute to the frailty phenotype 11,12. We have previously found that post-HCT delirium and falls, two common geriatric syndromes, significantly impacted long-term survival and had modifiable risk factors 13. Geriatric syndromes are ideally recognized through comprehensive geriatric assessment (GA), defined as a multidisciplinary process to assess medical, psychosocial, and functional limitations of an older adult 14. GA has emerged as an important risk stratification tool for survival in advanced hematologic malignancies, including allo-HCT 15–18.
Sarcopenia, loss of lean body muscle mass, is one of the most common geriatric syndromes and contributes to age-related functional decline and disability 19,20. Several methods, including direct measurement of muscle mass through imaging or function through beside assessment, have been utilized to define sarcopenia in different patient populations 20. Importantly, sarcopenia has been linked to treatment-related toxicities, healthcare resource utilization, and survival in multiple tumor types and transplant settings 21–26.
In patients with relapsed/refractory, aggressive or indolent lymphomas, RIC-based allo-HCT allows amplification of graft-versus-lymphoma effect and results in long-term disease control in a substantial portion of patients including those who are older 27,28. Allo-HCT has been increasingly utilized at many institutions including ours for relapsed/refractory lymphoma even in the era of chimeric antigen receptor-based therapy 29. Given that computerized tomography (CT) is a gold standard measure of body composition and sarcopenia 30,31, and that most lymphoma patients have staging scan prior to and following HCT, we examine the prevalence and impact of sarcopenia in older lymphoma allo-HCT patients.
MATERIAL AND METHODS
Patients, Geriatric Characteristics, and Transplant Care
This analysis included patients aged 50 years or older who underwent first allo-HCT for lymphoma using RIC-based regimen between 2008 and 2018 at our institution. We chose 50 years or older since this is the age when increased geriatric vulnerabilities are seen in patients undergoing transplant evaluation 15, and with available GA measures. A waiver of authorization for this retrospective review was obtained from the Institutional Review and Privacy Board. Pathologic diagnosis of lymphoma was confirmed at our institution. Pre-HCT assessment, transplant and post-transplant care, and disease monitoring followed standard institutional guidelines. Demographic data, disease and transplant characteristics, relapse/disease progression, cause of death, and survival were retrieved from our institutional database. Pre-transplant assessments of geriatric variables such as basic activities of daily living (Katz’s ADL), IADL (Lawton’s IADL), mood, nutrition, prior falls, weight loss (≥10 pounds in 3-month), and medication use were defined as previously 13. Potentially inappropriate medication (PIM) use was defined by the updated Beers Criteria 32. HCT-comorbidity index (HCT-CI) and revised disease risk index (DRI) were assigned according to published criteria 33,34.
Computerized Tomography (CT)-Based Identification of Sarcopenia
Cross-sectional images from a diagnostic CT or PET/CT images were assessed at 2-time points: baseline (up to 60-day before HCT) and within 180-day after HCT (closest to 100-day). Suboptimal images due to artefact were excluded. Evaluation of body composition was performed by two independent radiologists (LM and RN) using MIM software (supplemental method). Semiautomated threshold-based segmentation was used. Skeletal muscle and adipose tissue were defined by ranges of −29 to 150 HU and −190 to −30 HU, respectively. Radiologists were blinded to clinical information and independently identified lumbar vertebra and the following muscles; rectus abdominus, abdominal, psoas and paraspinal. Two adjacent axial images within the same series at the third lumbar vertebra were selected for the analysis of total muscle cross-sectional area (cm2) and of total fat (subcutaneous and visceral adipose tissues) cross-sectional area (cm2) and average of the results was calculated 30. Skeletal muscle cross-sectional area was normalized for stature and reported as skeletal muscle index in cm2/m2. Sarcopenia was defined as a skeletal muscle index <41 in women, <43 in men with BMI <25, and <53 in men with BMI ≥25 30. Total lean body mass (TLBM) and total body fat mass (TBFM) were calculated as TLBM (Kg)=0.3 × [skeletal muscle at L3(cm2)] +6.06 and TBFM (Kg)=0.042 × [fat tissue at L3(cm2)] +11.2 31.
Statistical Methods
Baseline characteristics in patients with and without pre-transplant sarcopenia were compared using Fisher’s exact tests for categorical variables and Wilcoxon rank sum tests for continuous. Kaplan-Meier survival curves and cumulative incidence function were generated to examine the survival and incidence of NRM with relapse-related death and relapse as competing risks. The log rank test was used to compare OS and PFS between groups. The Gray’s test was used to compare NRM between groups. Univariable Cox proportional hazards regression was used to analyze OS and PFS, and univariable Fine-Gray competing risks regression was used to analyze NRM. Multivariable models were built with all univariable covariates of p < 0.1. Wilcoxon signed rank tests were used to test for significant changes in anthropometric variables from pre-to-post-HCT time points and interobserver agreement was described using Cohen’s Kappa coefficient. The correlation between measurements was described using Kendall’s tau and graphed with Bland-Altman plots. All statistical computations were performed using SAS Software Version 9.4 (The SAS Institute, Cary, NC).
RESULTS
Patients Characteristics and Transplant Outcomes
Baseline characteristics of the cohort are listed in Table 1. The median age was 60.7 years (range, 50 −78.7 years). There was no significant difference in baseline HCT-CI, revised DRI, stem cell source, donor type, or CMV status except for slightly more females in the sarcopenic group (p=0.046). Ninety-seven percent of patients were Caucasian. Baseline geriatric deficits were common in domains of function, mobility, nutrition, mood, and medications and no significant difference was found among patients with and without sarcopenia (Table 1). With median follow-up of 4.12 years for survivors, the 5-year OS and PFS were 62% (95% Confidence Interval [CI] 53 – 71) and 56% (95% CI 47 – 65), respectively (Supplemental Figure A). The 3-year cumulative incidence of NRM and relapse/progression of disease were 21% (95% CI 14 – 28) and 18% (95% CI 12 – 24), respectively (Supplemental Figure B). Fifty-two patients died including 16 (31%) from relapse or disease progression; 25 (48%) from GVHD; 7 (13%) from organ failure; and 2 patients each (4%) from infection and others, respectively.
Table 1.
Baseline characteristics by pre-transplant sarcopenia status.
| All patients (n=146) | Normal (n=66) | Sarcopenic (n=80) | p-value | |
|---|---|---|---|---|
| Age (median, range) | 60.7 (50–78.7) | 58.9 (50–75.3) | 61.5 (50.2–78.7) | 0.09 |
| Sex (n, %) | 0.046 | |||
| Male | 102 (69.9) | 52 (78.8) | 50 (62.5) | |
| Female | 44 (30.1) | 14 (21.2) | 30 (37.5) | |
| Lymphoma (n, %) | 0.73 | |||
| Hodgkin's | 8 (5.5) | 3 (4.6) | 5 (6.3) | |
| Non-Hodgkin's | 138 (94.5) | 63 (95.5) | 75 (93.8) | |
| Disease risk index (n, %) | >0.99 | |||
| Low/intermediate | 142 (97.3) | 64 (97) | 78 (92.5) | |
| High/very high | 4 (2.7) | 2 (3) | 2 (2.5) | |
| HCT-CI (n, %) | >0.99 | |||
| 0–2 | 81 (55.5) | 37 (56.1) | 44 (55) | |
| ≥3 | 65 (44.5) | 29 (43.9) | 36 (45) | |
| Stem cell source | >0.99 | |||
| Peripheral blood | 136 (93.2) | 62 (93.9) | 74 (92.5) | |
| Bone marrow | 10 (6.8) | 4 (6.1) | 6 (7.5) | |
| Donor type | 0.84 | |||
| Matched | 118 (80.8) | 54 (81.8) | 64 (80) | |
| Alternative | 28 (19.2) | 12 (18.2) | 16 (20) | |
| Patient CMV | 0.07 | |||
| Positive | 66 (45.2) | 24 (36.4) | 42 (52.5) | |
| Negative | 80 (54.8) | 42 (63.6) | 38 (47.5) | |
| ADL/IADL | 0.54 | |||
| Impaired | 11 (7.5) | 5 (7.6) | 6 (7.5) | |
| Normal | 89 (61) | 37 (56.1) | 52 (65) | |
| Missing | 46 (31.5) | 24 (36.4) | 22 (27.5) | |
| PIM user | >0.99 | |||
| Yes | 70 (47.9) | 32 (48.5) | 38 (47.5) | |
| No | 76 (52.1) | 34 (51.5) | 42 (52.5) | |
| Depression | 0.66 | |||
| Yes | 24 (16.4) | 12 (18.2) | 12 (15) | |
| No | 122 (83.6) | 54 (81.8) | 68 (85) | |
| Prior fall | 0.66 | |||
| Yes | 24 (16.4) | 12 (18.2) | 12 (15) | |
| No | 122 (83.6) | 54 (81.8) | 68 (85) | |
| Weight loss | 0.09 | |||
| Yes | 14 (9.6) | 3 (4.5) | 11 (13.8) | |
| No | 132 (90.4) | 63 (95.5) | 69 (86.3) | |
Abbreviations: HCT-CI, Hematopoietic cell transplantation-comorbidity index; CMV, Cytomegalovirus; ADL, Activities of daily living; IADL, Instrumental activities of daily living; PIM, Potentially inappropriate medication.
Changes in Body Composition and Factors associated with Sarcopenia
Based on CT definition, 55% (80/146) and 70% (90/128) of patients were sarcopenic prior to and following transplant, respectively. Among those 90 sarcopenic patients post-HCT, 65 patients were also sarcopenic pre-HCT. As shown in Table 1, neither age nor pre-transplant patient- or disease-related factors were associated with sarcopenia except for the female gender (p = 0.046). Moreover, sarcopenia was not associated with geriatric deficits including functional limitation measured by ADL/IADL deficit or prior fall, depression, comorbidities (HCT-CI), or PIM. Of 128 patients who had both pre- and post-HCT imaging, the changes in body composition were significant. The median change in TLBM was −2.86 kilogram (range, −16.7 – 9.87, and p<0.001) and the median change in TBFM was −2.23 kilogram (range −14.2 – 5.67, and p<0.001).
Impact of Pre-HCT Sarcopenia
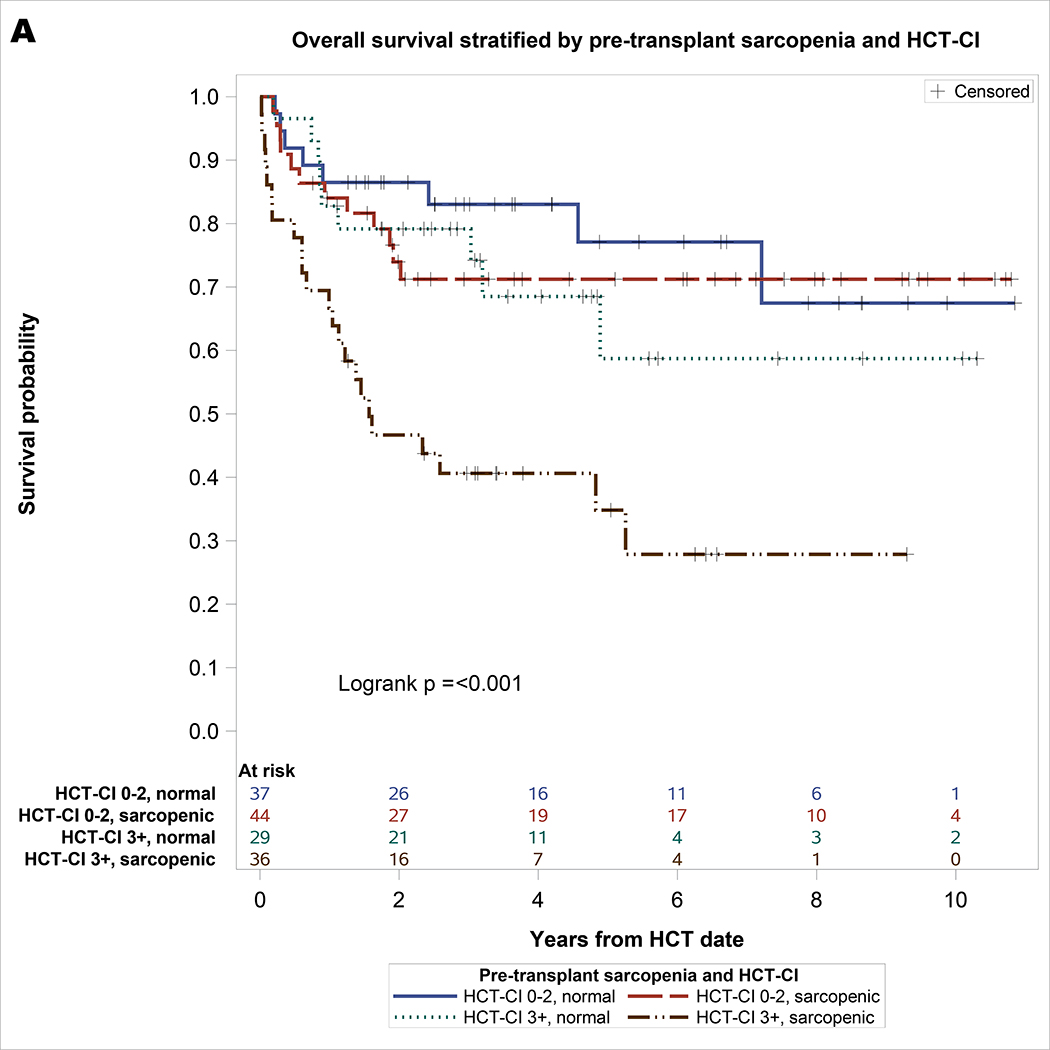
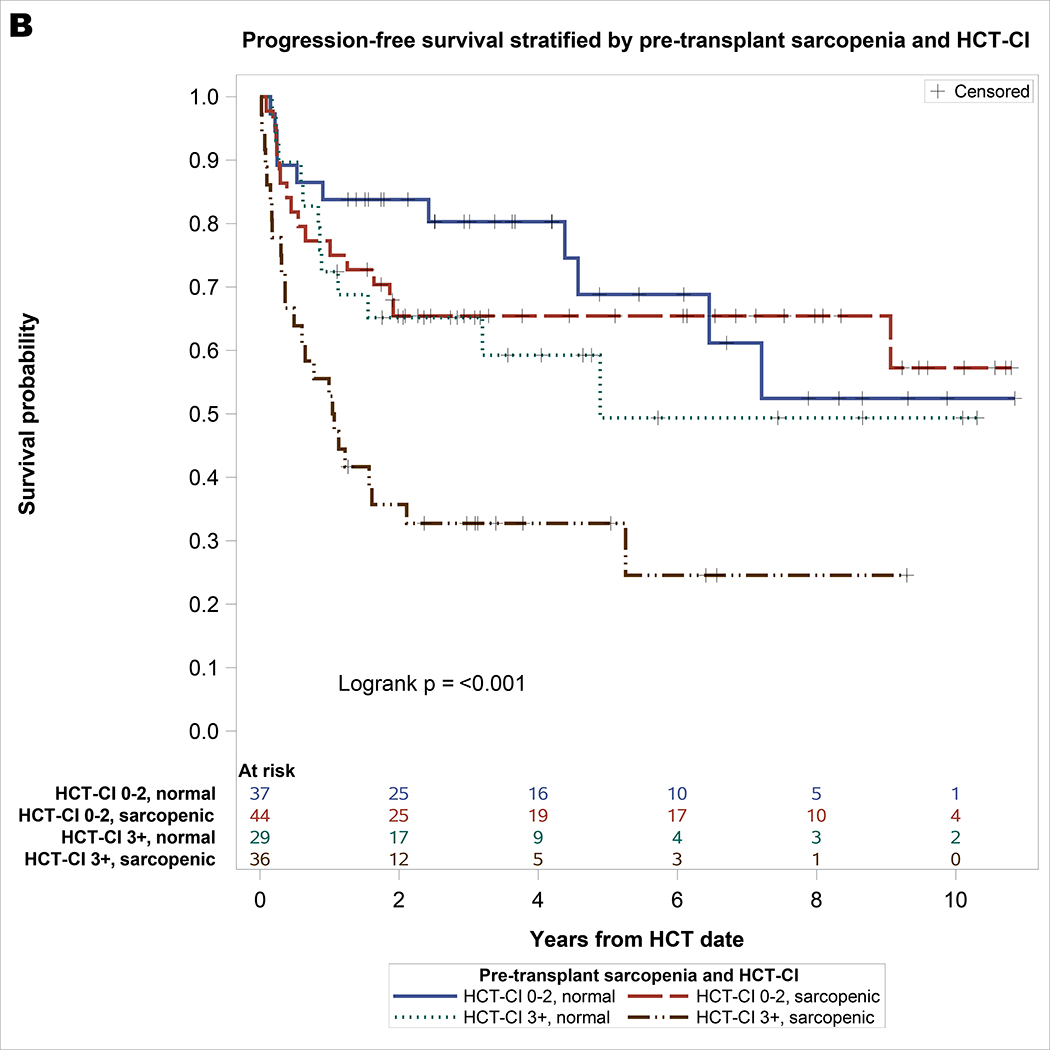
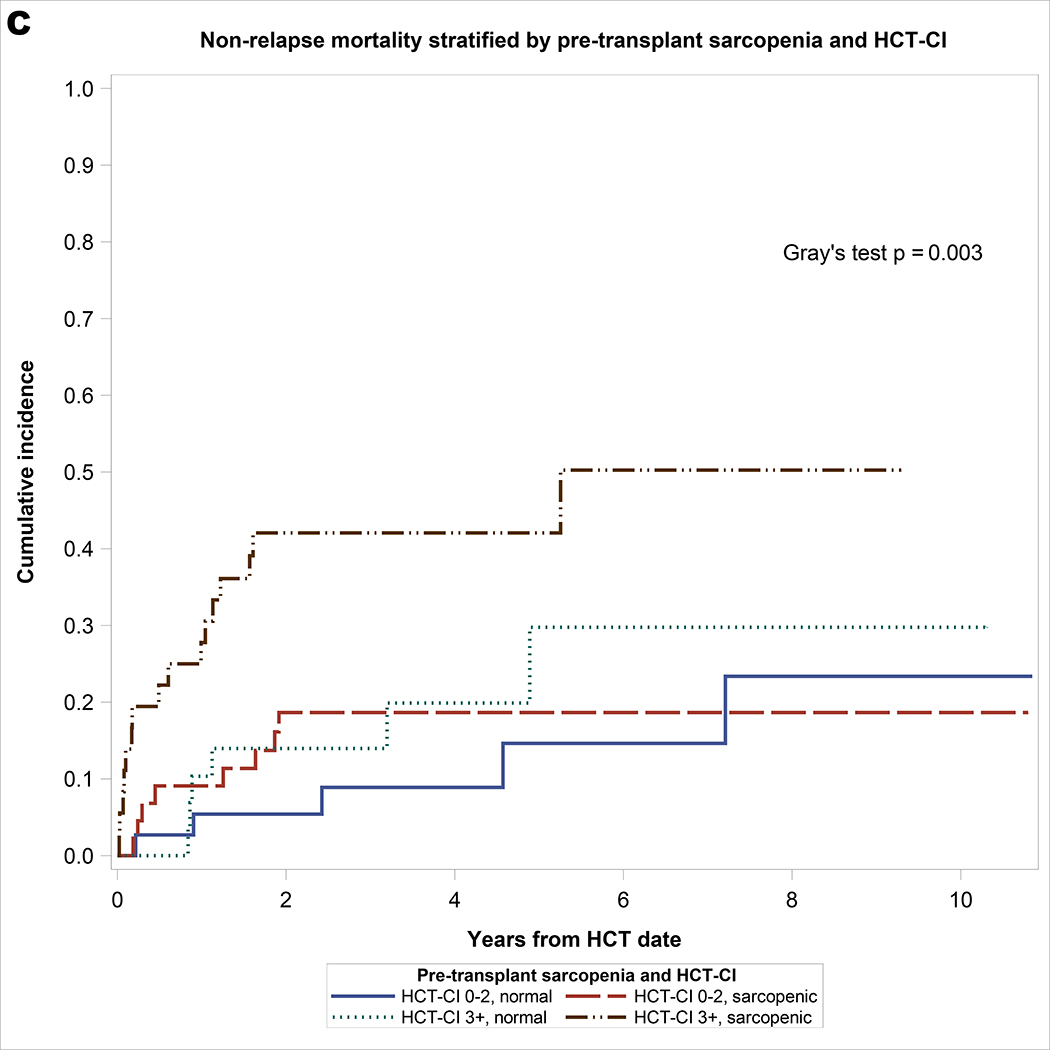
As shown in Table 2, significant univariable covariates associated with NRM included pre-transplant sarcopenia and HCT-CI. On multivariable analysis, both pre-transplant sarcopenia (hazard ratio [HR] = 2.06, 95% CI: 1.02–4.19, p = 0.044) and HCT-CI ≥3 (HR = 2.6, 95% CI: 1.31–5.16, p = 0.006) remained significantly associated with NRM. For univariable analysis of OS, significant covariates included sarcopenia and HCT-CI. On multivariable analysis, pre-transplant sarcopenia (HR = 2.12, 95% CI: 1.18–3.8, p = 0.01) and HCT-CI ≥3 (HR = 2.38, 95% CI: 1.35–4.19, p = 0.003) remained significantly associated with inferior OS. For univariable analysis of PFS, significant covariates included sarcopenia, HCT-CI, and prior fall. On multivariable analysis, pre-transplant sarcopenia (HR = 1.78, 95% CI: 1.07–2.98, p = 0.03), HCT-CI ≥3 (HR = 2.21, 95% CI: 1.33–3.67, p = 0.002), and prior fall (HR = 1.92, 95% CI: 1.07–3.46, p = 0.03) remained significantly associated with inferior PFS. As shown in Figure 1, the combination of sarcopenia and HCT-CI effectively stratify OS (log rank p <0.001), PFS (log rank p <0.001), and NRM (Gray’s p = 0.003). Patients with pre-HCT sarcopenia and HCT-CI ≥3 had the worst survival and highest incidence of NRM: 3-year OS of 41% (95% CI 24 – 57), PFS of 33% (95% CI 17 – 48), and NRM of 42% (95% CI 25 – 59), respectively.
Table 2.
Univariable and multivariable analyses of pre-transplant factors associated with OS, PFS, and NRM.
| Univariable Analysis |
Multivariable Analysis |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OS | PFS | NRM | OS | PFS | NRM | |||||||
| HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Sarcopenia | 0.03 | 0.05 | 0.06 | 0.01 | 0.03 | 0.044 | ||||||
| No | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||
| Yes | 1.93 (1.08–3.45) | 1.67 (1.00–2.79) | 1.97 (0.98–3.97) | 2.12 (1.18–3.80) | 1.78 (1.07–2.98) | 2.06 (1.02–4.19) | ||||||
| HCT-CI | 0.003 | 0.002 | 0.008 | 0.003 | 0.002 | 0.006 | ||||||
| 0–2 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||
| ≥3 | 2.38 (1.35–4.17) | 2.22 (1.35–3.67) | 2.51 (1.28–4.94) | 2.38 (1.35–4.19) | 2.21 (1.33–3.67) | 2.60 (1.31–5.16) | ||||||
| Prior fall | 0.10 | 0.02 | 0.23 | 0.12 | 0.03 | - | - | |||||
| No | Ref. | Ref. | Ref. | Ref. | Ref. | |||||||
| Yes | 1.72 (0.90–3.29) | 2.04 (1.14–3.65) | 1.61 (0.74–3.49) | 1.69 (0.88–3.25) | 1.92 (1.07–3.46) | |||||||
Abbreviations: OS, Overall survival; PFS, Progression free survival; NRM, Non-relapse mortality; HR, Hazard ratio; CI, Confidence interval; HCT-CI, Hematopoietic cell transplantation-comorbidity index; Ref., Reference.
Figure 1.
A. Overall survival stratified by pre-transplant sarcopenia and HCT-CI with at-risk table. B. Progression-free survival stratified by pre-transplant sarcopenia and HCT-CI with at-risk table. C. Non-relapse mortality stratified by pre-transplant sarcopenia and HCT-CI.
Impact of Post-HCT Sarcopenia
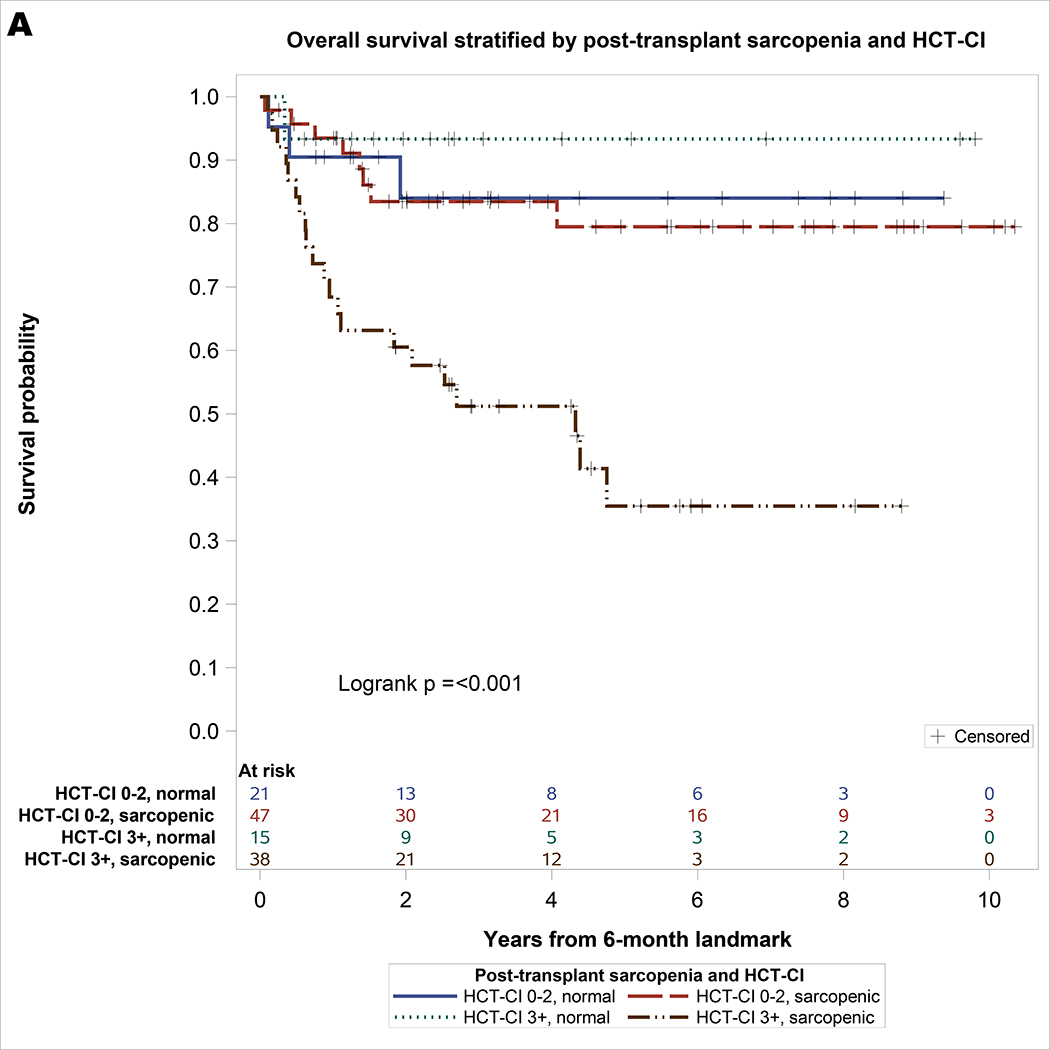
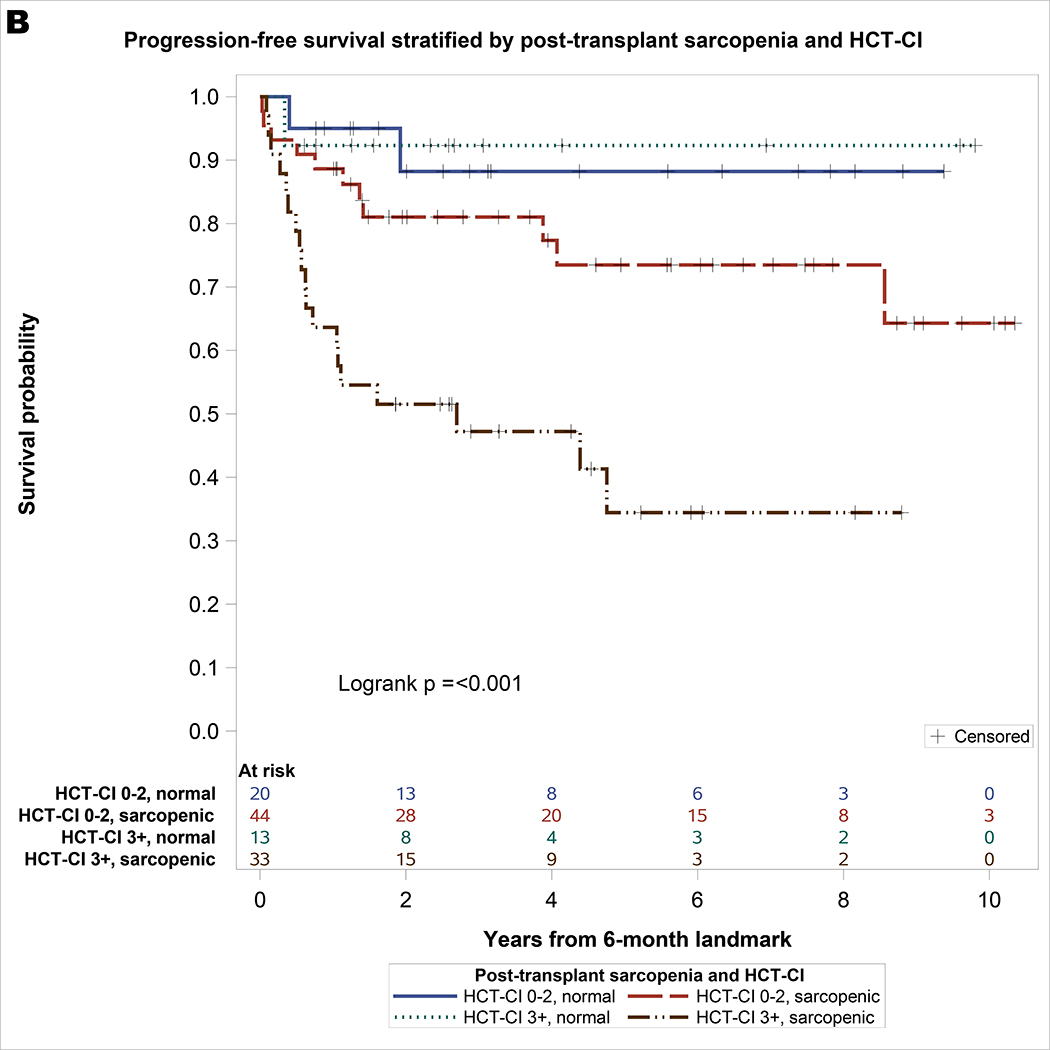
One hundred twenty-one patients survived for at least 6-month post-HCT and were included in the landmark analysis of OS. Of these, one hundred ten patients survived or relapsed beyond 6-month post-HCT and were included in the landmark analysis of PFS and NRM. As shown in Table 3, the only significant covariate for NRM was HCT-CI. For univariate analysis of OS, significant covariates included sarcopenia and HCT-CI. On multivariable analysis, post-HCT sarcopenia (HR = 3.12, 95% CI: 1.1–8.89, p = 0.03) and HCT-CI ≥3 (HR = 2.94, 95% CI: 1.42–6.07, p = 0.004) remained significantly associated with inferior OS. For univariable analysis of PFS, significant covariates included post-HCT sarcopenia, HCT-CI, grade 2–4 aGVHD, and age. On multivariable analysis, post-HCT sarcopenia (HR = 4.2, 95% CI: 1.27–13.9, p = 0.02) and HCT-CI ≥3 (HR = 2.65, 95% CI: 1.28–5.49, p = 0.009) remained significantly associated with inferior PFS. As shown in Figure 2, the combination of post-HCT sarcopenia and HCT-CI effectively stratify OS (log rank p <0.001) and PFS (log rank p <0.001). Patients with post-HCT sarcopenia and HCT-CI ≥3 had the lowest survival: 3-year OS of 51% (95% CI 35 – 68) and PFS of 47% (95% CI 30 – 65), respectively.
Table 3.
Univariable and multivariable 6-month landmark analyses of factors associated with OS, PFS, and NRM.
| Univariable Analysis |
Multivariable Analysis |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OS (n=121) | PFS (n=110) | NRM (n=110) | OS (n=121) | PFS (n=110) | NRM (n=110) | |||||||
| HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Sarcopenia | 0.03 | 0.01 | 0.19 | 0.03 | 0.02 | - | - | |||||
| No | Ref. | Ref. | Ref. | Ref. | Ref. | |||||||
| Yes | 3.12 (1.10–8.86) | 4.57 (1.40–15.0) | 2.27 (0.66–7.78) | 3.12 (1.10–8.89) | 4.20 (1.27–13.9) | |||||||
| HCT-CI | 0.004 | 0.006 | 0.002 | 0.004 | 0.009 | 0.002 | ||||||
| 0–2 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||
| ≥3 | 2.93 (1.42–6.05) | 2.68 (1.33–5.40) | 3.04 (1.22–7.55) | 2.94 (1.42–6.07) | 2.65 (1.28–5.49) | 3.04 (1.22–7.55) | ||||||
| aGVHD | 0.14 | 0.03 | 0.37 | - | - | 0.11 | - | - | ||||
| Grade 0–1 | Ref. | Ref. | Ref. | Ref. | ||||||||
| Grade 2–4 | 1.69 (0.84–3.37) | 2.13 (1.07–4.24) | 1.50 (0.62–3.63) | 1.76 (0.88–3.52) | ||||||||
| Age (per 10 years) | 1.04 (0.99–1.09) | 0.17 | 1.04 (0.99–1.09) | 0.10 | 1.03 (0.97–1.10) | 0.36 | - | - | 1.01 (0.96–1.06) | 0.75 | - | - |
Abbreviations: OS, Overall survival; PFS, Progression free survival; NRM, Non-relapse mortality; HR, Hazard ratio; CI, Confidence interval; HCT-CI, Hematopoietic cell transplantation-comorbidity index; Ref., Reference; aGVHD, Acute graft-versus-host disease.
Figure 2.
A. Landmark Analysis of overall survival stratified by post-transplant sarcopenia and HCT-CI with at-risk table. B. Progression-free survival stratified by post-transplant sarcopenia and HCT-CI with at-risk table.
DISCUSSION
In this study, we examined the incidence of sarcopenia prior to and following RIC allo-HCT for lymphoma and demonstrated its negative impact on allo-HCT outcomes independent of other geriatric vulnerabilities such as multi-morbidity or functional limitation. We found that the incidence of pre- and post-HCT sarcopenia defined radiographically was 55% and 70%, respectively, which is higher than what has been previously reported 24–26. This is likely due to the older age of our cohort given the increasing incidence of sarcopenia in older adults 19,35. Moreover, we did not identify an association of pre-HCT sarcopenia with any geriatric deficits or any disease-related factors except for gender, suggesting that sarcopenia is not a simple surrogate of disease burden, comorbidity, functional limitation, or nutritional status. This is consistent with previous findings in several geriatric oncology patient populations 23,35. Finally, our method of sarcopenia detection is well-validated based on published literature 30, 36–38.
Sarcopenia has profound negative impacts on the function, survival, and quality of life of general medical, surgical, and cancer patients 19,25,35,39. We have demonstrated here that both pre- and post-HCT sarcopenia were associated with significantly increased NRM and worsened PFS and OS. Interestingly, two other significant variables, HCT-CI and prior fall, are surrogates of geriatric comorbidity and functional limitation, respectively 14,33. We acknowledge that the mechanism by which sarcopenia directly contributes to mortality remains unclear and it is not related to body mass index (data not shown). It is possible that sarcopenia is the second “hit” that acts synergistically with multi-morbidity and functional limitation to amplify frailty and contribute to poor survival, consistent with the Rockwood deficit accumulation model 40. Our results yield significant insights on geriatric factors contributing to inferior outcomes; namely, pre-HCT geriatric comorbidities, functional status, sarcopenia, as well as the development of sarcopenia post-HCT. There are likely other potential post-transplant contributors to OS and NRM in older patients including organ toxicities, infections, and graft-versus-host disease, and we are actively examining these factors in relation to comorbidities and geriatric syndromes.
Our study has several limitations. First, given its retrospective design and the timing of imaging, the causal relationship of sarcopenia with induction/salvage chemotherapy, aGVHD, and steroid use cannot be reliably established. Second, we lack grading and functional assessment of sarcopenia and its impact on quality of life. Third, the heterogeneity in donors, GVHD prophylaxis, and post-transplant complications including acute and chronic GVHD may confound our findings. Finally, this is a single institutional study with a predominantly Caucasian population which may not be applicable to other ethnic populations or institutional settings. We expect that the upcoming Blood and Marrow Transplant Clinical Trials Network (BMT CTN 1704) CHARM study will help address many of these issues (NCT03992352).
Nevertheless, our results have important implications for the selection of older patients for potential curative allo-HCT, and for management interventions in patients with sarcopenia. It is conceivable that the true impact of sarcopenia, multi-comorbidity, and functional limitation on transplant outcomes should be evaluated by a prospectively designed, GA-adapted, interventional trial, where non-transplant options are chosen for overly frail patients with combined deficits as demonstrated here, or to develop management strategies based on GA findings to improve outcomes. Potential GA-directed, multidisciplinary intervention on sarcopenia could include intensified rehabilitation, medication management, and nutritional support 14. Such interventions have been shown to be feasible in small pilot trials 41,42. In conclusion, our results add to the growing literature on the impact of geriatric vulnerabilities on HCT outcomes and provide an entry point for prospective, sarcopenia-directed, interventional trials for older allo-HCT patients to improve their outcomes and quality of life.
Supplementary Material
Supplemental Figure. A. Overall and progression-free survival for all patients with at-risk table. B. Non-relapse mortality and relapse/progression of disease for all patients.
ACKNOWLEDGMENTS
This research was supported in part by NIH/NCI Program Project Grant P01 CA023766 and Cancer Center Support Grant P30 CA008748 to Memorial Sloan Kettering Cancer Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. RJL acknowledges support from the Elsa U. Pardee Foundation for Cancer Research, New York State Empire Clinical Research Investigator Program, Parker Institute for Cancer Immunotherapy at MSK, and the Dresner MDS Foundation. This work is presented in part at the 61st American Society of Hematology Annual Meeting & Exposition, December 6–9, 2019, Orlando, FL.
DECLARATION OF INTEREST
SAG—Advisory Board for Amgen, Actinuum, Celgene, Johnson & Johnson, Jazz pharmaceutical, Takeda, Novartis, Kite, Spectrum Pharma; Research funding from Amgen, Actinuum, Celgene, Johnson & Johnson, Miltenyi, Takeda.
GLS—Research funding from Janssen and Amgen.
MS—Consultancy and research funding: Angiocrine Bioscience, Inc.; Consultancy: McKinsey & Company; Consultancy on Advisory Boards for: Omeros Corporation and Kite Pharma.
PAH—Research support and consulting fees from Portola Pharmaceuticals, Inc. Consultancy on advisory boards for: Astra-Zeneca, Celgene, Karyopharm.
CSS—Consultant on advisory boards for: Juno Therapeutics, Sanofi Genzyme, Spectrum Pharmaceuticals, Novartis, Precision Biosciences, Kite, a Gilead Company and GSK. Research funds for investigator-initiated trials from: Juno Therapeutics and Sanofi-Genzyme.
MAP—Honoraria from Abbvie, Bellicum, Bristol-Myers Squibb, Incyte, Merck, Novartis, Nektar Therapeutics, and Takeda. He serves on DSMBs for Servier and Medigene, and the scientific advisory boards of MolMed and NexImmune. Research support for clinical trials from Incyte, Kite (Gilead) and Miltenyi Biotec.
IP – has received research support from Merck and serves on a Data and Safety Monitoring Board (DSMB) for ExCellThera.
REFERENCES
- 1.Giralt SA. Hematopoietic cell transplantation for older adults. In: Handbook of Geriatric Oncology - Practical Guide to Caring for the Older Cancer Patient. Demos Medical, 2019, pp 241–254. [Google Scholar]
- 2.D’Souza A Fretham C Current Uses and Outcomes of Hematopoietic Cell Transplantation (HCT): CIBMTR Summary Slides, 2018. CIBMTR; http://www.cibmtr.org. [Google Scholar]
- 3.Hahn T, Jr PL, Hassebroek A et al. Significant Improvement in Survival After Allogeneic Hematopoietic Cell Transplantation During a Period of Significantly Increased Use, Older Recipient Age, and Use of Unrelated Donors. Journal of Clinical Oncology 2013; 31: 2437–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Majhail NS, Chitphakdithai P, Logan B et al. Significant Improvement in Survival after Unrelated Donor Hematopoietic Cell Transplantation in the Recent Era. Biology of Blood and Marrow Transplantation 2015; 21: 142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Artz A Biologic vs physiologic age in the transplant candidate. Hematology 2016; 2016: 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elsawy M, Sorror M. Up-to-date tools for risk assessment before allogeneic hematopoietic cell transplantation. Bone marrow transplantation 2016; 51: 1283–1300. [DOI] [PubMed] [Google Scholar]
- 7.McClune BL, Weisdorf DJ, Pedersen TL et al. Effect of Age on Outcome of Reduced-Intensity Hematopoietic Cell Transplantation for Older Patients with Acute Myeloid Leukemia in First Complete Remission or With Myelodysplastic Syndrome. Journal of Clinical Oncology 2010; 28: 1878–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sorror ML, Brendmaier B, Storer BE et al. Long-term Outcomes Among Older Patients Following Nonmyeloablative Conditioning and Allogeneic Hematopoietic Cell Transplantation for Advanced Hematologic Malignancies. JAMA 2011; 306: 1874–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rashidi A, Ebadi M, Colditz GA, DiPersio JF. Outcomes of Allogeneic Stem Cell Transplantation in Elderly Patients with Acute Myeloid Leukemia: A Systematic Review and Meta-analysis. Biology of Blood and Marrow Transplantation 2016; 22: 651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric Syndromes: Clinical, Research, and Policy Implications of a Core Geriatric Concept. Journal of the American Geriatrics Society 2007; 55: 780–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kane RL, Shamliyan T, Talley K, Pacala J. The Association Between Geriatric Syndromes and Survival. Journal of the American Geriatrics Society 2012; 60: 896–904. [DOI] [PubMed] [Google Scholar]
- 12.Abel GA, Klepin HD. Frailty and the management of hematologic malignancies. Blood 2018; 131: 515–524. [DOI] [PubMed] [Google Scholar]
- 13.Lin RJ, Hilden PD, Elko TA et al. Burden and impact of multifactorial geriatric syndromes in allogeneic hematopoietic cell transplantation for older adults. Blood Advances 2019; 3: 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohile SG, Dale W, Somerfield MR et al. Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Chemotherapy: ASCO Guideline for Geriatric Oncology. Journal of Clinical Oncology 2018; 36: JCO.2018.78.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muffly LS, Kocherginsky M, Stock W et al. Geriatric assessment to predict survival in older allogeneic hematopoietic cell transplantation recipients. Haematologica 2014; 99: 1373–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deschler B, Ihorst G, Schnitzler S, Bertz H, Finke J. Geriatric assessment and quality of life in older patients considered for allogeneic hematopoietic cell transplantation: a prospective risk factor and serial assessment analysis. Bone marrow transplantation 2018; 53: 565–575. [DOI] [PubMed] [Google Scholar]
- 17.Lin RJ, Elko TA, Devlin S et al. Impact of geriatric vulnerabilities on allogeneic hematopoietic cell transplantation outcomes in older patients with hematologic malignancies. Bone Marrow Transplantation 2019; 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang L-W, Sheng Y, Andreadis C et al. Functional status as measured by geriatric assessment predicts inferior survival in older allogeneic hematopoietic cell transplant recipients. Biol Blood Marrow Transplantation 2019. doi: 10.1016/j.bbmt.2019.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet 2019; 393: 2636–2646. [DOI] [PubMed] [Google Scholar]
- 20.Cruz-Jentoft AJ, Bahat G, Bauer J et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019. doi: 10.1093/ageing/afz046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang M, Shen Y, Tan L, Li W. Prognostic value of sarcopenia in lung cancer: A systematic review and meta-analysis. Chest 2019; 156: 101–111. [DOI] [PubMed] [Google Scholar]
- 22.Caan BJ, Feliciano EM, Prado CM et al. Association of Muscle and Adiposity Measured by Computed Tomography with Survival in Patients with Nonmetastatic Breast Cancer. Jama Oncol 2018; 4: 798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shachar S, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur J Cancer 2016; 57: 58–67. [DOI] [PubMed] [Google Scholar]
- 24.Caram MV, Bellile EL, Englesbe MJ et al. Sarcopenia is associated with autologous transplant-related outcomes in patients with lymphoma. Leukemia Lymphoma 2015; 56: 2855–2862. [DOI] [PubMed] [Google Scholar]
- 25.Armenian SH, Xiao M, Teh J et al. Impact of Sarcopenia on Adverse Outcomes After Allogeneic Hematopoietic Cell Transplantation. J Natl Cancer I 2019. doi: 10.1093/jnci/djy231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeFilipp Z, Troschel FM, Qualls DA et al. Evolution of Body Composition Following Autologous and Allogeneic Hematopoietic Cell Transplantation: Incidence of Sarcopenia and Association with Clinical Outcomes. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation 2018. doi: 10.1016/j.bbmt.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 27.Fenske TS, Hamadani M, Cohen JB et al. Allogeneic Hematopoietic Cell Transplantation as Curative Therapy for Patients with Non-Hodgkin Lymphoma: Increasingly Successful Application to Older Patients. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation 2016; 22: 1543–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah NN, Ahn KW, Litovich C et al. Outcomes of Medicare-age eligible NHL patients receiving RIC allogeneic transplantation: a CIBMTR analysis. Blood advances 2018; 2: 933–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunter BD, Chen Y-B, Jacobson CA. Allogeneic Stem Cell Transplantation and Chimeric Antigen Receptor (CAR) T-Cell Therapy for the Treatment of Non-Hodgkin Lymphoma. Hematology/Oncology Clinics of North America 2019; 33: 687–705. [DOI] [PubMed] [Google Scholar]
- 30.Martin L, Birdsell L, MacDonald N et al. Cancer Cachexia in the Age of Obesity: Skeletal Muscle Depletion Is a Powerful Prognostic Factor, Independent of Body Mass Index. J Clin Oncol 2013; 31: 1539–1547. [DOI] [PubMed] [Google Scholar]
- 31.Mourtzakis M, Prado C, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiology Nutrition Metabolism 2008; 33: 997–1006. [DOI] [PubMed] [Google Scholar]
- 32.the Panel B American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. Journal of the American Geriatrics Society 2015; 63: 2227–46. [DOI] [PubMed] [Google Scholar]
- 33.Sorror ML. How I assess comorbidities before hematopoietic cell transplantation. Blood 2013; 121: 2854–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Armand P, Kim HT, Logan BR et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood 2014; 123: 3664–3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Otten L, Stobäus N, Franz K et al. Impact of sarcopenia on 1-year mortality in older patients with cancer. Age Ageing 2019; 48: 413–418. [DOI] [PubMed] [Google Scholar]
- 36.Fearon K, Strasser F, Anker SD et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011; 12: 489–495. [DOI] [PubMed] [Google Scholar]
- 37.van Vugt J, Levolger S, Gharbharan A et al. A comparative study of software programmes for cross‐sectional skeletal muscle and adipose tissue measurements on abdominal computed tomography scans of rectal cancer patients. J Cachexia Sarcopenia Muscle 2017; 8: 285–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perthen JE, Ali T, McCulloch D et al. Intra- and Interobserver Variability in Skeletal Muscle Measurements using Computed Tomography Images. Eur J Radiol 2018; 109: 142–146. [DOI] [PubMed] [Google Scholar]
- 39.Ryan AM, Prado CM, Sullivan ES, Power DG, Daly LE. Impact of weight loss and sarcopenia on response to chemotherapy, quality of life and survival. Nutrition 2019; 67–68: 110539. [DOI] [PubMed] [Google Scholar]
- 40.Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. SciencetificWorldJournal 2001, 1: 323–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanaka S, Imataki O, Kitaoka A et al. Clinical impact of sarcopenia and relevance of nutritional intake in patients before and after allogeneic hematopoietic stem cell transplantation. J Cancer Res Clin 2017; 143: 1083–1092. [DOI] [PubMed] [Google Scholar]
- 42.Skaarud K, Veierød M, Lergenmuller S, Bye A, Iversen P, Tjønnfjord G. Body weight, body composition and survival after 1 year: follow-up of a nutritional intervention trial in allo-HSCT recipients. Bone Marrow Transpl 2019; Aug 27: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure. A. Overall and progression-free survival for all patients with at-risk table. B. Non-relapse mortality and relapse/progression of disease for all patients.